Abstract
The herpes simplex virus type 1 (HSV-1) genome is contained in a capsid wrapped by a complex tegument layer and an external envelope. The poorly defined tegument plays a critical role throughout the viral life cycle, including delivery of capsids to the nucleus, viral gene expression, capsid egress, and acquisition of the viral envelope. Current data suggest tegumentation is a dynamic and sequential process that starts in the nucleus and continues in the cytoplasm. Over two dozen proteins are assumed to be or are known to ultimately be added to virions as tegument, but its precise composition is currently unknown. Moreover, a comprehensive analysis of all proteins found in HSV-1 virions is still lacking. To better understand the implication of the tegument and host proteins incorporated into the virions, highly purified mature extracellular viruses were analyzed by mass spectrometry. The method proved accurate (95%) and sensitive and hinted at 8 different viral capsid proteins, 13 viral glycoproteins, and 23 potential viral teguments. Interestingly, four novel virion components were identified (UL7, UL23, UL50, and UL55), and two teguments were confirmed (ICP0 and ICP4). In contrast, UL4, UL24, the UL31/UL34 complex, and the viral UL15/UL28/UL33 terminase were undetected, as was most of the viral replication machinery, with the notable exception of UL23. Surprisingly, the viral glycoproteins gJ, gK, gN, and UL43 were absent. Analyses of virions produced by two unrelated cell lines suggest their protein compositions are largely cell type independent. Finally, but not least, up to 49 distinct host proteins were identified in the virions.
Herpes simplex virus type 1 (HSV-1) is a multilayered particle composed of a DNA core surrounded by a capsid, a tegument, and finally an envelope. The tegument consists of several proteins that are critical for the virus. For instance, upon the virus’ entry into the cell, the tegument likely directs the virus to the nucleus (20, 33, 52, 90). There, the UL36 tegument protein anchors the capsid to the nuclear pore to enable viral DNA transfer into the nucleus (90). Three other teguments, namely, ICP0, ICP4, and UL48 (VP16), then play an essential role in initiating viral transcription (24). Meanwhile, the UL41 (VHS) tegument specifically degrades some mRNAs to the benefit of the virus (93, 94). During egress, passage of the newly assembled capsids across the two nuclear membranes relies on the UL31 (tegument)/UL34 (transmembrane protein) complex, as well as the US3 tegument (46, 81). Interestingly, despite the involvement of all three proteins in nuclear viral egress, only US3 is found in mature virions (81). Other teguments also participate in nuclear capsid egress, including UL48 (VP16), ICP34.5, UL36, UL37, and possibly UL51 (12, 45, 65, 71). The tegument further mediates the anterograde transport of newly assembled capsids (53, 101). Finally, several teguments are involved in the acquisition of the mature viral envelope, including UL36 and UL37 (19, 30), UL7 (29), UL11 (6, 47), UL20 (26), UL46 to 49 (31, 65), and perhaps UL51 (45, 71).
Given the multiple roles played by the tegument throughout the life cycle of the virus, its incorporation into mature extracellular virions is surely significant. So far, the HSV-1 teguments possibly present in mature extracellular virions include UL11 (54), UL13 (15, 74), UL14 (16), UL16 (61, 68), UL21 (5), UL36 (60), UL37 (57, 86), UL41 (25, 88), UL46 (110), UL47 (59, 110), UL48 (67), UL49 (23, 91), UL51 (17), US2 (in HSV-2; [37]), US3 (81), US10 (102), US11 (83), and ICP34.5 (34). In addition, conflicting reports have hinted at the presence of ICP0 (22, 39, 106, 107) and ICP4 (22, 58, 106, 108). Finally, a number of proteins with predicted transmembrane domains, but which are often referred to as teguments in the literature, are also found in virions. They include UL20 (99), UL56 (41, 42), and US9 (28). Despite this knowledge, the complete composition of a mature extracellular virion, including its tegument, awaits a comprehensive characterization.
Little is known so far about the presence and role of host proteins in HSV-1 virions. However, a variety of host proteins have been found in other herpesviruses (7, 9, 18, 21, 38, 40, 63, 96, 111). Though some of these proteins are uniquely incorporated in a given virus, cytoskeleton, heat shock, and other cellular proteins are shared by several members of the Herpesviridae family. Unfortunately, their roles have yet to be elucidated. As the presence of these proteins is unlikely anecdotic, it is of utmost interest to identify and characterize them.
Mass spectrometry is a powerful means with which to identify the protein composition of complex samples. Such a proteomics approach has been performed successfully for analyzing various herpesviruses, including human cytomegalovirus (HCMV) (7, 96), murine cytomegalovirus (MCMV) (40), Epstein-Barr virus (EBV) (38), Kaposi's sarcoma-associated virus (KSHV) (9, 111), rhesus monkey rhadinovirus (RRV) (72), murine gammaherpesvirus 68 (MHV68) (11), and very recently alcelaphine herpesvirus type 1 (21). HSV-1 is conspicuously absent from this list. As a first step toward elucidating the process of tegumentation and the role played by host proteins, we opted to analyze by mass spectrometry the composition of highly purified HSV-1 extracellular virions. This approach detected low-abundance proteins such as UL6 (12 copies), some of the smallest viral proteins (for example, US9; 10 kDa) as well as multimembrane-spanning proteins such as the gM glycoprotein.
Overall, this study successfully detected 37 of 40 known viral components and correctly diagnosed the absence of 21 nonstructural proteins, for an estimated 95.1% accuracy. This accuracy was accrued to 96.7% when we performed an in-depth multiple reaction monitoring (MRM) analysis, an approach that targets specific proteins by mass spectrometry. The data further revealed the presence of four novel virion components (UL7, UL23, UL50, and UL55) and confirmed the presence of ICP0 and ICP4 in mature virions. In contrast, the viral terminase (UL15/UL28/UL33), the UL31/UL34 complex, four different glycoproteins (gJ, gK, gN, and UL43), UL4, and UL24 were all absent. Finally, analysis of the host protein content revealed the potential incorporation of up to 49 distinct cellular proteins in extracellular virions.
MATERIALS AND METHODS
Cells and viruses.
HeLa and baby hamster kidney (BHK) cells were grown at 37°C in 5% CO2 in Dulbecco's modified Eagle's medium (DMEM; Sigma Aldrich) supplemented with 10% fetal bovine serum (Medicorp), 2 mM l-glutamine (Invitrogen), and antibiotics (100 U/ml penicillin and 100 μg/ml streptomycin [Invitrogen]). The wild-type HSV-1 strain F, provided by Beate Sodeik, was propagated on BHK cells and titrated on Vero cells as described previously (95).
Antibodies.
Primary antibodies were graciously provided by various sources (and diluted) as follows. Anti-HSV-1 Remus, a general HSV-1 polyclonal serum was from B. Sodeik (1:1,000); anti-UL4 (1:1,000), UL20 (1:4,000), US3 (1:4,000), and US11 (1:1,000) were from B. Roizman; anti-UL7 was from Y. Nishiyama (1:1,000); anti-UL15 (1:1,000), UL28 (1:5,000), UL31 (1:1,000), and UL33 (1:1,000) were from J. Baines; anti-UL23 was from J. Smiley (1:5,000); anti-UL24 was from A. Pearson and D. Coen (1:750); anti-UL34 was from R. Roller (1:1,000); and anti-US6 gD-DL6 was from R. J. Eisenberg and G. H. Cohen (1:2,000). Commercial antibodies against UL19 (1:1,000; Cedarlane), ICP0 (1:5,000; Abcam), and ICP4 (1:1,000; Abcam) were also used. Goat anti-mouse, -rabbit, or -rat, as well as donkey anti-chicken secondary, antibodies were used at a 1:20,000 dilution and were from Jackson ImmunoResearch or Cedarlane.
Determination of optimal infection kinetics.
HeLa cells were mock treated or infected with HSV-1 at a multiplicity of infection (MOI) of 5 at 37°C. Extracellular medium was collected at 9, 12, 16, and 24 h postinfection (hpi), concentrated for 1 h at 39,000 × g (rotor model SW41ti; Beckman), and finally resuspended in MNT buffer (30 mM MES, 100 mM NaCl, and 20 mM Tris-HCl [pH 7.4]). In parallel, cells were also collected by scraping them into phosphate-buffered saline (PBS), spinning the mixture at 300 × g for 5 min at 4°C, and then suspending viral pellets in MNT buffer. Five micrograms of each sample was loaded onto an 8% sodium dodecyl sulfate-polyacrylamide gel and analyzed by Western blotting using the Remus polyclonal antibody against HSV-1.
Purification of extracellular virions.
HeLa or BHK cells were grown on 500-cm2 dishes until 70% confluent. Cells were mock treated or infected with wild-type HSV-1 at an MOI of 5. At 6 hpi, the cells were washed twice with PBS, and the medium was replaced by serum-free DMEM until the extracellular medium was harvested at either 24 hpi (HeLa) or 16 hpi (BHK). The medium was then collected, clarified by centrifugation at 300 × g for 10 min, and treated with 50 μg/ml DNase I (Roche) for 30 min at 4°C. The sample was then filtered through a 0.45-μm filter to eliminate intact cells and large cellular debris. Extracellular virions were subsequently pelleted by centrifugation at 20,000 × g for 30 min at 4°C in a Beckman JA25.50 rotor. The viral pellet was resuspended in MNT buffer and was further purified by layering it over a 10% Ficoll 400 cushion centrifuged at 26,000 × g for 2 h at 4°C in an SW41ti rotor (92). The virus was washed with MNT buffer and concentrated by centrifugation at 20,000 × g for 30 min at 4°C. Purified viruses were stored at −80°C in MNT buffer.
Electron microscopy.
Sample purity was evaluated first by negative staining. Briefly, purified viruses were deposited on square 150-mesh copper grids coated with Formvar and carbonated (Canemco and Marivac). Excess liquid was blotted away with filter paper, and the samples were stained for contrast with 2% uranyl acetate (Canemco and Marivac). The grids were then washed in distilled water and dried on filter paper. Samples were examined with a Philips 300 transmission electron microscope (79).
Gel electrophoresis and Western blotting.
All samples were boiled for 10 min in loading buffer (50 mM Tris-HCl [pH 6.8], 2% SDS, 0.1% bromophenol blue, 10% glycerol, and 2% β-mercaptoethanol) and separated on 8, 10, 12, or 15% gels by SDS-PAGE. Proteins were electrophoretically transferred from the gels to polyvinylidene difluoride (PVDF) membranes, and the membranes were blocked for 1 h in blocking buffer (5% nonfat dry milk, 13.7 mM NaCl, 0.27 mM KCl, 0.2 mM KH2PO4, 1 mM Na2HPO4, and 0.1% Tween 20). All primary antibodies were diluted in blocking buffer and added to blots for 1 to 2 h. Blots were then washed three times in blocking buffer and probed with secondary antibodies conjugated to horseradish peroxidase. The detection was done on Kodak BioMax MR film and with a Super Signal West Pico chemiluminescent substrate (Pierce).
Silver staining.
