Abstract
The anterior heart field (AHF) comprises a population of mesodermal progenitor cells that are added to the nascent linear heart to give rise to the majority of the right ventricle, interventricular septum, and outflow tract in mammals and birds. The zinc finger transcription factor GATA4 functions as an integral member of the cardiac transcription factor network in the derivatives of the AHF. In addition to its role in cardiac differentiation, GATA4 is also required for cardiomyocyte replication, although the transcriptional targets of GATA4 required for proliferation have not been previously identified. In the present study, we disrupted Gata4 function exclusively in the AHF and its derivatives. Gata4 AHF knockout mice die by embryonic day 13.5 and exhibit hypoplasia of the right ventricular myocardium and interventricular septum and display profound ventricular septal defects. Loss of Gata4 function in the AHF results in decreased myocyte proliferation in the right ventricle, and we identified numerous cell cycle genes that are dependent on Gata4 by microarray analysis. We show that GATA4 is required for cyclin D2, cyclin A2, and Cdk4 expression in the right ventricle and that the Cyclin D2 and Cdk4 promoters are bound and activated by GATA4 via multiple consensus GATA binding sites in each gene's proximal promoter. These findings establish Cyclin D2 and Cdk4 as direct transcriptional targets of GATA4 and support a model in which GATA4 controls cardiomyocyte proliferation by coordinately regulating numerous cell cycle genes.
The cardiac lineage in mammals is initially specified from the anterior lateral mesoderm at embryonic day 7.5 (E7.5) in the mouse. The nascent cardiac mesoderm migrates anteriolaterally and fuses ventrally in the embryo to form a linear tube. The linear tube elongates through the addition of cells from the second heart field to the arterial and venous poles (1, 12, 28). A more restricted, anterior subset of these cells are added only to the arterial pole from the pharyngeal and splanchnic mesoderm. These cells, referred as the anterior heart field (AHF), give rise to the outflow tract, right ventricle, and ventricular septum (1, 9, 11, 27, 81). As cells from the AHF are added, the heart bends toward the ventral side, undergoes rightward looping, expands dramatically, and is eventually remodeled into the mature, four-chambered organ (13, 66).
Embryonic cardiomyocytes differentiate as they continue to proliferate (48, 52). At early stages in development, cardiomyocytes have a high proliferation rate, which decreases progressively in late gestation (67). The high rate of cell cycle activity during the early stages of cardiomyocyte differentiation contributes to the growth of the future chambers within the linear tube during looping morphogenesis (42). The trabecular myocardium has a high rate of proliferation at this stage. As ventricular volumes increase, the trabeculations become compressed within the ventricular wall, resulting in a significant increase in the thickness of the compact myocardium (66). The compact myocardium proliferates more rapidly than the trabecular myocardium after chamber maturation has occurred (84), and several cell cycle genes have been shown to play important roles in cardiomyocyte proliferation (51, 76). D-cyclins and their catalytic partners, cyclin-dependent kinases (Cdks), are key components of the cell cycle machinery that determine whether cells divide or remain quiescent (24). D-cyclins are regarded as sensors of the extracellular environment that link mitogenic pathways to the cell cycle machinery (35). Once D-cyclins are induced by mitogenic signals, they associate with Cdks, resulting in the phosphorylation of the retinoblastoma suppressor RB and RB-related proteins p107 and p130 (37). This phosphorylation causes the release of the E2F transcription factor and allows cells to progress from G1 to S phase (2, 3, 63, 68).
GATA transcription factors comprise an evolutionarily conserved family of zinc finger-containing proteins and recognize the consensus binding site WGATAR (53). There are six GATA factors; GATA1, -2, and -3 play key roles in hematopoiesis, and GATA4, -5, and -6 are important for development of multiple mesoderm- and endoderm-derived tissues, including heart and liver (5, 38). Gata4 is one of the earliest genes expressed in the cardiac crescent of the mouse, and Gata4-null mice die around E10 as a result of severe defects in the extraembryonic endoderm and display defects in heart and foregut morphogenesis (31, 39). In humans, GATA4 mutations are associated with defects in ventricular and atrial septation (22, 45). GATA4 regulates the expression of genes that are important for cardiac contraction as well as the expression of other cardiac transcription factor genes, such as Mef2c, Hand2, and Nkx2-5 (18, 33, 36, 65).
In addition to its role in cardiac differentiation, GATA4 is also an important regulator of apoptosis and cell proliferation (29, 47, 74, 82, 86). The balance of these two processes controls cardiomyocyte number and ultimately the function of the working myocardium, and several studies have shown the importance of GATA4 in myocardial development (47, 59, 62, 82, 86). Tetraploid complementation, which circumvents extraembryonic defects in Gata4-null mice, revealed a role for GATA4 in myocardial growth (82). A mutation in GATA4 that disrupts its interaction with its cofactor Friend-of-GATA 2 (FOG2) results in embryonic arrest around E12.5. Affected embryos displayed defects in vascular development and also had a thin ventricular wall (15). More-recent studies showed that conditional inactivation of Gata4 using Nkx2-5Cre resulted in embryonic lethality around E11.5, with decreased cardiomyocyte proliferation and major defects in the development of the right ventricle (86). However, the expression of Cre from the Nkx2-5Cre knock-in allele is broad, encompassing the derivatives of both the first and second heart fields, as well as the pharyngeal endoderm, which leaves open the possibility that signals downstream of GATA4 in the pharyngeal endoderm or from the first heart field may have affected the development of the right ventricle (86).
While previous studies have demonstrated a role for GATA4 in cardiomyocyte proliferation, the genes regulated by GATA4 that mediate this activity were not previously identified. In the present study, we used whole-genome microarray analysis to identify misexpressed genes in myocytes lacking Gata4 function. In addition, we address the function of GATA4 in a more restricted myocardial region than previous studies by inactivating Gata4 exclusively in the AHF and its derivatives in the outflow tract, right ventricle, and interventricular septum (81). Gata4-null cardiomyocytes show downregulation of a wide array of cell cycle-associated genes, consistent with significant alteration of proliferation. Cdk4, Cyclin D2, and Cyclin A2 were among the most dramatically downregulated genes in Gata4-null hearts, and we show that expression of all three cell cycle proteins is decreased specifically in the right ventricles of Gata4 AHF conditional knockout embryos. Furthermore, we show that GATA4 binds and directly activates the Cyclin D2 and Cdk4 promoters in vitro and in vivo, which establishes for the first time a direct regulatory relationship between GATA4 and these two components of the cell cycle machinery. The broad downregulation of cell cycle-associated genes provides an explanation for the profound proliferation defects in the hearts of mice lacking GATA4 function and suggests a coordinated, GATA-dependent program for myocyte proliferation. Given the broad overlap of GATA transcription factors with cyclin D2, Cdk4, and other cell cycle regulators, the studies presented here suggest the possibility that GATA transcription factors function generally to regulate G1/S transition and cellular proliferation.
MATERIALS AND METHODS
Generation of Gata4 AHF knockout mice.
Gata4flox/flox, Nkx2-5Cre, and Mef2c-AHF-Cre mice have been described previously (44, 59, 81, 86). Mice harboring the Gata4 floxed allele were crossed with Mef2c-AHF-Cre mice such that the second coding exon was removed specifically in the AHF by the action of Cre recombinase. The strategy for genotyping Gata4 wild-type and floxed alleles has been described previously (86). The Cre transgene was detected by PCR using the following primers: 5′-TGCCACGACCAAGTGACAGC-3′ and 5′-CCAGGTTACGGATATAGTTCATG-3′. To obtain Gata4flox/flox; Mef2c-AHF-CreTg/0 embryos, timed matings between Gata4flox/+; Mef2c-AHF-CreTg/0 male mice and Gata4flox/flox female mice were set up. All experiments using animals complied with federal and institutional guidelines and were reviewed and approved by University of California, San Francisco, Institutional Animal Care and Use Committee.
Immunohistochemistry and in situ hybridization.
Embryos collected at different stages were fixed in 4% paraformaldehyde, dehydrated with ethanol and xylene, and mounted in paraffin. Sections were cut at a thickness of 5 μm with a Leica RM 2155 microtome. Sections were dewaxed through a series of xylene and ethanol washes and counterstained with hematoxylin and eosin to visualize embryonic structures using standard procedures (25).
For immunohistochemistry, sections were dewaxed, incubated in phosphate-buffered saline (PBS) for 5 min, boiled in antigen retrieval solution (Biogenex), and blocked in 3% normal goat serum for 1 h. Incubation with primary rabbit anti-cyclin D2 (Santa Cruz; Sc-593), rabbit anti-Cdk4 (Santa Cruz; Sc-260), rabbit anti-cyclin A2 (Santa Cruz; Sc-751), mouse monoclonal anti-Ki67 (Novocastra), or rabbit anti-phospho-histone H3 (Upstate Laboratories; catalog no. 06-570) at a 1:300 dilution in each case was done overnight at 4°C in a humid chamber. Following incubation with the primary antibodies, sections were washed three times with PBS and incubated with one of the following secondary antibodies: Alexa Fluor 594 donkey anti-rabbit antibody (Invitrogen; no. A21207), Oregon Green 488 goat anti-rabbit antibody (Invitrogen; no. 0-11038), or biotinylated goat anti-mouse antibody (Vector Laboratories; BA-9200). Secondary antibodies were diluted 1:300 in 3% normal goat serum and were incubated with the slides at room temperature for 1 h. Slides were then washed three times in PBS, mounted using SlowFade Light antifade with DAPI (4′,6-diamidino-2-phenylindole; Molecular Probes), and photographed on a fluorescence microscope. For Ki67 and cyclin A2 detection, immunoperoxidase staining was performed using the Vectastain Elite ABC kit (Vector Laboratories; PK-6102) and developed using the peroxidase substrate diaminobenzidine (Vector Laboratories; SK-4100). Terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) assays were performed using the ApopTag kit from Chemicon (S-7110) by following the manufacturer's recommendations.
To measure DNA synthesis, 2 mg of 5-bromo-2-deoxyuridine (BrdU; Sigma; B9285) dissolved in saline was injected intraperitoneally into pregnant mice and the mice were euthanized 2 h later. Embryos collected from these mice were processed as described above, and the sections were dewaxed and treated with 1 M HCl for 7 min at 60°C. Antibody staining was performed using rat anti-BrdU (Serotec; MCA2060) and tetramethyl rhodamine isocyanate-conjugated anti-rat antibody (Sigma).
Whole-mount in situ hybridization was performed as described previously (64). A Gata4 in situ probe was generated from a pBluescript II SK(+) plasmid containing the first and second exons of the murine Gata4 gene, linearized with NotI, and transcribed with T3 polymerase.
Microarray.
RNA was isolated and pooled from four or five E9.5 hearts from mice with each of the following genotypes: Gata4flox/+; Nkx2-5+/+ (control; n = 3), Gata4flox/+; Nkx2-5Cre/+ (Gata4; Nkx2-5; doubly heterozygous, n = 3), and Gata4flox/flox; Nkx2-5Cre/+ (Gata4 CKONkx; n = 4). Total RNA (50 ng) was amplified and converted to cDNA using the Ovation RNA labeling kit (NuGen). The cDNA was then hybridized to Affymetrix GeneChip Mouse 430.2 microarrays, which have 45,101 probe sets. Gene expression data are available through the Gene Expression Omnibus database (accession number GSE9652). Probe sets with absent calls in nine or more samples were excluded. Comparisons between controls and the other two groups were made. Differentially expressed genes were defined as those for which the nominal P was <0.005. Gene set analysis was performed using the gene set enrichment analysis method with default parameters (http://www.broad.mit.edu/gsea). Cell cycle-related gene sets of size 10 to 250 were selected from the Molecular Signature Database (MSigDB). The C2 collection is available at http://www.broad.mit.edu/gsea/msigdb/collections.jsp#c2 (72).
EMSA.
DNA binding reactions were performed as described previously (19). Briefly, double-stranded oligonucleotides were labeled with [32P]dCTP, using Klenow fragments to fill in the overhanging 5′ ends, and purified on a nondenaturing polyacrylamide-Tris-borate-EDTA gel. Binding reaction mixtures were preincubated at room temperature in 1× binding buffer (40 mM KCl, 15 mM HEPES [pH 7.9], 1 mM EDTA, 0.5 mM dithiothreitol, 5% glycerol) containing recombinant protein, 1 μg of poly(dI-dC), and competitor DNA for 10 min prior to probe addition. Reaction mixtures were incubated for an additional 20 min at room temperature after probe addition and were then electrophoresed on a 6% nondenaturing polyacrylamide gel. The Gata4 cDNA was transcribed and translated using the TNT coupled transcription-translation system (Promega), as described in the manufacturer's directions. GATA4 protein was generated from pCITE-GATA4 plasmid, which has been described previously (18). The sense strand sequences of the mouse Cyclin D2 and Cdk4 GATA sites and mutant GATA sites used for the electrophoretic mobility shift assay (EMSA) were as follows: cyclin D2 Gata I, 5′-GGAACAGCTTGAAAGTTATCAGGAGTCTAAGCTTGAG-3′; cyclin D2 Gata Im, 5′-AACAGCTTGAAAGGTACCAGGAGTCTAAGCTTGAG-3′; cyclin D2 Gata II, 5′-GGGAGGGGCATAACCTTTATCCCTGGTTTGGCGAGGT-3′; cyclin D2 Gata IIm, 5′-GAGGGGCATAACCTCTAGACCTGGTTTGGCGAGGT-3′; cyclin D2 Gata III, 5′-GGACAGAATGTCAGAAAGGATAATCAATAGGAATCCAT-3′; cyclin D2 Gata IIIm, 5′-ACAGAATGTCAGAAAGGATCCTCAATAGGAATCCAT-3′; Cdk4 GataI/II, 5′-GGAATTACCTATACTAGTTATCTTTATCATTCACTTCAAAGGGC-3′; Cdk4 GataI/IIm, 5′-AATTACCTATACTAGTAAGCTTTATAATTCACTTCAAAGGGC-3′; Cdk4 GataIII, 5′-GGCAAGGGGTCACGTGGGATAGCAACAGGTCACCGTGG-3′; Cdk4 GataIIIm, 5′-CAAGGGGTCACGTGGGTTAACAACAGGTCACCGTGG-3′.
Cell culture, transfections, and reporter assays.
A 931-bp fragment containing 671 bp upstream and 260 bp downstream of the transcriptional start site from the mouse Cyclin D2 promoter region was amplified by PCR using the following primers: 5′-ACAGAAAGGTTTCTGCAGGAGGGTCATATTC-3′ and 5′-GCCAGCCGGCGTCGACTCGGTCCCGAC-3′. An 827-bp fragment containing 771 bp upstream and 56 bp downstream of the transcriptional start site from the mouse Cdk4 promoter region was amplified by PCR using the following primers: 5′-CTTTTAATATTCCGCGGGAGGTTTAC-3′ and 5′-GGGCAGCTGGATCCTTCGGGCCAGAC-3′. Cyclin D2 and Cdk4 PCR products were cloned into the pAUG-β-gal reporter vector (36). Plasmid pECE-GATA4-EnR has the Drosophila Engrailed repressor domain fused to the Gata4 cDNA and has been described previously (32). The expression plasmid pRK5-GATA4-VP16 contains the herpes simplex virus type 1 Vmw65.1 transcriptional activation domain fused in frame to the 3′ end of the GATA4 cDNA. Mutations of the GATA sites in the Cyclin D2 and Cdk4 promoters were introduced by PCR to create the mutant sequences in the EMSA oligonucleotides described above.
C3H10T1/2 was maintained in Dulbecco modified Eagle medium supplemented with 10% fetal bovine serum. P19CL6 cells were maintained in Dulbecco modified Eagle medium supplemented with 10% fetal bovine serum in the presence of 1% dimethyl sulfoxide (DMSO) for 7 days prior to transfection. Transient transfections were performed in 12-well plates using Fugene6 (Roche) for C3H10T1/2 cells and Lipofectamine XLT (Invitrogen) for P19CL6 cells, by following the manufacturer's recommendations. Each transfection mixture contained 0.5 μg of Cyclin D2 or Cdk4 reporter plasmids and 1.0 μg of repressor or activator plasmids. In transfections without an expression construct, the parent expression plasmid was added to keep the total amount of DNA in each transfection constant at 1.5 μg. Cells were cultured for 48 h after transfection and harvested, and cellular extracts were prepared by sonication and were normalized as described previously (14). Chemiluminescence β-galactosidase (β-Gal) assays were performed using the luminescent β-Gal detection system (Clontech) according to the manufacturer's recommendations, and relative light units were detected using a Tropix TR717 microplate luminometer (PE Applied Biosystems).
ChIP assays.
Chromatin immunoprecipitation (ChIP) assays were performed using the ChIP assay kit from Upstate Pharmaceuticals (catalog no. 17-295), in accordance with the recommendations of the manufacturer. Briefly, a 10-cm plate containing approximately 1 × 106 P19CL6 cells, which had been differentiated into cardiomyocytes by treatment with 1% DMSO for 7 days, was treated with 1% paraformaldehyde at 37°C for 10 min to cross-link protein-DNA complexes. Cells were then lysed and sonicated to shear the DNA into fragments of between 300 and 500 bp. The cleared supernatant was incubated with 4 μg of anti-GATA4 antibody (Santa Cruz; Sc-1237) or 4 μg of anti-goat immunoglobulin G (IgG) (Santa Cruz; Sc-2020) overnight at 4°C. The DNA fragments were then precipitated after incubating the lysate and antibody mixture with protein A-agarose beads for 1 h. Reaction mixtures were incubated with NaCl at 65°C for 4 h to reverse the cross-links, and DNA was recovered by phenol-chloroform extraction. The following primers were used to amplify the Cyclin D2 promoter, which contains three GATA sites, following ChIP: P1, 5′-CTCCACGCACGTGGCTCGGGGCGG-3′, and P2, 5′-TAGGGGAACCCACAAACCCCATGG-3′. Two different regions of the Cdk4 promoter, a distal region containing two GATA sites and a proximal region containing one GATA site, were amplified following ChIP using the following primers: P1, 5′-CATACAGTGGCTTATTATATTTCC-3′, P2, 5′-CTCCACCGCCATGGGGAAACATTC-3′, P3, 5′-GTTGGCCCGGTTGCCATGACACCG-3′, and P4, 5′-CTGGACACGTGATCTTCACCCTTG-3′. The Cyclin D2 second exon was amplified as a negative control in ChIP experiments using the following primers: 5′-GCGGCCTTAGTGTGATGGGGAAGG-3′ and 5′-TCGGACCCTACCCCACTCTTGATTG-3′. ChIP PCR products were confirmed by sequencing.
RESULTS
Inactivation of Gata4 in the AHF results in right ventricular hypoplasia and ventricular septal defects (VSDs).
To determine the role of the GATA4 specifically in the development of the right ventricle and outflow tract, we inactivated Gata4 in the progenitors of the right ventricle and outflow tract using Mef2c-AHF-Cre, which directs early excision in AHF progenitors in the splanchnic and pharyngeal mesoderm (4, 50, 81). This resulted in specific loss of Gata4 expression in AHF derivatives in the right ventricle and outflow tract ( Fig. 1A and B). These crosses did not produce any live Gata4 AHF knockout animals, indicating that GATA4 is required in the derivatives of the AHF for embryonic development (Table 1). At E10.5 and E11.5, Gata4 conditional knockout embryos were present at normal Mendelian frequencies (Table 1), and the appearance of AHF knockout embryos was normal at these stages (data not shown). However, by E13.5, all Gata4 AHF conditional knockout embryos exhibited cardiovascular congestion and vascular hemorrhage, and the majority of the embryos lacked a heartbeat at this stage (Fig. 1C and D).
FIG. 1.
Inactivation of Gata4 in the AHF results in lethality due to right ventricular hypoplasia and VSDs. (A and B) Whole-mount in situ hybridization showing expression of Gata4 mRNA in control (A) and Gata4 AHF knockout (B) hearts at E10.5. The excision of the Gata4 floxed allele by Mef2c-AHF-Cre results in loss of Gata4 mRNA in the right ventricle (RV) and outflow tract (OFT) in Gata4 AHF knockout embryos. LV, left ventricle. (C and D) Gata4 AHF knockout embryos (D) display obvious vascular hemorrhage (arrowheads) compared to littermate controls (C) at E13.5. (E to H) Hematoxylin- and eosin-stained transverse sections of littermate control (E) and Gata4 AHF knockout (F) embryos show that the formation of the ventricular septum (arrowheads) is aberrant at E13.5 in Gata4 AHF knockout embryos compared to controls. LA, left atrium; RA, right atrium. (G and H) The compact wall myocardium of the right ventricle (asterisks) is thinner at E13.5 in Gata4 AHF knockout embryos (H) than in littermate control embryos (G). Bars, 100 μm. Genotypes for control (Gata4flox/flox) and Gata4 AHF knockout (CreTg/0; Gata4flox/flox) embryos are indicated. n was 4 for each genotype.
TABLE 1.
Loss of Gata4 function in the AHF results in embryonic lethality by E13.5a
| Mouse genotype | No. of offspring at:
|
||||
|---|---|---|---|---|---|
| E10.5 (0.83, 0.841) | E11.5 (0.16, 0.980) | E12.5 (4.44, 0.216) | E13.5 (11.10, 0.011) | P0 (44.47, <0.0001) | |
| Gata4flox/+ | 28 | 13 | 33 | 17 | 44 |
| Gata4flox/flox | 28 | 12 | 24 | 16 | 35 |
| CreTg/0; Gata4flox/+ | 34 | 11 | 22 | 21 | 47 |
| CreTg/0; Gata4flox/flox | 29 | 12 | 19 | 4* | 0** |
Gata4flox/+; Mef2c-AHF-CreTg/0 mice were crossed to Gata4flox/flox mice, and the offspring were collected at the indicated developmental stages. Offspring of each genotype from E10.5 to E12.5 were present at normal Mendelian frequencies. By E13.5, most of the conditional knockout embryos (*) lacked a heartbeat. No Gata4 AHF knockouts were present at birth (P0; **). The χ2 and P values are in parentheses (χ2, P) after the developmental stage.
Histological analyses of knockout hearts at E12.5 to E13.5 did not reveal any obvious defects in outflow tract alignment, and the septation into the pulmonary trunk and aorta appeared to be normal (data not shown). However, the right ventricles of all AHF knockout embryos were obviously hypoplastic compared with those of littermate control embryos (Fig. 1E to H). The compact zone of the myocardium in knockout embryos contained fewer myocardial cell layers than those in control embryos, where the myocardial wall of the right ventricle was much thicker by this stage (Fig. 1G and H). In addition, the right ventricular trabecular myocardium of conditional knockout embryos appeared disorganized and not well connected with the compact myocardium (Fig. 1G and H). The formation of the ventricular septum, which was almost completed by E13.5 in control embryos, was delayed in all Gata4 AHF knockout embryos (Fig. 1E and F). As expected, no abnormalities were observed in the left ventricle, which is outside of the Mef2c-AHF-Cre expression domain (Fig. 1E and F).
Gata4 AHF knockout mice display myocardial proliferation defects in the right ventricle.
GATA4 has been implicated previously in both myocardial proliferation and apoptosis (74, 85, 86), and the myocardial hypoplasia observed in the right ventricles of Gata4 AHF knockout embryos could be explained by an increase in cell death or a decrease in proliferation. To determine whether cell death might be involved in the myocardial hypoplasia of Gata4 AHF knockout embryos, we performed TUNEL staining on cardiac sections from embryos at E10.5. We selected this developmental stage since it represents a time prior to embryonic lethality and hearts would be less likely to exhibit nonspecific apoptosis secondary to cardiac failure. Results from these experiments showed no differences in TUNEL staining between conditional knockout and control embryos (Fig. 2I and J).
FIG. 2.
Gata4 AHF knockout embryos have profound myocardial proliferation defects. (A to H) Immunohistochemical analyses of proliferation markers on transverse sections show that Gata4 AHF knockout embryos (B, D, F, and H) have reduced proliferation compared to control embryos (A, C, E, and G) at E10.5. (A and B) Gata4 AHF knockout embryos display decreased staining of the nuclear antigen Ki67 (brown) in the right ventricular myocardium and interventricular septum compared to control embryos (asterisks). (C and D) Closer view of the right ventricle (RV) shows that Ki67 staining in Gata4 AHF knockout hearts is reduced in the myocardium (myo) but not in other regions where Gata4 was not inactivated, such as the epicardium (epi). (E and F) BrdU incorporation is diminished in the myocardium of the right ventricle of Gata4 AHF knockout embryos compared to control embryos (asterisks). (G and H) Expression of the mitotic marker phospho-histone H3 (pHH3) is reduced in the right ventricle in Gata4 AHF knockout embryos compared to control littermates (arrowheads). No differences in the staining of any of these proliferation markers between Gata4 AHF knockout and control embryos was observed in the left ventricle (LV). (I and J) TUNEL staining on transverse sections of embryonic hearts shows no difference in apoptosis between Gata4 AHF knockout and control embryos at E10.5. Genotypes for control (Gata4flox/flox) and Gata4 AHF knockout (Gata4flox/flox; Mef2c-AHF-CreTg/0) embryos are indicated. (K and L) Quantification of BrdU (K)- and pHH3 (L)-labeled cells shows a significant decrease in proliferation in the right ventricle of conditional knockout (CKO) embryos compared to littermate controls. The total number of DAPI-labeled cells and the number of BrdU- or pHH3-labeled cells were determined by counting cells in a series of sections from three CKO and three control hearts. Data are presented as the mean percentages of cells labeled with BrdU or pHH3 plus standard errors of the means from three hearts of each genotype. P values were calculated using a two-tailed, unpaired t test.
To determine if inactivation of Gata4 in the AHF resulted in defective myocyte replication, we examined the expression of several markers of proliferation, including Ki67 and phospho-histone H3, and BrdU incorporation at E10.5 (Fig. 2). In each case, Gata4 AHF knockout embryos displayed significantly reduced expression of the proliferation markers in the right ventricle compared to littermate control embryos (Fig. 2A to H). Similarly, Gata4 AHF knockout embryos displayed reduced proliferation in the interventricular septum (Fig. 2A, B, and E to H), which is consistent with theVSDs observed in knockout embryos at E13.5 (Fig. 1F). The reduced proliferation in the right ventricular myocardium in AHF knockouts compared to littermate controls was especially evident when Ki67, which marks all stages of the cell cycle, was examined (Fig. 2, compare panels A and C to B and D). Quantification of BrdU-labeled and phospho-histone H3-labeled nuclei as a percentage of the total number of DAPI-stained nuclei showed that proliferation was significantly reduced in the right ventricle (Fig. 2K and L). By contrast, in the left ventricle, where Gata4 excision did not occur since the left ventricle is outside the expression domain of Mef2c-AHF-Cre, levels of expression of these markers in knockout and control embryos were the same (Fig. 2K and L). Taken together, the results presented in Fig. 2 demonstrate that GATA4 is required in the derivatives of the AHF for proliferation, which supports previous studies that demonstrated a role for GATA4 in cardiomyocyte proliferation (82, 86).
GATA4 regulates the expression of numerous cell cycle genes in the heart.
The defects in myocyte proliferation in Gata4 AHF knockout hearts (Fig. 2) suggested that GATA4 was likely to regulate one or more genes involved in the cell cycle. Therefore, to investigate further the molecular changes underlying these alterations in cardiomyocyte proliferation, we measured mRNA expression in E9.5 mouse hearts by microarray. To accomplish this, we used Nkx2-5Cre to inactivate Gata4 (Gata4 CKONkx). As in Gata4 AHF knockout hearts, cardiomyocyte proliferation was decreased in Gata4 CKONkx cardiomyocytes (86). However, the expression domain of Nkx2-5Cre is broader than that of Mef2c-AHF-Cre (44, 81), allowing us to use the entire hearts from Gata4 CKONkx embryos at E9.5 for microarray analyses.
We used the microarray expression data to determine if established sets of cell cycle genes showed statistically discordant differences between Gata4flox/+ (control) and Gata4flox/flox; Nkx2-5Cre/+ (Gata4 CKONkx) hearts. We used curated gene sets available from the Molecular Signature Database (http://www.broad.mit.edu/gsea) and the gene set enrichment analysis method (72). Seven out of 12 cell cycle-related gene sets, including the Brentani cell cycle gene set (10), were significantly altered in Gata4 conditional knockout hearts (P < 0.001; data not shown), suggesting that Gata4 inactivation leads to a broad, coordinate perturbation of genes involved in cell cycle regulation (Fig. 3). By comparison, no cell cycle gene sets were significantly altered between Gata4flox/+; Nkx2-5Cre/+ and control mice, indicating that double heterozygosity for Gata4 and Nkx2-5 does not result in significant alteration in the expression of cell cycle gene sets (data not shown).
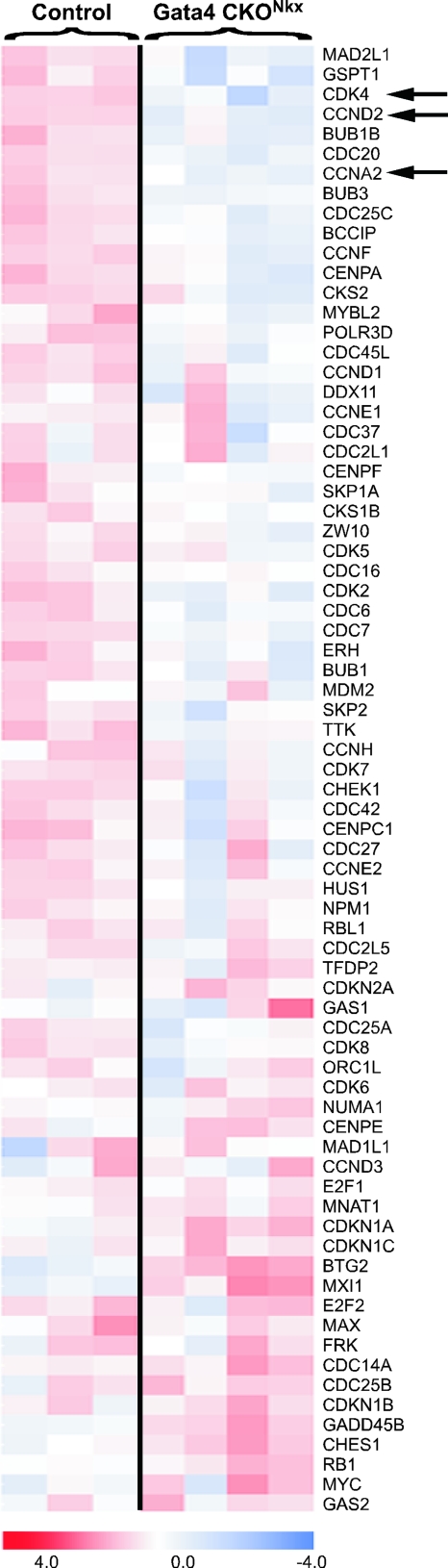
FIG. 3.
GATA4 regulates multiple cell cycle control genes. Affymetrix gene expression data were analyzed by gene set enrichment (72). Several sets of genes with known roles in cell cycle regulation showed statistically significant, concordant differences between control (Gata4flox/+) and Gata4 CKONkx (Gata4flox/flox; Nkx2-5Cre/+) hearts. The heat map of genes comprising the cell cycle gene set with the most significant statistical score (Brentani cell cycle gene set) is shown (10). Color indicates degree of upregulation (red) or downregulation (blue) relative to the mean expression across all samples (see the color scale at the bottom). Numerous cell cycle control genes were significantly downregulated in Gata4 CKONkx hearts compared to controls, including Cyclin D2 (CCND2), Cyclin A2 (CCNA2), and Cdk4, which are denoted by arrows.
Next, we looked for individual genes that when misregulated might contribute to abnormal expression of cell cycle gene sets and abnormal cardiomyocyte proliferation. We found that 1,302 probe sets were differentially expressed between control and Gata4 CKONkx embryos (P < 0.005). In contrast, only 68 probe sets were differentially expressed between Gata4flox/flox control and Gata4flox/+; Nkx2-5Cre/+ doubly heterozygous hearts, indicating that Gata4 inactivation rather than Nkx2-5 and Gata4 heterozygosity is responsible for the majority of the observed changes in gene expression (data not shown). Notably, many genes that are known to play fundamental roles in cellular proliferation, including several cyclin genes and numerous other cell cycle genes, were significantly downregulated in the absence of GATA4 function (Fig. 3). Interestingly, Cyclin D2 (CCND2) and Cyclin A2 (CCNA2) were among the most dramatically downregulated genes in the absence of GATA4 function in the heart (Fig. 3). Cyclin function depends on the activity of Cdks, including Cdk2 and Cdk4, and mice lacking multiple Cdk genes die during embryonic development with thin myocardial walls (8). Our microarray data indicated that several Cdk genes, including Cdk4, also had reduced expression in the absence of GATA4 (Fig. 3).
Gata4 is required for Cyclin D2, Cdk4, and Cyclin A2 expression in the right ventricle.
Because Cyclin D2, Cyclin A2, and Cdk4 were among the most significantly downregulated transcripts according to microarray data (Fig. 3), we further examined the expression of the corresponding gene products in Gata4flox/flox; Mef2c-AHF-CreTg/0 embryos by immunohistochemistry at E10.5. These analyses showed that the expression of all three cell cycle proteins was substantially reduced in the myocardium and endocardium of the right ventricle in Gata4 AHF knockouts (Fig. 4B, D, and F) compared with control embryos (Fig. 4A, C, and E). No differences in expression in the left ventricle between Gata4 AHF knockouts and littermate controls were observed, consistent with the specific inactivation of Gata4 in the AHF. Similarly, expression was unperturbed in the epicardial cell layer, where the Gata4 floxed allele also was not excised by Cre recombinase (Fig. 4). Taken together, these immunohistochemistry data strongly support our microarray studies, which indicate that GATA4 is required for expression of multiple cell cycle control genes. In particular, our results demonstrate the requirement of GATA4 function for cyclin D2, cyclin A2, and Cdk4 expression in myocytes in the embryonic right ventricle (Fig. 4).
FIG. 4.
Gata4 inactivation leads to decreased expression of cell cycle proteins. Immunohistochemical staining of transverse sections with anti-cyclin D2 (A and B), anti-Cdk4 (C and D), and anti-cyclin A2 (E and F) antibodies shows that the expression of all three cell cycle proteins is dramatically reduced in the right ventricular myocardium in Gata4 AHF knockout embryos (B, D, and F) compared to that in littermate control embryos (A, C, and E) at E10.5 (asterisks). In panels A to D, staining for cyclin D2 and Cdk4 is red and nuclei have been counterstained with DAPI (blue). In panels E and F, cyclin A2 is stained in brown. No differences in cyclin D2, Cdk4, or cyclin A2 protein expression between knockout and control embryos were observed in regions outside the Mef2c-AHF-Cre domain, such as the left ventricle (LV). Genotypes for control (Gata4flox/flox) and Gata4 AHF knockout (CreTg/0;Gata4flox/flox) embryos are indicated. RV, right ventricle.
GATA4 binds to the Cyclin D2 and Cdk4 promoters in vitro and in vivo.
To determine if the regulation of these cell cycle genes by GATA4 was direct, we examined the upstream regions for evolutionarily conserved GATA binding sites. These bioinformatic analyses identified three perfect consensus GATA sites upstream of both the Cyclin D2 and Cdk4 genes, and therefore we examined these two genes in detail to determine if they were regulated by direct GATA4 binding to their promoter regions in cardiomyocytes (Fig. 5). The Cyclin D2 promoter contains two conserved, consensus GATA sites at positions −558 and −525 relative to the transcriptional start site (Fig. 5A). These two sites, referred to as D2 Gata I and D2 Gata II, were each found to be bound efficiently by GATA4 by EMSA (Fig. 5C, lanes 2 and 6). Binding of GATA4 to these sites in the Cyclin D2 promoter was specific because it was efficiently competed by an excess of unlabeled self probe (Fig. 5C, lanes 3 and 7) but not by mutant versions of the Cyclin D2 Gata I or Gata II sites (Fig. 5C, lanes 4 and 8). In addition to these two conserved GATA sites, another candidate site at bp −299 (D2 Gata III) was also bound robustly by GATA4 (Fig. 5C, lane 10). Binding to this site was also specific as it was inhibited by the addition of excess unlabeled self probe but not by the addition of a mutant version of itself (Fig. 5C, lanes 11 and 12).
FIG. 5.
GATA4 binds directly to the Cyclin D2 and Cdk4 promoters in vivo and in vitro. (A and B) Schematic representations of the mouse Cyclin D2 and Cdk4 promoters. The Cyclin D2 construct encompasses nucleotides −671 to +260 relative to the transcriptional start site (bent arrow). The Cdk4 construct encompasses nucleotides −771 to +56 relative to the transcriptional start site (bent arrow). Boxes denote consensus GATA binding sites in the Cyclin D2 (Gata I [GI], Gata II, and Gata III) and Cdk4 (Gata I/II and Gata III) promoters. Arrowheads indicate the locations of primers used to amplify regions of the Cyclin D2 and Cdk4 promoters, containing consensus GATA sites, in ChIP assays. (C and D) Recombinant GATA4 proteins were transcribed and translated in vitro and used in EMSA with radiolabeled double-stranded oligonucleotides encompassing the CyclinD2 Gata I (C, lanes 1 to 4), Gata II (C, lanes 5 to 8), and Gata III (C, lanes 9 to 12) sites and the Cdk4 Gata I/II (D, lanes 1 to 4) and Gata III (D, lanes 5 to 8) sites. Lanes 1, 5, and 9 (C) and lanes 1 and 5 (D) contain reticulocyte lysate without recombinant GATA4 (−). GATA4 efficiently bound to all GATA sites in the Cyclin D2 and Cdk4 promoters in vitro. mI, mutant version of the Gata I site. (E) GATA4 binds to the endogenous Cyclin D2 and Cdk4 promoters in vivo. Differentiated P19CL6 cardiomyocytes were subjected to ChIP to detect endogenous GATA4 bound to the Cyclin D2 and Cdk4 promoters using anti-GATA4 antibody. Following ChIP, the Cyclin D2 promoter was detected using primers P1 and P2 (lanes 1 to 3) and the Cdk4 promoter was detected using primers P1 and P2 (lanes 4 to 6) and primers P3 and P4 (lanes 7 to 9). In addition, primers were used to detect the second exon of Cyclin D2 as a nonspecific control (lanes 10 to 12). PCR products were analyzed by agarose gel electrophoresis. Lanes 3, 6, 9, and 12 contain PCR products obtained following ChIP using anti-GATA4 antibody (α-G4). Lanes 1, 4, 7, and 10 contain PCR products obtained following ChIP using a nonspecific anti-IgG (α-IgG). Lanes 2, 5, 8, and 11 contain PCR products from input DNA (Inp) amplified prior to immunoprecipitation. ChIP products were detected only from promoter regions in samples where anti-GATA4 antibody was used. Sizes in bp are shown at the left.
Within the proximal Cdk4 promoter, a perfectly conserved GATA site (Cdk4 Gata III) is present at position −180 relative to the transcriptional start site (Fig. 5B). This GATA site was efficiently bound by GATA4 in EMSA (Fig. 5D, lane 6). The binding to this site was competed by an excess of unlabeled self probe but not by a mutant version of the self probe (Fig. 5D, lanes 7 and 8). In addition, the Cdk4 upstream region also contains two nonconserved, consecutive candidate sites at positions −607 and −601 relative to the transcriptional start site (Fig. 5B). These two Cdk4 GATA sites, referred as Cdk4 Gata I/II, were also found to be robustly bound by GATA4 by EMSA (Fig. 5D, lane 2). The binding to these sites was also specifically competed by an excess of unlabeled probe containing both sites but not by a probe in which both GATA sites were mutated (Fig. 5D, lanes 3 and 4).
The data presented in Fig. 5C and D demonstrate that the Cyclin D2 and Cdk4 promoter regions each contain multiple bona fide GATA sites that are efficiently bound in vitro by GATA4. To determine the ability of GATA4 to bind to the Cyclin D2 and Cdk4 promoters in cardiomyocytes, we performed ChIP assays with differentiated P19CL6 cardiomyocytes (Fig. 5E). P19CL6 is a clonal derivative from the pluripotent P19 mouse embryonal carcinoma cell line, which efficiently differentiates into functional, contractile cardiac myocytes in the presence of 1% DMSO, and these myocytes express numerous cardiac transcription factors, including GATA4, Nkx2-5, and MEF2C (40, 41, 54, 80, 83). Anti-GATA4 antibodies specifically precipitated DNA fragments encompassing the GATA sites in the endogenous Cyclin D2 promoter (Fig. 5E, lane 3). This product was specific to the GATA4 antibody since the addition of nonspecific anti-IgG in the ChIP reaction did not result in the detection of Cyclin D2 by PCR (Fig. 5E, lane 1). Similarly, the anti-GATA4 antibody specifically precipitated promoter fragments from the endogenous Cdk4 gene that encompassed the proximal Gata III site and the more distal Gata I and II sites (Fig. 5E, lanes 6 and 9). These results demonstrate that GATA4 directly interacts with the endogenous Cyclin D2 and Cdk4 promoters in cardiac myocytes via multiple bona fide, consensus GATA sites.
Transcriptional activation of the Cyclin D2 and Cdk4 promoters requires GATA sites.
The observations that cyclin D2 and Cdk4 expression required GATA4 and that GATA4 bound directly to the Cyclin D2 and Cdk4 promoters in vitro and in vivo suggested that the promoters of these two cell cycle genes might require GATA4 for activation. Therefore, we examined the requirement of the GATA sites in the Cyclin D2 and Cdk4 promoters for activation in P19CL6 cardiomyocytes in vivo by fusing the promoters to the lacZ reporter gene and testing them in a luminescent β-Gal assay (Fig. 6). Both the Cyclin D2 and Cdk4 promoters exhibited significant activation in differentiated P19CL6 cells compared to the parent reporter construct, pAUG-β-gal (Fig. 6A and B), due to the presence of endogenous GATA4 factors in P19CL6 cells (40, 41, 54, 80, 83). Consistent with this notion, we observed a dramatic increase in GATA4 protein in P19CL6 cells by Western blotting after 7 days of culture in the presence of DMSO (data not shown). Importantly, the activation of both the Cyclin D2 and Cdk4 promoters significantly decreased in P19CL6 cardiomyocytes when the GATA sites in the promoters were mutated, indicating that GATA factors are important in the transcriptional activation of both promoters (Fig. 6A and B).
FIG. 6.
The GATA sites in the Cyclin D2 and Cdk4 promoters are required for activation. (A and B) The Cyclin D2 (A) and Cdk4 (B) promoters were significantly activated by endogenous GATA factors in differentiated P19CL6 cardiomyocytes (lane 2) compared to the activity of the parent reporter construct, pAUG-β-gal (lane 1), and mutation of the GATA sites in each promoter significantly attenuated activity (lane 3). RLU, relative light units. (C) The Cyclin D2 promoter was significantly activated in C3H10T1/2 fibroblasts (lane 2) compared to the activity of the parent reporter (lane 1), and mutation of the GATA sites significantly attenuated promoter activation (lane 3). (D) GATA4-EnR inhibits activation of the Cyclin D2 promoter in C3H10T1/2 cells. Cotransfection of GATA4-EnR expression plasmid resulted in potent repression of the Cyclin D2 reporter construct (compare lanes 3 and 4). (E) Cotransfection of a GATA4-VP16 expression plasmid with the Cyclin D2-lacZ reporter plasmid resulted in potent transactivation of the Cyclin D2 promoter in C3H10T1/2 cells (lane 6). Mutation of the GATA sites (mGATA) in the Cyclin D2 promoter disrupted transactivation by GATA4-VP16 (lane 4). In all cases, the total amount of transfected plasmid DNA was held constant by addition of the appropriate amount of the parent expression plasmid. Error bars represent the standard errors of the means for at least three independent triplicate sets of transfections and analyses for each panel. P values were calculated by two-tailed, unpaired t test.
We also observed a significant level of activation of the Cyclin D2 promoter in C3H10T1/2 cells, suggesting that this fibroblast cell line also expresses GATA factors endogenously (Fig. 6C, lane 2). Consistent with this notion, the activation of the Cyclin D2 reporter by endogenous factors in C3H10T1/2 cells was also dependent on the presence of intact GATA sites (Fig. 6C, lane 3). In addition, the activity of the Cyclin D2 promoter in C3H10T1/2 was inhibited by coexpression of a repressor form of GATA4, GATA4-EnR, which has the repressor domain from the Drosophila Engrailed protein fused to the Gata4 cDNA (Fig. 6D). In spite of the activation of the Cyclin D2 promoter by endogenous GATA factors in C3H10T1/2 cells, the activation was significantly less in this fibroblast cell line than in differentiated P19CL6 cardiomyocytes, prompting us to test the ability of exogenous GATA4 to activate the Cyclin D2 promoter in this cell line (Fig. 6E). GATA4 has weak intrinsic transactivation ability and is widely appreciated for interacting with transcriptional coregulators to activate target genes in the heart (16, 20, 43, 71). Therefore, we used an activator form of GATA4, GATA4-VP16, to overcome the requirement for GATA4 cofactors that may not be abundant in C3H10T1/2 cells. GATA4-VP16 strongly transactivated the Cyclin D2-lacZ reporter construct (Fig. 6E, lane 6), and this activation was dependent on the presence of intact GATA binding sites since mutation of the three consensus GATA elements in the Cyclin D2 promoter ablated transactivation (Fig. 6E, lane 4).
Taken together, the data presented in Fig. 5 and 6 demonstrate that GATA4 is a direct transcriptional activator of the Cyclin D2 and Cdk4 genes through direct promoter binding and activation. These data support a model in which GATA4 regulates myocyte proliferation, at least in part, through direct regulation of Cyclin D2 and Cdk4.
DISCUSSION
GATA4 is an essential regulator of mesodermal and endodermal organ formation and is a key component of the core cardiac transcription factor network (38, 56, 57). Recent studies using conditional-inactivation approaches in mice have shown that Gata4 is required for proper cardiomyocyte proliferation, although the pathways downstream of GATA4 that control myocyte division have not been elucidated previously (85, 86). In this paper, we show that inactivation of Gata4 in the AHF, prior to the formation of the right ventricle, results in hypoplasia of the right ventricle and VSDs resulting from diminished cardiac proliferation. We also show for the first time that GATA4 regulates the expression of numerous cell cycle control genes, including Cyclin D2 and Cdk4, via direct promoter binding and activation. Interestingly, later inactivation of Gata4 using α-myosin heavy chain-Cre, which does not become fully active until after E10.5, when the right ventricle has already formed, does not result in loss of Cyclin D2 expression (86), suggesting a requirement for GATA4 regulation of cyclin D2 expression early in the development of the right ventricle.
Mouse models have been developed in order to understand the cell cycle and the interplay of cyclin/Cdk complexes. Cyclin D2 is a member of the D-cyclin family of cell cycle regulators (61). D-cyclins are intracellular sensors that integrate mitogenic signals to direct G1/S cell cycle transition (68). Three mammalian D-cyclins are expressed in overlapping patterns in all proliferating cell types (30). Consistent with the overlapping expression of D-cyclin proteins, mice lacking any single D-cyclin are viable and display only narrow, highly tissue-restricted phenotypes with no obvious cardiac defects (21, 26, 69, 70). However, compound mutation of all three D-cyclin genes results in embryonic lethality due to cellular proliferation defects, including reduced cardiomyocyte cell division (30). Similarly, individual knockout of either Cdk4 or Cdk2 did not reveal any obvious defects, and neither gene is required for viability in mice (7, 49, 60, 79). However, compound mutation of Cdk4 and Cdk2 results in impaired proliferation and heart growth (8). The microarray studies presented here demonstrate that GATA4 regulates a large number of cell cycle genes, including multiple cyclin and cyclin-dependent kinase genes (Fig. 3). These observations suggest that GATA4 controls cardiomyocyte proliferation through coordinate regulation of numerous cell cycle genes. In support of that notion, we show that GATA4 directly binds to and activates the Cyclin D2 and Cdk4 promoters (Fig. 5 and 6). It is likely that GATA4 also directly regulates other cell cycle genes.
GATA factors have an important function in either enhancing or inhibiting cell cycle progression in tissues other than the myocardium (73, 75, 78). For example, it has been proposed that GATA6 maintains the quiescent state of vascular smooth muscle cells, probably through induction of p21, a Cdk inhibitor (55). In pulmonary smooth muscle cells, GATA4 appears to be important for cell proliferation, and overexpression of a repressor form of GATA4 suppresses cyclin D2 expression (73), which supports the direct activation of Cyclin D2 by GATA4 observed in our studies. Similarly, GATA1 induces the sustained expression of cyclin D1 in a myeloid cell line (77), and GATA4 cooperates with the Kruppel-like factor KLF13 to activate Cyclin D1 in Xenopus laevis (46). All of these studies, taken together with the work presented here, support a model in which GATA factors may function generally as regulators of the cell cycle in multiple tissues. It will be interesting to determine if additional cell cycle genes are also direct transcriptional targets of GATA4 and other GATA factors in the heart and other tissues.
In addition to its role in proliferation, GATA4 is widely appreciated as a key regulator of cardiomyocyte differentiation through the activation of other transcription factor and downstream structural genes (38, 56, 57). Our data suggest that GATA4 is dispensable for myocyte specification and differentiation in the AHF and that it is not essential for the patterning or alignment of the outflow tracts. AHF-derived structures appear to be normal in Gata4 AHF knockouts except for a VSD, which is probably secondary to myocyte proliferation defects in the muscular septum. Lack of Gata4 in the endocardium has been previously shown to affect the proliferation of the membranous portion of the septum, also leading to VSDs (62). Gata4 AHF conditional knockout mice also have GATA4 depleted in the endocardium, which may contribute to the observed membranous VSDs in the conditional knockout mice described in the present study (Fig. 1). Previous work has shown that Gata4 is not broadly expressed in the pharyngeal mesoderm (86), which may explain why the outflow tracts form normally and have normal alignment in Gata4 AHF knockout embryos. Alternatively, Gata5 and Gata6 may be able to compensate for Gata4 in myocyte specification and differentiation in the AHF and its derivatives.
GATA4 may regulate the balance between differentiation and proliferation through cofactor interactions or by integrating and interpreting distinct upstream signals into unique outputs. Consistent with this idea, numerous GATA4 differentiation partners have been identified previously, including MEF2C, Nkx2-5, HAND2, SRF, and Tbx5 (6, 16, 20, 43, 58). Interestingly, Tbx5 regulates cell cycle genes that control G1/S phase transition in Xenopus (23). We show here that GATA4 also regulates numerous cell cycle genes, including several that control G1/S transition (Fig. 3). GATA4 interacts with Tbx5 in the activation of the Nppa, p204, and connexin40 promoters during cardiomyocyte differentiation in vitro (17, 22, 34, 58), and disruption of the Tbx5-GATA4 interaction in humans results in congenital septation defects (22). It will be important to determine if Tbx5 and GATA4 also cooperatively regulate cell cycle genes and whether other core cardiac transcription factors also participate in a complex with GATA4 for cell cycle control.
Acknowledgments
We thank Jeff Molkentin and Evie Dodou for providing plasmids used in these studies and Benoit Bruneau for helpful comments on the manuscript.
A.R. was supported in part by a postdoctoral fellowship from the American Heart Association, Western States Affiliate. S.W.K. and W.T.P. were supported by NIH SCCOR grant P01 HL074734. This work was supported by grants HL64658 and AR52130 from the NIH to B.L.B.
Footnotes
Published ahead of print on 30 June 2008.
REFERENCES
- 1.Abu-Issa, R., and M. L. Kirby. 2007. Heart field: from mesoderm to heart tube. Annu. Rev. Cell Dev. Biol. 2345-68. [DOI] [PubMed] [Google Scholar]
- 2.Adams, P. D. 2001. Regulation of the retinoblastoma tumor suppressor protein by cyclin/cdks. Biochim. Biophys. Acta 1471M123-M133. [DOI] [PubMed] [Google Scholar]
- 3.Adams, P. D., and W. G. Kaelin, Jr. 1995. Transcriptional control by E2F. Semin. Cancer Biol. 699-108. [DOI] [PubMed] [Google Scholar]
- 4.Ai, D., X. Fu, J. Wang, M. F. Lu, L. Chen, A. Baldini, W. H. Klein, and J. F. Martin. 2007. Canonical Wnt signaling functions in second heart field to promote right ventricular growth. Proc. Natl. Acad. Sci. USA 1049319-9324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arceci, R. J., A. A. King, M. C. Simon, S. H. Orkin, and D. B. Wilson. 1993. Mouse GATA-4: a retinoic acid-inducible GATA-binding transcription factor expressed in endodermally derived tissues and heart. Mol. Cell. Biol. 132235-2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Belaguli, N. S., J. L. Sepulveda, V. Nigam, F. Charron, M. Nemer, and R. J. Schwartz. 2000. Cardiac tissue enriched factors serum response factor and GATA-4 are mutual coregulators. Mol. Cell. Biol. 207550-7558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berthet, C., E. Aleem, V. Coppola, L. Tessarollo, and P. Kaldis. 2003. Cdk2 knockout mice are viable. Curr. Biol. 131775-1785. [DOI] [PubMed] [Google Scholar]
- 8.Berthet, C., K. D. Klarmann, M. B. Hilton, H. C. Suh, J. R. Keller, H. Kiyokawa, and P. Kaldis. 2006. Combined loss of Cdk2 and Cdk4 results in embryonic lethality and Rb hypophosphorylation. Dev. Cell 10563-573. [DOI] [PubMed] [Google Scholar]
- 9.Black, B. L. 2007. Transcriptional pathways in second heart field development. Semin. Cell Dev. Biol. 1867-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brentani, H., O. L. Caballero, A. A. Camargo, A. M. da Silva, W. A. da Silva, Jr., E. Dias Neto, M. Grivet, A. Gruber, P. E. Guimaraes, W. Hide, C. Iseli, C. V. Jongeneel, J. Kelso, M. A. Nagai, E. P. Ojopi, E. C. Osorio, E. M. Reis, G. J. Riggins, A. J. Simpson, S. de Souza, B. J. Stevenson, R. L. Strausberg, E. H. Tajara, S. Verjovski-Almeida, M. L. Acencio, M. H. Bengtson, F. Bettoni, W. F. Bodmer, M. R. Briones, L. P. Camargo, W. Cavenee, J. M. Cerutti, L. E. Coelho Andrade, P. C. Costa dos Santos, M. C. Ramos Costa, I. T. da Silva, M. R. Estecio, K. Sa Ferreira, F. B. Furnari, M. Faria, Jr., P. A. Galante, G. S. Guimaraes, A. J. Holanda, E. T. Kimura, M. R. Leerkes, X. Lu, R. M. Maciel, E. A. Martins, K. B. Massirer, A. S. Melo, C. A. Mestriner, E. C. Miracca, L. L. Miranda, F. G. Nobrega, P. S. Oliveira, A. C. Paquola, J. R. Pandolfi, M. I. Campos Pardini, F. Passetti, J. Quackenbush, B. Schnabel, M. C. Sogayar, J. E. Souza, S. R. Valentini, A. C. Zaiats, E. J. Amaral, L. A. Arnaldi, A. G. de Araujo, S. A. de Bessa, D. C. Bicknell, M. E. Ribeiro de Camaro, D. M. Carraro, H. Carrer, A. F. Carvalho, C. Colin, F. Costa, C. Curcio, I. D. Guerreiro da Silva, N. Pereira da Silva, M. Dellamano, H. El-Dorry, E. M. Espreafico, A. J. Scattone Ferreira, C. Ayres Ferreira, M. A. Fortes, A. H. Gama, D. Giannella-Neto, M. L. Giannella, R. R. Giorgi, G. H. Goldman, M. H. Goldman, C. Hackel, P. L. Ho, E. M. Kimura, L. P. Kowalski, J. E. Krieger, L. C. Leite, A. Lopes, A. M. Luna, A. Mackay, et al. 2003. The generation and utilization of a cancer-oriented representation of the human transcriptome by using expressed sequence tags. Proc. Natl. Acad. Sci. USA 10013418-13423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buckingham, M., S. Meilhac, and S. Zaffran. 2005. Building the mammalian heart from two sources of myocardial cells. Nat. Rev. Genet. 6826-835. [DOI] [PubMed] [Google Scholar]
- 12.Cai, C. L., X. Liang, Y. Shi, P. H. Chu, S. L. Pfaff, J. Chen, and S. Evans. 2003. Isl1 identifies a cardiac progenitor population that proliferates prior to differentiation and contributes a majority of cells to the heart. Dev. Cell 5877-889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Christoffels, V. M., P. E. Habets, D. Franco, M. Campione, F. de Jong, W. H. Lamers, Z. Z. Bao, S. Palmer, C. Biben, R. P. Harvey, and A. F. Moorman. 2000. Chamber formation and morphogenesis in the developing mammalian heart. Dev. Biol. 223266-278. [DOI] [PubMed] [Google Scholar]
- 14.Cripps, R. M., B. L. Black, B. Zhao, C. L. Lien, R. A. Schulz, and E. N. Olson. 1998. The myogenic regulatory gene Mef2 is a direct target for transcriptional activation by Twist during Drosophila myogenesis. Genes Dev. 12422-434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crispino, J. D., M. B. Lodish, B. L. Thurberg, S. H. Litovsky, T. Collins, J. D. Molkentin, and S. H. Orkin. 2001. Proper coronary vascular development and heart morphogenesis depend on interaction of GATA-4 with FOG cofactors. Genes Dev. 15839-844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dai, Y. S., P. Cserjesi, B. E. Markham, and J. D. Molkentin. 2002. The transcription factors GATA4 and dHAND physically interact to synergistically activate cardiac gene expression through a p300-dependent mechanism. J. Biol. Chem. 27724390-24398. [DOI] [PubMed] [Google Scholar]
- 17.Ding, B., C. J. Liu, Y. Huang, R. P. Hickey, J. Yu, W. Kong, and P. Lengyel. 2006. p204 is required for the differentiation of P19 murine embryonal carcinoma cells to beating cardiac myocytes: its expression is activated by the cardiac Gata4, Nkx2.5, and Tbx5 proteins. J. Biol. Chem. 28114882-14892. [DOI] [PubMed] [Google Scholar]
- 18.Dodou, E., M. P. Verzi, J. P. Anderson, S. M. Xu, and B. L. Black. 2004. Mef2c is a direct transcriptional target of ISL1 and GATA factors in the anterior heart field during mouse embryonic development. Development 1313931-3942. [DOI] [PubMed] [Google Scholar]
- 19.Dodou, E., S. M. Xu, and B. L. Black. 2003. mef2c is activated directly by myogenic basic helix-loop-helix proteins during skeletal muscle development in vivo. Mech. Dev. 1201021-1032. [DOI] [PubMed] [Google Scholar]
- 20.Durocher, D., F. Charron, R. Warren, R. J. Schwartz, and M. Nemer. 1997. The cardiac transcription factors Nkx2-5 and GATA-4 are mutual cofactors. EMBO J. 165687-5696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fantl, V., G. Stamp, A. Andrews, I. Rosewell, and C. Dickson. 1995. Mice lacking cyclin D1 are small and show defects in eye and mammary gland development. Genes Dev. 92364-2372. [DOI] [PubMed] [Google Scholar]
- 22.Garg, V., I. S. Kathiriya, R. Barnes, M. K. Schluterman, I. N. King, C. A. Butler, C. R. Rothrock, R. S. Eapen, K. Hirayama-Yamada, K. Joo, R. Matsuoka, J. C. Cohen, and D. Srivastava. 2003. GATA4 mutations cause human congenital heart defects and reveal an interaction with TBX5. Nature 424443-447. [DOI] [PubMed] [Google Scholar]
- 23.Goetz, S. C., D. D. Brown, and F. L. Conlon. 2006. TBX5 is required for embryonic cardiac cell cycle progression. Development 1332575-2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Golias, C. H., A. Charalabopoulos, and K. Charalabopoulos. 2004. Cell proliferation and cell cycle control: a mini review. Int. J. Clin. Pract. 581134-1141. [DOI] [PubMed] [Google Scholar]
- 25.Hogan, B., R. Beddington, F. Costantini, and E. Lacy. 1994. Manipulating the mouse embryo, 2nd ed. Cold Spring Harbor Laboratory Press, Plainview, NY.
- 26.Huard, J. M., C. C. Forster, M. L. Carter, P. Sicinski, and M. E. Ross. 1999. Cerebellar histogenesis is disturbed in mice lacking cyclin D2. Development 1261927-1935. [DOI] [PubMed] [Google Scholar]
- 27.Kelly, R. G., N. A. Brown, and M. E. Buckingham. 2001. The arterial pole of the mouse heart forms from Fgf10-expressing cells in pharyngeal mesoderm. Dev. Cell 1435-440. [DOI] [PubMed] [Google Scholar]
- 28.Kelly, R. G., and M. E. Buckingham. 2002. The anterior heart-forming field: voyage to the arterial pole of the heart. Trends Genet. 18210-216. [DOI] [PubMed] [Google Scholar]
- 29.Kim, Y., A. G. Ma, K. Kitta, S. N. Fitch, T. Ikeda, Y. Ihara, A. R. Simon, T. Evans, and Y. J. Suzuki. 2003. Anthracycline-induced suppression of GATA-4 transcription factor: implication in the regulation of cardiac myocyte apoptosis. Mol. Pharmacol. 63368-377. [DOI] [PubMed] [Google Scholar]
- 30.Kozar, K., M. A. Ciemerych, V. I. Rebel, H. Shigematsu, A. Zagozdzon, E. Sicinska, Y. Geng, Q. Yu, S. Bhattacharya, R. T. Bronson, K. Akashi, and P. Sicinski. 2004. Mouse development and cell proliferation in the absence of D-cyclins. Cell 118477-491. [DOI] [PubMed] [Google Scholar]
- 31.Kuo, C. T., E. E. Morrisey, R. Anandappa, K. Sigrist, M. M. Lu, M. S. Parmacek, C. Soudais, and J. M. Leiden. 1997. GATA4 transcription factor is required for ventral morphogenesis and heart tube formation. Genes Dev. 111048-1060. [DOI] [PubMed] [Google Scholar]
- 32.Liang, Q., L. J. De Windt, S. A. Witt, T. R. Kimball, B. E. Markham, and J. D. Molkentin. 2001. The transcription factors GATA4 and GATA6 regulate cardiomyocyte hypertrophy in vitro and in vivo. J. Biol. Chem. 27630245-30253. [DOI] [PubMed] [Google Scholar]
- 33.Lien, C. L., C. Wu, B. Mercer, R. Webb, J. A. Richardson, and E. N. Olson. 1999. Control of early cardiac-specific transcription of Nkx2-5 by a GATA-dependent enhancer. Development 12675-84. [DOI] [PubMed] [Google Scholar]
- 34.Linhares, V. L., N. A. Almeida, D. C. Menezes, D. A. Elliott, D. Lai, E. C. Beyer, A. C. Campos de Carvalho, and M. W. Costa. 2004. Transcriptional regulation of the murine Connexin40 promoter by cardiac factors Nkx2-5, GATA4 and Tbx5. Cardiovasc. Res. 64402-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matsushime, H., D. E. Quelle, S. A. Shurtleff, M. Shibuya, C. J. Sherr, and J. Y. Kato. 1994. D-type cyclin-dependent kinase activity in mammalian cells. Mol. Cell. Biol. 142066-2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McFadden, D. G., J. Charite, J. A. Richardson, D. Srivastava, A. B. Firulli, and E. N. Olson. 2000. A GATA-dependent right ventricular enhancer controls dHAND transcription in the developing heart. Development 1275331-5341. [DOI] [PubMed] [Google Scholar]
- 37.Meyerson, M., and E. Harlow. 1994. Identification of G1 kinase activity for cdk6, a novel cyclin D partner. Mol. Cell. Biol. 142077-2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Molkentin, J. D. 2000. The zinc finger-containing transcription factors GATA-4, -5, and -6. Ubiquitously expressed regulators of tissue-specific gene expression. J. Biol. Chem. 27538949-38952. [DOI] [PubMed] [Google Scholar]
- 39.Molkentin, J. D., Q. Lin, S. A. Duncan, and E. N. Olson. 1997. Requirement of the transcription factor GATA4 for heart tube formation and ventral morphogenesis. Genes Dev. 111061-1072. [DOI] [PubMed] [Google Scholar]
- 40.Monzen, K., Y. Hiroi, S. Kudoh, H. Akazawa, T. Oka, E. Takimoto, D. Hayashi, T. Hosoda, M. Kawabata, K. Miyazono, S. Ishii, Y. Yazaki, R. Nagai, and I. Komuro. 2001. Smads, TAK1, and their common target ATF-2 play a critical role in cardiomyocyte differentiation. J. Cell Biol. 153687-698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Monzen, K., I. Shiojima, Y. Hiroi, S. Kudoh, T. Oka, E. Takimoto, D. Hayashi, T. Hosoda, A. Habara-Ohkubo, T. Nakaoka, T. Fujita, Y. Yazaki, and I. Komuro. 1999. Bone morphogenetic proteins induce cardiomyocyte differentiation through the mitogen-activated protein kinase kinase kinase TAK1 and cardiac transcription factors Csx/Nkx-2.5 and GATA-4. Mol. Cell. Biol. 197096-7105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moorman, A. F., and V. M. Christoffels. 2003. Cardiac chamber formation: development, genes, and evolution. Physiol. Rev. 831223-1267. [DOI] [PubMed] [Google Scholar]
- 43.Morin, S., F. Charron, L. Robitaille, and M. Nemer. 2000. GATA-dependent recruitment of MEF2 proteins to target promoters. EMBO J. 192046-2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moses, K. A., F. DeMayo, R. M. Braun, J. L. Reecy, and R. J. Schwartz. 2001. Embryonic expression of an Nkx2-5/Cre gene using ROSA26 reporter mice. Genesis 31176-180. [DOI] [PubMed] [Google Scholar]
- 45.Nemer, G., F. Fadlalah, J. Usta, M. Nemer, G. Dbaibo, M. Obeid, and F. Bitar. 2006. A novel mutation in the GATA4 gene in patients with tetralogy of Fallot. Hum. Mutat. 27293-294. [DOI] [PubMed] [Google Scholar]
- 46.Nemer, M., and M. E. Horb. 2007. The KLF family of transcriptional regulators in cardiomyocyte proliferation and differentiation. Cell Cycle 6117-121. [DOI] [PubMed] [Google Scholar]
- 47.Oka, T., M. Maillet, A. J. Watt, R. J. Schwartz, B. J. Aronow, S. A. Duncan, and J. D. Molkentin. 2006. Cardiac-specific deletion of Gata4 reveals its requirement for hypertrophy, compensation, and myocyte viability. Circ. Res. 98837-845. [DOI] [PubMed] [Google Scholar]
- 48.Olson, E. N., and M. D. Schneider. 2003. Sizing up the heart: development redux in disease. Genes Dev. 171937-1956. [DOI] [PubMed] [Google Scholar]
- 49.Ortega, S., M. Malumbres, and M. Barbacid. 2002. Cyclin D-dependent kinases, INK4 inhibitors and cancer. Biochim. Biophys. Acta 160273-87. [DOI] [PubMed] [Google Scholar]
- 50.Park, E. J., L. A. Ogden, A. Talbot, S. Evans, C. L. Cai, B. L. Black, D. U. Frank, and A. M. Moon. 2006. Required, tissue-specific roles for Fgf8 in outflow tract formation and remodeling. Development 1332419-2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pasumarthi, K. B., and L. J. Field. 2002. Cardiomyocyte cell cycle regulation. Circ. Res. 901044-1054. [DOI] [PubMed] [Google Scholar]
- 52.Pasumarthi, K. B., H. Nakajima, H. O. Nakajima, M. H. Soonpaa, and L. J. Field. 2005. Targeted expression of cyclin D2 results in cardiomyocyte DNA synthesis and infarct regression in transgenic mice. Circ. Res. 96110-118. [DOI] [PubMed] [Google Scholar]
- 53.Patient, R. K., and J. D. McGhee. 2002. The GATA family (vertebrates and invertebrates). Curr. Opin. Genet. Dev. 12416-422. [DOI] [PubMed] [Google Scholar]
- 54.Peng, C. F., Y. Wei, J. M. Levsky, T. V. McDonald, G. Childs, and R. N. Kitsis. 2002. Microarray analysis of global changes in gene expression during cardiac myocyte differentiation. Physiol. Genomics 9145-155. [DOI] [PubMed] [Google Scholar]
- 55.Perlman, H., E. