Abstract
Glutamate is a critical neurotransmitter of the central nervous system (CNS) and also an important regulator of cell survival and proliferation. The binding of glutamate to metabotropic glutamate receptors induces signal transduction cascades that lead to gene-specific transcription. The transcription factor NF-κB, which regulates cell proliferation and survival, is activated by glutamate; however, the glutamate receptor-induced signaling pathways that lead to this activation are not clearly defined. Here we investigate the glutamate-induced activation of NF-κB in glial cells of the CNS, including primary astrocytes. We show that glutamate induces phosphorylation, nuclear accumulation, DNA binding, and transcriptional activation function of glial p65. The glutamate-induced activation of NF-κB requires calcium-dependent IκB kinase α (IKKα) and IKKβ activation and induces p65-IκBα dissociation in the absence of IκBα phosphorylation or degradation. Moreover, glutamate-induced IKK preferentially targets the phosphorylation of p65 but not IκBα. Finally, we show that the ability of glutamate to activate NF-κB requires cross-coupled signaling with the epidermal growth factor receptor. Our results provide insight into a glutamate-induced regulatory pathway distinct from that described for cytokine-induced NF-κB activation and have important implications with regard to both normal glial cell physiology and pathogenesis.
Glutamate, the major excitatory neurotransmitter of the central nervous system (CNS), regulates many physiological functions in the nervous system, including cell proliferation, differentiation, and survival (2, 16, 20). Glutamate activates neuronal and glial cells through ionotropic and metabotropic glutamate receptors (mGluRs) (13, 30). In neurons, glutamate is an essential signaling component regulating synaptic plasticity and other physiological processes (24). Glutamate-induced Ca2+ elevation is also believed to promote excitotoxic neuronal cell death associated with brain injury and disease (15). Recently, particular attention has been paid to the role of mGluRs in regulating glial cell functions such as proliferation, glutamate uptake, and modulation of neuronal activity (10, 35, 38).
mGluR5 is one of the most abundant and best-characterized glutamate receptors in glial cells. The stimulation of mGluR5 has been shown to promote cytosolic Ca2+ release, glutamate transporter 1 (GLT-1)/excitatory amino acid transporter 2 (EAAT2)-mediated glutamate uptake (37), immediate-early gene expression (6), and glial cell proliferation and survival (10). Additionally, mGluR5 expression is rapidly upregulated by treatment with growth factors and in response to injury (36). The signaling cascade initiated by mGluR stimulation has been recently investigated. In one study, it was shown that mGluR5 stimulation leads to the activation of extracellular signal-regulated kinase 1/2 and the epidermal growth factor receptor (EGFR) in astrocytes (23). Similarly to mGluR5, EGFR signaling promotes Ca2+ oscillation in astrocytes (22), and the EGFR is upregulated in astrocytes in response to injury (14). Interestingly, it was recently demonstrated that in the hippocampus, mGluR5 activates NF-κB, an evolutionarily conserved transcription factor that has critical roles in regulating cell survival, proliferation, and immune/inflammatory responses (8).
The mammalian NF-κB family consists of five members: RelA (RelA/p65), RelB, c-Rel, NF-κB1 (p105/p50), and NF-κB2 (p100/p52). These homo- and heterodimeric proteins share a conserved N-terminal Rel homology domain harboring motifs that mediate nuclear localization, DNA binding, and interaction with inhibitory IκB proteins in the cytoplasm. In the classical pathway of NF-κB activation, the signal-dependent phosphorylation of IκBα on Ser32/Ser36 by the IKK complex triggers IκBα polyubiquitination and degradation. NF-κB subsequently translocates to the nucleus and regulates the transcription of target genes (1). The IKK complex is comprised of two catalytic subunits, IKKα and IKKβ, as well as the regulatory subunit, NEMO/IKKγ (11). In addition to IκBα, IKK phosphorylates other proteins in the NF-κB signaling pathway, including p65 (28). However, IKK has not been demonstrated to preferentially phosphorylate distinct substrates in response to specific stimuli.
NF-κB is widely expressed throughout the CNS where it is involved in neuroinflammatory responses, as well as CNS-specific functions such as synaptic plasticity, long-term memory, and neurodegeneration (19, 21). NF-κB is activated by a wide variety of CNS-specific stimuli, including neurotrophins and neurotransmitters. In this regard, glutamate has been shown to activate NF-κB in neurons (9), and there are a few reports describing the glutamate-induced regulation of NF-κB in primary astrocytes (4, 5). However, the signaling pathways that regulate NF-κB in specific cells of the CNS are not well defined. We previously reported that NF-κB can regulate the expression of the glutamate transporter EAAT2/GLT-1 in glial cells (33), and we hypothesized that NF-κB-mediated regulation of EAAT2 may be part of a regulatory feedback mechanism in response to extracellular glutamate signaling.
In this study, we investigated the mechanisms whereby glutamate activates NF-κB activity in glial cells. We demonstrate that in glial cells of the CNS, glutamate activates NF-κB through mGluR5 in an IKKα/β-dependent manner that induces p65-IκBα dissociation but is not associated with IκBα phosphorylation or degradation. Notably, our data demonstrate that glutamate-induced IKK preferentially targets the phosphorylation of p65 but not IκBα in vitro. Our results also show that glutamate regulation of NF-κB requires calcium and cross-coupled signaling with the EGFR. These findings highlight the diversity of signaling pathways that modulate NF-κB activity and underscore the importance of cross talk among different classes of receptors to control gene expression.
MATERIALS AND METHODS
Cells and reagents.
H4 cells were obtained from ATCC and grown in Dulbecco's modified Eagle's medium plus 10% fetal bovine serum (FBS). U87-MG cells were obtained from ATCC and grown in minimal essential medium plus 10% FBS, nonessential amino acids, and Na-pyruvate. Primary mouse astrocytes were dissected from 3- to 5-day-old cortices and stained for immunoreactivity to glial fibrillary acidic protein (data not shown). Brain pieces were digested in 0.05% trypsin-EDTA (Gibco-BRL) at 37°C for 30 min, followed by repeated trituration to form single-cell suspensions. The cells were plated in Dulbecco's modified Eagle's medium plus 10% FBS (Gibco-BRL). The cells were highly enriched for glial fibrillary acidic protein immunostaining (data not shown). l-Glutamic acid and (RS)-2-chloro-5-hydroxyphenylglycine (CHPG) were purchased from Tocris Neurochemicals. Cultures were switched to 0.5% serum for 14 to 24 h before treatments with glutamate, CHPG, EGF, or tumor necrosis factor alpha (TNF-α). BAPTA-AM and MG-132 were used at final concentrations of 10 μM and 1 μM, respectively. Lapatinib and gefitinib were gifts from H. S. Earp (UNC-Chapel Hill).