Following electrophoresis, gels were fixed overnight in a 5% acetic acid-50% methanol solution. Gels were then extensively rinsed in distilled water for 30 min and incubated in 0.02% thiosulfate sodium solution for 1 min. Gels were briefly washed with distilled water for 2 min and incubated in 0.1% silver nitrate solution for 20 min. After this incubation, gels were again briefly washed in distilled water and then incubated in reaction buffer (0.04% formaldehyde and 2% carbonate sodium). The reaction was stopped by the addition of 5% acetic acid solution to the gels.
Mass spectrometry.
Purified virions were first acetone precipitated and solubilized in Laemmli buffer (2% SDS, 10% glycerol, 5% β-mercaptoethanol, 0.002% bromophenol blue, and 62.5 mM Tris-HCl [pH 6.8]). Twenty micrograms of sample was loaded onto 7 to 15% SDS-polyacrylamide gradient gels. Following electrophoresis, the gels were stained with Coomassie brilliant blue G (Sigma) and then subjected to automated band excision. Proteins from gel bands were subjected to reduction, cysteine-alkylation, and in-gel tryptic digestion by using an automated MassPrep workstation (Micromass). Extracted peptides were injected onto a Zorbax C18 (Agilent) desalting column and subsequently chromatographically separated on a Biobasic C18 Integrafrit (New Objective) capillary column, using a Nano high-performance liquid chromatography system (1100 series unit; Agilent). Eluted peptides were electrosprayed as they exited the column and analyzed on a QTRAP 4000 linear ion trap mass spectrometer (SCIEX/ABI). Individual sample tandem mass spectrometry spectra were peak listed and searched with Mascot (Matrix Science) software against a homemade database generated from the complete NCBI nonredundant protein data set and limited to the human HSV-1 taxonomy. To identify host proteins, a second search was performed against the nonredundant human NCBI database. Only peptides that were both unique and above the minimal score proposed by Mascot were considered. When indicated, the relative abundance of individual proteins was estimated by clustering the data and using the percentage of queries (NQPCT) score, a value that takes into consideration the number of peptides detected for a given protein as well as the size of that protein (10, 51). Note that the sum of all NQPCT is 100%, i.e., all proteins identified by Mascot. Given the semiquantitative nature of this approach, the proteins were grouped into four categories (<1%, low abundance; 1 to 4%, medium abundance; 5 to 10%, high abundance; and >10%, very high abundance).
Analysis by MRM.
To reevaluate more precisely the presence of UL20, UL43, gJ, gK, gN, US11, full-length UL26, VP21, and VP22a in the virions, the proteins were reanalyzed by MRM. This technique allows the detection of specific proteins of interest in complete mixtures and, thus, is a more sensitive method than classical mass spectrometry (3, 48). To this end, a transition table containing the predicted masses of tryptic peptides for UL20, UL43, gJ, gK, gN, US11, full-length UL26, VP21, and VP22a was generated. Appropriate bands from the above-described gels were reanalyzed as before by tandem mass spectrometry, except that only peptides matching the predicted masses were considered. To minimize the risk of missing a protein due to its unexpected migration by SDS-PAGE, adjacent bands were also analyzed.
RESULTS
Purification of extracellular virions.
Sample purity is most important for the accurate identification of protein content by mass spectrometry. One way to reduce cellular contamination is simply to harvest extracellular virions when viral release is significant but when cell lysis is minimal. To determine this window, HeLa cells were chosen because the infection proceeds relatively slowly in these cells compared to that in other cell types (data not shown). A single round of infection was, thus, monitored over a 24-h period, using an MOI of 5, a statistical condition under which 99.3% of all cells are infected (Poisson distribution). Viral release was evaluated by Western blotting using a general HSV-1 polyclonal antiserum. As shown in Fig. 1, viral release into the extracellular medium was detectable by 16 hpi but significantly increased by 24 hpi. By then, cell lysis was less than 5% (data not shown). Given these results, the latter time point was chosen to pursue the analysis.
FIG. 1.
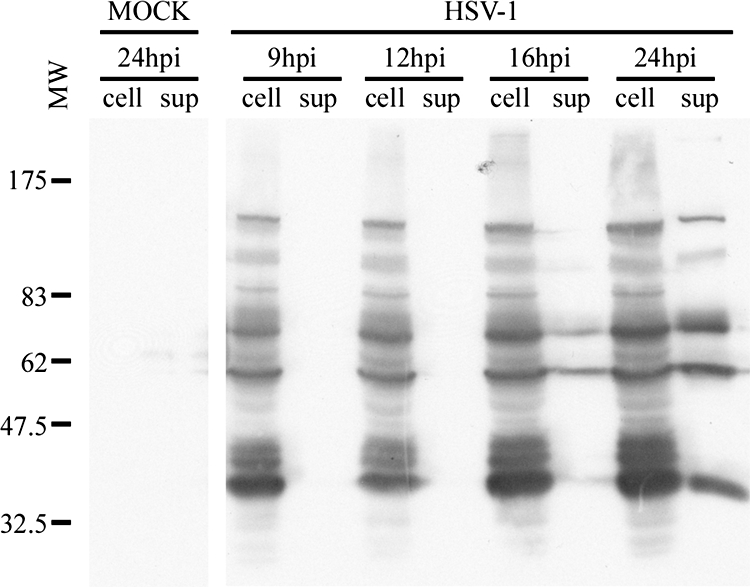
Kinetics of infection. HeLa cells were mock treated or infected with HSV-1 and the cells (cell) and extracellular medium (sup) were collected at 9, 12, 16, and 24 hpi. Once cells were concentrated by centrifugation, they were analyzed by Western blotting with the Remus polyclonal antibody against HSV-1. “Mock” denotes an uninfected control that was analyzed at 24 hpi. The molecular weights (MW) of the protein markers are indicated (103) to the left.
A scheme was next elaborated to purify the extracellular virions (Fig. 2). First, to reduce the contribution of proteins found in the tissue culture medium, the cells were washed twice at 6 hpi with PBS and fed with serum-free medium. The infection was then allowed to proceed for an additional 18 h, at which point the extracellular viruses were harvested. The sample was spun at low speed to remove intact cells and nuclei, treated with DNase I to reduce viscosity, and passed through a 0.45-μm filter to remove intact cells and large cellular debris. Extracellular viruses were spun at 20,000 × g, a condition that efficiently pellets the virions but minimizes contamination by small cellular organelles. The virus was then purified further over an adapted Ficoll 400 gradient. In typical 5 to 15% Ficoll gradients, the defective L particles migrate at the 5% mark, whereas the heavier mature virions remains near the bottom at 15% (92). Consequently, the virions were top loaded over a 10% Ficoll step gradient, the viral pellet was washed with MNT buffer, and the virus was finally concentrated by centrifugation at 20,000 × g.
FIG. 2.
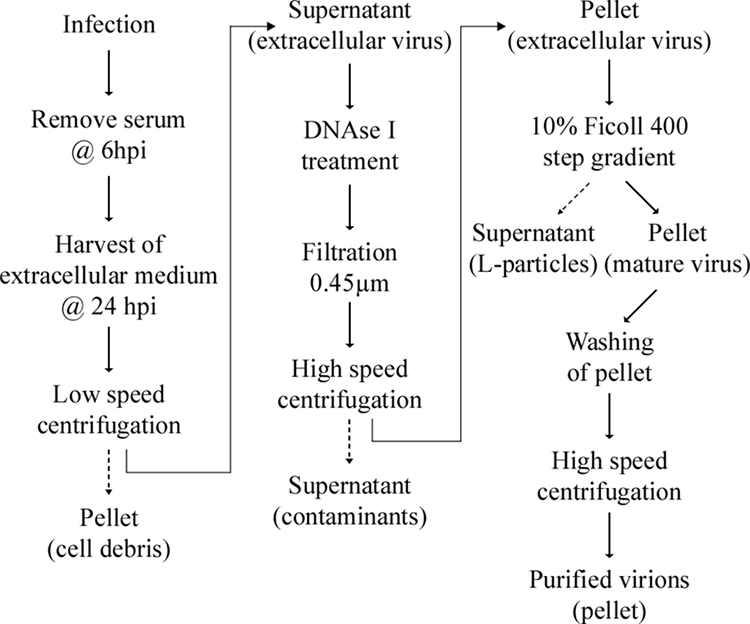
Purification scheme. Schematic description of the different steps used to purify extracellular HSV-1 virions. See text and Materials and Methods for details.
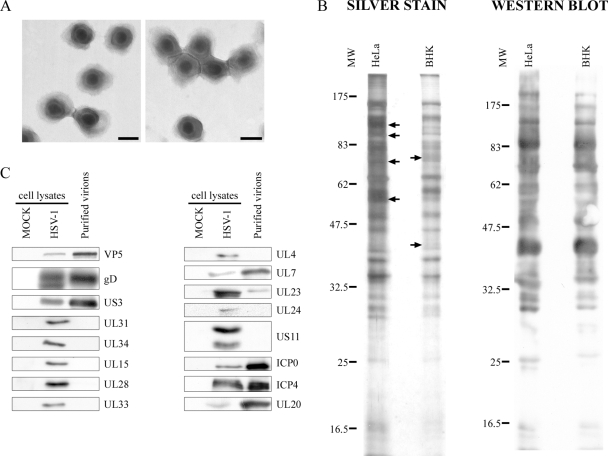
To monitor sample purity, negative staining was performed, and the samples were examined by electron microscopy (EM). As shown in Fig. 3, the viral preparation was completely devoid of cellular debris, L particles, nuclei, mitochondria, and even small vesicles. Note that all viruses shared the characteristics of mature virions with an envelope and a DNA core (Fig. 3). To determine the efficacy of the purification scheme, the virions were monitored by Western blots using a pan HSV-1 polyclonal antibody and by silver coloration. As controls, total cell lysates from mock-treated or HSV-1-infected cells were included. Figure 4A shows the strong enrichment of numerous viral bands compared to that of the unpurified extracellular medium (Fig. 4, compare lanes 3 and 4). Furthermore, the total protein pattern seen by silver staining was significantly different than that of mock or HSV cell control lysates (Fig. 4, see dots). The enrichment of virions was particularly evident in Western blots (Fig. 4b). Further purification of the virions by additional means such as immunoprecipitation against the viral glycoproteins, affinity chromatography using a heparin column, or other density gradients only reduced viral yield and did not improve sample purity, suggesting that the samples were already relatively pure (data not shown).
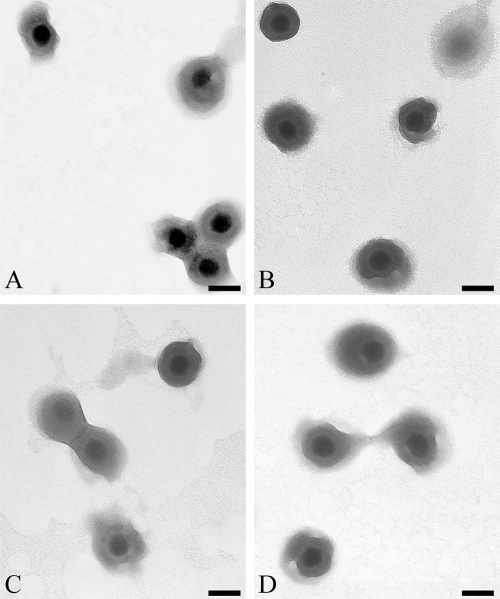
FIG. 3.
