Abstract
Although the best-defined function of type II major histocompatibility complex (MHC-II) is presentation of antigenic peptides to T lymphocytes, these molecules can also transduce signals leading alternatively to cell activation or apoptotic death. MHC-II is a heterodimer of two transmembrane proteins, each containing a short cytoplasmic tail that is dispensable for transduction of death signals. This suggests the function of an undefined MHC-II-associated transducer in signaling the death response. Here we describe a novel plasma membrane tetraspanner (MPYS) that is associated with MHC-II and mediates its transduction of death signals. MPYS is unusual among tetraspanners in containing an extended C-terminal cytoplasmic tail (∼140 amino acids) with multiple embedded signaling motifs. MPYS is tyrosine phosphorylated upon MHC-II aggregation and associates with inositol lipid and tyrosine phosphatases. Finally, MHC class II-mediated cell death signaling requires MPYS-dependent activation of the extracellular signal-regulated kinase signaling pathway.
The ability of major histocompatibility complex class II (MHC-II) to transmit signals was first recognized more than 20 years ago (6, 10). Depending upon the species and cell type, anti-MHC-II monoclonal antibody (MAb) stimulation induces tyrosine phosphorylation, calcium mobilization, cAMP production, and mitogen-activated protein kinase, AKT, and protein kinase C activation (for a review, see reference 1). In activated murine B cells, MHC class II signals trigger tyrosine phosphorylation and calcium mobilization via associated CD79a and -b (18). These responses are also induced by T-cell receptor (TCR) binding to the MHC-II/antigenic peptide complexes on B cells (18).
Several recent studies have shed light on the biological importance of MHC-II signaling, particularly MHC-II-mediated death signaling. It was found that cross-linking of MHC-II by MAb and via cognate MHC-II-TCR interaction can lead to Fas-independent antigen-presenting cell (APC) death (21, 25). Dendritic cells (DCs) undergo accelerated clearance from the lymphoid organs after interacting with antigen-specific T cells (15). Prolonged DC survival can lead to autoimmunity (9). Thus, it has been proposed that MHC-II-mediated death signaling functions to limit potentially dangerous uncontrolled immune responses by elimination of APCs after they have served their antigen-presenting purpose (25). Given the similar response of certain B cells to MHC-II aggregation, it seems likely that under certain circumstances they too are eliminated by this mechanism.
The ability to mediate cell death signaling makes MHC-II a candidate therapeutic target for treatment of certain malignancies (4). Anti-MHC-II MAbs (1D09C3 and Hu1D10) are being tested in clinical trials involving patients with refractory and relapsed non-Hodgkin's lymphoma or relapsed low-grade or follicular lymphoma (5, 7). These anti-MHC-II MAbs exhibit rapid and potent in vitro tumoricidal activity for lymphoma/leukemia cells with no long-lasting hematological toxicity in primates (21).
The molecular basis of MHC-II-mediated signaling of cell death is unknown. This response is independent of complement and Fc receptors (22). Effective humanized anti-MHC-II MAbs are all immunoglobulin G4 (IgG4) isotype antibodies (Abs) that are devoid of complement-dependent cytotoxicity and do not mediate Ab-dependent cell-mediated cytotoxicity (21). All of these MAbs induce death efficiently in lymphoma/leukemia tumor cells (21). Thus, death induced by these Abs, and by extension T-cell antigen receptors, is thought to be mediated by MHC-II signaling (20). Consistent with this possibility, protein kinase C activation is reportedly required for MHC-II-mediated death in Raji human B-cell lymphoma, mature DCs, and activated THP-1 monocytes but not in primary human plasmacytoid DCs (2, 12, 14, 27). MHC-II-mediated signaling of death also appears to occur independently of Src family kinase (14, 27) and caspase activation (8, 13) in Raji and Ramos cells. Reactive oxygen species production and Jun N-terminal protein kinase (JNK) activation have been implicated in MHC-II-mediated death in lymphomas line JVM-2 and GRANTA-519 (7). Thus, data are fragmentary, and there is no consensus regarding how MHC class II molecules transduce death signals.
Here we describe studies aimed at dissection of the molecular signaling pathways by which MHC class II molecules transduce signals leading to apoptotic death of B lymphoma cells. We report identification of a novel MHC-II-associated membrane protein, termed MPYS, and demonstrate that it mediates death signaling via activation of the extracellular signal-regulated kinase (ERK) pathway.
MATERIALS AND METHODS
Induction and assay of cell death.
K46 cells were suspended in 5% complete IMDM (catalog no. 10-016-cv; Mediatech Inc.) at a concentration of 106 cells/ml and then transferred to round-bottomed 96-well plates (100 μl/well) and cultured at 37°C for 1 h. Biotinylated MHC-II MAbs were added to the wells and incubated for 12 min. Avidin was then added to the wells, and cells were cultured for the indicated time. Propidium iodide (PI) (2 μg/ml) and annexin V-Alexa Fluor 488 (1 μl/100 μl cells) (A-13201; Molecular Probes) or DiOC6 (3) (50 nM) and PI (2 μg/ml) were used to measure apoptotic or dead cells by flow cytometry.
The ERK inhibitor PD98059 (513000; Calbiochem), AKT inhibitor LY294002 (440202; Calbiochem), and p38 inhibitor SB203580 (559389; Calbiochem) were used in this study. The inhibitor was added to cells at 5 × 106 cells/ml and cultured for 4 h. Samples were then split into 1 × 106 cells/ml, and cell death was induced and measured as describe above. To inhibit src kinase, PP2 (529573; Calbiochem) was added to cells and cultured for 30 min before cell death was induced as described above.
Immunoprecipitation and immunoblotting.
Cells were lysed in 0.33% CHAPS buffer (150 mM NaCl, 10 mM Tris [pH 7.5], 10 mM sodium pyrophosphate, 2 mM Na3VO4, 10 mM NaF, 0.4 mM EDTA, 1 mM phenylmethylsulfonylfluoride, and 1 μg/ml each of aprotinin, α1-antitrypsin, and leupeptin) (40 × 106/ml) at 4°C for 1.5 h or overnight. Cell lysates were centrifuged at 12,000 × g at 4°C for 20 min. Supernatants were analyzed on a 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gel. The gel was transferred to a polyvinylidene difluoride membrane, and proteins were probed with Abs and visualized using an Odyssey infrared imaging system (Li-Cor). The Abs used were 4G10 phosphotyrosine Ab, antihemagglutinin (anti-HA) MAb 16B12 (MMS-101P; Covance Research), antiactin Ab (I-19) (sc1616; Santa Cruz), anti-SHP-1 Ab (12660), anti-SHIP Ab (26), anti-CD22 Ab (12045), anti-JNK 1 Ab (c-17) (sc474; Santa Cruz), anti-pan-ERK (610123; Transduction Lab), and Abs from Cell Signaling Technology: anti-p38 (no. 9212), anti-p-P38 (no. 9211), anti-pERK (no. 9101), anti-AKT (no. 9272), anti-pJNK (no. 9251), and anti-pSer473-AKT (no. 9271).
Analysis of intracellular free calcium concentration.
Cells were loaded with Indo-1AM (Molecular Probes, Eugene, OR) for 30 min at 37°C as recommended by the manufacturer. Anti-MHC-II MAb (10 μg/ml) was then added to the suspension for another 12 min at 37°C before cells were washed and suspended in IMDM supplemented with 2% fetal calf serum. The cells (106 cells/ml) were analyzed before and after stimulation via cross-linking with avidin (20 μg/ml). Data were analyzed using the Flow-Jo software program (Tree Star, Inc., San Carlos, CA).
Nano-LC-MS/MS.
K46 cells (∼5 × 108) were lysed in 0.33% CHAPS buffer, and immunoprecipitation was performed as previously described (18). Proteins were eluted from Ab-bound beads with 0.1 M citric acid (pH 2.0). Eluates in citric acid were neutralized with 2 M Tris (pH 10.0). The combined eluates were buffer exchanged into 25 mM ammonium bicarbonate (40867; Fluka) using Zeba desalting spin columns (89890; Pierce). The protein ammonium bicarbonate solution was dried, reconstituted in 8.0 M urea (U-4883; Sigma), reduced in dithiothreitol (50 mM; D5545; Sigma), alkylated with iodoacetamide (100 mM; I1149; Sigma) and trypsin (1 μg; V511A; Promega), and digested overnight at 37°C. The digests were analyzed by reverse-phase nanospray liquid chromatography-tandem mass spectrometry (nano-LC-MS/MS) (Agilent 1100 high-performance liquid chromatography system and Agilent Ultra ion trap). Proteins were identified using the Spectrum Mill (Agilent) database search algorithm.
Molecular cloning of the murine MPYS gene.
Primers (MPY5 and MPY3) were designed to amplify the full-length mpys sequence from a K46 cDNA library (see Table S2 in the supplemental material). PCR was carried out using Ex Tag DNA polymerase (RR001; TaKaRa) and sequenced.
Making MPYS-EGFP, MPYS-HA, and MPYS-Flag constructs.
Primers (MPYS-EGFP-For and -Rev) were designed to include restriction enzyme sites in the end of the mpys coding sequence (see Table S2 in the supplemental material). The PCR product was cloned into the EcoRI-NotI sites of a pMXI-EGFP fusion construct. A reverse primer (HA-MPY-Rev) was designed to include an XhoI site and an HA tag sequence (see Table S2 in the supplemental material). HA-MPYS was amplified using the primers MPYS-EGFP-For and HA-MPY-Rev. The PCR product was cloned into the EcoRI-XhoI sites of a pMXI-IRES-EGFP vector. For the LEL-Flag construct, a Flag sequence was inserted between V73 and Q74 of MPYS. For the SEL-Flag construct, the Flag sequence was inserted between E149 and K150 of MPYS. The insertion was performed as described previously (30). The construct was confirmed by sequencing.
Generation of polyclonal anti-MPYS Abs.
Primers (GST-MPYS-For and -Rev) were designed to amplify cDNA sequence encoding the C-terminal 101 amino acids (aa) of MPYS (see Table S2 in the supplemental material). The PCR product was cloned into the BamHI-NotI sites of pGEX-5X-1 (27-4585-01; Amersham Biosciences) to make the peptide, which was used to immunize a rabbit. The polyclonal Ab was affinity purified using peptide-coupled Sepharose beads.
shRNA knockdown of MPYS expression.
Two short-hairpin RNAs (shRNAs) were designed to target sequences in exons 5 and 7 of mpys (sh5, 5′GGATCCGAATGTTCAATCA-3 ′; sh7, 5′GCACATTCGTCAGGAAGAA-3 ′, respectively), using the pSicoOligomaker 1.5 software program (28). The forward and reverse primers (1 μl each of 1 μg/μl) (see Table S2 in the supplemental material) were annealed (28), and the annealed oligonucleotides were ligated into pLL3.7 through HpaI and XhoI sites. An shRNA that targets the luciferase gene was used as a control (31). Lentiviruses were generated as previously described (28).
Cell surface biotinylation.
Cells (25 × 106 cells/ml) were suspended in phosphate-buffered saline. Sulfo-NHS-LC-Biotin (21335; Pierce) (0.5 mg/ml) was added, and cells were incubated with rotation for 30 min at room temperature. Glycine (50 mM) was added to the cell suspension for an additional 5 min to quench excess succinimidyl ester. The cells were then centrifuged and lysed in 0.33% CHAPS buffer.
Live-cell imaging.
K46 cells expressing green fluorescent protein (GFP)-tagged MPYS (MPYS-GFP) (5 × 104) were loaded into Lab-Tek chamber (177445; Nunc, Denmark) slides and allowed to adhere for 12 h. Cells were loaded with 2 μg/ml Hoechst 34580 (H21486; Invitrogen) and 1 μM dihydrorhodamine 6G (D633; Invitrogen) for 30 min. Images were collected using a 63× objective in an inverted Zeiss 200 M microscope.
Table S1 in the supplemental material lists proteins identified by nano-LC/MS/MS in the MHC-II complex with at least two unique peptides; Table S2 lists all the primers used in the study. Figure S1 in the supplemental material shows the mass spectra of peptides from MHC-II α/β chains, CD20, CD37, and MPYS identified in the MHC-II complex (panel A) and MHC-II α/β chains and MPYS in the MPYS complex (panel B).
Nucleotide sequence accession number.
The mouse mpys cDNA sequence (accession no. DQ910493) has been deposited in GenBank.
RESULTS
MHC-II MAb induces time and dosage-dependent death of K46 B lymphoma cells.
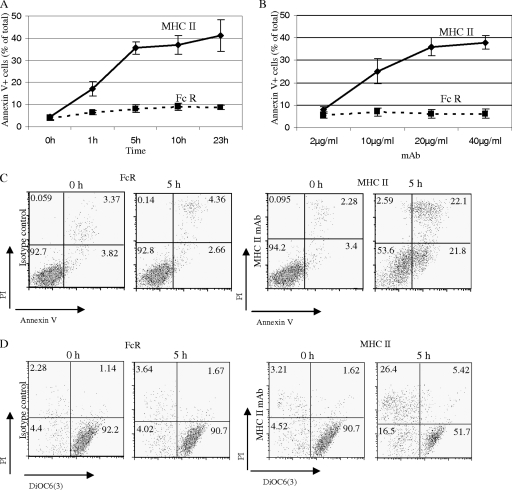
We studied MHC-II-induced cell death in a murine B lymphoma line, K46 (17). Cross-linking of MHC-II by MAbs in K46 cells has been shown to induce tyrosine phosphorylation, calcium flux, and phosphatidylinositol 3-kinase activation (3). As shown in Fig. 1A and B, MHC-II cross-linking by biotinylated I-Ab,d,q/Ed,k-reactive MAb M5/114 (rat IgG2b) and avidin induced time and dosage-dependent K46 B lymphoma exposure of phosphatidylserine as indicated by annexin V staining. The anti-Fc receptor MAb 2.4G2 (rat IgG2b) was used as an isotype control. This MHC-II MAb response, which suggests a commitment to cell death, was observed as early as 1 h and peaked at 5 h (Fig. 1A). The cell death response was also measured by dual staining with PI and DiOC6(3)/PI (Fig. 1C and D). PI staining reflects cell permeability, and loss of DiOC6(3) staining reflects mitochondrial membrane depolarization. The control 2.4G2 MAb did not induce cell death. Our findings demonstrate that MHC-II molecules transduce apoptotic cell death signals in K46 cells and this response involves depolarization of mitochondrial membranes.
FIG. 1.
