Abstract
The female genital tract is the major route of heterosexual human immunodeficiency virus (HIV) acquisition and transmission. Here, we investigated whether HIV-specific CD8 T-cell-mediated immune responses could be detected in the genital mucosa of chronically HIV-infected women and whether these were associated with either local mucosal HIV shedding or local immune factors. We found that CD8+ T-cell gamma interferon responses to Gag were detectable at the cervix of HIV-infected women but that the magnitude of genital responses did not correlate with those similarly detected in blood. This indicates that ex vivo HIV responses in one compartment may not be predictive of those in the other. We found that increased genital tumor necrosis factor alpha (TNF-α) and interleukin-10 (IL-10) levels correlated significantly with levels of Gag-specific CD8+ T cells at the cervix. Women who were detectably shedding virus in the genital tract had significantly increased cervical levels of TNF-α, IL-1β, IL-6, and IL-8 compared to women who were not detectably shedding virus. We were, however, unable to detect any association between the magnitude of cervical HIV-specific responses and mucosal HIV shedding. Our results support the hypothesis that proinflammatory cytokines in the female genital tract may promote HIV replication and shedding. In addition, we further show that inflammatory cytokines are associated with increased levels of HIV-specific CD8 effector cells at the genital mucosa but that these were not able to control genital HIV shedding.
The female genital tract plays an important role in human immunodeficiency virus type 1 (HIV-1) acquisition and transmission during heterosexual contact. Women are twice as likely to become infected with HIV during heterosexual sex as are men (37, 38), with transmission risks for both sexes increasing with higher plasma viral loads (9). Although plasma concentrations of HIV virions are a strong predictor of transmission rates, local mucosal factors such as genital inflammation and concurrent sexually transmitted infections are also strongly associated with both increased shedding of virus at the genital tract and greater susceptibility to infection via the genital mucosa (4, 6, 17, 30). Although immunological activation in response to invading pathogens is a crucial component of protective immunity, such responses may ironically also contribute to HIV pathogenesis by providing the virus with a steady supply of susceptible target cells (16).
Several lines of evidence implicate systemic HIV-specific CD8+ T lymphocytes in the control of HIV infection (8, 20). However, these HIV-specific CD8+ T cells inevitably fail to contain the virus, and all HIV-infected individuals eventually progress to AIDS in the absence of antiretroviral treatment. Mucosal HIV-specific CD8+ T cells have been detected in the semen and genital tracts of HIV-infected men and women, respectively. Importantly, these cells are demonstrably capable of cytokine production and cytotoxicity (5, 13-15, 21, 22, 25, 31-34, 39). Although no direct evidence exists for their role in local control of HIV infection, the presence of HIV-specific CD8+ T cells at the genital mucosa of exposed but uninfected commercial sex workers have implicated these cells in protection from HIV infection (14).
In the present study, we investigate the interplay between mucosal HIV-specific CD8 T cells, local HIV shedding in the female genital tract, and cervical inflammation. We evaluate the presence and magnitude of HIV-specific CD8+ T cells at the cervix. We measure mucosal and systemic levels of tumor necrosis factor alpha (TNF-α), interleukin-10 (IL-10), IL-1β, IL-6, IL-8, and IL-12p70 and relate these to the levels of HIV shedding in the genital tract. Although HIV-specific CD8 T cells are detectable in the cervixes of chronically HIV-infected women, we show that these cells probably do very little to prevent HIV transmission. We instead find evidence supporting the role of cervical inflammation as the central effector of both CD8+ T-cell recruitment and HIV shedding at the cervical mucosa.
MATERIALS AND METHODS
Description of study participants.
Fifty-one chronically HIV-1-infected women and 24 HIV-negative women attending community clinics in Cape Town, South Africa, were enrolled in the present study. Women who were menstruating at the time of sampling, who were postmenopausal or had undergone a hysterectomy, or who had vaginal discharge or visible or reported sexually transmitted infections were excluded from the study. The study was approved by the Research Ethics Committee of the University of Cape Town, South Africa, and informed written consent was obtained from all volunteers before initiation of the study. All women were naive to antiretroviral therapy.
Cervical mucosal sample collection and processing.
Cervical mucosal mononuclear cells (MMCs) were collected by using a Digene cervical sampler as described previously (24). The cervical cytobrush was inserted into the endocervical os (preferentially sampling from the transformation zone), rotated through 360° once, and immediately placed in 3 ml of cold transport medium (RPMI 1640 medium supplemented with 5 mM glutamine, amphotericin B, penicillin, streptomycin, and 10% fetal calf serum [FCS]). Cervical samples that had visible red blood cell contamination were discarded. Cervical cells were isolated within 4 h of collection by gently rotating the cytobrush against the sides of the tube to dislodge cells. The transport medium was flushed through the cytobrush bristles 30 times by using a sterile plastic Pasteur pipette to dislodge all cervical sample-derived cells. The cell suspension was transferred to a sterile 15-ml centrifuge tube, and the cells were pelleted at 250 × g for 10 min. The supernatant fraction was split into two equal aliquots and stored at −80°C until analysis for inflammatory cytokines and HIV shedding. Pelleted cells were resuspended in 2 ml of 10% FCS RPMI (transport medium). Cervical MMCs were counted by either (i) manual trypan staining on a Naubauer hemacytometer and light microscopy or (ii) using an automated Guava cell counter. At this point, cervical MMCs were rested for 16 h at 37°C and 5% CO2.
PBMC isolation.
Blood from HIV-infected women was collected by using standard venipunture into sterile ACD anticoagulated Vacutainer tubes (Becton Dickinson). Peripheral blood mononuclear cells (PBMC) were isolated by using Ficoll-Hypaque density gradient centrifugation using Leucosep tubes and counted using an automated Guava cell counter and Viacount staining (Guava). Cell concentrations were adjusted to 2 × 106 cells/ml, and the cells were rested for 16 h at 37°C and 5% CO2. All experiments were carried out on fresh PBMC.
