Abstract
Type I interferon (IFN-α/β) comprises a family of immunomodulatory cytokines that are critical for controlling viral infections. In cell culture, many RNA viruses trigger IFN responses through the binding of RNA recognition molecules (RIG-I, MDA5, and TLR-3) and induction of interferon regulatory factor IRF-3-dependent gene transcription. Recent studies with West Nile virus (WNV) have shown that type I IFN is essential for restricting infection and that a deficiency of IRF-3 results in enhanced lethality. However, IRF-3 was not required for optimal systemic IFN production in vivo or in vitro in macrophages. To begin to define the transcriptional factors that regulate type I IFN after WNV infection, we evaluated IFN induction and virus control in IRF-7−/− mice. Compared to congenic wild-type mice, IRF-7−/− mice showed increased lethality after WNV infection and developed early and elevated WNV burdens in both peripheral and central nervous system tissues. As a correlate, a deficiency of IRF-7 blunted the systemic type I IFN response in mice. Consistent with this, IFN-α gene expression and protein production were reduced and viral titers were increased in IRF-7−/− primary macrophages, fibroblasts, dendritic cells, and cortical neurons. In contrast, in these cells the IFN-β response remained largely intact. Our data suggest that the early protective IFN-α response against WNV occurs through an IRF-7-dependent transcriptional signal.
The production of type I interferon (IFN-α/β) after viral infection usually is triggered by the recognition of pathogen-associated molecular patterns (PAMP) on viral proteins or nucleic acids by specific host sensors known as pattern recognition receptors (PRR) (reviewed in reference 20 and 34). PRR for RNA viruses recognize single- or double-stranded RNA motifs on the cell surface, in endosomes (TLR3, TLR7, and TLR8), or in the cytoplasm (MDA5 and RIG-I) and activate the downstream transcriptional factors IRF-3 and IRF-7 to induce IFN gene transcription and protein production (reviewed in references 5 and 17).
IFN production and signaling generally are thought to occur through a two-step amplification model (27, 39, 40) in which IFN-β initially is produced in an IRF-3-dependent manner from virus-infected cells to trigger the expression of interferon-stimulated genes (ISGs), including IRF-7. The activation of IRF-7 then promotes additional IFN-α and IFN-β production via a positive feedback loop. More recently, a model has been proposed in which IRF-3 and IRF-7 both activate the initial phase of IFN-α and IFN-β gene expression in specific cell types (17-19).
Innate immune responses are required for the control of West Nile virus (WNV) infection (reviewed in reference 35 and 49). IFN-α and IFN-β gene induction is essential to the host response against WNV, as mice lacking the IFN-α/β receptor (IFN-αβR−/−) are vulnerable to WNV infection, with expanded tissue tropism, uncontrolled viral replication, rapid dissemination to the central nervous system (CNS), and uniform death (36). Studies in cell culture suggest that WNV triggers IFN production through processes involving RIG-I, MDA5 (13), and protein kinase R (PKR) (15). Pathogenic WNV strains disrupt IFN-α/β signaling by modulating JAK-STAT pathway signals, thereby attenuating specific ISG expression and the innate antiviral response, resulting in the enhanced spread of infection (10, 16, 21, 24). The WNV control of JAK-STAT signaling might be expected to impact the overall level of IRF-7 expression that is induced by the IFN response within infected cells.
WNV is a potent trigger of IRF-3 activation (4, 14, 41, 45), and its antiviral response limits the spread of infection. Accordingly, IRF-3−/− mice, similarly to IFN-αβR−/− mice, show greater WNV burden in the periphery, expanded tissue tropism, and early entry into the CNS, leading to uniform lethality after subcutaneous infection with low doses of virus (6). Somewhat surprisingly, and in contrast to that observed with encephalomyocarditis virus (EMCV) infection (40), an absence of IRF-3 in vivo did not impair the systemic IFN-α or IFN-β responses after WNV infection (4, 6). Ex vivo experiments in primary macrophages showed IRF-3-restricted WNV infection through an IFN-independent mechanism by regulating the basal expression of key host defense molecules, including ISG54, ISG56, RIG-I, and MDA5 (6). In contrast, IRF-3 directly regulated IFN-α/β gene expression and protein production in cortical neuron cultures. Thus, IRF-3 exerts its antiviral activity against WNV through IFN-dependent and IFN-independent pathways in a cell type-specific manner (4, 6).
IRF-7, which is downstream of the Myd88-dependent TLR7 and TLR8 responses, has been suggested as a master transcriptional regulator of type I IFN-dependent immune responses (19). Initial studies with Sendai virus in HeLa cells suggested that IRF-3 and IRF-7 coordinately induce IFN-β gene transcription after infection (50). Subsequent experiments in murine embryonic fibroblasts (MEF) showed that IRF-7 activates IFN-α genes through a positive feedback loop after IFN-β gene induction (23, 27, 39). However, the specific role of IRF-7 in regulating IFN-α/β gene expression in distinct tissue and cell types and its effect on viral replication remain controversial. Given that IRF-3 modulated WNV replication but was not required for systemic IFN production, we hypothesized that IRF-7 has a dominant regulatory role in specific cell types. Herein, we show that IRF-7−/− mice are vulnerable to WNV infection with uncontrolled viral replication and rapid mortality. Moreover, IRF-7 controls WNV replication in peripheral and CNS tissues in vivo and in primary cells ex vivo, in part by its ability to directly regulate IFN-α gene responses.
MATERIALS AND METHODS
Mouse experiments and quantitation of viral burden.
C57BL/6 wild-type inbred mice were commercially obtained (Jackson Laboratories, Bar Harbor, ME). The congenic, backcrossed IRF-7−/− mice were the generous gift of T. Taniguchi (Tokyo, Japan) (19). All mice were genotyped and bred in the animal facilities of the Washington University School of Medicine, and experiments were performed in accordance with Washington University animal studies guidelines. Eight- to 12-week-old mice were used for all in vivo studies. For peripheral infection, 102 PFU of WNV was diluted in Hanks balanced salt solution (HBSS) supplemented with 1% heat-inactivated fetal bovine serum (FBS) and inoculated by footpad injection in a volume of 50 μl. Intracranial (i.c.) inoculation was performed by injecting 101 PFU of WNV diluted in 10 μl of HBSS with 1% FBS.
Viruses.
The WNV strain (3000.0259) was isolated in New York in 2000 and passaged once in C6/36 cells to generate a stock virus that was used in all experiments.
Quantification of tissue viral burden and viremia.
To monitor viral spread in vivo, mice were infected with 102 PFU of WNV by footpad inoculation and sacrificed at days 1, 2, 4, 6, 8, and 10 after inoculation. For the subcutaneous infection experiments, the wild-type control data were from a previous publication on WNV and IRF-3−/− mice (6). Indeed, control experiments with wild-type mice were performed concurrently with IRF-3−/− and IRF-7−/− mice (although samples were processed at different times) to facilitate direct comparisons (Tables 1 and 2). In other experiments, mice were infected with 101 PFU of WNV by an i.c. route and sacrificed at days 2 and 4 after infection. After extensive cardiac perfusion with phosphate-buffered saline, organs were harvested, weighed, and homogenized, and virus was titrated by standard plaque assay as previously described (9). Viral burden also was measured by analyzing RNA levels using fluorogenic quantitative reverse transcription-PCR (qRT-PCR) as previously described (38).