Analysis of extracellular virions by EM. Purified virions from HeLa cells were negatively stained and analyzed by EM. Panels A to D show typical views of the sample. Note that the sample consisted exclusively of enveloped virions and was exempt of cellular debris and L particles. Bars represent 100 nm.
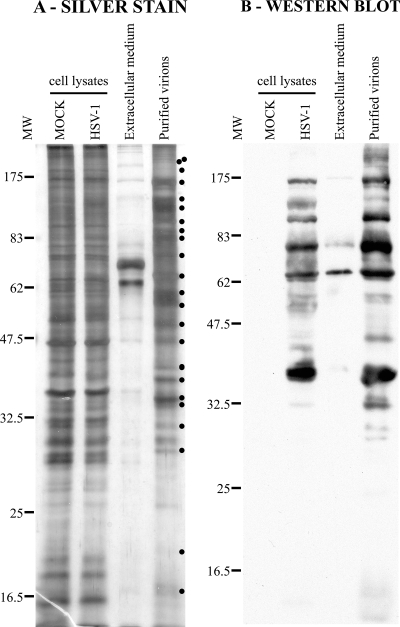
FIG. 4.
Analysis of purified virions by silver staining and Western blotting. (A) Five micrograms of unpurified (Extracellular medium) or purified virions from HeLa cells were loaded onto a 12% SDS-polyacrylamide gel and silver stained. As controls, equivalent amounts of total mock-treated and infected cell lysates were also loaded. Note the differences between the protein pattern of purified virions and that of control total cell lysates (dots at right). (B) The same samples were also analyzed by Western blotting with the Remus antibody, but in this case, 25 μg of control mock and infected total cell lysates was loaded. Viral enrichment of the purified virions was particularly evident compared to that of the unpurified sample. As before, the molecular weights (MW) of the protein markers are indicated at the left of the gels (103).
Viral content of extracellular virions.
One goal of this study was to identify the viral protein content of extracellular virions. Given their high purity, we pursued protein separation by SDS-PAGE, in-gel trypsin digestion, and tandem mass spectrometry (see Materials and Methods). To optimize viral protein identification, a database was generated using all human HSV-1 proteins present in the NCBI nonredundant protein data bank. These proteins were trypsin digested in silico, and the viral peptides found by mass spectrometry were identified with Mascot software against this homemade tryptic database. Over 400 highly confident unique peptides matched HSV-1 proteins (Table 1). For simplicity, they are organized according to the proposed localization of the proteins in virions (4, 62). Since the number of peptides is one measure of relative abundance (10, 21), albeit imperfect, the data suggested that the tegument is the major virion component and represented 54% of all peptides, by comparison to 27% for the capsid and 19% for the envelope. Similarly, 23 of the identified proteins were from the tegument, while 8 proteins matched capsid proteins, and 12 corresponded to the envelope.
TABLE 1.
Viral content of extracellular virions
| Protein group (expected in virions)a | Protein | MWb | Size (aa)c | No. of unique peptidesd | % Coveragee | % of totalf | Total no. of proteins |
|---|---|---|---|---|---|---|---|
| Capsid | |||||||
| UL6 (+) | 74.1 | 676 | 1 | 2.5 | |||
| UL15 (?) | Terminase | 80.9 | 735 | − | NA | ||
| UL17 (+) | 74.6 | 703 | 10 | 23.8 | |||
| UL18 (+) | VP23 | 34.3 | 318 | 15 | 65.7 | ||
| UL19 (+) | VP5/ICP5 | 149.1 | 1,374 | 54 | 59.5 | ||
| UL25 (+) | 62.7 | 580 | 8 | 19.5 | |||
| UL26 protease | VP24 (+) | 26.6 | 247 | 7 | 41.3 | ||
| VP21 (−) | 37.3 | 363 | − | NA | |||
| COOH termini (−) | 2.6 | 25 | − | NA | |||
| UL26.5 | VP22a (−) | 31.2 | 304 | − | NA | ||
| COOH termini (−) | 2.6 | 25 | − | NA | |||
| UL28 (?) | ICP18.5 | 85.6 | 785 | − | NA | ||
| UL33 (?) | 14.4 | 130 | − | NA | |||
| UL35 (+) | VP26 | 12.1 | 112 | 3 | 39.3 | ||
| UL38 (+) | VP19C | 50.3 | 465 | 9 | 23.9 | ||
| Total | 107 | 26.7 | 8 | ||||
| Envelope | |||||||
| UL1 (+) | gL | 24.9 | 224 | 4 | 24.6 | ||
| UL10 (+) | gM | 51.4 | 473 | 2 | 11.2 | ||
| UL20 (+) | 24.2 | 222 | − | NA | |||
| UL22 (+) | gH | 90.4 | 838 | 11 | 18.7 | ||
| UL27 (+) | gB | 100.3 | 904 | 22 | 30.2 | ||
| UL34 (−) | 29.8 | 275 | − | NA | |||
| UL43 (?) | 44.9 | 434 | − | NA | |||
| UL44 (+) | gC | 55.0 | 511 | 7 | 26.6 | ||
| UL45 (+) | 18.2 | 172 | 5 | 39.0 | |||
| UL49A (+) | gN | 9.2 | 91 | − | NA | ||
| UL53 (?) | gK | 37.6 | 338 | − | NA | ||
| UL56 (+) | 21.2 | 197 | 2 | 22.8 | |||
| US4 (+) | gG | 25.2 | 238 | 2 | 3.8 | ||
| US5 (?) | gJ | 9.6 | 92 | − | NA | ||
| US6 (+) | gD | 43.3 | 394 | 7 | 23.2 | ||
| US7 (+) | gI | 41.4 | 390 | 4 | 16.7 | ||
| US8 (+) | gE | 59.1 | 550 | 9 | 18.4 | ||
| US8A (?) | 16.8 | 159 | NA | ||||
| US9 (+) | 10.0 | 90 | 1 | 37.8 | |||
| Total | 76 | 19 | 12g | ||||
| Tegument | |||||||
| RL1 (+) | ICP34.5 | 26.2 | 248 | 1 | 3.4 | ||
| RL2 (?) | ICP0 | 78.5 | 776 | 10 | 21.3 | ||
| RS1 (?) | ICP4 | 132.8 | 1,298 | 20 | 22.6 | ||
| UL4 (−) | 78.2 | 718 | − | NA | |||
| UL7 (?) | 33.1 | 296 | 4 | 25.0 | |||
| UL11 (+) | 10.5 | 96 | 2 | 20.8 | |||
| UL13 (+) | 57.2 | 518 | 3 | 10.0 | |||
| UL14 (+) | 23.9 | 219 | 1 | 10.5 | |||
| UL16 (+) | 40.4 | 373 | 7 | 32.4 | |||
| UL21 (+) | 57.6 | 535 | 9 | 27.5 | |||
| UL23 (?) | TK | 41.0 | 376 | 1 | 4.4 | ||
| UL31 (−) | 34.0 | 306 | − | NA | |||
| UL36 (+) | Large tegument | 335.9 | 3,164 | 42 | 20.7 | ||
| UL37 (+) | ICP32 | 120.6 | 1,123 | 22 | 33.2 | ||
| UL41 (+) | Vhs | 54.9 | 489 | 9 | 30.1 | ||
| UL46 (+) | VP11/12 | 78.2 | 718 | 16 | 34.3 | ||
| UL47 (+) | VP13/14 | 73.8 | 693 | 25 | 51.2 | ||
| UL48 (+) | VP16/ICP25 | 54.3 | 490 | 15 | 45.5 | ||
| UL49 (+) | VP22 | 32.3 | 301 | 8 | 39.5 | ||
| UL50 (?) | dUTPase | 39.1 | 371 | 6 | 26.7 | ||
| UL51 (+) | 25.5 | 244 | 4 | 27.0 | |||
| UL55 (?) | 20.5 | 186 | 4 | 37.6 | |||
| US2 (+) | 32.5 | 291 | 5 | 15.6 | |||
| US3 (+) | 52.8 | 481 | 1 | 6.7 | |||
| US10 (+) | 34.1 | 312 | 3 | 13.7 | |||
| US11 (+) | 17.8 | 161 | − | NA | |||
| Total | 218 | 54.4 | 23 | ||||
| Other | |||||||
| UL2 (−) | 36.3 | 334 | − | NA | |||
| UL3 (−) | 25.6 | 235 | − | NA | |||
| UL5 (−) | 98.7 | 882 | − | NA | |||
| UL8 (−) | 79.9 | 750 | − | NA | |||
| UL9 (−) | 94.3 | 851 | − | NA | |||
| UL12 (−) | 67.5 | 626 | − | NA | |||
| UL24 (?) | 29.5 | 269 | − | NA | |||
| UL29 (−) | ICP8 | 128.4 | 1,196 | − | NA | ||
| UL30 (−) | 136.4 | 1,235 | − | NA | |||
| UL32 (−) | 64.0 | 596 | − | NA | |||
| UL39 (−) | ICP6/10 | 124.1 | 1,137 | − | NA | ||
| UL40 (−) | 38.0 | 340 | − | NA | |||
| UL42 (−) | 51.2 | 488 | − | NA | |||
| UL52 (−) | 114.4 | 1,058 | − | NA | |||
| UL54 (−) | ICP27 | 55.3 | 512 | − | NA | ||
| US1 (−) | ICP22 | 46.5 | 420 | − | NA | ||
| US12 (−) | ICP47 | 9.8 | 88 | − | NA |
Localization is based on that described in references 2 and 56. +, expected; −, not expected; ?, uncertain.
MW, estimated molecular weight (103).
aa, amino acids.
−, no peptides found.
Values shown are the percentages of coverage of proteins by peptides. NA, not applicable.
Relative peptide abundance.
Thirteen proteins if UL20 is included (Table 2, MRM data).
Validation of the proteomics approach.
One caveat of proteomics is the possible inclusion of false positives (contaminants) and false negatives (below the detection threshold). Fortunately, many components of HSV-1 virions have been identified by other means by several laboratories. This independent input represented an ideal opportunity to validate our approach. Thus, analysis of the literature revealed that 37 of the 40 anticipated capsid, tegument, and viral glycoproteins were detected (Table 1). This translates to 92.5% accuracy for true positives. Similarly, none of the 21 nonstructural viral proteins, i.e., not anticipated on mature virions, was detected (100% accuracy for true negatives). This value included the viral replication machinery, the UL31/UL34 complex, UL4, and VP22a. Altogether, this means a 95.1% accuracy rate (58 out of 61). Furthermore, the mass spectrometry appeared sufficiently sensitive to detect low-abundance proteins such as UL6, a protein present only in 12 copies in mature virions (69). US9 and UL11, the two smallest proteins expected in virions, were also detected (90 and 96 amino acids, respectively). In addition, numerous glycoproteins were also found, suggesting that hydrophobicity was not a major issue. Collectively, these data highlighted the remarkable accuracy and sensitivity of the approach.
