Abstract
Pituitary adenylate cyclase-activating polypeptide 38 (PACAP38) is a potent neuropeptide that acts through G-protein-coupled receptors. While it is well established that PACAP mediates both neurotrophic and neurodevelopmental effects, the signaling cascades that underlie these diverse actions remain incompletely characterized. Here we show that the Ras-related Rin GTP-binding protein, a GTPase that is expressed predominantly in neurons, is regulated by PACAP38 signaling, and loss-of-function analysis demonstrates that Rin makes an essential contribution to PACAP38-mediated pheochromocytoma cell differentiation. Rin is activated following stimulation of both Gsα and Giα cascades but does not rely upon cyclic AMP (cAMP)-, Ca2+-, or Epac-dependent signaling pathways. Instead, Rin is activated in a Src kinase-dependent manner. Surprisingly, Rin knockdown significantly inhibits PACAP38-mediated neurite outgrowth, without affecting mitogen-activated protein kinase signaling cascades. Instead, Rin loss attenuates PACAP38-mediated HSP27 activation by disrupting a cAMP-protein kinase A cascade. RNA interference-mediated HSP27 silencing suppresses both PACAP38- and Rin-mediated neurite outgrowth, while expression of a constitutively active Rin mutant increases both HSP27 protein and phospho-HSP27 levels, supporting a role for Rin-HSP27 signaling in neuronal differentiation. Together, these observations identify an unsuspected role for Rin in neuronal PACAP signaling and establish a novel Gα-Src-Rin-HSP27 signal transduction pathway as a critical element in PACAP38-mediated neuronal differentiation signaling.
The neuropeptide pituitary adenylate cyclase-activating polypeptide 38 (PACAP38) is widely expressed within the nervous system and is a member of the vasoactive intestinal peptide/secretin/glucagon polypeptide family (73, 77). PACAP38 binds and activates G-protein-coupled receptor (GPCR) family members to regulate a number of nerve cell functions, including differentiation, axonal and dendritic growth, and cell survival (79). In pheochromocytoma 12 (PC12) cells, PACAP38 exposure results in differentiation characterized by neurite elongation (13, 41, 63). Although a comprehensive understanding of the signaling network required to promote neuritogenesis following PACAP38 receptor stimulation is lacking, activation of adenylate cyclase and regulation of both extracellular signal-regulated kinase (ERK) and p38 mitogen-activated protein (MAP) kinase cascades have been shown to play critical roles (19, 35, 63, 72). While cyclic AMP (cAMP) signaling has classically been associated with activation of protein kinase A (PKA) and while cAMP analogues can induce PC12 cell differentiation (24), the neurotrophic effects of PACAP38 do not rely solely upon PKA signaling. Recent work has identified the cAMP-activated Epac guanine nucleotide exchange factors as crucial mediators of PKA-independent cAMP signaling (8, 67). Epac proteins are required for cAMP-mediated differentiation signaling (35), serving to link Gsα-coupled receptor signaling to both ERK and MAP kinase activation in neuronal cells (48), and for the activation of a second Rit-p38 MAP kinase signaling pathway (72). Thus, the cell-type-specific actions of cAMP are the result of both PKA-dependent and PKA-independent signaling cascades.
Recent studies have identified the Rin small GTP-binding protein as an important mediator of nerve growth factor (NGF)-dependent neuronal differentiation (30-32, 43, 68, 71, 74, 80). Rin is highly expressed in neurons and shares a conserved and unique effector domain with the closely related Rit and Drosophila Ric proteins (43, 68, 80). Rin fails to undergo posttranslational lipidation, a modification required for the association of the majority of Ras proteins with cellular membranes. Instead, Rin contains a conserved polybasic C-terminal domain that was recently shown to direct interactions with phosphatidylinositol lipids (27). We have shown that Rin is activated following NGF stimulation in pheochromocytoma cells and that Rin signaling plays a critical role in NGF-mediated neuronal differentiation (71, 74).
It is well established that neurotrophic factors act through cell surface receptors to activate often convergent signaling cascades to promote both neurite outgrowth in pheochromocytoma cells and elongation of axons and dendrites in neurons (64). Among the targets of these pathways are regulators of cytoskeleton dynamics, particularly Rho family GTPases (22). Another class of regulatory factors is the small heat shock proteins, including heat shock protein 27 (HSP27). HSP27 has reported roles in the regulation of apoptosis and in protein folding and interacts with both actin and tubulin (1, 2, 23, 39, 61). Phosphorylated HSP27 acts to promote actin polymerization and stress fiber formation and stabilizes the actin cytoskeleton (4, 23, 39, 40), while nonphosphorylated HSP27 inhibits in vitro actin polymerization (4, 56). HSP27 expression is developmentally regulated in dorsal root ganglion neurons, is upregulated in response to nerve injury and hyperthermia (11, 29, 37, 46, 78), and contributes to axonal outgrowth (81, 82). Indeed, missense mutations of HSP27 are associated with peripheral neuropathies (17).
In this report, we investigated the molecular events that mediate PACAP38-induced differentiation and the role of the Rin GTPase in this process. We demonstrate that Rin is activated by PACAP38 signaling, in a manner that depends upon a pertussis toxin (PTX)-sensitive Gα subunit signaling pathway but does not involve cAMP-, Ca2+-, or Epac-dependent signaling pathways. Instead, Rin activation is dependent upon a Gsα/Giα-mediated Src kinase signaling cascade. Importantly, Rin silencing blocked PACAP38-mediated neurite outgrowth, without altering either ERK or p38 MAP kinase signaling, but strongly inhibited HSP27 activation. Furthermore, expression of constitutively active Rin (RinQ78L) promoted increased phospho-HSP27 levels and resulted in a threefold increase in endogenous HSP27 protein levels, while HSP27 silencing inhibited both PACAP- and Rin-mediated neuritogenesis. Moreover, Rin knockdown attenuated PACAP38-induced cAMP production, and both RinQ78L-mediated neurite outgrowth and HSP27 phosphorylation were found to be cAMP- and PKA-dependent processes. Together, these observations indicate that PACAP38-mediated neuronal differentiation relies upon a novel Rin-HSP27 signaling cascade.
MATERIALS AND METHODS
Plasmids and reagents.
Epitope-tagged mouse and human Rin, Rap1A, and Ras and their mutants have been described previously (70-72). The expression vectors for Gi2αQ204L (QL), GqαQ209L (QL), GsαQ227L (QL), G12αQ229L (QL), dominant-negative c-Src (DN-Src) [pUse-c-Src (K296R/Y528F)], and constitutively active c-Src (CA-Src) [pUse-c-Src (Y529F)] were kindly provided by J. H. Kehrl (National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD) (69). Site-directed mutagenesis was used to generate Src mutants [pUse-c-Src (S17A) and pUse-CA-c-Src (S17A)]. The mutants were subsequently confirmed by sequencing. Constitutively active Epac2 (Epac2ΔCBR) was kindly provided by L. Quilliam (Indiana University School of Medicine, Indianapolis, IN) (47) and includes residues 430 to 994 of the full-length protein. PACAP38 was purchased from Bachem California (Torrance, CA). Antibodies against the following were purchased: Flag (Sigma, St. Louis, MO); phospho-specific ERK1/2, phospho-specific p38, phospho-specific HSP27, p38 MAPK, and HSP27 (Cell Signaling, Beverly, MA); and ERK1/2 and actin (Santa Cruz Biotechnology, Santa Cruz, CA). The MEK1/2 inhibitor PD98059, the Src kinase inhibitor PP2 and its inactive analogue (PP3), 2′,5′-dideoxyadenosine (ddA), H89, the inactive cAMP analogue adenosine 3′,5′-cyclic monophosphorothioate, Rp isomer (Rp-cAMP), 8-bromo-adenosine-3′,5′-cyclic monophosphate (8-Br-cAMP), 8-(p-chlorophenylthio)-2′-O-methyl-adenosine-3′,5′-cyclic monophosphate (8-CPT-2-Me-cAMP), and cholera toxin (CTX) were purchased from Calbiochem, La Jolla, CA; the p38 MAP kinase inhibitor SB203580 was purchased from Tocris, Ellisville, MO; and PTX, A23187, potassium chloride (KCl), and forskolin were purchased from Sigma, St. Louis, MO. Glutathione S-transferase-Raf-Rin/Ras interacting domain (GST-Raf-RID) glutathione-agarose beads were prepared from bacterially expressed GST-Raf-RID as described previously (65).
Cell lines, cell cultures, and transfections.
PC6 is a subline of PC12 cells that produces neurites in response to NGF but grows as well-isolated cells rather than in clumps (the generous gift of T. Vanaman, University of Kentucky, Lexington). The cells were maintained and transfected with Effectene (Qiagen) as described previously (71, 74).
RNA interference.
The mammalian expression vector pSUPER-GFP/Neo (OligoEngine) was used for expression of small interfering RNA (siRNA) in PC6 cells. The vector allows direct synthesis of siRNA transcripts by use of the RNA polymerase H1 promoter and coexpresses green fluorescent protein (GFP) to allow detection of transfected cells. The gene-specific insert sequence of rat HSP27 (GACCAAGGAAGGCGTGGTG [target sense]), which was separated by a 9-nucleotide noncomplementary spacer (TTCAAGAGA) from the reverse complement of the same HSP27-specific 19-nucleotide sequence, was synthesized and then subcloned into the BglII and HindIII sites of pSUPER-GFP/Neo to generate pSUPER-shHSP27-344 (shHSP27-344). Other rat target sense sequences used in this study included shHSP27-560 (TTTCGAGGCCCGTGCCCAA), shPACR1-384 (CTACTTCGATGCTTGTGGG), and shRin99 (AGCGCAGTCACAATGCAGT) (71). The resulting constructs were verified by DNA sequencing. A siRNA with no predicted target site in the rat genome (Scramber) was inserted into the same sites in pSUPER-GFP/Neo to generate pSUPER-Scramber (shCTR) and served as a negative control (71). To reconstitute the Rin deficiency in PC6 cells, PC6 cells expressing shRin99 or shCTR (1.5 μg) were cotransfected with 0.2 μg of pcDNA3.1-hRin-WT. To determine the effects of shHSP27 on the expression of endogenous HSP27 proteins, PC6 cells were transfected with 1.5 μg of shHSP27-344, shHSP27-560, or shCTR as a control. siRNA-transfected cells were enriched by G418 selection (400 μg/ml) for 60 h, and total cell lysates were prepared with kinase lysis buffer and subjected to immunoblotting with either anti-Rin or anti-HSP27 antibodies to determine the expression levels of overexpressed human Rin (hRin) or endogenous HSP27. Levels of actin in the lysates were monitored to demonstrate equal loading. To determine the efficacy of shPACR1-384 treatment, total RNA was isolated, using an RNeasy Mini kit (Qiagen), from PC6 cells transfected with shCTR or shPACR1-384 (1.5 μg) following G418 enrichment (400 μg/ml for 60 h). Total RNA (2 μg) was subjected to reverse transcription by use of an Omniscript reverse transcription kit (Qiagen) and the following primers used for PCR analysis: for rat PACR1, GCTGGCCCGCTCCACCCTACT and TCAGGTGGCCAAGTTGTCGGCC (358 bp); and for rat β-actin, GTTTGAGACCTTCAACACCC and ATACTCCTGCTTGCTGATCC (842 bp).
Neurite outgrowth.