MHC class II MAb induces time and dosage-dependent apoptosis of K46 B lymphoma cells. (A) K46 cells were treated with 15 μg/ml biotinylated anti-MHC class II (M5/114; rat IgG2b) MAb or its isotype control, biotinylated anti-mouse FcγRIIB MAb ((2.4G2; rat IgG2b), followed by 20 μg/ml avidin for the indicated time. Cell death was measured by annexin V/PI dual staining (n > 3). (B) Cells were treated as for panel A at the indicated Ab doses for 5 h. Cell death was measured as for panel A (n > 3). (C and D) Cells were treated with 15 μg/ml biotinylated M5/114 or 2.4G2 plus 20 μg/ml avidin for 5 h. Cell death was measured by annexin V/PI (C) or DiOC6/PI dual staining (D) (n > 3). Numerical annotation reflects the percentage of cells in each quadrant. Error bars represent standard deviations of triplicates.
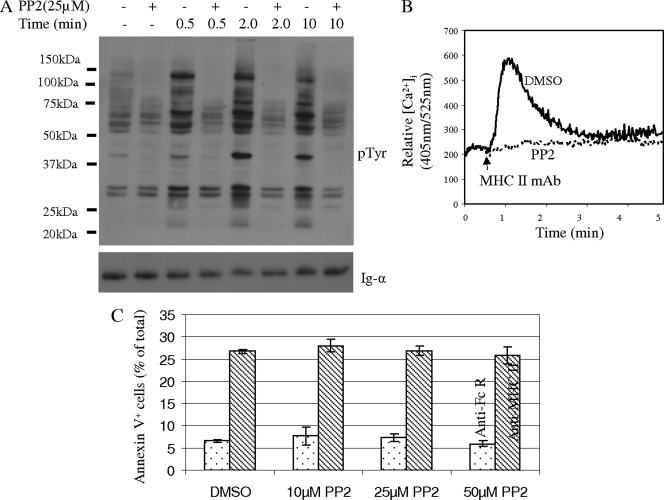
PP2 inhibits MHC-II MAb-induced tyrosine phosphorylation and calcium mobilization but not the death response.
Cross-linking of MHC-II by MAbs in K46 cells is known to induce protein tyrosine phosphorylation and calcium flux (3). To explore whether these signaling events are involved in the death response, we assessed the effect of the Src kinase inhibitor PP2 on induction of cell death. As expected, MHC-II MAb-induced tyrosine phosphorylation and calcium responses were blocked by PP2 treatment (Fig. 2a and b). However, MHC-II MAb-induced cell death remained largely intact after treatment with inhibitor (Fig. 2c). This finding is consistent with results in previous studies, which showed that tyrosine phosphorylation and calcium flux are not required for the MHC-II-mediated death response of human B cells (14, 16).
FIG. 2.
MHC-II signaling of cell death does not require activation of Src family tyrosine kinases. (A) K46 cells were treated with 25 μM PP2 or dimethylsulfoxide (DMSO) for 30 min at 37°C and then stimulated with biotin-MHC-II (M5.114; 20 μg/ml) and avidin (20 μg/ml) for the indicated times. SDS-PAGE-fractionated whole-cell lysates were probed with the indicated Abs (n = 3). (B) The intracellular free calcium concentration ([Ca2+]i) was measured before and during K46 stimulation with biotin-MHC-II (M5.114; 10 μg/ml) and avidin (10 μg/ml). Cells were pretreated with DMSO or PP2 as described for panel (A) (n > 3). (C) K46 cells treated as for panel A were stimulated with biotin-anti-MHC-II (M5.114; 10 μg/ml; shaded bars) or biotin-anti-FcγRIIB (2.4G2; 10 μg/ml; open bars) and avidin (10 μg/ml) for 5 h, and death was measured by annexin V-PI staining as described in Materials and Methods (n > 3). Error bars represent standard deviations of triplicates.
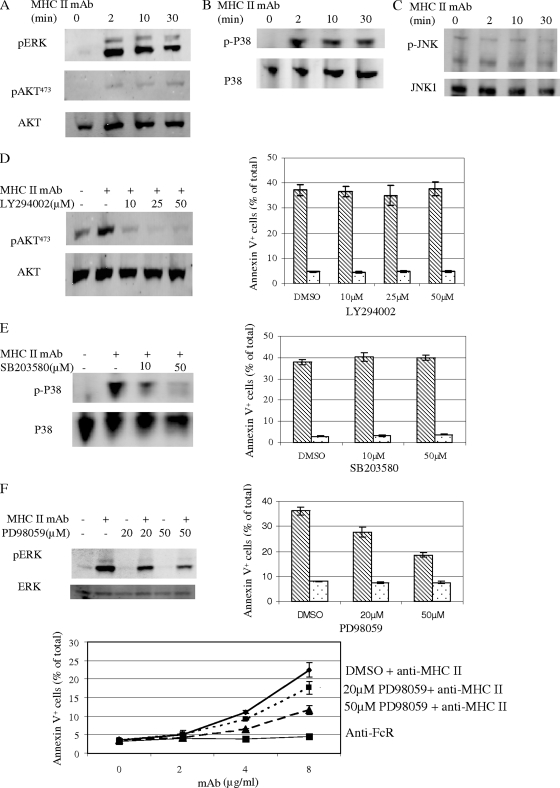
MHC-II MAb-induced ERK activation but not AKT and p38 activation is required for the death response.
MHC-II aggregation can lead to activation of AKT and mitogen-activated protein kinase kinases ERK, p38, and JNK in monocytes and human B cells (1). We investigated whether these signaling events are activated upon MHC-II MAb stimulation of K46 cells. Indeed, cross-linking of MHC-II by biotinylated M5/114 and avidin induced strong and sustained ERK and p38 activation in K46 cells (Fig. 3a and b). AKT was weakly activated by MHC-II cross-linking, as measured using phospho-AKT Ab blotting (Fig. 3A). There was weak basal JNK activation measured by phosphor-JNK Ab. However, MHC-II activation did not increase JNK phosphorylation (Fig. 3C). Thus, MHC-II cross-linking activates AKT, ERK, and p38 but not JNK in K46 cells.
FIG. 3.
ERK function is required for MHC-II-mediated cell death. (A to C) K46 cells were stimulated with biotin-MHC-II (M5/114; 20 μg/ml) plus avidin (20 μg/ml) for the indicated times and detergent lysates prepared. Transfers of SDS-PAGE-fractionated whole-cell lysates were probed with the indicated Abs (n = 4). (D to F) K46 cells were treated with the indicated doses of LY294002, SB203580, or PD98059 as described in Materials and Methods and then stimulated, lysed, and probed as for panels A to C. Cell death was stimulated and measured as for panel C of Fig. 2. Shaded bars denote biotin-anti-MHC-stimulated samples. Open bars denote biotin-anti-FcR-stimulated samples. Error bars represent standard deviations of triplicates (n > 3).
We then studied requirements for these signaling events in the cell death induced by MHC-II MAb. Treatment of K46 cells with LY294002 inhibited MHC-II-mediated AKT activation but had no effect on the death response (Fig. 3D). MHC-II-mediated p38 activation was completely inhibited by SB203580, but the inhibitor also had no effect on the death response (Fig. 3E). Interestingly, at 50 μM, the ERK-specific inhibitor PD98059 inhibited MHC-II-mediated cell death by ∼50% (Fig. 3F). However, this dose did not completely inhibit MHC-II-induced ERK activation, explaining the residual cell survival and implicating ERK in the cell death response (Fig. 3F).
In conclusion, although cross-linking MHC-II with MAb leads to activation of AKT, ERK, and p38, only ERK activation is required for the death response of these cells.