EMSAs and ChIP assays.
Electromobility shift assays (EMSAs) were performed as previously described (18). Briefly, 4 to 5 μg of nuclear extracts were prepared following cell stimulation and incubated with a radiolabeled DNA probe containing an NF-κB consensus site from the κ light-chain enhancer (Promega) or the EAAT2 promoter (33). For supershifts, 1 μl of anti-p65 antibody (SC-109; Santa Cruz) or 2 μl of anti-p50 antibody (SC-7178; Santa Cruz) was added to the binding reaction. Protein-DNA complexes were resolved on a nondenaturing polyacrylamide gel and visualized by autoradiography. Chromatin immunoprecipitation (ChIP) analysis was performed as previously described (33) with some modifications. Briefly, protein-DNA complexes were cross-linked with formaldehyde, and the cells were harvested and then either snapfrozen in dry ice/ethanol and stored at −80°C or immediately used. DNA from the cell lysates was sonicated to lengths of 500 to 1,000 bp, and 5 μg of a p65 antibody (SC-109; Santa Cruz) was used for immunoprecipitation overnight at 4°C. Primers (5′-ATGTCAGCTCTCGACGAAAATAGA-3′ [forward] and 5′-GGAGGGATTGCAAGGTTTAGC-3′ [reverse]) to amplify the EAAT2 −583 promoter region were designed using PrimerExpress (ABI).
Transient transfection reporter assay.
Cells were seeded in 24-well plates at 1 × 104 cells/well and transfected with Fugene (Roche) according to the manufacturer's protocol. A total of 500 ng of DNA was used for each well: 100 ng of EAAT2-luciferase reporter and 50 ng of pRL-TK (renilla reporter control) DNA were used for transfection. Medium containing 0.5% FBS and EGF (100 ng/ml), TNF-α (20 ng/ml), or glutamate (400 μM) was added to the wells for the next 24 to 48 h. The cells were harvested after 24 to 48 h of treatment. Luciferase assays were performed using a dual luciferase assay system (Promega), and activity was measured using an Lmax luminometer (Molecular Devices). All transfections were performed in triplicate.
Immunoprecipitation, Western blot analysis, and kinase assays.
The following antibodies were used for immunoprecipitation/Western blot analysis: phospho-Ser536 (CST#3031; Cell Signaling Technologies), total p65 (SC-8008; Santa Cruz), phospho-IKKα/β-Ser180/181 (CST#2681), total IKKα (Upstate 05-536), total IKKβ (Upstate 05-535), phospho-IκBα-Ser32/36 (CST#9246), total IκBα (SC-371), phospho-AKT (CST#9271), and total AKT (CST#9272). To detect phosphorylated EGFR, the total EGFR was immunoprecipitated with CST#2232. Immunoprecipitated lysates were subject to Western blot analysis with different phospho-EGFR antibodies: Y1173 (CST#4407) and Y845 (Upstate 07-820). Secondary immunoglobulin G (IgG)-horseradish peroxidase antibodies were obtained from Promega. For the Western blot analysis, 10 to 30 μg of protein was analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) using 4 to 12% Bis-Tris gels (Invitrogen). Protein was transferred onto polyvinylidene difluoride or nitrocellulose membranes, and Western blot assays were performed according to the specific manufacturer guidelines for each antibody. For IKK kinase assays, IKKα was immunoprecipitated from whole-cell lysates (Upstate 05-536) and incubated with recombinant glutathione S-transferase (GST)-IκBα or GST-p65 fragments and [γ-32P]ATP. The proteins were resolved by SDS-PAGE and analyzed by autoradiography. Nonsaturating exposures of Western blots were scanned into Adobe Photoshop, and the average pixel density was calculated using the NIH Scion software.
siRNA knockdown.
All small interfering RNAs (siRNAs) were purchased from Dharmacon. The cells were seeded in six-well plates at 1 × 105 cells/well. The following siRNAs were purchased from Dharmacon, and transfections were performed according to the manufacturer's protocol using Dharmacon transfection reagent 1: IKKα (M-003473-01), IKKβ (M-003503-00), EGFR (M-003114-01), and mGluR5 (M-005620-02). For control transfections, we used Dharmacon nontargeting siRNA control 2 (D-001210-02). The cells were serum starved for 14 to 16 h before treatments and harvesting. Western blot analysis was performed as described above.
Calcium and proliferation assays.
The cells were seeded in a 96-well plate at 1 × 103 cells/well. After overnight serum starvation, the cells were treated with dimethyl sulfoxide (DMSO), lapatinib (1 μM), gefitinib (1 μM), or compound A (1 μM) for 48 h. The level of intracellular calcium was measured using a Fluo-4 NW calcium assay kit (F36205; Invitrogen) according to the manufacturer's directions. The level of relative fluorescence was measured using an Optima-Fluor 96-well fluorometer. Cell metabolic activity was measured using an XTT/MTT assay cell titer kit (Promega) according to the manufacturer's protocol using a Vmax microplate reader (Molecular Devices).
RESULTS
Glutamate regulates NF-κB activity and phosphorylation in glial cells.
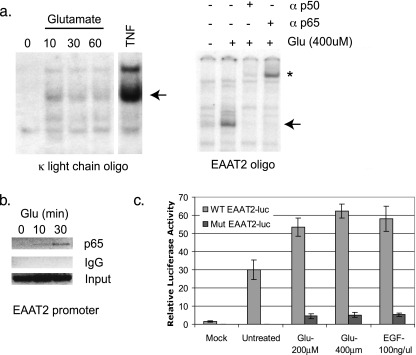
To investigate glutamate-induced responses in glial cells, we first examined the ability of glutamate to regulate NF-κB DNA binding. We found that the glutamate treatment of H4 glioma cells stimulated DNA binding to the κ enhancer light-chain NF-κB element (Fig. 1a) as well as to an NF-κB binding site in the promoter of EAAT2/GLT-1, a gene we previously demonstrated to be regulated in an NF-κB-dependent manner (33) (Fig. 1b). Supershift studies with specific antibodies demonstrate that the glutamate-induced complexes contain p65 and p50 subunits. Similar results were observed with U87-MG cells (data not shown). Next, we used ChIP assays to determine if glutamate induces the recruitment of NF-κB in vivo. The results from these experiments demonstrated the recruitment of p65 to the EAAT2 promoter with glutamate treatment for 10 and 30 min (Fig. 1b). Finally, we determined whether glutamate could activate a luciferase reporter construct driven by the promoter of EAAT2/GLT-1 (33). In H4 cells transiently transfected with an EAAT2-luciferase reporter containing wild-type NF-κB consensus sequences, glutamate treatment significantly increased luciferase activity to an extent similar to that seen with EGF. In contrast, the ability of glutamate to activate an EAAT2-luciferase reporter containing mutated NF-κB consensus sites was significantly diminished (Fig. 1c), demonstrating that the regulation of EAAT2 by glutamate is NF-κB dependent.