To determine the requirement of Rin for PACAP38-mediated neurite outgrowth, PC6 cells were transfected with shRin99 or shCTR after pretreatment with or without SB203580 (10 μM) or PD98059 (10 μM), using Effectene (Qiagen), and then replated at a 1:4 dilution and exposed simultaneously to PACAP38 (5 nM) to initiate differentiation and to G418 selection to eliminate untransfected cells. On days 3 and 7 after PACAP exposure, cells were fixed with methanol-acetone (3:1), and images of random fields were captured on an Axiovert 200 M phase-contrast microscope (Zeiss), using a ×20 objective lens and OpenLab 3.1.4 imaging software. We analyzed the percentage of neurite-bearing cells, neurite number per cell body, neurite length, and number of branch points per neurite in three separate experiments, as described previously (70-72). To determine whether the shRin99-mediated differentiation block could be recovered by the reintroduction of exogenous Rin, PC6 cells were transfected with 0.2 μg of empty pcDNA3.1 vector or pcDNA3.1-hRin-WT in the presence of 1.5 μg of shRin99 or shCTR, and neurite outgrowth was initiated and analyzed on day 3 as described above. To examine the requirement of Src and PKA signaling for PACAP38-mediated neuronal differentiation, PC6 cells seeded at a low density (1 × 103/cm2) were pretreated with PP2 (10 μM), PP3 (10 μM), H89 (10 μM), Rp-cAMP (50 μM), or dimethyl sulfoxide (DMSO) before initiation of neurite outgrowth by PACAP38 (10 nM), and the percentages of neurite-bearing cells were analyzed on days 3 and 7 as described above. To determine the requirement for HSP27 in Rin-mediated neurite outgrowth, PC6 cells were cotransfected with either 3×Flag-Rin-Q78L or empty 3×Flag vector and either shCTR, shHSP27-344, or shHSP27-560 (all vectors were used at 1.0 μg). To examine the role of HSP27 in PACAP38-mediated neurite outgrowth, PC6 cells expressing either shCTR, shHSP27-344, or shHSP27-560 were exposed to PACAP38 (10 nM) as indicated, while untreated transfected cells were used as a negative control for these studies. To explore the signaling pathways in Rin-mediated neurite outgrowth, PC6 cells were transfected with 3×Flag-Rin-Q78L (1.5 μg) after pretreatment with H89 (10 μM), Rp-cAMP (50 μM), PP2 (10 μM), PP3 (10 μM), or DMSO vehicle and then replated at a 1:4 dilution with complete Dulbecco's modified Eagle's medium (DMEM) containing G418 (400 mg/ml) in the presence of the appropriate inhibitors or vehicle as a control.
MAP kinase assay and immunoblotting.
PC6 cells seeded in six-well plates were transfected with shRin99 or shCTR as a control and then subjected to G418 selection for 48 h to remove untransfected cells. The remaining short hairpin RNA (shRNA)-expressing cells were starved for 5 h before stimulation with PACAP38 (5 nM) for the indicated duration. The phosphorylation levels of ERK1/2, p38 MAPK, and HSP27 (Ser 82) were determined by immunoblotting with phospho-specific antibodies. To determine the signaling pathways involved in Rin signaling, PC6 cells were pretreated with H89 (10 μM), Rp-cAMP (50 μM), PP2 (10 μM), PP3 (10 μM), or DMSO vehicle for 30 min before transfection with 3×Flag-Rin-Q78L and then were subjected to G418 (400 μg/ml) selection for 48 h. Cells were then starved for 5 h and analyzed by immunoblotting. To determine the signaling pathways that contribute to PACAP38 signaling, PC6 cells seeded at a density of 5 × 104/cm2 were allowed to grow for 48 h, subjected to serum starvation for 5 h, and subsequently treated with PP2 (10 μM), PP3 (10 μM), PTX (100 ng/ml), ddA (50 μM), Rp-cAMP (50 μM), or DMSO vehicle for 30 min prior to stimulation with PACAP38 (10 nM) for 20 min. NGF stimulation (100 ng/ml for 15 min) was used as a positive control.
Immunoblots were blocked in 1% casein (Sigma) in phosphate-buffered saline (PBS) supplemented with 0.1% Tween 20 (PBST) for 1 h at 25°C and then incubated with an appropriate dilution of the primary antibody in 1% casein or 5% bovine serum albumin in PBST for 1 to 2 h. The immunoblots were washed three times with PBST before the addition of a horseradish peroxidase (HRP)-conjugated secondary antibody (Zymed Laboratories Inc., San Francisco, CA) diluted 1:20,000 in 1% casein in PBST. The signal was detected by chemiluminescence (SuperSignal West Pico system; Pierce, Rockford, IL) as described previously (70-72). Stripping and reprobing of immunoblots to ensure equal expression of recombinant proteins were performed as described previously (71, 72).
cAMP assay.
cAMP assays were performed using a cAMP direct immunoassay kit (BioVision, Mountain View, CA) following the manufacturer's instructions. In brief, transfected PC6 cells were washed once with 1× PBS, incubated with 500 μl of 0.1 N HCl for 20 min at room temperature, and removed from the plate by repeated pipetting to achieve a uniform suspension. The collected fractions were centrifuged (13,000 rpm) at room temperature for 10 min, and the supernatants were transferred to fresh tubes. The resulting cell supernatant (100 μl) was neutralized with neutralizing buffer (50 μl) and acetylated at room temperature for 10 min, and assay buffer (845 μl) was added to each sample. Standard cAMP provided by the manufacturer was serially diluted with 0.1 N HCl to concentrations from 100 to 1.5625 fmol/50 μl and then neutralized and acetylated as described above to generate a standard curve. Acetylated standard (50 μl) or test samples (50 μl) were added to a protein A-coated assay plate, assay buffer (10 μl) containing anti-cAMP polyclonal antibody was added to each well (except for the 0-fmol standard), and the mixtures were incubated for 1 h at room temperature with agitation. Diluted cAMP-HRP (10 μl) was then added to each well, and the mixtures were incubated for a second time for 1 h at room temperature with agitation. Wells were washed five times with assay buffer (200 μl) before the addition of HRP developer (100 μl). Reaction mixtures were finally incubated for 1 h at room temperature with agitation, and reactions were stopped by the addition of 1 N HCl (100 μl) and read immediately at 450 nm, using a microplate reader (Bio-Rad). The cAMP levels were calculated using the cAMP standard curve after subtraction of the background reading (0-fmol cAMP well). Results are represented as changes in induction, calculated by dividing the cAMP amount in experimental wells by that in the negative control wells. To examine the role of Rin in PACAP38-mediated cAMP signaling, PC6 cells were transfected with empty 3×Flag vector, 3×Flag-Rin-S34N, pSuper-ShRin99, or 3×Flag-H-Ras-S17N (1.5 μg vector/transfection) and subjected to G418 selection for 60 h. Cells were subsequently starved with serum-free DMEM for 5 h and stimulated with PACAP38 (10 nM) for 0, 15, or 30 min. To determine the ability of Rin and Ras to stimulate cAMP signaling, PC6 cells were transfected with empty 3×Flag vector, 3×Flag-Rin-Q78L, or 3×Flag-H-Ras-Q61L (1.5 μg) and cultured for an additional 36 h. Cells were starved with serum-free DMEM for 5 h before being harvested and analyzed for cAMP production as described above.
Rin-GTP precipitation assays.
GST fusion proteins containing the Rin binding domain of Raf-RID (residues 1 to 140) were expressed and purified, and Rin activation was assessed essentially as described previously (71). In brief, PC6 cells seeded in six-well plates were transfected with 3×Flag-Rin-WT, using Effectene, and incubated for an additional 36 h to allow maximal gene expression. Cells were then starved in serum-free DMEM for an additional 5 h. Cell monolayers were washed once in ice-cold PBS and lysed in GST pull-down assay buffer (20 mM HEPES [pH 7.4], 250 mM NaCl, 50 mM KF, 50 mM β-glycerolphosphate, 1% Triton X-100, 10% glycerol, and 1× protease inhibitor cocktail) with sonication on ice. GST resin (10 μg of the appropriate fusion protein/20 μl glutathione beads) was added to Rin (200 μg)-expressing cell lysates in a total volume of 1 ml and incubated with rotation for 1 h at 4°C, and the resin was recovered in a 4°C microcentrifuge (5 min at 10,000 rpm). The GST-Raf-RID pellets were washed once with GST pull-down buffer, twice with GST pull-down buffer supplemented with 500 mM NaCl, and finally with two additional washes with ice-cold GST pull-down buffer. Bound GTP-Rin was detected by anti-Flag immunoblot analysis. To determine the specificity of isoform Gα proteins on Rin activation, PC6 cells were cotransfected with 3×Flag-Rin-WT and an expression vector for GsαQ227L (10, 20, or 50 ng), Gi2αQ204L (50, 100, or 200 ng), GqαQ209L (50, 100, or 200 ng), or G12αQ229L (100 ng). To determine the effects of PACAP38 on Rin activation, PC6 cells expressing 3×Flag-Rin-WT were stimulated with PACAP38 (10 nM) following serum starvation. To determine whether PACAP38-mediated Rin activation is Giα dependent, PC6 cells expressing 3×Flag-Rin-WT were stimulated with PACAP38 (10 nM) after pretreatment with or without PTX (100 ng/ml) for 30 min. To determine whether Rin activation by Gsα is mediated by cAMP, PC6 cells expressing 3×Flag-Rin-WT were starved with serum-free DMEM for 5 h and then stimulated with CTX (5 μg/ml) after pretreatment with or without ddA (50 μM) for 5 min or stimulated directly with 8-Br-cAMP (50 μM). To analyze the roles of Epac proteins in Rin activation, PC6 cells transfected with 3×Flag-Rin-WT were either coexpressed with constitutively active Epac2 (CA-Epac2) (20, 50, or 100 ng), alone or as a combination of 50 ng of CA-Epac2 and WT Rap1A (0, 20, 50, or 100 ng) or Rap1A-G12V (20, 50, or 100 ng), or stimulated with 8-CPT-2-Me-cAMP (25 μM). To determine if calcium signaling results in Rin activation, PC6 cells expressing 3×Flag-Rit-WT were stimulated with A23187 (2.5 μM), forskolin (10 μM), or potassium chloride (KCl; 50 mM or 100 mM). To determine the requirement for Src in PACAP38-mediated Rin activation, PC6 cells expressing 3×Flag-Rin-WT, with or without DN-Src (0.5 μg), were stimulated with PACAP38 (10 nM), cotransfected with CA-Src (20, 50, or 100 ng) or transfected with 3×Flag-Rin-WT after pretreatment with or without PP2 or PP3 (10 μM), and then stimulated with PACAP38. To examine the requirement for Src serine 17 phosphorylation in PACAP38-mediated Rin activation, PC6 cells were cotransfected with 3×Flag-Rin-WT and either Src-S17A (0.5 μg), prior to stimulation with PACAP38 (10 nM), or increasing amounts of CA-Src-S17A (10, 20, or 50 ng), and the amount of GTP-bound Rin was determined. To determine the requirement of PACAP receptor type I (PACR1) in PACAP38-mediated Rin activation, PC6 cells expressing 3×Flag-Rin-WT were cotransfected with either shCTR or shPACR1-384 (1.5 μg) and then stimulated with PACAP38 (10 nM) for 10, 30, or 60 min before GTP-bound Rin levels were determine by pull-down assay. All experiments were repeated three to six times.