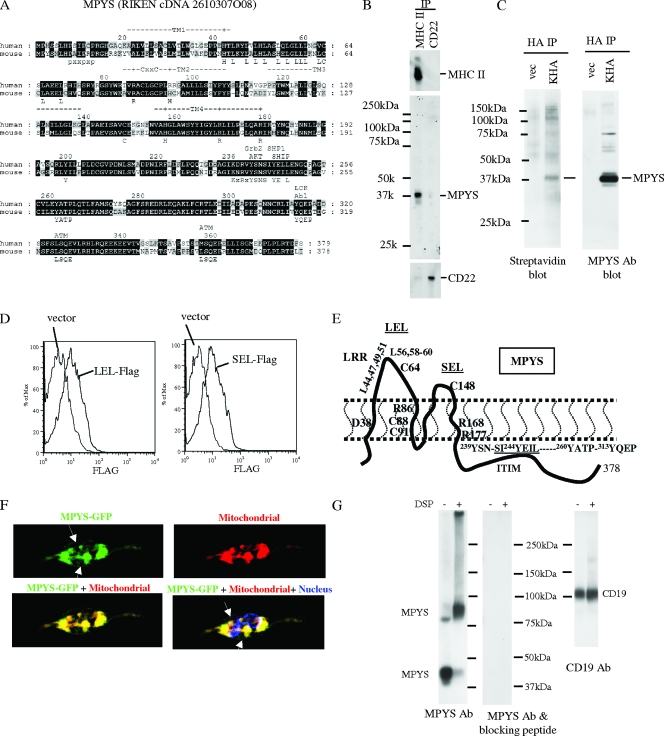
Identification of a novel MHC-II-associated membrane protein using nano-LC-MS/MS.
The signaling circuitry by which MHC-II aggregation activates ERK is unknown. MHC-II α and β chains contain cytoplasmic tails of only 12 and 18 amino acids, respectively, and deletion of these tails does not affect the death response (16). Thus, we postulated that MHC-II must transmit death signals through an associated cell surface protein(s). The B-cell-specific proteins CD19, CD20, and CD79a/b have been shown to be physically and functionally associated with MHC-II and therefore were candidates (18, 19). However, unlike the MHC-II death response, CD19, CD20, and CD79a/b expression is B cell specific, suggesting that these molecules are not likely to be involved in this response. MHC-II is also known to associate with tetraspanins, including CD9 and CD37 (19). However, these molecules have very short cytoplasmic tails (8 to ∼14 aa) that lack defined signaling motifs. Therefore, we hypothesized that a novel MHC-II-associated protein(s) might function in transduction of signals that mediate the death response. To explore this possibility, we undertook proteomic analysis of MHC-II-associated proteins. We prepared lysates of K46 cells using a mild detergent (CHAPS) that preserves weak protein-protein interactions and then immunoprecipitated MHC-II and associated proteins using anti-MHC-II MAb beads. We eluted proteins from the beads and identified them using nano-LC-MS/MS. Forty-one proteins were recovered from the MHC-II immunoprecipitates based on the detection of at least two unique peptides from each (see Table S1 in the supplemental material). MHC-II α and β chains were identified by 6 and 14 peptides, respectively, confirming the effectiveness of the affinity purification (see Fig. S1a in the supplemental material).
We were particularly interested in MHC-II-associated proteins containing a transmembrane domain(s) and cytoplasmic signaling motifs. A hypothetical protein, RIKEN cDNA 2610307O08, was implicated by three unique peptides predicted by its DNA sequence (see Fig S1a in the supplemental material). This hypothetical protein is predicted to contain four transmembrane domains by the SOSHI (http://bp.nuap.nagoya-u.ac.jp/sosui/), TMHMM (http://www.cbs.dtu.dk/services/TMHMM-2.0/), and TMpred (http://www.ch.embnet.org/software/TMPRED_form.html) software programs, along with multiple signaling motifs predicted by the ELM (http://elm.eu.org/) and Scansite (http://scansite.mit.edu) software programs, including an immunoreceptor tyrosine-based inhibitory motif (ITIM), SVY244EIL) (Fig. 4A). We designated it MPYS based on its N-terminal methionine-proline-tyrosine-serine amino acid sequence.
FIG. 4.
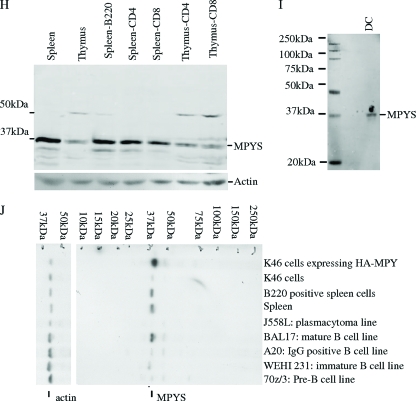
Identification of a novel MHC-II-associated membrane protein. MHC-II-associated proteins were isolated by coimmunoprecipitation from K46 cell CHAPS detergent lysates and analyzed using nano-LC-MS/MS. One candidate, designated MPYS, was identified based on detection of three peptides predicted by the DNA sequence. (A) Alignment of mouse and human MPYS orthologs with annotation of their predicted signaling motifs. (B) K46 cells were lysed in CHAPS buffer. MHC-II IP was accomplished using M5/114-coupled Sepharose beads; CD22 was immunoprecipitated using Cy34-coupled Sepharose beads. MPYS, MHC-II, and CD22 were detected with the indicated Abs (n > 3). (C) HA IP was performed using 1% NP-40 lysates of surface-biotinylated K46 cells (5 × 106) expressing HA-MPYS (KHA) or vector using HA MAb 16B12 (vec). Electrophoretic transfers were probed with indicated Abs (n > 3). (D) K46 cells expressing LEL-Flag, SEL-Flag, or vector were stained using biotin-Flag (M2; Sigma) and streptavidin-PE (n = 2). (E) A cartoon illustrates plasma membrane disposition of MPYS and its four transmembrane domains. Key residues conserved between human and mouse MPYS are annotated. (F) K46 cells expressing MPYS-GFP were stained with dihydrorodamine 6G (red) and Hoescht (blue). Arrows indicate MPYS-GFP localization on the cell surface (n = 3). (G) K46 cells were treated with dithiobis(succinimidyl)propionate (DSP) (+) or DMSO (−) and lysed in radioimmunoprecipitation assay buffer as described previously (16). Whole-cell lysates were run on 5% nonreducing gels, transferred, and probed with MPYS Ab and then stripped and reprobed with MPYS Ab plus the blocking peptide. Afterward, the blot was probed again with anti-CD19 Ab. (H and I) Transfers of whole-cell detergent lysates from AutoMACS (Miltenyi Biotec)-selected CD4+, CD8+, B220+ cells, and cultured BMDC were probed with anti-MPYS Ab (n = 3). (J) Transfers of whole-cell lysates from various B-lineage lymphomas were probed with anti-MPYS Ab (n = 3).
We cloned the mpys gene from a cDNA library produced from the murine K46 B lymphoma cell line (17). The gene encoded a 378-aa protein with a predicted mass of 42 kDa (Fig. 4A). The sequence does not show significant homology to known or predicted proteins, suggesting MPYS belongs to a novel, single-member class of proteins. Human MPYS is ∼80% homologous with mouse MPYS, and no invertebrate homologues of mpys were found in the database (Fig. 4A).
To begin to explore protein expression and function, we raised a polyclonal Ab against the C-terminal 101 aa of murine MPYS and used it to immunoprecipitate endogenous MPYS from K46 cell lysates. We then analyzed eluates by nano-LC-MS/MS. Ab reactivity with MPYS was confirmed by detection in eluates of 15 unique peptides covering more than 50% of the total amino acid sequence (see Fig. S1b in the supplemental material), including peptides from the N and C termini of the protein (see Fig. S1b in the supplemental material). Consistent with MPYS association, two and four peptides derived from MHC-II α and β chains, respectively, were found in the immunoprecipitate (see Fig. S1b in the supplemental material). This association was confirmed by immunoblotting (Fig. 4B).