Stimulation with HIV-1 Gag peptides and ex vivo intracellular cytokine staining.
PBMC (1 × 106 cell/ml) or cervical cells (∼0.15 × 106 to 1 × 106 lymphocytes/ml) were split into three BD Falcon tubes (500 μl/tube) and were stimulated with either a single pool of 121 HIV subtype C (Du422) Gag overlapping peptides (each peptide was used at a final concentration of 1 μg/ml; peptides were kindly provided by the NIH AIDS Reagent Repository) or staphylococcal enterotoxin B (10 μg/ml; Sigma-Aldrich; positive control) or were left untreated for 6 h at 37°C and 5% CO2. Brefeldin A (10 μg/ml; Sigma, St. Louis, MO) was added after the first hour. After stimulation, the cells were washed once by adding 2 ml of 10% FCS phosphate-buffered saline (PBS) (0.01% NaN3) and centrifuged (5 min, 300 × g, 1,300 rpm, room temperature). They were then stained with phenotypic markers allophycocyanin-labeled anti-CD3 (Becton Dickinson, San Jose, CA) and fluorescein isothiocyanate-labeled anti-CD8 (BD) for 30 min on ice. Cells were washed once by adding 2 ml of 10% FCS PBS (0.01% NaN3), centrifuged (5 min, 300 × g, 1,300 rpm, room temperature), fixed, and permeabilized by using BD CytoFix/CytoPerm for 10 min at room temperature and washed once with 0.1% saponin (Fluka) PBS (containing 5% FCS and 0.01% NaN3). Cells were stained with phycoerythrin-conjugated anti-gamma interferon (anti-IFN-γ; BD) for 30 min on ice. Cells were finally washed with 2 ml of 10% FCS PBS (0.01% NaN3), centrifuged (5 min, 300 × g, 1,300 rpm, room temperature), and fixed with BD Cell Fix. Cell fluorescence was measured by flow cytometry using a FACSCalibur (BD Immunocytometry Systems) with FlowJo (Tree Star, Inc.) used for data analysis. An average of 1,934 ± 3,167 events was acquired for each cervical cytobrush sample during ICS (data not shown).
Measurement of inflammatory cytokine levels.
Inflammatory cytokine (TNF-α, IL-10, IL-1β, IL-6, IL-8, and IL-12p70) levels in cervical supernatants (after removal of the cervical MMCs) and plasma samples from the 51 chronically HIV-infected women included in the present study were determined by using a human inflammation cytometric bead array kit (BD Immunocytometry Systems) according to the manufacturer's instructions. The limit of detection of this assay ranged between 1.9 and 7.2 pg/ml (average, 3.6 pg/ml). Cytokine values below the assay's limit of detection were reported as 1.6 pg/ml (the midpoint between the average lower level of detection 3.6 pg/ml and 0).
Determination of viral load in cervical supernatant and plasma.
Viral load was determined in cervical supernatants and plasma samples by using Nuclisens Easyq HIV-1 (version 1.2). The detection limit of this assay was 50 copies/ml. The cervical supernatant fraction was obtained after flushing the cervical cytobrush 30 times with 3 ml of transport medium and removal of the cells by centrifugation (250 × g for 10 min). Plasma was obtained from ACD anticoagulated whole blood after Ficoll density gradient centrifugation.
Statistical analysis.
Mann-Whitney U and Student unpaired t tests were performed for independent sample comparisons, and Spearman rank tests were applied for correlations, using GraphPad Prism version 5.0. All tests were two tailed, and P values of ≤0.05 were considered significant.
RESULTS
All 51 HIV-1-infected women included in the present study (Table 1) were chronically HIV-1 infected and had been seropositive for more than 3 years. The subjects had a mean CD4 count of 432 ± 181 (mean ± the standard deviation) cells/μl and an average HIV plasma viral load of 12,761 ± 18,163 RNA copies/ml. None were on antiretroviral therapy at the time of study.
TABLE 1.
Clinical details of chronically HIV-1-infected women included in the study
| Characteristic | Value |
|---|---|
| No. of subjects | 51 |
| Mean age in yr ± SD | 32.8 ± 6.2 |
| Absolute blood CD4 count (mean cells/ml ± SD) | 432.27 ± 181.4 |
| Plasma viral load (mean RNA copies/ml ± SD) | 12,761 ± 6,398.1 |
| No. (%) of women with detectable plasma viral load | 33 (64.7) |
| Range of plasma viral load in women with detectable levels (RNA copies/ml) | 80-72,000 |
| Cervical viral load in all women (mean RNA copies/ml ± SD) | 1,295.8 ± 6,398.1 |
| No. (%) of women with detectable cervical viral load | 20a (45.5) |
| Cervical viral load only in women with detectable levels (mean RNA copies/ml ± SD) | 3,030.6 ± 9,674.1 |
| Range of cervical viral load in women with detectable levels (RNA copies/ml) | 92-44,000 |
Cervical supernatant samples were only available for viral load assessment from 44 of 51 women, so the value is calculated as 20/44.
HIV-1 shedding in the female genital tract during chronic HIV infection.