FIG. 1.
Glutamate regulates NF-κB DNA binding and transcriptional activity. (a) EMSAs were performed using nuclear extracts from glutamate or TNF-treated H4 cells and radiolabeled oligonucleotides corresponding to an NF-κB consensus site from the κ light-chain enhancer (Promega) or the human EAAT2 promoter. Supershift assays were performed with the indicated antibodies. Arrows indicate p65-containing complexes. *, supershifted complexes. (b) ChIP assays were performed using IgG or p65 antibody in U87-MG cells that were untreated or treated with glutamate at the indicated time points. PCR analysis was performed using primers spanning the −583 NF-κB site of the EAAT2 promoter (see Materials and Methods). (c) A wild-type (WT) or NF-κB mutant (Mut) EAAT2-luciferase (luc) reporter was transfected into H4 cells and treated with glutamate or EGF at the indicated doses for 14 to 16 h. Whole-cell lysates were prepared and monitored for luciferase activity. The relative luciferase values represent units of luciferase normalized to renilla. The results from a representative experiment are shown. Error bars represent standard deviations (n = 3). Glu, glutamate.
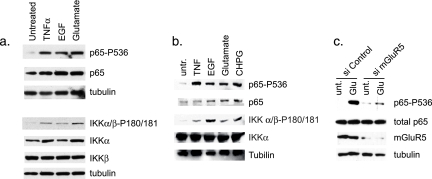
Upon stimulation with activating signals, many proteins in the NF-κB pathway, including IKKα/β, IκBα, and p65, undergo inducible, site-specific phosphorylation (8, 11). Having shown that glutamate can regulate NF-κB DNA binding and transcriptional activity, we next examined whether glutamate induces the phosphorylation of proteins in the NF-κB signaling pathway. Using Western blot analysis of whole-cell lysates isolated from H4 glial cells, glutamate was shown to induce the rapid phosphorylation of p65 at Ser536 (Fig. 2a), which is a marker of transcriptional activation. Additionally, glutamate induced the phosphorylation of IKKα/β at specific sites in the activation loop (IKKα-Ser180/IKKβ-Ser181) that are important for kinase activation, suggesting an involvement of an IKK-regulated pathway in the glutamate response (Fig. 2a). The treatment of glial cells with EGF also led to the phosphorylation of p65 and IKKα/β (Fig. 2a). Similar results were observed in primary murine astrocytes treated with glutamate and EGF (Fig. 2b).
FIG. 2.
Glutamate and mGluR5 stimulation induce the phosphorylation of p65 and IKK. Western blot analysis was performed on whole-cell lysates from H4 cells (a) and mouse primary astrocytes (b) treated with TNF-α, EGF, glutamate, or CHPG for 10 min using the indicated antibodies. Similar results were seen with U87-MG cells (data not shown). untr., untreated. (c) H4 cells were transfected with siRNA targeting mGluR5 and subsequently treated with glutamate (Glu) for 15 min. Western blot analysis was performed on whole-cell lysates using the indicated antibodies. unt., untreated.
It has been previously reported that mGluR5 can regulate EAAT2 expression in astrocytes (37). Therefore, we sought to determine whether the glutamate-induced regulation of NF-κB involved mGluR5. We found that primary astrocytes treated with the mGluR5-specific agonist CHPG showed increased phosphorylation of p65 and IKKα/β to a similar extent as treatment with glutamate (Fig. 2b). Using the siRNA-mediated knockdown of gene expression, we directly tested the role of mGluR5 in mediating glutamate induction of NF-κB. The results from these experiments revealed diminished glutamate-induced phosphorylation of p65 in the presence of siRNA targeting mGluR5 compared with a nontargeting control siRNA (Fig. 2c). These data demonstrate that the glutamate-induced regulation of NF-κB is mediated, at least partly, by mGluR5.
Glutamate activates NF-κB in the absence of IκB phosphorylation/degradation.
We next sought to determine whether the glutamate-induced activation of NF-κB involved IκBα, whose proteolytic regulation is an inherent component of the classical NF-κB signaling pathway (3, 28). Notably, we found that treatment with glutamate failed to induce the phosphorylation of IκBα at Ser32/Ser36 (Fig. 3a), which is typically required for subsequent IκBα proteolytic processing and NF-κB activation. Similarly, EGF treatment did not induce IκBα phosphorylation (Fig. 3a). Consistent with the lack of glutamate-induced IκBα-Ser32/Ser36 phosphorylation, IκBα protein levels remained stable in the presence of glutamate, whereas TNF-α induced both IκBα-Ser32/Ser36 phosphorylation and degradation (Fig. 3a). We confirmed that glutamate activates NF-κB in a manner that is independent of IκBα degradation by examining the ability of glutamate to regulate NF-κB DNA binding and p65 phosphorylation in the presence of proteosome inhibition. As expected, the proteosome inhibitor MG-132 abrogated the TNF-induced degradation of IκBα, resulting in the stabilization of total IκBα (Fig. 3b) and demonstrating the efficacy of proteosome inhibition. Furthermore, we observed that the pretreatment of U87-MG cells with MG-132 stabilized levels of basal phosphorylated and total IκBα. Moreover, our data revealed that glutamate-induced NF-κB DNA binding was only slightly affected in the presence of MG-132, whereas TNF-induced DNA binding was significantly diminished. Additionally, we observed diminished TNF-induced p65 phosphorylation in the presence of MG-132. However, EGF- and glutamate-induced p65 phosphorylation persisted under these conditions (Fig. 3b). These results demonstrate that the degradation of IκBα is not required for the regulation of NF-κB DNA binding and p65 phosphorylation induced by EGF and glutamate.
FIG. 3.
Glutamate/mGluR5 attenuates p65-IκBα interaction in the absence of IκBα phosphorylation/degradation. (a) Whole-cell lysates were isolated from H4 cells treated with TNF-α (10 ng/ml), EGF (100 ng/ml), or glutamate (400 μM) for 30 min. Western blot analysis was performed on 10 to 30 μg protein using antibodies to phospho-IκBα and total IκBα. (b) Untreated U87-MG cells or cells treated with 1 μM of MG-132 were stimulated with glutamate (400 μM), EGF (100 ng/ml), or TNF-α (20 ng/ml) for 15 min, and whole-cell lysates were harvested for Western blot analysis to examine phospho-IκBα-Ser32/36 and total IκBα to verify the efficacy of proteosome inhibition. Western blot analysis was also performed using antibodies to phospho-p65-Ser536 and total p65. Similarly treated cells were used to isolate nuclear fractions for EMSA using the κ light-chain enhancer NF-κB probe. (c) Antibodies to IgG or p65 (5 μg each) were used to immunoprecipitate endogenous proteins from 250 μg of U87-MG whole-cell lysates that were untreated (U) or treated with TNF-α (T; 20 ng/ml) or glutamate (G; 400 μM) for 30 min. Western blot analysis was performed on immunoprecipitates using antibodies to IκB, p50 (SC-7178; Santa Cruz) or p65 (SC-109; Santa Cruz). The pixel densities of IκBα and p65 Western blots were quantitated using NIH Scion software. Graphs represent the pixel density for IκBα normalized to p65 for each experimental condition. Unt, untreated; Glu, glutamate; IP, immunoprecipitate.
