Abstract
The lentiviral Nef protein has been studied extensively for its ability to induce the downregulation of several immunoreceptors on the surfaces of infected cells. However, Nef expression is unique in inducing highly effective upregulation of the major histocompatibility complex class II-associated chaperone invariant (Ii) chain complexes in different cell types. Under normal conditions, endocytosis of the Ii chain and other molecules, like the transferrin receptor and CD4, is rapid and AP-2 dependent. Human immunodeficiency virus type 1 (HIV-1) Nef expression strongly reduces the internalization of the Ii chain, enhances that of CD4, and does not modify transferrin uptake. The mutation of AP-2 binding motifs LL164 and DD174 in Nef leads to the inhibition of Ii chain upregulation. In AP-2-depleted cells, surface levels of the Ii chain are high and remain unmodified by Nef expression, further indicating that Nef regulates Ii chain internalization via the AP-2 pathway. Immunoprecipitation experiments revealed that the Ii chain can interact with Nef in a dileucine-dependent manner. Importantly, we have shown that Nef-induced CD4 downregulation and Ii chain upregulation are genetically distinguishable. We have identified natural nef alleles that have lost one of the two functions but not the other one. Moreover, we have characterized Nef mutant forms possessing a similar phenotype in the context of HIV-1 infection. Therefore, the Nef-induced accumulation of Ii chain complexes at the cell surface probably results from a complex mechanism leading to the impairment of AP-2-mediated endocytosis rather than from direct competition between Nef and the Ii chain for binding AP-2.
Nef is a highly conserved primate lentiviral accessory protein with diverse functions but with no enzymatic activity. Nef appears to be essential for human immunodeficiency virus (HIV) pathogenicity and viral replication in primary T cells (16) and helps to maintain the full infectivity of viral particles (41, 53). The capacity of Nef to modulate the surface expression of many immunoreceptors is likely to contribute to efficient viral spread, immune system escape, and disease progression. These receptors include major histocompatibility complex class I (MHC-I; in fact, HLA-A and HLA-B but not HLA-C) (8, 26), MHC-II and the class II-associated invariant (Ii) chain (50), CD4 (18), and CD28 (51), all of which play a role at the contact site between antigen-presenting cells and T cells. Nef expression in T lymphocytes prevents the correct formation of the immunological synapse at the interface with antigen-presenting cells (52). Thus, Nef-expressing cells can escape from the cytotoxic activities of antiviral CD8 T cells and NK and NKT cells.
Nef-expressing cells may also compromise the antiviral T helper response. Indeed, Nef impairs MHC-II presentation by affecting MHC-II intracellular trafficking (50). Nef possesses a dual effect leading to (i) reduced surface levels of mature—i.e., peptide-loaded—MHC-II molecules and (ii) increased levels of immature MHC-II molecules, which are functionally incompetent due to their association with the chaperone, the Ii chain. These surface-displayed Ii chains are associated with MHC-II α and β chains (50). The upregulation of surface Ii chain complexes requires smaller amounts of Nef than the downregulation of mature MHC-II molecules. The two functions are genetically separable (50) and are well conserved among nef alleles from primary HIV type 1 (HIV-1), HIV-2, and simian immunodeficiency virus (46).
Accumulating data suggest that Nef-mediated surface upregulation of the Ii chain plays an important role in viral replication and persistence in vivo. First, the level of upregulation is very high, an increase in expression of up to 50-fold, in contrast to that of DC-SIGN, another molecule upregulated by Nef but only by 2- to 3-fold (47). Second, the Ii chain upregulation has been documented to occur not only in HeLa-CIITA and MelJuSo cell lines (50) but also in the monocytic cell line THP-1, as well as in primary CD4+ T cells (24) and monocyte-derived macrophages, leading to similarly high levels of Ii chain expression (45). Third, in a previous study, the function of Ii chain surface upregulation was conserved among many nef alleles derived from progressing HIV-1-infected individuals but among nef alleles from only two of four long-term nonprogressors (46). Fourth, it has been implied previously that Nef-mediated Ii chain upregulation may impact the clinical course of pediatric AIDS progression (45). In fact, HIV-specific helper-T-cell responses are impaired in progressing HIV-1-infected individuals and are strong in long-term nonprogressors (38, 42). Thus, the capacity of Nef to affect MHC-II antigen presentation may impact the virus-specific T helper response and, thus, the control of the infection. On the other hand, strong Ii chain upregulation may contribute to the low level of generalized immune activation that is observed in nonpathogenic lentiviral infections and HIV-2 infections (45).
MHC-II molecules are synthesized in the endoplasmic reticulum, where their α and β subunits associate with the Ii chain to form nonameric (αβIi)3 complexes (2, 40). These complexes have to traffic through the Golgi apparatus and the endocytic pathway to undergo complete maturation. There, the Ii chain is degraded in a sequential manner, and the Ii chain and its fragments drive associated complexes to specialized endocytic compartments, where peptides are loaded onto MHC-II molecules, allowing the mature MHC-II molecules to reach the cell surface (48). The results from recent studies performed with HeLa-CIITA cells indicate that most newly synthesized αβIi complexes are initially transported to the cell surface, where they are rapidly internalized via a clathrin adaptor protein 2 (AP-2)-dependent pathway to reach the endosomes and lysosomes (14, 33). APs are heterotetrameric complexes involved in the vesicular transport of proteins. They bind to motifs present in the cytoplasmic tails of cargo proteins and to the clathrin heavy chain (15, 39). AP complexes promote the sorting of cargo proteins into nascent vesicles at specific intracellular locations. For example, AP-2, which has been extensively characterized, promotes the endocytosis of cargo proteins such as the transferrin receptor (TfR) and the Ii chain.
AP-2 also appears to play an important role for Nef. Mutagenesis studies of Nef combined with two-hybrid or pull-down experiments initially identified subunits of AP-1, AP-2, and AP-3 as binding partners of Nef to induce the downregulation of CD4 and MHC-I (3, 10, 20, 26, 36). Recent studies established that Nef can bind the AP-2 α-σ2 hemicomplexes through its dileucine and diacidic motifs, both of which are required for Nef-induced downregulation of CD4 (6, 13, 28). According to the currently accepted model, Nef promotes accelerated endocytosis of CD4 by serving as an AP cooperatively binding CD4 and AP-2. In addition, Nef expression inhibits CD4 recycling to the plasma membrane (37).
How Nef induces the strong upregulation of surface Ii chain complexes remains obscure. Pulse-chase analyses established that the rates of neosynthesis of the MHC-II α, β, and Ii chains are unchanged in the presence of Nef but that Ii chain degradation is impaired (49). Subcellular fractionation experiments performed with Nef-expressing cells revealed that the transport of immature αβIi complexes to lysosomes is delayed but that other lysosomal markers are correctly directed to their final destinations (49). In fact, Nef induces the accumulation of numerous multivesicular bodies containing abnormally high Ii chain levels (9, 49). Thus, Nef induces, through a posttranslational mechanism, the accumulation of αβIi complexes not only at the cell surface but also inside the cell.
In the present study, we investigated the mechanism involved in Nef-induced upregulation of the Ii chain. We showed that this effect of Nef requires the integrity of its dileucine and diacidic motifs, known to be essential for Nef association with AP-2 (28). Nef expression drastically reduced the rate of endocytosis of the Ii chain without affecting the kinetics of transferrin (Tf) uptake, while preventing Tf and anti-Ii chain monoclonal antibody (MAb) from being internalized in the same early compartments. Importantly, Nef-induced CD4 downregulation and Ii chain upregulation could be genetically distinguished, although both required the integrity of the AP-2 pathway. Rather than just competing for AP-2 binding, as we initially proposed (50), Nef appears to upregulate the Ii chain by a more complex mechanism that may involve Ii chain-Nef interaction, thus affecting the AP-2-dependent internalization of the Ii chain.
MATERIALS AND METHODS
Plasmids.