TABLE 1.
Viral burden in IRF-3−/− and IRF-7−/− animalsa
| Viremia or infection site | Change in viral burden
|
|||||
|---|---|---|---|---|---|---|
| IRF-3−/−
|
IRF-7−/−
|
|||||
| D2 | D4 | D6 | D2 | D4 | D6 | |
| Viremia | ↑11 | ↑5 | ↑10 | ↑57 | ↑11 | ↑21 |
| Lymph node | ↑2 | ↑5 | NDb | ↑22 | ↑16 | ND |
| Spleen | ↑8 | ↑10 | ↑32 | ↑400 | ↑80 | ↑56 |
| Kidney | No change | No change | ↑4 | No change | ↑250 | ↑100 |
| Brain | No change | ↑25 | ↑20 | No change | ↑320 | ↑500 |
| Spinal Cord | No change | No change | ↑20 | No change | ↑16 | ↑5,000 |
Data are expressed as the n-fold increase (↑) or decrease (↓) in viral burden compared to that of wild-type tissues, as determined in this paper or in reference 6. D2, D4, D6, day 2, day 4, and day 6, respectively.
ND, not determined.
TABLE 2.
IFN production in IRF-3−/− and IRF-7−/− cellsa
| Cell type | Change in IFN production
|
|||||
|---|---|---|---|---|---|---|
| IRF-3−/−
|
IRF-7−/−
|
|||||
| 24 h | 48 h | 72 h | 24 h | 48 h | 72 h | |
| Macrophages | ||||||
| Virus production | ↑16 | ↑63 | ↑8 | No change | ↑16 | ↑12 |
| IFN-α mRNA induction | ↑5 | No change | NDb | ↓7 | ↓ 9 | ND |
| IFN-β mRNA induction | ↑14 | ↑6 | ND | ↑2 | ↑7 | ND |
| Bone marrow-DC | ||||||
| Virus production | ↑6 | ↑13 | ↑10 | ↑3 | ↑2 | ↑4 |
| IFN-α production | No change | No change | No change | No change | ↓16 | ↓20 |
| IFN-β production | No change | No change | No change | No change | ↓2 | ↓2 |
| MEFc | ||||||
| Virus production | No change | ↑16 | ↑95 | No change | ↑7 | ↑11 |
| IFN-α production | No change | No change | ND | No change | ↓9 | ND |
| IFN-β production | No change | No change | ND | No change | No change | ND |
| Cortical neurons | ||||||
| Virus production | No change | ↑5 | ND | ↑7 | ↑12 | ND |
| IFN-α mRNA induction | ↓100 | ↓5 | ND | ↓140 | ↓140 | ND |
| IFN-β mRNA induction | ↓115 | ↓3 | ND | ↓3 | No change | ND |
Data are expressed as the n-fold increase (↑) or decrease (↓) in virus production or IFN gene expression compared to that of wild-type cells, as determined in this paper or in reference 6.
ND, not determined.
The data for IRF-3−/− MEF are from an unpublished source.
Quantification of IFN activity. (i) L929 bioassay.
The relative levels of biologically active IFN in serum were determined using an EMCV L929 cytopathic effect bioassay as described previously (6). The specificity of the assay for type I IFN was confirmed using a previously described neutralizing anti-IFN-α/β receptor monoclonal antibody (MAb; MAR1-5A3) (44).
(ii) IFN-α and IFN-β mRNA qRT-PCR assay.
Total RNA was isolated from primary cells using the RNeasy kit according to the manufacturer's instructions (Qiagen). During the isolation, to remove any contaminating DNA, samples were treated with RNase-free DNase (Qiagen). IFN-α and IFN-β mRNA were amplified and quantified from total RNA by qRT-PCR using previously published primer sets (36). To analyze the relative change (n-fold) in the induction of IFN-α and IFN-β mRNA, 18S rRNA expression levels also were determined for normalization using the threshold cycle method as described previously (25).
(iii) IFN-α and IFN-β protein ELISA.
To measure levels of secreted IFN-α and IFN-β protein in cell supernatants, a commercial capture enzyme-linked immunosorbent assay (ELISA) with a standard curve was used according to the manufacturer's instructions (PBL Biomedical Laboratories, NJ).
Macrophage and dendritic cell infection.
Macrophages and myeloid dendritic cells (mDC) were generated as described previously (6). Briefly, cells were isolated from the bone marrow of wild-type or IRF-7−/− mice and cultured for 7 days either in the presence of 40 ng/ml macrophage colony-stimulating factor (PeproTech Inc., Rocky Hill, NJ) to generate macrophages or with 20 ng/ml granulocyte-macrophage colony-stimulating factor and 20 ng/ml interleukin-4 (PeproTech Inc., Rocky Hill, NJ) to generate mDC. Multistep virus growth curves were performed after infection at a multiplicity of infection (MOI) of 0.01 for macrophages and 0.001 for mDC. Supernatants were titrated by plaque assay on BHK21-15 cells (9). To test for the induction of IFN-α and IFN-β after WNV infection, 5 × 105 macrophages or 2 × 105 mDC were infected at an MOI of 0.1. IFN-α and IFN-β mRNA were quantified by qRT-PCR, and protein was measured from supernatants as described above.
MEF infection.
MEF were generated from day-14 wild-type or IRF-7−/− embryos and maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% FBS according to established protocols. Multistep virus growth curves and IFN-α and IFN-β gene induction and protein secretion assays were performed as described above after infection at MOIs of 0.001 and 0.1, respectively.
Primary neuron infection.
Primary cortical neurons were prepared from day-15 wild-type and IRF-7−/− mouse embryos as described previously (22, 37). Neurons were seeded in 24-well poly-d-lysine/laminin-coated plates in DMEM containing 5% heat-inactivated FBS and 5% horse serum for 24 h. Cortical neurons then were cultured for 4 days with Neurobasal medium containing B27 and l-glutamine (Invitrogen). Multistep virus growth curves and IFN-α and IFN-β gene induction and protein secretion assays were performed as described above after infection at MOIs of 0.001 and 0.1, respectively.
Immunoblotting.
MEF or macrophages (106 cells) were lysed in radioimmunoprecipitation assay buffer (10 mM Tris, 150 mM NaCl, 0.02% sodium azide, 1% sodium deoxycholate, 1% Triton X-100, 0.1% sodium dodecyl sulfate [SDS], pH 7.4) with protease inhibitors (Sigma) and 1 mM okadaic acid (Sigma). Samples (30 μg) were resolved on 10% SDS-polyacrylamide gels. Following transfer, membranes were blocked with 5% nonfat dried milk overnight at 4°C. Membranes were probed with the following panel of monoclonal or polyclonal antibodies: anti-WNV (Centers for Disease Control and Prevention), anti-tubulin (Santa Cruz Biotechnology), anti-PKR (Santa Cruz Biotechnology), and anti-mouse ISG56 and anti-mouse ISG54 (gifts from G. Sen, Cleveland, OH). The RIG-I antibody was described previously (12). Blots were incubated with peroxidase-conjugated secondary antibodies (Jackson Immunoresearch) and visualized using ECL-plus immunoblotting reagents (Amersham Biosciences).
Statistical analysis.
