Abstract
Plasmacytoid dendritic cells (PDC) are major producers of type I interferons (IFN) in response to human immunodeficiency virus type 1 (HIV-1) infection. To better define the underlying mechanisms, we studied the magnitude of alpha IFN (IFN-α) induction by recombinant viruses containing changes in the Env protein that impair or disrupt CD4 binding or expressing primary env alleles with differential coreceptor tropism. We found that the CD4 binding affinity but not the viral coreceptor usage is critical for the attachment of autofluorescing HIV-1 to PDC and for subsequent IFN-α induction. Our results illustrate the importance of the gp120-CD4 interaction in determining HIV-1-induced immune stimulation via IFN-α production.
Plasmacytoid dendritic cells (PDC) have been identified as major type I interferon (IFN)-producing cells in the blood (8, 46). They are positive for HLA-DR, BDCA2, and BDCA4 (14) but negative for CD11c and lineage markers (20). In addition, PDC express the human immunodeficiency virus (HIV) receptor CD4 and the coreceptors CXCR4 (X4) and CCR5 (R5) (39). Stimulation with interleukin 3 and CD40 ligand promotes their differentiation into mature dendritic cells (25). Upon virus exposure, PDC maturation induces a potent Th1 polarization via stimulation of naïve T cells, linking innate and adaptive immunity (7, 29, 41). By orchestrating the early immune response, PDC play an important role in the host defense against viral and bacterial infections (3, 21).
In acute and chronic HIV type 1 (HIV-1) infection, PDC counts (1, 9, 13, 18, 19, 37, 43, 47) and function (1, 4, 9, 12, 18, 19, 45) are severely reduced, reflecting the clinical status of infected patients and predicting immunological control of HIV-1 replication (36). Upon HIV-1 stimulation, PDC upregulate the chemokine receptor 7, which promotes migration to secondary lymphatic tissue (23, 27, 42, 49). PDC can be infected by HIV-1 in vivo and in vitro, but lytic replication was observed only after CD40 ligation and alpha IFN (IFN-α) neutralization (12, 22, 23, 39, 44). Infection was reported to be more efficient with R5-tropic than with X4-tropic viral strains (6). PDC secrete high amounts of IFN-α upon exposure to high-titered infectious virus (22, 23, 49), inactivated HIV-1 particles (26), and HIV-1-infected cells (42). Experiments using monoclonal antibodies to CD4, soluble CD4, neutralizing antibodies to gp120, and viral entry inhibitors of CD4-gp120 binding (2, 27, 42, 49) provide indirect evidence that the CD4 receptor on PDC is involved in this process. HIV coreceptors appear to play a minor role, based on studies using antibodies to CXCR4 and coreceptor antagonists (27, 42).
We used recombinant viruses to directly assess the relevance of the interaction of HIV-1 gp120 with the CD4 receptor on PDC for virion attachment and subsequent IFN-α induction. Furthermore, the role of HIV-1 coreceptors was investigated, because the switch of R5-tropic to X4-tropic viruses frequently accompanies progression of disease in HIV-infected individuals (24, 48).
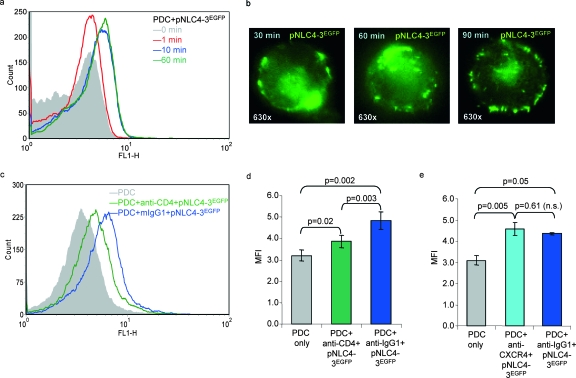
To evaluate binding of HIV-1 particles to PDC, these cells were purified from peripheral blood mononuclear cells (PBMC) of HIV-uninfected volunteers, using the BDCA4 cell isolation kit (Miltenyi Biotec, Bergisch Gladbach, Germany) as described previously (30, 42). The median purity assessed by BDCA2/CD4 staining was 97.0% in 10 preparations (interquartile range, 95.76 to 97.44%) using a three-color FACSCalibur with CellQuest 3.3 software (Becton Dickinson, Heidelberg, Germany). PDC were cultivated in RPMI 1640 medium containing 10% heat-inactivated fetal calf serum (Cambrex, Verviers, Belgium), antibiotics, and 20 ng/ml interleukin 3 (R&D Systems, Wiesbaden, Germany). Autofluorescing viral particles were generated by transfection of 293T cells with equimolar amounts of pNLC4-3 and pNLC4-3EGFP and pelleting the supernatants through a 20% (wt/wt) sucrose cushion (90 min, 130,000 × g at 4°C) (33). Fluorescence-activated cell sorting (FACS) analyses revealed a rapid attachment of HIV to PDC within 10 min of incubation (Fig. 1a). Time-lapse microscopy showed a corona of autofluorescing viral particles over an extended period of time (Fig. 1b). The attachment of HIV to PDC (Fig. 1c) was significantly impaired by anti-CD4, compared to the isotype control in six separate experiments (P = 0.003) (Fig. 1d), whereas anti-CXCR4 had no effect (Fig. 1e). A significant number of particles attached to PDC despite the presence of anti-CD4 (P = 0.02) (Fig. 1d), confirming data from the work of Martinelli et al. (31) and suggesting a role for other PDC surface receptors, e.g., the mannose receptor (32), the C-type lectin BDCA2 (15), and Fc receptors, involved in capturing antibody-opsonized antigens.