Bicistronic cytomegalovirus-based pCG expression vectors containing an internal ribosome entry site (IRES) and coexpressing the nef allele of HIV-1 NL4-3 or NA7 and green fluorescent protein (GFP) were called Nef NL4-3 or Nef NA7, respectively (21). The negative control was called Nef-STOP and was the same as plasmid Nef NL4-3 but with a premature stop codon at the beginning of the nef gene. Mutants of Nef NL4-3 or Nef NA7 carrying an alanine mutation in the dileucine motif (positions 164 and 165) or in the diacidic motif (positions 174 and 175) were also used (29, 46). The plasmid pSP72 (Promega) was used as a DNA carrier in transfection experiments. The plasmid pSUPER AP-2-μ2 (targeting μ2 [GTGGATGCCTTTCGGGTCA]) has been described previously (14). The vector pSUPER CTL has no target sequence and was used as a control (4). The pDsRed2-C1 plasmid was from Clontech. Two CD8 chimeras were constructed, and the corresponding coding sequences were cloned into pIRESneo2 (Clontech): CD8-LL contained the luminal and transmembrane domains of CD8 fused to the cytoplasmic tail KRLKRRRIPAEAAALLAV, whereas CD8-AA contained the tail KRLKRRRIPAAAAAAAAV instead. The A83L and LIH100IYY changes in the sequence encoded by the nef gene of the proviral backbone containing pBR-NL4-3 and an IRES and expressing enhanced GFP (eGFP; the pBR-NL4-3 IRES-eGFP backbone) were introduced via splice overlap extension PCR, and the mutant sequences were cloned by using unique HpaI and MluI restriction sites flanking the nef open reading frame essentially as described previously (44, 46). Sequence analysis confirmed that the recombinant proviral constructs contained the correct nef genes and verified the absence of undesired changes. The two nef mutant forms, designated A83L and LIH100IYY, were also subcloned between the XbaI and MluI sites of the pCG-IRES-GFP plasmid (46).
Cell culture, transfections, and infections.
We used HeLa, HeLa-CIITA, HeLa-P4, Jurkat, 293T, and THP-1 cells. HeLa-CIITA cells are HeLa cells expressing the class II transactivator (CIITA) in a stable manner (50), whereas HeLa-P4 cells stably express CD4. HeLa-CIITA cells were transfected with plasmids by electroporation using a Bio-Rad gene pulser II as described previously (49). Briefly, we transfected 8 × 106 cells at 230 V and 975 μF with up to 15 μg of the various Nef vectors mixed with 15 μg of pSP72, giving a total of 30 μg of DNA per electroporation procedure. Usually, cells were recovered 24 h later for further analysis. In flow cytometry experiments described below (see Fig. 3 and the corresponding legend), cells were first transfected with 27.5 μg of pSUPER CTL or pSUPER AP-2-μ2 mixed with 2.5 μg of pDsRed2-C1. Three days later, cells were subjected to a second round of transfection with Nef plasmids by the calcium phosphate precipitation technique. Cells were collected 24 h later for further analysis. Jurkat cells were maintained and transfected as described previously (46). The transfection of HeLa cells with small interfering RNA (siRNA) against pSUPER AP-2-μ2 was performed as described previously (35). The isolation and culture of human peripheral blood mononuclear cells (PBMC), as well as 293T cells and cells of the monocytic cell line THP-1, have already been described (44, 45). Vesicular stomatitis virus G protein-pseudotyped HIV NL4-3 IRES-eGFP viruses were generated by CaCl2 transfection of 293T cells (44, 45). Supernatants of transfected 293T cells were harvested and evaluated using a p24 enzyme-linked immunosorbent assay kit provided by the AIDS and Cancer Virus Program of the NCI, Fredrick, MD. Cells were infected using 50 ng of p24 and analyzed by flow cytometry 3 days later as described previously (45, 46).
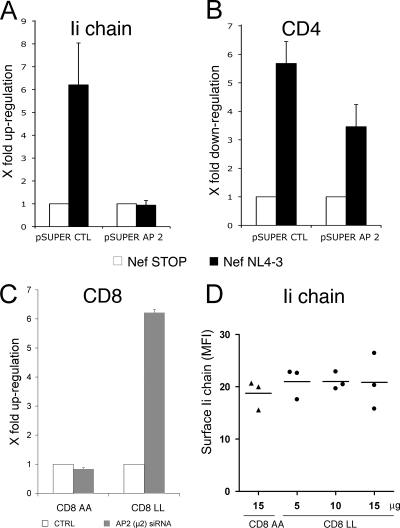
FIG. 3.
Nef acts on the AP-2-dependent pathway to induce Ii chain upregulation. (A and B) HeLa-CIITA or HeLa-P4 cells were cotransfected with pSUPER CTL or pSUPER AP-2-μ2 and limiting amounts of pDsRed2-C1. Two days later, cells were transfected with the bicistronic plasmid Nef NL4-3 or Nef-STOP. After 24 h, cells were collected, stained for surface Ii chain or CD4 by using specific MAbs and Alexa 647-labeled secondary antibodies, and analyzed by flow cytometry. Doubly positive cells (DsRed+ GFP+) were gated and analyzed for FL4 staining. Within each group, corresponding to pSUPER CTL or pSUPER AP-2-μ2, MFI values obtained in FL4 with Nef-expressing cells were normalized with MFI from the respective control cells (Nef-STOP-transfected cells) and expressed as the degrees of Ii chain upregulation (A) or CD4 downregulation (B) (n-fold). Data from three independent experiments were used to calculate means and their standard errors. (C) HeLa cells stably expressing CD8-LL or CD8-AA chimeras were transfected with siRNA against pSUPER AP-2-μ2. After 72 h, the cells were harvested, labeled for cell surface CD8 by using a MAb and anti-mouse Alexa 647 antibody, and analyzed by flow cytometry. The mean FL4 fluorescence of siRNA-treated cells was normalized with the fluorescence of the respective mock-treated controls (CTRL) and is expressed as the degree of CD8 upregulation (n-fold). (D) The expression of a CD8 chimera containing a dileucine signal of endocytosis does not induce cell surface accumulation of the Ii chain. Indicated doses of plasmids encoding CD8-LL or CD8-AA were introduced into HeLa-CIITA cells by electroporation. Twenty-four hours later, cells were collected, stained for surface Ii chain, and analyzed by flow cytometry. MFI values and their means obtained in three independent experiments are shown.
Antibodies and reagents.
The following MAbs were used (see references in reference 49): anti-TfR (from Santa Cruz Biotech), anti-Nef MATG, anti-Ii chain PIN1 and BU45 or By2 coupled to phycoerythrin (PE) (from Santa Cruz Biotech), anti-α-tubulin (from Oncogene), and anti-CD55 (clone BRIC 230; from the International Blood Group Reference Laboratory). From BD Biosciences, we used the antibodies anti-2 (AP-50) subunit, anti-clathrin heavy chain (clone 23), PE-conjugated anti-CD4 MAb (clone SK3), and allophycocyanin-conjugated anti-CD8 (clone RPA-T8). We also used the anti-CD4 MAb 13B8.2 (12), the MHC-I-specific MAb W6/32, and the MAb L243, which is specific for mature MHC-II molecules. PE-conjugated donkey anti-mouse F(ab′)2 fragments (from Jackson ImmunoResearch Laboratories) or Alexa Fluor 647-conjugated goat or chicken anti-mouse F(ab′)2 fragments (from Molecular Probes) were used as secondary reagents. Human Tf (from Sigma-Aldrich) was coupled to Alexa Fluor 647 by using a protein-labeling kit from Molecular Probes.
Flow cytometry.
For surface staining, cells were detached and stained with various mouse MAbs in fluorescence-activated cell sorter buffer (phosphate-buffered saline supplemented with 3% fetal calf serum and 0.05% azide) on ice. Dead cells were excluded by gating based on forward and side light scattering. Events corresponding to at least 5,000 live cells positive for GFP or for both GFP and DsRed2 per sample were accumulated. Flow cytometry was performed on a FACSCalibur machine, with analysis by CellQuest software (BD Biosciences).