For in vitro experiments, an unpaired two-tailed t test was used to determine statistically significant differences. For viral burden analysis, differences in log titers were analyzed by the Mann-Whitney test. Kaplan-Meier survival curves were analyzed by the log rank test. All data were analyzed using Prism software (GraphPadPrism4, San Diego, CA).
RESULTS
IRF-7 is required for control of lethal WNV infection.
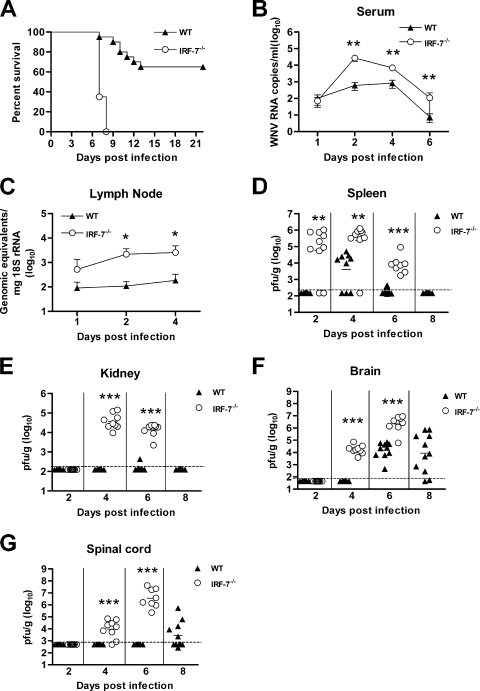
Mice lacking IFN-α/β receptors are vulnerable to WNV infection due to rapid and overwhelming viral replication and altered tissue tropism (36). IRF-3−/− mice also showed uniform mortality but, surprisingly, had a largely intact systemic IFN response (6). To better understand the regulation of type I IFN and the modulation of WNV infection in vivo, we infected IRF-7−/− C57BL/6 mice with a highly pathogenic strain. After footpad inoculation with 102 PFU of a New York strain of WNV, IRF-7−/− mice showed an increased rate and severity of clinical signs and symptoms of illness, including hunchback posture, weight loss, fur ruffling, and reduced activity. Whereas wild-type mice show 65% survival and a mean time of death of 10.7 ± 2 days after inoculation with 102 PFU of WNV, IRF-7−/− mice were more vulnerable, with a 0% survival rate and a mean time of death of 7.4 ± 0.5 days (P < 0.0001) (Fig. 1A). In comparison, in studies performed in parallel, congenic IRF-3−/− mice had a mean time of death of 9.3 ± 1 days after WNV infection at this dose (6). Thus, WNV infection causes a more severe phenotype in IRF-7−/− mice than IRF-3−/− mice, and signaling through IRF-7 is absolutely essential for protection.
FIG. 1.
Survival and viral burden analysis for wild-type (WT) and IRF-7−/− C57BL/6 mice. (A) Eight- to 12-week-old mice were inoculated with 102 PFU of WNV by footpad injection and were monitored for mortality for 21 days. Survival differences were statistically significant (wild-type mice, n = 20; IRF-7−/− mice, n = 20; P < 0.0001). (B to G) Viral burden in peripheral and CNS tissues after WNV infection. WNV RNA in serum (B) and draining lymph node (C) and infectious virus in the spleen (D), kidney (E), brain (F), and spinal cord (G) were determined from samples harvested on days 1, 2, 4, 6, and 8 using qRT-PCR (B and C) or viral plaque assay (D to G). Data are shown as viral RNA equivalents or PFU per gram of tissue for 10 to 12 mice per time point. For all viral data, the solid line represents the median PFU per gram at the indicated time point, and the dotted line represents the limit of the sensitivity of the assay. Asterisks indicate values that are statistically significant (*, P < 0.05; **, P < 0.005; ***, P < 0.0001) compared to results for wild-type mice. As discussed in Materials and Methods, the control wild-type viral burden and survival data in this figure have been reported in a prior publication (6).
IRF-7−/− mice show expanded tissue tropism and enhanced WNV replication.
As IRF-7 has been identified as a transcriptional regulator of the type I IFN response (19), we hypothesized that the observed increased lethality would correlate with higher viral burdens in tissue. To evaluate this, IRF-7−/− and wild-type mice were infected with 102 PFU of WNV, and the viral burden was measured by fluorogenic qRT-PCR or viral plaque assay at days 1, 2, 4, and 6 postinfection in serum, peripheral organs (draining lymph nodes, spleen, and kidney), and the CNS (brain and spinal cord).
(i) Viral RNA in serum and lymph node.
Within 2 days of WNV infection, 50-fold higher (P < 0.005) levels of viral RNA were detected in serum of IRF-7−/− mice. Increased RNA levels (9- to 15-fold; P < 0.005) persisted in IRF-7−/− mice through day 6 (Fig. 1B), after which the mice succumbed to infection. In the draining popliteal lymph node, increased WNV replication also was observed at day 1 after infection and remained elevated at days 2 and 4 (15- to 22-fold; P < 0.05) (Fig. 1C). Based on virological analysis, IRF-7 has a key role in restricting WNV replication soon after infection.
(ii) Viral RNA in spleen and kidney.
Consistent with prior studies (6, 38), WNV was not detected in the spleen by plaque assay at day 2 after infection in wild-type mice (n = 10). In contrast, 80% (8 of 10) of IRF-7−/− mice had a measurable infection at day 2 (mean titer of 104.8 PFU/g; P < 0.005) (Fig. 1D). By day 4, which corresponds to the peak of splenic infection in wild-type animals, higher WNV titers were observed in IRF-7−/− mice (mean titer of 105.4 PFU/g for IRF-7−/− mice and 103.6 PFU/g for wild-type mice; P < 0.005). Elevated levels of infectious WNV (mean titer of 103.9 PFU/g; P < 0.0001) still were detected at day 6 in all (8 of 8) IRF-7−/− mice, whereas WNV was cleared from the spleen in the majority (3 of 10 remained positive) of the wild-type animals. The kidney in wild-type C57BL/6 mice is relatively resistant to WNV infection, as the virus is not usually detected in this organ. Nonetheless, similarly to that observed with IFN-α/βR−/− mice (36), significant WNV replication was detected in the kidneys of IRF-7−/− mice (at day 4, 104.6 PFU/g; day 6, 104.1 PFU/g) (Fig. 1E). Thus, IRF-7-dependent signaling pathways modulate WNV infection in peripheral tissues and restrict tissue tropism.
(iii) Viral RNA in the CNS.
WNV spread to the CNS more rapidly and replicated to higher levels in IRF-7−/− mice. Infectious WNV was present in the brains of all IRF-7−/− mice (9 of 9) at day 4 after infection (104.3 PFU/g), whereas in wild-type mice WNV was not detected until day 6. IRF-7−/− mice also averaged significantly higher (∼200-fold) viral titers than wild-type mice in the brain on day 6 after infection (106.4 PFU/g for IRF-7−/− mice and 104.1 PFU/g for wild-type mice; P < 0.0001), corresponding with the increased mortality rate (Fig. 1F). A similar pattern of early infection in the spinal cord was observed, with 78% (7 of 9) and 100% (8 of 8) of IRF-7−/− mice having detectable viral loads at day 4 (104.0 PFU/g) and at day 6 (106.5 PFU/g), respectively. In contrast, none of the wild-type mice had measurable WNV in the spinal cord at these time points. These data show that an absence of IRF-7 signaling resulted in early spread to and sustained WNV replication in the CNS.
