Abstract
Hepatitis A virus (HAV) is an enterically transmitted virus that replicates predominantly in hepatocytes within the liver before excretion via bile through feces. Hepatocytes are polarized epithelial cells, and it has been assumed that the virus load in bile results from direct export of HAV via the apical domain of polarized hepatocytes. We have developed a subclone of hepatocyte-derived HepG2 cells (clone N6) that maintains functional characteristics of polarized hepatocytes but displays morphology typical of columnar epithelial cells, rather than the complex morphology that is typical of hepatocytes. N6 cells form microcolonies of polarized cells when grown on glass and confluent monolayers of polarized cells on semipermeable membranes. When N6 microcolonies were exposed to HAV, infection was restricted to peripheral cells of polarized colonies, whereas all cells could be infected in colonies of nonpolarized HepG2 cells (clone C11) or following disruption of tight junctions in N6 colonies with EGTA. This suggests that viral entry occurs predominantly via the basolateral plasma membrane, consistent with uptake of virus from the bloodstream after enteric exposure, as expected. Viral export was also found to be markedly vectorial in N6 but not C11 cells. However, rather than being exported from the apical domain as expected, more than 95% of HAV was exported via the basolateral domain of N6 cells, suggesting that virus is first excreted from infected hepatocytes into the bloodstream rather than to the biliary tree. Enteric excretion of HAV may therefore rely on reuptake and transcytosis of progeny HAV across hepatocytes into the bile. These studies provide the first example of the interactions between viruses and polarized hepatocytes.
Hepatitis A virus (HAV) is an enterically transmitted Picornavirus that is endemic in developing countries, and in many developed countries it remains a significant cause of sporadic hepatitis associated with poor hygiene and food-borne outbreaks. Infection results in life-long immunity, and several highly effective vaccines based on inactivated virus are available, the use of which has dramatically reduced the incidence of hepatitis A, largely in developed countries such as the United States (15, 18, 72, 78). Although HAV has been shown to infect enterocyte-derived cells in culture (4, 59), very little or no virus is detectable in the gastrointestinal cells of experimentally infected animals (1, 34). Viral replication predominantly occurs in hepatocytes: the likely source of both the significant acute-phase viremia and the high titers of virus found in feces which are responsible for transmission to new hosts. Large amounts of HAV are detectable in the stool for some weeks after infection, prior to significant hepatocyte death, suggesting the virus is actively secreted from the liver.
Cell culture-adapted strains, infectious cDNA clones of HAV, and a variety of primate models have revealed many details of the replication cycle of the virus (12, 21, 43, 49), but relatively little is known regarding the interactions between HAV and hepatocytes in vivo. Notably, both gastrointestinal epithelial cells and hepatocytes are polarized (Fig. 1). In the case of simple columnar cells such as respiratory and enteric epithelia (Fig. 1B), it is recognized that cell polarity plays a major role in viral pathogenesis (8, 10, 13, 65, 71). Blank et al. have shown that HAV both infects and is secreted from polarized enterocyte-derived Caco-2 cells via the apical domain (adjoining the gastrointestinal lumen) (4). Thus, in order to reach the liver, progeny virus may enter the bloodstream through other mechanisms, such as transcytosis or via intestinal M-cells, as has been shown for poliovirus and reovirus (2, 4, 5, 30, 37, 41, 75).
FIG. 1.
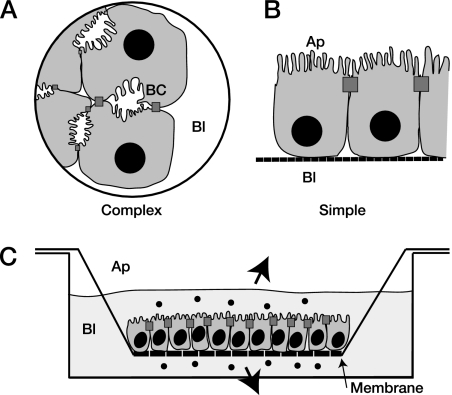
Epithelial cell polarity. A. Hepatocytes in vivo and in vitro develop complex polarity, such that adjacent apical (Ap) surfaces will form bile canaliculae (BC). B. Other polarized epithelial cells grow with a simple (columnar) orientation of polarity. C. The apical and basolateral domains are both accessible when cells, such as those in panel B, are grown as a confluent monolayer on semipermeable membranes, with tight junctions between cells preventing lateral diffusion of substrates.
Hepatocytes are also polarized epithelial cells, with distinct apical and basolateral domains facing the bile canaliculi and the hepatic sinusoid, respectively (Fig. 1A) (74). Tight junctions between hepatocytes prevent the lateral diffusion of substrates and form a barrier between blood and bile. The apical plasma membrane has a high concentration of lipids and is rich in glycosylphosphatidylinositol-related proteins and glycosphingolipids (31, 40, 76). Intracellular microtubules form the cytoskeleton along which the majority of substrates are transported (16). Interactions of hepatotropic viruses with these cells have been difficult to study due to the complex orientation and morphology of hepatocyte polarity (Fig. 1A). Studies of virus interactions with polarized cells commonly use cell monolayers grown on semipermeable membranes, providing separate access to both the apical and basolateral domains of the polarized cells (Fig. 1C), but hepatocytes do not share the columnar morphology of simple epithelial cells. The apical surfaces of adjacent hepatocytes form bile canaliculi in vivo and bile cysts or ductules in culture (Fig. 1A) and are thus not readily accessible for exposure to, or sampling of, viruses. Also, primary hepatocytes and immortalized hepatic cell lines are poorly and variably polarized in culture and do not form confluent monolayers when grown on membranes.
Despite this, the polarized trafficking of endogenous substrates and proteins in hepatocytes has been studied in detail using a variety of models, including hepatocyte couplets sandwiched in a matrix (24), the hybrid rat hepatocyte-human fibroblast WIF-B cell line (27, 51), and human hepatocyte-derived HepG2 cells. Studies of such cells have revealed significant differences in protein trafficking between hepatocytes and other polarized epithelia. In particular, hepatocytes are largely deficient in the machinery to traffic endogenously synthesized proteins directly from the endoplasmic reticulum to the apical domain of the cell for release, with the vast majority of proteins (such as albumin) being trafficked to the basolateral domain for release into the bloodstream, while proteins destined for the apical domain must then traverse the cell via the process of transcytosis (reviewed by Mostov et al. [38]).
In working with HepG2 cells maintained in our laboratory, we observed by immunofluorescence staining occasional foci that exhibited a honeycomb pattern of distribution of the tight junction protein zonula occludens 1 (ZO-1), typical of columnar polarized epithelia. We therefore isolated clonal cell populations from HepG2 and from HepG2-derived C3A cells, producing a well-polarized clone (N6) that displays the morphology of polarized columnar epithelia and has the ability to form confluent monolayers on semipermeable membranes, together with a nonpolarized clone (C11) to serve as a control. In preliminary experiments, the N6 cells were shown to export albumin preferentially into the basolateral domain, while C11 cells export albumin equally to both apical and basolateral domains (57). These cells appeared promising for studies of virus-polarized hepatocyte interactions.
It has been shown that enterocyte-derived Caco-2 cells support HAV replication, with almost exclusive export of progeny virus into the apical domain (4). This direction of secretion in hepatocytes would result in the direct release of virus into the bile and gastrointestinal tract, as might be expected for enteric excretion (4). Indeed, there is good evidence in many primate studies showing HAV antigens present in the bile of infected animals with acute hepatitis A (26, 29, 34, 34, 42, 48). However, in liver cells, direct apical transportation from the Golgi complex runs counter to the direction of export of most de novo-synthesized substrates. Additionally, Caco-2 cells also are most efficiently infected from the apical (lumenal) surface, while hepatocytes are more likely to be exposed to HAV from the basolateral (sinusoidal) surface through the circulation. We wished to examine whether the vectorial nature of entry and export of HAV was the same in polarized hepatocytes and gastrointestinal cells or whether liver cells employed a distinct pathway.