Biochemical analyses.
For Western blotting, cells were lysed in NP-40 lysis buffer (0.5% NP-40, 50 mM Tris-HCl [pH 7.4], 5 mM MgCl2) supplemented with 1 mM phenylmethylsulfonyl fluoride. Cell lysates were diluted in reducing Laemmli buffer and heated at 95°C for 5 min before analysis by sodium dodecyl sulfate-polyacrylamide gel electrophoresis in precast 12% acrylamide gels in MOPS (morpholinepropanesulfonic acid) buffer (Invitrogen). The proteins were then transferred onto a polyvinylidene difluoride membrane (Millipore). The membrane was incubated in blocking solution, followed by primary antibody, and finally by horseradish peroxidase-labeled goat anti-mouse or anti-rabbit immunoglobulin G secondary antibody (Jackson ImmunoResearch Laboratories). Antibody binding was detected by chemiluminescence using the SuperSignal West Pico chemiluminescent substrate (Pierce). Coimmunoprecipitation experiments were performed essentially as described previously (50) by using protein G-Sepharose beads covalently coupled to the Ii chain-specific MAb PIN1.
Endocytosis assays.
Endocytosis assays were performed essentially as described previously (14). Cells were harvested and incubated with Tf-Alexa Fluor 647 or with the anti-Ii chain MAb By2-PE for 30 min at 4°C, washed, and shifted to 37°C for various periods of time in culture medium supplemented with 20 mM HEPES. The medium was removed by washing, and half the samples were washed in 25 mM glycine-HCl-125 mM NaCl (pH 2.8) and rapidly neutralized with 25 mM Tris (pH 10). Samples were then washed and analyzed by flow cytometry. Mean fluorescence intensities (MFI) on GFP-positive cells in FL2 or FL4 were determined. The ratio of the intracellular MFI (acid wash resistant) to the total MFI at each time point was plotted as a function of time. With the anti-Ii chain MAb, total MFI remained stable over time. In contrast, with Tf-Alexa Fluor 647, total MFI decreased over time, reflecting Tf recycling and detachment from its receptor at low pHs.
Immunofluorescence microscopy.
Immunofluorescent stainings were performed as described previously (49), and the results were analyzed using an LSM Axiovert 220 confocal microscope with a 63×, 1.4-numerical-aperture oil immersion lens objective (Carl Zeiss MicroImaging, Inc.).
RESULTS
Nef expression reduces the rate of uptake of an Ii chain-specific MAb.
To investigate the mechanism underlying the Nef-induced cell surface expression of the Ii chain, we first established our experimental model using HeLa-CIITA cells transfected with either the bicistronic plasmid Nef NL4-3 (expressing Nef and GFP) or the negative control plasmid Nef-STOP (expressing GFP only). Levels of various markers on the surfaces of GFP+ cells were measured by flow cytometry analysis 24 h after transfection. As expected, Nef increased the surface expression of the Ii chain in a dose-dependent manner (Fig. 1A). Nef NA7 was generally more potent in inducing Ii chain upregulation than Nef NL4-3. Both corresponding alleles encode a dileucine motif in the flexible loop (positions 164 and 165) that is well conserved among products of HIV-1 nef alleles. The mutation of the dileucine motif coding sequence in both alleles abolished the upregulation of the Ii chain (Fig. 1A) but did not affect the Nef-induced downregulation of surface MHC-I (data not shown), indicating that the mutant alleles were still functional. Surface levels of the TfR in the various transfected cell populations remained roughly unchanged (data not shown).
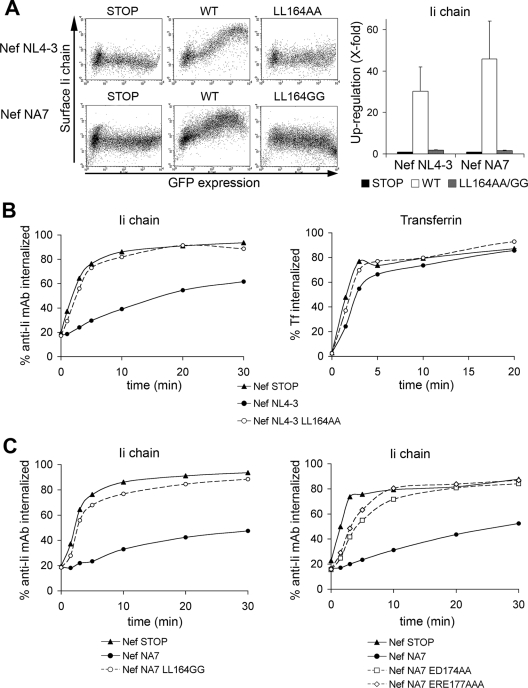
FIG. 1.
Nef expression slows down the internalization of the Ii chain in a dileucine-dependent manner. (A) HeLa-CIITA cells were transfected with the indicated bicistronic plasmid, Nef NL4-3 or Nef NA7 (expressing Nef and GFP), or Nef-STOP (negative control expressing GFP only), collected 24 h later, stained for surface Ii chain, and analyzed by flow cytometry. (Left panels) Representative dot plot analyses are shown. (Right panel) The Nef-induced upregulation of the Ii chain on GFP+ cells was quantified, and the results were normalized using the Nef-STOP control as a reference. Data from four experiments were used to calculate means and their standard errors. WT, wild type. (B and C) Kinetics of internalization of Tf and the Ii chain. HeLa-CIITA cells were transfected with the indicated bicistronic plasmid, Nef NL4-3 or Nef NA7, or Nef-STOP. After 24 h, cells were collected and allowed to bind at 0°C with either the Ii chain-specific PE-labeled MAb or Alexa Fluor 647-labeled Tf. Cells were then shifted to 37°C for the lengths of time indicated. Ligands remaining on the cell surface were released with an acid wash of half of each fraction. Flow cytometry analysis of the cells allowed the measurement of the MFI on GFP+ cells. For each sample, total MFI and acid wash-resistant MFI were used to determine the percentage of internalized ligand. Data presented are representative of at least three independent experiments.
Given that surface αβIi complexes are endocytosed through an AP-2-dependent pathway in HeLa-CIITA cells (14, 33) and that Nef binds to AP-2, we next evaluated the effects of Nef on the internalization of the Ii chain. We monitored the fate of αβIi complexes on the cell surface by means of an uptake assay using a MAb specific for the luminal side of the Ii chain and directly coupled to PE. As a control, we monitored in parallel the uptake of Tf. Quantification was performed with transfected cells gated on the basis of their levels of GFP expression. In control cells (transfected with Nef-STOP), anti-Ii chain MAb uptake was rapid, with 50% of the MAb taken up in 3 min, and reached a plateau of around 90% after 10 min (Fig. 1B). The expression of Nef NL4-3 or Nef NA7 led to a severe reduction of the rate of anti-Ii chain MAb uptake, which reached only 40 to 50% after 20 min (Fig. 1B and C). The mutation of the dileucine motif coding sequence in both alleles restored a normal rate of MAb uptake (Fig. 1B and C). The LL164GG mutation of Nef NA7 and the ED174AA and EDE177AAA mutations of the two acidic motifs also abolished the upregulation of the Ii chain (Fig. 1C). In all cell populations, the uptake of Tf was rapid and remained roughly unmodified in the presence of the various Nef proteins (Fig. 1B and data not shown), as previously reported (17, 23, 30). Finally, as previously shown (37), we observed that Nef expression induces an enhanced rate of CD4 internalization (see Fig. S1D in the supplemental material).