RESULTS
Role for Rin in PACAP38-mediated neurite outgrowth.
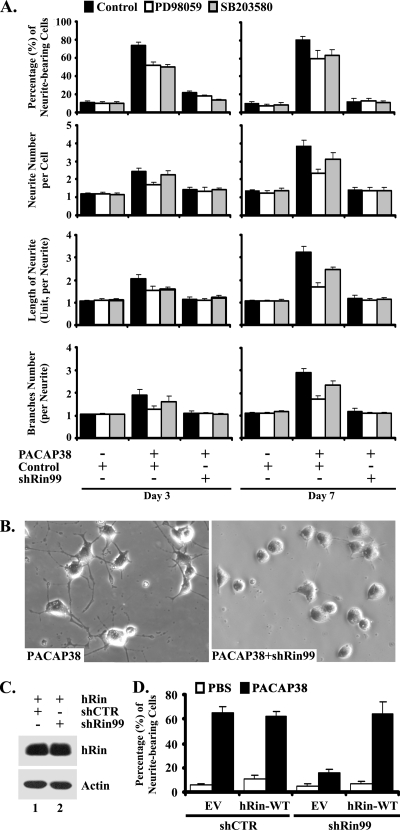
PACAP38 activates PACR1 to regulate a complex signaling network and to promote neurite outgrowth in pheochromocytoma cells (19, 28, 41, 63), including activation of the Rit GTPase (72). Since we have shown that Rin is rapidly activated following NGF stimulation of PC6 cells (a PC12 subline) and that Rin silencing inhibits NGF-induced neurite elongation by attenuating p38 MAP kinase activation (71), we wished to examine the requirement for Rin signaling in PACAP38-mediated differentiation. To directly assess whether Rin signaling is involved in PACAP38-mediated neurite outgrowth, we used siRNA-mediated RNA interference (16) to selectively inhibit the expression of endogenous Rin. We previously developed the shRNA shRin99 and demonstrated that it potently and specifically reduces rat Rin protein levels >80% when transfected into PC6 cells, whereas a control shRNA with no predicted target in the rat genome (shCTR) has no effect on Rin expression (71). As shown in Fig. 1, shRin99-induced silencing potently inhibited PACAP38-dependent neurite outgrowth, resulting in a >75% reduction in the percentage of PACAP38-stimulated cells displaying neurites. Rin silencing also resulted in decreased neurite length (64%), neurite branching (58%), and neurite number per cell body (63%) (Fig. 1A). Surprisingly, this inhibition was even greater than that induced by pharmacological blockade of either MEK/ERK (10 μM PD98059) (22.8% reduction of neurite-bearing cells at day 7) or p38 (10 μM SB203580) MAP kinase (20.9% reduction of neurite-bearing cells at day 7) signaling (Fig. 1A). Treatment of shRin99-transfected cells with either PD98059 or SB203580 had no significant additional inhibitory effect on neuritogenesis (Fig. 1A). However, the same inhibitors resulted in a significant decrease in neurite outgrowth in PACAP38-stimulated shCTR-transfected PC6 cells, indicating that both MEK/ERK and p38 MAP kinase cascades are required for PACAP38-mediated neurite outgrowth. To control against nonspecific cellular effects, we reconstituted the Rin deficiency by cotransfecting PC6 cells with wild-type (WT) hRin and shRNA expression vectors. As expected, hRin escaped shRin99-mediated gene silencing (Fig. 1C) and restored PACAP38-mediated PC6 cell differentiation (Fig. 1D). Taken together, these data suggest that Rin plays a central role in PACAP38-dependent neuronal differentiation.
FIG. 1.
PACAP38-mediated neuronal differentiation is attenuated by knockdown of endogenous Rin. (A and B) PC6 cells were pretreated with SB203580 (10 μM) (gray bars) or PD98059 (10 μM) (white bars) or left untreated (black bars) for 30 min, as indicated, and then transfected with shCTR or shRin99 (1.5 μg). Transfected cells were replated (1:4 dilution) onto 35-mm dishes, placed under G418 (400 μg/ml) selection, and then subjected to PACAP38-mediated (5 nM) neurite initiation, as indicated. On days 3 and 7 following PACAP38 stimulation, neurite outgrowth was analyzed as described in Materials and Methods. The percentage of neurite-bearing cells, neurite number per cell, neurite length, and branch number per neurite are shown as means ± standard deviations (SD) for three independent experiments. (B) Representative microphotographs from random fields on day 7. (C) hRin escapes shRin99-mediated silencing. PC6 cells were transfected with shRin99 (1.5 μg) together with WT hRin (200 ng) and subjected to G418 (400 μg/ml) selection for 60 h. The levels of hRin expression were analyzed by immunoblotting with anti- Rin monoclonal antibody. Actin levels were used as a loading control. (D) WT hRin restores PACAP38-mediated neuritogenesis in shRin99-expressing PC6 cells. PC6 cells expressing shRin99 or shCTR vector were cotransfected with either vector expressing WT hRin (200 ng) or empty vector control (EV), replated at a 1:4 dilution, and subjected to G418 (400 μg/ml) selection. Neurite outgrowth was initiated immediately after replating of cells, with (black bars) or without (white bars) PACAP38 (10 nM). The percentage of neurite-bearing cells was calculated at day 3 and is presented as the mean ± SD for three experiments.
PACAP38 activates Rin in a Gsα-mediated but cAMP-independent manner.
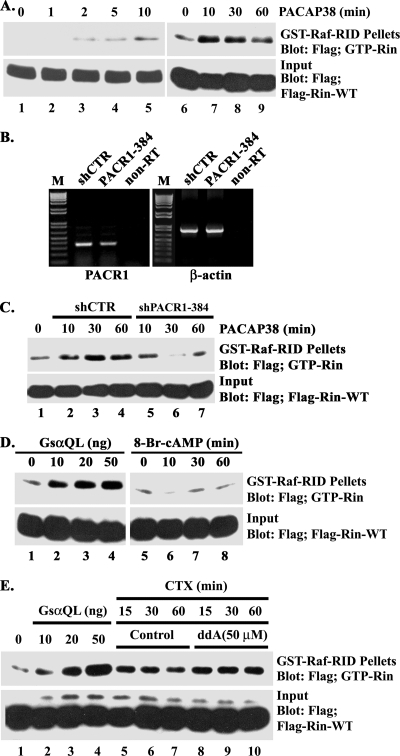
We reasoned that if Rin serves as an important mediator of PACAP38-mediated signaling, it must be activated following PACAP38 stimulation. To examine Rin activation, we utilized a GST fusion protein containing the RID of the Raf kinase in a pull-down assay to monitor the cellular GTP-binding status of Rin following PACAP38 stimulation (74). PC6 cells transiently transfected with Flag-tagged Rin were incubated in serum-deficient medium for 5 h, and pull-down experiments were performed on cell lysates prepared at various times following PACAP38 stimulation. Although Rin protein levels were constant (Fig. 2A) and serum-starved PC6 cells contain barely detectable levels of GTP-Rin, PACAP38 stimulation led to a rapid increase in the level of GTP-Rin. Activation of Rin was detected within 2 min following PACAP38 stimulation and had begun to return to basal levels within 60 min (Fig. 2A).
FIG. 2.
PACAP38-mediated Rin activation is Gsα dependent but cAMP independent. (A) PACAP38 activates Rin in a transient manner. PC6 cells were transfected with 3×Flag-Rin-WT (1 μg), and 36 h later, the cells were starved with serum-free DMEM for 5 h before stimulation with PACAP38 (10 nM) for 1, 2, 5, or 10 min (lanes 2 to 5) or for 10, 30, or 60 min (lanes 7 to 9). GTP-Rin levels were determined by GST-Raf-RID pull-down assay as described in Materials and Methods. (B) shPACR1-384 mediates partial silencing of PACR1. PC6 cells were transfected with either shCTR or shPACR1-384 (1.5 μg) and subsequently subjected to G418 (400 μg/ml) selection for 60 h. Total RNA was isolated and utilized for reverse transcription-PCR as described in Materials and Methods. (C) PACR1 silencing attenuates PACAP38-mediated Rin activation. PC6 cells were cotransfected with 3×Flag-Rin-WT (0.5 μg) and either shCTR or shPACR1-384 (1.5 μg) and stimulated with PACAP38 (10 nM) for 10, 30, or 60 min following starvation (in serum-free DMEM for 5 h). GTP-bound Rin levels were determined using GST-Raf-RID precipitation as described in Materials and Methods. (D) Gsα activates Rin. PC6 cells were cotransfected with 3×Flag-Rin-WT in the presence (lanes 2 to 4) or absence (lanes 1 and 5 to 8) of the indicated amount of GsαQ227L, starved with serum-free DMEM for 5 h, and subsequently stimulated with (lanes 6 to 8) or without (lanes 1 to 5) 8-Br-cAMP (50 μM for 10, 30, or 60 min). GTP-Rin levels were determined by GST-Raf-RID pull-down assay as described for panel A. (E) Rin activation is not adenylyl cyclase dependent. PC6 cells were transfected with 3×Flag-Rin-WT together with GsαQ227L (lanes 2 to 4) or were stimulated with CTX (5 μg/ml for 15, 30, or 60 min) after being pretreated with (lanes 8 to 10) or without (lanes 5 to 7) ddA (50 μM). Rin-GTP was recovered with GST-Raf-RID and detected by anti-Flag immunoblotting. The expression of recombinant Rin present in each lysate was also determined by immunoblot analysis. The data are from one experiment that was representative of the three experiments performed.
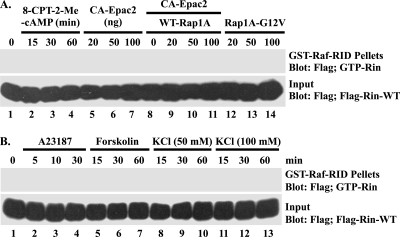
In PC12 cells, PACAP38 has been shown to activate PACR1 and to promote neuronal differentiation (63). While PACR1 activates a number of signaling pathways, including the phospholipase C and phosphatidylinositol 3-kinase pathways, and stimulates L-type Ca2+ channel activation (77), it predominantly activates the Gsα-adenylate cyclase-cAMP signaling pathway. To confirm that the effects of PACAP38 were PACR1 dependent, we developed an shRNA vector (shPACR1-384) which resulted in a partial knockdown of endogenous PACR1 in transiently transfected PC6 cells (Fig. 2B) and impaired PACAP38-mediated Rin activation (Fig. 2C). Consistent with a role for Gsα signaling in PACAP38-mediated Rin activation, cells expressing a GTPase-deficient activated mutant of Gsα (GsαQ227L) resulted in dose-dependent Rin activation (Fig. 2D and E). Furthermore, treatment of PC6 cells with CTX, which is a known activator of Gsα proteins (10), increased GTP-Rin in a time-dependent fashion (Fig. 2E). Surprisingly, CTX-mediated Rin activation was not inhibited by the direct adenylate cyclase inhibitor ddA (50 μM) (Fig. 2E). Moreover, addition of 8-Br-cAMP, a nonhydrolyzable cAMP analog, did not result in Rin activation (Fig. 2D). We have recently shown that PACAP38-mediated Rit activation involves a cAMP-Epac signaling pathway (72). To confirm that a similar pathway was not involved in Rin activation, we examined the ability of the membrane-permeating Epac-selective cAMP analog 8-CPT-2′-O-Me-cAMP, expression of CA-Epac2, and either activated Rap1A (a known cellular target of the Epac guanine nucleotide exchange factors) (7) or coexpressed CA-Epac2 and Rap1A to activate Rin. As illustrated in Fig. 3A, elevated Epac-Rap1 signaling did not promote Rin activation. Taken together, these data suggest that Gsα-mediated Rin activation occurs in a cAMP/Epac-independent manner.
