Abstract
The role of clathrin light chain phosphorylation in regulating clathrin function has been examined in Saccharomyces cerevisiae. The phosphorylation state of yeast clathrin light chain (Clc1p) in vivo was monitored by [32P]phosphate labeling and immunoprecipitation. Clc1p was phosphorylated in growing cells and also hyperphosphorylated upon activation of the mating response signal transduction pathway. Mating pheromone-stimulated hyperphosphorylation of Clc1p was dependent on the mating response signal transduction pathway MAP kinase Fus3p. Both basal and stimulated phosphorylation occurred exclusively on serines. Mutagenesis of Clc1p was used to map major phosphorylation sites to serines 52 and 112, but conversion of all 14 serines in Clc1p to alanines [S(all)A] was necessary to eliminate phosphorylation. Cells expressing the S(all)A mutant Clc1p displayed no defects in Clc1p binding to clathrin heavy chain, clathrin trimer stability, sorting of a soluble vacuolar protein, or receptor-mediated endocytosis of mating pheromone. However, the trans-Golgi network membrane protein Kex2p was not optimally localized in mutant cells. Furthermore, pheromone treatment exacerbated the Kex2p localization defect and caused a corresponding defect in Kex2p-mediated maturation of the α-factor precursor. The results reveal a novel requirement for clathrin during the mating response and suggest that phosphorylation of the light chain subunit modulates the activity of clathrin at the trans-Golgi network.
INTRODUCTION
In eukaryotic cells, the assembly of clathrin coats on specific membrane organelles leads to the formation of vesicles that engage in selective intracellular protein transport (Schmid, 1997). At the plasma membrane clathrin participates in receptor-mediated endocytosis, whereas at the Golgi complex clathrin is involved in the sorting of newly synthesized lysosomal proteins to the lysosome and localization of trans-Golgi network (TGN) resident membrane proteins (Pearse and Robinson, 1990; Wilsbach and Payne, 1993a; Schmid, 1997). Clathrin coats comprise individual clathrin molecules, termed triskelions, that associate to form a polyhedral lattice (Kirchhausen and Harrison, 1981; Ungewickell and Branton, 1981). The clathrin triskelion itself is a protracted three-legged structure composed of three heavy chain subunits (HCs) of 180 kDa and three light chains (LCs) of 30 kDa (Schmid, 1997). The extended HC molecules constitute the main structural component of the coat lattice. In mammalian cells two forms of HC have been identified. The first is ubiquitously expressed and has been extensively characterized (Schmid, 1997). Recently, a second form has been discovered in humans that shows restricted tissue distribution limited primarily to skeletal muscle (Brodsky, 1997). In contrast, the yeast Saccharomyces cerevisiae contains only a single clathrin heavy chain gene (CHC1) encoding an HC that shares 50% amino acid identity with the mammalian ubiquitous HC (Payne and Schekman, 1985; Lemmon et al., 1991). There are two forms of LC in mammalian cells, LCa and LCb, which have an identity of 60% (Brodsky et al., 1991). Yeast has one gene (CLC1) encoding an LC, which, although only 18% identical to mammalian LCs, shares many properties with LCa and LCb such as size, acidic composition, acid and heat stability, and binding to calcium and calmodulin (Nathke et al., 1988; Silveira et al., 1990; our unpublished results).
The LC subunit has been proposed to regulate clathrin function based on several potential regulatory features, including phosphorylation, calcium binding, and interactions with calmodulin, the Hsp70-uncoating ATPase, and the HC trimerization domain (Brodsky et al., 1991; Pishvaee et al., 1997). In yeast, deletion of CLC1 (clc1Δ) causes defects in receptor-mediated endocytosis and localization of resident TGN membrane proteins (Chu et al., 1996; Huang et al., 1997). These defects are similar to those observed in chc1 mutant strains, consistent with the idea that the HC and LC subunits act in concert within the cell. The protein trafficking defects in clc1Δ cells can be attributed to the decreased stability of clathrin trimers, altered Chc1p membrane association, and loss of clathrin-coated vesiculation that occur in the absence of Clc1p (Chu et al., 1996; Huang et al., 1997). These results point to the critical role that Clc1p plays in clathrin trimer formation or stability and in trimer association with membranes. Interestingly, Clc1p also appears to have functions independent of Chc1p, because overexpression of Clc1p can suppress the growth defect caused by deletion of CHC1 in the appropriate strain background (Huang et al., 1997).
One possible mechanism for regulating clathrin function is through phosphorylation of LC. LC has been shown to be phosphorylated in vivo in rat liver, rat reticulocytes, and Chinese hamster ovary cells (Cantournet et al., 1987; Bar-Zvi et al., 1988; Corvera and Capocasale, 1990). In vitro, LCb can be phosphorylated by casein kinase II, a kinase that copurifies with coated vesicles (Schook and Puszkin, 1985; Usami et al., 1985; Bar-Zvi and Branton, 1986; Cantournet et al., 1987; Merrese et al., 1990). The sites of in vitro phosphorylation have been mapped to serines at positions 11 and 13 located within casein kinase II consensus recognition sequences (Hill et al., 1988). LCa is also phosphorylated, but to a lesser extent than LCb. LCa phosphorylation occurs on as yet unidentified serine residues (Wilde and Brodsky, 1996) and, in a regulated manner, on tyrosine residues (Mooibroek et al., 1992). Little data are available that address the function of LC phosphorylation. In rat reticulocytes, phosphorylated LC was detected in both assembled and soluble clathrin. However, the level of LC phosphorylation in the assembled fraction was slightly higher than in the soluble fraction, perhaps implicating phosphorylation in regulating the assembly state of clathrin (Bar-Zvi et al., 1988). In vitro, phosphorylated LCb has been reported to activate a phosphatase that acts on a 50-kDa coated vesicle protein, presumably a member of the clathrin adaptor complex, although the effect of this activation was not established (Hanson et al., 1990). LCa can be phosphorylated in response to epidermal growth factor (EGF) stimulation by the EGF receptor-associated tyrosine kinase (Mooibroek et al., 1992), but the consequences of this modification were not determined. Thus, the function of LC phosphorylation remains obscure.
We have applied biochemical and genetic approaches to investigate the effects of phosphorylation on LC function in yeast. Here we show that yeast Clc1p is constitutively phosphorylated in vivo at multiple serines scattered throughout the protein. Unexpectedly, activation of the mating response pathway results in Clc1p hyperphosphorylation. Mutagenesis of all 14 serine residues to eliminate phosphorylation resulted in no detectable differences in clathrin trimer stability, cell growth, receptor-mediated endocytosis, or vacuolar protein targeting. However, resident TGN protein localization was affected by these mutations, particularly in the presence of pheromone. These results suggest that Clc1p phosphorylation plays a modulatory role that becomes more important when pheromone activation of the mating response signal transduction pathway elicits the complex program of cellular changes that allow mating.
MATERIALS AND METHODS
Plasmids
Plasmid constructions were carried out using standard molecular biology techniques (Sambrook et al., 1989). Site-directed mutagenesis was done as described by Kunkel et al. (1987). All mutations were introduced onto the plasmid pBKSCLC. Sequential mutagenesis and subcloning were used to convert multiple serine to alanine residues to create the S(52,112)A and S(all)A clc1 mutants. pRSCLC05 wild-type and mutant versions were created by inserting a 1.5-kbp SmaI–SacI fragment containing wild-type or mutant CLC1 from pBKSCLC into pRS305 digested with XhoI–SacI.
Yeast Strains and Media
Yeast strains used in this study are listed in Table 1. GPY680, 681, and 682 were generated by transformation of YPH499, YDM200, and YDM600 (Ma et al., 1995) with the plasmid pJGsst1 (Reneke et al., 1988) digested with SalI and EcoRI to disrupt the SST1 gene. GPY915 and 916 were derived similarly from YDH6 and YDH8. GPY1034-19D was obtained as a meiotic progeny of the diploid formed by mating GPY74-15C and GPY986-2A. GPY1946, 1947, and 1949 were generated by transformation of GPY986-2A with pRSCLC05 linearized by digestion with PstI for wild-type CLC1 and the S(52,112)A clc1 mutant and with BstXI for the S(all)A clc1 mutant to integrate wild-type or mutant versions of the CLC1 gene at the LEU2 locus. To obtain these strains without pgalCLCURA3 (Chu et al., 1996), cells were selected by growth on media containing 5-fluoro-orotic acid. GPY1950, 1954, 1970, 1971, and 1973 were generated in the same way from GPY1034-19D.
Table 1.