To evaluate the cell surface expression of MPYS, we expressed mpys-HA in K46 cells. These cells are designated KHA. Anti-HA immunoprecipitation (IP) of lysates of surface-biotinylated K46 cells, followed by SDS-PAGE, transfer, and avidin blotting, revealed that MPYS is biotinylated (Fig. 4c) and therefore is on the cell surface.
To confirm the predicted topology of the MPYS protein, we made two Flag-tagged mpys constructs. One has a Flag tag inserted in the predicted large extracellular loop (LEL-Flag). The other has a Flag tag in the predicted small extracellular loop (SEL-Flag). We expressed these two constructs in K46 cells and stained the cells with anti-Flag Ab. As shown in Fig. 4D, the Flag-tagged MPYS proteins were detected on the cell surface. On the contrary, our own polyclonal Ab, which is against the predicted cytoplasmic tail of MPYS, did not stain intact cells (data not shown). These data suggest that the predicted four-transmembrane topology is likely correct (Fig. 4E).
To investigate MPYS distribution in cells, we made a MPYS-GFP fusion construct and expressed it in K46 cells. Confocal microscopy showed that while some MPYS was found on the cell surface, a large proportion was actually localized to mitochondria (Fig. 4F).
The transmembrane region of MPYS contains four charged residues and two cysteines. Such residues often mediate inter- or intraprotein interactions. To determine if MPYS can form protein complexes, we used the membrane-permeative chemical cross-linker dithiobis(succinimidyl)propionate to form covalent bonds between neighboring proteins and performed SDS-PAGE analysis on K46 whole-cell lysates on a nonreducing SDS-PAGE gel. In addition to the monomer, MPYS immunoblotting revealed an ∼80-kDa band, a band double the size of the MPYS monomer (Fig. 4G). This suggested that most MPYS exists as a dimer within cells. The blot was stripped and reblotted with MPYS Abs in the presence of a blocking peptide (the 101 aa used to generate the MPYS Ab). As show in Fig. 4G, no protein bands were recognized, indicating that MPYS Ab blotting is specific. Sequential blotting for CD19 provided an additional control for equivalent loading (Fig. 4G).
To assess tissue distribution of MPYS, we probed SDS-PAGE fractionated and transferred lysates of various B and T cells (Fig. 4H). Anti-MPYS reacted with a predominant 40-kDa species in spleen and thymus tissue (Fig. 4H). Splenocytes have higher MPYS expression than thymocytes, which is consistent with higher level-expression in B cells (Fig. 4H). MPYS was also present in dendritic cells (Fig. 4I).
To assess potential changes in MPYS expression during B-cell development and differentiation, whole-cell lysates from B-lineage tumors were probed with MPYS Ab. MPYS Ab recognized a 40-kDa band that was enhanced in K46 cells expressing the HA-tagged mpys gene (Fig. 4J). MPYS was highly expressed in cells representing mature stages of B cells (Bal17 line) but weakly expressed in pre-B cells (70Z/3 line), immature B cells (WEHI 231 line), and memory B-cell stages (A20 line). It was not detected in plasma cells (J558L line) (Fig. 4J). Thus, it appears to be expressed throughout the B lineage prior to the plasma cell stage but occurs at highest levels in mature B cells.
MPYS possesses inhibitory signaling function.
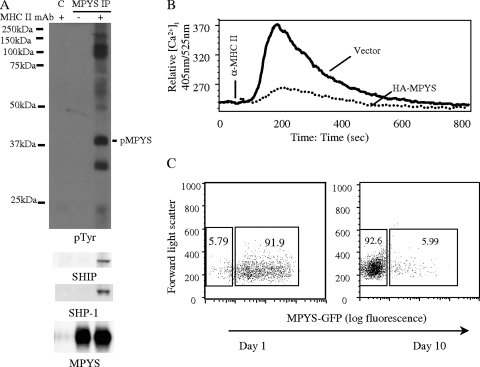
The cytoplasmic tail of MPYS contains ITIMs, motifs known to recruit the inhibitory signaling effectors SHP-1 and SHIP (11). To assess the ability of MPYS to serve an inhibitory function, K46 cells were stimulated with anti-MHC-II MAb for 2 min before cells were lysed and MPYS was immunoprecipitated, fractionated by SDS-PAGE, and subjected to immunoblotting analysis. Antiphosphotyrosine blotting revealed that MPYS is tyrosine phosphorylated upon MHC-II cross-linking (Fig. 5A). Reprobing the blot with anti-SHP-1 and SHIP Abs showed that phosphorylated MPYS bound SHP-1 and SHIP (Fig. 5A). Notably, a 32-kDa unknown tyrosine-phosphorylated protein was also associated with MPYS. Thus, MPYS engages negative signaling effectors when tyrosine is phosphorylated, consistent with its ITIM.
FIG. 5.
MPYS negatively regulates MHC-II signaling. (A) K46 cells were stimulated using biotinylated anti-MHC-II (M5/114, 20 μg/ml) and avidin (40 μg/ml) for 2 min. Cells were lysed in CHAPS buffer. MPYS was immunoprecipitated using MPYS polyclonal Ab and protein G. Normal rabbit serum IgGs and protein G were used as a control IP in the lane designated C. The blot was probed with the indicated Abs (n = 3). (B) K46 cells expressing vector alone or HA-MPYS were stimulated and calcium mobilization measured as for Fig. 2B (n > 3). (C) Sorted GFP-positive MPYS-GFP-expressing A20 cells were cultured and GFP expression levels monitored on the indicated days (n = 5).
Studies of various inhibitory receptors suggest that SHP-1 and SHIP recruitment results in inhibition of calcium mobilization (11). To assess whether MPYS mediates this function, we overexpressed MPYS in K46 cells (Fig. 4J). MPYS overexpression led to dramatically reduced MHC-II-mediated calcium mobilization (Fig. 5B). These data suggest that MPYS act in feedback regulation of some MHC-II signals, i.e., those that lead to calcium mobilization.
We also found that cells overexpressing MPYS tend to be lost from populations during culture, suggesting MPYS may have a negative effect on cell growth. To further explore this possibility, we expressed an MPYS-GFP fusion construct in A20 cells. Sorted MPYS-GFP-positive A20 B lymphoma cells lost MPYS-GFP expression within 10 days (Fig. 5C). In contrast, expression of GFP alone in A20 was maintained indefinitely (data not shown). These data indicate that MPYS functions as a negative regulator of cell growth/viability.
Knockdown of MPYS expression in K46 cells inhibits MHC class II aggregation-induced cell death and ERK activation.