FIG. 3.
Rin is not activated by either Epac2 or calcium signaling. (A) Epac and Rap fail to activate Rin. PC6 cells expressing 3×Flag-Rin-WT were either stimulated with 8-CPT-2-Me-cAMP (25 μM) (lanes 2 to 4) for 15, 30, or 60 min or coexpressed with CA-Epac2 (20, 50, or 100 ng) (lanes 5 to 7), CA-Epac2 (50 ng) (lanes 8 to 11) and WT Rap1A (20, 50, or 100 ng) (lanes 9 to 11), or Rap1A-G12V (20, 50, or 100 ng) (lanes 12 to 14). The GTP-Rin levels were determined as described in Materials and Methods. (B) Rin activation is not calcium dependent. PC6 cells expressing 3×Flag-Rin-WT were starved with serum-free DMEM for 5 h before stimulation with A23187 (2.5 μM) (lanes 2 to 4) for 5, 10, or 30 min or with forskolin (10 μM) (lanes 5 to 7) or KCl (50 mM or 100 mM, as indicated) (lanes 8 to 13) for 15, 30, or 60 min. Cellular Rin-GTP levels were analyzed using GST-Raf-RID pull-down assay as described in Materials and Methods.
Both Giα and Gsα signaling pathways contribute to PACAP38-mediated Rin activation.
Because PACR1 signaling has been shown to stimulate Ca2+-dependent events (63, 77) and previous work has suggested a role for Ca2+-calmodulin in Rin regulation (31), we next examined the contribution of Ca2+ to PACAP38-dependent Rin activation. PC6 cells transiently transfected with Flag-tagged WT Rin were incubated in serum-deficient medium for 5 h prior to treatment with the calcium ionophore A23187 (2.5 μM), KCl (50 and 100 mM) to induce calcium entry via depolarization, or forskolin (10 μM) to stimulate cAMP signaling as a negative control. Figure 3B shows that GTP-Rin levels were not elevated following A23187, KCl, or forskolin treatment.
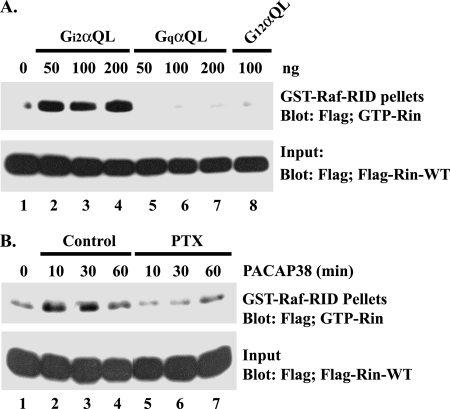
Although PACAP38-GPCR signaling has been described as predominantly stimulating Gsα-cAMP signaling, studies have also reported PACAP38-mediated activation of other Gα family-regulated signaling pathways (53). To determine whether these alternative Gα-dependent signaling pathways were involved in Rin activation, PC6 cells were cotransfected with 3×Flag-Rin-WT and constitutively active Giα, Gqα, and G12α. Coexpression of GTPase-deficient GiαQ204L, but not GqαQ209L or G12αQ229L, resulted in Rin activation (Fig. 4A). Moreover, treatment of PC6 cells with PTX, an inhibitor of Giα signaling (58), potently suppressed PACAP38-mediated Rin activation (Fig. 4B), implicating Giα signaling in PACAP38-dependent Rin activation.
FIG. 4.
Giα signaling activates Rin. (A) Giα activates Rin. PC6 cells were transiently transfected with 3×Flag-Rin-WT and GiαQ204L, GqαQ209L, or G12αQ229L expression vectors and cultured for 36 h to allow for protein expression. Prior to the preparation of whole-cell lysates, cells were serum starved for 5 h, and the level of GTP-Rin was determined as described in Materials and Methods. (B) PACAP38 activates Rin in a PTX-sensitive manner. Transiently transfected PC6 cells expressing 3×Flag-Rin-WT were starved in serum-free DMEM for 5 h and subsequently pretreated with PTX (100 ng/ml) or DMSO for 30 min prior to PACAP38 (10 nM) stimulation. Rin-GTP was recovered by GST-Raf-RID pull-down assay as described in Materials and Methods.
Src is required for PACAP38-mediated Rin activation and neurite outgrowth.
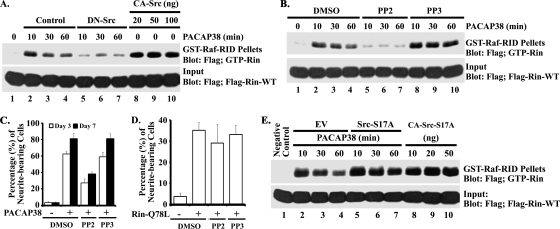
Recent studies indicate that Giα and Gsα activate a Src-dependent signaling pathway to regulate both neurite outgrowth and Rap1 activation (60), and they are known to be stimulated in PACAP38-treated PC12 cells (41, 44). To determine whether PACAP38-mediated Rin activation involves a similar pathway, we next examined whether Rin was regulated in a Src-dependent manner. Consistent with a role for Src signaling in PACAP38-mediated Rin activation, expression of a dominant inhibitory Src mutant (DN-Src [SrcK296R/Y528F]) suppressed PACAP38-mediated Rin activation, while expression of an activated Src (CA-Src [SrcY529F]) mutant alone resulted in strongly elevated Rin-GTP levels in PC6 cells (Fig. 5A). Furthermore, treatment with the Src inhibitor PP2 (10 μM), but not with the inactive PP3 isomer (10 μM), potently inhibited PACAP38-mediated Rin activation (Fig. 5B). Src inhibition also disrupted PACAP38-dependent neurite elongation (Fig. 5C). However, PP2 treatment did not inhibit RinQ78L-mediated (Fig. 5D) neurite outgrowth, suggesting that Src functions as an upstream regulator of Rin.
FIG. 5.
Src activates Rin. (A) PC6 cells were transfected with 3×Flag-Rin-WT in the presence (lanes 5 to 7) or absence (lanes 2 to 4) of DN-Src (0.5 μg) and then stimulated with PACAP38 (10 nM) for 10, 30, or 60 min or cotransfected with 3×Flag-Rin-WT and CA-Src (20, 50, or 100 ng) (lanes 8 to 10). Whole-cell lysates were prepared, and GTP-Rin levels were determined by GST-Raf-RID pull-down assay as described in Materials and Methods. (B) PACAP38 activates Rin in a PP2-dependent manner. PC6 cells were pretreated with PP2 or PP3 (10 μM) for 30 min before being transiently transfected with 3×Flag-Rin-WT. Cells were grown for an additional 36 h to allow for Flag-Rin expression, serum starved in the presence of PP2 or PP3 (10 μM), and stimulated with PACAP38 (10 nM) for 10, 30, or 60 min. GTP-Rin levels were determined as described above. (C) PACAP38-mediated neurite outgrowth is PP2 sensitive. PC6 cells plated at low density were exposed to PACAP38 (10 nM) to induce neurite outgrowth after pretreatment with either PP2 (10 μM) or PP3 (10 μM), and the percentage of neurite-bearing cells was determined. (D) PP2 does not inhibit Rit-mediated neurite outgrowth. PC6 cells were pretreated with either PP2 (10 μM) or PP3 (10 μM) for 30 min prior to transfection with empty Flag vector or 3×Flag-RinQ78L (1.5 μg), and neurite outgrowth was determined as described in Materials and Methods. (E) Phosphorylation of Src serine 17 is not required for PACAP38- and Src-mediated Rin activation. PC6 cells were cotransfected with 3×Flag-Rin-WT (1 μg) and either Src-S17A (0.5 μg) (lanes 5 to 7), the indicated amount of CA-Src-S17A (lanes 8 to 10), or empty Flag vector (EV) as a negative control. Cultures were serum starved for 5 h before stimulation with (lanes 2 to 7) or without (lanes 1 and 8 to 10) PACAP38 (10 nM). GTP-Rin was recovered by GST-Raf-RID pull-down assay and analyzed as described in Materials and Methods.
Recent studies suggest that PKA-dependent phosphorylation of Src at residue serine 17 (S17) plays a critical role in Rap1 GTPase activation in response to both elevated cAMP in a wide range of cells and NGF stimulation of PC12 cells (60). To determine whether a similar mechanism might contribute to PACAP-mediated Rin activation, we generated a Src mutant incapable of being phosphorylated by PKA (SrcS17A). As shown in Fig. 5E, expression of SrcS17A had no effect on PACAP38-dependent Rin activation. Furthermore, expression of an activated SrcS17A mutant (CA-SrcY529F/S17A) resulted in strongly elevated Rin-GTP levels (Fig. 5E). Taken together, these data indicate that PKA-mediated Src phosphorylation is not required for PACAP38-dependent Rin activation.
Loss of Rin does not alter MAP kinase signaling but downregulates HSP27 phosphorylation.
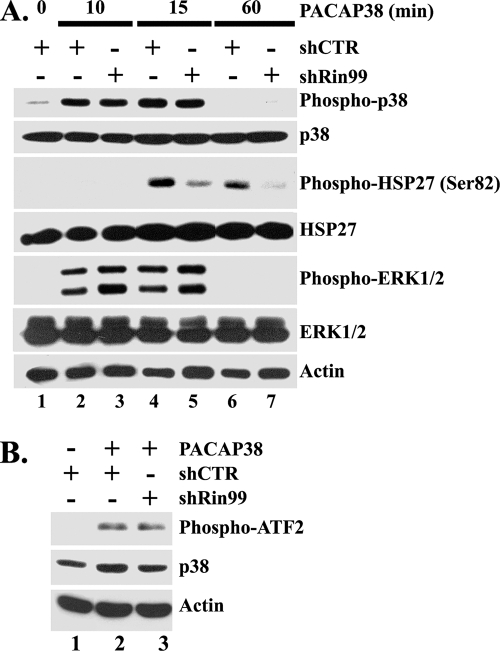
Since PACAP38-mediated neurite outgrowth is associated with sustained activation of MAP kinase cascades (19, 63), we next examined whether siRNA-mediated Rin knockdown would alter PACAP38-induced MAP kinase signaling. Kinase activation in shRin99- or shCTR-transfected PC6 cells was monitored by immunoblotting with phospho-specific antibodies following PACAP38 stimulation. Surprisingly, Rin silencing had no obvious effect on PACAP38-mediated ERK or p38 MAP kinase activation (Fig. 6A), and a coupled kinase assay was used to confirm that p38 signaling did not rely upon Rin (Fig. 6B). However, Rin silencing inhibited activation of HSP27, a protein with known roles in both neurite outgrowth and survival (3, 81), in response to PACAP38 treatment (Fig. 6A). This alteration was not a consequence of reduced HSP27 levels, since protein levels remained constant during these studies (Fig. 6A). These data suggest that Rin signaling may contribute to PACAP38-mediated neurite outgrowth, in part through regulation of HSP27 activation.
FIG. 6.