An important aspect is whether the sample consisted only of mature viruses. As indicated above, EM analysis suggested that the sample was relatively pure (Fig. 3). Another way to probe this issue is to examine the UL26 protease, the UL26.5 (preVP22a) scaffold, and their respective cleavage products. Though full-length versions of these proteins are present in immature capsids, the former is cleaved into VP21 and VP24, while the second is processed into VP22a. Incidentally, all but VP24 are removed from mature capsids and are, thus, useful markers (reviewed by Baines et al. [4]). Interestingly, mass spectrometry identified seven peptides matching VP24, but none corresponded to the full-length UL26, VP21, preVP22a, or processed VP22a (Table 1). Consequently, only mature virions were detected in the sample.
To further validate the mass spectrometric approach, control proteins were examined by Western blotting. These proteins included the VP5 major capsid protein, the gD glycoprotein expected of mature envelope virions, and the US3 tegument as positive controls. In contrast, UL31 and UL34 acted as negative controls as they are known to be absent on mature virions (81). As noted in Fig. 5, the results were all fully consistent with the mass spectrometry data; i.e., the virions were VP5, gD, and US3 positive but UL31 and UL34 negative. Once again, the data strongly suggested that mass spectrometry was sensitive and correctly identified the viral protein content of mature virions. It was, unfortunately, not possible to evaluate the presence of gJ, gK, and gN in mature virions by Western blotting due to the lack of appropriate antibodies.
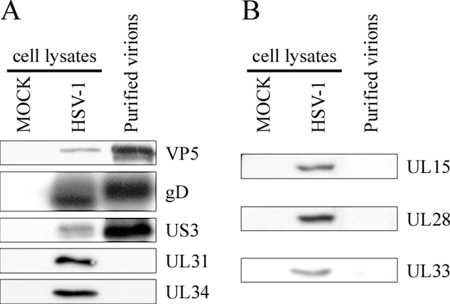
FIG. 5.
Western blotting analysis of the capsid. (A) Five micrograms of purified virions from HeLa cells was separated by SDS-PAGE, transferred to PVDF membranes, and probed with antibodies against the major VP5 capsid, a control glycoprotein D envelope protein (gD), the serine/threonine viral kinase (US3), and the UL31/UL34 complex. Fifteen micrograms of total mock-infected or infected cell lysates was also included as an antibody control. (B) As described above, except that the viral terminase components (UL15, UL28, UL33) were analyzed with specific antibodies.
Encapsidation of the viral DNA requires the participation of the tripartite UL15/UL28/UL33 terminase (104, 105). It has been suggested that it remains associated with mature C capsids (4). However, the present study failed to detect these proteins by mass spectrometry. To independently validate the data, these proteins were probed with the more sensitive Western blotting technique using sera specific for each of the terminase subunits. Once again, the results fully matched those obtained by mass spectrometry in that none of these proteins was detected, despite being detected in control-infected lysates (Fig. 5).
Analysis of teguments.
Having established the validity of the approach, it was possible to specifically examine the tegument. Surprisingly, four novel virion components were identified by mass spectrometry. They include UL7, UL23 (TK), UL50, and UL55 (Table 1). Based on the well-characterized capsid constituents and the lack of a predicted transmembrane domain in these proteins (data not shown), the novel virion components were tentatively assigned to the tegument layer. However, biochemical confirmation of this assignment will be required. In contrast, UL4, UL24, and US11 were not detected. This was also the case for UL20, a predicted transmembrane protein often referred to as a tegument (see below). To confirm these findings and probe the apparent discrepancy between these results and that reported for US11 (83), the samples were once again analyzed by Western blotting, with the exception of UL50 and UL55, for which no antibodies are available. As seen in Fig. 6A, the results perfectly matched the mass spectrometry data. Hence, UL7 and UL23 were detected, while UL4, UL24, and US11 were absent.
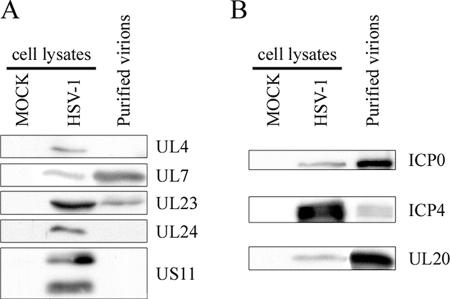
FIG. 6.
Western blot analysis of the tegument. Five micrograms of purified virions from HeLa cells was separated by SDS-PAGE, transferred to PVDF membranes, and probed with antibodies against UL4, UL7, UL23, UL24, and US11 (A) or ICP0, ICP4 and UL20 (B). Fifteen micrograms of mock-infected or infected cell lysates was included as an antibody control.
Incorporation of ICP0 and ICP4.
ICP0 and ICP4 have been considered by many as minor components of mature virions (22, 87, 107, 108). However, Roizman and colleagues failed to detect ICP0 on virions by immuno-EM (39). Furthermore, Courtney and his team reported the cell type-specific inclusion of ICP0 and ICP4 in some virions (106). In addition, others have argued that ICP4 may instead be present in contaminating L particles (56, 92). In this study, ICP0 and ICP4 were readily identified in a highly enriched virion preparation (Table 1) that contained no noticeable L particles (Fig. 3). To confirm these findings, we next monitored ICP0 and ICP4 by Western blotting. As shown in Fig. 6B, both proteins were once again detected. We conclude that mature extracellular virions do indeed contain ICP0 and ICP4.
Incorporation of UL20/gK in virions.
Our proteomics data suggested the absence of gK and UL20 from mature viruses (Table 1). This was unexpected given that UL20 and gK have been reported on herpesvirus virions (27, 44, 99). However, in support of our initial results, gK was not detected in HSV-1 virions in at least one study (36). To sort out this issue, we probed the highly purified virions by Western blotting. Unfortunately, three distinct antibodies against gK could not detect the native protein in control infected cell lysates (data not shown). Consequently, only UL20 was followed more closely. To our surprise, UL20 was detected by Western blotting on the purified virions (Fig. 6B) in contrast to the proteomics results (Table 1). Given the specificity of the antibody used (data not shown), it could only be that UL20 is truly on mature virions but was simply not detected by mass spectrometry.
MRM analysis.
Low-abundance peptides can at times be masked in mass spectrometry by more abundant ones, which can lead to false-negative results. However, it is possible to look for specific peptides by using MRM. This is a well-established method that essentially instructs the mass spectrometer to look for proteins based on the predicted masses of their tryptic peptides (3, 48). In doing so, the instrument ignores all other proteins and makes it possible to detect rarer peptides. Thus, to independently test for proteins that are not identified by standard mass spectrometry, a table with their predicted tryptic fragments was generated, and the mass of appropriately sized, hydrophilic and non-N-glycosylated peptides signaled to the mass spectrometer so it could detect them in the sea of peptides. This analysis was performed for the problematic gK, UL20, and US11 proteins (see above), as well as for UL43, gJ, gN, full-length UL26, VP21, and preVP22a, all molecules that were undetected by conventional proteomics in this study. To reduce the chances of missing a protein because it unexpectedly runs on SDS-PAGE, adjacent bands were also analyzed. Table 2 shows that among all these proteins, only UL20 was found by this more sensitive approach. We conclude that UL20 is indeed present on mature virions, while UL43, gJ, gK, gN, and US11 are likely absent. Furthermore, the full-length UL26, VP21, and preVP22a markers of immature capsids were all absent, and this reiterated the sole presence of mature virions in the sample. Naturally, we cannot exclude the possibility that all undetected proteins were below the resolution limit of this study.
TABLE 2.
MRM analysis
| Protein analyzed | Comment | MWa | Size (aa)b | Unique peptidesc | % Coveraged |
|---|---|---|---|---|---|
| UL20 | 24.2 | 222 | DDLPLVDR | 3 | |
| UL26 | Full length | 66.5 | 635 | − | NA |
| VP21 | 37.3 | 363 | − | NA | |
| COOH termini | 2.6 | 25 | − | NA | |
| UL26.5 | pre-VP22a | 33.7 | 329 | − | NA |
| COOH termini | 2.6 | 25 | − | NA | |
| UL43 | 44.9 | 434 | − | NA | |
| UL49A | gN | 9.2 | 91 | − | NA |
| UL53 | gK | 37.6 | 338 | − | NA |
| US5 | gJ | 9.6 | 92 | − | NA |
| US11 | 17.8 | 161 | − | NA |
MW, estimated molecular weight (103).
aa, amino acids.
Minus sign indicates that none were detected.
Value shown is the percentage of coverage of protein by polypeptides detected by tandem mass spectrometry. NA, not applicable.
Cell type-specific incorporation of viral proteins.
To determine whether the composition of the virions may be cell type related, extracellular virions were isolated from the unrelated BHK cell line, using the same protocol as that for HeLa cells, except they were harvested slightly earlier (see Materials and Methods). EM analysis of these virions once again reiterated the purity of the sample (Fig. 7A). To get a quick overall picture of the protein composition of the virions, viral preparations from HeLa and BHK cells were compared by Western blotting with the Remus polyclonal HSV-1 antibody and by silver staining. Figure 7B shows that the protein patterns were overall very similar, with few distinct bands. To confirm these findings, several viral proteins were specifically probed by Western blotting to evaluate potential differences between the two virion preparations (Fig. 7C). The results essentially revealed identical expression patterns for BHK- and HeLa-derived virions. Though a full mass spectrometry analysis may be useful, the present data nonetheless strongly suggest that the protein composition of the virions is largely independent of the cell type.
FIG. 7.
Characterization of virions purified from BHK cells. (A) Virions were purified from BHK cells by the same procedure as that used for HeLa cells, negatively stained, and examined by EM. The viruses were all enveloped and exempt of cellular debris and L particles. Bars represent 100 nm. (B) Five micrograms of purified virions from HeLa and BHK cells was loaded onto a 12% SDS-polyacrylamide gel, and the overall protein composition was determined by silver staining (left panel). In contrast, viral enrichment was examined by Western blotting using a polyclonal HSV-1 antibody (right panel). Note the similar protein pattern (arrows indicate differences). (C) To more specifically probe differences between HeLa and BHK virions, 15 μg of mock-infected or infected cell lysates (antibody controls) and 5 μg of purified virions from BHK cells were separated by SDS-PAGE, transferred to PVDF membranes, and immunoblotted for various viral proteins as indicated. MW, molecular weight (103).
Incorporation of host proteins.
The purity of the sample and the accuracy, sensitivity, and strong validation of our approach allowed us to probe host proteins present in HSV-1 virions. To this end, the nonredundant human NCBI database was digested in silico and used to reanalyze the purified virions by mass spectrometry. As described before, only significant and unique peptides were considered. Remarkably, 85 highly confident unique peptides, corresponding to up to 49 distinct cell-derived proteins, were identified in mature extracellular HSV-1 virions (Table 3). In all cases, only one to four peptides were identified for each of these proteins, suggesting they may be minor components of the virions.
TABLE 3.
