Abstract
We studied the susceptibility of human embryonic stem cell-derived oligodendrocyte progenitor cells to infection with JC virus, the causative agent of progressive multifocal leukoencephalopathy (PML). A human embryonic stem cell line, H7, was used to derive an enriched population of cells expressing the oligodendrocyte progenitor cell-specific marker NG2. These cells expressed the 5HT2a receptor (5HT2aR) for JC virus and were highly susceptible to infection. Infection was reduced by treatment with anti-5HT2aR antibodies and by the 5HT2aR antagonists ritanserin and ketanserin. This is the first demonstration that human embryonic stem cell-derived oligodendrocyte progenitor cells are susceptible to JC virus infection and indicates that cells poised to replenish mature oligodendrocytes in PML lesions may also be a target of viral infection.
The ubiquitous human polyomavirus JC virus (JCV) causes a relatively rare but fatal central nervous system (CNS)-demyelinating disease known as progressive multifocal leukoencephalopathy (PML) (1, 20). Seroepidemiological studies indicate that 70% of the human population worldwide is infected with JCV (2, 3, 9). The mode of virus transmission is unknown, and no clinical illness has been associated with primary infection. Like all polyomaviruses, infection with JCV is associated with the establishment of lifelong persistent infection. PML occurs predominately in immunosuppressed patients, with the majority of cases occurring in the setting of human immunodeficiency virus infection (7). PML has also been reported in patients being treated with natalizumab, a drug designed to inhibit leukocyte trafficking into inflamed tissue (8, 11, 23). PML is thought to develop following reactivation of the virus and dissemination from peripheral sites to the CNS, where the primary targets are astrocytes and oligodendrocytes (13). Others have suggested that reactivation of latent JCV within the CNS can also contribute to the development and progression of PML (24). The mechanism by which JCV becomes reactivated and traffics to the CNS is unclear.
Infection of glial cells by JCV is dependent on virus binding to a receptor complex that includes α(2,3) or α(2,6)-linked sialic acid and the 5HT2a receptor (5HT2aR) (5, 6, 10, 12). Recently, human brain microvascular endothelial cells were shown to be susceptible to JCV infection independent of the 5HT2aR component, indicating that at least some cell types do not require this receptor (4).
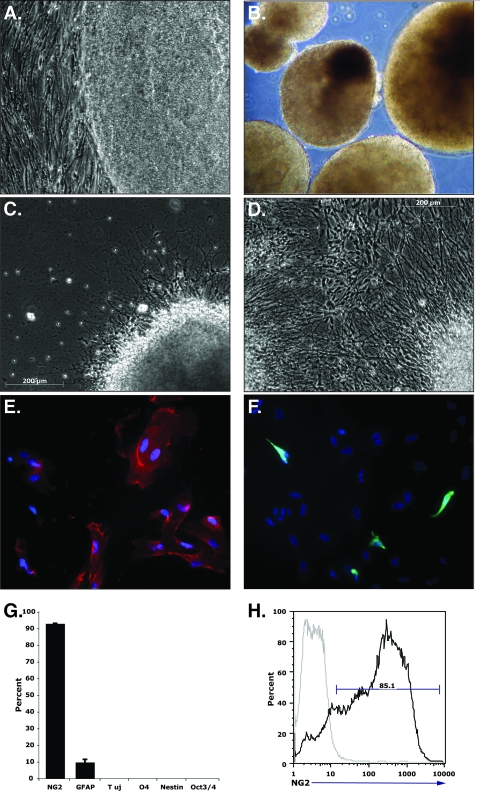
In this report, we sought to determine whether oligodendrocyte progenitor cells (OPCs) were susceptible to JCV infection and to what extent, if any, infection was dependent on the 5HT2aR. To test the susceptibility of human OPCs to JCV, we derived an enriched population from the H7 human embryonic stem cell (hESC) line by using a previously described 42-day differentiation protocol (17, 21). In brief, hESCs were expanded on a feeder-free, 1:30 growth factor-reduced Matrigel substrate (BD Biosciences, San Diego, CA) in hESC growth media supplemented with 10 ng/ml human recombinant basic fibroblast growth factor (Chemicon, Temecula, CA). hESCs were fed daily and passaged at 70% confluence (Fig. 1A). On day 1 of the OPC differentiation, hESC colonies were dissociated from the adherent substrate with collagenase IV (Invitrogen, San Diego, CA) and seeded in ultralow-binding 75-cm2 tissue culture flasks to facilitate embryoid body formation. Embryoid bodies were grown in suspension for 28 days in a series of specific media for defined periods of time (Fig. 1B) (17). On day 28, embryoid bodies were seeded on Matrigel-coated plates overnight, gently shaken to dislodge nonadherent cells, and cultured in glial restrictive media with 20 