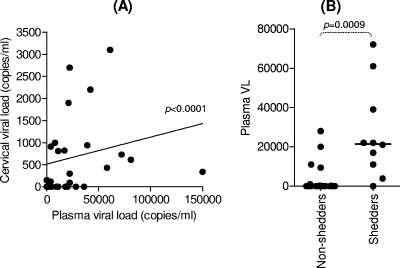
Of the 51 women included, 18 (35.3%) had no detectable virus in their plasma. The remaining 33 women with detectable HIV RNA in their plasma samples had viral loads that ranged from 80 to 72,000 RNA copies/ml. Viral loads were determined from cervical supernatants of 44 of 51 of these women. Of the 44 women, 20 (45.5%) had detectable HIV RNA in their cervical supernatant samples and were therefore classified as shedding virus. In these 20 women, the mean HIV cervical viral load was 3,030.6 ± 9,674.1 RNA copies/ml (ranging from 92 to 44,000 copies/ml). Importantly, there was a significant correlation between plasma and cervical viral loads (Fig. 1A; P < 0.0001). Accordingly, we found that women who were shedding HIV at the cervical mucosa (i.e., those with detectable cervical HIV RNA) had significantly higher plasma viral loads than women who where not shedding virus at the cervix (Fig. 1B; P = 0.0009).
FIG. 1.
Correlation between plasma viral load and HIV shedding at the cervical mucosa during chronic HIV infection. (A) Matching cervical and plasma viral loads were correlated by using the Spearman rank test. (B) Comparison of plasma viral load in women who had detectable HIV in their cervical supernatants (shedders) versus those that did not (nonshedders). Each data point represents an individual's plasma viral load. The line indicates the median viral load for each group. The Mann-Whitney U test was used to compare the plasma viral load between shedders and nonshedders.
Magnitude of HIV-1 Gag-specific CD8 T-cell responses at the cervix in HIV-infected women.
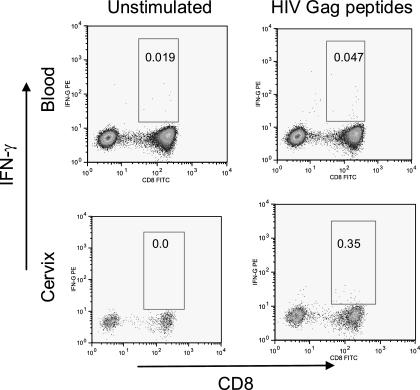
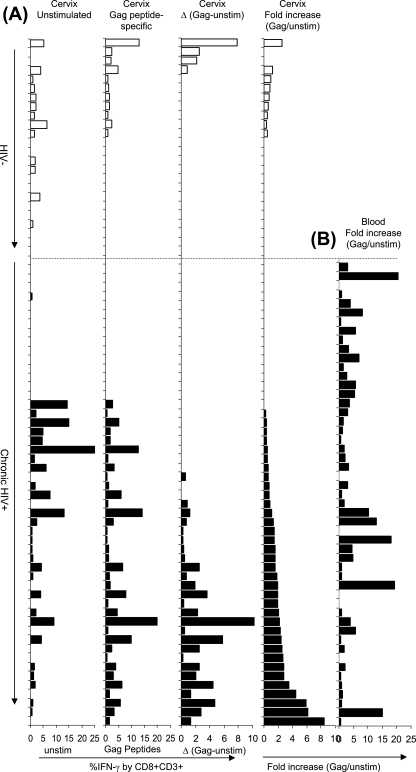
A number of studies have demonstrated the existence of HIV-specific CD8+ T cells at the cervical mucosa of HIV-infected women (13-15, 21, 22, 32, 39). We investigated the magnitude of ex vivo HIV Gag-specific IFN-γ-producing CD8+ T-cell responses at the genital mucosa in HIV-infected and uninfected women. Figure 2 shows a representative plot of IFN-γ production by CD8+ T cells isolated from both the blood and cervix of an HIV-infected woman. We found that the background frequency of IFN-γ-producing CD8+ T cells at the cervix (unstimulated) was significantly higher than the frequencies observed in matching blood samples: 0.65% (interquartile range [IQR] = 0 to 3.63) background IFN-γ at the cervix compared to 0.1% (IQR = 0.03 to 0.35) in the blood (P = 0.001). We did not observe any significant difference between background frequencies of IFN-γ+ cells among cervical T cells derived from HIV-positive and HIV-negative women (Fig. 3A). Both the frequency and the fold increase in HIV Gag peptide-specific CD8 responses were significantly higher in chronically HIV-infected women than in HIV-negative women (Fig. 3A, P = 0.04 for % IFN-γ+ to Gag [second panel in Fig. 3A], P = 0.02 for Δ in % IFN-γ+ to Gag [Gag-unstim; third panel in Fig. 3A], and P = 0.002 for the fold increase in IFN-γ+ responses to Gag [Gag/unstim; fourth panel in Fig. 3A]; t test). In HIV-infected women, the Gag-specific IFN-γ response frequency by CD8+ T cells ranged from undetectable (0% above background; 25 of 51 participants) to 10.8% above background. A similar analysis in uninfected women showed that Gag-specific IFN-γ responses ranged from undetectable (20 of 24 participants) to 2.5% above background.
FIG. 2.
Representative plot showing ex vivo intracellular IFN-γ production by blood (top panels)- and cervical mucosa (bottom panels)-derived CD8+ CD3+ cells in response to HIV Gag. Mononuclear cells were either not stimulated (left panels) or stimulated ex vivo with a single pool of HIV-1 subtype C Gag peptides (right panels). Events were acquired by using a BD FACSCaliber flow cytometer; compensation and analysis were performed by using FlowJo software.
FIG. 3.