Potential cellular proteins associated with extracellular virions identified by mass spectrometry
| Protein name | MWa | Size (aa)b | Number of unique peptides | % Coveragec |
|---|---|---|---|---|
| 14-3-3 protein | 28.2h | 248 | 1d | 4.0 |
| 14-3-3 protein epsilon | 29.2 | 255 | 3 | 16.9 |
| 14-3-3 protein gamma | 28.3 | 247 | 1 | 5.7 |
| 14-3-3 protein zeta/delta | 27.7 | 245 | 2 | 12.7 |
| Actin | 41.8h | 375 | 3e | 16.3 |
| Annexin A1 | 38.7 | 346 | 1 | 4.6 |
| Annexin A2 | 38.6 | 339 | 3 | 13.6 |
| Annexin A5 | 35.9 | 320 | 1 | 5.0 |
| Arf1/Arf 3 | 20.7 | 181 | 3f | 26.0 |
| Arf1/Arf3/Arf5 | 20.6 | 181 | 2f | 11.0 |
| Arf1/Arf3/Arf4/Arf5 | 20.6 | 181 | 2f | 16.0 |
| Arf4 | 20.5 | 180 | 1 | 10.0 |
| ATP-dependent RNA helicase DDX3X | 73.2 | 662 | 2 | 3.3 |
| Casein kinase 2 | 45.1 | 391 | 1 | 4.6 |
| Cofilin 1 | 18.5 | 166 | 1 | 7.4 |
| Cyclophilin A | 18.0 | 164 | 8 | 64.8 |
| Cystein-glycin-rich protein 1 | 20.6 | 193 | 1 | 11.4 |
| Eukaryotic translation initiation factor 4H (eIF4H) | 27.4 | 248 | 4 | 28.2 |
| GAPDHi | 36.1 | 335 | 1 | 4.2 |
| Growth factor receptor bound protein 2 | 25.2 | 217 | 1 | 6.5 |
| HSP70 | 70.4 | 641 | 3 | 7.8 |
| Keratin 1 | 66.0 | 644 | 1 | 2.5 |
| Keratin 10 | 59.5 | 593 | 1 | 2.0 |
| Macrophage migration inhibitory factor | 12.5 | 115 | 1 | 9.5 |
| Membrane attack complex inhibition factor (CD59) | 14.2 | 128 | 1 | 9.4 |
| Nucleoside diphosphate kinase A/B | 17.3 | 152 | 1 | 11.8 |
| Peroxiredoxin-1 | 22.1 | 199 | 2 | 10.6 |
| Peroxiredoxin-2 | 21.9 | 198 | 1 | 5.5 |
| Profilin-1 | 15.1 | 140 | 4 | 28.6 |
| Programmed cell death protein 6 | 21.9 | 191 | 2 | 10.5 |
| Rab | 24.0h | 215 | 3g | 15.3 |
| Rab2A/Rab 2B/Rab4B | 23.8 | 214 | 1 | 6.1 |
| Rab5 | 23.6 | 215 | 1 | 5.1 |
| Rab6 | 25.2 | 223 | 3 | 21.1 |
| Rab7A | 23.5 | 207 | 1 | 6.8 |
| Rab10 | 22.5 | 200 | 1 | 5.5 |
| Rab11 | 24.5 | 217 | 2 | 11.1 |
| Rab15 | 24.4 | 212 | 1 | 5.2 |
| Rab33B | 25.7 | 229 | 1 | 4.8 |
| Rab35 | 23.0 | 201 | 1 | 5.5 |
| Rab-like protein 3 | 26.4 | 236 | 1 | 4.7 |
| S100 calcium protein binding A11 | 11.7 | 105 | 2 | 25.7 |
| Sec14-like protein 4 | 46.6 | 406 | 1 | 2.2 |
| Tetraspanin 13 | 22.2 | 204 | 1 | 10.3 |
| Transferrin receptor protein 1 (CD71) | 84.9 | 760 | 1 | 1.2 |
| Translocase of inner mitochondrial membrane 50 (TIMM50) | 39.6 | 353 | 1 | 3.1 |
| Triosephosphatase isomerase | 26.7 | 249 | 1 | 11.7 |
| Ubiquitin C | 8.6 | 76 | 2 | 32.9 |
| Ubiquitin-conjugating enzyme E2 L3 | 17.9 | 154 | 1 | 14.3 |
| Total peptides | 85 |
MW, estimated molecular weight (103).
aa, amino acids.
Percentage of coverage of protein by polypeptides detected by tandem mass spectrometry.
Shared among the 14-3-3 protein family members.
Shared among the actin family members.
Shared among the indicated Arf proteins.
Shared among the Rab protein family members.
MW was averaged for these family members.
GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
DISCUSSION
Efficacy of the proteomic approach.
Three criteria are essential for proteomics studies, namely, purity, sensitivity, and accuracy. First, an examination of sample purity by EM revealed a homogeneous virion preparation free of cellular debris, small contaminating vesicles, and L particles (Fig. 3). Importantly, the last were not expected as the sample was passed over a Ficoll gradient known to effectively segregate them (92). Moreover, the purified sample was highly infectious (data not shown). Contamination by other components was also deemed nonsignificant based on the lack of intracellular naked capsids, mitochondria, nuclei, and other organelles that would result from extensive cell lysis (Fig. 3). In addition, pre-VP22a, VP21, or full-length UL26, all markers of immature capsids (reviewed by Baines et al. [4]), were absent from our sample (Tables 1 and 2). Similarly, the absence of UL31 and UL34, known to interact solely with nuclear capsids, is additional evidence that cell lysis and cellular contamination were minimal. Finally, further purification of the virions by additional means did not improve sample purity, suggesting that the virions were already pure (data not shown). Second, sensitivity was very high as the assay readily detected both low-copy (e.g., UL6) and low-molecular-weight proteins (e.g., UL11 and US9). Moreover, this extended to hydrophobic proteins, such as the multimembrane-spanning gM glycoprotein. Third, accuracy was very significant, with an estimated 95.1% overall rate. The similar detection of the viral proteins in the two unrelated HeLa and BHK cell lines is further evidence of the accuracy of the data (Fig. 7). Finally, the results were validated by Western blotting against several individual proteins. The proteomics approach was, thus, an accurate and efficient means with which to address HSV-1 virion composition.
Transient interactions with the capsid.
HSV-1 assembles its new capsids in the nucleus. Their maturation implies the packaging of monomeric viral genomes into procapsids, with the participation of the viral UL15/UL28/UL33 terminase (reviewed in reference 4). While these proteins are components of immature capsids (100), it has been suggested they may be incorporated in very low copy numbers in nucleus- and cell-associated C capsids (8, 109). Functionally, the incorporation of the terminase onto nuclear capsids makes good sense given its role in DNA packaging (4). However, there is no current evidence for its role in mature virions or for unpacking of the viral genome at the nuclear pore following entry. The lack of detection of the terminase by mass spectrometry in this study suggests that it may be absent in mature extracellular virions (Table 1). Although we cannot rule out that the terminase is below the detection threshold of the mass spectrometry approach used, the absence of signal for all three terminase subunits by the more sensitive Western blotting analysis is consistent with the former interpretation (Fig. 5). Moreover, these findings were reproducible for virions produced in two unrelated cell lines (Fig. 5 and 7). In addition, the sizes of UL15, UL28, and UL33 (1,931, 785, and 130 amino acids, respectively) are well beyond the size of other viral proteins detected in this study (for instance, US9 has 90 amino acids, while UL11 is 96 amino acids long). Taken together, the most straightforward interpretation is that the terminase is not incorporated in mature extracellular virions. Mechanistically, this implies that the complex dissociates at some point from the capsids, highlighting a potential dynamic interaction. The precise moment at which these proteins are released is most interesting but beyond the scope of the present study.
The UL31 tegument and the UL34 envelope protein bind one another and facilitate egress from the nucleus of newly formed capsids (50, 80, 81, 84). Interestingly, UL34 is the substrate of yet another necessary tegument, the US3 viral kinase (46, 78, 81, 98). The detection in this study of US3 and the absence of the UL31/UL34 partners on mature extracellular virions are consistent with findings from previous reports (81). The absence of UL31/UL34, as for the viral terminase (see above), hints at transitory interactions of viral proteins with the capsids in the nucleus that do not persist later on. While the incorporation of US3 in the extracellular virions may be merely fortuitous, a more appealing scenario is that it interacts with other targets at subsequent steps of the viral life cycle. This is in line with the numerous US3 substrates already discovered to date (13, 49, 64, 66, 75, 77, 78, 89).
Missing glycoproteins.
Standard mass spectrometry and the more sensitive MRM approach failed to detect gJ, gK, gN, and UL43 on mature virions (Tables 1 and 2). As for detection of gK, this is controversial, as some reports claim its presence in mature virions (27, 44), while others failed to see it (36). Similarly, UL43 and gJ were reportedly present on PRV and HSV-1 virions, respectively (32, 43). These discrepancies may be related to the hydrophobicity of these proteins, since such proteins tend to aggregate upon boiling (40) and may never reach the mass spectrometer. Unfortunately, it was not possible to probe their presence on virions by Western blotting for lack of appropriate antibodies. Consequently, we cannot say with certainty whether these gJ, gK, gN, and UL43 proteins are truly absent.
The detection of gM but the absence of gN on mature extracellular virions (Tables 1 and 2) was surprising given that the two proteins form a complex and that gN is needed for the proper localization of gM at the trans-Golgi network (14). This is even more puzzling as gN was previously reported in [35S]methionine- labeled mature virions, based on its molecular weight (1). Regrettably, it was not possible to probe for gN by Western blotting for lack of antibodies against the protein. Although we cannot completely rule out that gN was simply undetected in this study, it may dissociate from gM prior to final envelopment of the capsids. As proposed for HCMV, it may also be that gM exists in two forms, either by itself or in complex with gN, and that free gM is preferentially incorporated into virions (96). These suggestions are in agreement with a recent proteomics study of alcelaphine herpesvirus type 1 that found gM but not gN in the virions (21).
Composition of the tegument.
Little information is available concerning the incorporation of UL7, UL23, UL50, and UL55 in HSV-1. Some laboratories have reported their analyses with related herpesviruses. Hence, UL7 was found in HSV-2 virions (70), while UL23 was detected in KHSV and alcelaphine herpesvirus-1 virions (21, 111). In contrast, UL55 is seemingly absent from mature HSV-2 (103). The current report clearly shows that all four proteins constitute novel components of the virions. At present, their inclusion as teguments is circumstantial and based on their absence from the well-characterized capsids and the lack of transmembrane domains (data not shown). Biochemical studies to confirm their exact location in the virus will be needed. It will also be most interesting to elucidate the functional relevance of these proteins in virions.
A number of studies point to ICP0 and ICP4 as minor components of the tegument (22, 107, 108). However, a recent study failed to detect ICP0 on virions by immuno-EM analysis (39). Furthermore, it has also been reported that ICP4 may instead be preferentially incorporated in L particles (56, 92), which typically outnumber viable virions in the extracellular medium. Similarly, conflicting reports either support the presence of UL24 protein in herpesvirus virions (11, 35) or failed to detect it (76). Given the lack of noticeable L particles in our viral preparation (Fig. 3), the detection of ICP0 and ICP4 by both proteomics and Western blotting and the absence of UL24 by these techniques (Table 1 and Fig. 6 and 7), we conclude that ICP0 and ICP4 are components of extracellular virions but that UL24 is most likely absent.