IFN-α/β levels in circulation are blunted in IRF-7−/− mice.
We hypothesized that the enhanced replication and spread of WNV in IRF-7−/− mice was due to the blunted production of type I IFN. To evaluate this, IRF-7−/− and wild-type mice were infected with WNV, and the relative levels of biologically active type I IFN in serum was monitored using a validated EMCV-L929 cell protection bioassay (1). Type I IFN protective activity in the serum of infected wild-type mice peaked at 72 h and then slightly decreased at 96 h (Fig. 2A). The specificity of the assay for measuring type I IFN activity was confirmed with a neutralizing MAb against the IFN-α/β receptor (Fig. 2B). In IRF-7−/− mice, the relative levels of IFN at 48 h were slightly but not significantly reduced (1.5-fold reduction; P > 0.2). However, by 72 and 96 h after infection, serum IFN activity was reduced in IRF-7−/− mice (up to 8-fold; P < 0.05) despite the higher viral loads.
FIG. 2.
Relative type I IFN levels in sera of wild-type (WT) and IRF-7−/− mice infected with WNV. (A) Mice were inoculated with 102 PFU of WNV by footpad injection and sacrificed at the indicated times. Relative type I IFN activity was determined from sera collected on days 1 to 4 after WNV infection by an EMCV bioassay in L929 cells. Data reflect the averages from serum samples harvested from 5 to 10 mice per time point and are shown as the percentages of cells protected from lysis by EMCV (see Materials and Methods). Asterisks indicate differences that are statistically significant (*, P < 0.05; **, P < 0.005). (B) Specificity of bioassay for type I IFN. L929 cells either were untreated or pretreated with a neutralizing MAb (10 μg/ml) against the IFN-α/β receptor. Sera from WNV-infected mice obtained at the indicated times were added to cells. After subsequent infection with EMCV, the cytopathic effect was measured 7 h later.
IRF-7 restricts WNV infection, IFN induction, and ISG regulation in MEF.
To begin to investigate the cell-specific roles of IRF-7, we assayed WNV infection and IFN production in wild-type and IRF-7−/− MEF, a primary cell that has been used extensively in virus infection-host immune response assays (13, 27, 39). Whereas virologic analysis showed no significant difference in WNV burden at 24 h after infection, modestly enhanced viral replication was observed in IRF-7−/− MEF at 48 (a sixfold increase; P < 0.001) and 72 h postinfection (a fourfold increase; P < 0.001) (Fig. 3A). Thus, IRF-7 signaling partially controls WNV infection in MEF.
FIG. 3.
IRF-7 limits WNV replication and controls IFN-α induction and early host defense activation in primary MEF. (A) MEF generated from wild-type (WT) or IRF-7−/− mice were infected at an MOI of 0.001, and virus production was evaluated at the indicated times postinfection by plaque assay. Values are averages from quadruplicate samples generated from at least three independent experiments. The dotted line represents the limit of the sensitivity of the assay. (B) Whole-cell lysates were generated at the indicated times from wild-type or IRF-7−/− MEF that were uninfected (U) or infected with WNV (W). Protein levels of IRF-7, PKR, ISG54, RIG-I, ISG56, and tubulin were examined by immunoblot analysis. (C and D) Total RNA from uninfected and WNV-infected MEF was harvested and analyzed for IFN-α (C) and IFN-β (D) mRNA expression by qRT-PCR. Data are normalized to values for 18S rRNA and are expressed as the relative (n-fold) increase above the level of RNA from uninfected controls. Average values are averages from duplicate samples from at least three independent experiments. (E and F) Accumulation of IFN-α (E) and IFN-β (F) protein in supernatants of WNV-infected MEF as determined by ELISA. The data are the averages from at least three independent experiments performed in quadruplicate (***, P < 0.0001).
As we previously showed that increased replication could result from altered antiviral protein expression, we examined both the basal and induced expression of host defense molecules by immunoblotting. In wild-type MEF, IRF-7 was not basally expressed. The infection of the cells with WNV rapidly induced IRF-7 protein production, as high levels were detected within 24 h (Fig. 3B). IRF-7 expression in wild-type MEF was transient and was no longer detected 48 h postinfection. These results are consistent with a WNV-imposed block of JAK-STAT signaling that occurs during infection and attenuates the expression of ISGs, possibly including IRF-7 (10, 16, 21, 24, 29), and also may reflect the rapid degradation of IRF-7 that occurs after its activation (19). Whereas the expression of PKR in resting and WNV-infected IRF-7−/− MEF did not differ from that of wild-type cells, the induction of RIG-I, ISG54, and ISG56 after WNV infection was blunted in IRF-7−/− MEF during the first 24 h.
To assess whether this phenotype also correlated with a defect in the IFN response, IFN-α/β gene transcription (Fig. 3C and D) and protein secretion (Fig. 3E and F) were measured. In wild-type MEF, IFN-α/β mRNA levels rapidly increased at 24 h (80- and 1,000-fold, respectively), and by 48 h, levels of secreted IFN-α (∼780 pg/ml) and IFN-β (∼3,300 pg/ml) were easily measured. In contrast, in IRF-7−/− MEF increases in IFN-α mRNA and protein were virtually abolished at all time points, whereas IFN-β mRNA and protein levels were largely sustained after WNV infection. Thus, IRF-7 modestly restricts WNV infection in MEF and is required for the induction of IFN-α but is almost completely dispensable for the stimulation of IFN-β.
IRF-7 restricts WNV infection and IFN induction in macrophages.
To explore further the cellular basis for the virologic and immunologic phenotypes in IRF-7−/− mice, we next investigated the effect of IRF-7 on WNV infection in macrophages, a cell type that is permissive for WNV infection in vivo (33, 35). Although no difference was observed at 24 h, enhanced replication was detected in IRF-7−/− macrophages beginning at 48 h and gradually increased at 72 h, resulting in a ∼20-fold increase (P ≤ 0.005) (Fig. 4A). In comparison, IRF-3−/− macrophages display significantly increased WNV replication at 24 h after infection (6) (Table 2). Thus, although IRF-7-dependent signals limit WNV replication in macrophages, they do so at a later stage of infection relative to that of IRF-3.
FIG. 4.
IRF-7 modulates WNV infection and regulates IFN-α induction in primary macrophages. (A) Bone marrow-derived macrophages (BM-Mφ) generated from wild-type or IRF-7−/− mice were infected at an MOI of 0.01, and virus production was evaluated at the indicated times postinfection by plaque assay. Values are averages from quadruplicate samples generated from at least three independent experiments (**, P < 0.005). The dotted line represents the limit of the sensitivity of the assay. (B) Whole-cell lysates were generated at the indicated times from wild-type (WT) or IRF-7−/− macrophages that were uninfected (U) or infected with WNV (W). Protein levels of IRF-7, PKR, ISG54, RIG-I, and tubulin were examined by immunoblot analysis. (C and D) The induction of IFN-α (C) and IFN-β (D) mRNA in WNV-infected macrophages was analyzed by qRT-PCR as described in the legend to Fig. 3.