ng/ml epidermal growth factor for 7 days. Within 24 h of plating, cells began migrating out from the adherent embryoid bodies (Fig. 1C), and by day 5, the flasks were nearly 100% confluent (Fig. 1D). On day 35, cells were trypsinized and plated into 150-cm2 flasks for 1 h at 37°C to remove contaminating cell types. The remaining nonadherent cells were then plated onto Matrigel-coated 24-well tissue culture plates and cultured for 7 days in the continued presence of epidermal growth factor. Cells were infected with JCV (strain Mad-1SVEΔ) on the final day of differentiation (day 42). In parallel, cells were also plated onto Permanox Lab-Tek chamber slides (Nunc, Rochester, NY) for immunocytochemical analysis. Immuocytochemical staining showed that 92.9% ± 0.2% of the cells were positive for the OPC-specific marker, the NG2 glycoprotein (Fig. 1E and G). Cells expressing the astrocyte-specific glial fibrillary acidic protein were detected within the differentiated population at a frequency of 9.7% ± 1.8% (Fig. 1F and G). Cells expressing the neuronal marker class III β-tubulin Tuj1, the early glial progenitor marker nestin, and the mature oligodendrocyte marker O4 were not detected (Fig. 1G), which is consistent with previous findings (17). Negative staining for the hESC-specific Oct3/4 indicated that there were no detectable undifferentiated hESCs remaining in the culture (Fig. 1G).
FIG. 1.
(A to D) Phase-contrast micrographs depicting H7 hESCs at different stages of differentiation. (E) H7 cells at 42 days postdifferentiation, stained with the OPC-specific marker NG2 (red). (F) The same cultures stained with the astrocyte-specific marker glial fibrillary acidic protein. (G) Quantitation of the percentages of cells staining positive for differentiation markers. (H) Flow cytometric analysis for NG2 expression at 42 days postdifferentiation.
Flow cytometric analysis was used to confirm the purity of the hESC-derived OPC population. Cells (5 × 105) were incubated in blocking buffer (0.1% bovine serum albumin and 2.5 mg/ml anti-CD16/CD32) for 20 min at 4°C. Cells were then incubated with allophycocyanin-conjugated NG2 antibody (BD Pharmingen, San Diego, CA) and with an isotype-matched, nonreactive allophycocyanin-conjugated antibody as a negative control for 30 min at 4°C. The cells were then washed and analyzed on a FACStar flow cytometer (BD Biosciences, Mountain View, CA). These data verified that the majority of the cells (85.1%) were indeed OPCs (Fig. 1H).
We next asked whether parallel cultures of hESC-derived OPCs expressed the 5HT2aR by reverse transcriptase PCR (RT-PCR). RNA was extracted from control SVG-A cells and from the OPC-enriched H7 cells by using the Qiagen RNeasy midi kit. RNA (1 μg) from each was used to generate cDNA (iScript cDNA synthesis kit; Bio-Rad). Primers and RT-PCR cycling conditions have been described previously (4). GAPDH (glyceraldehyde-3-phosphate dehydrogenase) was used as an internal control, and all reactions were run with and without RT as a negative control. The amplicons were run out on a 3% agarose gel and visualized using ethidium bromide staining. The OPCs expressed similar amounts of the 5HT2aR mRNA as control SVG-A cells (Fig. 2).
FIG. 2.
RT-PCR analysis of 5HT2aR expression in SVG-A cells and in cells differentiated to oligodendrocyte progenitors (OPCs). RNA was extracted from SVG-A cells and from the H7-derived progenitors. The RNA was reverse transcribed and cDNA amplified with 5HT2aR-specific primers. GAPDH was used as an internal control. 5HT2aR-specific products (141 bp) were amplified from both the SVG-A- and H7-derived progenitor populations only when reverse transcriptase was included in the reaction. The GAPDH bands are 96 bp, making them easily distinguishable on the gel.