The reduced rate of the anti-Ii chain MAb uptake observed in Nef-expressing cells may reflect a true reduction of the rate of Ii chain endocytosis or an increase of the rate of Ii chain recycling to the plasma membrane. To distinguish between these two possibilities, we performed recycling assays using the anti-Ii chain MAb and Tf-Alexa Fluor 647 simultaneously. We also measured in parallel the recycling of CD4 in HeLa-P4 cells by using a CD4-specific MAb. The recycling of the anti-Ii chain MAb in both control and Nef-expressing cells was hardly detectable (see Fig. S1 in the supplemental material). In contrast, the recycling of Tf and of CD4 in control cells occurred as expected, reaching levels of recycled Tf of 40% and of recycled CD4 of 20% after 10 min, but was significantly reduced in the presence of Nef (see Fig. S1 in the supplemental material), as shown previously (references 30 and 37, respectively).
Taken together, our data indicate that Nef acts differently on three transmembrane proteins, which are all endocytosed through an AP-2-dependent mechanism: Nef slows down the internalization of the Ii chain but not that of the TfR and enhances the internalization of CD4. In addition, Nef reduces the rate of TfR and CD4 recycling.
In Nef-expressing cells, the internalized Tf and Ii chain do not colocalize.
These results prompted us to analyze the fate of the internalized Tf and anti-Ii chain MAb early (5 min) after endocytosis in control cells (transfected with Nef-STOP) and in cells transfected with a Nef construct (Nef NL4-3 or the corresponding dileucine mutant construct Nef LL164AA) and identified by their levels of GFP expression. Confocal analyses of control cells and of cells expressing the dileucine mutant protein showed the codistribution of the two markers, indicating that the Ii chain and Tf were directed to the early endosome (Fig. 2). Nef-expressing cells exhibited very intense Ii chain labeling, mostly at the plasma membrane and on its extensions or ruffles. Notably, most of the Ii chain labeling did not codistribute with Tf labeling (Fig. 2). Closer examination suggested that Ii chain staining tended to concentrate in some areas of the plasma membrane. However, this pattern may reflect antibody-induced concentration of the Ii chain. Classical immunofluorescence analysis of permeabilized Nef-expressing cells generated a too-strong signal for the Ii chain at the plasma membrane for the precise evaluation of Ii chain distribution. To circumvent these problems, semithin cryosections (between 0.5 and 1 μm thick) of HeLa-CIITA cells expressing Nef or not were stained for the Ii chain by using immunofluorescence. As expected, the Ii chain was mainly in intracellular vesicles of control cells, whereas Nef-expressing cells also exhibited very intense labeling for the Ii chain at their plasma membranes (see Fig. S2 in the supplemental material). In contrast, the staining patterns of the glycosylphosphatidylinositol-anchored protein CD55 in the two cell populations appeared to be very similar. Moreover, the Ii chain staining pattern in Nef-expressing cells appeared to be discontinuous, suggesting that the Ii chain was present in patches rather than in a homogeneous distribution.
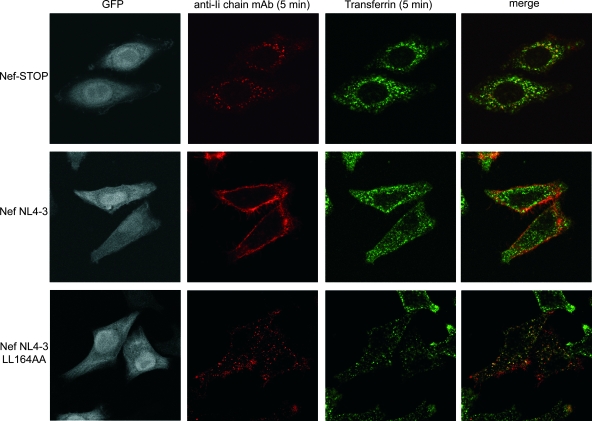
FIG. 2.
Distribution of internalized anti-Ii chain MAb and Tf in the presence of Nef. HeLa-CIITA cells transfected with the bicistronic plasmid Nef NL4-3 (expressing Nef and GFP) or Nef-STOP (negative control expressing GFP only) were allowed to bind at 0°C with either Tf-Alexa Fluor 647 or the Ii chain-specific MAb. Cells were then shifted to 37°C for 5 min and fixed, and the Ii chain-specific MAb was revealed with Cy3-labeled secondary antibodies. Confocal sections of the indicated stainings are presented together with their overlays.
We concluded that Nef expression prevents the rapid access of Ii chain complexes, but not of Tf, to the early endosomes. This effect of Nef is also dependent on its dileucine motif.
Nef-mediated Ii chain upregulation depends on the AP-2 pathway.
Given that surface αβIi complexes are endocytosed through an AP-2-dependent mechanism and that Nef has the capacity to bind AP-2, we next tested the involvement of AP-2 in the Nef-induced upregulation of the Ii chain. The depletion of HeLa-CIITA cells of functional AP-2 was obtained by transfection with limiting amounts of pDsRed2 mixed with a pSUPER plasmid carrying short hairpin RNAs specific for the pSUPER AP-2-μ2 mRNAs (14). Two days later, cells were transfected either with Nef NL4-3 (a bicistronic plasmid expressing Nef and GFP) or with Nef-STOP (expressing GFP only). Ii chain levels on the surfaces of GFP-DsRed2 doubly positive cells were measured by flow cytometry analysis 24 h after transfection. The same experimental design was validated in parallel with HeLa-P4 cells, in which we checked that AP-2 depletion partially impeded Nef-mediated downregulation of CD4 (Fig. 3B), as previously reported (6). The efficiency of the depletions was also verified by analyzing the expression level of pSUPER AP-2-μ2 by Western blotting (see Fig. S3 in the supplemental material). In cells expressing the Nef-STOP plasmid, the knockdown of pSUPER AP-2-μ2 expression induced high surface levels of the Ii chain (a 15-fold increase compared to the levels in pSUPER CTL cells), attesting to the efficiency of depletion (see Fig. S3 in the supplemental material). However, the expression of Nef in AP-2-depleted cells did not modify the high levels of surface Ii chain (Fig. 3A; data have been normalized to the results for the respective control [Nef-STOP-transfected cells]).
The lack of synergistic, and even additive, effects of AP-2 knockdown and Nef expression on Ii chain surface expression suggested that Nef acts on the AP-2 pathway to induce the upregulation of the Ii chain.
The expression of a dileucine-based endocytosis motif does not lead to the upregulation of the Ii chain on the cell surface.
One way to explain our data is to propose that Nef and the Ii chain compete through their respective dileucine motifs for binding to AP-2. A shortage of AP-2 would lead to reduced AP-2-mediated endocytosis of the Ii chain. If this were true, then the expression of any surface protein carrying a classical dileucine motif able to recruit AP-2 should lead to Ii chain upregulation. This possibility was tested using a CD8 chimera, termed CD8-LL, which carried a bona fide endocytosis dileucine motif, or its mutated version, termed CD8-AA. The endocytosis of CD8-LL was shown to be dependent on the presence of AP-2, whereas CD8-AA remained on the surfaces of HeLa cells (Fig. 3C). HeLa-CIITA cells were also transfected with both chimeras and checked for CD8 as well as Ii chain surface expression by flow cytometry. In all cases, and even with increasing amounts of the CD8-LL plasmid, Ii chain levels remained low, as those in the control cells expressing CD8-AA (Fig. 3D).
Thus, the CD8-LL chimera cannot increase Ii chain surface expression, suggesting that Nef-induced upregulation of the Ii chain does not result only from competition for AP-2 binding. However, we cannot rule out that the CD8-LL chimera lacks high enough affinity to compete efficiently with the Ii chain for binding AP-2.
Nef can interact with the Ii chain in a dileucine-dependent manner.