FIG. 1.
Effect of CD4 and CXCR4 on the attachment of HIV-1 to PDC. (a and b) Time-lapse experiments evaluating the attachment of autofluorescing HIV particles (pNLC4-3EGFP) (33) to PDC after different incubation periods (minutes) using FACS analysis (data are representative of four independent experiments) (a) and immunofluorescence microscopy (b). (c) Blocking of the attachment of pNLC4-3EGFP to PDC using monoclonal antibodies to CD4 (Leu3a) compared to an isotype control (mIgG1). (d and e) Effect of anti-CD4 (d) and anti-CXCR4 (e) antibodies on the attachment of pNLC4-3EGFP to PDC, shown as means and standard errors of six and three separate experiments, respectively, comparing the mean fluorescence intensities (MFI) after 10 minutes of incubation. n.s., not significant.
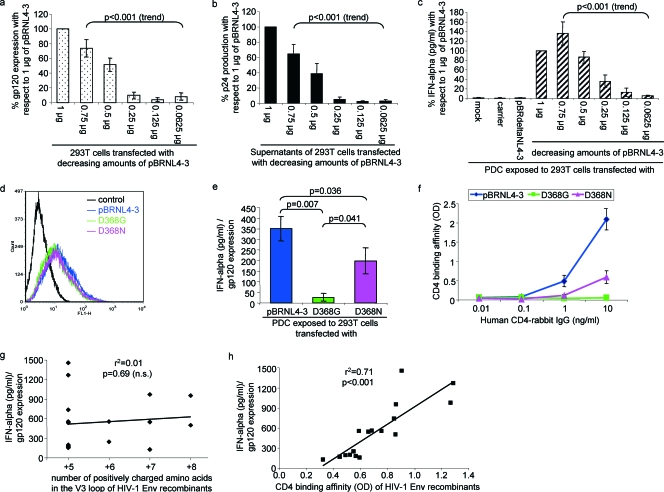
To assess the effect of Env expression and virus production on IFN-α induction, 293T cells were transfected with decreasing amounts of pBRNL4-3 using FuGENE 6 and FuGENE HD transfection reagents (Roche, Mannheim, Germany). Expression levels of gp120 were evaluated using the monoclonal antibody 2G12 and a fluorescein isothiocyanate-conjugated goat anti-human polyclonal immunoglobulin G (IgG) (heavy plus light) secondary antibody (Dianova, Hamburg, Germany). Production of p24 antigen was measured using the Murex HIV antigen monoclonal antibody kit (Abbott, Wiesbaden, Germany). A total of 6 × 104 transfected 293T cells were cocultured with 1 × 104 PDC/well in 96-well flat-bottomed plates, each coculture being set up in duplicate at least. Supernatants harvested after 24 h were assayed for IFN-α 2a/2b using an enzyme-linked immunosorbent assay module set (Bender Medsystems, Vienna, Austria). A decrease in gp120 expression (Fig. 2a) paralleled a reduced p24 antigen production by the transfected 293T cells (Fig. 2b) and a decline in IFN-α induction in the cocultures (Fig. 2c) (all P < 0.001 for trend). Because the effect saturated at 0.75 μg of transfected DNA, we generally used 0.5 μg in subsequent experiments. To exclude the possibility that IFN-α was induced by vector sequences, pBRNL4-3 was sequentially restricted using SstI, BamHI, and NheI. The resulting pBRdeltaNL4-3 plasmid, containing 607 bp of the 5′ long terminal repeat and 146 bp of the 3′ long terminal repeat in addition to the vector sequences, was not stimulatory (Fig. 2c).
FIG. 2.