Yeast strains used in this study
| Strain | Genotype | Source |
|---|---|---|
| GPY74-15C | MATa ura3-52 leu2-3,112 trp1-289 his4-519 sst1-3 | Rad et al., 1995 |
| GPY449 | MATα ura3-52 leu2-3,112 trp1 can1 pep4::LEU2 | Tan et al., 1993 |
| LSY89.2-4C | MATα ura3-52 leu2-3,112 trp1 clc1Δ::URA3 | L. Silveira |
| SM1581 | MATa ura3 leus his4 trp1 can1 pSM219 | S. Michaelis |
| FC279 | MATa ura3-52 leu2-3,112 trp1-289 his2 ace1 sst1::LEU2 | Chang and Herskowitz, 1990 |
| FC280 | MATa ura3-52 leu2-3,112 trp1-289 his2 ace1 sst1::LEU2 far1::URA3 | Chang and Herskowitz, 1990 |
| RD680 | MATa ura3-52 leu2-3,112 reg1-501 pep4-3 prd-1-1122 GAL+ gal-STE4::LEU2 | I. Herskowitz |
| YPH499 | MATa ura3-52 leu2-Δ1 trp1-Δ63 his3-Δ200 ade2-101 lys-801 | Sikorski and Hieter, 1989 |
| YDM200 | MATa ura3-52 leu2-Δ1 trp1-Δ63 his3-Δ200 ade2-101 lys2-801 fus3::LEU2 | D. Ma and J. Thorner |
| YDM600 | MATa ura3-52 leu2-Δ1 trp1-Δ63 his3-Δ200 ade2-101 lys2-801 kss1::hisg | D. Ma and J. Thorner |
| GPY680 | MATa ura3-52 leu2-Δ1 trp1-Δ63 his3-Δ200 ade2-101 lys2-801 sst1::LYS2 | This study |
| GPY681 | MATa ura3-52 leu2-Δ1 trp1-Δ63 his3-Δ200 ade2-101 lys2-801 fus3::LEU2 sst1::LYS2 | This study |
| GPY682 | MATa ura3-52 leu2-Δ1 trp1-Δ63 his3-Δ200 ade2-101och lys2-801 kss1::hisg sst1::LYS2 | This study |
| YDH6 | MATa ura3-52 leu2-Δ1trp1-Δ1 ade2-101 lys2-801 cka1-Δ1::HIS3 cka2-Δ1::TRP1 pCEN6/LEU2CKA2 | Hanna et al., 1995 |
| YDH8 | MATa ura3-52 leu2-Δ1trp1-Δ1 ade2-101 lys2-801 cka1-Δ1::HIS3 cka2-Δ1::TRP1 pCEN6/LEU2cka2-8 | Hanna et al., 1995 |
| GPY915 | MATa ura3-52 leu2-Δ1 trp1-Δ1 ade2-101 lys2-801 cka1-Δ1::HIS3 cka2-Δ1::TRP1 pCEN6/LEU2CKA2 sst1::URA3 | This study |
| GPY916 | MATa ura3-52 leu2-Δ 1trp1-Δ1 ade2-101 lys2-801 cka1-Δ1::HIS3 cka2-Δ1::TRP1 pCEN6/LEU2cka2-8 sst1::URA3 | This study |
| GPY986-2A | MATα ura3-52 leu2-3,112 trp1-Δ200 ade2-101 suc2-Δ9 clc1::HIS3 pgalCLCURA3 | Chu et al., 1996 |
| GPY1034-19D | MATa ura3-52 leu2-3,112 trp1 his4-519 and/or his3-Δ200 sst1-3 ade2-101 clc1Δ::HIS3 pgalCLCURA3 | This study |
| GPY1080 | MATa ura3-52 leu2-3,112 trp1 his4-519 and/or his3-Δ200 sst1-3 ade2-101 clc1Δ::HIS3 pRSCLC14 (TRP1) | This study |
| GPY1081 | MATa ura3-52 leu2-3,112 trp1 his4-519 and/or his3-Δ200 sst1-3 ade2-101 clc1Δ::HIS3 pgalCLCURA3 pRS314 | This study |
| GPY1118 | MATa leu2-3,112 ura3-52 his4-519 trp1 can1 pep4::LEU2 YEpCHCCLCURA3 | Chu et al., 1996 |
| GPY1946 | MATα ura3-52 leu2-3,112::pRSCLC05 trp1-Δ200 ade2-101 suc2-Δ9 clc1::HIS3 | This study |
| GPY1947 | MATα ura3-52 leu2-3,112PPpRSCLC05(S52,112A) trp1-Δ200 ade2-101 suc2-Δ9 clc1::HIS3 | This study |
| GPY1949 | MATα ura3-52 leu2-3,112::pRSCLC05(SallA) trp1-Δ200 ade2-101 suc2-Δ9 clc1::HIS3 | This study |
| GPY1954 | MATa ura3-52 leu2-3,11::pRSCLC14(S52T) trp1 his4-519 and/or his3-Δ200 sst1-3 ade2-101 clc1Δ::HIS3 | This study |
| GPY1955 | MATa ura3-52 leu2-3,112::pRSCLC14(S112T) trp1 his4-519 and/or his3Δ200 sst1-3 ade2-101 clc1Δ::HIS3 | This study |
| GPY1970 | MATa ura3-52 leu2-3,112::pRSCLC05 trp1 his4-519 and/or his3-Δ200 sst1-3 ade2-101 clc1Δ::HIS3 | This study |
| GPY1971 | MATa ura3-52 leu2-3,112::pRSCLC05(S52,112A) trp1 his4-519 and/or his3-Δ200 sst1-3 ade2-101 clc1Δ::HIS3 | This study |
| GPY1973 | MATa ura3-52 leu2-3,112::pRSCLC05(SallA) trp1 his4-519 and/or his3-Δ200 sst1-3 ade2-101 clc1Δ::HIS3 | This study |
Standard media preparation (Difco, Detroit, MI) and yeast growth were conducted as described by Sherman et al. (1974). DNA transformations were performed by the lithium acetate procedure (Ito et al., 1982; Gietz and Schiestl, 1995).
SD is 0.67% yeast nitrogen base without amino acids (Difco) and 2% dextrose. SDYE is SD supplemented with 0.2% yeast extract. SDCAA medium is SD containing 5 mg/ml vitamin assay casamino acid mix (Difco) with 15 μg/ml adenine and 20 μg/ml methionine, histidine, uracil, and tryptophan. SDCAA-ura is SDCAA without uracil. SDCAA-trp is SDCAA without tryptophan. YP medium is 1% bacto-yeast extract and 2% bactopeptone. YPD is YP with 2% dextrose. YP-phosphate was prepared according to the method of Rubin (1975). Cell densities in liquid culture were measured in a 1-cm plastic cuvette using a Beckman (Fullerton, CA) DU-62 spectrophotometer. One A500 unit is equivalent to 2.3 × 107 cells/ml.
Size Exclusion Chromatography, Coimmunoprecipitations, and Immunoblotting
Preparation of cellular lysates, size exclusion chromatography, and coimmunoprecipitation with Clc1p antibodies were carried out as previously described (Chu et al., 1996; Pishvaee et al., 1997). Immunoblotting was carried out according to the method of Burnette (1981) with secondary antibodies coupled to alkaline phosphatase (ALP; Bio-Rad, Richmond, CA). Antibodies were visualized using color development for ALP. Immunoblot signals were quantitated using a Molecular Dynamics (Sunnyvale, CA) densitometer.
Radiolabeling and Immunoprecipitations
In general for in vivo phosphate labeling, sst1Δ cells were grown in YP-phosphate plus 2% dextrose to midlogarithmic phase at 30°C. Cells were then incubated with or without 2.5 μM α-factor for 20 min at 30°C. Fifty microcuries of [32P]inorganic phosphate (Pi) were added per 1 × 107 cells for 20 min at 30°C. Cells were lysed in 2% SDS and 0.2-ml glass beads in the presence of 10 μM sodium orthovandate. For Gal-Ste4p experiments using the strain RD680, cells were shifted to 2% galactose-containing media for 1 h in lieu of α-factor addition before labeling. To test the role of casein kinase II in Clc1p phosphorylation, cells were grown on agar medium at 24°C and then resuspended in YP plus 1 M sorbitol. Unbudded cells were isolated by centrifugation for 3 min at 1000 × g and then washed in YPD. Cells were then shifted to 24°C for 90 min or 37°C for 60 min before treatment with or without 2.5 μM α-factor for 30 min. Labeling time was 30 min.
Immunoprecipitations were performed as described by Seeger and Payne (1992b) using affinity-purified polyclonal antisera against Clc1p, or Kss1p antibodies (a gift from J. Thorner, University of California, Berkeley, CA). Quantitation of 32P incorporated into Clc1p was accomplished using a Molecular Dynamics PhosphorImager. In all cases, the level of phosphorylation was normalized to protein recovery as measured by immunoblotting. Phosphoamino acid analysis was conducted as described by Boyle et al. (1991) and visualized using either autoradiography or a Molecular Dynamics PhosphorImager.
For metabolic labeling of α-factor, cells were grown to midlogarithmic phase in SDYE at 30°C. Cells were then resuspended in SDYE or preconditioned a-factor–containing medium for 1 h. Preconditioned medium was prepared by growing SM1581 in SDCAA-ura overnight at 30°C. SM1581 overexpresses a-factor from a multicopy plasmid carrying the MFA1 gene (pSM219; a gift from Susan Michaelis, Johns Hopkins University, Baltimore, MD). The medium was removed from cells, and 2% dextrose and 2% YE were added. Cells were labeled at 30°C. Labeling and immunoprecipitation of α-factor was performed as described by Seeger and Payne (1992b), except that the labeling period was 30 instead of 10 min.
Metabolic labeling of Kex2p was performed according to the method of Wilsbach and Payne (1993b), except that labeling time was 15 instead of 20 min. Kex2p and the various forms of α-factor were quantified using a Molecular Dynamics PhosphorImager.
Endocytosis assays were performed as described by Chu et al. (1996).
RESULTS
Clc1p Is Phosphorylated In Vivo and Hyperphosphorylated in the Presence of Mating Pheromone
To determine whether Clc1p is phosphorylated in vivo in yeast, incorporation of 32P-labeled Pi into Clc1p was analyzed. Label was added to cells grown to midlogarithmic phase in medium depleted of phosphate. Clc1p was immunoprecipitated from these cells, subjected to SDS-PAGE and immunoblotting, and then analyzed by phosphoimaging. Incorporation of [32P]phosphate into immunoprecipitated material indicated that Clc1p is phosphorylated in vivo (Figure 1A, lane 1). Two control experiments established the specificity of the immunoprecipitation. First, no signal was detected in clc1Δ cells, which harbor a disruption of the CLC1 gene (Figure 1A, lane 3). Second, overexpression of clathrin trimers by introduction of CLC1 and CHC1 genes together on a multicopy plasmid resulted in an increased signal (Figure 1A, lane 2). These data demonstrate that Clc1p is phosphorylated in growing cells.
Figure 1.
Phosphorylation and hyperphosphorylation of Clc1p in vivo. (A) Phosphorylation of Clc1p in vivo. Wild-type cells (GPY449, lane 1), wild-type cells overexpressing Clc1p and Chc1p (GPY1118, lane 2), and clc1Δ cells (LSY89.1, lane 3) were grown to midlogarithmic phase and labeled with [32P]Pi at 30°C for 1 h. Clc1p was immunoprecipitated, separated by SDS-PAGE, and analyzed by autoradiography. (B) Clc1p is hyperphosphorylated in the presence of mating pheromone. MATa sst1Δ cells (GPY74-15) were grown at 30°C for 30 min without (lane 1) or with (lane 2) 2.5 μM α-factor. Cells were labeled with [32P]Pi for 30 min, and Clc1p was immunoprecipitated, separated by SDS-PAGE, and transferred to nitrocellulose. After determination of radiolabel incorporation through phosphoimaging, protein content was determined by probing with polyclonal Clc1p antibodies and densitometry. Label incorporated was then normalized to protein content. Values obtained for α-factor–treated cells are divided by values obtained from untreated cells to determine “fold induction” values. (C) Clc1p hyperphosphorylation requires activation of the mating response pathway. Cells (RD680) harboring a galactose-inducible copy of Ste4p were incubated with 2% glucose (lane 1) or 2% galactose (lane 2) for 1 h before incubation with [32P]Pi for 30 min at 30°C. Clc1p was analyzed as described in B. (D) Clc1p hyperphosphorylation is not dependent on cell cycle arrest. Wild-type (WT; FC279, lanes 1 and 2) or far1Δ (FC279, lanes 3 and 4) cells were labeled, and Clc1p was analyzed as described in B.
We also observed that treatment of cells with the mating pheromone α-factor caused an increase in the amount of phosphate incorporated into Clc1p. For this experiment, cells were used that contain a disrupted version of the SST1 gene. SST1 encodes a secreted protease that degrades α-factor, so the sst1Δ disruption confers increased sensitivity to the pheromone (Chan and Otte, 1982; MacKay et al., 1988). When sst1Δ mating type a (MATa) cells were treated with α-factor for 30 min before a 30-min labeling period in the continued presence of pheromone, Clc1p phosphorylation increased up to fivefold over levels in untreated cells (Figure 1B). This hyperphosphorylation was evident as quickly as 5 min after pheromone addition and was sustained until pheromone was removed (our unpublished data). The magnitude of Clc1p hyperphosphorylation varied from experiment to experiment. We found that the genetic background of different strains and the specific biological activity of the commercial α-factor preparations contributed to the variability. Nevertheless, in every case, α-factor–treated cells incorporated more [32P]phosphate into Clc1p than untreated cells. Furthermore, similar increases in phosphorylation were also observed in MATα cells treated with a-factor–containing medium (our unpublished data).
The Mating Response Signal Transduction Pathway Is Responsible for Clc1p Hyperphosphorylation
Mating pheromones trigger a well-defined signal transduction pathway to elicit the mating response by binding to plasma membrane receptors that are members of the G protein–coupled receptor family (Sprague and Thorner, 1992). Pheromone binding to the receptors catalyzes nucleotide exchange on the heterotrimeric G protein α subunit (Gpa1p), causing release of the Gβγ subunit complex (consisting of Ste4p and Ste18p, respectively). Free βγ complexes then stimulate a kinase cascade that results in activation of a MAP kinase module. This mating response signal transduction pathway also can be activated in the absence of pheromone by overexpression of the Gβ subunit (Cole et al., 1990; Nomoto et al., 1990; Whiteway et al., 1990). It is thought that high levels of Gβ compete with endogenous βγ complexes for binding to α subunits, thereby increasing levels of free βγ to stimulate the pathway. To avoid variation caused by different preparations of α-factor and to obtain independent evidence that Clc1p is a target for the mating response kinase pathway, we examined Clc1p phosphorylation in a strain capable of overexpressing the Gβ subunit from a galactose-inducible copy of STE4. Cells carrying an integrated copy of STE4 under control of the GAL1 promoter were incubated for 60 min in galactose to induce expression or in glucose to maintain repression. After a 30-min labeling period with 32P, immunoprecipitation of Clc1p revealed hyperphosphorylation in galactose-treated cells (Figure 1C, lane 2) compared with the glucose-treated control (Figure 1C, lane 1). Together, the results shown in Figure 1 indicate that Clc1p is constitutively phosphorylated in growing cells and is hyperphosphorylated upon activation of the mating response pathway.
Cell Cycle Arrest Is Not Necessary for Clc1p Hyperphosphorylation
Pheromone triggering of the mating response pathway leads not only to morphological changes and transcriptional activation but also to arrest of cells in the G1 phase of the cell cycle (Sprague and Thorner, 1992). It was possible that the hyperphosphorylation of Clc1p could be a direct consequence of arrest in G1 and, therefore, a secondary effect of mating pathway activation. To test this possibility, Clc1p hyperphosphorylation was examined in a strain unable to undergo pheromone-induced cell cycle arrest because of disruption of the FAR1 gene. FAR1 encodes a cyclin-dependent kinase inhibitor that is necessary for G1 arrest in response to mating pheromones (Chang and Herskowitz, 1990; Peter et al., 1993). When treated with pheromone, cells carrying a FAR1 disruption (far1Δ) exhibit morphological changes and transcriptional activation but continue to proceed through the cell cycle (Chang and Herskowitz, 1990). Wild-type and congenic far1Δ cells were incubated with or without α-factor and labeled with 32P. As shown in Figure 1D, the absence of Far1p (Figure 1D, lanes 3 and 4) did not inhibit the increased phosphorylation of Clc1p stimulated by α-factor treatment. Therefore, cell cycle arrest is not required for pheromone-stimulated hyperphosphorylation of Clc1p, suggesting that hyperphosphorylation is directly dependent on the mating response pathway.
Clc1p Hyperphosphorylation Is Dependent on the Fus3p MAP Kinase
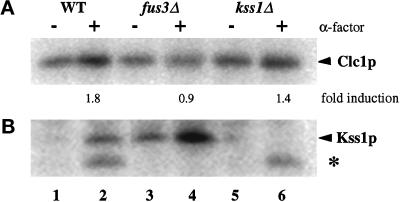
The mating response pathway propagates the pheromone-induced signal through two partially redundant MAP kinases, Fus3p and Kss1p (Sprague and Thorner, 1992). Cells lacking either kinase alone will respond to pheromone, but cells lacking both are unresponsive (Elion et al., 1991a,b). Cells carrying disruptions of either FUS3 and KSS1 were examined for Clc1p hyperphosphorylation. In the experiment shown in Figure 2, α-factor stimulated Clc1p phosphorylation by 1.8-fold in the congenic wild-type strain (Figure 2A, lanes 1 and 2). Because the MAP kinases are activated by phosphorylation, immunoprecipitates of Kss1p were analyzed in parallel. Indicative of mating response pathway activation, Kss1p displayed a strong increase in phosphorylation in the cells treated with α-factor (Figure 2B, lanes 1 and 2). A faster-migrating band of undetermined identity that correlates with the presence and size of Fus3p was also detected by our methods in the samples from the α-factor–treated cells precipitated with antibodies against Kss1p (Figure 2B, asterisk). This cross-reacting band also served as a useful control for pathway activation. Disruption of FUS3 completely eliminated pheromone-dependent Clc1p hyperphosphorylation (Figure 2A, lanes 3 and 4), although activation of the pathway was apparent from the increased level of Kss1p phosphorylation (Figure 3B, lanes 3 and 4). Disruption of KSS1 had only a moderate effect on the level of Clc1p hyperphosphorylation (Figure 2A, lanes 5 and 6). Qualitatively similar results were obtained in multiple experiments, although quantitative variations occurred in the level of pheromone-stimulated Clc1p hyperphosphorylation in wild-type and kss1Δ strains. Importantly, in each experiment Clc1p hyperphosphorylation was not observed in fus3Δ cells. These results demonstrate that Clc1p hyperphosphorylation is dependent on the Fus3p kinase and suggest that either Fus3p or a downstream kinase is responsible for modifying Clc1p in response to pheromone.
Figure 2.
Clc1p hyperphosphorylation depends on Fus3p. Congenic wild-type (GPY680, WT, lanes 1 and 2), fus3Δ (GPY681, lanes 3 and 4), or kss1Δ (GPY682, lanes 5 and 6) cells were grown to midlogarithmic phase, incubated with or without 2.5 μM α-factor for 30 min, and labeled with [32P]Pi for 30 min. (A) Immunoprecipitation of Clc1p. (B) Immunoprecipitation of Kss1p. Asterisk, cross-reacting Fus3-dependent phosphoprotein.
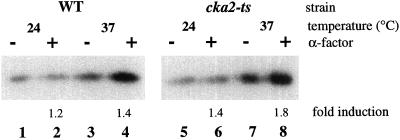
Figure 3.
Casein kinase II is not responsible for Clc1p phosphorylation. Clc1p phosphorylation in a cka1Δ cka2Δ strain carrying either a wild-type (GPY987, WT) or temperature-sensitive (GPY988, cka2-ts) copy of CKA2. Wild-type cells were grown at 24°C and then incubated at the permissive temperature (24°C) for 90 min or the nonpermissive temperature (37°C) for 60 min. Cells were then treated with or without 2.5 μM α-factor for 30 min and labeled with [32P]Pi for 30 min.
Casein Kinase II Is Not Responsible for Constitutive Phosphorylation of Clc1p
Casein kinase II associates with clathrin-coated vesicles in mammalian cells and can phosphorylate LCb in vitro (Schook and Puszkin, 1985; Bar-Zvi and Branton, 1986; Merrese et al., 1990). The availability of yeast strains with mutant forms of casein kinase II allowed us to investigate the role of this enzyme in the in vivo phosphorylation of Clc1p. Yeast casein kinase II is a complex of two catalytic subunits (α and α′) and two regulatory subunits (β and β′) encoded by the genes CKA1, CKA2, CKB1, and CKB2, respectively (Glover et al., 1994). Simultaneous disruption of both of the catalytic genes (cka1Δ cka2Δ) is lethal, whereas single disruptions have no deleterious effects (Chen-Wu et al., 1988; Padmanabha et al., 1990; Bidwai et al., 1995). Strains with conditional alleles of different subunits have been used to demonstrate that casein kinase II functions in cell cycle progression during both G1 and G2-M transitions, cell polarity, and ion homeostasis (Bidwai et al., 1995; Hanna et al., 1995; Rethinaswamy et al., 1998). To assess the role of casein kinase II in Clc1p phosphorylation, cka1Δ cka2Δ strains sustained by either wild-type (CKA2) or temperature-sensitive alleles of CKA2 (cka2-ts) were incubated at permissive (24°C) or nonpermissive (37°C) temperature for 60 min before a 30-min 32P-labeling period. Immunoprecipitation of Clc1p revealed no significant reduction in the amount of label incorporated into Clc1p in cka2-ts cells compared with cells with wild-type CKA2 at either temperature (Figure 3, odd-numbered lanes). Analysis of pheromone-stimulated Clc1p hyperphosphorylation in these strains was complicated by the relatively low level of hyperphosphorylation exhibited by the wild-type strain. However, no consistent effect of the temperature-sensitive mutation on pheromone-stimulated hyperphosphorylation was observed (Figure 3, even-numbered lanes). Although it is possible that casein kinase II may be a minor contributor to Clc1p phosphorylation, it does not appear to be the kinase responsible for the bulk of the modification.
Serines 52 and 112 Are Major Phosphorylation Sites of Clc1p
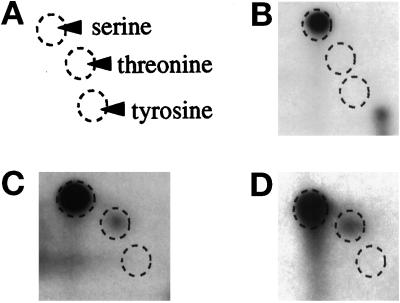
The sites of phosphorylation in Clc1p were characterized by subjecting in vivo–labeled Clc1p to phosphoamino acid analysis. Labeled Clc1p was immunoprecipitated and subjected to acid hydrolysis. Two-dimensional thin-layer cellulose electrophoresis of the hydrolysate showed that phosphorylation occurred solely on serine residues (Figure 4, A and B). There are 14 serine residues present in Clc1p (Figure 5). Because serine kinases will often also recognize threonine, a site-directed strategy to convert each serine to threonine was adopted (Kennelly, 1994). Phosphoamino acid analysis of each of the 14 individual S to T clc1 mutants detected phosphorylation of threonines only at positions 52 and 112 (Figure 4, C and D; our unpublished results). In these two mutants, the level of threonine phosphorylation relative to serine phosphorylation did not change in cells treated with α-factor (our unpublished results). This result suggests that the pheromone-stimulated Clc1p kinase(s) recognizes both serine and threonine. The absence of phosphorylation of threonine replacements at sites other than 52 and 112 may be accounted for by kinase(s) that show an especially strong predilection for serines over threonines in a specific context (Kemp et al., 1977; Tuazon and Traugh, 1991).
Figure 4.
Clc1p is phosphorylated on serines, primarily at residues 52 and 112. Cells expressing wild-type Clc1p (GPY1080, B), Clc1p S52T (GPY1954, C) or Clc1p S112T (GPY1955, D) were labeled as described in Figure 1A. Immunoprecipitated Clc1p was subjected to acid hydrolysis and two-dimensional thin-layer cellulose chromatography electrophoresis to separate labeled amino acids. (A) Positions of phosphoamino acid standards.
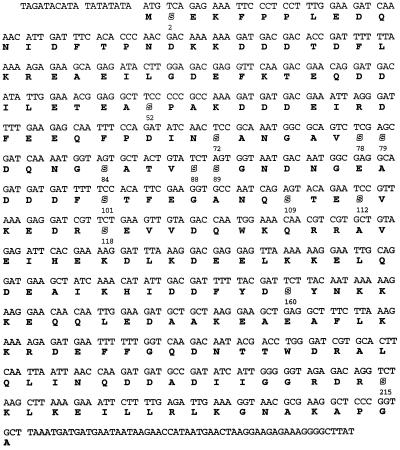
Figure 5.
Amino Acid sequence of Clc1p. Serine residues are shown in outline.
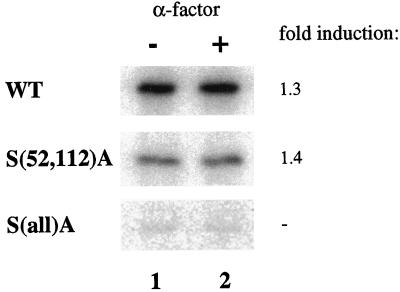
To further characterize phosphoacceptor sites, the 14 serine residues were also mutated to alanines. Single substitutions did not result in dramatic decreases in the amount of 32P incorporated into Clc1p, even when serine 52 or 112 was changed (our unpublished results). When both serines 52 and 112 were converted to alanine in combination, the level of Clc1p phosphorylation dropped to a level approximately one-third that of wild-type Clc1p, but the degree of hyperphosphorylation was not significantly affected [Figure 6, S(52A,112)A]. Combinations of the S(52,112)A mutations with various S to A changes at other sites revealed that Clc1p is phosphorylated to a small extent on many serine residues throughout the protein. Because of the large number of serine phosphoacceptor sites, and the slight changes in phosphorylation levels resulting from each mutation, we were unable to ascertain exact sites of phosphorylation. Mutagenesis of all serine residues to alanines was necessary to essentially eliminate phosphorylation of Clc1p [Figure 6, S(all)A]. Our results indicate that Clc1p is phosphorylated on multiple serines throughout the protein, with serines 52 and 112 constituting major sites of phosphorylation.
Figure 6.
S to A mutations in Clc1p reduce phosphorylation. Cells expressing wild-type Clc1p (GPY1970), Clc1p S(52,112)A (GPY1971), or Clc1p S(all)A (GPY1973) integrated at the LEU2 locus were analyzed as described in Figure 1B.
S(all)A Clc1p Associates with HC in Stable Clathrin Trimers
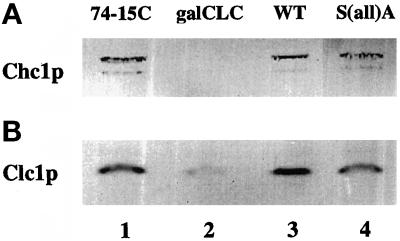
Because the S(52,112)A mutant retained a substantial level of phosphorylation, we focused on cells expressing the S(all)A mutant to investigate the structural and functional roles of phosphorylation. For this approach, a congenic set of mutant and wild-type strains was constructed. Each strain contained a disrupted version of the endogenous CLC1 gene and either a single copy of the S(all)A mutant or wild-type CLC1 integrated at the LEU2 locus (see MATERIALS AND METHODS). Because steady-state levels of clathrin heavy chain and the stability of clathrin trimers are dependent on Clc1p (Chu et al., 1996; Huang et al., 1997), we analyzed the levels of Clc1p and Chc1p by immunoblotting. Densitometric quantitation of multiple immunoblots demonstrated that steady-state levels of expression from the LEU2-integrated wild-type Clc1p are approximately twofold higher than from the endogenous CLC1 (an example is shown in Figure 7B, lane 1 vs. 3). The levels of Clc1p protein expressed from the LEU2-integrated S(all)A Clc1p were decreased by approximately twofold compared with that from the integrated wild-type CLC1 (Figure 7B, lane 3 vs. 4), resulting in levels comparable with those in cells with only the endogenous CLC1 (Figure 7B, lane 1 vs. 4). The cause of the differences in Clc1p levels is not clear; the decreased quantity of mutant Clc1p may be due to instability of the mutant protein or, alternatively, due to decreased synthesis caused by mutagenesis, perhaps from the presence of nonideal codons. It is unlikely that the differences detected by immunoblotting reflect differential antibody recognition of mutant and wild-type Clc1p antibodies, because bacterially expressed S(all)A Clc1p was detected by α-Clc1p antibodies as effectively as bacterially expressed wild-type Clc1p (our unpublished results).