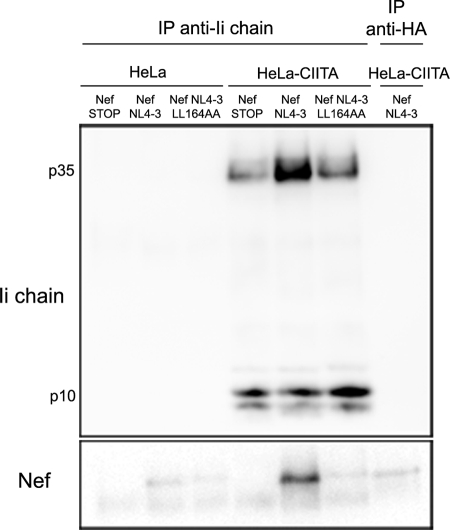
One of the hypotheses to explain the specificity of Nef-mediated Ii chain upregulation is direct binding to the Ii chain. This possibility was tested by performing coimmunoprecipitation experiments using anti-Ii chain MAb on lysates from cells transfected with Nef NL4-3, its dileucine mutant form, or the Nef-STOP control plasmid. Transfections were carried out either with regular HeLa cells as a control, since these cells do not express the Ii chain and MHC-II, or with HeLa-CIITA cells. Immunoprecipitates were analyzed by Western blotting for the presence of the Ii chain and Nef. As expected, neither the Ii chain nor Nef was detectable in immunoprecipitates from HeLa cells (Fig. 4). Fractions immunoprecipitated from Nef-expressing cells by using an irrelevant antibody (specific for hemagglutinin) were free of both proteins (Fig. 4). In contrast, Ii chain p35, as well as its degradation product p10, was present in anti-Ii chain immunoprecipitates from HeLa-CIITA cells, but Nef was coprecipitated only when cells were transfected with Nef NL4-3 and not when they were transfected with Nef-STOP or with the Nef dileucine mutant construct (Fig. 4).
FIG. 4.
Nef can interact with the Ii chain in a dileucine-dependent manner. Immunoprecipitations were performed with HeLa or HeLa-CIITA cells transfected with Nef-STOP, Nef NL4-3, or the Nef LL164AA construct by using the Ii chain-specific MAb PIN1 covalently linked to protein A beads. Immunoprecipitates (IP) were eluted at 95°C and revealed with either anti-Nef or anti-Ii MAbs and peroxidase-labeled secondary antibodies by Western blotting.
We concluded that the Ii chain can bind Nef in a dileucine-dependent manner.
Nef-induced Ii chain upregulation and CD4 downregulation are genetically distinguishable.
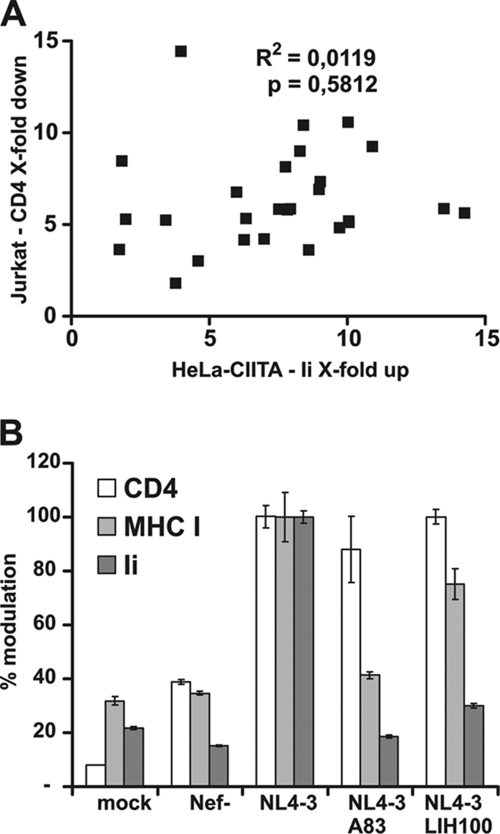
According to the currently accepted model, Nef accelerates the endocytosis of CD4 by bridging the CD4 cytoplasmic tail to AP-2 (see reference 27). Thus, Nef-mediated modulation of the Ii chain and that of CD4 are both mediated by an AP-2-dependent mechanism. If Ii chain upregulation resulted only from AP-2 competition with Nef, then both functions should tend to segregate together among naturally occurring nef alleles. We screened our collection of 27 pediatric nef alleles (45) for their efficiency in modulating CD4 and the Ii chain. CD4 downregulation was monitored in Jurkat cells and Ii chain upregulation was monitored in HeLa-CIITA cells. The data were plotted, and we performed a correlation analysis (Fig. 5A). It revealed that there was no correlation between CD4 downregulation and Ii chain upregulation induced by Nef. Indeed, some Nef forms had greatly reduced activity toward the Ii chain on the cell surface but still efficiently downregulated CD4 on the cell surface (Fig. 5A). Conversely, some other nef alleles exhibited opposite capacities (Fig. 5A).
FIG. 5.
Genetic dissociation of Nef-induced CD4 downregulation and Ii chain upregulation. (A) Twenty-seven pediatric nef alleles were tested for their abilities to downregulate CD4 in Jurkat cells and to upregulate the Ii chain in HeLa-CIITA cells. Data obtained from at least three independent experiments were normalized. Each dot represents one of the 27 nef alleles. (B) Vesicular stomatitis virus G protein-pseudotyped HIV-1 NL4-3 IRES-eGFP viruses containing either the NL4-3 wild-type nef gene or its two mutant forms A83L and LIH100IYY were tested for CD4 and MHC-I downmodulation on phytohemagglutinin-activated PBMC and Ii chain upregulation on THP-1 cells. Results from at least three independent experiments were normalized by taking the effects observed with Nef NL4-3 as 100%.
During evolution, these alleles may have selected for other mechanisms to compensate for the partial loss of function. We therefore screened our collection of Nef NL4-3 mutant forms in the context of HIV-1 infection. Thus, PBMC and THP-1 cells were infected with HIV NL4-3 IRES-eGFP viruses carrying various mutated nef genes. We thus identified two Nef mutant proteins, the A83L and LIH100IYY proteins, which had lost most of the capacity to upregulate Ii chain surface expression but kept their capacity to downregulate CD4 and, partially, MHC-I (Fig. 5B). Along the same line, we previously reported a similar phenotype for the NL4-3 PP75/78AA Nef mutant protein (50) and an inverse phenotype for the NA7 RD35AA Nef mutant protein (46).
Taken together, these findings demonstrated that Nef-induced CD4 downregulation and Ii chain upregulation can be genetically separated and argue against a model in which Nef-mediated upregulation of the Ii chain results only from the competition of Nef with the Ii chain for AP-2 recruitment.
DISCUSSION
Among the various surface molecules modulated by Nef, the MHC-II-associated chaperone Ii chain appears to be special. While most of the surface molecules modulated by Nef are downregulated, the Ii chain and a couple of other type II transmembrane proteins are upregulated (41). Even at low doses of Nef, the Ii chain starts to accumulate, together with associated MHC-II α and β chains, at the cell surface (50). This function is highly conserved among nef alleles from lentiviruses and has been observed previously in primary cells (45). Here, we have shown that αβIi complexes accumulate at the surfaces of Nef-expressing HeLa-CIITA cells, probably as a result of impaired endocytosis. In the absence of Nef, most of the newly synthesized αβIi complexes are rapidly targeted to the plasma membrane, where they are endocytosed through an AP-2-dependent mechanism (14, 33). AP-2 also appears to be involved in the Nef-mediated inhibition of the αβIi complex endocytosis since we have shown here that Nef has no additive effect on the Ii chain levels on the surfaces of AP-2-depleted cells. In addition, Nef-mediated inhibition of Ii chain endocytosis relies on two motifs (LL164 and ED174) present in the Nef C-terminal loop, which have been characterized to be involved in the interaction between Nef and AP-2 (28). It is thus tempting to propose, as initially suggested (50), that Nef and the Ii chain compete for AP-2 binding.