IRF-3 restricts WNV replication in macrophages by regulating the basal expression of host defense molecules (6). To test whether IRF-7 inhibited WNV infection through a similar mechanism, we performed immunoblot analysis. IRF-7 rapidly accumulated in wild-type macrophages following WNV infection, with levels peaking at 24 h and again falling by 48 h after infection (Fig. 4B). In contrast to MEF, resting macrophages basally expressed IRF-7, which could contribute to the relatively low permissiveness of these cells to WNV. Immunoblot analysis before or after infection showed similar expression levels of ISG54 among wild-type and IRF-7−/− macrophages, with modestly reduced levels of RIG-I in the latter (Fig. 4B). Basal PKR production was somewhat reduced in IRF-7−/− macrophages, whereas infection-induced levels were comparable to those of wild-type cells. As a deficiency of IRF-7 did not extensively alter the expression profile of host defense genes, we hypothesized that it might restrict viral replication in macrophages by directly modulating the IFN response. To evaluate this, we measured IFN-α and IFN-β mRNA levels after WNV infection (Fig. 4C and D). Whereas IFN-α mRNA levels in wild-type macrophages gradually increased throughout the time course, IFN-α gene induction in IRF-7−/− macrophages was blunted (five- to sixfold reduction at 24 and 48 h; P < 0.0001). In comparison, the induction of IFN-β mRNA remained largely intact in IRF-7−/− macrophages: at 24 h, a similar ∼10-fold induction of IFN-β mRNA levels was observed, and by 48 h, fivefold higher IFN-β mRNA levels were apparent in IRF-7−/− macrophages (P < 0.0001), possibly because of the higher viral burden that may trigger increased IFN-β expression. Overall, these experiments suggest that IRF-7 restricts WNV replication in macrophages only after the first 24 h through an IFN-α-dependent, but IFN-β-independent, mechanism.
IRF-7-dependent regulation of the IFN response in mDC.
mDC may be early targets for flavivirus infection in vivo (7, 28, 35, 48) and are important for inducing innate and adaptive antiviral immune responses. To further evaluate the role of IRF-7 in regulating host defense responses, we infected wild-type and IRF-7−/− bone marrow-derived CD11c+ mDC with WNV and assayed viral replication and IFN production (Fig. 5). Multistep viral growth curve analysis showed a small (two- to threefold; P < 0.05) yet statistically significantly higher viral burden in IRF-7−/− mDC than in wild-type cells (Fig. 5A). An analysis of IFN gene induction revealed a pattern consistent with that of other cell types (Fig. 5B and C): whereas both IFN-α and IFN-β mRNA levels rapidly increased (∼7,000- and ∼1,500-fold, respectively) in wild-type mDC, IRF-7−/− mDC showed blunted IFN-α gene induction (∼25-fold less; P < 0.0001) yet relatively intact IFN-β mRNA levels (∼2-fold less; P < 0.05). Consistently with this, infected IRF-7−/− mDC failed to secrete detectable IFN-α but produced only slightly reduced amounts of IFN-β (1.5- to 2-fold reduction; P < 0.001) (Fig. 5D and E). These data suggest that in mDC, IRF-7 contributes slightly to IFN-β production but is essential for the expression and production of IFN-α.
FIG. 5.
IRF-7 restricts WNV infection and regulates IFN responses in mDC. (A) Bone marrow-derived mDC (BM-DC) generated from wild-type (WT) or IRF-7−/− mice were infected at an MOI of 0.001, and virus production was evaluated at the indicated times postinfection by plaque assay. Values are averages from quadruplicate samples generated from at least three independent experiments. (B and C) The induction of IFN-α (B) and IFN-β (C) mRNA in WNV-infected mDC was analyzed by qRT-PCR as described in the legend to Fig. 3. *, P < 0.05; **, P < 0.005; ***, P < 0.0001. (D and E) The accumulation of IFN-α (D) and IFN-β (E) protein in supernatants of WNV-infected mDC was determined by ELISA. The data are the averages from at least three independent experiments performed in quadruplicate. The dotted lines represent the limit of sensitivity of the individual assays.
IRF-7 controls WNV replication and induces IFN in primary cortical neurons.
Neurons produce type I IFN after infection by WNV and by other RNA viruses (8, 32). To evaluate whether IRF-7 limits WNV replication and/or regulates antiviral responses in a key CNS target cell, we cultured wild-type and IRF-7−/− cortical neurons (98 to 99% purity) and performed multistep growth curve and IFN induction analyses (Fig. 6). IRF-7−/− cortical neurons produced higher levels of WNV at 24 h that increased by 48 h after infection (∼7-fold [P < 0.05] and ∼12-fold, [P < 0.005], respectively) (Fig. 6A). Associated with this, markedly decreased levels of IFN-α mRNA (∼150-fold decrease at 24 and 48 h postinfection) were observed in WNV-infected IRF-7−/− cortical neurons (Fig. 6B). In contrast, levels of IFN-β mRNA were normal or higher in WNV-infected IRF-7−/− cortical neurons than in wild-type cells (Fig. 6C). Overall, these experiments show that IRF-7 limits WNV replication in cortical neuron cultures and regulates the antiviral IFN-α response.
FIG. 6.
IRF-7 restricts WNV infection and modulates IFN induction in primary cortical neurons. (A) Primary cortical neurons generated from wild-type (WT) or IRF-7−/− mice were infected at an MOI of 0.001, and virus production was evaluated at the indicated times by plaque assay. Values are averages from triplicate samples generated from three independent experiments. Asterisks indicate values that are statistically significant (*, P < 0.05; **, P < 0.005). The dotted line represents the limit of the sensitivity of the assay. (B and C) The induction of IFN-α (B) and IFN-β mRNA (C) in WNV-infected cortical neurons was analyzed by qRT-PCR as described in the legend to Fig. 3.
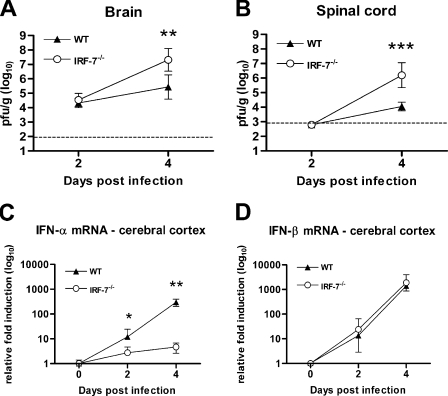
IRF-7 controls WNV replication in the CNS.
In vivo experiments were performed to support our results with primary cortical neuron cultures. Although CNS viral titers after peripheral WNV infection in IRF-7−/− mice were elevated, it was unclear whether this effect was due directly to an increased permissiveness of IRF-7−/− neurons or indirectly through greater CNS dissemination associated with the elevated and sustained viremia. To address this, wild-type and IRF-7−/− mice were infected with 101 PFU of WNV via an i.c. route, and the viral burdens in the brain and spinal cord were measured on days 2 and 4 (Fig. 7A). Whereas no significant differences were observed at day 2, IRF-7−/− mice showed ∼50-fold higher average viral burdens at day 4 after infection in the brain (107.3 PFU/g and 105.6 PFU/g for IRF-7−/− and wild-type mice, respectively; P < 0.005) and spinal cord (106.2 PFU/g and 104.0 PFU/g for IRF-7−/− and wild-type mice, respectively; P < 0.0001). These results suggest that IRF-7 directly limits WNV infection in the brain and spinal cord. To ascertain whether increased CNS titers in IRF-7−/− mice correlated with a difference in the IFN response, we analyzed IFN-α and IFN-β gene expression in the cerebral cortex at day 4 after i.c. infection (Fig. 7B and C). Levels of IFN-β mRNA in the cerebral cortex of IRF-7−/− and wild-type mice were similar. In contrast, IFN-α mRNA levels were ∼60-fold lower in IRF-7−/− mice at day 4 (P < 0.005). These experiments establish that in the brain, IFN-α but not IFN-β gene induction requires signaling through IRF-7.