Effect of virus replication, CD4 binding affinity, and coreceptor tropism on PDC IFN-α induction. (a to c) Transfection of decreasing amounts of pBRNL4-3 DNA resulted in parallel decreases of gp120 expression levels (a), p24 antigen production (b), and IFN-α (c) induction in PDC cocultures. Data represent three to six separate experiments. Statistical analysis was performed using one-way analysis of variance for trend analysis. Controls included unstimulated PDC (mock), calf thymus DNA (carrier) substituting for the total amount of transfected DNA, and pBRdeltaNL4-3, deleted for all sequences coding for retroviral genes. (d) Expression of gp120 in transfected 293T cells using pBRNL4-3 mutants with either completely abolished (D368G) or reduced (D368N) CD4 affinity. Controls were pBRNL4-3-transfected 293T cells incubated with the fluorescein isothiocyanate-conjugated secondary antibody but without 2G12. One representative FACS histogram out of three separate experiments is shown. (e) IFN-α induction by transfected 293T cells using PDC obtained from four different donors. Data are shown as means and standard errors normalized for gp120 expression. (f) CD4 binding affinities of the respective viruses, determined in three separate experiments. (g and h) Linear regression analysis of the number of positively charged amino acids in the V3 loop (Table 2) (g) and the mean CD4 binding affinity of 17 HIV-1 V3 loop and Env recombinants with respect to the mean induction of IFN-α production (h), normalized for gp120 expression in five separate experiments (n.s., not significant). OD, optical density.
The effect of Env alone was assessed using a full-length HIV-1 Env plasmid (pBRenv). Transfected 293T cells induced slightly more IFN-α than did unstimulated PDC in five experiments (690 ± 275 pg/ml versus 114 ± 82 pg/ml, respectively; P = 0.13, nonsignificant). Notably, full-length pBRNL4-3 transfected in parallel induced significantly more IFN-α than did pBRenv after normalizing for gp120 expression (P = 0.03) (data not shown). These data indicate that viral proteins other than gp120, viral nucleic acids (5), and/or particle formation are required for efficient IFN-α induction.
To further evaluate the role of CD4 in IFN-α induction, we used pBRNL4-3 mutants with changes in the gp120 protein that impair (D368N) or disrupt (D368G) CD4 binding and infectivity (35). While gp120 expression levels were comparable (Fig. 2d), IFN-α induction was significantly different (P < 0.05, Fig. 2e). As expected, pBRNL4-3 showed a significantly higher CD4 binding affinity than did D368N (P = 0.05) and D368G (P = 0.02) (Fig. 2f), when assessed using an affinity-purified CD4-rabbit IgG protein produced from stably transfected 293T cells as described previously (35). Thus, binding of HIV to CD4 on PDC seems critical for IFN-α induction.
To assess the impact of HIV-1 gp120 coreceptor tropism on IFN-α induction, pBRNL4-3 recombinants containing various V3 loops (38) were used. Each individual clone was analyzed as two separate vector preparations using the Qiagen Midiprep kit (Qiagen, Hilden, Germany) or the High Pure plasmid isolation kit (Invitrogen, Karlsruhe, Germany). In addition, we generated proviral pBRNL4-3 constructs expressing primary env genes amplified from PBMC samples of four individuals in a cohort followed by the New England Hemophilia Center at the University of Massachusetts Memorial Health Center, Worcester, MA. These included sequential samples from two individuals obtained before and after disease progression (P8 and SP13) and two cross-sectional samples obtained during the chronic phase of infection (P13 and P18) (Table 1). No participant received highly active antiretroviral therapy over this time frame (1983 to 1993). All of them gave informed consent for these studies, with the approval of the Institutional Review Board on the conduct of research on human subjects at the University of Massachusetts Medical School. Sequential primary full-length env genes were cloned in bulk into pBRNL4-3 to ensure that they were representative of the virus quasispecies for each patient and time point. In addition, two individual clones were isolated from two patient samples (P8-83-K16 and P18-86-K52). This set of coreceptor mutants (n = 17), covering a broad coreceptor range, induced different IFN-α levels in PDC cocultures with transfected 293T cells (Table 2). The efficiency of IFN-α induction did not correlate with the increase of positively charged amino acids in the V3 loop (r2 = 0.01, P = 0.69, Fig. 2g), but with the CD4 binding affinities of these viruses (r2 = 0.71, P < 0.001, Fig. 2h). These data are in agreement with a model of receptor-mediated endocytosis, in which CD4 serves as the anchor molecule for the attachment and endocytosis of RNA-containing virions into PDC (5).
TABLE 1.
Characteristics of clinical samples with respect to age and CD4+ cell count of the HIV-1-infected individuals and replication capacities of recombinant viruses in different coreceptor-expressing cell linesa
| Patient code | Age (yr) | Peripheral CD4+ cells
|
Infection of cell line
|
|||
|---|---|---|---|---|---|---|
| % | Absolute count (cells/μl) | P4-CCR5 | R5.3 | X4.15 | ||
| SP13-84 | 6 | 53 | 1,622 | ++ | +++ | − |
| SP13-93 | 14 | 6 | 66 | + | +++ | ++ |
| P8-83 | 43 | 26 | 526 | ++ | +++ | − |
| P8-89 | 49 | 1 | 12 | − | ++ | − |
| P13-85 | 9 | 16 | 598 | + | +++ | − |
| P18-86 | 17 | 10 | 456 | + | +++ | + |
SP13-84 and SP13-93 as well as P8-83 and P8-89 represent sequential virus isolates from two untreated HIV-1-infected individuals, who progressed to severe immunodeficiency during the observation period. P13-85 and P18-86 were obtained as cross-sectional samples. Full-length env genes of these isolates were directly amplified from PBMC by PCR and cloned in bulk into pBRNL4-3. Virus stocks, obtained after transfection of 293T cells, were used to infect cell lines expressing the human CD4 receptor in addition to either both HIV coreceptors (P4-CCR5) or CCR5 (R5.3) or CXCR4 (X4.15). Virus replication was evaluated using β-galactosidase activity (11, 34). The symbols represent highly efficient (+++), efficient (++), low (+), and no significant (−) replication or entry.