To address how glutamate activates NF-κB in the absence of IκBα degradation, we characterized the effect of glutamate on the interaction between p65 and IκBα. Examining the interactions between endogenous proteins using coimmunoprecipitation experiments from whole-cell H4 glioma lysates revealed that the interaction between p65 and IκBα was reduced by approximately 50% in glutamate-treated cells compared to untreated cells. As expected, and as a control, glutamate did not significantly affect the interaction between p65 and p50 (Fig. 3c). In additional control experiments, TNF-α treatment significantly attenuated the interaction between p65 and IκB but not between p65 and p50 (Fig. 3c). Similar results were seen with the U87-MG glioblastoma cells (data not shown). These data indicate that glutamate-induced signaling leads to reduced interaction between p65 and IκBα.
Glutamate induces p65 nuclear accumulation and enhanced transactivation potential.
Based on the finding that glutamate induces the dissociation of p65-IκBα complexes, we next examined whether glutamate induces the accumulation of nuclear p65. The levels of p65 were examined in cytoplasmic and nuclear subcellular fractions of U87-MG cells treated with glutamate, EGF, or CHPG. The results from these experiments demonstrate that both glutamate and EGF induce the nuclear accumulation of p65 at 10 and 60 min (Fig. 4a). The TNF-induced nuclear accumulation of p65 at 10 min is shown as a positive control, and the purity of nuclear and cytoplasmic fractions was verified by examining the subcellular localization of β-tubulin and lamin A/C (Fig. 4a and b). Additionally, we observed that CHPG induced the accumulation of phosphorylated p65-Ser536 in nuclear fractions starting at 5 min and persisting over the course of 1 h (Fig. 4b). Nuclear levels of phosphorylated p65-Ser536 accumulation coincided with its loss in cytoplasmic fractions from 10 to 60 min. We also observed an increase in total nuclear p65 with CHPG treatment at 10 and 30 min, which diminished at 60 min (Fig. 4b). Similar results were observed with H4 cells (data not shown). Overall, these data indicate that glutamate and mGluR5 stimulation results in the nuclear accumulation of p65.
FIG. 4.
Glutamate induces p65 nuclear accumulation and transactivation. Nuclear and cytoplasmic fractions were isolated from U87-MG cells treated with glutamate (Glu; 400 μM), EGF (100 ng/ml), or TNF-α (20 ng/ml) (a) or CHPG (200 μM) (b) at the indicated time points. Western blot analysis was performed using the indicated antibodies. β-Tubulin and lamin A/C were used as controls for loading and subcellular fractionation. Unt., untreated. (c) Glutamate increases p65 transcriptional activation. H4 cells were transiently transfected with a Gal4-luciferase reporter (Gal4-luc) and either Gal4-Elk1(TAD) or Gal4-p65(TAD) fusion proteins. Cells were serum starved overnight and stimulated with glutamate (Glu; 400 μM), EGF (100 ng/ml), or TNF-α (20 ng/ml) for 14 to 16 h and monitored for luciferase and renilla activity. Relative luciferase values represent units of luciferase normalized to renilla. Error bars represent standard deviations (n = 3). unt, untreated.
Based on the result that glutamate induces the phosphorylation of p65 at Ser536, we next used a Gal4-luciferase-based reporter assay to determine whether glutamate could therefore regulate p65 transcriptional activity. In this assay, a fusion protein containing the Gal4 DNA binding domain linked to the p65 transcriptional activation domain (TAD) is cotransfected with a luciferase reporter under the control of Gal4 regulatory sites (Gal4-luciferase). Since DNA binding is mediated by the Gal4 regulatory elements, this assay specifically monitors the ability of the p65 TAD to induce Gal4-luciferase activity. In transiently transfected glial cells, both Gal4-p65 and a control fusion protein, Gal4-Elk1, very modestly increased the activity of the Gal4-luciferase reporter. Importantly, while treatment with glutamate had little effect on Gal4-Elk1, it significantly potentiated the activity of Gal4-p65 (Fig. 4c). In further experiments, it was found that EGF and TNF-α treatment increased the activity of Gal4-p65 but not Gal4-Elk1 (Fig. 4c). These results demonstrate that, in addition to inducing the nuclear accumulation of p65, the glutamate treatment of glial cells regulates p65 through its TAD.
IKK is required for the glutamate/CHPG induction of RelA/p65 phosphorylation.
Interestingly, our results have revealed that glutamate treatment increases IKKα/β and p65 phosphorylation, although this does not correlate with the increased phosphorylation/degradation of IκBα. Therefore, we examined the ability of glutamate receptor-induced IKK to target the phosphorylation of both p65 and IκBα in vitro. IKK complexes were immunoprecipitated from untreated U87-MG cells, or cells treated with glutamate or TNF-α and used to perform in vitro kinase assays with a purified GST-p65314 to 551 corresponding to the transactivation domain of p65, or GST-IκBα1 to 50 recombinant proteins as substrates. We found that while TNF-α was able to potently stimulate the IKK-mediated phosphorylation of both p65 and IκBα, the mGluR5 agonist CHPG preferentially stimulated the phosphorylation of p65 (Fig. 5a). These results suggest that glutamate receptor stimulation signals NF-κB in a manner that is distinct from the classical activation pathway and demonstrate the ability for distinct signals to differentially specify IKK targets.
FIG. 5.
IKK is required for glutamate/CHPG induction of p65 phosphorylation. (a) IKKα was immunoprecipitated from untreated (Untr.) and TNF- or CHPG-treated U87-MG cells and incubated with GST-IκBα or GST-p65 and [γ-32P]ATP. Proteins were resolved by SDS-PAGE and visualized by autoradiography. (b) U87-MG cells were pretreated with DMSO or compound A (Cmpd A) for 1 h, and cells were subsequently treated with glutamate (Glu; 400 μM), CHPG (200 μM), or TNF-α (10 ng/ml) for 10 min. Whole-cell lysates were isolated, and Western blot analysis was performed using the indicated antibodies. untr., untreated. (c) H4 cells were transfected with siRNA targeting IKKα. Twenty-four hours after transfection, the cells were serum starved for 14 to 16 h and subsequently treated with CHPG or TNF-α for 10 min. Whole-cell lysates were isolated, and Western blot analysis was performed with the indicated antibodies. (d) H4 cells were transfected with siRNA targeting IKKβ and analyzed as described for panel b.