FIG. 7.
IRF-7 controls viral replication and IFN-α induction in the CNS after i.c. WNV infection. (A and B) Wild-type (WT) or IRF-7−/− mice were inoculated with 101 PFU of WNV. Brains (A) and spinal cords (B) were harvested at the indicated time points, and viral titers were determined as described in the legend to Fig. 1. (C and D) The induction of IFN-α (C) and IFN-β (B) mRNA in WNV-infected cerebral cortices was analyzed by qRT-PCR as described in the legend to Fig. 3. *, P < 0.05; **, P < 0.005.
DISCUSSION
Protection against viral infections in mice requires an intact type I IFN signaling pathway to limit replication. Different cell types secrete various amounts of IFN and, thus, play distinct roles in protecting against viral infection (11, 47). Nonetheless, the cell-specific mechanism(s) for IFN induction remains incompletely characterized. Here, we demonstrate that IRF-7 is an essential transcriptional regulator of the early IFN-α response after WNV infection. Mice lacking IRF-7 showed a rapid, uniformly lethal infection, with higher viral burden, expanded tissue tropism, and earlier entry in the CNS. Correspondingly, the systemic type I IFN production in the blood was blunted in IRF-7−/− mice despite an increased viral burden. A deficiency of IRF-7 preferentially depressed the induction and accumulation of IFN-α, with the relative sparing of the IFN-β response in primary MEF, macrophages, mDC, and cortical neurons. Our data are most consistent with a model in which IRF-7 protects against WNV largely through its ability to induce IFN-α-dependent antiviral responses.
Recent studies using cell cultures have made progress in elucidating how antiviral pathways become activated. IFN gene induction is downstream of PRR, which detect viral nucleic acids and PAMPs. For positive-strand RNA viruses, RIG-I, MDA, and TLR3 recognize double-stranded RNA in the cytoplasm and endosome and induce IFN-β expression via the nuclear localization of the transcription factors IRF-3 and, possibly, IRF-7 (13, 18, 26). Although IRF-3 and IRF-7 appear to have overlapping transcriptional activities, studies suggest they may differentially activate IFN-α, IFN-β, and specific ISGs (30, 40, 43). These activities may be influenced by unique expression patterns; whereas IRF-3 often is constitutively expressed in many cells, the basal expression of IRF-7 may be restricted (31). Our experiments demonstrate that macrophages also constitutively express IRF-7. The basal expression of IRF-7 and other host defense molecules by macrophages may account for the relatively efficient control of WNV infection compared to that of other cell types.
Our experiments with mice and primary cells demonstrate that IRF-7 is essential for the regulation of the early induction of IFN-α. These findings are consistent with those of a prior study in which depressed systemic type I IFN levels and blunted IFN-α gene induction were observed in IRF-7−/− mice and primary MEF, mDC, and plasmacytoid DC after herpes simplex virus and EMCV infections (19). Beyond cell type-specific antiviral pathways, different viruses may themselves stimulate unique innate immune response patterns. In contrast to our results with WNV, IFN-β gene induction was markedly reduced in IRF-7−/− MEF after infection with herpes simplex virus, vesicular stomatitis virus, or EMCV and was lost in IRF-3−/− and IRF-7−/− MEF (19). In the context of these pathogens, IRF-3 and IRF-7 both may regulate IFN-β gene expression. While it is generally believed that IRF-3 regulates the early phase of IFN-β induction after RNA virus infection (40), in vivo WNV may not follow this paradigm. A direct comparison of the virologic and IFN phenotypes after WNV infection in different tissues and cell types (Tables 1 and 2) shows that in several cell types (macrophages, mDC, and MEF), only a deficiency of IRF-7 resulted in decreased IFN gene expression. This was unexpected, as IRF-7 signaling is thought to be downstream of IRF-3 activation and, thus, IRF-3−/− cells should have blunted levels of both IFN-α and IFN-β. Thus, in several cell types, (i) IRF-3 or IRF-7 by itself can induce IFN-β at wild-type levels or (ii) additional undefined signaling pathways stimulate IFN-β expression after WNV infection. Future studies with IRF-3−/− and IRF-7−/− mice and additional primary cells are planned to determine whether these two transcriptional activators solely determine type I IFN activation after WNV infection or whether other IRF proteins (e.g., IRF-1, IRF-5, or IRF-8) contribute (2, 3, 42, 46).
In addition to diverse IFN induction pathways, distinct cell types have different capacities to respond to IFN-α or IFN-β. For example, central and peripheral neuron subtypes showed inherent differences in the ability of IFN-α or IFN-β pretreatment to limit WNV infection (38). Consistently with this, there was no absolute correlation between the degree of IFN-α production and changes in viral replication. The increase in late-phase WNV replication in IRF-7−/− macrophages correlated with reduced levels of IFN-α, whereas smaller virological differences were observed in IRF-7−/− mDC and MEF despite a complete loss of IFN-α production. The exact contribution of individual IFN (e.g., IFN-α versus IFN-β) in regulating viral infection in vivo remains an unanswered question. The ability of WNV to evade IFN in part through the viral suppression of JAK-STAT signaling has been defined as a virulence determinant; attenuated ISG expression releases WNV from antiviral processes imposed by IFN (16, 21). Since IRF-7 is an ISG in many cell types, it is possible that the WNV regulation of IFN signaling confers altered levels of IRF-7 induction or abundance. It is interesting that the reduced levels of IRF-7 that occurred at 48 h after WNV infection in macrophages and fibroblasts (Fig. 3B and 4B) temporally associate with the WNV-imposed suppression of JAK-STAT signaling (21). Thus, altered levels of IRF-7 may further support WNV infection and spread among cells and tissues.
While this and other studies highlight the importance of IRF-7 in peripheral innate immune responses, little is known about its function in the brain. Our studies suggest an independent role for IRF-7 in regulating viral infection and IFN production in the CNS. Viral burden in the brain and spinal cord after i.c. infection was greater in IRF-7−/− mice at time points that precede the development of an adaptive immune response. Moreover, IFN-α induction in the cerebral cortex and primary cortical neurons was IRF-7 dependent, suggesting that IRF-7 plays a dominant role in the CNS and restricts WNV through IFN-dependent mechanisms. In contrast, IFN-β gene induction after WNV infection in cortical neurons apparently was not regulated by IRF-7. These results complement previous studies with WNV-infected IRF-3−/− neurons that showed blunted IFN-α and IFN-β gene responses (Table 2). Thus, the regulation of IFN-α and IFN-β in neurons appears to follow the more canonical, or two-step, model in which IRF-3 acts as a primary transcriptional activator and IRF-7 amplifies the response.