TABLE 2.
Characteristics of clinical coreceptor mutants with respect to IFN-α induction and CD4 affinity
| Clone | V3 loop sequencea | Charge | Tropism | IFN-α inductionb | CD4 affinityc |
|---|---|---|---|---|---|
| Consensus | CTRPNNNTRKSIHIGPGRAFYTTGEIIGDIRQAHC | ||||
| V3 loop recombinants | |||||
| 011jr101 | ------------p---------------g------ | +5 | R5 | 1,455 ± 488 | 0.904 ± 0.221 |
| YU2 | ------------n------l--------------- | +5 | R5 | 1,270 ± 183 | 1.287 ± 0.244 |
| 92ht593.1 | --------s-r-s-------ra-.k---n------ | +7 | R5X4 | 973 ± 224 | 1.266 ± 0.199 |
| P34-S | ------his-r-s-------ra-er------k--- | +8 | X4 | 952 ± 236 | 0.862 ± 0.115 |
| 92th014.12 | -------------1-----w----q---------- | +5 | R5 | 740 ± 190 | 0.848 ± 0.207 |
| 92ug037.8 | -----------vr----qt--a--d---------- | +5 | R5 | 559 ± 133 | 0.685 ± 0.203 |
| 93br029.2 | ------------q------------------k--- | +6 | R5 | 556 ± 151 | 0.590 ± 0.159 |
| 93br020.17 | ----------r-sl----v---a--------k--- | +7 | R5X4 | 553 ± 127 | 0.734 ± 0.186 |
| 005pf130 | ----------g-n---------------------- | +5 | R5 | 540 ± 208 | 0.658 ± 0.124 |
| P51-Sc | ----g-k--r-ms-------ia-rq------k--- | +8 | X4 | 502 ± 144 | 0.861 ± 0.156 |
| Env recombinants | |||||
| Bulk constructs | |||||
| SP13-84-K1 | ----------------------------------- | +5 | R5 | 177 ± 39 | 0.566 ± 0.222 |
| SP13-84-K2 | ----------------------------------- | +5 | R5 | 177 ± 39 | 0.566 ± 0.222 |
| SP13-93-K1 | -i----------------------q------k--- | +7 | R5X4 | 128 ± 33 | 0.321 ± 0.038 |
| SP13-93-K2 | -i----------------------q------k--- | +7 | R5X4 | 128 ± 33 | 0.321 ± 0.038 |
| P8-83-K1 | ----s-------t---------------------- | +5 | R5 | 188 ± 87 | 0.489 ± 0.097 |
| P8-83-K2 | ------------t---------------------- | +5 | R5 | 188 ± 87 | 0.489 ± 0.097 |
| P8-89 | NA | +5 | R5 | 168 ± 43 | 0.448 ± 0.183 |
| P13-85-K4 | ---------e---m---k------d------k--- | +5 | R5 | 158 ± 31 | 0.594 ± 0.181 |
| P13-85-K10 | ---------e---m---k------d------k--- | +5 | R5 | 158 ± 31 | 0.594 ± 0.181 |
| Clonal constructs | |||||
| P8-83-K16 | ------------t---------------------- | +5 | R5 | 197 ± 42 | 0.524 ± 0.066 |
| P18-86-K52 | ------------t-----------a--------y- | +6 | R5X4 | 249 ± 47 | 0.549 ± 0.109 |
All sequences are presented in comparison to the V3 consensus sequence (uppercase), starting with Env296. Dashes indicate amino acid identity. Amino acids Env306 and Env322, reported previously to be crucial for coreceptor tropism (40), are labeled in bold uppercase, and candidate mutations for enhanced IFN-α induction (Env315L and Env324N/G) are labeled in bold lowercase. For P8-89, no sequences were available (NA).
IFN-α induction (pg/ml) by transfected 293T cells is presented as mean and standard error after normalization for gp120 expression. SP13-84, SP13-93, P8-83, P8-89, and P13-85 were measured as bulk preparations, but sequences are given for individual clones (K numbers) derived from the bulk preparations. IFN-α production was also determined for two individual clones (P8-83-K16 and P18-86-K52). Data for V3 loop recombinants are from five separate experiments, data for bulk construct Env recombinants are from 3 to 11 separate experiments, and data for clonal construct Env recombinants are from 10 separate experiments.
CD4 binding affinity of human CD4 rabbit IgG (10 ng/ml) is displayed as mean and standard error of the optical density value, determined in three to six separate analyses.