Rin is involved in PACAP38-mediated HSP27 activation. (A) Loss of Rin downregulates PACAP38-mediated HSP27 but not p38 MAPK or ERK1/2 phosphorylation. PC6 cells were transfected with 1.5 μg of shCTR (lanes 1, 2, 4, and 6) or shRin99 (lanes 3, 5, and 7) and subjected to G418 selection for 48 h. The cells were subjected to serum starvation (serum-free DMEM) for 5 h prior to stimulation with PACAP38 (5 nM). Whole-cell lysates were prepared, and the levels of phosphorylated ERK1/2, p38 MAPK, and HSP27 (Ser82) were determined by immunoblotting with appropriate phospho-specific antibodies. The expression of endogenous ERK, p38 MAPK, HSP27, and actin was examined by Western blotting. (B) Loss of Rin does not attenuate PACAP38-induced p38 activation. PC6 cells expressing either shRin99 or shCTR (1.5 μg) were enriched by G418 (400 μg/ml) selection and serum starved with serum-free DMEM for 5 h prior to stimulation with PACAP38 (10 nM) for 15 min. Cell lysates were subsequently prepared (200 μg) and subjected to anti-phospho-p38(T180/Y182) immunoprecipitation. The resulting precipitates were subjected to in vitro p38 kinase assay as described previously (71).
HSP27 contributes to PACAP38- and Rin-mediated neurite outgrowth.
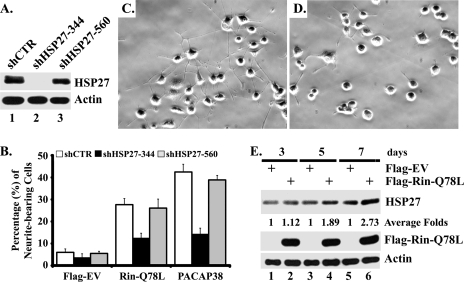
While HSP27 has been found to promote neuronal survival (3, 14, 54), the requirement for HSP27 in PACAP-mediated pheochromocytoma cell differentiation remains unexplored. To directly assess the contribution of HSP27 to this process, we used siRNA-mediated RNA interference (16) to selectively knock down endogenous HSP27 in PC6 cells. As shown in Fig. 7A, shHSP27-344, but not shHSP27-560 or shCTR, resulted in the silencing of HSP27 in PC6 cells. In addition, knockdown of endogenous HSP27 potently inhibited PACAP38-mediated neurite outgrowth (Fig. 7B to D), resulting in a blockade that equaled that seen following Rin silencing (Fig. 1). Endogenous HSP27 was also required for RinQ78L-mediated neurite elongation (Fig. 7B). PC12 cells have been found to express elevated levels of HSP27 following PACAP stimulation (42), suggesting that increased HSP27 expression may contribute to PACAP-induced differentiation. As shown in Fig. 7E, expression of activated Rin also results in increased HSP27 protein expression in PC6 cells (2.73-fold induction at day 7), supporting the notion that Src-Rin-HSP27 signaling contributes to PACAP38-mediated differentiation.
FIG. 7.
Rin induces neurite outgrowth through HSP27. (A) shRNA interference silences HSP27 expression. PC6 cells were transfected with either shCTR, shHSP27-344, or shHSP27-560 (1.5 μg) and subjected to G418 (400 μg/ml) selection for 60 h. Whole-cell lysates were prepared and subjected to immunoblotting with anti-HSP27 antibody to detect expression of endogenous HSP27 protein. (B to D) Loss of HSP27 attenuates both RinQ78L- and PACAP38-mediated neurite outgrowth. PC6 cells expressing shCTR, shHSP27-344, or shHSP27-560 (1 μg) were cotransfected with 3×Flag-Rin-Q78L (1 μg) or treated with PACAP38 (10 nM) to promote neurite elongation. Empty 3×Flag vector-transfected cells served as a control. The transfected cells were enriched by G418 (400 μg/ml) selection, and neurite outgrowth was analyzed as described in Materials and Methods. The percentages of neurite-bearing cells are shown as means ± SD for two experiments performed in triplicate. Representative micrographs of PACAP38-stimulated shCTR (C)- and shHSP27-344 (D)-expressing PC6 cells from day 7 are shown. (E) Rin-Q78L upregulates endogenous HSP27 expression. PC6 cells transfected with either empty 3×Flag vector (lanes 1, 3, and 5) or 3×Flag-Rin-Q78L (lanes 2, 4, and 6) were subjected to G418 (400 μg/ml) selection for the indicated times. Whole-cell lysates were prepared, and HSP27, 3×Flag-Rin-Q78L, and actin expression was examined by immunoblotting. HSP27 levels were determined by densitometry and normalized to cellular actin levels, and the average amount of induction of HSP27 was calculated for each time point.
Rin signaling contributes to PACAP38-mediated cAMP production.
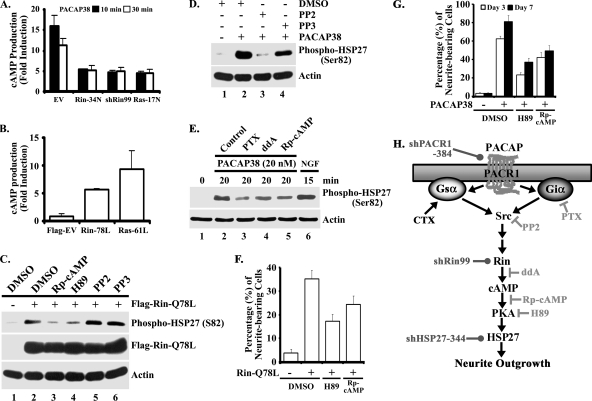
HSP27 is subject to complex phosphorylation, and p38 MAP kinase signaling has been reported to play a central role in this process (18, 66, 75). However, recent reports indicate that both PKC and PKA contribute to HSP27 phosphorylation (6, 33, 55). Since Rin signaling is required for PACAP38-mediated HSP27 phosphorylation (Fig. 6A) but does not alter p38 MAP kinase activity (Fig. 6A and B), we predicted that Rin might control a cAMP-PKA signaling pathway to regulate HSP27 activation. To address this issue, we examined whether Rin could modulate PACAP38-dependent cAMP production. As shown in Fig. 8A, PACAP38 stimulation of empty vector-transfected PC6 cells resulted in a potent increase in cellular cAMP levels within 10 min (∼15-fold induction), with cAMP levels beginning to return to basal levels by 30 min. Expression of dominant-negative Rin or Ras mutants significantly inhibited PACAP38-mediated cAMP production (Fig. 8A). In contrast, expression of either a constitutively active Rin (RinQ78L) or Ras (RasQ61L) mutant in PC6 cells resulted in potent stimulation of cAMP levels (fivefold and eightfold, respectively) (Fig. 8B). Importantly, shRin99-mediated Rin knockdown also resulted in a potent inhibition of PACAP38-mediated cAMP production (Fig. 8A). Taken together, these results suggest that Rin contributes to PACAP38-mediated cAMP signaling.
FIG. 8.
Rin activates HSP27 and induces neuronal differentiation via cAMP-PKA signaling pathways. (A) Rin silencing inhibits PACAP38-mediated cAMP production. PC6 cells transiently transfected with 3×Flag empty vector, 3×Flag-RinS34N, shRin99, or 3×Flag-H-RasS17N (1.5 μg) were subjected to G418 (400 μg/ml) selection. Following incubation in serum-free DMEM (5 h), cells were stimulated with PACAP38 (10 nM) for the indicated times, and cAMP levels were assayed as described in Materials and Methods. The data are reported as means ± SD for three experiments performed in triplicate. (B) Rin stimulates cAMP production. PC6 cells were transiently transfected with 3×Flag empty vector, 3×Flag-RinQ78L, or 3×Flag-H-RasQ61L (1.5 μg), and cAMP levels were determined at 36 h posttransfection. The data are presented as means ± SD for two experiments performed in duplicate. (C) Rin signaling to HSP27 is Rp-cAMP and H89 sensitive but PP2 insensitive. PC6 cells pretreated with Rp-cAMP (50 μM) (lane 3), H89 (10 μM) (lane 4), PP2 (10 μM) (lane 5), PP3 (10 μM) (lane 6), or vehicle (DMSO) (lanes 1 and 2) were transfected with 3×Flag-RinQ78L (1.5 μg) (lanes 2 to 6) or empty 3×Flag vector (lane 1). Whole-cell lysates were prepared following serum starvation (5 h), and the phosphorylation status of HSP27 was determined by immunoblotting with phospho-specific HSP27 (Ser82) antibody. The results are representative of three independent experiments. (D) PP2 inhibits PACAP38-mediated HSP27 phosphorylation. PC6 cells seeded at a density of 5 × 104 cells/cm2 were allowed to grow for 48 h, starved in serum-free DMEM for 5 h, and subsequently treated with either PP2 or PP3 (10 μM) prior to stimulation with PACAP38 (10 nM) for 20 min. Detergent-soluble cell lysates were prepared, and phosphorylation levels of HSP27 (Ser 82) were determined by phospho-specific immunoblotting. Actin levels were used to ensure equal loading. The results are representative of three individual experiments. (E) PACAP38-mediated HSP27 activation requires Giα, adenylyl cyclase, and PKA. PC6 cells were maintained and treated as described for panel D except for pretreatment with PTX (100 ng/ml), ddA (50 μM), or Rp-cAMP (50 μM) for 30 min. NGF stimulation (100 ng/ml for 15 min) was used as a positive control. HSP27 activation was determined as described for panel D. (F) Rin-Q78L-mediated neurite outgrowth is Rp-cAMP and H89 sensitive. PC6 cells were pretreated with either Rp-cAMP (50 μM), H89 (10 μM), or vehicle control (DMSO) prior to transient transfection with 3×Flag-Rin-Q78L, and neurite outgrowth was analyzed as described in Materials and Methods. The results are presented as means ± SD for two experiments performed in quadruplicate. (G) PACAP38-mediated neurite outgrowth in PC6 cells is Rp-cAMP and H89 sensitive. PC6 cells seeded at a density of 1 × 103/cm2 were pretreated with either Rp-cAMP (50 μM), H89 (10 μM), or vehicle control (DMSO) prior to exposure to PACAP38 (10 nM), and neurite outgrowth was analyzed as described in Materials and Methods. The results are presented as means ± SD for two experiments performed in quadruplicate. (H) Schematic of the putative PACAP-Src-Rin-HSP27 signal transduction cascade. The sites of action for the various pharmacological inhibitors (⊣), selective activators (→), and the targets of shRNA interference silencing reagents (•) are indicated.
Rin-induced neurite outgrowth requires PKA-HSP27 signaling.
The ability of Rin to modulate cellular cAMP levels suggested that regulation of a cAMP-PKA-HSP27 signaling cascade might contribute to neuritogenesis. Consistent with this proposed signaling pathway, treatment with the known PKA inhibitors H89 (10 μM) and Rp-cAMP (50 μM), but not inhibition of Src signaling using PP2 (10 μM), decreased RinQ78L-mediated activation of HSP27, as measured by anti-phospho-specific antibody immunoblotting (Fig. 8C). Inhibition of Giα (PTX at 100 ng/ml), Src (PP2 at 10 μM), adenylate cyclase (ddA at 50 μM), or PKA (Rp-cAMP at 50 μM) also inhibited PACAP38-mediated HSP27 activation (Fig. 8D and E). Importantly, PKA signaling was also necessary for RinQ78L- and PACAP38-mediated PC6 cell neurite outgrowth. As shown in Fig. 8F and G, addition of H89 or Rp-cAMP attenuated both Rin- and PACAP38-mediated neuritogenesis, supporting the notion that a novel Rin-PKA-HSP27 signaling pathway contributes to PACAP38-dependent neuronal differentiation (Fig. 8H).