However, not all the AP-2 users are affected by Nef expression, and a certain specificity seems to apply. Strikingly, the kinetics of Tf uptake appears to be largely unmodified by Nef. The TfR is a transmembrane type II protein and the prototypic receptor using the AP-2 pathway for its endocytosis. While the Ii chain contains two dileucine-based endocytosis signals in its short cytoplasmic tail (11), the TfR cytoplasmic tail contains a tyrosine-based signal for endocytosis (39). Competition for the endocytic machinery between transmembrane receptors carrying similar motifs has been evaluated previously in transient transfection experiments with HeLa cells (32). Results from this previous study indicated that tyrosine-based motifs are able to compete with each other but not with dileucine-based motifs (32). Similarly, dileucine-based motifs are able to compete with each other. Therefore, it is possible that Nef affects receptors carrying dileucine- but not tyrosine-based endocytosis motifs. Results obtained with DC-SIGN support this interpretation. DC-SIGN contains two endocytosis motifs, one dileucine based and the other tyrosine based. However, only the dileucine motif is required for constitutive DC-SIGN endocytosis, as well as for Nef-induced decrease of DC-SIGN endocytosis (47). Moreover, the Nef dileucine motif is also required for Nef-mediated DC-SIGN upregulation. This situation suggests again that Nef can upregulate surface molecules recruiting the AP-2 pathway for their endocytosis through dileucine-based motifs but not tyrosine-based motifs.
Explaining the consequences of Nef expression on surface receptors through its capacity to compete for AP-2 still fails to account for the different observations. Indeed, a model for Nef activity should explain the selectivity of Nef effects: inducing strong upregulation of the Ii chain, not affecting Tf uptake, and promoting the endocytosis and downregulation of CD4, another receptor carrying a dileucine motif in its cytoplasmic tail. Moreover, our data raise several arguments against the simple model in which Nef and the Ii chain would compete to recruit AP-2.
(i) Using bicistronic vectors encoding Nef and GFP, we found that as soon as GFP and, thus, Nef expression was detectable, then Ii chain accumulation at the cell surface was observed (Fig. 1). The lack of a threshold suggests the absence of AP-2 titration by Nef.
(ii) We have shown that the expression of a CD8 chimera carrying a dileucine motif in its cytoplasmic tail did not affect Ii chain expression on the surfaces of HeLa-CIITA cells. However, this negative result may have several origins, such as CD8-LL's not being a good competitor for binding AP-2 compared to the Ii chain.
(iii) Most importantly, Ii chain upregulation was genetically distinguishable from CD4 downregulation. Indeed, we characterized Nef alleles and Nef mutant forms having reduced capacities to modulate one of the surface molecules but not the other one. CD4 downregulation by Nef results in part from Nef's bridging AP-2 to the CD4 cytoplasmic tail and thus enhancing the CD4 rate of endocytosis (37). If Ii chain upregulation resulted only from the titration of AP-2 by Nef, then both functions of Nef should segregate together.
These observations do not rule out that the accumulation of the Ii chain at the cell surface is due in part to competition between Nef and the Ii chain for binding AP-2, but they strongly suggest that there is a more specific mechanism involved. One of the hypotheses to explain the specificity of Nef-mediated Ii chain upregulation is direct binding to the Ii chain. We were able to coimmunoprecipitate wild-type Nef, but not the LL164AA Nef mutant protein, and a MAb specific for the Ii chain (Fig. 4). However, Nef was also found in immunoprecipitates from cells expressing the two Nef mutant forms LIH100IYY and A83L (data not shown), which have lost most of their capacity to upregulate the Ii chain but have kept their capacity to downregulate CD4 and, partially, MHC-I (Fig. 5B). Therefore, the presence of Nef in the Ii chain immunoprecipitates does not always correlate with its capacity to upregulate the surface Ii chain level. This situation appears to be very similar to that observed for CD4: Nef was found previously to interact with a 13-residue peptide from the cytoplasmic tail of CD4 through its WL58 motif (22), but there are many Nef mutant forms which have lost the capacity to downregulate CD4 but still posses an intact WL58 motif and thus should be able to interact with CD4 (31). Conversely, Nef can bind to mutant forms of CD4 without inducing their downmodulation (1, 7). Therefore, our data are still compatible with the idea that Nef can impede the correct recruitment of AP-2 to the Ii chain by binding to the short cytoplasmic tail of the Ii chain. In contrast, Nef would not interfere with the TfR recruitment of AP-2, possibly because the tyrosine-based motif of the TfR has better affinity for AP-2 than Nef.
Regarding CD4 downregulation, earlier studies established that the dileucine motif of Nef is strictly required for CD4 downregulation and for AP-2 binding (10, 20). This motif is also required to target Nef to clathrin-coated pits independently of CD4 expression (5). The cytoplasmic tail of CD4 also contains a dileucine motif, which is not required for Nef binding but is critical for CD4 downregulation (1, 7). Taken together, these data indicate that Nef-mediated downregulation of CD4 may involve clathrin or AP-2 and the cytoplasmic tail of CD4 but that the exact nature of the interactions between Nef and this machinery remains to be established. It seems likely that Nef somehow cooperates with CD4 to allow more efficient recruitment of AP-2 to the CD4 cytoplasmic tail. This cooperation may participate in the Nef-induced inhibition of CD4 recycling if, for instance, CD4 bound to the Nef-AP-2 complex cannot follow the recycling route. However, a similar mechanism cannot account for the inhibitory effect of Nef on Tf recycling because Tf uptake remains roughly normal in the presence of Nef. This finding suggests that Nef exerts a more direct effect on the recycling machinery.
Finally, the great sensitivity of the Ii chain complexes to Nef expression may be related to the nonameric structure of the (αβIi)3 complexes. The short cytoplasmic tails of both MHC-II chains and especially a leucine-based motif in the β chain are necessary for the internalization of mature MHC-II molecules (48). Therefore, taking into account the two motifs present in the Ii chain short cytoplasmic tail, the (αβIi)3 complexes carry 9 to 12 endocytic motifs in the vicinity of nine transmembrane domains probably packed closely together (see reference 11). It is thus reasonable to assume that such a nonameric structure should have an impact on the biophysical properties of the complexes during intracellular trafficking. This conclusion is supported by previous reports indicating that Ii chain expression alone induces modifications of the endocytic pathway, leading to large compartments called macrosomes (25) and delayed transport within the endocytic pathway (19). These modifications are not observed when the whole MHC-II machinery is expressed, as it is in the case of HeLa-CIITA cells. Of note, we observed that the Ii chain at the plasma membranes of Nef-expressing cells appeared to be concentrated in patches, which may be due to Nef's impeding of AP-2 recruitment by αβIi complexes. Nef expression also leads to general alterations of the endocytic pathway (43), inducing in MHC-II+ cells the accumulation of multivesicular bodies containing large amounts of the Ii chain, which are delayed in their access to lysosomes (49). Nef expression is also known to inhibit the recycling of CD4 (37) and Tf (30) and to inhibit Shiga toxin retrograde transport from the plasma membrane to the endoplasmic reticulum (23). The complex interactions among Nef, the Ii chain complexes, and the endocytic pathway clearly need to be better understood to approach the mechanism involved in the Nef-induced upregulation of the Ii chain.
In the course of the final revision of our report, results from a study suggesting that Nef competes with the Ii chain for AP-2, leading to Ii chain upregulation at the cell surface, were described (34). Our findings suggest that Nef-induced CD4 downregulation and Ii chain upregulation rely on overlapping but not identical surfaces of Nef and argue against a simple model in which Nef effects on the Ii chain would result only from Nef competition with the Ii chain for AP-2 recruitment. Nef appears to impair Ii chain AP-2-dependent endocytosis through an additional mechanism(s), which remains to be elucidated.
Supplementary Material
Acknowledgments
We thank Sebastian Amigorena and Ana-Maria Lennon-Dumenil for discussions.
This work was supported by a grant and a fellowship from Agence Nationale de Recherche contre le SIDA to P.B. and to H.T., respectively. F.-X.G. was supported by a grant from Ensemble contre le SIDA. H.T. was initially supported by a fellowship from INSERM region Ile de France. F.K. and M.S. are supported by the Deutsche-Forschungs-Gemeinschaft and the Wilhelm-Sander-Foundation.