When the V3 loop sequences of the HIV-1 Env recombinants were aligned with respect to their IFN-α induction, potential mutations (Env315L and Env324N/G) corresponding to the highest IFN-α induction were evident (Table 2). Env324N has been described as a naturally occurring mutation in isolates with a syncytium-inducing, high-replication phenotype (10). It has to be noted, however, that CD4 does not make any major contacts with the V3 loop directly. Therefore, the relevance of these mutations for the IFN-α induction needs to be evaluated in further studies.
The HIV-1 pBRNL4-3 recombinants containing full-length primary env genes (SP13-84, SP13-93, P8-83, P13-85, P8-83-K16, and P18-86-K52) induced significantly lower IFN-α levels than pBRNL4-3, expressing a T-cell-line adapted env gene (P < 0.05, Table 2). To assess whether the capacity to induce IFN-α production may change during disease progression, sequential virus isolates from early and late stages of HIV-1 infection were studied. Both patients experienced a substantial CD4+ cell decline during the observation period, and the R5 tropism changed to R5X4 in one patient (SP13) (Table 1). No significant differences in IFN-α induction were detected (P8-83 versus P8-89, P = 0.91; SP13-84 versus SP13-93, P = 0.38), suggesting that the ability of Env to induce IFN-α production does not change during AIDS progression. However, these results are preliminary and a larger number of sequential HIV-1 isolates must be analyzed to corroborate this conclusion.
In summary, our data are direct evidence that HIV-1 attachment to PDC and the magnitude of IFN-α induction critically depend on the gp120-CD4 binding affinity. Another important factor appears to be the extent of virus replication. Recently, viral pathogenicity and disease progression have been linked to immune stimulation caused by HIV-1 infection. Infection-associated immune activation may involve the induction of tumor necrosis factor-related apoptosis-inducing ligand expression in response to PDC-derived IFN-α secretion, resulting in death receptor 5 (DR5)-mediated apoptosis of CD4+ cells exposed to either infectious or noninfectious HIV-1 (16, 26, 27). Thus, most of the bystander cell killing may be caused by this immunopathogenic mechanism (26, 28). The results of our study using HIV-1 recombinant viruses support the conclusion that virus entry inhibitors disrupting the interaction between gp120 and CD4 (17) may be promising candidates to prevent HIV-1-induced IFN-α production, immune stimulation, and progression of disease.
Acknowledgments
We thank Bernhard Fleckenstein for continuous support. The autofluorescing pNLC4-3EGFP construct was kindly provided by Barbara Müller and Hans-Georg Kräusslich, University of Heidelberg, Heidelberg, Germany, and the sodium-azide free anti-CD4 (Leu3a) was provided by Becton Dickinson (San Jose, CA). The gp120 (2G12) and CXCR4 (12G5) antibodies were kindly contributed by the National Institutes of Health, AIDS Research and Reference Reagent Program, Bethesda, MD. We thank John Sullivan and Doreen Brettler for their leadership of the hemophilia cohort study at the University of Massachusetts Memorial Health Center, Worcester, MA, and all volunteers at the Institute of Virology, Erlangen, for donating blood.
This work was supported by the German Research Foundation (grants SCHM1702/1-1; SFB466, Project A12; SCHM1702/2-1), the Akademie der Wissenschaften und Literatur zu Mainz, and the German Competence Network for HIV/AIDS (to B.S.); the Wilhelm Sander Foundation, the German Competence Network for HIV/AIDS, and NIH grant 1R01AI067057-01A2 (to F.K.); grant support ID NIH 40880 to Robert W. Doms' laboratory at the Department of Microbiology, University of Pennsylvania, Philadelphia, PA; and the hemophilia grant (NIH HL-42257) and the UMass Center for AIDS Research (P30 AI-42845) (to T.C.G.). Sabrina Haupt was supported by the graduate college GRK 1071 (“Viruses of the Immune System”).
We have no financial conflict of interest.
Footnotes
Published ahead of print on 25 June 2008.