In summary, IRF-7 is essential for restricting WNV infection and inducing IFN-α-dependent antiviral responses. A deficiency of IRF-7 did not dramatically alter the primary IFN-β response but rather more specifically abrogated IFN-α induction. This study, combined with previous work, suggests that cell- and tissue-specific roles of IRF-3 and IRF-7 regulate antiviral programs against WNV and, likely, other viruses. An enhanced understanding of the molecular mechanisms of the earliest protective antiviral immune response may provide novel strategies for therapeutic intervention against viral pathogens.
Acknowledgments
We thank A. Blasius and M. Colonna for experimental advice and T. Taniguchi for the IRF-7−/− mice. We appreciate the critical reading of the manuscript by M. Colonna.
This work was supported by a New Scholar Award in Global Infectious Disease from the Ellison Medical Foundation (M.S.D.), a predoctoral fellowship from the Howard Hughes Medical Institute (M.A.S.), and by NIH AI057568 (M.G.).
The authors report no conflicts of interest.
Footnotes
Published ahead of print on 18 June 2008.
REFERENCES
- 1.Austin, B. A., C. James, R. H. Silverman, and D. J. Carr. 2005. Critical role for the oligoadenylate synthetase/RNase L pathway in response to IFN-beta during acute ocular herpes simplex virus type 1 infection. J. Immunol. 1751100-1106. [DOI] [PubMed] [Google Scholar]
- 2.Barnes, B. J., A. E. Field, and P. M. Pitha-Rowe. 2003. Virus-induced heterodimer formation between IRF-5 and IRF-7 modulates assembly of the IFNA enhanceosome in vivo and transcriptional activity of IFNA genes. J. Biol. Chem. 27816630-16641. [DOI] [PubMed] [Google Scholar]
- 3.Barnes, B. J., J. Richards, M. Mancl, S. Hanash, L. Beretta, and P. M. Pitha. 2004. Global and distinct targets of IRF-5 and IRF-7 during innate response to viral infection. J. Biol. Chem. 27945194-45207. [DOI] [PubMed] [Google Scholar]
- 4.Bourne, N., F. Scholle, M. C. Silva, S. L. Rossi, N. Dewsbury, B. Judy, J. B. De Aguiar, M. A. Leon, D. M. Estes, R. Fayzulin, and P. W. Mason. 2007. Early production of type I interferon during West Nile virus infection: role for lymphoid tissues in IRF3-independent interferon production. J. Virol. 819100-9108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Colonna, M. 2007. TLR pathways and IFN-regulatory factors: to each its own. Eur. J. Immunol. 37306-309. [DOI] [PubMed] [Google Scholar]
- 6.Daffis, S., M. A. Samuel, B. C. Keller, M. Gale, Jr., and M. S. Diamond. 2007. Cell-specific IRF-3 responses protect against West Nile virus infection by interferon-dependent and independent mechanisms. PLoS. Pathog. 3e106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davis, C. W., H. Y. Nguyen, S. L. Hanna, M. D. Sanchez, R. W. Doms, and T. C. Pierson. 2006. West Nile virus discriminates between DC-SIGN and DC-SIGNR for cellular attachment and infection. J. Virol. 801290-1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Delhaye, S., S. Paul, G. Blakqori, M. Minet, F. Weber, P. Staeheli, and T. Michiels. 2006. Neurons produce type I interferon during viral encephalitis. Proc. Natl. Acad. Sci. USA 1037835-7840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diamond, M. S., B. Shrestha, A. Marri, D. Mahan, and M. Engle. 2003. B cells and antibody play critical roles in the immediate defense of disseminated infection by West Nile encephalitis virus. J. Virol. 772578-2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Evans, J. D., and C. Seeger. 2007. Differential effects of mutations in NS4B on WNV replication and inhibition of interferon signaling. J. Virol. 8111809-11816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fitzgerald-Bocarsly, P., and D. Feng. 2007. The role of type I interferon production by dendritic cells in host defense. Biochimie 89843-855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fredericksen, B. L., and M. Gale, Jr. 2006. West Nile virus evades activation of interferon regulatory factor 3 through RIG-I-dependent and -independent pathways without antagonizing host defense signaling. J. Virol. 802913-2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fredericksen, B. L., B. C. Keller, J. Fornek, M. G. Katze, and M. Gale, Jr. 2008. Establishment and maintenance of the innate antiviral response to West Nile virus involves both RIG-I and MDA5 signaling through IPS-1. J. Virol. 82609-616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fredericksen, B. L., M. Smith, M. G. Katze, P. Y. Shi, and M. Gale. 2004. The host response to West Nile virus infection limits spread through the activation of the interferon regulatory factor 3 pathway. J. Virol. 787737-7747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gilfoy, F. D., and P. W. Mason. 2007. West Nile virus-induced IFN production is mediated by the double-stranded RNA-dependent protein kinase, PKR. J. Virol. 8111148-11158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guo, J. T., J. Hayashi, and C. Seeger. 2005. West Nile virus inhibits the signal transduction pathway of alpha interferon. J. Virol. 791343-1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hiscott, J. 2007. Triggering the innate antiviral response through IRF-3 activation. J. Biol. Chem. 28215325-15329. [DOI] [PubMed] [Google Scholar]
- 18.Honda, K., and T. Taniguchi. 2006. IRFs: master regulators of signalling by Toll-like receptors and cytosolic pattern-recognition receptors. Nat. Rev. Immunol. 6644-658. [DOI] [PubMed] [Google Scholar]
- 19.Honda, K., H. Yanai, H. Negishi, M. Asagiri, M. Sato, T. Mizutani, N. Shimada, Y. Ohba, A. Takaoka, N. Yoshida, and T. Taniguchi. 2005. IRF-7 is the master regulator of type-I interferon-dependent immune responses. Nature 434772-777. [DOI] [PubMed] [Google Scholar]
- 20.Kawai, T., and S. Akira. 2006. Innate immune recognition of viral infection. Nat. Immunol. 7131-137. [DOI] [PubMed] [Google Scholar]
- 21.Keller, B. C., B. L. Fredericksen, M. A. Samuel, R. E. Mock, P. W. Mason, M. S. Diamond, and M. Gale, Jr. 2006. Resistance to alpha/beta interferon is a determinant of West Nile virus replication fitness and virulence. J. Virol. 809424-9434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klein, R. S., E. Lin, B. Zhang, A. D. Luster, J. Tollett, M. A. Samuel, M. Engle, and M. S. Diamond. 2005. Neuronal CXCL10 directs CD8+ T-cell recruitment and control of West Nile virus encephalitis. J. Virol. 7911457-11466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Levy, D. E., I. Marie, E. Smith, and A. Prakash. 2002. Enhancement and diversification of IFN induction by IRF-7-mediated positive feedback. J. Interferon Cytokine Res. 2287-93. [DOI] [PubMed] [Google Scholar]