Oddly, US11 was not detected in this study (Tables 1 and 2 and Fig. 6 and 7), in sharp contrast to a report by Roller and colleagues, who estimated 600 to 1,000 copies of US11 in virions (83). The significance of this discrepancy is unclear at the moment. Given the overall homology between various HSV-1 strains (90 to 99% identity at the amino acid level; S. Loret, G. Guay, and R. Lippé, unpublished observations), it is unlikely to explain such disagreement. Note that US11, like UL20, migrated at the expected molecular weight in our study (data not shown) and cannot be responsible for the lack of US11 detection by MRM.
Incorporation of host proteins in virions.
The excellent sample purity and accuracy of the method made it possible to evaluate the presence of host protein components. The present study reveals a heteroclite collection of up to 49 distinct potential cellular proteins incorporated into mature virions. Interestingly, a variety of transport, cytoskeletal, enzymatic and other proteins were identified (Table 3). The low number of peptides identified for these proteins suggests that they are minor virion components. While some of these proteins were found on other herpesviruses (7, 9, 18, 21, 38, 40, 63, 96, 111), many are seemingly specific to HSV-1. It will now be important to functionally validate their presence and ultimately address their putative functions for the virus.
Relative abundance of virion components.
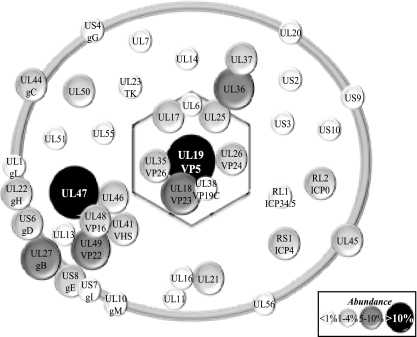
It should be pointed out that quantitative mass spectrometry of complex protein mixtures is still being actively pursued by mass spectrometry specialists (2, 73, 82, 85). At issue is the different behavior of different proteins and peptides during separation, ionization, and solvation, not including relative abundance and potential unpredictable posttranslational modifications. This means some peptides are never detected, while others are under- or overrepresented. This naturally has an impact on the lack of detection of some proteins in our study. To satisfy ourselves of these quantitative limitations, a plot of the relative abundance of the viral proteins detected in our study (measured by NQCPT or the number of unique peptides) against published copy numbers was drawn in an effort to determine the exact copy number or molar ratios of the viral components. At best, this “standard curve” had a correlation coefficient of R2 = 0.42 when normalized for the size of the proteins (data not shown), confirming the semiquantitative nature of our results. For this reason, the contribution of the viral components can only roughly be estimated. Figure 8 shows the results of this evaluation. Although it is not depicted in Fig. 8, all but one host protein from Table 3 fall within the two lowest abundance brackets.
FIG. 8.
Schematic representation of mature extracellular virions. The viral composition of the capsid, tegument, and envelope is indicated. The diameter of each circle indicates the relative abundance of the proteins based on their NQPCT score (see Materials and Methods). Given the semiquantitative nature of this score, they are grouped into low abundance (<1% of total proteins), medium abundance (1 to 4%), high abundance (5 to 10%), and very high abundance (>10%). The potential cellular components identified in Table 3 have been omitted since they are in need of validation. However, all but one of them (profilin-1) falls within the first two lowest abundance brackets.
Concluding remarks.
Mass spectrometry is a powerful method to identify complex protein samples. Recently, the proteomics of several herpesviruses, including HCMV (7, 96), MCMV (40), EBV (38), KSHV (9, 111), RRV (72), MHV68 (11), and alcelaphine herpesvirus 1 (21), were reported and reviewed (55, 97). These studies revealed the important contribution of the teguments to the total mass of the virions, identified novel open reading frames, highlighted the presence of various cellular components in the virions, and examined virulence genes. The present study is the first ever to complete an analysis of HSV-1 virions. It corroborates the importance of the tegument (54% of all peptides found by mass spectrometry), identifies four novel virion components, confirms the presence of two controversial teguments, and hints at the surprising absence of a number of viral glycoproteins. It also raises several interesting questions, for instance, the potential dynamic interaction of the terminase with the capsids, the putative existence of distinct gM complexes in infected cells (alone or with gN), the precise localization of the novel UL7, UL23, UL50, and UL55 virion components, and the role of the thymidine kinase in virions. As the sequential process of capsid tegumentation is currently under intense scrutiny, this study should prove useful by identifying the final outcome of tegumentation in mature virions. Finally, the work presented here hints at 8 virally encoded capsid proteins, 13 glycoproteins (including UL20), 23 teguments, and up to 49 distinct host proteins.
Acknowledgments
We thank Beate Sodeik, Bernard Roizman, Joel Baines, Y. Nishiyama, Angela Pearson, Don Coen, Jim Smiley, Gary Cohen, Roselyn Eisenberg, David Johnson, and Richard Roller for the generous gifts of reagents. We also thank Sylvie Laboissière and her team at McGill University and the staff at Genome Quebec Innovation Centre Proteomics Platform for help with mass spectrometry and analysis of the data. Finally, we are indebted to Joël Lanoix, Sylvie Laboissière, and Marcos Di Falco for the critical reading of the manuscript.
Footnotes
Published ahead of print on 2 July 2008.
REFERENCES
- 1.Adams, R., C. Cunningham, M. D. Davison, C. A. MacLean, and A. J. Davison. 1998. Characterization of the protein encoded by gene UL49A of herpes simplex virus type 1. J. Gen. Virol. 79813-823. [DOI] [PubMed] [Google Scholar]
- 2.America, A. H., and J. H. Cordewener. 2008. Comparative LC-MS: a landscape of peaks and valleys. Proteomics 8731-749. [DOI] [PubMed] [Google Scholar]
- 3.Anderson, L., and C. L. Hunter. 2006. Quantitative mass spectrometric multiple reaction monitoring assays for major plasma proteins. Mol. Cell. Proteomics 5573-588. [DOI] [PubMed] [Google Scholar]
- 4.Baines, J., and C. Duffy. 2006. Nucleocapsid assembly and envelopment of herpes simplex virus, p. 175-204. In R. M. Sandri-Goldin (ed.), Alpha herpesviruses: molecular and cellular biology. Caister Academic Press, Norfolk, United Kingdom.
- 5.Baines, J. D., A. H. Koyama, T. Huang, and B. Roizman. 1994. The UL21 gene products of herpes simplex virus 1 are dispensable for growth in cultured cells. J. Virol. 682929-2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baines, J. D., and B. Roizman. 1992. The UL11 gene of herpes simplex virus 1 encodes a function that facilitates nucleocapsid envelopment and egress from cells. J. Virol. 665168-5174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baldick, C. J., Jr., and T. Shenk. 1996. Proteins associated with purified human cytomegalovirus particles. J. Virol. 706097-6105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beard, P. M., and J. D. Baines. 2004. The DNA cleavage and packaging protein encoded by the UL33 gene of herpes simplex virus 1 associates with capsids. Virology 324475-482. [DOI] [PubMed] [Google Scholar]
- 9.Bechtel, J. T., R. C. Winant, and D. Ganem. 2005. Host and viral proteins in the virion of Kaposi's sarcoma-associated herpesvirus. J. Virol. 794952-4964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blondeau, F., B. Ritter, P. D. Allaire, S. Wasiak, M. Girard, N. K. Hussain, A. Angers, V. Legendre-Guillemin, L. Roy, D. Boismenu, R. E. Kearney, A. W. Bell, J. J. Bergeron, and P. S. McPherson. 2004. Tandem MS analysis of brain clathrin-coated vesicles reveals their critical involvement in synaptic vesicle recycling. Proc. Natl. Acad. Sci. USA 1013833-3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bortz, E., J. P. Whitelegge, Q. Jia, Z. H. Zhou, J. P. Stewart, T. T. Wu, and R. Sun. 2003. Identification of proteins associated with murine gammaherpesvirus 68 virions. J. Virol. 7713425-13432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brown, S. M., A. R. MacLean, J. D. Aitken, and J. Harland. 1994. ICP34.5 influences herpes simplex virus type 1 maturation and egress from infected cells in vitro. J. Gen. Virol. 753679-3686. [DOI] [PubMed] [Google Scholar]
- 13.Cartier, A., E. Broberg, T. Komai, M. Henriksson, and M. G. Masucci. 2003. The herpes simplex virus-1 Us3 protein kinase blocks CD8T cell lysis by preventing the cleavage of Bid by granzyme B. Cell Death Differ. 101320-1328. [DOI] [PubMed] [Google Scholar]
- 14.Crump, C. M., B. Bruun, S. Bell, L. E. Pomeranz, T. Minson, and H. M. Browne. 2004. Alphaherpesvirus glycoprotein M causes the relocalization of plasma membrane proteins. J. Gen. Virol. 853517-3527. [DOI] [PubMed] [Google Scholar]
- 15.Cunningham, C., A. J. Davison, A. Dolan, M. C. Frame, D. J. McGeoch, D. M. Meredith, H. W. Moss, and A. C. Orr. 1992. The UL13 virion protein of herpes simplex virus type 1 is phosphorylated by a novel virus-induced protein kinase. J. Gen. Virol. 73303-311. [DOI] [PubMed] [Google Scholar]
- 16.Cunningham, C., A. J. Davison, A. R. MacLean, N. S. Taus, and J. D. Baines. 2000. Herpes simplex virus type 1 gene UL14: phenotype of a null mutant and identification of the encoded protein. J. Virol. 7433-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Daikoku, T., K. Ikenoya, H. Yamada, F. Goshima, and Y. Nishiyama. 1998. Identification and characterization of the herpes simplex virus type 1 UL51 gene product. J. Gen. Virol. 793027-3031. [DOI] [PubMed] [Google Scholar]
- 18.del Rio, T., T. H. Ch'ng, E. A. Flood, S. P. Gross, and L. W. Enquist. 2005. Heterogeneity of a fluorescent tegument component in single pseudorabies virus virions and enveloped axonal assemblies. J. Virol. 793903-3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Desai, P. J. 2000. A null mutation in the UL36 gene of herpes simplex virus type 1 results in accumulation of unenveloped DNA-filled capsids in the cytoplasm of infected cells. J. Virol. 7411608-11618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dohner, K., A. Wolfstein, U. Prank, C. Echeverri, D. Dujardin, R. Vallee, and B. Sodeik. 2002. Function of dynein and dynactin in herpes simplex virus capsid transport. Mol. Biol. Cell 132795-2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dry, I., D. M. Haig, N. F. Inglis, L. Imrie, J. P. Stewart, and G. C. Russell. 2008. Proteomic analysis of pathogenic and attenuated alcelaphine herpesvirus 1. J. Virol. 825390-5397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elliott, G., W. Hafezi, A. Whiteley, and E. Bernard. 2005. Deletion of the herpes simplex virus VP22-encoding gene (UL49) alters the expression, localization, and virion incorporation of ICP0. J. Virol. 799735-9745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Elliott, G. D., and D. M. Meredith. 1992. The herpes simplex virus type 1 tegument protein VP22 is encoded by gene UL49. J. Gen. Virol. 73723-726. [DOI] [PubMed] [Google Scholar]