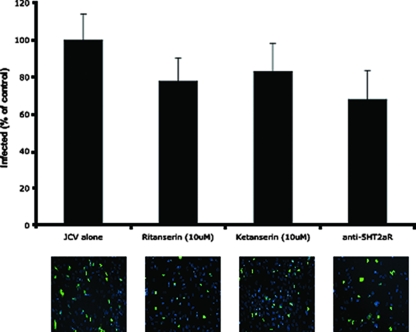
We then challenged the OPCs with JCV in the presence and absence of 5HT2aR antagonists. OPCs that had been plated as described above were treated in triplicate with either ketanserin or ritanserin for 24 h prior to infection. One hour prior to infection, additional cells were treated in triplicate with anti-5HT2a antibody (USB Scientific). Cells were then infected with JCV (strain Mad-1SVEΔ) for 1 h at 37°C in the continued presence of drug or antibody. At 72 h postinfection, cells were fixed in 2% paraformaldehyde and permeabilized with Triton X-100, and infection was scored by indirect immunofluorescence analysis of T antigen. The OPCs were as susceptible to JCV infection as the SVG-A glial cell line, with 25% of the cells staining positive for T antigen (Fig. 3). The majority of these cells were OPCs, but, as expected, a small percentage of astrocytes in the OPC-enriched cultures was also infected, as determined by morphological analysis (not shown). The 5HT2aR antagonists and the anti-5HT2aR antibody all modestly reduced infection at doses that were not toxic to the cells (Fig. 3). This level of reduction is similar to what is seen when these low doses of antagonists are used on SVG-A cells (6, 18). The hESC-derived OPCs were more prone to drug toxicity than the established SVG-A cell line was, and higher doses of drug could not be used reliably on these cells.
FIG. 3.
OPCs were treated in triplicate with ritanserin, ketanserin, or anti-5HT2aR antibody as indicated and then challenged with JCV. The data are plotted as percentages of the untreated infected control. Representative micrographs showing JCV T-antigen-positive cells (green). The cells were counterstained with DAPI (4′,6-diamidino-2-phenylindole).
The data clearly show that OPCs derived from hESC cultures are susceptible to JCV infection. These data are consistent with previous data showing susceptibility of nestin-positive glial progenitors derived from human fetal brain to infection with JCV (14-16). Interestingly, the human OPCs express the 5HT2aR for JCV, and susceptibility to infection can be reduced by pretreatment with 5HT2aR antagonists. Inhibition is not as pronounced as in SVG-A cells, and this could be due to a difference in the kinetics of receptor downregulation and recycling or to dosing.
It is also important to note that oligodendrocyte precursor populations expressing the NG2 glycoprotein have been shown to proliferate extensively in response to spinal cord injury in rat models and are capable of replacing damaged oligodendrocytes (19, 22). PML lesions rarely regress, and this could be due at least in part to the destruction of precursor cells by the virus. Experiments are ongoing to better define the mechanisms involved in JCV infection of these precursors as well as in fully differentiated oligodendrocytes.
Acknowledgments
We thank Tammy Glass, Wendy Virgadamo, and Heather Forand for administrative assistance. H7 cells were a kind gift from Hans Keirstead, Reeve-Irvine Research Center, University of California, Irvine, CA.
This work was supported by grants from the National Cancer Institute (R01 CA71878) and the National Multiple Sclerosis Society to W.J.A., by a California Institute of Regenerative Medicine (CIRM) Leon J. Thal grant (RS1-409) to T.E.L., and by a CIRM postdoctoral fellowship (T1-00008) to C.S.
Footnotes
Published ahead of print on 25 June 2008.
REFERENCES
- 1.Astrom, K., E. Mancall, and E. P. Richardson, Jr. 1958. Progressive multifocal leukoencephalopathy. Brain 8193-127. [DOI] [PubMed] [Google Scholar]
- 2.Bofill-Mas, S., M. Formiga-Cruz, P. Clemente-Casares, F. Calafell, and R. Girones. 2001. Potential transmission of human polyomaviruses through the gastrointestinal tract after exposure to virions or viral DNA. J. Virol. 7510290-10299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bofill-Mas, S., S. Pina, and R. Girones. 2000. Documenting the epidemiologic patterns of polyomaviruses in human populations by studying their presence in urban sewage. Appl. Environ. Microbiol. 66238-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chapagain, M. L., S. Verma, F. Mercier, R. Yanagihara, and V. R. Nerurkar. 2007. Polyomavirus JC infects human brain microvascular endothelial cells independent of serotonin receptor 2A. Virology 36455-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dugan, A. S., M. L. Gasparovic, and W. J. Atwood. 2008. Direct correlation between sialic acid binding and infection of cells by two human polyomaviruses (JC virus and BK virus). J. Virol. 822560-2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fonseca-Elphick, G., W. Querbes, J. A. Jordan, G. V. Gee, S. Eash, K. Manley, A. Dugan, M. Stanifer, A. Bhatnagar, W. K. Kroeze, B. L. Roth, and W. J. Atwood. 2004. The human polyomavirus, JCV, uses serotonin receptors to infect cells. Science 3061241-1420. [DOI] [PubMed] [Google Scholar]