Footnotes
Published ahead of print on 2 July 2008.
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1.Bentham, M., S. Mazaleyrat, and M. Harris. 2003. The di-leucine motif in the cytoplasmic tail of CD4 is not required for binding to human immunodeficiency virus type 1 Nef, but is critical for CD4 down-modulation. J. Gen. Virol. 842705-2713. [DOI] [PubMed] [Google Scholar]
- 2.Bijlmakers, M. J., P. Benaroch, and H. L. Ploegh. 1994. Assembly of HLA DR1 molecules translated in vitro: binding of peptide in the endoplasmic reticulum precludes association with invariant chain. EMBO J. 132699-2707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bresnahan, P. A., W. Yonemoto, S. Ferrell, D. Williams-Herman, R. Geleziunas, and W. C. Greene. 1998. A dileucine motif in HIV-1 Nef acts as an internalization signal for CD4 downregulation and binds the AP-1 clathrin adaptor. Curr. Biol. 81235-1238. [DOI] [PubMed] [Google Scholar]
- 4.Brummelkamp, T. R., R. Bernards, and R. Agami. 2002. A system for stable expression of short interfering RNAs in mammalian cells. Science 296550-553. [DOI] [PubMed] [Google Scholar]
- 5.Burtey, A., J. Z. Rappoport, J. Bouchet, S. Basmaciogullari, J. Guatelli, S. M. Simon, S. Benichou, and A. Benmerah. 2007. Dynamic interaction of HIV-1 Nef with the clathrin-mediated endocytic pathway at the plasma membrane. Traffic 861-76. [DOI] [PubMed] [Google Scholar]
- 6.Chaudhuri, R., O. W. Lindwasser, W. J. Smith, J. H. Hurley, and J. S. Bonifacino. 2007. Downregulation of CD4 by human immunodeficiency virus type 1 Nef is dependent on clathrin and involves direct interaction of Nef with the AP2 clathrin adaptor. J. Virol. 813877-3890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cluet, D., C. Bertsch, C. Beyer, L. Gloeckler, M. Erhardt, J. P. Gut, J. L. Galzi, and A. M. Aubertin. 2005. Detection of human immunodeficiency virus type 1 Nef and CD4 physical interaction in living human cells by using bioluminescence resonance energy transfer. J. Virol. 798629-8636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohen, G. B., R. T. Gandhi, D. M. Davis, O. Mandelboim, B. K. Chen, J. L. Strominger, and D. Baltimore. 1999. The selective downregulation of class I major histocompatibility complex proteins by HIV-1 protects HIV-infected cells from NK cells. Immunity 10661-671. [DOI] [PubMed] [Google Scholar]
- 9.Costa, L. J., N. Chen, A. Lopes, R. S. Aguiar, A. Tanuri, A. Plemenitas, and B. M. Peterlin. 2006. Interactions between Nef and AIP1 proliferate multivesicular bodies and facilitate egress of HIV-1. Retrovirology 333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Craig, H. M., T. R. Reddy, N. L. Riggs, P. P. Dao, and J. C. Guatelli. 2000. Interactions of HIV-1 Nef with the μ subunits of adaptor protein complexes 1, 2, and 3: role of the dileucine-based sorting motif. Virology 2719-17. [DOI] [PubMed] [Google Scholar]
- 11.Cresswell, P. 1996. Invariant chain structure and MHC class II function. Cell 84505-507. [DOI] [PubMed] [Google Scholar]
- 12.Dhiver, C., D. Olive, S. Rousseau, C. Tamalet, M. Lopez, J. R. Galindo, M. Mourens, M. Hirn, J. A. Gastaut, and C. Mawas. 1989. Pilot phase I study using zidovudine in association with a 10-day course of anti-CD4 monoclonal antibody in seven AIDS patients. AIDS 3835-842. [DOI] [PubMed] [Google Scholar]
- 13.Doray, B., I. Lee, J. Knisely, G. Bu, and S. Kornfeld. 2007. The γ/σ1 and α/σ2 hemicomplexes of clathrin adaptors AP-1 and AP-2 harbor the dileucine recognition site. Mol. Biol. Cell 181887-1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dugast, M., H. Toussaint, C. Dousset, and P. Benaroch. 2005. AP2 clathrin adaptor complex, but not AP1, controls the access of the major histocompatibility complex (MHC) class II to endosomes. J. Biol. Chem. 28019656-19664. [DOI] [PubMed] [Google Scholar]
- 15.Edeling, M. A., C. Smith, and D. Owen. 2006. Life of a clathrin coat: insights from clathrin and AP structures. Nat. Rev. Mol. Cell Biol. 732-44. [DOI] [PubMed] [Google Scholar]
- 16.Foster, J. L., and J. V. Garcia. 2007. Role of Nef in HIV-1 replication and pathogenesis. Adv. Pharmacol. 55389-409. [DOI] [PubMed] [Google Scholar]
- 17.Foti, M., A. Mangasarian, V. Piguet, D. P. Lew, K. H. Krause, D. Trono, and J. L. Carpentier. 1997. Nef-mediated clathrin-coated pit formation. J. Cell Biol. 13937-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garcia, J. V., and A. D. Miller. 1991. Serine phosphorylation-independent downregulation of cell-surface CD4 by nef. Nature 350508-511. [DOI] [PubMed] [Google Scholar]
- 19.Gorvel, J. P., J. M. Escola, E. Stang, and O. Bakke. 1995. Invariant chain induces a delayed transport from early to late endosomes. J. Biol. Chem. 2702741-2746. [DOI] [PubMed] [Google Scholar]
- 20.Greenberg, M., L. DeTulleo, I. Rapoport, J. Skowronski, and T. Kirchhausen. 1998. A dileucine motif in HIV-1 Nef is essential for sorting into clathrin-coated pits and for downregulation of CD4. Curr. Biol. 81239-1242. [DOI] [PubMed] [Google Scholar]
- 21.Greenberg, M. E., A. J. Iafrate, and J. Skowronski. 1998. The SH3 domain-binding surface and an acidic motif in HIV-1 Nef regulate trafficking of class I MHC complexes. EMBO J. 172777-2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grzesiek, S., S. J. Stahl, P. T. Wingfield, and A. Bax. 1996. The CD4 determinant for downregulation by HIV-1 Nef directly binds to Nef. Mapping of the Nef binding surface by NMR. Biochemistry 3510256-10261. [DOI] [PubMed] [Google Scholar]
- 23.Johannes, L., V. Pezo, F. Mallard, D. Tenza, A. Wiltz, A. Saint-Pol, J. Helft, C. Antony, and P. Benaroch. 2003. Effects of HIV-1 Nef on retrograde transport from the plasma membrane to the endoplasmic reticulum. Traffic 4323-332. [DOI] [PubMed] [Google Scholar]
- 24.Keppler, O. T., N. Tibroni, S. Venzke, S. Rauch, and O. T. Fackler. 2006. Modulation of specific surface receptors and activation sensitization in primary resting CD4+ T lymphocytes by the Nef protein of HIV-1. J. Leukoc. Biol. 79616-627. [DOI] [PubMed] [Google Scholar]
- 25.Lagaudriere-Gesbert, C., S. L. Newmyer, T. F. Gregers, O. Bakke, and H. L. Ploegh. 2002. Uncoating ATPase Hsc70 is recruited by invariant chain and controls the size of endocytic compartments. Proc. Natl. Acad. Sci. USA 99515-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Le Gall, S., L. Erdtmann, S. Benichou, C. Berlioz-Torrent, L. Liu, R. Benarous, J. M. Heard, and O. Schwartz. 1998. Nef interacts with the mu subunit of clathrin adaptor complexes and reveals a cryptic sorting signal in MHC I molecules. Immunity 8483-495. [DOI] [PubMed] [Google Scholar]
- 27.Lindwasser, O. W., R. Chaudhuri, and J. S. Bonifacino. 2007. Mechanisms of CD4 downregulation by the Nef and Vpu proteins of primate immunodeficiency viruses. Curr. Mol. Med. 7171-184. [DOI] [PubMed] [Google Scholar]