Next, the role of IKK in the glutamate-induced activation of NF-κB was confirmed using pharmacological inhibition and siRNA knockdown strategies. In H4 cells pretreated with compound A, a well-established specific inhibitor of IKKβ (39), we observed not only the loss of basal NF-κB phosphorylation but also the greatly diminished induction of p65-Ser536 phosphorylation by glutamate, CHPG, or TNF-α (Fig. 5b). Next, to determine whether IKK is required for the glutamate-induced regulation of NF-κB, the expression of IKKα and IKKβ was inhibited by siRNA. In these experiments, the >90% knockdown of both IKKα and IKKβ subunits was obtained with specific siRNA transfection (Fig. 5c and d). Notably, the loss of IKKα suppressed glutamate receptor- and TNF-α-induced p65 phosphorylation (Fig. 5c), while the loss of IKKβ strongly suppressed both basal and glutamate-induced p65-Ser536 phosphorylation (Fig. 5d). The loss of IKKβ significantly reduced but did not completely suppress TNF-α-induced p65-Ser536 phosphorylation. These results demonstrate that the IKK complex, utilizing both IKKα and IKKβ, is a primary mediator of glutamate-induced NF-κB and suggest that IKKα/β regulates the p65 transactivation function and is not associated with the induction of IκBα phosphorylation and degradation.
The EGFR signaling pathway is required for the glutamate-mediated regulation of NF-κB activity.
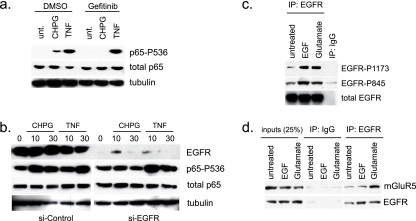
Thus far, we have observed the similar activation of NF-κB in glial cells in response to treatment with either EGF or glutamate. Interestingly, similarly to mGluR5, EGFR signaling promotes calcium oscillation and its expression is increased in astrocytes in response to injury (14, 22). Moreover, previous studies have shown that mGluR5 stimulation activates EGFR in astrocytes (23), suggesting that there is cross talk between these two signaling pathways. Therefore, we examined the role of EGFR in glutamate signaling to NF-κB using the pharmacological inhibition of EGFR or the siRNA-mediated knockdown of EGFR expression. We found that the pretreatment of glial cells with the EGFR-specific inhibitor gefitinib abrogated CHPG- but not TNF-α-induced p65 phosphorylation (Fig. 6a). Furthermore, the loss of EGFR expression by siRNA knockdown completely abolishes the ability of CHPG, but not TNF-α, to induce p65 phosphorylation (Fig. 6b). Taken together, our results establish a requirement for EGFR in mGluR5 signaling to NF-κB, which is distinct from TNF-α signaling.
FIG. 6.
The EGFR signaling pathway is required for the glutamate regulation of NF-κB activity. (a) H4 cells were pretreated with DMSO or gefitinib for 1 h and subsequently treated with CHPG (200 μM) or TNF-α (10 ng/ml) for 10 min. Western blot analysis was performed on whole-cell lysates using the indicated antibodies. unt., untreated. (b) H4 cells were transfected with siRNA targeting the EGFR. Twenty-four hours after transfection, the cells were serum starved for 14 to 16 h and subsequently treated with CHPG or TNF-α for 10 min. Whole-cell lysates were isolated, and Western blot analysis was performed with the indicated antibodies. (c and d) EGFR or IgG was immunoprecipitated from whole-cell lysates prepared from untreated or EGF- or glutamate-treated H4 cells. Cells were treated for 10 min. Western blot analysis was performed with the indicated antibodies. IP, immunoprecipitate.
To further characterize the potential cross-coupling between mGluR5 and EGFR, we examined whether glutamate-induced signals lead to EGFR phosphorylation, an indicator of EGFR activation. Total EGFR was immunoprecipitated from cells treated with either EGF or glutamate, and phosphospecific antibodies were used to examine the phosphorylation status of EGFR. The results from these experiments demonstrated that glutamate induces the phosphorylation of EGFR at Tyr1173 and Tyr845 to a similar extent as that seen with EGF treatment (Fig. 6c). Based on these results, we asked whether EGFR and mGluR5 could form a complex in cells. In unstimulated cells, a weak interaction was observed between EGFR and mGluR5, and this interaction was robustly enhanced by glutamate treatment (Fig. 6d). Importantly, EGF failed to potentiate interaction between EGFR and mGluR5, demonstrating that the increased interaction is specific for glutamate signaling. We failed to observe an association between EGFR and mGluR3 (data not shown). These data suggest that functional cross talk between EGFR and mGluR5 is important for the regulation of NF-κB activity in glial cells.
Inhibition of the EGFR attenuates glial cell proliferation and mGluR5-mediated calcium mobilization.
Interestingly, glutamate and EGFR have been shown to induce calcium release within cells (12, 22). To determine whether calcium-regulated signaling is required for glutamate-induced NF-κB activity in glial cells, we examined the effects of the cell-permeable calcium chelator BAPTA-AM on glutamate-induced NF-κB phosphorylation. BAPTA-AM treatment significantly attenuated CHPG-induced p65-Ser536 phosphorylation (Fig. 7a), as well as glutamate-induced IKKα/β phosphorylation (Fig. 7b). As an additional control, H4 cells were treated with N,N,N′,N′-tetrakis(2-pyridylmethyl)ethylenediamine (TPEN), a high-affinity, heavy metal chelator that has low affinity for calcium. While TPEN increased basal p65-Ser536 phosphorylation, it did not prevent the additional phosphorylation of p65 with either CHPG or TNF-α treatment (Fig. 7a). These results demonstrate that the effects of glutamate on NF-κB activation are mediated by calcium.
FIG. 7.
Intracellular calcium is required for CHPG-mediated p65 phosphorylation. (a) U87-MG cells were pretreated with DMSO, BAPTA-AM (10 μM), or TPEN (10 μM) for 1 h and subsequently treated with glutamate (Glu; 400 μM) or EGF (100 ng/ml) for 10 min. Whole-cell lysates were prepared for Western blot analysis with the indicated antibodies. unt., untreated. (b) H4 cells were pretreated with DMSO or BAPTA-AM (10 μM) for 1 h and subsequently treated with glutamate (Glu; 400 μM) or TNF-α (10 ng/ml) for 10 min. Whole-cell lysates were prepared for Western blot analysis with the indicated antibodies to examine phosphorylated and total IKKα/β. The pixel densities of the phospho-IKKα/β bands were determined using NIH Scion software and normalized to untreated DMSO (lane 1) or untreated BAPTA-AM (lane 5) to examine n-fold induction.