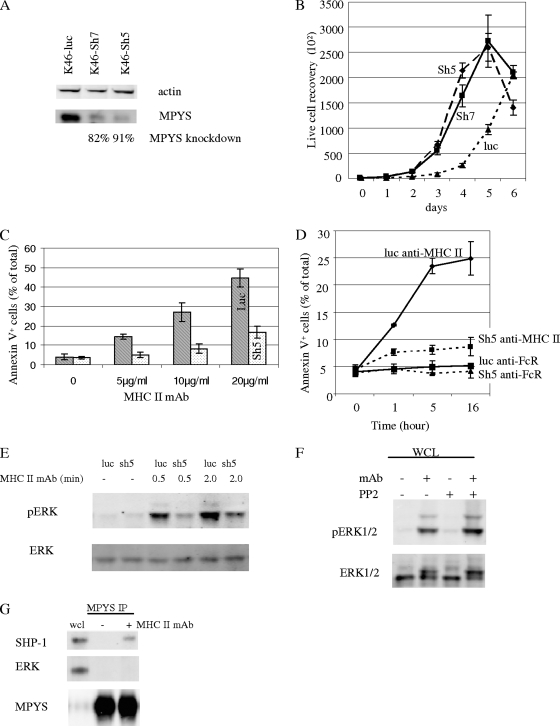
To further study the role of MPYS in MHC-II signaling, an shRNA targeting exon 5 (sh5) or exon 7 (sh7) of the mpys gene was prepared and used to knock down MPYS expression in K46 cells. Cells expressing sh5 RNA displayed a >90% reduction in MPYS expression, while in cells expressing sh7-RNA MPYS, expression decreased by >80% (Fig. 6A). Surface expression of IgM, MHC-II, CD19, CD45, CD80, and CD86 was not altered by shRNA expression (data not shown). Contrary to the case with MPYS overexpression (Fig. 5C), MPYS knockdown increased the growth rate of K46 cells (Fig. 6B).
FIG. 6.
Knockdown of MPYS expression in K46 cells inhibits MHC class II-mediated cell death and ERK activation. (A) K46 cells expressing the control shRNA targeting the luciferase gene (luc) or exon 5 of the mpys gene (sh5) or exon 7 of the mpys gene (sh7) were lysed, SDS-PAGE fractionated, transferred, and blotted with indicated Abs (n = 4). (B) K46 cells expressing different shRNA constructs were cultured, and the cell growth rate was measured using trypan blue (15250061; Invitrogen) (n = 3). (C) K46 cells expressing luc shRNA (luc) or mpys-sh5 shRNA (sh5) were stimulated with biotin-m5/114 and avidin for 5 h in cells and death measured by annexin V staining (n = 3). (D) Time course of cell death induced in shRNA-expressing populations by biotin-m5/114 (10 μg/ml) and avidin (20 μg/ml) (n = 3). Biotinylated anti-FcR MAb 2.4G2 (10 μg/ml) and avidin (20 μg/ml) were used as the isotype control (n = 3). (E) K46 cells expressing luc shRNA or sh5 shRNA were activated as for Fig. 3A. The blot was probed with the indicated Abs (n = 3). (F) Cells were treated with PP2 (10 μM) for 2 min as for Fig. 2 and were then stimulated and analyzed as for Fig. 3F. (G) MPYS IP was done as for Fig. 5A. The blot was probed with indicated Abs (n = 2). Error bars in all panels represent standard deviations of triplicate assays. Data shown are representative of at least three experiments.
We next examined the role of MPYS in the death response by assessing anti-MHC-II induction of death in K46 cells expressing MPYS knockdown constructs. K46 cells expressing a control luciferase shRNA (luc) or MPYS sh5 knockdown shRNA (sh5) were stimulated with biotinylated MAb M5/114 and avidin, and cell death was measured by annexin V/PI dual staining (Fig. 6C and D). Biotinylated MAb 2.4G2 was used as an isotype control. MHC-II MAb-induced cell death was reduced significantly in K46 cells expressing the sh5 MPYS construct (where MPYS expression is diminished by >90%) (Fig. 6C and D). An effect of MPYS knockdown was noted at all doses of anti-MHC-II MAb used (Fig. 6C). Similar results were observed in K46 cells expressing the sh7 MPYS knockdown construct (data not shown). The fact that two shRNAs, targeting different regions of mpys, caused similar outcomes argues strongly that this is not an off-target effect. Similar results were observed when other anti-MHC-II Abs were used for stimulation (data not shown). We conclude that MPYS expression is essential for anti-MHC-II MAb induction of B-cell death.
To extend earlier findings that MHC-II-mediated activation of ERK is required for the death response, we investigated the effect of MPYS knockdown on ERK activation. We probed fractionated and transferred lysates of luc or sh5 shRNA-expressing K46 cells using phospho-ERK Ab. MPYS knockdown inhibited MHC-II MAb-induced ERK activation (Fig. 6E). These findings indicate that MHC class II signaling of cell death is dependent on MPYS-linked ERK activation.
MHC-II-mediated, MPYS-dependent activation of ERK is Src family kinase independent.
If, as shown in Fig. 2, anti-MHC class II-induced death is not dependent on Src family kinase-mediated tyrosine phosphorylation but is ERK dependent, one would predict that ERK activation by class II signals should not be inhibited by PP2. As shown in Fig. 6F, this is the case. Taken together, these data indicate that the death response is mediated by Src family kinase-independent activation of MPYS and downstream ERK. Further, PP2-sensitive anti-class II-activated signaling events, including calcium mobilization and MPYS tyrosine phosphorylation and association with SHIP-1 and SHP-1, are not required for the death response (Fig. 2; also data not shown).
Finally, we addressed whether the MPYS dependence of ERK activation reflects a requirement that MPYS interact directly with ERK. Cells were activated with MHC-II MAb before being lysed and subjected to MPYS immunoprecipitation. Immunoprecipitates were analyzed by SDS-PAGE and ERK immunoblotting. Under conditions in which we observed SHP-1 recruitment to MPYS, we did not detect recruitment of ERK (Fig. 6G).
MHC-II-independent aggregation of MPYS leads to cell death signaling.
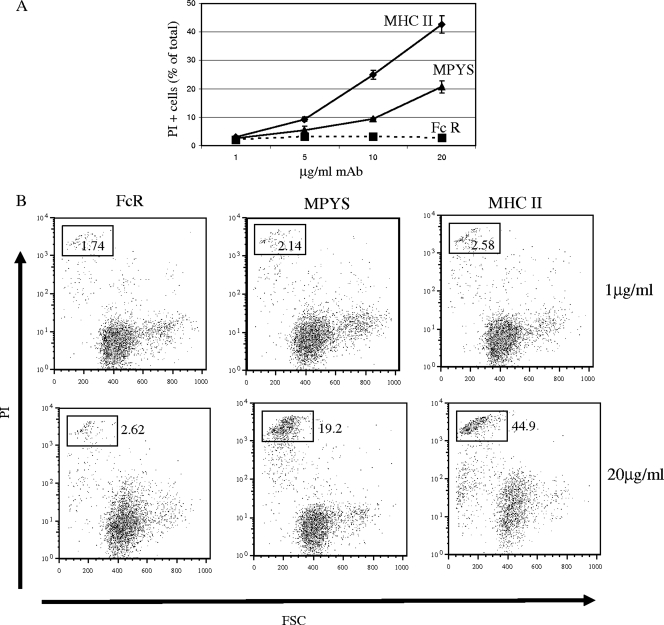
To test the ability of MPYS to transduce death signals when aggregated independently, we stimulated K46 cells expressing Flag-tagged MPYS (LEL-MPYS) with anti-Flag and measured death by PI staining. As shown in Fig. 7, anti-Flag treatment led to a 10-fold increase in PI staining of cells within 15 h of stimulation. This response, while clearly significant and Ab dose dependent, was somewhat less than that induced by anti-MHC-II. We conclude that MPYS may function in isolation as a transducer of death signals and thus could mediate death signaling in MHC-II-negative cells, such as T cells.
FIG. 7.
Aggregation of surface MPYS induces K46 cell death. (A) K46 cells expressing the Flag-tagged-MPYS (LEL-MPYS) were stimulated for 15 h with different doses of 2.4G2 anti-FcR MAb (FcR), anti-Flag MAb (MPYS), or D3.137 anti-MHC-II MAb (MHC II). Cell death was measured by PI staining and forward light scatter properties. Shown are dead cells as a percentage of the total (n = 2). (B) Cytograms displaying PI staining as a function of forward light scatter of cells described for panel A (n = 2).