- 7.Holman, R. C., T. J. Torok, E. D. Belay, R. S. Janssen, and L. B. Schonberger. 1998. Progressive multifocal leukoencephalopathy in the United States, 1979-1994: increased mortality associated with HIV infection. Neuroepidemiology 17303-309. [DOI] [PubMed] [Google Scholar]
- 8.Kleinschmidt-DeMasters, B. K., and K. L. Tyler. 2005. Progressive multifocal leukoencephalopathy complicating treatment with natalizumab and interferon beta-1a for multiple sclerosis. N. Engl. J. Med. 353369-374. [DOI] [PubMed] [Google Scholar]
- 9.Knowles, W. A., P. Pipkin, N. Andrews, A. Vyse, P. Minor, D. W. Brown, and E. Miller. 2003. Population-based study of antibody to the human polyomaviruses BKV and JCV and the simian polyomavirus SV40. J. Med. Virol. 71115-123. [DOI] [PubMed] [Google Scholar]
- 10.Komagome, R., H. Sawa, T. Suzuki, Y. Suzuki, S. Tanaka, W. J. Atwood, and K. Nagashima. 2002. Oligosaccharides as receptors for JC virus. J. Virol. 7612992-13000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Langer-Gould, A., S. W. Atlas, A. J. Green, A. W. Bollen, and D. Pelletier. 2005. Progressive multifocal leukoencephalopathy in a patient treated with natalizumab. N. Engl. J. Med. 353375-381. [DOI] [PubMed] [Google Scholar]
- 12.Liu, C. K., G. Wei, and W. J. Atwood. 1998. Infection of glial cells by the human polyomavirus JC is mediated by an N-linked glycoprotein containing terminal α(2-6)-linked sialic acids. J. Virol. 724643-4649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Major, E. O., K. Amemiya, C. S. Tornatore, S. A. Houff, and J. R. Berger. 1992. Pathogenesis and molecular biology of progressive multifocal leukoencephalopathy, the JC virus-induced demyelinating disease of the human brain. Clin. Microbiol. Rev. 549-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Messam, C. A., J. Hou, J. W. Berman, and E. O. Major. 2002. Analysis of the temporal expression of nestin in human fetal brain derived neuronal and glial progenitor cells. Brain Res. Dev. Brain Res. 13487-92. [DOI] [PubMed] [Google Scholar]
- 15.Messam, C. A., J. Hou, R. M. Gronostajski, and E. O. Major. 2003. Lineage pathway of human brain progenitor cells identified by JC virus susceptibility. Ann. Neurol. 53636-646. [DOI] [PubMed] [Google Scholar]
- 16.Messam, C. A., J. Hou, and E. O. Major. 2000. Coexpression of nestin in neural and glial cells in the developing human CNS defined by a human-specific anti-nestin antibody. Exp. Neurol. 161585-596. [DOI] [PubMed] [Google Scholar]
- 17.Nistor, G. I., M. O. Totoiu, N. Haque, M. K. Carpenter, and H. S. Keirstead. 2005. Human embryonic stem cells differentiate into oligodendrocytes in high purity and myelinate after spinal cord transplantation. Glia 49385-396. [DOI] [PubMed] [Google Scholar]
- 18.O'Hara, B. A., and W. J. Atwood. 2008. Interferon beta1-a and selective anti-5HT(2a) receptor antagonists inhibit infection of human glial cells by JC virus. Virus Res. 13297-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rabchevsky, A. G., P. G. Sullivan, and S. W. Scheff. 2007. Temporal-spatial dynamics in oligodendrocyte and glial progenitor cell numbers throughout ventrolateral white matter following contusion spinal cord injury. Glia 55831-843. [DOI] [PubMed] [Google Scholar]
- 20.Richardson, E. P., Jr. 1961. Progressive multifocal leukoencephalopathy. N. Engl. J. Med. 265815-823. [DOI] [PubMed] [Google Scholar]
- 21.Thomson, J. A., J. Itskovitz-Eldor, S. S. Shapiro, M. A. Waknitz, J. J. Swiergiel, V. S. Marshall, and J. M. Jones. 1998. Embryonic stem cell lines derived from human blastocysts. Science 2821145-1147. [DOI] [PubMed] [Google Scholar]
- 22.Tripathi, R., and D. M. McTigue. 2007. Prominent oligodendrocyte genesis along the border of spinal contusion lesions. Glia 55698-711. [DOI] [PubMed] [Google Scholar]
- 23.Van Assche, G., M. Van Ranst, R. Sciot, B. Dubois, S. Vermeire, M. Noman, J. Verbeeck, K. Geboes, W. Robberecht, and P. Rutgeerts. 2005. Progressive multifocal leukoencephalopathy after natalizumab therapy for Crohn's disease. N. Engl. J. Med. 353362-368. [DOI] [PubMed] [Google Scholar]
- 24.White, F. A., III, M. Ishaq, G. L. Stoner, and R. J. Frisque. 1992. JC virus DNA is present in many human brain samples from patients without progressive multifocal leukoencephalopathy. J. Virol. 665726-5734. [DOI] [PMC free article] [PubMed] [Google Scholar]