REFERENCES
- 1.Almeida, M., M. Cordero, J. Almeida, and A. Orfao. 2005. Different subsets of peripheral blood dendritic cells show distinct phenotypic and functional abnormalities in HIV-1 infection. AIDS 19261-271. [PubMed] [Google Scholar]
- 2.Ankel, H., M. R. Capobianchi, C. Castilletti, and F. Dianzani. 1994. Interferon induction by HIV glycoprotein 120: role of the V3 loop. Virology 20534-43. [DOI] [PubMed] [Google Scholar]
- 3.Barchet, W., M. Cella, and M. Colonna. 2005. Plasmacytoid dendritic cells—virus experts of innate immunity. Semin. Immunol. 17253-261. [DOI] [PubMed] [Google Scholar]
- 4.Barron, M. A., N. Blyveis, B. E. Palmer, S. MaWhinney, and C. C. Wilson. 2003. Influence of plasma viremia on defects in number and immunophenotype of blood dendritic cell subsets in human immunodeficiency virus 1-infected individuals. J. Infect. Dis. 18726-37. [DOI] [PubMed] [Google Scholar]
- 5.Beignon, A. S., K. McKenna, M. Skoberne, O. Manches, I. Dasilva, D. G. Kavanagh, M. Larsson, R. J. Gorelick, J. D. Lifson, and N. Bhardwaj. 2005. Endocytosis of HIV-1 activates plasmacytoid dendritic cells via Toll-like receptor-viral RNA interactions. J. Clin. Investig. 1153265-3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cameron, P. U., A. J. Handley, D. C. Baylis, A. E. Solomon, N. Bernard, D. F. Purcell, and S. R. Lewin. 2007. Preferential infection of dendritic cells during human immunodeficiency virus type 1 infection of blood leukocytes. J. Virol. 812297-2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cella, M., F. Facchetti, A. Lanzavecchia, and M. Colonna. 2000. Plasmacytoid dendritic cells activated by influenza virus and CD40L drive a potent TH1 polarization. Nat. Immunol. 1305-310. [DOI] [PubMed] [Google Scholar]
- 8.Cella, M., D. Jarrossay, F. Facchetti, O. Alebardi, H. Nakajima, A. Lanzavecchia, and M. Colonna. 1999. Plasmacytoid monocytes migrate to inflamed lymph nodes and produce large amounts of type I interferon. Nat. Med. 5919-923. [DOI] [PubMed] [Google Scholar]
- 9.Chehimi, J., D. E. Campbell, L. Azzoni, D. Bacheller, E. Papasavvas, G. Jerandi, K. Mounzer, J. Kostman, G. Trinchieri, and L. J. Montaner. 2002. Persistent decreases in blood plasmacytoid dendritic cell number and function despite effective highly active antiretroviral therapy and increased blood myeloid dendritic cells in HIV-infected individuals. J. Immunol. 1684796-4801. [DOI] [PubMed] [Google Scholar]
- 10.de Jong, J. J., A. de Ronde, W. Keulen, M. Tersmette, and J. Goudsmit. 1992. Minimal requirements for the human immunodeficiency virus type 1 V3 domain to support the syncytium-inducing phenotype: analysis by single amino acid substitution. J. Virol. 666777-6780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Detheux, M., L. Standker, J. Vakili, J. Munch, U. Forssmann, K. Adermann, S. Pohlmann, G. Vassart, F. Kirchhoff, M. Parmentier, and W. G. Forssmann. 2000. Natural proteolytic processing of hemofiltrate CC chemokine 1 generates a potent CC chemokine receptor (CCR)1 and CCR5 agonist with anti-HIV properties. J. Exp. Med. 1921501-1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Donaghy, H., B. Gazzard, F. Gotch, and S. Patterson. 2003. Dysfunction and infection of freshly isolated blood myeloid and plasmacytoid dendritic cells in patients infected with HIV-1. Blood 1014505-4511. [DOI] [PubMed] [Google Scholar]
- 13.Donaghy, H., A. Pozniak, B. Gazzard, N. Qazi, J. Gilmour, F. Gotch, and S. Patterson. 2001. Loss of blood CD11c(+) myeloid and CD11c(−) plasmacytoid dendritic cells in patients with HIV-1 infection correlates with HIV-1 RNA virus load. Blood 982574-2576. [DOI] [PubMed] [Google Scholar]
- 14.Dzionek, A., A. Fuchs, P. Schmidt, S. Cremer, M. Zysk, S. Miltenyi, D. W. Buck, and J. Schmitz. 2000. BDCA-2, BDCA-3, and BDCA-4: three markers for distinct subsets of dendritic cells in human peripheral blood. J. Immunol. 1656037-6046. [DOI] [PubMed] [Google Scholar]
- 15.Dzionek, A., Y. Sohma, J. Nagafune, M. Cella, M. Colonna, F. Facchetti, G. Gunther, I. Johnston, A. Lanzavecchia, T. Nagasaka, T. Okada, W. Vermi, G. Winkels, T. Yamamoto, M. Zysk, Y. Yamaguchi, and J. Schmitz. 2001. BDCA-2, a novel plasmacytoid dendritic cell-specific type II C-type lectin, mediates antigen capture and is a potent inhibitor of interferon alpha/beta induction. J. Exp. Med. 1941823-1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Esser, M. T., J. W. Bess, Jr., K. Suryanarayana, E. Chertova, D. Marti, M. Carrington, L. O. Arthur, and J. D. Lifson. 2001. Partial activation and induction of apoptosis in CD4+ and CD8+ T lymphocytes by conformationally authentic noninfectious human immunodeficiency virus type 1. J. Virol. 751152-1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Este, J. A., and A. Telenti. 2007. HIV entry inhibitors. Lancet 37081-88. [DOI] [PubMed] [Google Scholar]