EGFR activation has also been shown to promote calcium oscillations (22) and the transactivation of mGluRs (23). To address whether EGFR signaling to IKKα/β and NF-κB is required for glutamate-induced calcium mobilization, glial cells were treated with CHPG at the indicated concentrations and calcium mobilization was monitored using a fluorescent calcium indicator. We found that CHPG treatment greatly enhanced the calcium response, which was significantly blunted by treatment with the EGFR/ErbB2 inhibitor lapatinib (Fig. 8a). Similar results were seen with gefitinib (data not shown). These results demonstrate that glutamate receptor-induced Ca2+ release is dependent on EGFR-dependent signaling. Finally, there is strong evidence that altered calcium homeostasis is an important factor in neuronal death following injury (15), and glutamate has been shown to regulate glial cell proliferation and survival (2, 10, 27). To determine whether the calcium mobilization induced by glutamate signaling through mGluR5 and EGFR affects U87-MG cell proliferation, we examined cellular metabolic activity, which is directly proportional to the number of living cells in culture. We found that glutamate, CHPG, and EGF treatment increased glial cell viability/proliferation over 24 to 48 h (Fig. 8b). Importantly, the cotreatment of cells with lapatinib or gefitinib decreased both EGF- and glutamate-induced cell viability/proliferation (Fig. 8b). Similarly, the inhibition of IKKβ activity using compound A blocked both glutamate- and CHPG-induced cell proliferation. Together these data establish that EGFR and IKK signaling are required for the mGluR5 induction of cell proliferation/survival.
FIG. 8.
EGFR inhibition blocks cell proliferation and calcium signaling. (a) H4 cells were pretreated with DMSO or lapatinib (1 μM) for 1 h and subsequently treated with CHPG at the indicated doses for 10 min. Calcium mobilization was assayed using the Fluo4NW kit (Invitrogen). Error bars indicate the standard deviations (n = 3). (b) H4 cells were pretreated with DMSO, lapatinib, gefitinib, or compound A (Cmpd A) (1 μM each) for 1 h and subsequently treated with glutamate (Glu; 400 μM), CHPG (200 μM), or EGF (100 ng/ml) for 24 h. Cell metabolic activity was measured using a colorimetric assay (Promega). Error bars indicate the standard deviations (n = 4). (c) Model for glutamate signaling to NF-κB. Glutamate binding to mGluR5 triggers association with and transactivation of the EGFR leading to calcium mobilization, calcium-dependent IKKα/β phosphorylation, and dissociation of the p65-IκBα complexes. Glutamate also leads to p65 phosphorylation and enhanced p65 transcriptional activation. Circled P, Phosphorylation sites.
DISCUSSION
NF-κB plays essential roles in the CNS, controlling synaptic plasticity, cell survival/death, and neurodegeneration (17). Therefore, dissecting the regulation of NF-κB is important toward understanding signaling dynamics and disease mechanisms in the CNS. Glutamate is an important signaling molecule that has effects on both neurons and astrocytes and can promote cell death and proliferation (2, 10). Here we have studied the signaling pathways controlled by glutamate that lead to NF-κB activation. We have shown that the stimulation of glial cells with glutamate leads to mGluR5 engagement of EGFR resulting in calcium release and, ultimately, the IKK-dependent activation of p65 to promote cell proliferation/survival (Fig. 8c). The results demonstrate that glutamate, like TNF-α, leads to the activation of IKKα/β. However, glutamate does not lead to the phosphorylation and degradation of IκB, which is associated with classical signaling to NF-κB. Indeed, experiments using the proteosome inhibitor MG-132 demonstrate that the degradation of IκB is not required for the glutamate-induced activation of NF-κB DNA binding, as well as p65 phosphorylation. Moreover, we find that glutamate signals to IKK in a calcium-dependent manner, leading to the preferential phosphorylation of p65, not IκB. These data clearly show that distinct signals can differentially specify the phosphorylation of IKK substrates and shed new light on our fundamental understanding of the diverse signaling pathways that regulate NF-κB activation in different cell types.
mGluR5 belongs to the G protein-coupled receptor (GPCR) family, several members of which have been shown to activate NF-κB (32). Recently, GPCR stimulation has been shown to regulate NF-κB activity in a manner that requires CARD and MAGUK domain-containing protein 3 (CARMA3) (7). Interestingly, Grabiner et al. reported that the loss of CARMA3 does not affect GPCR-induced IKKα/β phosphorylation but is associated with the loss of the GPCR-induced phosphorylation of both p65 and IκBα. Their findings suggest that the induction of IKK phosphorylation is not necessarily sufficient to induce its kinase activity. However, the uncoupling of IKK phosphorylation and its kinase activity observed in CARMA3 null cells affected both IκBα and p65 phosphorylation, whereas our findings describe the glutamate receptor-induced IKK phosphorylation that specifically targets the phosphorylation of p65 but not IκBα. We postulate that signal-dependent IKK substrate specificity may be achieved by the formation of distinct IKK complexes, possibly involving the signal-specific posttranslational modification of IKK and/or its substrates to alter kinase-substrate affinity. In this regard, the exact molecular mechanisms leading from different stimuli to the activation of the IKK complex are not clear but, in light of our findings, suggest that there are likely different modes of activating IKK.
Interestingly, the data demonstrate that, similarly to glutamate, EGF induces NF-κB activation without concomitant IκBα phosphorylation/degradation. It has been reported that EGF induces tyrosine phosphorylation of IκBα, thereby causing its degradation through a ubiquitination-independent pathway (31). However, in preliminary experiments, we were unable to detect the tyrosine phosphorylation of IκBα in the glutamate-induced NF-κB activation pathway (data not shown). It has also been reported that p65 phosphorylated on Ser536 does not interact with and is not regulated by IκBα (29), suggesting that the glutamate-induced phosphorylation of p65-Ser536 may cause dissociation from IκBα and subsequent p65-P536 nuclear accumulation. Alternatively, glutamate may regulate another protein that disrupts the p65 or IκBα complex, thereby releasing p65 to regulate gene expression. In this regard, the phosphorylation of p65 at Thr254 has been reported to mediate the association with Pin1, causing the dissociation of p65 from IκBα (26). However, in the preliminary data, we have not observed the glutamate-induced phosphorylation of p65-Thr254. We are currently investigating the mechanism(s) by which glutamate induces p65-IκBα dissociation.