- 24.Liu, W. J., X. J. Wang, V. V. Mokhonov, P. Y. Shi, R. Randall, and A. A. Khromykh. 2005. Inhibition of interferon signaling by the New York 99 strain and kunjin subtype of West Nile virus involves blockage of STAT1 and STAT2 activation by nonstructural proteins. J. Virol. 791934-1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Livak, K. J., and T. D. Schmittgen. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25402-408. [DOI] [PubMed] [Google Scholar]
- 26.Loo, Y. M., J. Fornek, N. Crochet, G. Bajwa, O. Perwitasari, L. Martinez-Sobrido, S. Akira, M. A. Gill, A. Garcia-Sastre, M. G. Katze, and M. Gale, Jr. 2008. Distinct RIG-I and MDA5 signaling by RNA viruses in innate immunity. J. Virol. 82335-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marié, I., J. E. Durbin, and D. E. Levy. 1998. Differential viral induction of distinct interferon-alpha genes by positive feedback through interferon regulatory factor-7. EMBO J. 176660-6669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martina, B. E., P. Koraka, P. van den Doel, G. F. Rimmelzwaan, B. L. Haagmans, and A. D. Osterhaus. 2008. DC-SIGN enhances infection of cells with glycosylated West Nile virusin vitro and virus replication in human dendritic cells induces production of IFN-alpha and TNF-alpha. Virus Res. 13564-71. [DOI] [PubMed] [Google Scholar]
- 29.Muñoz-Jordán, J. L., M. Laurent-Rolle, J. Ashour, L. Martinez-Sobrido, M. Ashok, W. I. Lipkin, and A. Garcia-Sastre. 2005. Inhibition of alpha/beta interferon signaling by the NS4B protein of flaviviruses. J. Virol. 798004-8013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakaya, T., M. Sato, N. Hata, M. Asagiri, H. Suemori, S. Noguchi, N. Tanaka, and T. Taniguchi. 2001. Gene induction pathways mediated by distinct IRFs during viral infection. Biochem. Biophys. Res. Commun. 2831150-1156. [DOI] [PubMed] [Google Scholar]
- 31.Prakash, A., E. Smith, C. K. Lee, and D. E. Levy. 2005. Tissue-specific positive feedback requirements for production of type I interferon following virus infection. J. Biol. Chem. 28018651-18657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Préhaud, C., F. Megret, M. Lafage, and M. Lafon. 2005. Virus infection switches TLR-3-positive human neurons to become strong producers of beta interferon. J. Virol. 7912893-12904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rios, M., M. J. Zhang, A. Grinev, K. Srinivasan, S. Daniel, O. Wood, I. K. Hewlett, and A. I. Dayton. 2006. Monocytes-macrophages are a potential target in human infection with West Nile virus through blood transfusion. Transfusion 46659-667. [DOI] [PubMed] [Google Scholar]
- 34.Saito, T., and M. Gale, Jr. 2007. Principles of intracellular viral recognition. Curr. Opin. Immunol. 1917-23. [DOI] [PubMed] [Google Scholar]
- 35.Samuel, M. A., and M. S. Diamond. 2006. Pathogenesis of West Nile virus infection: a balance between virulence, innate and adaptive immunity, and viral evasion. J. Virol. 809349-9360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Samuel, M. A., and M. S. Diamond. 2005. Type I IFN protects against lethal West Nile virus infection by restricting cellular tropism and enhancing neuronal survival. J. Virol. 7913350-13361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Samuel, M. A., J. D. Morrey, and M. S. Diamond. 2007. Caspase-3 dependent cell death of neurons contributes to the pathogenesis of West Nile virus encephalitis. J. Virol. 812614-2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Samuel, M. A., K. Whitby, B. C. Keller, A. Marri, W. Barchet, B. R. G. Williams, R. H. Silverman, M. Gale, and M. S. Diamond. 2006. PKR and RNAse L contribute to protection against lethal West Nile virus infection by controlling early viral spread in the periphery and replication in neurons. J. Virol. 807009-7019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sato, M., N. Hata, M. Asagiri, T. Nakaya, T. Taniguchi, and N. Tanaka. 1998. Positive feedback regulation of type I IFN genes by the IFN-inducible transcription factor IRF-7. FEBS Lett. 441106-110. [DOI] [PubMed] [Google Scholar]
- 40.Sato, M., H. Suemori, N. Hata, M. Asagiri, K. Ogasawara, K. Nakao, T. Nakaya, M. Katsuki, S. Noguchi, N. Tanaka, and T. Taniguchi. 2000. Distinct and essential roles of transcription factors IRF-3 and IRF-7 in response to viruses for IFN-alpha/beta gene induction. Immunity 13539-548. [DOI] [PubMed] [Google Scholar]
- 41.Scherbik, S. V., B. M. Stockman, and M. A. Brinton. 2007. Differential expression of interferon (IFN) regulatory factors and IFN-stimulated genes at early times after West Nile virus infection of mouse embryo fibroblasts. J. Virol. 8112005-12018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schmitz, F., A. Heit, S. Guggemoos, A. Krug, J. Mages, M. Schiemann, H. Adler, I. Drexler, T. Haas, R. Lang, and H. Wagner. 2007. Interferon-regulatory-factor 1 controls Toll-like receptor 9-mediated IFN-beta production in myeloid dendritic cells. Eur. J. Immunol. 37315-327. [DOI] [PubMed] [Google Scholar]
- 43.Servant, M. J., B. Tenoever, and R. Lin. 2002. Overlapping and distinct mechanisms regulating IRF-3 and IRF-7 function. J. Interferon Cytokine Res. 2249-58. [DOI] [PubMed] [Google Scholar]
- 44.Sheehan, K. C., K. S. Lai, G. P. Dunn, A. T. Bruce, M. S. Diamond, J. D. Heutel, C. Dungo-Arthur, J. A. Carrero, J. M. White, P. J. Hertzog, and R. D. Schreiber. 2006. Blocking monoclonal antibodies specific for mouse IFN-alpha/beta receptor subunit 1 (IFNAR-1) from mice immunized by in vivo hydrodynamic transfection. J. Interferon Cytokine Res. 26804-819. [DOI] [PubMed] [Google Scholar]
- 45.Silva, M. C., A. Guerrero-Plata, F. D. Gilfoy, R. P. Garofalo, and P. W. Mason. 2007. Differential activation of human monocyte-derived and plasmacytoid dendritic cells by West Nile virus generated in different host cells. J. Virol. 8113640-13648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tailor, P., T. Tamura, H. J. Kong, T. Kubota, M. Kubota, P. Borghi, L. Gabriele, and K. Ozato. 2007. The feedback phase of type I interferon induction in dendritic cells requires interferon regulatory factor 8. Immunity 27228-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tailor, P., T. Tamura, and K. Ozato. 2006. IRF family proteins and type I interferon induction in dendritic cells. Cell Res. 16134-140. [DOI] [PubMed] [Google Scholar]
- 48.Tassaneetrithep, B., T. Burgess, A. Granelli-Piperno, C. Trumpfheller, J. Finke, W. Sun, M. Eller, K. Pattanapanyasat, S. Sarasombath, D. Birx, R. M. Steinman, S. Schlesinger, and M. Marovich. 2003. DC-SIGN (CD209) mediates dengue virus infection of human dendritic cells. J. Exp. Med. 197823-829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang, T., and E. Fikrig. 2004. Immunity to West Nile virus. Curr. Opin. Immunol. 16519-523. [DOI] [PubMed] [Google Scholar]
- 50.Wathelet, M. G., C. H. Lin, B. S. Parekh, L. V. Ronco, P. M. Howley, and T. Maniatis. 1998. Virus infection induces the assembly of coordinately activated transcription factors on the IFN-beta enhancer in vivo. Mol. Cell 1507-518. [DOI] [PubMed] [Google Scholar]