Comparison between ex vivo IFN-γ production by cervical mucosa-derived CD8+ T cells from HIV-infected and uninfected women. (A, first panel) Background frequencies of IFN-γ+ CD8+ CD3+ T cells from HIV-negative (clear bars [n = 24]) and HIV-infected (solid bars [n = 51]) women. (A, second panel) HIV-1 Gag peptide-specific frequencies of IFN-γ+ T cells (without correction for background) in HIV-negative and HIV-positive women. (A, third panel) ΔHIV-1 Gag peptide-specific frequencies of IFN-γ+ T cells (after correction for background percentage IFN-γ production by unstimulated cells). (A, fourth panel) Fold increase in IFN-γ production by cervical CD8+ T cells in response to HIV Gag peptides (expressed as a ratio to unstimulated cells). (B) Fold increase in IFN-γ production by blood CD8+ T cells from HIV-infected women only (n = 51) in response to HIV Gag peptides (expressed as a ratio to unstimulated cells). Each bar represents an individual woman's response at the cervix or in blood. The order of the donors (and bars) is the same in each panel. The donor's have been stratified according to their hierarchy in fold increase Gag responses at the cervix.
No correlation between HIV-specific response magnitudes at the cervix and in blood.
We observed no correlation between net percent IFN-γ response magnitudes (data not shown) or fold increase (Fig. 3B; R = −0.03; P = 0.8271; Spearman rank test) when we compared blood and cervical T-cell responses, indicating that an HIV-specific response in blood does not necessarily reflect a similar ex vivo response at the cervix.
Impact of HIV immune responses at the cervix on HIV shedding at the genital mucosa.
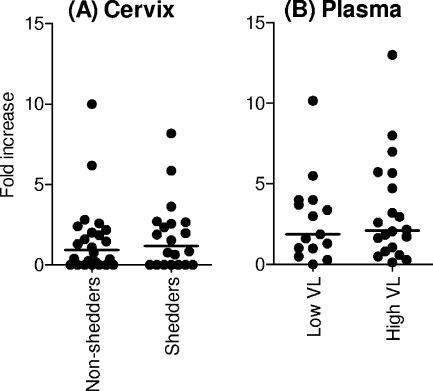
Cellular and humoral immune responses against HIV have been detected at the cervix and in semen of both HIV exposed and uninfected (highly exposed but persistently seronegative [HEPS]) and HIV-infected individuals (14, 15, 18, 34). It has therefore been suggested that such responses may be important correlates of protection against HIV locally. We thus investigated the relationship between mucosal HIV-specific CD8+ T cells responses and HIV shedding at the genital tract (Fig. 4). We found no significant association between the presence and magnitude of cervical CD8+ HIV-specific IFN-γ responses and cervical viral load (Fig. 4A), suggesting that the presence of local HIV-specific CD8+ T-cell responses had no clear impact on HIV shedding. Similarly, we observed no association between the magnitude of Gag-specific HIV responses in blood and either systemic viral loads (Fig. 4B) or genital shedding (data not shown).
FIG. 4.
Relationship between the magnitude of ex vivo HIV-specific IFN-γ production by cervical mucosa- and blood-derived CD8+ T cells and genital HIV shedding (A) or plasma viral load (B). (A) Women with detectable cervical viral load (detection limit of 80 RNA copies/ml) were ranked as shedders, while women with no detectable cervical viral load were ranked as nonshedders. (B) Women with plasma viral loads of >500 RNA copies/ml were ranked as high VL, while women with plasma viral loads of <500 RNA copies/ml were ranked as low VL. Each dot represents an individual's CD8 T-cell response magnitude (fold increase) to Gag peptides. Horizontal lines represent the median for each group. P values of ≤0.05 were considered significant, and the Mann-Whitney U test was applied to compare groups.
Cervical inflammation and HIV-specific CD8 T-cell responses at the genital mucosa.
To explore the hypothesis that HIV shedding is directly proportional to the level of cervical inflammation, we investigated the level of various inflammatory cytokines (IL-12, TNF-α, IL-10, IL-1β, IL-6, and IL-8) at the cervix and in plasma from chronically HIV-infected women (Table 2). We observed significantly elevated levels of TNF-α (P = 0.0025), IL-10 (P = 0.016), IL-1β (P < 0.0001), IL-6 (P < 0.0001), and IL-8 (P < 0.0001) at the cervix of each of these HIV-1 chronically infected women compared to matched plasma samples.
TABLE 2.
Comparison of inflammatory cytokine levels at the cervix and in blood
| Cytokine | Cytokine level (pg/ml) in:
|
P | |||
|---|---|---|---|---|---|
| Cervix
|
Blood
|
||||
| Median | IQR | Median | IQR | ||
| IL-12 | 6.5 | 0.0-8.6 | 6.3 | 0.0-7.0 | NSa |
| TNF-α | 4.4 | 3.8-5.5 | 3.8 | 3.5-4.0 | 0.0025 |
| IL-10 | 4.3 | 0.0-6.3 | 3.8 | 0.0-4.3 | 0.0159 |
| IL-1β | 42.0 | 13.0-220.8 | 4.9 | 4.6-5.5 | <0.0001 |
| IL-6 | 25.3 | 10.3-160.7 | 0.0 | 0.0-5.6 | <0.0001 |
| IL-8 | 1,291.2 | 343.6-4,305.3 | 8.1 | 7.5-9.3 | <0.0001 |
NS, not significant.
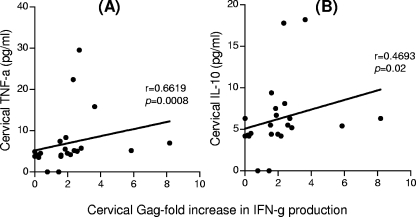
We examined the relationship between inflammatory cytokine levels at the cervix and in plasma and the magnitude of HIV-specific CD8+ T-cell responses (Fig. 5). We found a significant positive association between the magnitude of cervical HIV-specific CD8+ T-cell responses and the levels of TNF-α (P = 0.0008, r = 0.6619) and IL-10 (P = 0.0276, r = 0.4693) in cervical supernatants (Fig. 5).
FIG. 5.
Correlation between cervical TNF-α (A) and IL-10 (B) inflammatory cytokine levels and the magnitude of HIV Gag peptide-specific IFN-γ responses. The Spearman rank test was used to test for correlation. The magnitude of Gag-specific responses has been expressed as a ratio to unstimulated cells (fold increase).