DISCUSSION
Numerous studies have characterized trophic factors, including growth factors, cytokines, and hormones, that are capable of regulating both axonal and dendritic growth and the differentiation of neural tumor cells. Among these many factors, PACAP38 has been shown to have particularly prominent and widespread effects within the nervous system (25, 73, 77). For example, PACAP38 treatment increases cell survival and neurite elongation in both primary neurons (21, 49) and PC12 cells (13, 63) and regulates dendritic growth in cultured sympathetic neurons (15). Recent studies have demonstrated a role for Rin signaling in PC12 cell differentiation (71, 74), motivating studies to explore the role of Rin signaling in PACAP38-dependent neuronal differentiation. In this report, we describe a novel PACAP38 signaling pathway in which Gsα/Giα-dependent activation of a Src family kinase leads to stimulation of Rin signaling to promote neurite elongation via PKA-dependent HSP27 activation.
To determine whether Rin signaling was involved in transducing PACAP38-mediated neuronal differentiation signals, we investigated in vivo Rin regulation. When PC6 cells are stimulated with PACAP38, Rin is rapidly activated (Fig. 2A). These studies provide the first demonstration that Rin can be activated by G-protein-coupled receptor signaling pathways and establish Rin as a direct downstream target of PACAP38 signaling. More importantly, loss-of-function analysis directly implicates Rin signaling in PACAP38-induced differentiation (Fig. 1). Thus, Rin plays a critical role in the process of neurite outgrowth, acting downstream of both GPCR (this study)- and NGF-TrkA (71)-dependent signaling cascades. The molecular mechanisms that regulate neuritogenesis remain incompletely characterized, although members of both Ras and Rho GTPase subfamilies have established roles in neuronal development and regeneration (22), and studies presented here suggest that HSP27 activation may underlie the contribution of Rin signaling to neurite outgrowth (Fig. 6 and 7). We recently completed studies exploring the role of Rit signaling in the regulation of axonal and dendritic growth in primary neurons (45). To more firmly define a role for Rin in PACAP38-mediated differentiation signaling, it will be necessary to undertake a similarly detailed analysis of Rin function in primary neurons.
Surprisingly, while GTP loading of Rin is stimulated by expression of activated Gsα (Fig. 2D and E), Rin activation did not rely upon Epac-cAMP signaling (Fig. 3A), the pathway that regulates the closely related Rit GTPase following PACAP exposure (72). Neither stimulation with 8-bromo-cAMP (Fig. 2D), a membrane-permeating cAMP analog, nor that with forskolin (Fig. 3B), a direct adenylyl cyclase activator, resulted in Rin activation. Furthermore, GTP-bound Rin levels were not elevated following exposure to the Epac-specific cAMP analog 8-CPT-2-Me-cAMP or by constitutively activated Epac2 (Fig. 3A). The inability of cAMP signaling to activate Rin prompted us to explore other potential signaling pathways. These studies found that Giα, but not G12α or Gqα, signaling was also capable of inducing Rin activation (Fig. 4). While neither cAMP nor calcium alone stimulated Rin activation (Fig. 2 and 3), previous studies had identified Src family tyrosine kinases as a common downstream target of Giα/Gsα signaling (26, 44, 60). Consistent with a role for Src in PACAP38-mediated Rin signaling, expression of activated Src was sufficient to induce Rin activation (Fig. 5A), while a dominant-negative Src mutant or pharmacological blockade of Src family kinase signaling inhibited PACAP38-mediated Rin stimulation (Fig. 5A and B). In addition, treatment with the Src kinase inhibitor PP2 potently blocked PACAP38-mediated neuritogenesis but not RinQ78L-mediated neurite outgrowth (Fig. 5C and D). These data indicate that Rin is activated downstream of PACAP in a Giα/Gsα/Src-dependent fashion. While the molecular mechanisms by which GPCR signaling promotes Src activation remain relatively poorly understood, Huang and colleagues (50) found that both Giα and Gsα directly bind the catalytic domain to stimulate Src kinase activity. Interestingly, Kim et al. recently identified a direct interaction between the closely related Rit GTPase and both Goα and Giα (36). While the ability of Rin to serve as a Gα binding partner remains to be proven and is the focus of ongoing studies, these data suggest that a higher-order signaling complex containing Gα, Src, and Rin proteins might contribute to PACAP-mediated Rin activation. In addition, because of the prominent role of Src kinase signaling in a variety of neuronal differentiation cascades, including a critical role in the cross talk between GPCR and TrkA signaling pathways (44), further studies are required to determine whether Src-mediated Rin signaling contributes to these pathways.
How Src signaling results in Rin activation remains uncharacterized and is the target of ongoing studies. There is a large body of literature reporting Src family kinase-mediated regulation of Ras family GTPases. Several common molecular mechanisms emerge from these studies, including direct phosphorylation and activation of either individual guanine nucleotide exchange factors or their adaptor proteins, modulation of GAP protein activity, and Src-mediated activation of receptor tyrosine kinases downstream of GPCRs to allow cross talk (5, 20, 26, 34, 44, 57, 83). However, authentic Rin regulatory proteins have yet to be defined. Thus, an important goal of ongoing studies is to identify these regulatory proteins and examine their ability to be modulated by Src signaling.
Previous work has demonstrated that Ras family GTPases contribute to PACAP38-mediated neuronal differentiation, including roles for both Ras and Rap in the activation of ERK MAP kinase signaling and, more recently, our studies demonstrating a central role for the Rit GTPase in PACAP-mediated p38 MAP kinase activation (19, 63, 72, 76). Indeed, our previous analysis of Rin signaling in NGF-mediated neurite outgrowth found that Rin was required for p38 signaling but only modestly contributed to ERK MAP kinase signaling downstream of the TrkA receptor (71). Thus, the finding that Rin knockdown had no effect on either ERK or p38 MAP kinase signaling was unexpected. Instead, these studies found that Rin function was necessary for HSP27 activation in response to PACAP38 (Fig. 6A) in a cAMP-PKA-dependent manner (Fig. 8C). Rin signaling is also critical for NGF-dependent HSP27 activation (71), suggesting that Rin-mediated HSP27 activation might be central to its role in both NGF- and PACAP-driven neuronal differentiation.
HSP27 directs both cytoskeleton remodeling and survival in neurons (3, 81), and RNA interference-mediated silencing studies demonstrate a role for HSP27 in PACAP-mediated neurite elongation (Fig. 7). In addition to regulating the actin cytoskeleton (23, 38, 40), HSP27 has been reported to interact with both neurofilaments and microtubules in a phosphorylation-dependent manner (62). Thus, increased levels of phosphorylated HSP27 might stabilize the actin cytoskeleton, as well as neurofilaments and microtubules, to promote neurite outgrowth (59). HSP27 was identified recently as a protein whose expression was upregulated following PACAP stimulation of PC12 cells (42), and it was suggested to contribute to differentiation. The finding that activated Rin promotes a similar effect in PC6 cells (Fig. 7E) indicates that Rin signaling contributes to both the phosphorylation state and cellular expression of HSP27. Studies are under way to more fully elucidate the nature of the signaling pathways regulated by Rin, including the mechanisms of HSP27 regulation.
HSP27 phosphorylation has been reported to be controlled predominantly by the p38 MAP kinase cascade (18, 66, 75), although PKC, PKG, and PKA kinase pathways have all been reported to phosphorylate HSP27 (9, 33, 52). While activation of the p38 MAP kinase cascade likely regulates HSP27 after heat shock and other stresses, it appears that activation of cAMP-PKA signaling downstream of Rin is involved in PACAP38-dependent HSP27 activation. Rin signaling contributes to PACAP38-mediated cAMP production in PC6 cells (Fig. 8A), and expression of activated Rin alone is sufficient to promote elevated cAMP levels (Fig. 8B), perhaps by regulating the activity of cellular adenylyl cyclases. Alternatively, Rin signaling may control the activity of phosphodiesterase enzymes, thus modulating the concentration and kinetics of cAMP production following PACAP38 stimulation. Consistent with an important role for cAMP in Rin signaling, inhibition of PKA blocked both RinQ78L- and PACAP38-mediated HSP27 phosphorylation (Fig. 8C and E) and neurite outgrowth (Fig. 8F and G). Recent studies have found that PKA phosphorylation of Src mediates Rap1 activation in response to cAMP signaling in PC12 cells (60), suggesting that PKA signaling might be required for Rin activation. However, expression of a Src mutant incapable of PKA phosphorylation did not disrupt PACAP-dependent Rin activation (Fig. 5E), supporting a role for PKA solely as a downstream component of PACAP-Src-Rin signaling.
In summary, the loss-of-function analysis presented here indicates that Rin is a central participant in PACAP38-mediated signal transduction. The marked effects of Rin loss on PACAP38-mediated HSP27 activation, cAMP-PKA signaling, and neuritogenesis suggest that a novel Src-Rin-HSP27 pathway contributes to PACAP38-dependent neuronal differentiation. Studies are ongoing to examine the effects of selective Rin knockdown in primary neurons to further explore the importance of this pathway. Previous work indicating a prominent role for HSP27 in antiapoptotic signaling in neurons suggests that future studies must assess the potential role of Rin-HSP27 signaling in neuronal survival. Targeted inactivation of the PACAP and PACR1 genes in mice results in complex behavioral and neurological changes involving alterations in learning and memory and dysregulation of cellular stress responses, among others (25). These defects likely result from the inactivation of canonical PACAP38-PACR1 signaling pathways, but also from the loss of secondary cascades. For example, PACAP38-Src signaling has been implicated in the modulation of NMDA receptor function (51), PACAP38 signaling transactivates the TrkA receptor (44), and IGF-1 signals in part though PACR1 activation (12). As these investigations proceed, it will be necessary to consider the potential contribution of Rin signaling to these physiological processes.
Acknowledgments
This work was supported by Public Health Service grant NS045103 (to D.A.A.) from the National Institute of Neurological Disorders and Stroke and by grant P20RR20171 from the COBRE Program of the National Center for Research Resources, a component of the National Institutes of Health (NIH).
Footnotes
Published ahead of print on 9 June 2008.