Using polarized N6 cells, we show that both entry and export of HAV occur preferentially via the basolateral domain. As this would not result in direct export to the gastrointestinal tract, it suggests that transcytosis of virus from the blood, through hepatocytes to the bile, and possibly also across the gastrointestinal mucosal epithelia, is the likely source of enteric virus excreted by the host.
MATERIALS AND METHODS
Cells.
African Green monkey kidney cells (BSC-1) and feline renal epithelial cells (CRFK) were grown in minimal essential medium (MEM) supplemented with 5% and 10% fetal bovine serum (FBS), respectively, 2 mM l-glutamine, 10 mM Tris, 100 U penicillin, and 100 μg streptomycin per ml and maintained in serum-free medium for growth and quantification of HAV and feline calicivirus (FCV), respectively. HepG2 cells (maintained at the Burnet Institute for more than 10 years), HepG2-derived C3A cells (American Type Culture Collection), and their subclones (C11 and N6) were passaged in the same medium with the addition of 15 mM HEPES. Single-cell suspensions of the HepG2-derived cells were obtained by incubation of semiconfluent cultures in Hanks balanced salt solution (Ca2+ and Mg2+ free) containing 3 mM EGTA for 20 min, resulting in disruption of cell-cell contacts before trypsinization. Concentrated cell suspensions were then passed three times through a 25-gauge needle before dilution and seeding. For growth of monolayers on porous supports we used Transwell-COL tissue culture inserts (Costar) with a 4.7-cm2 growth area and 3.0-μm pore size. These inserts were shown to allow relatively free diffusion of HAV and FCV in the absence of cells (approximately 30% per hour from the basolateral to apical chamber [results not shown]). The inserts were seeded at a density of 5.0 × 104 cells per insert and then incubated at 37°C for up to 28 days. Transwell cultures were grown in Williams medium E (Invitrogen) containing 10% FBS and supplemented as above, with the addition of 1% dimethyl sulfoxide, which promoted development of polarity in terminal experiments but was unsuitable for passaging of cells. Cell microcolonies were obtained by seeding at low density (approximately 1.5 × 103 cells per cm2) on glass coverslips, followed by incubation for 10 to 14 days. Individual cell colonies contained at least 15 cells at the time of infection.
Cell polarity.
The development of cell polarity in many other cultured epithelial cell lines, such as MDCK cells, is assessed by measurement of the transepithelial resistance (TER) of membrane-grown cultures (25, 50). It has not been reported whether polarized hepatocytes exhibit this characteristic. Hepatocytes are considered a relatively leaky epithelia, with studies showing that molecules up to 50 kDa can pass into bile (and up to 270 kDa in cholestasis due to bile duct ligation) (45). This implies that conventional methods for determination of cell polarity, such as measurement of TER, are potentially unreliable as indicators of functional polarity in these cells. Similarly, Stevenson et al. showed that even in MDCK cell lines I and II, TER measurements differed by 100-fold, despite no differences in tight junctional organization or other markers of polarity (60). However, it is known that polarized hepatocytes preferentially secrete albumin via the basolateral domain (46, 47, 52, 67, 68), and vectorial secretion of human serum albumin (HSA), a molecule of 66 kDa, was therefore used to assess functional polarity of the HepG2-derived cells. A capture enzyme-linked immunosorbent assay format was developed to allow quantification of human albumin in culture samples containing large amounts of bovine albumin in supplemented medium. Ninety-six-well plates (Immunosorb; Nunc) were coated overnight at 4°C with 0.05% goat anti-HSA antibody (Calbiochem-Novabiochem) in 0.1 M NaHCO3 pH 9.6. Plates were washed with phosphate-buffered saline-0.05% Tween 20 (PBST) and then blocked with 2% skim milk powder in PBST. Samples (100 μl) were added to the plate in duplicate for 1 h and washed in PBST, and bound HSA was detected with 100 μl of 0.01% mouse anti-HSA (Zymed) with 2% FBS for a further hour. Immune complexes were detected with 0.02% horseradish peroxidase-conjugated sheep anti-mouse immunoglobulin G (IgG; FAb fragment; Amersham) and tetramethylbenzidine substrate (Chemicon), and absorbance was read at 450 nm and 620 nm. Standard curves for HSA were prepared using serial twofold dilutions of HSA (Sigma) starting at 0.5 μg/ml. Culture supernatants were diluted to fit in the linear range of the assay. Absorbance values were then translated to concentrations using the equation generated by the standard curve, and absolute amounts of secretion were corrected for the relative volumes of apical (1.5 ml) and basolateral (2.6 ml) culture reservoirs. Percent basolateral export was calculated against the total (apical plus basolateral) export of albumin per hour. Individual transwell cultures were considered to be sufficiently polarized when more than 75% of albumin was secreted into the basolateral culture reservoir over a 1-h period, typically observed around 14 to 21 days after seeding.
Viruses and infection.
The HM175A.2 strain of HAV was grown in BSC-1 cells and purified from cell lysates as previously described (3). Concentrated virus was diluted with serum-free MEM, extracted with an equal volume of chloroform, and diluted in MEM containing 1% FCS to 108 infectious particles per ml. Virus was applied to HepG2-derived cells for 1 h at 37°C, and cells were then washed with MEM and incubated in fresh medium. Infectious HAV was assessed by radioimmunofocus assay or infrared fluorescent immunofocus assay in BSC-1 cells as previously described (14). Passive diffusion of FCV (which does not replicate in HepG2 cells) was used as a measure of monolayer integrity in transwells, with infectious FCV measured by plaque assay on CRFK cells using MEM containing 10 mM MgCl2 and 0.4% SeaPlaque agarose (FMC Bioproducts) at 37°C for 48 h. Cells were then fixed in 4% formaldehyde for 60 min, and plaques were visualized by staining with crystal violet.
Indirect immunofluorescence.
Cells were washed with serum-free MEM and fixed in Kryofix (43% ethanol, 7% polyethylene glycol 300) at −20°C. Cells were stained with monoclonal antibodies to HAV (K24F2; CSL, Australia) or to CD26 (aminopeptidase N; Serotec), or with rabbit antibodies to ZO-1 (Zymed) or to low-density lipoprotein receptor (Maine Biotechnology Services) diluted in PBS containing 1% FBS. Immune complexes were detected with Alexa-labeled secondary antibodies (Alexa 568 goat anti-mouse or Alexa 488 goat anti-rabbit; Molecular Probes). Where indicated, nuclei were counterstained with TOTO-3 (Molecular Probes). Cells were examined using a Bio-Rad MRC 1024 Nikon scanning confocal microscope with Lasersharp software using a 20× or 40× objective.
Electron microscopy.
N6 cells grown on transwell inserts were fixed with glutaraldehyde in PBS for 2 h at room temperature. Inserts were resin embedded and then sectioned and stained with uranyl acetate and lead citrate before examination.
Data analysis.
All experiments were performed in duplicate at least three times. Error values given are the standard errors of the means in each experiment. We used the two-tailed Student's t test, with a P level of <0.05 considered a significant difference between values.
RESULTS
Development of polarized hepatocytes.
Primary hepatocytes and hepatocyte-derived cell lines such as HepG2 can sometimes develop cell polarity, but in these cases they generally show a complex, liver-like morphology, such as bile cysts, and fail to form confluent monolayers when grown on semipermeable membrane inserts (11, 39, 62). The HepG2 line used in our laboratory also showed formation of bile cysts when stained by indirect immunofluorescence for the tight junction-associated protein ZO-1 (Fig. 2A; see also Fig. 4A), but areas of cells were also observed with the honeycomb-like pattern of ZO-1 staining that is typical of simple, columnar polarized epithelial cells. This HepG2 cell line as well as the HepG2-derived clone C3A were further subcloned, resulting in a number of separate clones from both lines that demonstrated strong contact inhibition and lack of stratification similar to highly polarized Caco-2 cells, while other HepG2 subclones demonstrated stratified growth.