Finally, our studies demonstrate that mGluR5 signaling to NF-κB in glial cells requires cooperation with the EGFR. Previously, it was reported that glutamate induces an association between the EGFR and mGluR5 (23). Our data (Fig. 6) indicate that the interaction between the EGFR and mGluR5 increases following the treatment of glial cells with glutamate but not in response to EGF. Importantly, pharmacological inhibition studies indicate that a critical role for the EGFR in this response is to control calcium release and NF-κB activation. Our studies indicate that in response to mGluR5 signaling in glial cells, EGFR-induced calcium release activates NF-κB through IKKα/β to promote cell survival and proliferation. Our data also suggest that cross talk between EGFR and mGluR5 may be significant in glioblastoma, where increased EGFR expression as well as constitutive NF-κB activation has been observed (25). It is possible that aberrant NF-κB activity observed in glioblastoma cells, which use glutamate as a cell survival factor (34), is due to altered signaling by mGluR5 and EGFR. Notably, the interaction between EGFR and mGluR5 is enhanced specifically with glutamate, but not EGF, treatment. These findings are significant because they demonstrate that glioblastoma cells may use alternative mechanisms to activate EGFR to drive oncogenesis and, hence, account for the lack of efficacy of current therapeutic strategies that target EGFR activation.
Overall, these findings have important implications for new therapeutic strategies for glioblastoma, which currently has limited, dismal treatment options. The data presented here clearly demonstrate that the glutamate regulation of NF-κB is distinct from that of the canonical NF-κB activation pathway, highlighting the diversity and complexity of signaling to NF-κB by different stimuli. Our findings also underscore the emerging relevance of cross talk among different classes of receptors as a means of combinatorial receptor signaling to control gene expression.
Acknowledgments
We thank Peter Lipscomb for assistance with Western blots and tissue culture and Marty Mayo for generously providing GST-p65 recombinant protein. We also thank Matthew Morrison and Cell Signaling Technology for providing reagents and technical expertise for Western blots. We are grateful to Daniela Basseres, Brian Bednarsky, and Willie Wilson for critical reading of the manuscript.
This research was supported by NIH grants to R.S. (KO1-CA118274) and A.S.B. (AI35098, CA75080, and CA73756). A.S.B. is an investigator for the Samuel Waxman Cancer Research Foundation.
We declare no competing financial interests.
Footnotes
Published ahead of print on 9 June 2008.
REFERENCES
- 1.Bonizzi, G., and M. Karin. 2004. The two NF-kappaB activation pathways and their role in innate and adaptive immunity. Trends Immunol. 25280-288. [DOI] [PubMed] [Google Scholar]
- 2.Brazel, C. Y., J. L. Nunez, Z. Yang, and S. W. Levison. 2005. Glutamate enhances survival and proliferation of neural progenitors derived from the subventricular zone. Neuroscience 13155-65. [DOI] [PubMed] [Google Scholar]
- 3.Buss, H., A. Dorrie, M. L. Schmitz, E. Hoffmann, K. Resch, and M. Kracht. 2004. Constitutive and interleukin-1-inducible phosphorylation of p65 NF-{kappa}B at serine 536 is mediated by multiple protein kinases including I{kappa}B kinase (IKK)-{alpha}, IKK{beta}, IKK{epsilon}, TRAF family member-associated (TANK)-binding kinase 1 (TBK1), and an unknown kinase and couples p65 to TATA-binding protein-associated factor II31-mediated interleukin-8 transcription. J. Biol. Chem. 27955633-55643. [DOI] [PubMed] [Google Scholar]
- 4.Caccamo, D., A. Campisi, M. Curro, M. Aguennouz, G. Li Volti, R. Avola, and R. Ientile. 2005. Nuclear factor-kappab activation is associated with glutamate-evoked tissue transglutaminase up-regulation in primary astrocyte cultures. J. Neurosci. Res. 82858-865. [DOI] [PubMed] [Google Scholar]
- 5.Caccamo, D., A. Campisi, H. Marini, E. B. Adamo, G. Li Volti, F. Squadrito, and R. Ientile. 2005. Glutamate promotes NF-kappaB pathway in primary astrocytes: protective effects of IRFI 016, a synthetic vitamin E analogue. Exp. Neurol. 193377-383. [DOI] [PubMed] [Google Scholar]
- 6.Edling, Y., M. Ingelman-Sundberg, and A. Simi. 2007. Glutamate activates c-fos in glial cells via a novel mechanism involving the glutamate receptor subtype mGlu5 and the transcriptional repressor DREAM. Glia 55328-340. [DOI] [PubMed] [Google Scholar]
- 7.Grabiner, B. C., M. Blonska, P. C. Lin, Y. You, D. Wang, J. Sun, B. G. Darnay, C. Dong, and X. Lin. 2007. CARMA3 deficiency abrogates G protein-coupled receptor-induced NF-{kappa}B activation. Genes Dev. 21984-996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hayden, M. S., and S. Ghosh. 2004. Signaling to NF-kappaB. Genes Dev. 182195-2224. [DOI] [PubMed] [Google Scholar]
- 9.Kaltschmidt, B., D. Widera, and C. Kaltschmidt. 2005. Signaling via NF-kappaB in the nervous system. Biochim. Biophys. Acta 1745287-299. [DOI] [PubMed] [Google Scholar]
- 10.Kanumilli, S., and P. J. Roberts. 2006. Mechanisms of glutamate receptor induced proliferation of astrocytes. Neuroreport 171877-1881. [DOI] [PubMed] [Google Scholar]
- 11.Karin, M., and Y. Ben-Neriah. 2000. Phosphorylation meets ubiquitination: the control of NF-[kappa]B activity. Annu. Rev. Immunol. 18621-663. [DOI] [PubMed] [Google Scholar]
- 12.Kawabata, S., R. Tsutsumi, A. Kohara, T. Yamaguchi, S. Nakanishi, and M. Okada. 1996. Control of calcium oscillations by phosphorylation of metabotropic glutamate receptors. Nature 38389-92. [DOI] [PubMed] [Google Scholar]
- 13.Kondoh, T., T. Nishizaki, H. Aihara, and N. Tamaki. 2001. NMDA-responsible, APV-insensitive receptor in cultured human astrocytes. Life Sci. 681761-1767. [DOI] [PubMed] [Google Scholar]