- 28.Lindwasser, O. W., W. J. Smith, R. Chaudhuri, P. Yang, J. H. Hurley, and J. S. Bonifacino. 2008. A diacidic motif in human immunodeficiency virus type 1 Nef is a novel determinant of binding to AP-2. J. Virol. 821166-1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lubben, N. B., D. A. Sahlender, A. M. Motley, P. J. Lehner, P. Benaroch, and M. S. Robinson. 2007. HIV-1 Nef-induced down-regulation of MHC class I requires AP-1 and clathrin but not PACS-1 and is impeded by AP-2. Mol. Biol. Cell 183351-3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Madrid, R., K. Janvier, D. Hitchin, J. Day, S. Coleman, C. Noviello, J. Bouchet, A. Benmerah, J. Guatelli, and S. Benichou. 2005. Nef-induced alteration of the early/recycling endosomal compartment correlates with enhancement of HIV-1 infectivity. J. Biol. Chem. 2805032-5044. [DOI] [PubMed] [Google Scholar]
- 31.Mangasarian, A., V. Piguet, J. K. Wang, Y. L. Chen, and D. Trono. 1999. Nef-induced CD4 and major histocompatibility complex class I (MHC-I) down-regulation are governed by distinct determinants: N-terminal alpha helix and proline repeat of Nef selectively regulate MHC-I trafficking. J. Virol. 731964-1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marks, M. S., L. Woodruff, H. Ohno, and J. S. Bonifacino. 1996. Protein targeting by tyrosine- and di-leucine-based signals: evidence for distinct saturable components. J. Cell Biol. 135341-354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McCormick, P. J., J. A. Martina, and J. S. Bonifacino. 2005. Involvement of clathrin and AP-2 in the trafficking of MHC class II molecules to antigen-processing compartments. Proc. Natl. Acad. Sci. USA 1027910-7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mitchell, R. S., R. Chaudhuri, O. W. Lindwasser, K. A. Tanaka, D. Lau, R. Murillo, J. S. Bonifacino, and J. C. Guatelli. 2008. Competition model for upregulation of the major histocompatibility complex class II-associated invariant chain by human immunodeficiency virus type 1 Nef. J. Virol. 827758-7767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Motley, A., N. A. Bright, M. N. Seaman, and M. S. Robinson. 2003. Clathrin-mediated endocytosis in AP-2-depleted cells. J. Cell Biol. 162909-918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Piguet, V., Y. L. Chen, A. Mangasarian, M. Foti, J. L. Carpentier, and D. Trono. 1998. Mechanism of Nef-induced CD4 endocytosis: Nef connects CD4 with the mu chain of adaptor complexes. EMBO J. 172472-2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Piguet, V., F. Gu, M. Foti, N. Demaurex, J. Gruenberg, J. L. Carpentier, and D. Trono. 1999. Nef-induced CD4 degradation: a diacidic-based motif in Nef functions as a lysosomal targeting signal through the binding of beta-COP in endosomes. Cell 9763-73. [DOI] [PubMed] [Google Scholar]
- 38.Pitcher, C. J., C. Quittner, D. M. Peterson, M. Connors, R. A. Koup, V. C. Maino, and L. J. Picker. 1999. HIV-1-specific CD4+ T cells are detectable in most individuals with active HIV-1 infection, but decline with prolonged viral suppression. Nat. Med. 5518-525. [DOI] [PubMed] [Google Scholar]
- 39.Robinson, M. S. 2004. Adaptable adaptors for coated vesicles. Trends Cell Biol. 14167-174. [DOI] [PubMed] [Google Scholar]
- 40.Roche, P. A., M. S. Marks, and P. Cresswell. 1991. Formation of a nine-subunit complex by HLA class II glycoproteins and the invariant chain. Nature 354392-394. [DOI] [PubMed] [Google Scholar]
- 41.Roeth, J. F., and K. L. Collins. 2006. Human immunodeficiency virus type 1 Nef: adapting to intracellular trafficking pathways. Microbiol. Mol. Biol. Rev. 70548-563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rosenberg, E. S., J. M. Billingsley, A. M. Caliendo, S. L. Boswell, P. E. Sax, S. A. Kalams, and B. D. Walker. 1997. Vigorous HIV-1-specific CD4+ T cell responses associated with control of viremia. Science 2781447-1450. [DOI] [PubMed] [Google Scholar]
- 43.Sanfridson, A., S. Hester, and C. Doyle. 1997. Nef proteins encoded by human and simian immunodeficiency viruses induce the accumulation of endosomes and lysosomes in human T cells. Proc. Natl. Acad. Sci. USA 94873-878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schindler, M., J. Munch, O. Kutsch, H. Li, M. L. Santiago, F. Bibollet-Ruche, M. C. Muller-Trutwin, F. J. Novembre, M. Peeters, V. Courgnaud, E. Bailes, P. Roques, D. L. Sodora, G. Silvestri, P. M. Sharp, B. H. Hahn, and F. Kirchhoff. 2006. Nef-mediated suppression of T cell activation was lost in a lentiviral lineage that gave rise to HIV-1. Cell 1251055-1067. [DOI] [PubMed] [Google Scholar]
- 45.Schindler, M., S. Wildum, N. Casartelli, M. Doria, and F. Kirchhoff. 2007. Nef alleles from children with non-progressive HIV-1 infection modulate MHC-II expression more efficiently than those from rapid progressors. AIDS 211103-1107. [DOI] [PubMed] [Google Scholar]
- 46.Schindler, M., S. Wurfl, P. Benaroch, T. C. Greenough, R. Daniels, P. Easterbrook, M. Brenner, J. Munch, and F. Kirchhoff. 2003. Down-modulation of mature major histocompatibility complex class II and up-regulation of invariant chain cell surface expression are well-conserved functions of human and simian immunodeficiency virus nef alleles. J. Virol. 7710548-10556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sol-Foulon, N., A. Moris, C. Nobile, C. Boccaccio, A. Engering, J. P. Abastado, J. M. Heard, Y. van Kooyk, and O. Schwartz. 2002. HIV-1 Nef-induced upregulation of DC-SIGN in dendritic cells promotes lymphocyte clustering and viral spread. Immunity 16145-155. [DOI] [PubMed] [Google Scholar]
- 48.Stumptner-Cuvelette, P., and P. Benaroch. 2002. Multiple roles of the invariant chain in MHC class II function. Biochim. Biophys. Acta 15421-13. [DOI] [PubMed] [Google Scholar]
- 49.Stumptner-Cuvelette, P., M. Jouve, J. Helft, M. Dugast, A. S. Glouzman, K. Jooss, G. Raposo, and P. Benaroch. 2003. Human immunodeficiency virus-1 Nef expression induces intracellular accumulation of multivesicular bodies and major histocompatibility complex class II complexes: potential role of phosphatidylinositol 3-kinase. Mol. Biol. Cell 144857-4870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stumptner-Cuvelette, P., S. Morchoisne, M. Dugast, S. Le Gall, G. Raposo, O. Schwartz, and P. Benaroch. 2001. HIV-1 Nef impairs MHC class II antigen presentation and surface expression. Proc. Natl. Acad. Sci. USA 9812144-12149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Swigut, T., N. Shohdy, and J. Skowronski. 2001. Mechanism for down-regulation of CD28 by Nef. EMBO J. 201593-1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thoulouze, M. I., N. Sol-Foulon, F. Blanchet, A. Dautry-Varsat, O. Schwartz, and A. Alcover. 2006. Human immunodeficiency virus type-1 infection impairs the formation of the immunological synapse. Immunity 24547-561. [DOI] [PubMed] [Google Scholar]
- 53.Wei, B. L., V. K. Arora, J. L. Foster, D. L. Sodora, and J. V. Garcia. 2003. In vivo analysis of Nef function. Curr. HIV Res. 141-50. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.