- 24.Everett, R. D. 2000. ICP0, a regulator of herpes simplex virus during lytic and latent infection. Bioessays 22761-770. [DOI] [PubMed] [Google Scholar]
- 25.Fenwick, M. L., and R. D. Everett. 1990. Inactivation of the shutoff gene (UL41) of herpes simplex virus types 1 and 2. J. Gen. Virol. 712961-2967. [DOI] [PubMed] [Google Scholar]
- 26.Foster, T. P., J. M. Melancon, J. D. Baines, and K. G. Kousoulas. 2004. The herpes simplex virus type 1 UL20 protein modulates membrane fusion events during cytoplasmic virion morphogenesis and virus-induced cell fusion. J. Virol. 785347-5357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Foster, T. P., G. V. Rybachuk, and K. G. Kousoulas. 2001. Glycoprotein K specified by herpes simplex virus type 1 is expressed on virions as a Golgi complex-dependent glycosylated species and functions in virion entry. J. Virol. 7512431-12438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frame, M. C., D. J. McGeoch, F. J. Rixon, A. C. Orr, and H. S. Marsden. 1986. The 10K virion phosphoprotein encoded by gene US9 from herpes simplex virus type 1. Virology 150321-332. [DOI] [PubMed] [Google Scholar]
- 29.Fuchs, W., H. Granzow, R. Klopfleisch, B. G. Klupp, D. Rosenkranz, and T. C. Mettenleiter. 2005. The UL7 gene of pseudorabies virus encodes a nonessential structural protein which is involved in virion formation and egress. J. Virol. 7911291-11299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fuchs, W., H. Granzow, B. G. Klupp, M. Kopp, and T. C. Mettenleiter. 2002. The UL48 tegument protein of pseudorabies virus is critical for intracytoplasmic assembly of infectious virions. J. Virol. 766729-6742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fuchs, W., H. Granzow, and T. C. Mettenleiter. 2003. A pseudorabies virus recombinant simultaneously lacking the major tegument proteins encoded by the UL46, UL47, UL48, and UL49 genes is viable in cultured cells. J. Virol. 7712891-12900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ghiasi, H., A. B. Nesburn, S. Cai, and S. L. Wechsler. 1998. The US5 open reading frame of herpes simplex virus type 1 does encode a glycoprotein (gJ). Intervirology 4191-97. [DOI] [PubMed] [Google Scholar]
- 33.Granzow, H., B. G. Klupp, and T. C. Mettenleiter. 2005. Entry of pseudorabies virus: an immunogold-labeling study. J. Virol. 793200-3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harland, J., P. Dunn, E. Cameron, J. Conner, and S. M. Brown. 2003. The herpes simplex virus (HSV) protein ICP34.5 is a virion component that forms a DNA-binding complex with proliferating cell nuclear antigen and HSV replication proteins. J. Neurovirol. 9477-488. [DOI] [PubMed] [Google Scholar]
- 35.Hong-Yan, Z., T. Murata, F. Goshima, H. Takakuwa, T. Koshizuka, Y. Yamauchi, and Y. Nishiyama. 2001. Identification and characterization of the UL24 gene product of herpes simplex virus type 2. Virus Genes 22321-327. [DOI] [PubMed] [Google Scholar]
- 36.Hutchinson, L., C. Roop-Beauchamp, and D. C. Johnson. 1995. Herpes simplex virus glycoprotein K is known to influence fusion of infected cells, yet is not on the cell surface. J. Virol. 694556-4563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jiang, Y. M., H. Yamada, F. Goshima, T. Daikoku, S. Oshima, K. Wada, and Y. Nishiyama. 1998. Characterization of the herpes simplex virus type 2 (HSV-2) US2 gene product and a US2-deficient HSV-2 mutant. J. Gen. Virol. 792777-2784. [DOI] [PubMed] [Google Scholar]
- 38.Johannsen, E., M. Luftig, M. R. Chase, S. Weicksel, E. Cahir-McFarland, D. Illanes, D. Sarracino, and E. Kieff. 2004. Proteins of purified Epstein-Barr virus. Proc. Natl. Acad. Sci. USA 10116286-16291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kalamvoki, M., J. Qu, and B. Roizman. 2008. Translocation and colocalization of ICP4 and ICP0 in cells infected with herpes simplex virus 1 mutants lacking glycoprotein E, glycoprotein I, or the virion host shutoff product of the UL41 gene. J. Virol. 821701-1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kattenhorn, L. M., R. Mills, M. Wagner, A. Lomsadze, V. Makeev, M. Borodovsky, H. L. Ploegh, and B. M. Kessler. 2004. Identification of proteins associated with murine cytomegalovirus virions. J. Virol. 7811187-11197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kehm, R., H. R. Gelderblom, and G. Darai. 1998. Identification of the UL56 protein of herpes simplex virus type 1 within the virion by immuno electron microscopy. Virus Genes 1749-53. [DOI] [PubMed] [Google Scholar]
- 42.Kehm, R., E. Lorentzen, A. Rosen-Wolff, and G. Darai. 1994. In vitro expression of UL56 gene of herpes simplex virus type 1; detection of UL56 gene product in infected cells and in virions. Virus Res. 3355-66. [DOI] [PubMed] [Google Scholar]
- 43.Klupp, B. G., J. Altenschmidt, H. Granzow, W. Fuchs, and T. C. Mettenleiter. 2005. Identification and characterization of the pseudorabies virus UL43 protein. Virology 334224-233. [DOI] [PubMed] [Google Scholar]
- 44.Klupp, B. G., J. Baumeister, P. Dietz, H. Granzow, and T. C. Mettenleiter. 1998. Pseudorabies virus glycoprotein gK is a virion structural component involved in virus release but is not required for entry. J. Virol. 721949-1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Klupp, B. G., H. Granzow, R. Klopfleisch, W. Fuchs, M. Kopp, M. Lenk, and T. C. Mettenleiter. 2005. Functional analysis of the pseudorabies virus UL51 protein. J. Virol. 793831-3840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Klupp, B. G., H. Granzow, and T. C. Mettenleiter. 2001. Effect of the pseudorabies virus US3 protein on nuclear membrane localization of the UL34 protein and virus egress from the nucleus. J. Gen. Virol. 822363-2371. [DOI] [PubMed] [Google Scholar]
- 47.Kopp, M., H. Granzow, W. Fuchs, B. G. Klupp, E. Mundt, A. Karger, and T. C. Mettenleiter. 2003. The pseudorabies virus UL11 protein is a virion component involved in secondary envelopment in the cytoplasm. J. Virol. 775339-5351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kuhn, E., J. Wu, J. Karl, H. Liao, W. Zolg, and B. Guild. 2004. Quantification of C-reactive protein in the serum of patients with rheumatoid arthritis using multiple reaction monitoring mass spectrometry and 13C-labeled peptide standards. Proteomics 41175-1186. [DOI] [PubMed] [Google Scholar]
- 49.Leach, N., S. L. Bjerke, D. K. Christensen, J. M. Bouchard, F. Mou, R. Park, J. Baines, T. Haraguchi, and R. J. Roller. 2007. Emerin is hyperphosphorylated and redistributed in herpes simplex virus type 1-infected cells in a manner dependent on both UL34 and US3. J. Virol. 8110792-10803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liang, L., M. Tanaka, Y. Kawaguchi, and J. D. Baines. 2004. Cell lines that support replication of a novel herpes simplex virus 1 UL31 deletion mutant can properly target UL34 protein to the nuclear rim in the absence of UL31. Virology 32968-76. [DOI] [PubMed] [Google Scholar]
- 51.Liu, H., R. G. Sadygov, and J. R. Yates III. 2004. A model for random sampling and estimation of relative protein abundance in shotgun proteomics. Anal. Chem. 764193-4201. [DOI] [PubMed] [Google Scholar]
- 52.Luxton, G. W., S. Haverlock, K. E. Coller, S. E. Antinone, A. Pincetic, and G. A. Smith. 2005. Targeting of herpesvirus capsid transport in axons is coupled to association with specific sets of tegument proteins. Proc. Natl. Acad. Sci. USA 1025832-5837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Luxton, G. W., J. I. Lee, S. Haverlock-Moyns, J. M. Schober, and G. A. Smith. 2006. The pseudorabies virus VP1/2 tegument protein is required for intracellular capsid transport. J. Virol. 80201-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.MacLean, C. A., B. Clark, and D. J. McGeoch. 1989. Gene UL11 of herpes simplex virus type 1 encodes a virion protein which is myristylated. J. Gen. Virol. 703147-3157. [DOI] [PubMed] [Google Scholar]
- 55.Maxwell, K. L., and L. Frappier. 2007. Viral proteomics. Microbiol. Mol. Biol. Rev. 71398-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McLauchlan, J., C. Addison, M. C. Craigie, and F. J. Rixon. 1992. Noninfectious L-particles supply functions which can facilitate infection by HSV-1. Virology 190682-688. [DOI] [PubMed] [Google Scholar]
- 57.McLauchlan, J., K. Liefkens, and N. D. Stow. 1994. The herpes simplex virus type 1 UL37 gene product is a component of virus particles. J. Gen. Virol. 752047-2052. [DOI] [PubMed] [Google Scholar]
- 58.McLauchlan, J., and F. J. Rixon. 1992. Characterization of enveloped tegument structures (L particles) produced by alphaherpesviruses: integrity of the tegument does not depend on the presence of capsid or envelope. J. Gen. Virol. 73269-276. [DOI] [PubMed] [Google Scholar]
- 59.McLean, G., F. Rixon, N. Langeland, L. Haarr, and H. Marsden. 1990. Identification and characterization of the virion protein products of herpes simplex virus type 1 gene UL47. J. Gen. Virol. 712953-2960. [DOI] [PubMed] [Google Scholar]
- 60.McNabb, D. S., and R. J. Courtney. 1992. Analysis of the UL36 open reading frame encoding the large tegument protein (ICP1/2) of herpes simplex virus type 1. J. Virol. 667581-7584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Meckes, D. G., Jr., and J. W. Wills. 2007. Dynamic interactions of the UL16 tegument protein with the capsid of herpes simplex virus. J. Virol. 8113028-13036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mettenleiter, T. C. 2004. Budding events in herpesvirus morphogenesis. Virus Res. 106167-180. [DOI] [PubMed] [Google Scholar]
- 63.Michael, K., B. G. Klupp, T. C. Mettenleiter, and A. Karger. 2006. Composition of pseudorabies virus particles lacking tegument protein US3, UL47, or UL49 or envelope glycoprotein E. J. Virol. 801332-1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Morris, J. B., H. Hofemeister, and P. O'Hare. 2007. Herpes simplex virus infection induces phosphorylation and delocalization of emerin, a key inner nuclear membrane protein. J. Virol. 814429-4437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mossman, K. L., R. Sherburne, C. Lavery, J. Duncan, and J. R. Smiley. 2000. Evidence that herpes simplex virus VP16 is required for viral egress downstream of the initial envelopment event. J. Virol. 746287-6299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mou, F., T. Forest, and J. D. Baines. 2007. US3 of herpes simplex virus type 1 encodes a promiscuous protein kinase that phosphorylates and alters localization of lamin A/C in infected cells. J. Virol. 816459-6470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Naldinho-Souto, R., H. Browne, and T. Minson. 2006. Herpes simplex virus tegument protein VP16 is a component of primary enveloped virions. J. Virol. 802582-2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nalwanga, D., S. Rempel, B. Roizman, and J. D. Baines. 1996. The UL 16 gene product of herpes simplex virus 1 is a virion protein that colocalizes with intranuclear capsid proteins. Virology 226236-242. [DOI] [PubMed] [Google Scholar]