Cervical inflammation and HIV shedding at the female genital tract.
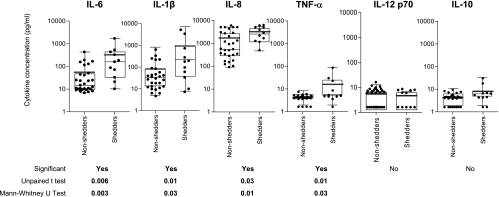
We next examined whether there were any associations between inflammatory cytokine levels and HIV shedding at the cervix (Fig. 6). Women who were found to be shedding HIV at the genital tract were found to have significantly elevated levels of TNF-α (P = 0.026), IL-1β (P = 0.0316), IL-6 (P = 0.0027), and IL-8 (P = 0.0148) compared to women who were not shedding virus (Fig. 6; all P values were calculated by using the Mann-Whitney U test). Although both the mean and median TNF-α, IL-1β, IL-6, and IL-8 concentrations (Fig. 6) were shown to be significantly different in shedders and nonshedders, it should be noted that the distributions of these cytokine concentrations were partially overlapping. Plasma inflammatory cytokine concentrations, generally at or below the detection limit of our assay, were not associated with plasma viral load or cervical shedding (data not shown).
FIG. 6.
Relationship between inflammatory cytokine levels in the cervical supernatant and genital tract HIV shedding in chronically HIV-infected women. Women with detectable cervical viral load (detection limit of 80 RNA copies/ml) were ranked as shedders, while women with no detectable cervical viral load were ranked as nonshedders. Each dot represents an individual woman's cytokine concentration in cervical supernatant and actual data has been plotted on a log scale. Horizontal bold lines represent mean values for each group. Box-and-whisker plots represent the median (center line), 25th and 75th percentiles. P values of ≤0.05 were considered significant and were calculated by using the Mann-Whitney U-test to compare medians, and the Student t test was used to compare the means of each group as indicated.
DISCUSSION
We have found that, in HIV-infected women, the immunological microenvironment of the female genital tract has a number of features that distinguish it from that found in blood. The cervical environment is defined by high frequencies of nonspecific activation, clearly detectable HIV-specific CD8+ T cells, and increased inflammatory cytokine concentrations, with the latter feature being associated with HIV shedding. Ours is the first reported investigation of potential associations between mucosal HIV-specific CD8+ T-cell responses, inflammation and HIV shedding in the female genital tract. Although we have not detected an association between either the presence or the magnitude of HIV-specific T-cell responses at the cervix and local HIV shedding, we have shown an association (i) between mucosal inflammation and the presence and magnitude of HIV-specific CD8+ T-cell mucosal responses and (ii) between mucosal inflammation and local HIV shedding. These data support our hypothesis that immune activation in the female genital tract is a driver of both local HIV shedding and HIV-specific CD8+ T-cell recruitment and is therefore likely to play a major role in HIV transmission.
A significant positive correlation exists between plasma and cervical viral loads, and we have found that elevated plasma viremia likely predicts HIV shedding in the female genital tract. Although positive correlations between plasma viral loads and genital shedding have been detected previously (2, 26, 27, 36), this association is still somewhat controversial, with some studies failing to find any significant correlation between HIV viral loads in the blood and at the genital mucosa (34, 36). Local factors in the genital tract may influence viral replication. Indeed, cytomegalovirus reactivation in the genital tract correlated strongly with high semen viral loads, even in the presence of undetectable viral load (VL) in blood (33).
Immune activation and inflammation are crucial features in driving HIV replication and pathogenesis. Our data suggest that a generalized activation of the cervix during chronic HIV infection occurs in some women, as marked by inflammatory cytokine production, and the likely activated CD4+ T cells would make the female genital tract a “hot-bed” of viral replication. In support of this, we have found that women with elevated levels of TNF-α, IL-1β, IL-6, and IL-8 in their genital tracts were more likely to be shedding HIV at their cervical mucosa than women with reduced levels of these cytokines. The finding that elevated inflammation and increased viral loads was not evident in the blood compartment supports the notion that HIV infectiousness may well be related to the level of cervical inflammation in some women. It is known that TNF-α, IL-1β, and IL-6 are proinflammatory cytokines that can help stimulate HIV replication (1) and, along with IL-8, a chemokine known to attract neutrophils to sites of inflammation (7), the cervix is likely to be a hostile activated microenvironment. The association between generalized mucosal cytokine activation and increased mucosal viral loads has also previously been observed in the context of HIV-1 infections (16, 19, 28). It may even be argued that the likely chronic proinflammatory microenvironment occurring in the female genital tract during HIV infection may damage the epithelial barrier, augmenting microbial translocation and later causing systemic immune activation (3, 12).
As in previous studies investigating mucosal HIV-specific CD8+ T-cell responses during HIV infection (5, 13-15, 21, 22, 25, 31-34, 39), we found that mucosal HIV-1 specific CD8+ T cells responses in the female genital tract are directly detectable ex vivo. A formidable technical hurdle is to discriminate between in vivo T-cell activation and the presence of HIV-specific immunity. It is also possible that the methodology used in preparing for intracellular signals may lead to cell exhaustion and death, since these cells are highly activated in situ. Bearing this in mind, our observation that no correlation existed between T-cell responses detected at the cervix with those in the blood does infer that responses in one compartment are not predictive of a response in another. We found instead that the magnitude of CD8+ T-cell responses detected at the cervix of chronically HIV-infected women correlated significantly with increased concentrations of cervical TNF-α and IL-10.
Our results indicate that cervical Gag-specific CD8+ T-cell responses may not be involved in controlling genital HIV replication or shedding. It is, however, possible that cervical HIV-specific CD8 T cells in vivo may be present in larger numbers or have enhanced functionality at the mucosa compared to the ex vivo analysis performed in the present study. Our data suggest that cervical inflammation is responsible for driving HIV shedding at the cervix or vice versa. Although several recent studies have shown that ulcerative and inflammatory sexually transmitted infections and resultant inflammation are associated with enhanced HIV-1 shedding (6), the cause and effect relationship is unclear, since others have shown a significant increase in inflammation at mucosal surfaces after HIV infection and replication (29). It is becoming clear that relying solely on IFN-γ production by CD8 T cells as a functional readout may not be enough to identify possible correlates of viral control (10, 11, 23), which thus begs for more functional phenotypic data to be accrued before concluding that HIV-specific CD8+ T cells have no impact on HIV shedding at the cervix.
Previous studies of HEPS women (14) described a relative enrichment of HIV-specific cervical responses in HIV-resistant compared to HIV-infected women and argued that the presence of local mucosal HIV-specific CD8 T-cell responses (in the absence of detectable HIV infection) may be important in providing protection at the genital mucosa against HIV infection in these women. The overall magnitude of HIV-specific responses at the cervix of HIV-resistant sex workers was twofold lower than the magnitude of responses measured in the HIV-infected cohort (but significantly higher than responses detected at the cervixes of HIV-negative women). Although protection from HIV infection (HEPS study [14]) and protection against HIV shedding are different, the HEPS study argues strongly for an important protective role for local CD8 T cells in HIV acquisition at the genital mucosa. The role of CD8 T cells in protection from HIV shedding was not evident in the present study.
The many differences between the blood and cervical compartments are likely to have an important influence on HIV pathogenesis and transmission. Many biological factors could contribute to the complexity of HIV disease in the female genital tract compartment, such as other local immune factors, the presence of sexually transmitted infections, and hormonal changes over the menstrual cycle. The high level of inflammation (regardless of the cause) in the female genital tract may contribute to high levels of HIV replication and could result in HIV-specific immune responses to increased local levels of HIV that are barely effective. Although various factors involved in HIV shedding in the female genital tract have been identified, it remains to be determined precisely how these factors interact to influence the rates of HIV transmission both between individual sexual partners and at the population level. Unraveling these potentially complex interactions to identify the prime factor(s) driving these associations would be a major advance. It may, for example, be important to determine whether anti-inflammatory interventions might reduce transmission risks in discordant couples. If cervical inflammation is a key driver of HIV shedding in women, then provision of anti-inflammatory preventative or therapaeutic treatments—for example, in microbicide formulations—may be crucial in lowering high transmission rates. Clearly, the present study highlights the fact that control of inflammation and sexually transmitted infections in the female genital tract should be a major component of HIV prevention programs. In addition, although we show here that CD8+ T cells do not correlate with local viral control during chronic HIV infection, this does not preclude that such cells, if induced by vaccination prior to HIV acquisition, could not contain HIV locally.
Acknowledgments
This study was supported by grants from the Wellcome Trust and South African AIDS Vaccine Initiative (MRC South Africa). J.S.P., A.B., and N.N.N. received Fogarty International training fellowships. J.S.P. is the recipient of a Wellcome Trust Intermediate Fellowship in Infectious Diseases.
We thank Janine Jones for collecting all of the specimens and the women who kindly participated in the study. We thank Darren Martin for reviewing the manuscript and for his constructive comments.
Footnotes
Published ahead of print on 18 June 2008.
REFERENCES
- 1.Al-Harthi, L., K. A. Roebuck, H. Kessler, and A. Landay. 1997. Inhibition of cytokine-driven human immunodeficiency virus type 1 replication by protease inhibitor. J. Infect. Dis. 1761175-1179. [DOI] [PubMed] [Google Scholar]
- 2.Bourlet, T., C. Cazorla, P. Berthelot, F. Grattard, F. Cognasse, A. Fresard, C. Defontaine, F. R. Lucht, C. Genin, and B. Pozzetto. 2001. Compartmentalization of HIV-1 according to antiretroviral therapy: viral loads are correlated in blood and semen but poorly in blood and saliva. AIDS 15284-285. [DOI] [PubMed] [Google Scholar]