REFERENCES
- 1.Arya, R., M. Mallik, and S. C. Lakhotia. 2007. Heat shock genes—integrating cell survival and death. J. Biosci. 32595-610. [DOI] [PubMed] [Google Scholar]
- 2.Beere, H. M. 2004. “The stress of dying”: the role of heat shock proteins in the regulation of apoptosis. J. Cell Sci. 1172641-2651. [DOI] [PubMed] [Google Scholar]
- 3.Benn, S. C., D. Perrelet, A. C. Kato, J. Scholz, I. Decosterd, R. J. Mannion, J. C. Bakowska, and C. J. Woolf. 2002. Hsp27 upregulation and phosphorylation is required for injured sensory and motor neuron survival. Neuron 3645-56. [DOI] [PubMed] [Google Scholar]
- 4.Benndorf, R., K. Hayess, S. Ryazantsev, M. Wieske, J. Behlke, and G. Lutsch. 1994. Phosphorylation and supramolecular organization of murine small heat shock protein HSP25 abolish its actin polymerization-inhibiting activity. J. Biol. Chem. 26920780-20784. [PubMed] [Google Scholar]
- 5.Bernards, A., and J. Settleman. 2004. GAP control: regulating the regulators of small GTPases. Trends Cell Biol. 14377-385. [DOI] [PubMed] [Google Scholar]
- 6.Bitar, K. N. 2002. HSP27 phosphorylation and interaction with actin-myosin in smooth muscle contraction. Am. J. Physiol. Gastrointest. Liver Physiol. 282G894-G903. [DOI] [PubMed] [Google Scholar]
- 7.Bos, J. L. 2003. Epac: a new cAMP target and new avenues in cAMP research. Nat. Rev. Mol. Cell Biol. 4733-738. [DOI] [PubMed] [Google Scholar]
- 8.Bos, J. L., J. de Rooij, and K. A. Reedquist. 2001. Rap1 signalling: adhering to new models. Nat. Rev. Mol. Cell Biol. 2369-377. [DOI] [PubMed] [Google Scholar]
- 9.Butt, E., D. Immler, H. E. Meyer, A. Kotlyarov, K. Laass, and M. Gaestel. 2001. Heat shock protein 27 is a substrate of cGMP-dependent protein kinase in intact human platelets: phosphorylation-induced actin polymerization caused by HSP27 mutants. J. Biol. Chem. 2767108-7113. [DOI] [PubMed] [Google Scholar]
- 10.Cassel, D., and Z. Selinger. 1977. Mechanism of adenylate cyclase activation by cholera toxin: inhibition of GTP hydrolysis at the regulatory site. Proc. Natl. Acad. Sci. USA 743307-3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Costigan, M., R. J. Mannion, G. Kendall, S. E. Lewis, J. A. Campagna, R. E. Coggeshall, J. Meridith-Middleton, S. Tate, and C. J. Woolf. 1998. Heat shock protein 27: developmental regulation and expression after peripheral nerve injury. J. Neurosci. 185891-5900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Delcourt, N., E. Thouvenot, B. Chanrion, N. Galeotti, P. Jouin, J. Bockaert, and P. Marin. 2007. PACAP type I receptor transactivation is essential for IGF-1 receptor signalling and antiapoptotic activity in neurons. EMBO J. 261542-1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deutsch, P. J., and Y. Sun. 1992. The 38-amino acid form of pituitary adenylate cyclase-activating polypeptide stimulates dual signaling cascades in PC12 cells and promotes neurite outgrowth. J. Biol. Chem. 2675108-5113. [PubMed] [Google Scholar]
- 14.Dodge, M. E., J. Wang, C. Guy, S. Rankin, M. Rahimtula, and K. M. Mearow. 2006. Stress-induced heat shock protein 27 expression and its role in dorsal root ganglion neuronal survival. Brain Res. 106834-48. [DOI] [PubMed] [Google Scholar]
- 15.Drahushuk, K., T. D. Connell, and D. Higgins. 2002. Pituitary adenylate cyclase-activating polypeptide and vasoactive intestinal peptide inhibit dendritic growth in cultured sympathetic neurons. J. Neurosci. 226560-6569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elbashir, S. M., J. Harborth, K. Weber, and T. Tuschl. 2002. Analysis of gene function in somatic mammalian cells using small interfering RNAs. Methods 26199-213. [DOI] [PubMed] [Google Scholar]
- 17.Evgrafov, O. V., I. Mersiyanova, J. Irobi, L. Van Den Bosch, I. Dierick, C. L. Leung, O. Schagina, N. Verpoorten, K. Van Impe, V. Fedotov, E. Dadali, M. Auer-Grumbach, C. Windpassinger, K. Wagner, Z. Mitrovic, D. Hilton-Jones, K. Talbot, J. J. Martin, N. Vasserman, S. Tverskaya, A. Polyakov, R. K. Liem, J. Gettemans, W. Robberecht, P. De Jonghe, and V. Timmerman. 2004. Mutant small heat-shock protein 27 causes axonal Charcot-Marie-Tooth disease and distal hereditary motor neuropathy. Nat. Genet. 36602-606. [DOI] [PubMed] [Google Scholar]
- 18.Freshney, N. W., L. Rawlinson, F. Guesdon, E. Jones, S. Cowley, J. Hsuan, and J. Saklatvala. 1994. Interleukin-1 activates a novel protein kinase cascade that results in the phosphorylation of Hsp27. Cell 781039-1049. [DOI] [PubMed] [Google Scholar]
- 19.Gerdin, M. J., and L. E. Eiden. 2007. Regulation of PC12 cell differentiation by cAMP signaling to ERK independent of PKA: do all the connections add up? Sci. STKE 2007pe15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Giglione, C., S. Gonfloni, and A. Parmeggiani. 2001. Differential actions of p60c-Src and Lck kinases on the Ras regulators p120-GAP and GDP/GTP exchange factor CDC25Mm. Eur. J. Biochem. 2683275-3283. [DOI] [PubMed] [Google Scholar]
- 21.Gonzalez, B. J., M. Basille, D. Vaudry, A. Fournier, and H. Vaudry. 1997. Pituitary adenylate cyclase-activating polypeptide promotes cell survival and neurite outgrowth in rat cerebellar neuroblasts. Neuroscience 78419-430. [DOI] [PubMed] [Google Scholar]
- 22.Govek, E. E., S. E. Newey, and L. Van Aelst. 2005. The role of the Rho GTPases in neuronal development. Genes Dev. 191-49. [DOI] [PubMed] [Google Scholar]
- 23.Guay, J., H. Lambert, G. Gingras-Breton, J. N. Lavoie, J. Huot, and J. Landry. 1997. Regulation of actin filament dynamics by p38 MAP kinase-mediated phosphorylation of heat shock protein 27. J. Cell Sci. 110357-368. [DOI] [PubMed] [Google Scholar]
- 24.Hansen, T. O., J. F. Rehfeld, and F. C. Nielsen. 2000. Cyclic AMP-induced neuronal differentiation via activation of p38 mitogen-activated protein kinase. J. Neurochem. 751870-1877. [DOI] [PubMed] [Google Scholar]
- 25.Hashimoto, H., N. Shintani, and A. Baba. 2006. New insights into the central PACAPergic system from the phenotypes in PACAP- and PACAP receptor-knockout mice. Ann. N. Y. Acad. Sci. 107075-89. [DOI] [PubMed] [Google Scholar]
- 26.He, J. C., I. Gomes, T. Nguyen, G. Jayaram, P. T. Ram, L. A. Devi, and R. Iyengar. 2005. The G alpha(o/i)-coupled cannabinoid receptor-mediated neurite outgrowth involves Rap regulation of Src and Stat3. J. Biol. Chem. 28033426-33434. [DOI] [PubMed] [Google Scholar]
- 27.Heo, W. D., T. Inoue, W. S. Park, M. L. Kim, B. O. Park, T. J. Wandless, and T. Meyer. 2006. PI(3,4,5)P3 and PI(4,5)P2 lipids target proteins with polybasic clusters to the plasma membrane. Science 3141458-1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hernandez, A., B. Kimball, G. Romanchuk, and M. W. Mulholland. 1995. Pituitary adenylate cyclase-activating peptide stimulates neurite growth in PC12 cells. Peptides 16927-932. [DOI] [PubMed] [Google Scholar]
- 29.Hopkins, D. A., J. C. Plumier, and R. W. Currie. 1998. Induction of the 27-kDa heat shock protein (Hsp27) in the rat medulla oblongata after vagus nerve injury. Exp. Neurol. 153173-183. [DOI] [PubMed] [Google Scholar]
- 30.Hoshino, M., and S. Nakamura. 2002. The Ras-like small GTP-binding protein Rin is activated by growth factor stimulation. Biochem. Biophys. Res. Commun. 295651-656. [DOI] [PubMed] [Google Scholar]
- 31.Hoshino, M., and S. Nakamura. 2003. Small GTPase Rin induces neurite outgrowth through Rac/Cdc42 and calmodulin in PC12 cells. J. Cell Biol. 1631067-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hoshino, M., T. Yoshimori, and S. Nakamura. 2005. Small GTPase proteins Rin and Rit bind to PAR6 GTP-dependently and regulate cell transformation. J. Biol. Chem. 28022868-22874. [DOI] [PubMed] [Google Scholar]
- 33.Huang, S. Y., M. L. Tsai, G. Y. Chen, C. J. Wu, and S. H. Chen. 2007. A systematic MS-based approach for identifying in vitro substrates of PKA and PKG in rat uteri. J. Proteome Res. 62674-2684. [DOI] [PubMed] [Google Scholar]
- 34.Kawakatsu, T., H. Ogita, T. Fukuhara, T. Fukuyama, Y. Minami, K. Shimizu, and Y. Takai. 2005. Vav2 as a Rac-GDP/GTP exchange factor responsible for the nectin-induced, c-Src- and Cdc42-mediated activation of Rac. J. Biol. Chem. 2804940-4947. [DOI] [PubMed] [Google Scholar]
- 35.Kiermayer, S., R. M. Biondi, J. Imig, G. Plotz, J. Haupenthal, S. Zeuzem, and A. Piiper. 2005. Epac activation converts cAMP from a proliferative into a differentiation signal in PC12 cells. Mol. Biol. Cell 165639-5648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim, S. H., S. Kim, and S. H. Ghil. 2008. Rit contributes to neurite outgrowth triggered by the alpha subunit of Go. Neuroreport 19521-525. [DOI] [PubMed] [Google Scholar]
- 37.Krueger-Naug, A. M., D. A. Hopkins, J. N. Armstrong, J. C. Plumier, and R. W. Currie. 2000. Hyperthermic induction of the 27-kDa heat shock protein (Hsp27) in neuroglia and neurons of the rat central nervous system. J. Comp. Neurol. 428495-510. [DOI] [PubMed] [Google Scholar]
- 38.Landry, J., and J. Huot. 1999. Regulation of actin dynamics by stress-activated protein kinase 2 (SAPK2)-dependent phosphorylation of heat-shock protein of 27 kDa (Hsp27). Biochem. Soc. Symp. 6479-89. [PubMed] [Google Scholar]
- 39.Lavoie, J. N., E. Hickey, L. A. Weber, and J. Landry. 1993. Modulation of actin microfilament dynamics and fluid phase pinocytosis by phosphorylation of heat shock protein 27. J. Biol. Chem. 26824210-24214. [PubMed] [Google Scholar]
- 40.Lavoie, J. N., H. Lambert, E. Hickey, L. A. Weber, and J. Landry. 1995. Modulation of cellular thermoresistance and actin filament stability accompanies phosphorylation-induced changes in the oligomeric structure of heat shock protein 27. Mol. Cell. Biol. 15505-516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lazarovici, P., H. Jiang, and D. Fink, Jr. 1998. The 38-amino-acid form of pituitary adenylate cyclase-activating polypeptide induces neurite outgrowth in PC12 cells that is dependent on protein kinase C and extracellular signal-regulated kinase but not on protein kinase A, nerve growth factor receptor tyrosine kinase, p21(ras) G protein, and pp60(c-src) cytoplasmic tyrosine kinase. Mol. Pharmacol. 54547-558. [DOI] [PubMed] [Google Scholar]