- 14.Liu, B., H. Chen, T. G. Johns, and A. H. Neufeld. 2006. Epidermal growth factor receptor activation: an upstream signal for transition of quiescent astrocytes into reactive astrocytes after neural injury. J. Neurosci. 267532-7540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mattson, M. P. 2007. Calcium and neurodegeneration. Aging Cell 6337-350. [DOI] [PubMed] [Google Scholar]
- 16.Mattson, M. P. 2003. Excitotoxic and excitoprotective mechanisms: abundant targets for the prevention and treatment of neurodegenerative disorders. Neuromolecular Med. 365-94. [DOI] [PubMed] [Google Scholar]
- 17.Mattson, M. P., and M. K. Meffert. 2006. Roles for NF-kappaB in nerve cell survival, plasticity, and disease. Cell Death Differ. 13852-860. [DOI] [PubMed] [Google Scholar]
- 18.Mayo, M. W., J. L. Norris, and A. S. Baldwin. 2001. Ras regulation of NF-kappa B and apoptosis. Methods Enzymol. 33373-87. [DOI] [PubMed] [Google Scholar]
- 19.Meffert, M. K., J. M. Chang, B. J. Wiltgen, M. S. Fanselow, and D. Baltimore. 2003. NF-kappa B functions in synaptic signaling and behavior. Nat. Neurosci. 61072-1078. [DOI] [PubMed] [Google Scholar]
- 20.Melchiorri, D., I. Cappuccio, C. Ciceroni, P. Spinsanti, P. Mosillo, I. Sarichelou, P. Sale, and F. Nicoletti. 2007. Metabotropic glutamate receptors in stem/progenitor cells. Neuropharmacology 53473-480. [DOI] [PubMed] [Google Scholar]
- 21.Memet, S. 2006. NF-kappaB functions in the nervous system: from development to disease. Biochem. Pharmacol. 721180-1195. [DOI] [PubMed] [Google Scholar]
- 22.Morita, M., N. Kozuka, R. Itofusa, M. Yukawa, and Y. Kudo. 2005. Autocrine activation of EGF receptor promotes oscillation of glutamate-induced calcium increase in astrocytes cultured in rat cerebral cortex. J. Neurochem. 95871-879. [DOI] [PubMed] [Google Scholar]
- 23.Peavy, R. D., M. S. Chang, E. Sanders-Bush, and P. J. Conn. 2001. Metabotropic glutamate receptor 5-induced phosphorylation of extracellular signal-regulated kinase in astrocytes depends on transactivation of the epidermal growth factor receptor. J. Neurosci. 219619-9628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Petroff, O. A. 2002. GABA and glutamate in the human brain. Neuroscientist 8562-573. [DOI] [PubMed] [Google Scholar]
- 25.Raychaudhuri, B., Y. Han, T. Lu, and M. A. Vogelbaum. 2007. Aberrant constitutive activation of nuclear factor kappaB in glioblastoma multiforme drives invasive phenotype. J. Neurooncol. 8539-47. [DOI] [PubMed] [Google Scholar]
- 26.Ryo, A., F. Suizu, Y. Yoshida, K. Perrem, Y. C. Liou, G. Wulf, R. Rottapel, S. Yamaoka, and K. P. Lu. 2003. Regulation of NF-kappaB signaling by Pin1-dependent prolyl isomerization and ubiquitin-mediated proteolysis of p65/RelA. Mol. Cell 121413-1426. [DOI] [PubMed] [Google Scholar]
- 27.Rzeski, W., L. Turski, and C. Ikonomidou. 2001. Glutamate antagonists limit tumor growth. Proc. Natl. Acad. Sci. USA 986372-6377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sakurai, H., H. Chiba, H. Miyoshi, T. Sugita, and W. Toriumi. 1999. IkappaB kinases phosphorylate NF-kappaB p65 subunit on serine 536 in the transactivation domain. J. Biol. Chem. 27430353-30356. [DOI] [PubMed] [Google Scholar]
- 29.Sasaki, C. Y., T. J. Barberi, P. Ghosh, and D. L. Longo. 2005. Phosphorylation of RelA/p65 on serine 536 defines an I{kappa}B{alpha}-independent NF-{kappa}B pathway. J. Biol. Chem. 28034538-34547. [DOI] [PubMed] [Google Scholar]
- 30.Seifert, G., and C. Steinhauser. 2001. Ionotropic glutamate receptors in astrocytes. Prog. Brain Res. 132287-299. [DOI] [PubMed] [Google Scholar]
- 31.Sethi, G., K. S. Ahn, M. M. Chaturvedi, and B. B. Aggarwal. 2007. Epidermal growth factor (EGF) activates nuclear factor-kappaB through IkappaBalpha kinase-independent but EGF receptor-kinase dependent tyrosine 42 phosphorylation of IkappaBalpha. Oncogene 267324-7332. [DOI] [PubMed] [Google Scholar]
- 32.Shi, C. S., and J. H. Kehrl. 2001. PYK2 links G(q)alpha and G(13)alpha signaling to NF-kappa B activation. J. Biol. Chem. 27631845-31850. [DOI] [PubMed] [Google Scholar]
- 33.Sitcheran, R., P. Gupta, P. B. Fisher, and A. S. Baldwin. 2005. Positive and negative regulation of EAAT2 by NF-kappaB: a role for N-myc in TNFalpha-controlled repression. EMBO J. 24510-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takano, T., J. H. Lin, G. Arcuino, Q. Gao, J. Yang, and M. Nedergaard. 2001. Glutamate release promotes growth of malignant gliomas. Nat. Med. 71010-1015. [DOI] [PubMed] [Google Scholar]
- 35.Teichberg, V. I. 1991. Glial glutamate receptors: likely actors in brain signaling. FASEB J. 53086-3091. [DOI] [PubMed] [Google Scholar]
- 36.Ulas, J., T. Satou, K. J. Ivins, J. P. Kesslak, C. W. Cotman, and R. Balazs. 2000. Expression of metabotropic glutamate receptor 5 is increased in astrocytes after kainate-induced epileptic seizures. Glia 30352-361. [PubMed] [Google Scholar]
- 37.Vermeiren, C., M. Najimi, N. Vanhoutte, S. Tilleux, I. de Hemptinne, J. M. Maloteaux, and E. Hermans. 2005. Acute up-regulation of glutamate uptake mediated by mGluR5a in reactive astrocytes. J. Neurochem. 94405-416. [DOI] [PubMed] [Google Scholar]
- 38.Winder, D. G., and P. J. Conn. 1996. Roles of metabotropic glutamate receptors in glial function and glial-neuronal communication. J. Neurosci. Res. 46131-137. [DOI] [PubMed] [Google Scholar]
- 39.Ziegelbauer, K., F. Gantner, N. W. Lukacs, A. Berlin, K. Fuchikami, T. Niki, K. Sakai, H. Inbe, K. Takeshita, M. Ishimori, H. Komura, T. Murata, T. Lowinger, and K. B. Bacon. 2005. A selective novel low-molecular-weight inhibitor of IkappaB kinase-beta (IKK-beta) prevents pulmonary inflammation and shows broad anti-inflammatory activity. Br. J. Pharmacol. 145178-192. [DOI] [PMC free article] [PubMed] [Google Scholar]