- 3.Brenchley, J. M., D. A. Price, T. W. Schacker, T. E. Asher, G. Silvestri, S. Rao, Z. Kazzaz, E. Bornstein, O. Lambotte, D. Altmann, B. R. Blazar, B. Rodriguez, L. Teixeira-Johnson, A. Landay, J. N. Martin, F. M. Hecht, L. J. Picker, M. M. Lederman, S. G. Deeks, and D. C. Douek. 2006. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat. Med. 121365-1371. [DOI] [PubMed] [Google Scholar]
- 4.Coleman, J. S., J. Hitti, E. A. Bukusi, C. Mwachari, A. Muliro, R. Nguti, R. Gausman, S. Jensen, D. Patton, D. Lockhart, R. Coombs, and C. R. Cohen. 2007. Infectious correlates of HIV-1 shedding in the female upper and lower genital tracts. AIDS 21755-759. [DOI] [PubMed] [Google Scholar]
- 5.Critchfield, J. W., D. Lemongello, D. H. Walker, J. C. Garcia, D. M. Asmuth, R. B. Pollard, and B. L. Shacklett. 2007. Multifunctional human immunodeficiency virus (HIV) gag-specific CD8+ T-cell responses in rectal mucosa and peripheral blood mononuclear cells during chronic HIV type 1 infection. J. Virol. 815460-5471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cummins, J. E., L. Christensen, J. L. Lennox, T. J. Bush, Z. Wu, D. Malamud, T. Evans-Strickfaden, A. Siddig, A. M. Caliendo, C. E. Hart, and C. S. Dezzutti. 2006. Mucosal innate immune factors in the female genital tract are associated with vaginal HIV-1 shedding independent of plasma viral load. AIDS Res. Hum. Retrovir. 22788-795. [DOI] [PubMed] [Google Scholar]
- 7.Dinarello, C. A. 2000. Proinflammatory cytokines. Chest 118503-508. [DOI] [PubMed] [Google Scholar]
- 8.Edwards, B. H., A. Bansal, S. Sabbaj, J. Bakari, M. J. Mulligan, and P. A. Goepfert. 2002. Magnitude of functional CD8+ T-cell responses to the gag protein of human immunodeficiency virus type 1 correlates inversely with viral load in plasma. J. Virol. 762298-2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fiore, J. R., B. Suligoi, A. Saracino, M. Di Stefano, R. Bugarini, A. Lepera, A. Favia, L. Monno, G. Angarano, and G. Pastore. 2003. Correlates of HIV-1 shedding in cervicovaginal secretions and effects of antiretroviral therapies. AIDS 172169-2176. [DOI] [PubMed] [Google Scholar]
- 10.Harari, A., V. Dutoit, C. Cellerai, P. A. Bart, R. A. Du Pasquier, and G. Pantaleo. 2006. Functional signatures of protective antiviral T-cell immunity in human virus infections. Immunol. Rev. 211236-254. [DOI] [PubMed] [Google Scholar]
- 11.Harari, A., S. Petitpierre, F. Vallelian, and G. Pantaleo. 2004. Skewed representation of functionally distinct populations of virus-specific CD4 T cells in HIV-1-infected subjects with progressive disease: changes after antiretroviral therapy. Blood 103966-972. [DOI] [PubMed] [Google Scholar]
- 12.Haynes, B. F. 2006. Gut microbes out of control in HIV infection. Nat. Med. 121351-1352. [DOI] [PubMed] [Google Scholar]
- 13.Ibarrondo, F. J., P. A. Anton, M. Fuerst, H. L. Ng, J. T. Wong, J. Matud, J. Elliott, R. Shih, M. A. Hausner, C. Price, L. E. Hultin, P. M. Hultin, B. D. Jamieson, and O. O. Yang. 2005. Parallel human immunodeficiency virus type 1-specific CD8+ T-lymphocyte responses in blood and mucosa during chronic infection. J. Virol. 794289-4297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaul, R., F. A. Plummer, J. Kimani, T. Dong, P. Kiama, T. Rostron, E. Njagi, K. S. MacDonald, J. J. Bwayo, A. J. McMichael, and S. L. Rowland-Jones. 2000. HIV-1-specific mucosal CD8+ lymphocyte responses in the cervix of HIV-1-resistant prostitutes in Nairobi. J. Immunol. 1641602-1611. [DOI] [PubMed] [Google Scholar]
- 15.Kaul, R., P. Thottingal, J. Kimani, P. Kiama, C. W. Waigwa, J. J. Bwayo, F. A. Plummer, and S. L. Rowland-Jones. 2003. Quantitative ex vivo analysis of functional virus-specific CD8 T lymphocytes in the blood and genital tract of HIV-infected women. AIDS 171139-1144. [DOI] [PubMed] [Google Scholar]
- 16.Lawn, S. D., S. T. Butera, and T. M. Folks. 2001. Contribution of immune activation to the pathogenesis and transmission of human immunodeficiency virus type 1 infection. Clin. Microbiol. Rev. 14753-777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.LeGoff, J., H. A. Weiss, G. Gresenguet, K. Nzambi, E. Frost, R. J. Hayes, D. C. Mabey, J. E. Malkin, P. Mayaud, and L. Belec. 2007. Cervicovaginal HIV-1 and herpes simplex virus type 2 shedding during genital ulcer disease episodes. AIDS 211569-1578. [DOI] [PubMed] [Google Scholar]
- 18.Mazzoli, S., L. Lopalco, A. Salvi, D. Trabattoni, S. Lo Caputo, F. Semplici, M. Biasin, C. Bl, A. Cosma, C. Pastori, F. Meacci, F. Mazzotta, M. L. Villa, A. G. Siccardi, and M. Clerici. 1999. Human immunodeficiency virus (HIV)-specific IgA and HIV neutralizing activity in the serum of exposed seronegative partners of HIV-seropositive persons. J. Infect. Dis. 180871-875. [DOI] [PubMed] [Google Scholar]
- 19.McGowan, I., J. Elliott, M. Fuerst, P. Taing, J. Boscardin, M. Poles, and P. Anton. 2004. Increased HIV-1 mucosal replication is associated with generalized mucosal cytokine activation. J. Acquir. Immune Defic. Syndr. 371228-1236. [DOI] [PubMed] [Google Scholar]
- 20.McMichael, A. J., and S. L. Rowland-Jones. 2001. Cellular immune responses to HIV. Nature 410980-987. [DOI] [PubMed] [Google Scholar]