- 18.Feldman, S., D. Stein, S. Amrute, T. Denny, Z. Garcia, P. Kloser, Y. Sun, N. Megjugorac, and P. Fitzgerald-Bocarsly. 2001. Decreased interferon-alpha production in HIV-infected patients correlates with numerical and functional deficiencies in circulating type 2 dendritic cell precursors. Clin. Immunol. 101201-210. [DOI] [PubMed] [Google Scholar]
- 19.Finke, J. S., M. Shodell, K. Shah, F. P. Siegal, and R. M. Steinman. 2004. Dendritic cell numbers in the blood of HIV-1 infected patients before and after changes in antiretroviral therapy. J. Clin. Immunol. 24647-652. [DOI] [PubMed] [Google Scholar]
- 20.Fitzgerald-Bocarsly, P. 2002. Natural interferon-alpha producing cells: the plasmacytoid dendritic cells. BioTechniques 2224-29. [PubMed] [Google Scholar]
- 21.Fitzgerald-Bocarsly, P., and D. Feng. 2007. The role of type I interferon production by dendritic cells in host defense. Biochimie 89843-855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fong, L., M. Mengozzi, N. W. Abbey, B. G. Herndier, and E. G. Engleman. 2002. Productive infection of plasmacytoid dendritic cells with human immunodeficiency virus type 1 is triggered by CD40 ligation. J. Virol. 7611033-11041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fonteneau, J. F., M. Larsson, A. S. Beignon, K. McKenna, I. Dasilva, A. Amara, Y. J. Li, J. D. Lifson, D. R. Littman, and N. Bhardwaj. 2004. Human immunodeficiency virus type 1 activates plasmacytoid dendritic cells and concomitantly induces the bystander maturation of myeloid dendritic cells. J. Virol. 785223-5232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fouchier, R. A., M. Groenink, N. A. Kootstra, M. Tersmette, H. G. Huisman, F. Miedema, and H. Schuitemaker. 1992. Phenotype-associated sequence variation in the third variable domain of the human immunodeficiency virus type 1 gp120 molecule. J. Virol. 663183-3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grouard, G., M. C. Rissoan, L. Filgueira, I. Durand, J. Banchereau, and Y. J. Liu. 1997. The enigmatic plasmacytoid T cells develop into dendritic cells with interleukin (IL)-3 and CD40-ligand. J. Exp. Med. 1851101-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Herbeuval, J. P., J. C. Grivel, A. Boasso, A. W. Hardy, C. Chougnet, M. J. Dolan, H. Yagita, J. D. Lifson, and G. M. Shearer. 2005. CD4(+) T-cell death induced by infectious and noninfectious HIV-1: role of type 1 interferon-dependent, TRAEL/DR5-mediated apoptosis. Blood 1063524-3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Herbeuval, J. P., A. W. Hardy, A. Boasso, S. A. Anderson, M. J. Dolan, M. Dy, and G. M. Shearer. 2005. Regulation of TNF-related apoptosis-inducing ligand on primary CD4(+) T cells by HIV-1: role of type IIFN-producing plasmacytoid dendritic cells. Proc. Natl. Acad. Sci. USA 10213974-13979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Herbeuval, J. P., and G. M. Shearer. 2007. HIV-1 immunopathogenesis: how good interferon turns bad. Clin. Immunol. 123121-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kadowaki, N., S. Antonenko, J. Y. N. Lau, and Y. J. Liu. 2000. Natural interferon alpha/beta-producing cells link innate and adaptive immunity. J. Exp. Med. 192219-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kittan, N. A., A. Bergua, S. Haupt, N. Donhauser, P. Schuster, K. Korn, T. Harrer, and B. Schmidt. 2007. Impaired plasmacytoid dendritic cell innate immune responses in patients with herpes virus-associated acute retinal necrosis. J. Immunol. 1794219-4230. [DOI] [PubMed] [Google Scholar]
- 31.Martinelli, E., C. Cicala, D. Van Ryk, D. J. Goode, K. Macleod, J. Arthos, and A. S. Fauci. 2007. HIV-1 gp120 inhibits TLR9-mediated activation and IFN-alpha secretion in plasmacytoid dendritic cells. Proc. Natl. Acad. Sci. USA 1043396-3401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Milone, M. C., and P. Fitzgerald-Bocarsly. 1998. The mannose receptor mediates induction of IFN-alpha in peripheral blood dendritic cells by enveloped RNA and DNA viruses. J. Immunol. 1612391-2399. [PubMed] [Google Scholar]