DISCUSSION
The heterodimeric MHC class II protein complex mediates binding of antigenic peptides and presentation of these peptides to CD4+ T cells for their consequent activation, proliferation, and differentiation. It is becoming increasingly clear that under some circumstances, it is important that cells presenting antigen be eliminated once they have served their function. Available evidence indicates that this may occur by TCR-induced MHC class II-mediated induction of apoptotic death. Importantly, such a mechanism would ensure elimination of only APC that had presented specific, potentially offensive antigens and would function to terminate responses to those antigens. Although this is not surprising for dendritic cells, it seems somewhat counterintuitive that such a mechanism would eliminate B-cell APC, some of whose daughters would be expected to become Ab-secreting cells. Nonetheless, there is compelling evidence that some ex vivo B cells die an apoptotic death following aggregation of their MHC class II (23). This fate may be reserved for a certain B-cell subpopulation whose continued antigen presentation and/or Ab production might be disadvantageous for the animal. For example, this mechanism may be important in elimination of autoreactive B cells. Indeed, one report has described T-cell-contact-induced death of anergic B cells (24).
This study focused on understanding how MHC class II molecules transduce apoptotic signals. This response is known not to be dependent on the short, 12- and 18-aa cytoplasmic tails of MHC-II (16), suggesting the function of an MHC-II-associated transmembrane protein in transduction of these signals. Although multiple candidate-associated molecules have been advanced, for reasons discussed earlier none of these are likely possibilities. Therefore, we embarked on a search for associated proteins that mediate MHC class II transduction of death signals. Nano-LC-MS/MS analysis of MHC class II-associated proteins revealed a previously unknown membrane tetraspanner that is associated with class II and expressed in both mature B cells and dendritic cells (Fig. 4): cells that undergo apoptotic death in response class II aggregation. Consistent with a transmembrane signaling function, the protein, termed MPYS, is found on the cell surface and contains multiple sites of predicted protein-protein interaction in its 140-aa cytoplasmic tail (Fig. 4).
Based on these features, we explored the role of MPYS in signaling. MPYS was tyrosine phosphorylated following MHC class II aggregation (Fig. 5). Consistent with its content of ITIMs, this phosphorylation was associated with its binding of SHP-1 and SHIP-1 (Fig. 5). Consistent with the previously demonstrated role of Src family kinases in tyrosine phosphorylation of ITIMs (29), the Src inhibitor PP2 blocked MPYS tyrosine phosphorylation and recruitment of SHP-1 and SHIP-1 (data not shown). Suggesting that inhibitory signaling by these molecules is operative during class II signaling, MPYS overexpression inhibited class II calcium signaling and placed cells at a competitive disadvantage in terms of growth (Fig. 5). Conversely, MPYS knockdown promoted growth (Fig. 6). Taken together, these data indicate that at least two signaling pathways emanate from MHC class II on these cells. One of them acts through Ig-α/β and SRC family kinase activation to mediate calcium mobilization (18) (Fig. 2 and 8). A second, acting through MPYS tyrosine phosphorylation and recruitment of SHP-1 and/or SHIP-1, mediates inhibition of calcium signaling and cell growth.
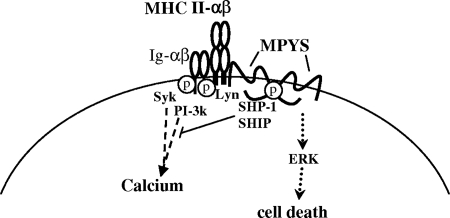
FIG. 8.
Model: MPYS and ERK mediate MHC-II signaling-induced cell death.
In parallel experiments, MPYS expression was found to be required for MHC class II transduction of signals leading to ERK activation and apoptotic death. These responses were found to be linked, since the ERK inhibitor PD98059 blocked both ERK phosphorylation and the cell death response (Fig. 3). Parenthetically, the class II death response was not affected by SB203580 or Ly294002, blockers of the Akt and p38 responses that also accompany class II signaling (Fig. 3). Surprisingly, however, neither MHC class II-mediated activation of ERK nor apoptosis was inhibited by the Src family tyrosine kinase inhibitor PP2 (Fig. 6 and 2). These findings indicate that a third signaling pathway emanates from MHC class II (Fig. 8). This pathway involves tyrosine phosphorylation-independent, but MPYS-dependent, ERK activation and leads to cell death. It can be activated by “direct” Ab-mediated aggregation of MPYS (7), suggesting that this signaling pathway is autonomous.
The role of MPYS in death responses involving mitochondrial membrane depolarization is particularly interesting in view of the demonstrated localization of MPYS both on the cell membranes and in mitochondria (Fig. 4). It is tempting to speculate that MPYS function somehow involves shuttling between these subcellular compartments. More studies will be required to address this question.
Supplementary Material
Acknowledgments
We thank Ryan Young and Yosef Refaeli for providing the shRNA knockdown protocol and the pLL3.7 vector, Aimee Pugh-Bernard for the K46 cDNA library, and Rick Willis for bone marrow-derived dendritic cells.
This work is supported by a grant from the National Institute of Allergy and Infectious Diseases, 5R01AI020519-22, to J.C.C. J.C.C. is an Ida and Cecil Green Professor of Immunology. The CNRU Mass Spectrometry Core is supported by NIH/NIDDK grant P30 DK048520.
We have no conflicting interests to declare.
Footnotes
Published ahead of print on 16 June 2008.