- 21.Musey, L., Y. Ding, J. Cao, J. Lee, C. Galloway, A. Yuen, K. R. Jerome, and M. J. McElrath. 2003. Ontogeny and specificities of mucosal and blood human immunodeficiency virus type 1-specific CD8+ cytotoxic T lymphocytes. J. Virol. 77291-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Musey, L., Y. Hu, L. Eckert, M. Christensen, T. Karchmer, and M. J. McElrath. 1997. HIV-1 induces cytotoxic T lymphocytes in the cervix of infected women. J. Exp. Med. 185293-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pantaleo, G., and R. A. Koup. 2004. Correlates of immune protection in HIV-1 infection: what we know, what we don't know, what we should know. Nat. Med. 10806-810. [DOI] [PubMed] [Google Scholar]
- 24.Passmore, J. A., V. C. Burch, E. G. Shephard, D. J. Marais, B. Allan, P. Kay, R. C. Rose, and A. L. Williamson. 2002. Single-cell cytokine analysis allows detection of cervical T-cell responses against human papillomavirus type 16 L1 in women infected with genital HPV. J. Med. Virol. 67234-240. [DOI] [PubMed] [Google Scholar]
- 25.Quayle, A. J., W. M. Coston, A. K. Trocha, S. A. Kalams, K. H. Mayer, and D. J. Anderson. 1998. Detection of HIV-1-specific CTLs in the semen of HIV-infected individuals. J. Immunol. 1614406-4410. [PubMed] [Google Scholar]
- 26.Quinn, T. C., M. J. Wawer, N. Sewankambo, D. Serwadda, C. Li, F. Wabwire-Mangen, M. O. Meehan, T. Lutalo, R. H. Gray, et al. 2000. Viral load and heterosexual transmission of human immunodeficiency virus type 1. N. Engl. J. Med. 342921-929. [DOI] [PubMed] [Google Scholar]
- 27.Rebbapragada, A., C. Wachihi, C. Pettengell, S. Sunderji, S. Huibner, W. Jaoko, B. Ball, K. Fowke, T. Mazzulli, F. A. Plummer, and R. Kaul. 2007. Negative mucosal synergy between herpes simplex type 2 and HIV in the female genital tract. AIDS 21589-598. [DOI] [PubMed] [Google Scholar]
- 28.Reka, S., M. L. Garro, and D. P. Kotler. 1994. Variation in the expression of human immunodeficiency virus RNA and cytokine mRNA in rectal mucosa during the progression of infection. Lymphokine Cytokine Res. 13391-398. [PubMed] [Google Scholar]
- 29.Sankaran, S., M. D. George, E. Reay, M. Guadalupe, J. Flamm, T. Prindiville, and S. Dandekar. 2008. Rapid onset of intestinal epithelial barrier dysfunction in primary HIV infection is driven by an imbalance between immune response and mucosal repair and regeneration. J. Virol. 82538-545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sha, B. E., M. R. Zariffard, Q. J. Wang, H. Y. Chen, J. Bremer, M. H. Cohen, and G. T. Spear. 2005. Female genital-tract HIV load correlates inversely with Lactobacillus species but positively with bacterial vaginosis and Mycoplasma hominis. J. Infect. Dis. 19125-32. [DOI] [PubMed] [Google Scholar]
- 31.Shacklett, B. L., T. J. Beadle, P. A. Pacheco, J. H. Grendell, P. A. Haslett, A. S. King, G. S. Ogg, P. M. Basuk, and D. F. Nixon. 2000. Characterization of HIV-1-specific cytotoxic T lymphocytes expressing the mucosal lymphocyte integrin CD103 in rectal and duodenal lymphoid tissue of HIV-1-infected subjects. Virology 270317-327. [DOI] [PubMed] [Google Scholar]
- 32.Shacklett, B. L., S. Cu-Uvin, T. J. Beadle, C. A. Pace, N. M. Fast, S. M. Donahue, A. M. Caliendo, T. P. Flanigan, C. C. Carpenter, and D. F. Nixon. 2000. Quantification of HIV-1-specific T-cell responses at the mucosal cervicovaginal surface. AIDS 141911-1915. [DOI] [PubMed] [Google Scholar]
- 33.Sheth, P. M., A. Danesh, A. Sheung, A. Rebbapragada, K. Shahabi, C. Kovacs, R. Halpenny, D. Tilley, T. Mazzulli, K. MacDonald, D. Kelvin, and R. Kaul. 2006. Disproportionately high semen shedding of HIV is associated with compartmentalized cytomegalovirus reactivation. J. Infect. Dis. 19345-48. [DOI] [PubMed] [Google Scholar]
- 34.Sheth, P. M., A. Danesh, K. Shahabi, A. Rebbapragada, C. Kovacs, R. Dimayuga, R. Halpenny, K. S. Macdonald, T. Mazzulli, D. Kelvin, M. Ostrowski, and R. Kaul. 2005. HIV-specific CD8+ lymphocytes in semen are not associated with reduced HIV shedding. J. Immunol. 1754789-4796. [DOI] [PubMed] [Google Scholar]
- 35.Reference deleted.
- 36.Speck, C. E., R. W. Coombs, L. A. Koutsky, J. Zeh, S. O. Ross, T. M. Hooton, A. C. Collier, L. Corey, A. Cent, J. Dragavon, W. Lee, E. J. Johnson, R. R. Sampoleo, and J. N. Krieger. 1999. Risk factors for HIV-1 shedding in semen. Am. J. Epidemiol. 150622-631. [DOI] [PubMed] [Google Scholar]
- 37.Tachet, A., E. Dulioust, D. Salmon, M. De Almeida, S. Rivalland, L. Finkielsztejn, I. Heard, P. Jouannet, D. Sicard, and C. Rouzioux. 1999. Detection and quantification of HIV-1 in semen: identification of a subpopulation of men at high potential risk of viral sexual transmission. AIDS 13823-831. [DOI] [PubMed] [Google Scholar]
- 38.UNAIDS. 2006. Report on the Global AIDS epidemic. http://www.unaids.org/en/HIV_data/2006GlobalReport/default.asp. UNAIDS, New York, NY.
- 39.White, H. D., L. K. Musey, M. M. Andrews, G. R. Yeaman, L. R. DeMars, P. D. Manganiello, A. L. Howell, C. R. Wira, W. R. Green, and M. J. McElrath. 2001. Human immunodeficiency virus-specific and CD3-redirected cytotoxic T lymphocyte activity in the human female reproductive tract: lack of correlation between mucosa and peripheral blood. J. Infect. Dis. 183977-983. [DOI] [PubMed] [Google Scholar]