Figure 7.
Steady-state levels of Chc1p in Clc1p mutants are not significantly altered. Protein extracts were prepared from GPY74-15C (which carries endogenous CLC1, lane 1) or GPY1034-19D (clc1Δ cells containing pgalCLCURA3, lane 2), GPY1970 (wild-type [WT] CLC1 integrated at the LEU2 locus, lane 3), and GPY1973 [S(all)A clc1 integrated at the LEU2 locus, lane 4]. Growth of GPY1034-19D cells in 2% glucose repressed Clc1p expression. Extracts were subjected to SDS-PAGE, transferred to nitrocellulose, and probed with either monoclonal antibodies to Chc1p or polyclonal antibodies to Clc1p. The lower band corresponds to a Chc1p degradation product.
Importantly, the level of Chc1p in mutant cells remained equal to those of wild-type strains, indicating that the slight fluctuation in Clc1p protein levels between strains has no deleterious effects on Chc1p stability (Figure 7A, lanes 1, 3, and 4). By comparison, the steady-state level of Chc1p was undetectable when Clc1p levels were dramatically reduced by glucose repression of cells expressing CLC1 under control of the GAL1 promoter (Figure 7, A and B, lane 2). In addition, when Clc1p was visualized by immunofluorescence, the only difference detected between wild-type and S(all)A strains was a slightly decreased signal for the S(all)A mutant (our unpublished results). Punctate distribution of Clc1p throughout the cytoplasm was consistent between strains in the absence or presence of pheromone.
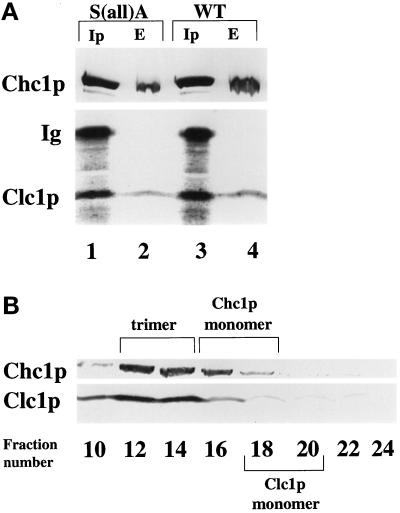
A coimmunoprecipitation protocol was used to examine the effects of the S(all)A mutations on Clc1p binding to Chc1p. In native conditions, antibodies specific for Clc1p will coprecipitate Chc1p (Pishvaee et al., 1997). However, in this assay, recovery of Chc1p is substoichiometric, because antibody binding to Clc1p appears to destabilize the Chc1p–Clc1p interactions. This destabilizing effect makes coprecipitation of Chc1p highly sensitive to mutations that perturb Clc1p binding to Chc1p; single amino acid changes in either Chc1p or Clc1p can abolish Chc1p coprecipitation (Pishvaee et al., 1997; our unpublished observations). Extracts of wild-type and S(all)A cells were incubated with Clc1p antibodies, and the resulting precipitates were analyzed by immunoblotting with Clc1p and Chc1p antibodies. As controls, the starting extracts were analyzed directly for levels of Chc1p and Clc1p (Figure 8A, lanes 2 and 4). No difference was evident in the levels of Chc1p coprecipitated with Clc1p from the two extracts (Figure 8A, lanes 1 and 3).
Figure 8.
S(all)A Clc1p associates with Chc1p in stable trimers. (A) Antibodies specific for Clc1p were used to coimmunoprecipitate Chc1p (Ip lanes) from extracts of S(all)A mutant cells (GPY1973, lane 1) or wild-type (WT) cells (GPY1970, lane 3). Total extracts (E lanes) representing ∼20% of the extract used for coimmunoprecipitation were analyzed directly in lanes 2 (GPY1973) and 4 (GPY1970). The band marked Ig corresponds to Ig heavy chain from the immunoprecipitates that reacts with the secondary antibody used in the immunoblot. (B) Sepharose CL4B column chromatography of extract from GPY1973 cells expressing S(all)A Clc1p. Extracts were prepared according to MATERIALS AND METHODS and fractionated by Sepharose CL4B column chromatography. Selected fractions were precipitated with 10% trichloroacetic acid and analyzed by immunoblotting with monoclonal antibodies to Chc1p or polyclonal antibodies to Clc1p. Column fraction numbers are indicated at the top of the panels. Wild-type clathrin displayed the same fractionation pattern.
Because Clc1p is necessary for the stability of clathrin trimers (Chu et al., 1996; Huang et al., 1997), we monitored the status of trimerization by size exclusion chromatography on Sepharose CL4B (Figure 8B). Both Chc1p and Clc1p in the S(all)A Clc1p strain coeluted in fractions in which wild-type clathrin trimers elute (Chu et al., 1996; Pishvaee et al., 1997). Thus mutagenesis of the 14 serines in Clc1p to alanines, and the concomitant loss of phosphorylation, does not overtly affect the association of Clc1p with Chc1p to form stable clathrin trimers.
A TGN Membrane Protein Localization Defect Caused by S(all)A Clc1p
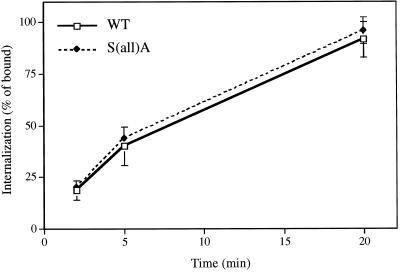
Clathrin-mediated transport processes were assessed in the S(all)A mutant cells to ascertain whether the absence of Clc1p phosphorylation affects clathrin function. Previous analyses of clc1Δ strains determined that Clc1p is required for efficient receptor-mediated endocytosis (Chu et al., 1996; Huang et al., 1997). To determine whether Clc1p phosphorylation affects this process, we monitored 35S-labeled α-factor internalization in S(all)A mutant and wild-type strains. No difference was apparent in the rate or extent of α-factor uptake over a 20-min time course in the two strains (Figure 9).
Figure 9.
Internalization of α-factor is normal in S(all)A Clc1p cells. Uptake of radiolabeled α-factor was measured as described in MATERIALS AND METHODS in congenic clc1Δ cells containing an integrated copy of wild-type (WT) CLC1 (GPY1970) or S(all)A clc1 (GPY1973). Each experiment was performed in duplicate, and the values at each time point were averaged. Data are the mean ± SD for three experiments.
Clathrin-mediated functions at the Golgi complex include sorting of vacuolar proteins to the vacuole and localization of resident TGN membrane proteins (Payne and Schekman, 1989; Seeger and Payne, 1992a,b). We found that the S(all)A Clc1p had no adverse effect on the sorting or delivery of carboxypeptidase Y, a vacuolar protein, to the vacuole (our unpublished results). Clathrin is also required for the efficient localization of TGN proteins such as the Kex2p endoprotease (Payne and Schekman, 1989; Seeger and Payne, 1992b). Kex2p initiates proteolytic maturation of the α-factor precursor as the precursor passes through the TGN (Fuller et al., 1988). In cells with compromised clathrin function, Kex2p is mislocalized to the cell surface and is less stable than in wild-type cells (Payne and Schekman, 1989; Seeger and Payne, 1992b; Redding et al., 1996b). The consequence of this mislocalization is a decrease in the efficiency of α-factor precursor maturation leading to secretion of the intact precursor. Thus, defects in Kex2p localization can be conveniently monitored by evaluating the form of α-factor that is secreted into the culture medium. After metabolic labeling with [35S]methionine, only mature α-factor was immunoprecipitated from the medium of wild-type and S(all)A mutant cells (Figure 10, lanes 1 and 2). Because pheromone treatment stimulates Clc1p hyperphosphorylation, we considered the possibility that phosphorylated Clc1p might be more important in cells undergoing a mating response. To test this idea, maturation of α-factor was investigated in cells treated with pheromone. Because α-factor is synthesized only in MATα cells, we treated S(all)A or wild-type MATα strains with preconditioned medium from an a-factor–overproducing strain. A 1-h pretreatment with a-factor–conditioned media resulted in a small but reproducible α-factor maturation defect in the S(all)A mutant (Figure 10, lanes 3 and 4).
Figure 10.
Maturation of α-factor precursor is incomplete in S(all)A Clc1p cells treated with pheromone. GPY1946 (WT CLC1, lanes 1 and 3) and GPY1949 [S(all)A clc1; lanes 2 and 4] were grown at 30°C, incubated without (lanes 1 and 2) or with (lanes 3 and 4) a-factor for 1 h, and metabolically labeled with [35S]cysteine/methionine for 30 min. α-Factor was immunoprecipitated from the culture supernatant and subjected to SDS-PAGE and phosphoimaging.
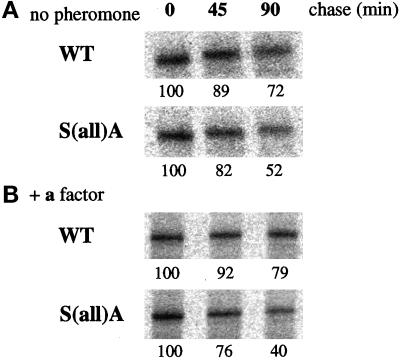
Based on the results from the α-factor maturation experiments, the status of Kex2p was investigated directly by measuring Kex2p stability with a pulse–chase immunoprecipitation protocol. Wild-type and S(all)A cells were pulse labeled with [35S]methionine/cysteine for 15 min and then subjected to a chase with unlabeled amino acids for 45 or 90 min. Kex2p stability was decreased in the S(all)A mutant cells; at the 90-min chase point the level of Kex2p in mutant cells declined to approximately half the starting level, whereas the level in wild-type cells only dropped to 70% (Figure 11A). When cells were treated with pheromone before the pulse–chase analysis, the stability of Kex2p in the mutant cells was slightly, but reproducibly, decreased even further (Figure 11B). Although small, the difference between Kex2p stability in mutant and wild-type cells and between mutant cells with or without pheromone treatment was observed in three separate experiments. These results suggest that mutation of Clc1p serines to alanines results in a subtle defect in TGN protein localization that is exacerbated in cells undergoing the mating response.