- 69.Newcomb, W. W., R. M. Juhas, D. R. Thomsen, F. L. Homa, A. D. Burch, S. K. Weller, and J. C. Brown. 2001. The UL6 gene product forms the portal for entry of DNA into the herpes simplex virus capsid. J. Virol. 7510923-10932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nozawa, N., T. Daikoku, Y. Yamauchi, H. Takakuwa, F. Goshima, T. Yoshikawa, and Y. Nishiyama. 2002. Identification and characterization of the UL7 gene product of herpes simplex virus type 2. Virus Genes 24257-266. [DOI] [PubMed] [Google Scholar]
- 71.Nozawa, N., Y. Kawaguchi, M. Tanaka, A. Kato, H. Kimura, and Y. Nishiyama. 2005. Herpes simplex virus type 1 UL51 protein is involved in maturation and egress of virus particles. J. Virol. 796947-6956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.O'Connor, C. M., and D. H. Kedes. 2006. Mass spectrometric analyses of purified rhesus monkey rhadinovirus reveal 33 virion-associated proteins. J. Virol. 801574-1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ong, S. E., and M. Mann. 2005. Mass spectrometry-based proteomics turns quantitative. Nat. Chem. Biol. 1252-262. [DOI] [PubMed] [Google Scholar]
- 74.Overton, H. A., D. J. McMillan, L. S. Klavinskis, L. Hope, A. J. Ritchie, and P. Wong-kai-in. 1992. Herpes simplex virus type 1 gene UL13 encodes a phosphoprotein that is a component of the virion. Virology 190184-192. [DOI] [PubMed] [Google Scholar]
- 75.Park, R., and J. D. Baines. 2006. Herpes simplex virus type 1 infection induces activation and recruitment of protein kinase C to the nuclear membrane and increased phosphorylation of lamin B. J. Virol. 80494-504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pearson, A., and D. M. Coen. 2002. Identification, localization, and regulation of expression of the UL24 protein of herpes simplex virus type 1. J. Virol. 7610821-10828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Poon, A. P., and B. Roizman. 2007. Mapping of key functions of the herpes simplex virus 1 US3 protein kinase: the US3 protein can form functional heteromultimeric structures derived from overlapping truncated polypeptides. J. Virol. 811980-1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Purves, F. C., D. Spector, and B. Roizman. 1991. The herpes simplex virus 1 protein kinase encoded by the US3 gene mediates posttranslational modification of the phosphoprotein encoded by the UL34 gene. J. Virol. 655757-5764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rémillard-Labrosse, G., G. Guay, and R. Lippé. 2006. Reconstitution of herpes simplex virus type 1 nuclear capsid egress in vitro. J. Virol. 809741-9753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Reynolds, A. E., B. J. Ryckman, J. D. Baines, Y. Zhou, L. Liang, and R. J. Roller. 2001. UL31 and UL34 proteins of herpes simplex virus type 1 form a complex that accumulates at the nuclear rim and is required for envelopment of nucleocapsids. J. Virol. 758803-8817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Reynolds, A. E., E. G. Wills, R. J. Roller, B. J. Ryckman, and J. D. Baines. 2002. Ultrastructural localization of the herpes simplex virus type 1 UL31, UL34, and US3 proteins suggests specific roles in primary envelopment and egress of nucleocapsids. J. Virol. 768939-8952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Righetti, P. G., and E. Boschetti. 14 May 2008, posting date. The proteominer and the Fortyniners: searching for gold nuggets in the proteomic arena. Mass Spectrom. Rev. doi: 10.1002/mas.20178. [DOI] [PubMed]
- 83.Roller, R. J., and B. Roizman. 1992. The herpes simplex virus 1 RNA binding protein US11 is a virion component and associates with ribosomal 60S subunits. J. Virol. 663624-3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Roller, R. J., Y. Zhou, R. Schnetzer, J. Ferguson, and D. DeSalvo. 2000. Herpes simplex virus type 1 UL34 gene product is required for viral envelopment. J. Virol. 74117-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Schmidt, A., N. Gehlenborg, B. Bodenmiller, L. N. Mueller, D. Campbell, M. Mueller, R. Aebersold, and B. Domon. May 29, 2008, posting date. An integrated, directed mass spectrometric approach for in-depth characterization of complex peptide mixtures. Mol. Cell. Proteomics. [Epub ahead of print.] [DOI] [PMC free article] [PubMed]
- 86.Schmitz, J. B., A. G. Albright, P. R. Kinchington, and F. J. Jenkins. 1995. The UL37 protein of herpes simplex virus type 1 is associated with the tegument of purified virions. Virology 2061055-1065. [DOI] [PubMed] [Google Scholar]
- 87.Sedlackova, L., and S. A. Rice. 2008. Herpes simplex virus type 1 immediate-early protein ICP27 is required for efficient incorporation of ICP0 and ICP4 into virions. J. Virol. 82268-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Smibert, C. A., D. C. Johnson, and J. R. Smiley. 1992. Identification and characterization of the virion-induced host shutoff product of herpes simplex virus gene UL41. J. Gen. Virol. 73467-470. [DOI] [PubMed] [Google Scholar]
- 89.Smith-Donald, B. A., and B. Roizman. 2008. The interaction of herpes simplex virus 1 regulatory protein ICP22 with the cdc25C phosphatase is enabled in vitro by viral protein kinases US3 and UL13. J. Virol. 824533-4543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sodeik, B., M. W. Ebersold, and A. Helenius. 1997. Microtubule-mediated transport of incoming herpes simplex virus 1 capsids to the nucleus. J. Cell Biol. 1361007-1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Spear, P. G., and B. Roizman. 1972. Proteins specified by herpes simplex virus. V. Purification and structural proteins of the herpesvirion. J. Virol. 9143-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Szilagyi, J. F., and C. Cunningham. 1991. Identification and characterization of a novel non-infectious herpes simplex virus-related particle. J. Gen. Virol. 72661-668. [DOI] [PubMed] [Google Scholar]
- 93.Taddeo, B., and B. Roizman. 2006. The virion host shutoff protein (UL41) of herpes simplex virus 1 is an endoribonuclease with a substrate specificity similar to that of RNase A. J. Virol. 809341-9345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Taddeo, B., W. Zhang, and B. Roizman. 2006. The U(L)41 protein of herpes simplex virus 1 degrades RNA by endonucleolytic cleavage in absence of other cellular or viral proteins. Proc. Natl. Acad. Sci. USA 1032827-2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Turcotte, S., J. Letellier, and R. Lippé. 2005. Herpes simplex virus type 1 capsids transit by the trans-Golgi network, where viral glycoproteins accumulate independently of capsid egress. J. Virol. 798847-8860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Varnum, S. M., D. N. Streblow, M. E. Monroe, P. Smith, K. J. Auberry, L. Pasa-Tolic, D. Wang, D. G. Camp, 2nd, K. Rodland, S. Wiley, W. Britt, T. Shenk, R. D. Smith, and J. A. Nelson. 2004. Identification of proteins in human cytomegalovirus (HCMV) particles: the HCMV proteome. J. Virol. 7810960-10966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Viswanathan, K., and K. Fruh. 2007. Viral proteomics: global evaluation of viruses and their interaction with the host. Expert Rev. Proteomics 4815-829. [DOI] [PubMed] [Google Scholar]
- 98.Wagenaar, F., J. M. Pol, B. Peeters, A. L. Gielkens, N. de Wind, and T. G. Kimman. 1995. The US3-encoded protein kinase from pseudorabies virus affects egress of virions from the nucleus. J. Gen. Virol. 761851-1859. [DOI] [PubMed] [Google Scholar]
- 99.Ward, P. L., G. Campadelli-Fiume, E. Avitabile, and B. Roizman. 1994. Localization and putative function of the UL20 membrane protein in cells infected with herpes simplex virus 1. J. Virol. 687406-7417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wills, E., L. Scholtes, and J. D. Baines. 2006. Herpes simplex virus 1 DNA packaging proteins encoded by UL6, UL15, UL17, UL28, and UL33 are located on the external surface of the viral capsid. J. Virol. 8010894-10899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wolfstein, A., C. H. Nagel, K. Radtke, K. Dohner, V. J. Allan, and B. Sodeik. 2006. The inner tegument promotes herpes simplex virus capsid motility along microtubules in vitro. Traffic 7227-237. [DOI] [PubMed] [Google Scholar]
- 102.Yamada, H., T. Daikoku, Y. Yamashita, Y. M. Jiang, T. Tsurumi, and Y. Nishiyama. 1997. The product of the US10 gene of herpes simplex virus type 1 is a capsid/tegument-associated phosphoprotein which copurifies with the nuclear matrix. J. Gen. Virol. 782923-2931. [DOI] [PubMed] [Google Scholar]
- 103.Yamada, H., Y. M. Jiang, S. Oshima, T. Daikoku, Y. Yamashita, T. Tsurumi, and Y. Nishiyama. 1998. Characterization of the UL55 gene product of herpes simplex virus type 2. J. Gen. Virol. 791989-1995. [DOI] [PubMed] [Google Scholar]
- 104.Yang, K., F. Homa, and J. D. Baines. 2007. Putative terminase subunits of herpes simplex virus 1 form a complex in the cytoplasm and interact with portal protein in the nucleus. J. Virol. 816419-6433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yang, K., A. P. Poon, B. Roizman, and J. D. Baines. 2008. Temperature-sensitive mutations in the putative herpes simplex virus type 1 terminase subunits pUL15 and pUL33 preclude viral DNA cleavage/packaging and interaction with pUL28 at the nonpermissive temperature. J. Virol. 82487-494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yang, T. Y., and R. J. Courtney. 1995. Influence of the host cell on the association of ICP4 and ICP0 with herpes simplex virus type 1. Virology 211209-217. [DOI] [PubMed] [Google Scholar]
- 107.Yao, F., and R. J. Courtney. 1992. Association of ICP0 but not ICP27 with purified virions of herpes simplex virus type 1. J. Virol. 662709-2716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yao, F., and R. J. Courtney. 1989. A major transcriptional regulatory protein (ICP4) of herpes simplex virus type 1 is associated with purified virions. J. Virol. 633338-3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yu, D., and S. K. Weller. 1998. Herpes simplex virus type 1 cleavage and packaging proteins UL15 and UL28 are associated with B but not C capsids during packaging. J. Virol. 727428-7439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhang, Y., and J. L. McKnight. 1993. Herpes simplex virus type 1 UL46 and UL47 deletion mutants lack VP11 and VP12 or VP13 and VP14, respectively, and exhibit altered viral thymidine kinase expression. J. Virol. 671482-1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhu, F. X., J. M. Chong, L. Wu, and Y. Yuan. 2005. Virion proteins of Kaposi's sarcoma-associated herpesvirus. J. Virol. 79800-811. [DOI] [PMC free article] [PubMed] [Google Scholar]