- 33.Muller, B., J. Daecke, O. T. Fackler, M. T. Dittmar, H. Zentgraf, and H. G. Krausslich. 2004. Construction and characterization of a fluorescently labeled infectious human immunodeficiency virus type 1 derivative. J. Virol. 7810803-10813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Munch, J., L. Standker, S. Pohlmann, F. Baribaud, A. Papkalla, O. Rosorius, R. Stauber, G. Sass, N. Heveker, K. Adermann, S. Escher, E. Kluver, R. W. Doms, W. G. Forssmann, and F. Kirchhoff. 2002. Hemofiltrate CC chemokine 1[9-74] causes effective internalization of CCR5 and is a potent inhibitor of R5-tropic human immunodeficiency virus type 1 strains in primary T cells and macrophages. Antimicrob. Agents Chemother. 46982-990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Otto, C., B. A. Puffer, S. Pohlmann, R. W. Doms, and F. Kirchhoff. 2003. Mutations in the C3 region of human and simian immunodeficiency virus envelope have differential effects on viral infectivity, replication, and CD4-dependency. Virology 315292-302. [DOI] [PubMed] [Google Scholar]
- 36.Pacanowski, J., L. Develioglu, I. Kamga, M. Sinet, M. Desvarieux, P. M. Girard, and A. Hosmalin. 2004. Early plasmacytoid dendritic cell changes predict plasma HIV load rebound during primary infection. J. Infect. Dis. 1901889-1892. [DOI] [PubMed] [Google Scholar]
- 37.Pacanowski, J., S. Kahi, M. Baillet, P. Lebon, C. Deveau, C. Goujard, L. Meyer, E. Oksenhendler, M. Sinet, and A. Hosmalin. 2001. Reduced blood CD123(+) (lymphoid) and CD11c(+) (myeloid) dendritic cell numbers in primary HIV-1 infection. Blood 983016-3021. [DOI] [PubMed] [Google Scholar]
- 38.Papkalla, A., J. Munch, C. Otto, and F. Kirchhoff. 2002. Nef enhances human immunodeficiency virus type 1 infectivity and replication independently of viral coreceptor tropism. J. Virol. 768455-8459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Patterson, S., A. Rae, N. Hockey, J. Gilmour, and F. Gotch. 2001. Plasmacytoid dendritic cells are highly susceptible to human immunodeficiency virus type 1 infection and release infectious virus. J. Virol. 756710-6713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Resch, W., N. Hoffman, and R. Swanstrom. 2001. Improved success of phenotype prediction of the human immunodeficiency virus type 1 from envelope variable loop 3 sequence using neural networks. Virology 28851-62. [DOI] [PubMed] [Google Scholar]
- 41.Rissoan, M. C., V. Soumelis, N. Kadowaki, G. Grouard, F. Briere, M. R. de Waal, and Y. J. Liu. 1999. Reciprocal control of T helper cell and dendritic cell differentiation. Science 2831183-1186. [DOI] [PubMed] [Google Scholar]
- 42.Schmidt, B., B. M. Ashlock, H. Foster, S. H. Fujimura, and J. A. Levy. 2005. HIV-infected cells are major inducers of plasmacytoid dendritic cell interferon production, maturation, and migration. Virology 343256-266. [DOI] [PubMed] [Google Scholar]
- 43.Schmidt, B., S. H. Fujimura, J. N. Martin, and J. A. Levy. 2006. Variations in plasmacytoid dendritic cell (PDC) and myeloid dendritic cell (MDC) levels in HIV-infected subjects on and off antiretroviral therapy. J. Clin. Immunol. 2655-64. [DOI] [PubMed] [Google Scholar]
- 44.Schmidt, B., I. Scott, R. G. Whitmore, H. Foster, S. Fujimura, J. Schmitz, and J. A. Levy. 2004. Low-level HIV infection of plasmacytoid dendritic cells: onset of cytopathic effects and cell death after PDC maturation. Virology 329280-288. [DOI] [PubMed] [Google Scholar]
- 45.Siegal, F. P., P. Fitzgerald-Bocarsly, B. K. Holland, and M. Shodell. 2001. Interferon-alpha generation and immune reconstitution during antiretroviral therapy for human immunodeficiency virus infection. AIDS 151603-1612. [DOI] [PubMed] [Google Scholar]
- 46.Siegal, F. P., N. Kadowaki, M. Shodell, P. A. Fitzgerald-Bocarsly, K. Shah, S. Ho, S. Antonenko, and Y. J. Liu. 1999. The nature of the principal type 1 interferon-producing cells in human blood. Science 2841835-1837. [DOI] [PubMed] [Google Scholar]
- 47.Soumelis, V., I. Scott, F. Gheyas, D. Bouhour, G. Cozon, L. Cotte, L. Huang, J. A. Levy, and Y. J. Liu. 2001. Depletion of circulating natural type 1 interferon-producing cells in HIV-infected AIDS patients. Blood 98906-912. [DOI] [PubMed] [Google Scholar]
- 48.Speck, R. F., K. Wehrly, E. J. Platt, R. E. Atchison, I. F. Charo, D. Kabat, B. Chesebro, and M. A. Goldsmith. 1997. Selective employment of chemokine receptors as human immunodeficiency virus type 1 coreceptors determined by individual amino acids within the envelope V3 loop. J. Virol. 717136-7139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yonezawa, A., R. Morita, A. Takaori-Kondo, N. Kadowaki, T. Kitawaki, T. Hori, and T. Uchiyama. 2003. Natural alpha interferon-producing cells respond to human immunodeficiency virus type 1 with alpha interferon production and maturation into dendritic cells. J. Virol. 773777-3784. [DOI] [PMC free article] [PubMed] [Google Scholar]