Figure 11.
Kex2p stability is decreased in the S(all)A Clc1p mutant. Congenic strains containing an integrated copy of wild-type (WT) CLC1 (GPY1946) or S(all)A clc1 (GPY1949) were grown at 30°C, incubated without (A) or with (B) a-factor, and metabolically labeled with [35S]cysteine/methionine for 15 min. Excess unlabeled amino acids were added, and an aliquot of cells was harvested from each sample at 0-, 45-, or 90-min intervals (chase). Cells were lysed, and Kex2p was immunoprecipitated and subjected to SDS-PAGE and phosphoimaging. Numbers below each lane correspond to the relative percentage of Kex2p remaining, normalized to the 0-min chase value.
DISCUSSION
Yeast clathrin light chain, like its mammalian counterparts, is a phosphoprotein. This finding has allowed a genetic approach to probe the functional significance of clathrin light chain phosphorylation in vivo. Our results indicate that Clc1p is constitutively phosphorylated in growing cells and undergoes hyperphosphorylation upon activation of the mating response MAP kinase signal transduction pathway. Elimination of Clc1p phosphorylation by mutagenesis of serine residues revealed only a modest defect in the clathrin-mediated localization of a TGN membrane protein. However, in cells expressing the phosphorylation-deficient Clc1p mutant, activation of the mating response pathway exacerbates the TGN membrane protein localization defect. These results suggest a role for Clc1p phosphorylation in modulating clathrin function at the TGN.
Both basal and pheromone-stimulated phosphorylation of Clc1p occurs exclusively on serine residues, with major sites mapped to serines at positions 52 and 112. Mutagenesis of S52 and S112 to alanines revealed that a substantial amount of both basal and stimulated phosphorylation, approximately one-third of wild-type levels, occurs at multiple other phosphoacceptor sites. Phenotypic analysis of S(52,112)A cells did not reveal defects in clathrin-dependent transport pathways, even in cells treated with pheromones (our unpublished observations). Thus, all 14 serine residues in Clc1p were converted to alanine to eliminate phosphorylation. Cells expressing this mutant exhibited a defect in localization of the TGN membrane protein Kex2p, suggesting a role for Clc1p phosphorylation in this process. However, the extent of this mutagenesis raises concern that any mutant phenotype could be due to a general perturbation of Clc1p structure by the 14 alanine residues rather than due to a specific effect on phosphate modification. This concern is allayed in part by the naturally resilient structure of wild-type Clc1p; both yeast and mammalian Clcs remain soluble and functional after incubation at 100°C (Silveira et al., 1990; Brodsky et al., 1991). Furthermore, the effect of the S(all)A mutations was relatively specific. Except for defective localization of Kex2p, the mutant Clc1p did not alter growth, receptor-mediated internalization, or vacuolar protein sorting. Additionally, the mutations did not affect Clc1p binding to heavy chain, clathrin trimer stability, light chain heat stability, or binding to calmodulin (Figures 8 and 9; our unpublished results). These properties argue that the mutations did not have a nonspecific, global effect on Clc1p structure and support our interpretation that the mutant phenotype can be attributed to an absence of Clc1p phosphorylation.
Mislocalization of Kex2p in cells expressing the S(all)A Clc1p provides an indication that Clc1p phosphorylation plays a role in clathrin-mediated localization of membrane proteins in the TGN. The prevailing model for TGN membrane protein localization in yeast suggests that the steady-state distribution of proteins to the TGN involves dynamic cycling between the TGN and a prevacuolar endosomal compartment (Wilsbach and Payne, 1993a; Nothwehr and Stevens, 1994). Clathrin has been proposed to act in this pathway at the TGN by collecting TGN membrane proteins into clathrin-coated vesicles targeted to the endosomes (Wilsbach and Payne, 1993a). In this scenario, phosphorylation of Clc1p could contribute to Kex2p localization in several ways. Phosphorylated Clc1p could slow the assembly of clathrin coats at the TGN, thereby increasing the residence time of Kex2p in the TGN. Phospho-Clc1p could also slow egress of Kex2p from the TGN by shifting the assembled state of clathrin from polyhedral cages into hexagonal planar coats. Planar coats could serve as a stable matrix to anchor Kex2p in the TGN. Alternatively, phospho-Clc1p could promote uncoating of TGN-derived, clathrin-coated vesicles to increase the rate of delivery to endosomes. Fractionation experiments to assess the relative distribution of phospho-Clc1p in soluble and assembled clathrin pools did not reveal significant enrichment in either fraction (our unpublished observations). Additional experiments will be necessary to distinguish between the various models for the role of Clc1p phosphorylation.
Pheromone-stimulated hyperphosphorylation of Clc1p revealed a possible link between modification of Clc1p and clathrin function during the mating response. Consistent with this possibility, the Kex2p localization defect in S(all)A mutant cells was enhanced by pheromone treatment. Although the decline in Kex2p stability caused by pheromone appears small, studies of localization-defective forms of Kex2p indicate that slight changes in Kex2p stability can have profound consequences on the mating process (Redding et al., 1996a). The enhanced localization defect in S(all)A cells treated with pheromones implies that the complex cellular changes that occur during mating place an increased demand on clathrin function at the TGN. The basis for this heightened requirement for clathrin is currently unclear. Possibilities include increased membrane flux through the TGN or recruitment of clathrin to other transport steps such as endocytosis.
One change known to occur upon activation of the mating response pathway is an increase in transcription of the genes encoding precursor α-factor (Sprague and Thorner, 1992). The resulting rise in levels of α-factor precursor traversing the TGN might challenge the processing capacity of Kex2p. Such a situation could underlie the α-factor maturation defect that accompanies the small decline in Kex2p stability in pheromone-treated S(all)A clc1 cells. This line of reasoning suggests one way in which functional requirements for clathrin could change during mating: to counter the increase in substrate, the level of Kex2p resident in the TGN could be increased through modulation of the clathrin-mediated localization mechanism.
Our results provide some insight into the kinase(s) responsible for basal or pheromone-stimulated Clc1p phosphorylation. From analysis of cells expressing a temperature-sensitive form of casein kinase II, we conclude that this kinase, which has been implicated in phosphorylation of mammalian LCb, does not contribute significantly to yeast Clc1p phosphorylation or hyperphosphorylation. Preliminary results also eliminate protein kinase C as a Clc1p kinase (our unpublished data). We have observed that disruption of the mating response pathway MAP kinase Fus3p essentially abolished pheromone-stimulated hyperphosphorylation. In contrast, disruption of the partly redundant MAP kinase Kss1p had lesser effects. These results are consistent with recent findings indicating that Fus3p is the MAP kinase dedicated to the mating response, whereas Kss1p principally is involved in the signal transduction pathway mediating invasive growth (Cook et al., 1997; Madhani et al., 1997; Tedford et al., 1997). The strong dependence of Clc1p hyperphosphorylation on Fus3p identifies Clc1p as a target of the mating branch of the MAP kinase cascade and a potentially direct substrate for Fus3p. However, preliminary results from in vitro kinase assays did not show Clc1p phosphorylation by Fus3p (Bardwell, Chu, Payne, and Thorner, unpublished results). Thus it is possible that an additional kinase (or kinases) acts as an intermediate between Fus3p and Clc1p. Given the rapidity of pheromone-stimulated Clc1p hyperphosphorylation, such a kinase is likely to be directly activated by the MAP kinase cascade rather than to require transcriptional induction through activation of the mating pathway transcription factor Ste12p. The same argument can be applied against the possibility that pheromone-dependent cross-activation of the cell integrity MAP kinase Mpk1p is responsible for Clc1p hyperphosphorylation, because cross-activation requires new transcription and protein synthesis (Buehrer and Errede, 1997). There is also recent evidence that the mating response pathway and the osmoregulatory MAP kinase pathway share a common MAP kinase kinase kinase, but cross-activation of the osmoregulatory pathway by pheromone does not occur, making it unlikely that osmoregulatory pathway kinases are involved in Clc1p modification (Posas and Saito, 1997). Thus, the kinase mediating pheromone-elicited Clc1p hyperphosphorylation awaits identification. Nonetheless, hyperphosphorylation of Clc1p represents a novel connection between the mating pathway MAP kinase pathway and membrane trafficking machinery.
Like yeast Clc1p, mammalian LCa and LCb are phosphorylated on serine residues (Usami et al., 1985; Hill et al., 1988; Wilde and Brodsky, 1996). Differential phosphorylation of the mammalian LCs has been proposed to provide a regulatory distinction between the two subunits (Brodsky et al., 1991). LCb is phosphorylated in vitro by a coated vesicle-associated casein kinase II-like activity on serines 11 and 13, whereas the phospho-acceptor sites on LCa have not been determined (Schook and Puszkin, 1985; Usami et al., 1985; Bar-Zvi and Branton, 1986; Cantournet et al., 1987; Hill et al., 1988). The absence of specific N-terminal phosphorylation sites in yeast Clc1p and the casein kinase II independence of Clc1p phosphorylation resemble the properties of LCa, which lacks serines 11 and 13 and is not a substrate for casein kinase II in vitro (Bar-Zvi and Branton, 1986). Another possible analogy between Clc1p and LCa, phosphorylation induced by extracellular stimuli, is provided by the observation that LCa, but not LCb, is subject to ligand-activated phosphorylation by the EGF receptor in vitro (Mooibroek et al., 1992). These similarities suggest that yeast Clc1p may reflect regulatory features of LCa more closely than LCb.
In mammalian cells, phosphorylation of other clathrin coat subunits has been observed (Keen and Black, 1986; Bar-Zvi et al., 1988; Corvera and Capocasale, 1990; Wilde and Brodsky, 1996). In particular, phosphorylation of the adaptor (AP) complexes has been studied. Adaptor complexes play a central role in coat assembly by mediating clathrin binding to membranes through a direct interaction between the AP β subunits and clathrin (Schmid, 1997). Phosphorylation of the AP β subunits inhibits clathrin binding, thus potentially promoting coat disassembly (Wilde and Brodsky, 1996). Together with our results, these findings argue that the multiple phosphorylation targets in clathrin coats provide an important regulatory framework for modulating clathrin-mediated protein traffic.
ACKNOWLEDGMENTS
We thank Matthias Peter, Ira Herskowitz, Lee Bardwell, Jean Cook McGowen, Jeremy Thorner, Susan Michaelis, and Claiborne Glover for strains and Steven Clarke and Leonard Rome for use of equipment. We are grateful to Jeremy Thorner, Lee Bardwell, Ray Deshaies, Matthias Peter, and Sandra Schmid for helpful discussions and to Elena Smirnova and Jim Howard for insightful comments on the manuscript. We also thank members of the Payne laboratory for assistance and input on this work. This work was supported by US Public Health Service National Research Award GM-07185 and a University of California Los Angeles dissertation year fellowship to D.C. and National Institutes of Health grant GM-39040 and American Heart Association grant 96006850 to G.P.
REFERENCES
- Bar-Zvi D, Branton D. Clathrin-coated vesicles contain two protein kinase activities. Phosphorylation of clathrin β-light chain by casein kinase II. J Biol Chem. 1986;261:9614–9621. [PubMed] [Google Scholar]
- Bar-Zvi D, Mosley ST, Branton D. In vivo phosphorylation of clathrin-coated vesicle proteins from rat reticulocytes. J Biol Chem. 1988;163:4408–4415. [PubMed] [Google Scholar]
- Bidwai AP, Reed JC, Glover CV. Cloning and disruption of CKB1, the gene encoding the 38-kDa β subunit of Saccharomyces cerevisiae casein kinase II (CKII). Deletion of CKII regulatory subunits elicits a salt-sensitive phenotype. J Biol Chem. 1995;270:10395–10404. doi: 10.1074/jbc.270.18.10395. [DOI] [PubMed] [Google Scholar]
- Boyle WJ, van der Geer P, Hunter T. Phosphopeptide mapping and phosphoamino acid analysis by two-dimensional separation on thin layer cellulose plates. Methods Enzymol. 1991;201:110–149. doi: 10.1016/0076-6879(91)01013-r. [DOI] [PubMed] [Google Scholar]
- Brodsky FM. New fashions in vesicle coats. Trends Cell Biol. 1997;7:175–179. doi: 10.1016/S0962-8924(97)01038-6. [DOI] [PubMed] [Google Scholar]
- Brodsky FM, Hill BL, Acton SL, Nathke I, Wong DH, Ponnambalam S, Parham P. Clathrin light chains: arrays of protein motifs that regulate coated-vesicle dynamics. Trends Biochem Sci. 1991;16:208–213. doi: 10.1016/0968-0004(91)90087-c. [DOI] [PubMed] [Google Scholar]
- Buehrer BM, Errede B. Coordination of the mating and cell integrity mitogen-activated protein kinase pathways in Saccharomyces cerevisiae. Mol Cell Biol. 1997;17:6517–6525. doi: 10.1128/mcb.17.11.6517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnette WN. “Western blotting”: electrophoretic transfer of proteins from SDS–polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981;112:195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Cantournet B, Creuzet C, Komano O, Loeb J. Clathrin β-light chain of rat liver coated vesicles is phosphorylated in vitro and in vivo. FEBS Lett. 1987;220:143–148. doi: 10.1016/0014-5793(87)80892-x. [DOI] [PubMed] [Google Scholar]
- Chan RK, Otte CA. Physiological characterization of Saccharomyces cerevisiae mutants supersensitive to G1 arrest by a factor and α factor pheromones. Mol Cell Biol. 1982;2:21–29. doi: 10.1128/mcb.2.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang F, Herskowitz I. Identification of a gene necessary for cell cycle arrest by a negative growth factor of yeast: FAR1 is an inhibitor of a G1 cyclin, CLN2. Cell. 1990;63:999–1011. doi: 10.1016/0092-8674(90)90503-7. [DOI] [PubMed] [Google Scholar]
- Chen-Wu JL, Padmanabha R, Glover CV. Isolation, sequencing, and disruption of the CKA1 gene encoding the α subunit of yeast casein kinase II. Mol Cell Biol. 1988;8:4981–4990. doi: 10.1128/mcb.8.11.4981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu DS, Pishvaee B, Payne GS. The light chain subunit is required for clathrin function in Saccharomyces cerevisiae. J Biol Chem. 1996;271:33123–33130. doi: 10.1074/jbc.271.51.33123. [DOI] [PubMed] [Google Scholar]
- Cole GM, Stone DE, Reed SI. Stoichiometry of G protein subunits affects the Saccharomyces cerevisiae mating pheromone signal transduction pathway. Mol Cell Biol. 1990;10:510–517. doi: 10.1128/mcb.10.2.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook JG, Bardwell L, Thorner J. Inhibitory and activating functions for MAPK Kss1 in the S. cerevisiae filamentous-growth signaling pathway. Nature. 1997;390:85–88. doi: 10.1038/36355. [DOI] [PubMed] [Google Scholar]
- Corvera S, Capocasale RJ. Enhanced phosphorylation of a coated vesicle polypeptide in response to insulin stimulation of rat adipocytes. J Biol Chem. 1990;265:15963–15969. [PubMed] [Google Scholar]
- Elion EA, Brill JA, Fink GR. Functional redundancy in the yeast cell cycle: FUS3 and KSS1 have both overlapping and unique functions. Cold Spring Harbor Symp Quant Biol. 1991a;56:41–49. doi: 10.1101/sqb.1991.056.01.007. [DOI] [PubMed] [Google Scholar]
- Elion EA, Brill JA, Fink GR. FUS3 represses CLN1 and CLN2 and in concert with KSS1 promotes signal transduction. Proc Natl Acad Sci USA. 1991b;88:9392–9396. doi: 10.1073/pnas.88.21.9392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller RS, Sterne RE, Thorner J. Enzymes required for yeast prohormone processing. Annu Rev Physiol. 1988;50:345–362. doi: 10.1146/annurev.ph.50.030188.002021. [DOI] [PubMed] [Google Scholar]
- Gietz RD, Schiestl RH. Transforming Yeast with DNA. Methods Mol Cell Biol. 1995;5:255–269. [Google Scholar]
- Glover CV, Bidwai AP, Reed JC. Structure and function of Saccharomyces cerevisiae casein kinase II. Cell & Mol Biol Res. 1994;40:481–488. [PubMed] [Google Scholar]
- Hanna DE, Rethinaswamy A, Glover CV. Casein kinase II is required for cell cycle progression during G1 and G2/M in Saccharomyces cerevisiae. J Biol Chem. 1995;270:25905–25914. doi: 10.1074/jbc.270.43.25905. [DOI] [PubMed] [Google Scholar]
- Hanson VG, Schook WJ, Puszkin S. Novel regulatory role of phosphorylated clathrin light chain β in bovine brain coated vesicles. J Neurochem. 1990;54:46–50. doi: 10.1111/j.1471-4159.1990.tb13281.x. [DOI] [PubMed] [Google Scholar]
- Hill BL, Drickamer K, Brodsky FM. Identification of the phosphorylation sites of clathrin light chain LCb. J Biol Chem. 1988;263:5499–5501. [PubMed] [Google Scholar]
- Huang KM, Gullberg L, Nelson KK, Stefan CJ, Blumer K, Lemmon SK. Novel functions of clathrin light chains: clathrin heavy chain trimerization is defective in light chain-deficient yeast. J Cell Sci. 1997;110:899–910. doi: 10.1242/jcs.110.7.899. [DOI] [PubMed] [Google Scholar]
- Ito H, Fukada Y, Murata K, Kimura A. Transformation of intact yeast cells with alkali cations. J Bacteriol. 1982;153:163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keen JH, Black MM. The phosphorylation of coated membrane proteins in intact neurons. J Cell Biol. 1986;102:1325–1333. doi: 10.1083/jcb.102.4.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp BE, Graves DJ, Benjamini E, Drebs EG. Role of multiple basic residues in determining the substrate specificity of cyclic AMP-dependent protein kinases. J Biol Chem. 1977;252:4888–4894. [PubMed] [Google Scholar]
- Kennelly PJ. Identification of sites of serine and threonine phosphorylation via site-directed mutagenesis—site transformation versus site elimination. Anal Biochem. 1994;219:384–386. doi: 10.1006/abio.1994.1285. [DOI] [PubMed] [Google Scholar]
- Kirchhausen T, Harrison SC. Protein organization in clathrin trimers. Cell. 1981;23:755–761. doi: 10.1016/0092-8674(81)90439-6. [DOI] [PubMed] [Google Scholar]
- Kunkel TA, Roberts JD, Zakour RA. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Lemmon SK, Pellicena-Palle A, Conley K, Freund CL. Sequence of clathrin heavy chain from Saccharomyces cerevisae and requirement of the COOH terminus for clathrin function. J Cell Biol. 1991;112:65–80. doi: 10.1083/jcb.112.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma D, Cook JG, Thorner J. Phosphorylation and localization of Kss1, a MAP kinase of the Saccharomyces cerevisiae pheromone response pathway. Mol Biol Cell. 1995;6:889–909. doi: 10.1091/mbc.6.7.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKay VL, Welch SK, Insley MY, Manney TR, Holly J, Saari GC, Parker ML. The Saccharomyces cerevisiae BAR1 gene encodes an exported protein with homology to pepsin. Proc Natl Acad Sci USA. 1988;85:55–59. doi: 10.1073/pnas.85.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhani HD, Styles CA, Fink GR. MAP kinases with distinct inhibitory functions impart signaling specificity during yeast differentiation. Cell. 1997;91:673–684. doi: 10.1016/s0092-8674(00)80454-7. [DOI] [PubMed] [Google Scholar]
- Merrese S, Ludwig T, Frank R, Hoflack B. Phosphorylation of the cytoplasmic domain of the bovine cation-independent mannose 6-phosphate receptor. J Biol Chem. 1990;265:18833–18842. [PubMed] [Google Scholar]
- Mooibroek MJ, Michiel DF, Wang JH. Differential in vitro phosphorylation of clathrin light chains by the epidermal growth factor receptor-associated protein tyrosine kinase and a pp60c-src-related spleen tyrosine kinase. Arch Biochem Biophys. 1992;292:448–455. doi: 10.1016/0003-9861(92)90015-o. [DOI] [PubMed] [Google Scholar]
- Nathke I, Parham P, Brodsky FM. The calcium binding site of clathrin light chains. J Biol Chem. 1988;265:18621–18627. [PubMed] [Google Scholar]
- Nomoto S, Nakayama N, Arai K, Matsumoto K. Regulation of the yeast pheromone response pathway by G protein subunits. EMBO J. 1990;9:691–696. doi: 10.1002/j.1460-2075.1990.tb08161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nothwehr SF, Stevens TH. Sorting of membrane proteins in the yeast secretory pathway. J Biol Chem. 1994;269:10185–10188. [PubMed] [Google Scholar]
- Padmanabha R, Chen-Wu JL, Hanna DE, Glover CV. Isolation, sequencing, and disruption of the yeast CKA2 gene: casein kinase II is essential for viability in Saccharomyces cerevisiae. Mol Cell Biol. 1990;10:4089–4099. doi: 10.1128/mcb.10.8.4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne G, Schekman R. A test of clathrin function in protein secretion and cell growth. Science. 1985;230:1009–1014. doi: 10.1126/science.2865811. [DOI] [PubMed] [Google Scholar]
- Payne GS, Schekman R. Clathrin: a role in the intracellular retention of a Golgi membrane protein. Science. 1989;245:1358–1365. doi: 10.1126/science.2675311. [DOI] [PubMed] [Google Scholar]
- Pearse BMF, Robinson MS. Clathrin, adaptors, and sorting. Annu Rev Cell Biol. 1990;6:151–171. doi: 10.1146/annurev.cb.06.110190.001055. [DOI] [PubMed] [Google Scholar]
- Peter M, Gartner A, Horecka J, Ammerer G, Herskowitz I. FAR1 links the signal transduction pathway to the cell cycle machinery in yeast. Cell. 1993;73:747–760. doi: 10.1016/0092-8674(93)90254-n. [DOI] [PubMed] [Google Scholar]
- Pishvaee B, Munn A, Payne GS. A novel structural model for regulation of clathrin function. EMBO J. 1997;16:2227–2239. doi: 10.1093/emboj/16.9.2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posas F, Saito H. Osmotic activation of the HOG MAPK pathway via Ste11p MAPKKK: scaffold role of Pbs2p MAPKK. Science. 1997;276:1702–1705. doi: 10.1126/science.276.5319.1702. [DOI] [PubMed] [Google Scholar]
- Redding K, Brickner J, Marschall LG, Nichols JW, Fuller RS. Allele-specific suppression of a defective trans-Golgi network (TGN) localization signal in Kex2p identifies three genes involved in localization of TGN transmembrane proteins. Mol Cell Biol. 1996a;16:6206–6217. doi: 10.1128/mcb.16.11.6208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redding K, Seeger M, Payne G, Fuller RS. The effects of clathrin inactivation on localization of Kex2 protease are independent of the TGN localization signal in the cytosolic tail of Kex2p. Mol Biol Cell. 1996b;7:1667–1677. doi: 10.1091/mbc.7.11.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reneke JE, Blumer KJ, Courchesne WE, Thorner J. The carboxy-terminal segment of the yeast α-factor receptor is a regulatory domain. Cell. 1988;55:221–234. doi: 10.1016/0092-8674(88)90045-1. [DOI] [PubMed] [Google Scholar]
- Rethinaswamy A, Birnbaum MJ, Glover CV. Temperature-sensitive mutations of the CKA1 gene reveal a role for casein kinase II in maintenance of cell polarity in Saccharomyces cerevisiae. J Biol Chem. 1998;273:5869–5877. doi: 10.1074/jbc.273.10.5869. [DOI] [PubMed] [Google Scholar]
- Rubin GM. Preparation of RNA and ribosomes from yeast. Methods Cell Biol. 1975;12:45–64. doi: 10.1016/s0091-679x(08)60951-6. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- Schmid SL. Clathrin-coated vesicle formation and protein sorting: an integrated process. Annu Rev Biochem. 1997;66:511–548. doi: 10.1146/annurev.biochem.66.1.511. [DOI] [PubMed] [Google Scholar]
- Schook WJ, Puszkin S. Brain clathrin light chain 2 can be phosphorylated by a coated vesicle kinase. Proc Natl Acad Sci USA. 1985;82:8039–8043. doi: 10.1073/pnas.82.23.8039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeger M, Payne GS. A role for clathrin in the sorting of vacuolar proteins in the Golgi complex of yeast. EMBO J. 1992a;11:2811–2818. doi: 10.1002/j.1460-2075.1992.tb05348.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeger M, Payne GS. Selective and immediate effects of clathrin heavy chain mutations on Golgi membrane protein retention in Saccharomyces cerevisiae. J Cell Biol. 1992b;118:531–540. doi: 10.1083/jcb.118.3.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman F, Fink G, Lawrence C. Methods in Yeast Genetics. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; 1974. [Google Scholar]
- Sikorski RS, Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989;122:19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silveira LA, Wong DH, Masiarz FR, Schekman R. Yeast clathrin has a distinctive light chain that is important for cell growth. J Cell Biol. 1990;111:1437–1449. doi: 10.1083/jcb.111.4.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprague GF, Thorner JW. Pheromone response and signal transduction during the mating process of Saccharomyces cerevisiae. In: Jones EW, Pringle JR, Broach JR, editors. The Molecular and Cellular Biology of the Yeast Saccharomyces. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; 1992. pp. 657–774. [Google Scholar]
- Tedford K, Kim S, Sa D, Stevens K, Tyers M. Regulation of the mating pheromone and invasive growth responses in yeast by two MAP kinase substrates. Curr Biol. 1997;7:228–238. doi: 10.1016/s0960-9822(06)00118-7. [DOI] [PubMed] [Google Scholar]
- Tuazon PT, Traugh JA. Casein kinase I and II-multiple serine protein kinases: structure, function, and regulation. Adv Second Messenger Phoshoprotein Res. 1991;23:123–164. [PubMed] [Google Scholar]
- Ungewickell E, Branton D. Assembly units of clathrin coats. Nature. 1981;289:420–422. doi: 10.1038/289420a0. [DOI] [PubMed] [Google Scholar]
- Usami M, Takahashi A, Kadota T, Katoda K. Phosphorylation of a clathrin light chain of coated vesicles in the presence of histones. J Biochem. 1985;97:1819–1822. doi: 10.1093/oxfordjournals.jbchem.a135243. [DOI] [PubMed] [Google Scholar]
- Whiteway M, Hougan L, Thomas DY. Overexpression of the STE4 gene leads to mating response in haploid Saccharomyces cerevisiae. Mol Cell Biol. 1990;10:217–222. doi: 10.1128/mcb.10.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilde A, Brodsky FM. In vivo phosphorylation of adaptors regulates their interaction with clathrin. J Cell Biol. 1996;135:635–645. doi: 10.1083/jcb.135.3.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilsbach K, Payne GS. Dynamic retention of TGN proteins in Saccharomyces cerevisiae. Trends Cell Biol. 1993a;3:426–431. doi: 10.1016/0962-8924(93)90031-u. [DOI] [PubMed] [Google Scholar]
- Wilsbach K, Payne GS. Vps1p, a member of the dynamin GTPase family, is necessary for Golgi membrane protein retention in S. cerevisiae. EMBO J. 1993b;8:3049–3059. doi: 10.1002/j.1460-2075.1993.tb05974.x. [DOI] [PMC free article] [PubMed] [Google Scholar]