FIG. 2.
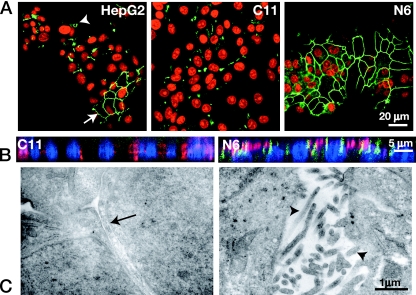
Polarized HepG2 cells. A. ZO-1 (green) staining of HepG2 cells shows a population of cells (nuclei, red) with mixed polarity, with areas of bile cyst formation (arrowhead) and areas of intracellular tight junction formation (arrow). Subcloning yielded populations of cells with a nonpolarized phenotype (C11) and polarized phenotype (N6), showing the extensive honeycomb distribution of ZO-1 (green) in the latter. B. x-z section of cells stained for the apically distributed protein CD26 (red) and the tight junction protein ZO-1 (green). The N6 cells show predominantly apical localization of the CD26 protein at the same level as the intracellular tight junctions, while the C11 cells have no detectable ZO-1 staining and a widespread intracellular CD26 distribution. C. The ultrastructure of N6 cells was examined by electron microscopy. These cells display many of the basic features of polarized epithelial cells. The apical surfaces show extensive villi (arrowheads), and intracellular tight junction complexes (arrow) can be seen.
FIG. 4.
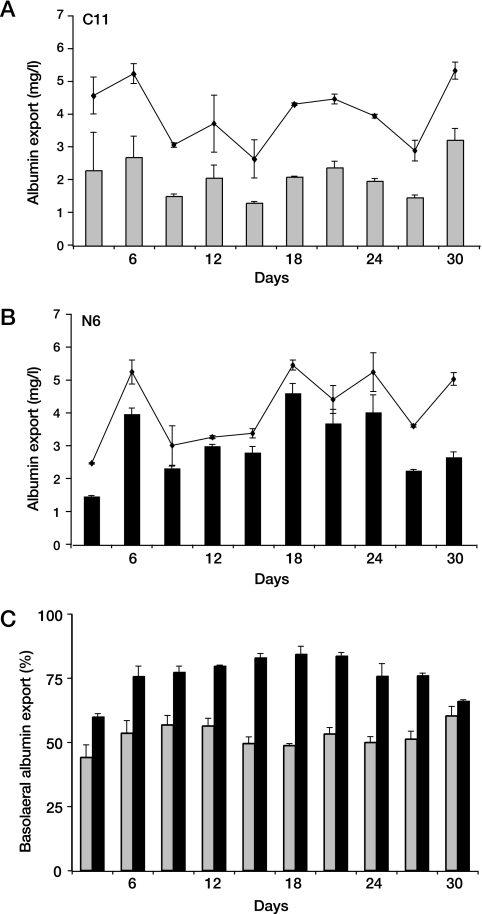
Export of albumin from subcloned cells. Cells grown on semipermeable membranes for 30 days were examined for albumin export into the apical and basolateral domains. (A and B) C11 (A) and N6 (B) cells export similar amounts of total albumin (triangles) but differ significantly in amounts of basolateral albumin (bars) secreted per hour. (C) Percent basolateral albumin export (of total export) is shown. C11 cells (gray bars) exported albumin equally into the apical and basolateral domains. Polarized N6 cells (black bars) show a gradual increase in percent basolateral albumin export, which is maximal at over 75% between days 14 and 24.
We investigated the polarized distribution of intracellular proteins in these cell lines. Figure 2A shows polarized (N6) and nonpolarized (C11) subclones cultured on semipermeable membranes, such that the apical surfaces are superior and the basolateral surfaces are attached to the membrane. A honeycomb pattern of ZO-1 distribution at intracellular tight junctions is visible in the polarized N6 cells (as seen for regions in the uncloned HepG2 cells), but ZO-1 does not show a regular pattern of accumulation in the nonpolarized C11 cells, which also lack the bile cysts that were often present in the uncloned HepG2 cells (Fig. 2A) and some other subclones (results not shown).
N6 and C11 cells grown on semipermeable membranes as above were fixed and stained for CD26, an apically distributed protein, together with ZO-1 to identify tight junctions and with TOTO-3 to identify nuclei. The x-z sections of cell monolayers were collected by confocal microscopy. CD26 was localized to the apical domains of the polarized N6 cells, while in the nonpolarized C11 cells, a nonvectorial distribution of this protein was observed (Fig. 2B). ZO-1 distribution at the tight junctions is seen in the polarized cells while barely detectable in C11 cells. Sequential x-y sections of N6 cells grown on glass coverslips confirmed that CD26 was restricted to the uppermost cell surface, with the apical domain bounded by tight junctions (Fig. 3). We also examined the distribution of a basolateral protein, the low-density lipoprotein receptor, and found corollary evidence of vectorial distribution of this marker on the basolateral (membrane-associated) domain (results not shown). These results confirm that the polarized N6 cells show columnar morphology with apical and basolateral disposition, as for simple polarized epithelia.
FIG. 3.
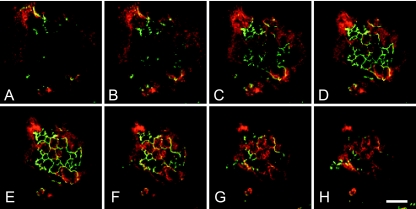
Vectorial distribution of CD26 in polarized hepatocytes. The images are a Z-series of 2-μm sections from basolateral (A) to apical (H) through a colony of N6 cells. Polarized central cells of the colony show ZO-1 staining (green). Nonpolarized peripheral cells display intracellular CD26 staining (red) throughout the cytoplasm, while the central cells show marked apical distribution of protein, confirming that these cells are functionally polarized. Bar, 30 μm.
A hallmark of the apical surface of epithelial cells is the formation of microvilli, which markedly increase the surface area of the apical plasma membrane (35, 77). We examined the cellular ultrastructure of the N6 cells by thin section electron microscopy. These cells showed tight junctional complexes segregating the apical and basolateral plasma membranes and significant apical microvilli formation (Fig. 2C).
Functional polarity of cell lines.
As cells were grown on transwell filters, we needed to determine the degree of leakage due to potential breaches of monolayer integrity. Initial studies with [3H]inulin (5 kDa) showed 1.5 to 2% diffusion per hour in both polarized N6 and nonpolarized C11 monolayers, compared with 60% diffusion per hour across blank membranes, and 0.5% per hour across Caco-2 monolayers. This result is not surprising, however, as hepatocytes are generally considered a relatively leaky epithelia, with studies showing that molecules up to 50 kDa can pass into bile (and up to 270 kDa in cholestasis due to bile duct ligation) (45). Thus, we determined monolayer integrity in the N6 and C11 cells using diffusion of FCV rather than inulin.
The leakiness of hepatocytes as epithelial cells (45) further implies that conventional methods of determining cell polarity, such as TER measurement, may not be reliable as indicators of functional polarity in these cells. We found average TER measurements of 56 to 96 Ω·cm2 in confluent monolayers of membrane-grown N6 cells compared with a mean of 279 Ω·cm2 in well-polarized Caco-2 cell monolayers in the same system. These low TER measurements did not correlate with development of tight junctions or vectorial export of albumin as alternative markers of polarity. Thus, we used vectorial export of albumin, a macromolecule of 66 kDa, as a marker of monolayer polarity in individual transwell cultures.