- 42.Lebon, A., D. Seyer, P. Cosette, L. Coquet, T. Jouenne, P. Chan, J. Leprince, A. Fournier, H. Vaudry, B. J. Gonzalez, and D. Vaudry. 2006. Identification of proteins regulated by PACAP in PC12 cells by 2D gel electrophoresis coupled to mass spectrometry. Ann. N. Y. Acad. Sci. 1070380-387. [DOI] [PubMed] [Google Scholar]
- 43.Lee, C. H. J., N. G. Della, C. E. Chew, and D. J. Zack. 1996. Rin, a neuron-specific and calmodulin-binding small G-protein, and Rit define a novel subfamily of ras proteins. J. Neurosci. 166784-6794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee, F. S., R. Rajagopal, A. H. Kim, P. C. Chang, and M. V. Chao. 2002. Activation of Trk neurotrophin receptor signaling by pituitary adenylate cyclase-activating polypeptides. J. Biol. Chem. 2779096-9102. [DOI] [PubMed] [Google Scholar]
- 45.Lein, P. J., X. Guo, G. X. Shi, M. Moholt-Siebert, D. Bruun, and D. A. Andres. 2007. The novel GTPase Rit differentially regulates axonal and dendritic growth. J. Neurosci. 274725-4736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lewis, S. E., R. J. Mannion, F. A. White, R. E. Coggeshall, S. Beggs, M. Costigan, J. L. Martin, W. H. Dillmann, and C. J. Woolf. 1999. A role for HSP27 in sensory neuron survival. J. Neurosci. 198945-8953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li, Y., S. Asuri, J. F. Rebhun, A. F. Castro, N. C. Paranavitana, and L. A. Quilliam. 2006. The RAP1 guanine nucleotide exchange factor Epac2 couples cyclic AMP and Ras signals at the plasma membrane. J. Biol. Chem. 2812506-2514. [DOI] [PubMed] [Google Scholar]
- 48.Lin, S. L., N. N. Johnson-Farley, D. R. Lubinsky, and D. S. Cowen. 2003. Coupling of neuronal 5-HT7 receptors to activation of extracellular-regulated kinase through a protein kinase A-independent pathway that can utilize Epac. J. Neurochem. 871076-1085. [DOI] [PubMed] [Google Scholar]
- 49.Lioudyno, M., Y. Skoglosa, N. Takei, and D. Lindholm. 1998. Pituitary adenylate cyclase-activating polypeptide (PACAP) protects dorsal root ganglion neurons from death and induces calcitonin gene-related peptide (CGRP) immunoreactivity in vitro. J. Neurosci. Res. 51243-256. [DOI] [PubMed] [Google Scholar]
- 50.Ma, Y. C., J. Huang, S. Ali, W. Lowry, and X. Y. Huang. 2000. Src tyrosine kinase is a novel direct effector of G proteins. Cell 102635-646. [DOI] [PubMed] [Google Scholar]
- 51.Macdonald, D. S., M. Weerapura, M. A. Beazely, L. Martin, W. Czerwinski, J. C. Roder, B. A. Orser, and J. F. MacDonald. 2005. Modulation of NMDA receptors by pituitary adenylate cyclase activating peptide in CA1 neurons requires G alpha q, protein kinase C, and activation of Src. J. Neurosci. 2511374-11384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Maizels, E. T., C. A. Peters, M. Kline, R. E. Cutler, Jr., M. Shanmugam, and M. Hunzicker-Dunn. 1998. Heat-shock protein-25/27 phosphorylation by the delta isoform of protein kinase C. Biochem. J. 332703-712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McCulloch, D. A., C. J. MacKenzie, M. S. Johnson, D. N. Robertson, P. J. Holland, E. Ronaldson, E. M. Lutz, and R. Mitchell. 2002. Additional signals from VPAC/PAC family receptors. Biochem. Soc. Trans. 30441-446. [DOI] [PubMed] [Google Scholar]
- 54.Mearow, K. M., M. E. Dodge, M. Rahimtula, and C. Yegappan. 2002. Stress-mediated signaling in PC12 cells—the role of the small heat shock protein, Hsp27, and Akt in protecting cells from heat stress and nerve growth factor withdrawal. J. Neurochem. 83452-462. [DOI] [PubMed] [Google Scholar]
- 55.Meier, M., G. L. King, A. Clermont, A. Perez, M. Hayashi, and E. P. Feener. 2001. Angiotensin AT(1) receptor stimulates heat shock protein 27 phosphorylation in vitro and in vivo. Hypertension 381260-1265. [DOI] [PubMed] [Google Scholar]
- 56.Miron, T., K. Vancompernolle, J. Vandekerckhove, M. Wilchek, and B. Geiger. 1991. A 25-kD inhibitor of actin polymerization is a low molecular mass heat shock protein. J. Cell Biol. 114255-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mitin, N., K. L. Rossman, and C. J. Der. 2005. Signaling interplay in Ras superfamily function. Curr. Biol. 15R563-R574. [DOI] [PubMed] [Google Scholar]
- 58.Moss, J. 1987. Signal transduction by receptor-responsive guanyl nucleotide-binding proteins: modulation by bacterial toxin-catalyzed ADP-ribosylation. Clin. Res. 35451-458. [PubMed] [Google Scholar]
- 59.Mounier, N., and A. P. Arrigo. 2002. Actin cytoskeleton and small heat shock proteins: how do they interact? Cell Stress Chaperones 7167-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Obara, Y., K. Labudda, T. J. Dillon, and P. J. Stork. 2004. PKA phosphorylation of Src mediates Rap1 activation in NGF and cAMP signaling in PC12 cells. J. Cell Sci. 1176085-6094. [DOI] [PubMed] [Google Scholar]
- 61.Ohtsuka, K., and T. Suzuki. 2000. Roles of molecular chaperones in the nervous system. Brain Res. Bull. 53141-146. [DOI] [PubMed] [Google Scholar]
- 62.Perng, M. D., L. Cairns, P. van Den IJssel, A. Prescott, A. M. Hutcheson, and R. A. Quinlan. 1999. Intermediate filament interactions can be altered by HSP27 and alphaB-crystallin. J. Cell Sci. 1122099-2112. [DOI] [PubMed] [Google Scholar]
- 63.Ravni, A., S. Bourgault, A. Lebon, P. Chan, L. Galas, A. Fournier, H. Vaudry, B. Gonzalez, L. E. Eiden, and D. Vaudry. 2006. The neurotrophic effects of PACAP in PC12 cells: control by multiple transduction pathways. J. Neurochem. 98321-329. [DOI] [PubMed] [Google Scholar]
- 64.Reichardt, L. F. 2006. Neurotrophin-regulated signalling pathways. Philos. Trans. R. Soc. Lond. B 3611545-1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rosario, M., H. F. Paterson, and C. J. Marshall. 2001. Activation of the Ral and phosphatidylinositol 3′ kinase signaling pathways by the Ras-related protein TC21. Mol. Cell. Biol. 213750-3762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rouse, J., P. Cohen, S. Trigon, M. Morange, A. Alonso-Llamazares, D. Zamanillo, T. Hunt, and A. R. Nebreda. 1994. A novel kinase cascade triggered by stress and heat shock that stimulates MAPKAP kinase-2 and phosphorylation of the small heat shock proteins. Cell 781027-1037. [DOI] [PubMed] [Google Scholar]
- 67.Seino, S., and T. Shibasaki. 2005. PKA-dependent and PKA-independent pathways for cAMP-regulated exocytosis. Physiol. Rev. 851303-1342. [DOI] [PubMed] [Google Scholar]
- 68.Shao, H., K. Kadono-Okuda, B. S. Finlin, and D. A. Andres. 1999. Biochemical characterization of the Ras-related GTPases Rit and Rin. Arch. Biochem. Biophys. 371207-219. [DOI] [PubMed] [Google Scholar]
- 69.Shi, C. S., and J. H. Kehrl. 2004. Pyk2 amplifies epidermal growth factor and c-Src-induced Stat3 activation. J. Biol. Chem. 27917224-17231. [DOI] [PubMed] [Google Scholar]
- 70.Shi, G. X., and D. A. Andres. 2005. Rit contributes to nerve growth factor-induced neuronal differentiation via activation of B-Raf-extracellular signal-regulated kinase and p38 mitogen-activated protein kinase cascades. Mol. Cell. Biol. 25830-846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shi, G. X., J. Han, and D. A. Andres. 2005. Rin GTPase couples nerve growth factor signaling to p38 and b-Raf/ERK pathways to promote neuronal differentiation. J. Biol. Chem. 28037599-37609. [DOI] [PubMed] [Google Scholar]
- 72.Shi, G. X., H. Rehmann, and D. A. Andres. 2006. A novel cAMP-dependent Epac-Rit signaling pathway contributes to PACAP38-mediated neuronal differentiation. Mol. Cell. Biol. 269136-9147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Somogyvari-Vigh, A., and D. Reglodi. 2004. Pituitary adenylate cyclase activating polypeptide: a potential neuroprotective peptide. Curr. Pharm. Des. 102861-2889. [DOI] [PubMed] [Google Scholar]
- 74.Spencer, M. L., H. Shao, H. M. Tucker, and D. A. Andres. 2002. Nerve growth factor-dependent activation of the small GTPase Rin. J. Biol. Chem. 27717605-17615. [DOI] [PubMed] [Google Scholar]
- 75.Stokoe, D., K. Engel, D. G. Campbell, P. Cohen, and M. Gaestel. 1992. Identification of MAPKAP kinase 2 as a major enzyme responsible for the phosphorylation of the small mammalian heat shock proteins. FEBS Lett. 313307-313. [DOI] [PubMed] [Google Scholar]
- 76.Stork, P. J., and J. M. Schmitt. 2002. Crosstalk between cAMP and MAP kinase signaling in the regulation of cell proliferation. Trends Cell Biol. 12258-266. [DOI] [PubMed] [Google Scholar]
- 77.Vaudry, D., B. J. Gonzalez, M. Basille, L. Yon, A. Fournier, and H. Vaudry. 2000. Pituitary adenylate cyclase-activating polypeptide and its receptors: from structure to functions. Pharmacol. Rev. 52269-324. [PubMed] [Google Scholar]
- 78.Wagstaff, M. J., Y. Collaco-Moraes, J. Smith, J. S. de Belleroche, R. S. Coffin, and D. S. Latchman. 1999. Protection of neuronal cells from apoptosis by Hsp27 delivered with a herpes simplex virus-based vector. J. Biol. Chem. 2745061-5069. [DOI] [PubMed] [Google Scholar]
- 79.Waschek, J. A. 2002. Multiple actions of pituitary adenylyl cyclase activating peptide in nervous system development and regeneration. Dev. Neurosci. 2414-23. [DOI] [PubMed] [Google Scholar]
- 80.Wes, P. D., M. Yu, and C. Montell. 1996. RIC, a calmodulin-binding Ras-like GTPase. EMBO J. 155839-5848. [PMC free article] [PubMed] [Google Scholar]
- 81.Williams, K. L., M. Rahimtula, and K. M. Mearow. 2006. Heat shock protein 27 is involved in neurite extension and branching of dorsal root ganglion neurons in vitro. J. Neurosci. Res. 84716-723. [DOI] [PubMed] [Google Scholar]
- 82.Williams, K. L., M. Rahimtula, and K. M. Mearow. 2005. Hsp27 and axonal growth in adult sensory neurons in vitro. BMC Neurosci. 624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wolf, R. M., J. J. Wilkes, M. V. Chao, and M. D. Resh. 2001. Tyrosine phosphorylation of p190 RhoGAP by Fyn regulates oligodendrocyte differentiation. J. Neurobiol. 4962-78. [DOI] [PubMed] [Google Scholar]