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Al-Daccak, R., N. Mooney, and D. Charron. 2004. MHC class II signaling in antigen-presenting cells. Curr. Opin. Immunol. 16108-113. [DOI] [PubMed] [Google Scholar]
- 2.Bertho, N., V. M. Blancheteau, N. Setterblad, B. Laupeze, J. M. Lord, B. Drenou, L. Amiot, D. J. Charron, R. Fauchet, and N. Mooney. 2002. MHC class II-mediated apoptosis of mature dendritic cells proceeds by activation of the protein kinase C-delta isoenzyme. Int. Immunol. 14935-942. [DOI] [PubMed] [Google Scholar]
- 3.Bobbitt, K. R., and L. B. Justement. 2000. Regulation of MHC class II signal transduction by the B cell coreceptors CD19 and CD22. J. Immunol. 1655588-5596. [DOI] [PubMed] [Google Scholar]
- 4.Bridges, S. H., A. M. Kruisbeek, and D. L. Longo. 1987. Selective in vivo antitumor effects of monoclonal anti-I-A antibody on B cell lymphoma. J. Immunol. 1394242-4249. [PubMed] [Google Scholar]
- 5.Brown, K. S., D. J. Levitt, M. Shannon, and B. K. Link. 2001. Phase II trial of Remitogen (humanized 1D10) monoclonal antibody targeting class II in patients with relapsed low-grade or follicular lymphoma. Clin. Lymphoma 2188-190. [DOI] [PubMed] [Google Scholar]
- 6.Cambier, J. C., M. K. Newell, L. B. Justement, J. C. McGuire, K. L. Leach, and Z. Z. Chen. 1987. Ia binding ligands and cAMP stimulate nuclear translocation of PKC in B lymphocytes. Nature 327629-632. [DOI] [PubMed] [Google Scholar]
- 7.Carlo-Stella, C., M. Di Nicola, M. C. Turco, L. Cleris, C. Lavazza, P. Longoni, M. Milanesi, M. Magni, M. Ammirante, A. Leone, Z. Nagy, W. R. Gioffre, F. Formelli, and A. M. Gianni. 2006. The anti-human leukocyte antigen-DR monoclonal antibody 1D09C3 activates the mitochondrial cell death pathway and exerts a potent antitumor activity in lymphoma-bearing nonobese diabetic/severe combined immunodeficient mice. Cancer Res. 661799-1808. [DOI] [PubMed] [Google Scholar]
- 8.Carmagnat, M., B. Drenou, H. Chahal, J. M. Lord, D. Charron, J. Estaquier, and N. A. Mooney. 2006. Dissociation of caspase-mediated events and programmed cell death induced via HLA-DR in follicular lymphoma. Oncogene 251914-1921. [DOI] [PubMed] [Google Scholar]
- 9.Chen, M., Y. H. Wang, Y. Wang, L. Huang, H. Sandoval, Y. J. Liu, and J. Wang. 2006. Dendritic cell apoptosis in the maintenance of immune tolerance. Science 3111160-1164. [DOI] [PubMed] [Google Scholar]
- 10.Chen, Z. Z., K. M. Coggeshall, and J. C. Cambier. 1986. Translocation of protein kinase C during membrane immunoglobulin-mediated transmembrane signaling in B lymphocytes. J. Immunol. 1362300-2304. [PubMed] [Google Scholar]
- 11.Dal Porto, J. M., S. B. Gauld, K. T. Merrell, D. Mills, A. E. Pugh-Bernard, and J. Cambier. 2004. B cell antigen receptor signaling 101. Mol. Immunol. 41599-613. [DOI] [PubMed] [Google Scholar]
- 12.Drenou, B., L. Amiot, N. Setterblad, S. Taque, V. Guilloux, D. Charron, R. Fauchet, and N. Mooney. 2005. MHC class II signaling function is regulated during maturation of plasmacytoid dendritic cells. J. Leukoc. Biol. 77560-567. [DOI] [PubMed] [Google Scholar]
- 13.Drenou, B., V. Blancheteau, D. H. Burgess, R. Fauchet, D. J. Charron, and N. A. Mooney. 1999. A caspase-independent pathway of MHC class II antigen-mediated apoptosis of human B lymphocytes. J. Immunol. 1634115-4124. [PubMed] [Google Scholar]
- 14.Guo, W., J. G. Castaigne, N. Mooney, D. Charron, and R. Al-Daccak. 2003. Signaling through HLA-DR induces PKC beta-dependent B cell death outside rafts. Eur. J. Immunol. 33928-938. [DOI] [PubMed] [Google Scholar]
- 15.Ingulli, E., A. Mondino, A. Khoruts, and M. K. Jenkins. 1997. In vivo detection of dendritic cell antigen presentation to CD4(+) T cells. J. Exp. Med. 1852133-2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jin, L., J. C. Stolpa, R. M. Young, A. E. Pugh-Bernard, Y. Refaeli, and J. C. Cambier. 2008. MHC class II structural requirements for the association with Igα/β, and signaling of calcium mobilization and cell death. Immunol. Lett. 116184-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim, K. J., F. I. Weinbaum, and R. Asofsky. 1978. Functional characteristics of BALB/c T cell lines: suppression in the mixed lymphocyte response and cell-mediated lysis. J. Immunol. 1212299-2304. [PubMed] [Google Scholar]
- 18.Lang, P., J. C. Stolpa, B. A. Freiberg, F. Crawford, J. Kappler, A. Kupfer, and J. C. Cambier. 2001. TCR-induced transmembrane signaling by peptide/MHC class II via associated Ig-alpha/beta dimers. Science 2911537-1540. [DOI] [PubMed] [Google Scholar]
- 19.Leveille, C., R. Al-Daccak, and W. Mourad. 1999. CD20 is physically and functionally coupled to MHC class II and CD40 on human B cell lines. Eur. J. Immunol. 2965-74. [DOI] [PubMed] [Google Scholar]
- 20.Longo, D. L. 2002. DR's orders: human antibody kills tumors by direct signaling. Nat. Med. 8781-783. [DOI] [PubMed] [Google Scholar]
- 21.Nagy, Z. A., B. Hubner, C. Lohning, R. Rauchenberger, S. Reiffert, E. Thomassen-Wolf, S. Zahn, S. Leyer, E. M. Schier, A. Zahradnik, C. Brunner, K. Lobenwein, B. Rattel, M. Stanglmaier, M. Hallek, M. Wing, S. Anderson, M. Dunn, T. Kretzschmar, and M. Tesar. 2002. Fully human, HLA-DR-specific monoclonal antibodies efficiently induce programmed death of malignant lymphoid cells. Nat. Med. 8801-807. [DOI] [PubMed] [Google Scholar]
- 22.Nagy, Z. A., and N. A. Mooney. 2003. A novel, alternative pathway of apoptosis triggered through class II major histocompatibility complex molecules. J. Mol. Med. 81757-765. [DOI] [PubMed] [Google Scholar]
- 23.Newell, M. K., J. VanderWall, K. S. Beard, and J. H. Freed. 1993. Ligation of major histocompatibility complex class II molecules mediates apoptotic cell death in resting B lymphocytes. Proc. Natl. Acad. Sci. USA 9010459-10463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rathmell, J. C., S. E. Townsend, J. C. Xu, R. A. Flavell, and C. C. Goodnow. 1996. Expansion or elimination of B cells in vivo: dual roles for CD40- and Fas (CD95)-ligands modulated by the B cell antigen receptor. Cell 87319-329. [DOI] [PubMed] [Google Scholar]
- 25.Setterblad, N., V. Blancheteau, A. Delaguillaumie, F. Michel, S. Becart, G. Lombardi, O. Acuto, D. Charron, and N. Mooney. 2004. Cognate MHC-TCR interaction leads to apoptosis of antigen-presenting cells. J. Leukoc. Biol. 751036-1044. [DOI] [PubMed] [Google Scholar]
- 26.Tamir, I., J. C. Stolpa, C. D. Helgason, K. Nakamura, P. Bruhns, M. Daeron, and J. C. Cambier. 2000. The RasGAP-binding protein p62dok is a mediator of inhibitory FcγRIIB signals in B cells. Immunity 12347-358. [DOI] [PubMed] [Google Scholar]
- 27.Truman, J. P., M. L. Ericson, C. J. Choqueux-Seebold, D. J. Charron, and N. A. Mooney. 1994. Lymphocyte programmed cell death is mediated via HLA class II DR. Int. Immunol. 6887-896. [DOI] [PubMed] [Google Scholar]
- 28.Ventura, A., A. Meissner, C. P. Dillon, M. McManus, P. A. Sharp, L. Van Parijs, R. Jaenisch, and T. Jacks. 2004. Cre-lox-regulated conditional RNA interference from transgenes. Proc. Natl. Acad. Sci. USA 10110380-10385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vivier, E., and M. Daeron. 1997. Immunoreceptor tyrosine-based inhibition motifs. Immunol. Today 18286-291. [DOI] [PubMed] [Google Scholar]
- 30.Wang, W., and B. A. Malcolm. 1999. Two-stage PCR protocol allowing introduction of multiple mutations, deletions and insertions using QuikChange site-directed mutagenesis. BioTechniques 26680-682. [DOI] [PubMed] [Google Scholar]
- 31.Wille, L., M. L. Kemp, P. Sandy, C. L. Lewis, and D. A. Lauffenburger. 2007. Epi-allelic Erk1 and Erk2 knockdown series for quantitative analysis of T cell Erk regulation and IL-2 production. Mol. Immunol. 443085-3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.