Cells were cultured as monolayers on semipermeable membranes, and human albumin secretion into the apical and basolateral culture reservoirs was determined by enzyme-linked immunosorbent assay, as a measure of functional polarity over time (Fig. 4). Monolayers of polarized cells secreted albumin predominantly into the basolateral reservoir, while nonpolarized cells secreted albumin equally into both reservoirs. The duration to reach peak polarity was between 12 and 21 days, with later deterioration of vectorial export as cells began to age. Peak vectorial secretion of albumin by N6 cells to the basolateral reservoir reached 80 to 90%, and 75% was selected as the minimum level for use in subsequent experiments. Assuming that the polarized cells secrete 100% of albumin into the basolateral reservoir, while the remaining cells secrete equally into both reservoirs, this represents a minimum of 50% fully polarized cells. Cell culture inserts were monitored from 14 days after seeding until reaching this level of vectorial albumin secretion. Albumin secretion was also monitored during the period of viral export.
Vectorial entry of HAV.
The most common route of transmission of HAV is through oral ingestion. It has been presumed that the virus enters the bloodstream through the gut and is transported to the liver in the portal circulation to enter hepatocytes. In this case virus would be exposed to the basolateral domain of hepatocytes, but HAV infection of Caco-2 cells has been reported to be more efficient via the apical domain (4).
To examine the vectorial infection of hepatocytes with HAV, monolayers of N6 cells were grown on transwell inserts and exposed to equal concentrations of virus from either the apical or basolateral compartment for 1 h, and total intracellular virus yield was measured after 24 h. Basolateral infection of N6 cells resulted in around twofold higher yields of virus than apical infection ([15.6 ± 0.2] × 103 versus [8.0 ± 3.3] × 103 virus particles/ml), despite a diffusion rate for virus from the basolateral compartment across blank membranes of only about 30% under these conditions, which would not affect infection from the apical compartment. This demonstrates that HAV infection of polarized hepatocytes from the basolateral domain is highly efficient, in contrast to that of Caco-2 cells (4). However, the cells were never 100% polarized in the vectorial albumin excretion assay, and we considered it likely that the virus yield following apical exposure may represent preferential infection of the nonpolarized cells in the monolayer, rather than bidirectional infection of polarized cells. To further examine vectorial entry of HAV into hepatocytes, microcolonies were obtained by seeding the cell lines at low density onto glass coverslips. Because tight junctions form only at the points of cell-cell contact, the central cells of colonies have the capacity to become polarized (with the basolateral domains in contact with the glass coverslip and apical domains exposed to the medium), whereas tight junction formation is incomplete in peripheral cells, preventing the polarized sorting of molecules, including viral receptors.
Microcolonies of HepG2, N6, and C11 cells were exposed to HAV for 1 h at 37°C and then washed and incubated for 24 h to allow a single round of virus replication. Coverslips were then fixed and stained for HAV and ZO-1 protein and visualized by confocal microscopy. In N6 microcolonies, HAV replication was limited to the peripheral nonpolarized cells, whereas cells throughout C11 and HepG2 microcolonies were infected (Fig. 5). This suggests that infection of polarized hepatocytes is occurring via the basolateral domain only. However, it must be noted that the N6 cells demonstrate strong contact inhibition and the central cells of colonies are not actively dividing, whereas C11 cells show less contact inhibition and continued cell division. To exclude the possibility that the sparing of central cells in N6 microcolonies from HAV infection was due to lack of cell division, cells were treated with 3 mM EGTA for 10 min at 37°C to reversibly disrupt tight junctions before exposure to HAV (Fig. 6A). Under these conditions, central N6 cells were also infected with HAV, confirming that the productive entry of HAV is restricted to the basolateral domain of polarized hepatocytes (Fig. 6). Immunofluorescence staining of cells for ZO-1 showed that they have largely regained tight junction architecture within 1 h and remain confluent, precluding cell division during EGTA treatment (Fig. 6B).
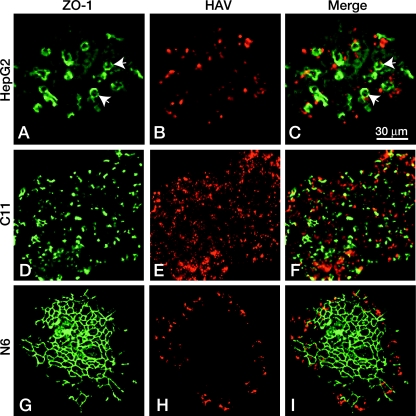
FIG. 5.
HAV infects hepatocytes from the basolateral domain. Cells grown in microcolonies were infected with HAV and examined for HAV (red) and ZO-1(green) expression by immunofluorescence. (A to C) Uncloned HepG2 cells show occasional bile cyst formation (arrows) at the apical domain of adjacent cells, indicating these cells are polarized, but as basolateral domains are facing both the glass and the media, cells throughout the colony are infected. (D to F) Similarly, C11 cells demonstrate that infection occurs readily throughout the colonies of cells that are not polarized. (G to I) The basolateral domains of N6 cells are adherent to the glass except at the periphery of the colony. Central polarized cells, with extensive tight junction formation as shown by ZO-1 staining, are unable to be infected with HAV, while peripheral cells are readily infected. Bar, 30 μm.
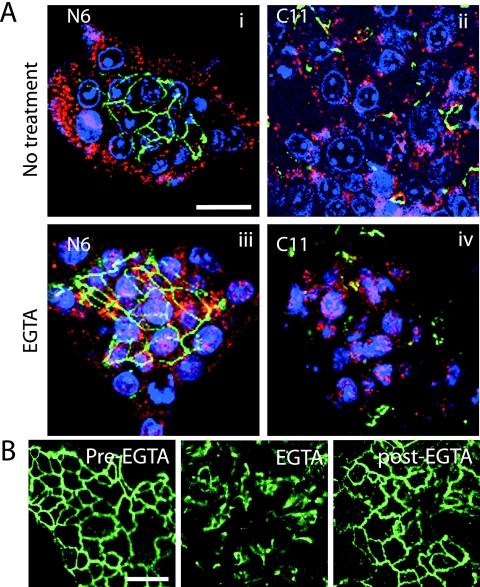
FIG. 6.
Disruption of tight junctions of polarized hepatocytes permits infection. (A) N6 and C11 cells grown in microcolonies were treated with EGTA for 10 min to disrupt the tight junctions and then infected with HAV. Cells were stained for ZO-1 (green), HAV (red), and nuclei (blue). Before EGTA treatment, only peripheral cells of the N6 colony (panel i), but all cells of the C11 colony (ii), were infectible with virus. Once tight junctions were disrupted, N6 cells also showed extensive viral infection throughout the colony despite reformation of tight junctions postinfection (iii). (B) N6 cells were untreated (pre-EGTA) or treated with EGTA for 10 min (EGTA) or incubated with normal medium for a further hour after EGTA treatment (post-EGTA) and then fixed and stained for ZO-1. Tight junctions were disrupted by the EGTA treatment but reformed within 1 h. Bar, 20 μm.
Vectorial export of HAV.
Vectorial export of HAV from the apical domain of Caco-2 cells has been described elsewhere (4), and apical release from hepatocytes would result in direct excretion into bile and gastrointestinal tract, providing virus for enteric transmission to new hosts. However, it is known that hepatocytes transport most newly synthesized macromolecules to the basolateral domain and into the plasma. To determine whether HAV release from hepatocytes is vectorial, N6 and C11 cells were grown on semipermeable membranes until N6 cells were optimally polarized, and duplicate cultures were then infected with HAV for 1 h via the basolateral domain, extensively washed, and then incubated for 24 h. Transwells were then transferred to prewarmed fresh medium, and total apical and basolateral supernatants were collected at 1 and 2 h for measurement of newly released HAV. Paracellular diffusion was measured by addition of FCV to the apical medium and titration of apical and basolateral supernatants.
Albumin export from the N6 cells was predominantly into the basolateral domain, indicating maintenance of functional polarity after infection with HAV, and export from C11 cells was equally into both domains as expected (Fig. 7). The cell monolayers demonstrated very low paracellular diffusion of FCV from apical to basolateral domains (0.1%/h), and diffusion was therefore considered to have no influence on the measure of HAV released. Additionally, we note that since there was no loss of FCV particles from the inoculum, viral adhesion or intracellular or paracellular sequestration did not take place. More than 90% of newly produced HAV was exported via the basolateral domain of N6 cells, while nonpolarized C11 cells exported virus equally into both apical and basolateral reservoirs (Fig. 7). This demonstrates that HAV undergoes vectorial export via the basolateral domain of polarized hepatocytes, consistent with the major pathway of export from these cells but in contrast to apical viral export from enteric epithelia.
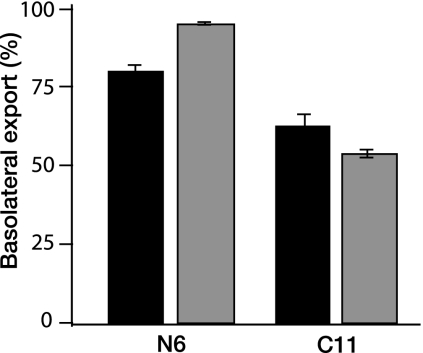
FIG. 7.
Vectorial export of hepatitis A virus. N6 and C11 cells grown on semipermeable membranes were assayed for viral export into the apical and basolateral domains. Both albumin (black) and HAV (gray) are exported predominantly into the basolateral domain by polarized N6 cells, indicating vectorial export of these substrates in polarized hepatocytes. Nonpolarized C11 cells exported about 50% of both the albumin and virus into the basolateral domain.
DISCUSSION
Hepatocyte polarity is essential to the maintenance of protein, carbohydrate, and lipid homeostasis in the body. Loss of tissue polarity likely contributes significantly to the pathogenesis of hepatic disorders, including cholestasis and cirrhosis (45, 61, 64). However, the role of hepatocyte polarity in the pathogenesis and transmission of hepatotropic viruses has not been examined previously, in contrast to the extensive studies of virus interactions with polarized mucosal epithelia for many viruses with enteric, sexual, or respiratory transmission (6, 7, 32, 55, 65, 66). This is largely a consequence of the lack of hepatocyte cell culture models suitable for study of virus entry and export, due to the complex morphology of most polarized hepatocyte-derived cell lines in vitro, reflecting in turn the complex morphology that is typical of hepatocytes in vivo.
HepG2 cells are a well-differentiated human hepatoma cell line that expresses many of the same biosynthetic and metabolic functions as liver cells, including the processing of bile and lipids, and HepG2 cells have been extensively used for the study of hepatocyte polarity (17, 33, 69, 76). However these cells typically demonstrate the complex morphology of hepatocytes, with their apical surfaces forming bile canaliculae or bile cysts, rather than having the columnar orientation of simple polarized epithelial cells. In this study, we derived a novel clone of HepG2 cells that provides the first practicable system for the study of viral interactions with polarized hepatocytes. The columnar orientation of the N6 cells provides access to both apical and basolateral plasma membranes when the cells are grown on semipermeable cell culture inserts, allowing the study of vectorial entry and export of viruses. This simple morphology also provides a significant advantage to the study of hepatocyte transcytosis. We utilized the N6 and C11 cells to study interactions of HAV with polarized hepatocytes. Previous studies suggested that infection of hepatocytes was primarily mediated through Kupffer cells and did not determine whether viral entry into hepatocytes was vectorial (53). Our results suggest that productive entry of HAV into polarized hepatocytes can occur directly, predominantly across the basolateral domain (Fig. 5). In contrast, a previous study of HAV interactions with polarized enteric epithelia under comparable conditions (Caco-2 cells) demonstrated preferential uptake via the apical domain (4). However, these contrasting routes of preferential entry are each consistent with the expected route of exposure to HAV during transmission: through the gut lumen to enterocytes, and through the bloodstream to hepatocytes.
The vectorial entry of HAV into hepatocytes shown here suggests that entry may be mediated by a basolaterally localized receptor. One specific receptor for HAV, HAVCR-1, has been found in a number of cell lines that support viral replication, and its human homologue, huHAVCR-1, has been identified as a receptor for HAV also (23, 28, 54). The vectorial distribution of the huHAVCR-1 glycoprotein in polarized cells, and more specifically hepatocytes, is not known. Other receptors for HAV have also been described, possibly conveying some viral tissue specificity. The asialoglycoprotein receptor, which is predominantly found on the basolateral plasma membrane of hepatocytes, has also been suggested as a possible receptor for HAV (20, 58). Further studies are required to determine what receptor(s) is responsible for the highly vectorial infection of hepatocytes by HAV.
A further unresolved question concerns the mechanism by which HAV gains access to the bloodstream to initiate infection. As HAV is generally transmitted via ingestion, it has been presumed that virus is transmitted to the portal circulation across the gastrointestinal epithelia, and thus to the liver. While significant HAV replication can be demonstrated in enterocyte-derived Caco-2 cells, export of virus from these cells was exclusively via the apical domain, and thus back into the gut lumen (4). In addition, detection of HAV in gut epithelia has been inconsistent, and intravenous inoculation of HAV does not reduce the incubation period for infection (1, 34, 44). Together, these observations suggest that trafficking of HAV to the bloodstream may be independent of replication in the gastrointestinal mucosa. Apical to basolateral transcytosis of HAV, mediated by the polymeric immunoglobulin receptor, has been shown to occur in polarized epithelial MDCK cells, and a similar IgA-mediated mechanism may be used by HAV in gut epithelia to achieve access to the circulation (19). IgA is a natural ligand of HAVCR1, and the association of IgA with HAVCR1 enhances virus-receptor interactions (63). It is of note, however, that virions would have to avoid uncoating in susceptible enteric cells in order to reach the bloodstream intact for transmission to the liver, and virus-specific IgA would need to have survived along with the virus in the environment (and the gut) to effect this function. An alternative mechanism may be transcytosis via intestinal M cells, as has been described for both poliovirus and reovirus (41, 75). Whatever mechanisms are responsible, they appear to be highly efficient for HAV, since it is so readily transmitted and invariably infects the liver.
Hepatocytes transport most newly synthesized substrates from the Golgi complex to the basolateral domain, where they may remain as resident plasma membrane proteins, to be secreted into the bloodstream or undergo transcytosis to the apical plasma membrane (47, 73). Direct apical export is seen only for some lipid substrates (56, 70). Our observation of almost exclusive basolateral export of HAV from polarized hepatocytes is therefore not surprising and readily accounts for the significant viremia observed during acute HAV infection, but this does not explain the presence of virus in bile (48) or excretion of very large amounts of virus in feces to support enteric transmission. Blank and colleagues previously showed that export of HAV from Caco-2 cells is exclusively via the apical domain and suggested that this was also likely to be true in hepatocytes, leading to direct excretion of virus via the apical domain to bile ducts and the gastrointestinal tract (4). However, our results clearly show that virus is exported in the opposite direction in hepatocytes and highlight that the findings of studies for viruses in other polarized epithelia cannot be extrapolated to the same viruses in polarized hepatocytes.
How then does HAV reach the gut from hepatocytes for excretion? Since HAV particles have been found in the bile and feces of infected animals, and the liver is the main organ of replication for HAV, a mechanism of trafficking of virus from the blood to the bile, against the default basolateral transportation of HAV in hepatocytes, is strongly implied by this study. It has been suggested that uptake of HAV in primary hepatocytes and in HepG2 cells can occur via the asialoglycoprotein receptor mediated by specific immunoglobulin (20), although the importance of this mechanism for infection is unclear, since antibody is not essential for infection of these cells. Conversely, IgA is exported from the liver into the gastrointestinal tract as bound ligand-immunoglobulin complexes in the bile, and specific IgA may therefore facilitate excretion of HAV from the body of infected hosts (9). It will be important to use appropriate cell models to study the potential role of these and other mechanisms in enteric excretion of HAV. The novel N6 polarized hepatocyte cell line provides a powerful tool for investigating virus-cell interactions for hepatotropic viruses. In this context it is notable that the tight junction protein claudin-1 has been reported to have activity as a cellular receptor for hepatitis C virus (22). Mee and colleagues have very recently investigated the role of cell polarity in HCV infection of Caco-2 cells (36), but the pronounced differences between simple epithelia and hepatocytes, well known for protein trafficking and as shown here for virus entry and export, argue that such studies may need to be confirmed in the relevant target cells. This study shows that both infection and export of HAV occur vectorially in polarized hepatocytes and provides new insights into the pathogenesis and transmission of this virus. Additionally, the development of a novel polarized hepatocyte cell line that simplifies the study of vectorial substrate movement in these cells provides a new tool for investigating virus-host interactions in the liver.
Acknowledgments
This study was supported by a Project Grant and a Senior Research Fellowship (D.A.A.) from the National Health and Medical Research Council of Australia.
Footnotes
Published ahead of print on 25 June 2008.
REFERENCES
- 1.Asher, L. V., L. N. Binn, T. L. Mensing, R. H. Marchwicki, R. A. Vassell, and G. D. Young. 1995. Pathogenesis of hepatitis A in orally inoculated owl monkeys (Aotus trivirgatus). J. Med. Virol. 47260-268. [DOI] [PubMed] [Google Scholar]
- 2.Bhat, P., and D. A. Anderson. 2007. Hepatitis B virus translocates across a trophoblastic barrier. J. Virol. 817200-7207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bishop, N. E., D. L. Hugo, S. V. Borovec, and D. A. Anderson. 1994. Rapid and efficient purification of hepatitis a virus from cell culture. J. Virol. Methods 47203-216. [DOI] [PubMed] [Google Scholar]
- 4.Blank, C. A., D. A. Anderson, M. Beard, and S. M. Lemon. 2000. Infection of polarized cultures of human intestinal epithelial cells with hepatitis A virus: vectorial release of progeny virions through apical cellular membranes. J. Virol. 746476-6484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bomsel, M. 1997. Transcytosis of infectious human immunodeficiency virus across a tight human epithelial cell line barrier. Nat. Med. 342-47. [DOI] [PubMed] [Google Scholar]
- 6.Bose, S., A. Malur, and A. K. Banerjee. 2001. Polarity of human parainfluenza virus type 3 infection in polarized human lung epithelial A549 cells: role of microfilament and microtubule. J. Virol. 751984-1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brock, S. C., J. R. Goldenring, and J. E. J. Crowe. 2003. Apical recycling systems regulate directional budding of respiratory syncytial virus from polarized epithelial cells. Proc. Natl. Acad. Sci. USA 10015143-15148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brock, S. C., J. M. Heck, P. A. McGraw, and J. E. J. Crowe. 2005. The transmembrane domain of the respiratory syncytial virus F protein is an orientation-independent apical plasma membrane sorting sequence. J. Virol. 7912528-12535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown, W. R., and T. M. Kloppel. 1989. The liver and IgA: immunological, cell biological and clinical implications. Hepatology 9763-784. [DOI] [PubMed] [Google Scholar]
- 10.Bugarcic, A., and J. A. Taylor. 2006. Rotavirus nonstructural glycoprotein NSP4 is secreted from the apical surfaces of polarized epithelial cells. J. Virol. 8012343-12349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chiu, J. H., C. P. Hu, W. Y. Lui, S. C. Lo, and C. M. Chang. 1990. The formation of bile canaliculi in human hepatoma cell lines. Hepatology 11834-842. [DOI] [PubMed] [Google Scholar]
- 12.Cohen, J. I., J. R. Ticehurst, S. M. Feinstone, B. Rosenblum, and R. H. Purcell. 1987. Hepatitis A virus cDNA and its RNA transcripts are infectious in cell culture. J. Virol. 613035-3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Connolly-Andersen, A.-M., K.-E. Magnusson, and A. Mirazimi. 2007. Basolateral entry and release of Crimean-Congo hemorrhagic fever virus in polarized MDCK-1 cells. J. Virol. 812158-2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Counihan, N. A., L. M. Daniel, J. Chojnacki, and D. A. Anderson. 2006. Infrared fluorescent immunofocus assay (IR-FIFA) for the quantitation of noncytopathic and minimally cytopathic viruses. J. Virol. Methods 13362-69. [DOI] [PubMed] [Google Scholar]
- 15.Craig, A. S., B. Watson, T. K. Zink, J. P. Davis, C. Yu, and W. Schaffner. 2007. Hepatitis A outbreak activity in the United States: responding to a vaccine-preventable disease. Am. J. Med. Sci. 334180-183. [DOI] [PubMed] [Google Scholar]
- 16.Crawford, J. M., C. A. Berken, and J. L. Gollan. 1988. Role of the hepatocyte microtubular system in the excretion of bile salts and biliary lipid: implications for intracellular vesicular transport. J. Lipid Res. 29144-156. [PubMed] [Google Scholar]
- 17.de Marco, M. C., F. Martin-Belmonte, L. Kremer, J. P. Albar, I. Correas, J. P. Vaerman, M. Marazuela, J. A. Byrne, and M. A. Alonso. 2002. MAL2, a novel raft protein of the MAL family, is an essential component of the machinery for transcytosis in hepatoma HepG2 cells. J. Cell Biol. 15937-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dominguez, A., M. Bruguera, P. Plans, J. Espunes, J. Costa, A. Plasencia, and L. Salleras. 2007. Declining hepatitis A seroprevalence in adults in Catalonia (Spain): a population-based study. BMC Infect. Dis. 773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dotzauer, A., M. Brenner, U. Gebhardt, and A. Vallbracht. 2005. IgA-coated particles of hepatitis A virus are translocalized antivectorially from the apical to the basolateral site of polarized epithelial cells via the polymeric immunoglobulin receptor. J. Gen. Virol. 862747-2751. [DOI] [PubMed] [Google Scholar]
- 20.Dotzauer, A., U. Gebhardt, K. Bieback, U. Gottke, A. Kracke, J. Mages, S. M. Lemon, and A. Vallbracht. 2000. Hepatitis A virus-specific immunoglobulin A mediates infection of hepatocytes with hepatitis A virus via the asialoglycoprotein receptor. J. Virol. 7410950-10957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Emerson, S. U., M. Lewis, S. Govindarajan, M. Shapiro, T. Moskal, and R. H. Purcell. 1992. cDNA clone of hepatitis A virus encoding a virulent virus: induction of viral hepatitis by direct nucleic acid transfection of marmosets. J. Virol. 666649-6654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Evans, M. J., T. von Hahn, D. M. Tscherne, A. J. Syder, M. Panis, B. Wolk, T. Hatziioannou, J. A. McKeating, P. D. Bieniasz, and C. M. Rice. 2007. Claudin-1 is a hepatitis C virus co-receptor required for a late step in entry. Nature 446801-805. [DOI] [PubMed] [Google Scholar]
- 23.Feigelstock, D., P. Thompson, P. Mattoo, Y. Zhang, and G. G. Kaplan. 1998. The human homolog of HAVcr-1 codes for a hepatitis A virus cellular receptor. J. Virol. 726621-6628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Graf, J., A. Gautam, and J. L. Boyer. 1984. Isolated rat hepatocyte couplets: a primary secretory unit for electrophysiologic studies of bile secretory function. Proc. Natl. Acad. Sci. USA 816516-6520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grasset, E., M. Pinto, E. Dussaulx, A. Zweibaum, and J. F. Desjeux. 1984. Epithelial properties of human colonic carcinoma cell line Caco-2: electrical parameters. Am. J. Physiol. 247C260-C267. [DOI] [PubMed] [Google Scholar]
- 26.Huang, S. N., D. Lorenz, and R. J. Gerety. 1979. Electron and immunoelectron microscopic study on liver tissues of marmosets infected with hepatitis A virus. Lab. Investig. 4163-71. [PubMed] [Google Scholar]
- 27.Ihrke, G., E. B. Neufeld, T. Meads, M. R. Shanks, D. Cassio, M. Laurent, T. A. Schroer, R. E. Pagano, and A. L. Hubbard. 1993. WIF-B cells: an in vitro model for studies of hepatocyte polarity. J. Cell Biol. 1231761-1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaplan, G., A. Totsuka, P. Thompson, T. Akatsuka, Y. Moritsugu, and S. M. Feinstone. 1996. Identification of a surface glycoprotein on African Green monkey kidney cells as a receptor for hepatitis A virus. EMBO J. 154282-4296. [PMC free article] [PubMed] [Google Scholar]
- 29.Krawczynski, K. K., D. W. Bradley, B. L. Murphy, J. W. Ebert, T. E. Anderson, I. L. Doto, A. Nowoslawski, W. Duermeyer, and J. E. Maynard. 1981. Pathogenetic aspects of hepatitis A virus infection in enterally inoculated marmosets. Am. J. Clin. Pathol. 76698-706. [DOI] [PubMed] [Google Scholar]
- 30.Lagaye, S., M. Derrien, E. Menu, C. Coito, E. Tresoldi, P. Mauclere, G. Scarlatti, G. Chaouat, F. Barre-Sinoussi, and M. Bomsel. 2001. Cell-to-cell contact results in a selective translocation of maternal human immunodeficiency virus type 1 quasispecies across a trophoblastic barrier by both transcytosis and infection. J. Virol. 754780-4791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lisanti, M. P., I. W. Caras, M. A. Davitz, and E. Rodriguez-Boulan. 1989. A glycophospholipid membrane anchor acts as an apical targeting signal in polarized epithelial cells. J. Cell Biol. 1092145-2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maidji, E., E. Percivalle, G. Gerna, S. Fisher, and L. Pereira. 2002. Transmission of human cytomegalovirus from infected uterine microvascular endothelial cells to differentiating/invasive placental cytotrophoblasts. Virology 30453-69. [DOI] [PubMed] [Google Scholar]
- 33.Maier, O., and D. Hoekstra. 2003. Trans-Golgi network and subapical compartment of HepG2 cells display different properties in sorting and exiting of sphingolipids. J. Biol. Chem. 278164-173. [DOI] [PubMed] [Google Scholar]
- 34.Mathiesen, L. R., A. M. Moller, R. H. Purcell, W. T. London, and S. M. Feinstone. 1980. Hepatitis A virus in the liver and intestine of marmosets after oral inoculation. Infect. Immun. 2845-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McAteer, J. A., G. S. Dougherty, K. D. J. Gardner, and A. P. Evan. 1986. Scanning electron microscopy of kidney cells in culture: surface features of polarized epithelia. Scan. Electron Microsc. 19861135-1150. [PubMed] [Google Scholar]
- 36.Mee, C. J., J. Grove, H. J. Harris, K. Hu, P. Balfe, and J. A. McKeating. 2008. Effect of cell polarization on hepatitis C virus entry. J. Virol. 82461-470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meng, G., X. Wei, X. Wu, M. T. Sellers, J. M. Decker, Z. Moldoveanu, J. M. Orenstein, M. F. Graham, J. C. Kappes, J. Mestecky, G. M. Shaw, and P. D. Smith. 2002. Primary intestinal epithelial cells selectively transfer R5 HIV-1 to CCR5+ cells. Nat. Med. 8150-156. [DOI] [PubMed] [Google Scholar]
- 38.Mostov, K. E., M. Verges, and Y. Altschuler. 2000. Membrane traffic in polarized epithelial cells. Curr. Opin. Cell Biol. 12483-490. [DOI] [PubMed] [Google Scholar]
- 39.Musat, A. I., C. A. Sattler, G. L. Sattler, and H. C. Pitot. 1993. Reestablishment of cell polarity of rat hepatocytes in primary culture. Hepatology 18198-205. [PubMed] [Google Scholar]
- 40.Nyasae, L. K., A. L. Hubbard, and P. L. Tuma. 2003. Transcytotic efflux from early endosomes is dependent on cholesterol and glycosphingolipids in polarized hepatic cells. Mol. Biol. Cell 142689-2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ouzilou, L., E. Caliot, I. Pelletier, M.-C. Prevost, E. Pringault, and F. Colbere-Garapin. 2002. Poliovirus transcytosis through M-like cells. J. Gen. Virol. 832177-2182. [DOI] [PubMed] [Google Scholar]
- 42.Pinto, M. A., R. S. Marchevsky, M. L. Baptista, M. A. de Lima, M. Pelajo-Machado, C. L. Vitral, C. F. Kubelka, J. W. Pissurno, M. S. Franca, H. G. Schatzmayr, and A. M. C. Gaspar. 2002. Experimental hepatitis A virus (HAV) infection in Callithrix jacchus: early detection of HAV antigen and viral fate. Exp. Toxicol. Pathol. 53413-420. [DOI] [PubMed] [Google Scholar]
- 43.Purcell, R. H., and S. U. Emerson. 2001. Animal models of hepatitis A and E. ILAR J. 42161-177. [DOI] [PubMed] [Google Scholar]
- 44.Purcell, R. H., D. C. Wong, and M. Shapiro. 2002. Relative infectivity of hepatitis A virus by the oral and intravenous routes in 2 species of nonhuman primates. J. Infect. Dis. 1851668-1671. [DOI] [PubMed] [Google Scholar]
- 45.Rahner, C., B. Stieger, and L. Landmann. 1996. Structure-function correlation of tight junctional impairment after intrahepatic and extrahepatic cholestasis in rat liver. Gastroenterology 1101564-1578. [DOI] [PubMed] [Google Scholar]
- 46.Saucan, L., and G. E. Palade. 1992. Differential colchicine effects on the transport of membrane and secretory proteins in rat hepatocytes in vivo: bipolar secretion of albumin. Hepatology 15714-721. [DOI] [PubMed] [Google Scholar]
- 47.Saucan, L., and G. E. Palade. 1994. Membrane and secretory proteins are transported from the Golgi complex to the sinusoidal plasmalemma of hepatocytes by distinct vesicular carriers. J. Cell Biol. 125733-741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schulman, A. N., J. L. Dienstag, D. R. Jackson, J. H. Hoofnagle, R. J. Gerety, R. H. Purcell, and L. F. Barker. 1976. Hepatitis A antigen particles in liver, bile, and stool of chimpanzees. J. Infect. Dis. 13480-84. [DOI] [PubMed] [Google Scholar]
- 49.Shaffer, D. R., E. A. Brown, and S. M. Lemon. 1994. Large deletion mutations involving the first pyrimidine-rich tract of the 5′ nontranslated RNA of human hepatitis A virus define two adjacent domains associated with distinct replication phenotypes. J. Virol. 685568-5578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shah, D., and W. C. Shen. 1994. The establishment of polarity and enhanced transcytosis of transferrin receptors in enterocyte-like Caco-2 cells. J. Drug Target. 293-99. [DOI] [PubMed] [Google Scholar]
- 51.Shanks, M. R., D. Cassio, O. Lecoq, and A. L. Hubbard. 1994. An improved polarized rat hepatoma hybrid cell line. Generation and comparison with its hepatoma relatives and hepatocytes in vivo. J. Cell Sci. 107813-825. [DOI] [PubMed] [Google Scholar]
- 52.Shiao, Y. J., and J. E. Vance. 1993. Sphingomyelin transport to the cell surface occurs independently of protein secretion in rat hepatocytes. J. Biol. Chem. 26826085-26092. [PubMed] [Google Scholar]
- 53.Shibayama, T., H. Kojima, M. Ashida, S. Hirose, A. Sato, T. Kamimura, C. Hamada, Y. Shimizu, S. Suzuki, and F. Ichida. 1985. Localization of hepatitis A virus in marmoset liver tissue during the acute phase of experimental infection. Gastroenterol. Jpn. 20564-572. [DOI] [PubMed] [Google Scholar]
- 54.Silberstein, E., L. Xing, W. van de Beek, J. Lu, H. Cheng, and G. G. Kaplan. 2003. Alteration of hepatitis A virus (HAV) particles by a soluble form of HAV cellular receptor 1 containing the immunoglobin-and mucin-like regions. J. Virol. 778765-8774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sinn, P. L., G. Williams, S. Vongpunsawad, R. Cattaneo, and P. B. J. McCray. 2002. Measles virus preferentially transduces the basolateral surface of well differentiated human airway epithelia. J. Virol. 762403-2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Slimane, T. A., G. Trugnan, S. C. D. Van IJzendoorn, and D. Hoekstra. 2003. Raft-mediated trafficking of apical resident proteins occurs in both direct and transcytotic pathways in polarized hepatic cells: role of distinct lipid microdomains. Mol. Biol. Cell 14611-624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Snooks, M., and D. Anderson. 2005. Vectorial binding of hepatitis A and E viruses to polarized hepatocytes, p. 277-279. In A. Jilbert, E. Grgacic, K. Vickery, and Y. Cossart (ed.), Viral hepatitis and liver disease. Proceedings of the 11th International Symposium on Viral Hepatitis and Liver Disease. Australian Centre for Hepatitis Virology, Sydney, Australia.
- 58.Spiess, M. 1990. The asialoglycoprotein receptor: a model for endocytic transport receptors. Biochemistry 2910009-10018. [DOI] [PubMed] [Google Scholar]
- 59.Stapleton, J., J. Frederick, and B. Meyer. 1991. Hepatitis A virus attachment to cultured cell lines J. Infect. Dis. 1641098-1103. [DOI] [PubMed] [Google Scholar]
- 60.Stevenson, B. R., J. M. Anderson, D. A. Goodenough, and M. S. Mooseker. 1988. Tight junction structure and ZO-1 content are identical in two strains of Madin-Darby canine kidney cells which differ in transepithelial resistance. J. Cell Biol. 1072401-2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stieger, B., and L. Landmann. 1996. Effects of cholestasis on membrane flow and surface polarity in hepatocytes. J. Hepatol. 24(Suppl. 1)128-134. [PubMed] [Google Scholar]
- 62.Talamini, M. A., B. Kappus, and A. Hubbard. 1997. Repolarization of hepatocytes in culture. Hepatology 25167-172. [DOI] [PubMed] [Google Scholar]
- 63.Tami, C., E. Silberstein, M. Manangeeswaran, G. J. Freeman, S. E. Umetsu, R. H. DeKruyff, D. T. Umetsu, and G. G. Kaplan. 2007. Immunoglobulin A (IgA) is a natural ligand of hepatitis A virus cellular receptor 1 (HAVCR1), and the association of IgA with HAVCR1 enhances virus receptor interactions. J. Virol. 813437-3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Torok, N. J., E. M. Larusso, and M. A. McNiven. 2001. Alterations in vesicle transport and cell polarity in rat hepatocytes subjected to mechanical or chemical cholestasis. Gastroenterology 1211176-1184. [DOI] [PubMed] [Google Scholar]
- 65.Tseng, C.-T. K., J. Tseng, L. Perrone, M. Worthy, V. Popov, and C. J. Peters. 2005. Apical entry and release of severe acute respiratory syndrome associated coronavirus in polarized Calu-3 lung epithelial cells. J. Virol. 799470-9479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tugizov, S. M., J. W. Berline, and J. M. Palefsky. 2003. Epstein-Barr virus infection of polarized tongue and nasopharyngeal epithelial cells. Nat. Med. 9307-314. [DOI] [PubMed] [Google Scholar]
- 67.Tuma, P. L., C. M. Finnegan, J. H. Yi, and A. L. Hubbard. 1999. Evidence for apical endocytosis in polarized hepatic cells: phosphoinositide 3-kinase inhibitors lead to the lysosomal accumulation of resident apical plasmamembrane proteins. J. Cell Biol. 1451089-1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tuma, P. L., L. K. Nyasae, and A. L. Hubbard. 2002. Nonpolarized cells selectively sort apical proteins from cell surface to a novel compartment, but lack apical retention mechanisms. Mol. Biol. Cell 133400-3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tyteca, D., S. C. D. van Ijzendoorn, and D. Hoekstra. 2005. Calmodulin modulates hepatic membrane polarity by protein kinase C-sensitive steps in the basolateral endocytic pathway. Exp. Cell Res. 310293-302. [DOI] [PubMed] [Google Scholar]
- 70.van IJzendoorn, S. C., M. M. Zegers, J. W. Kok, and D. Hoekstra. 1997. Segregation of glucosylceramide and sphingomyelin occurs in the apical to basolateral transcytotic route in HepG2 cells. J. Cell Biol. 137347-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vidricaire, G., and M. J. Tremblay. 2007. A clathrin, caveolae, and dynamin independent endocytic pathway requiring free membrane cholesterol drives HIV-1 internalization and infection in polarized trophoblastic cells. J. Mol. Biol. 3681267-1283. [DOI] [PubMed] [Google Scholar]
- 72.Wasley, A., A. Fiore, and B. P. Bell. 2006. Hepatitis A in the era of vaccination. Epidemiol. Rev. 28101-111. [DOI] [PubMed] [Google Scholar]
- 73.Wilton, J. C., and G. M. Matthews. 1996. Polarized membrane traffic in hepatocytes. Bioessays 18229-236. [DOI] [PubMed] [Google Scholar]
- 74.Wisher, M. H., and W. H. Evans. 1975. Functional polarity of the rat hepatocyte surface membrane. isolation and characterization of plasma-membrane subfractions from the blood-sinusoidal, bile-canalicular and contiguous surfaces of the hepatocyte. Biochem. J. 146375-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wolf, J. L., D. H. Rubin, R. Finberg, R. S. Kauffman, A. H. Sharpe, J. S. Trier, and B. N. Fields. 1981. Intestinal M cells: a pathway for entry of reovirus into the host. Science 212471-472. [DOI] [PubMed] [Google Scholar]
- 76.Wustner, D., S. Mukherjee, F. R. Maxfield, P. Muller, and A. Herrmann. 2001. Vesicular and nonvesicular transport of phosphatidylcholine in polarized HepG2 cells. Traffic 2277-296. [DOI] [PubMed] [Google Scholar]
- 77.Zaal, K. J., J. W. Kok, R. Sormunen, S. Eskelinen, and D. Hoekstra. 1994. Intracellular sites involved in the biogenesis of bile canaliculi in hepatic cells. Eur. J. Cell Biol. 6310-19. [PubMed] [Google Scholar]
- 78.Zhou, F., A. Shefer, C. Weinbaum, M. McCauley, and Y. Kong. 2007. Impact of hepatitis A vaccination on health care utilization in the United States, 1996-2004. Vaccine 253581-3587. [DOI] [PubMed] [Google Scholar]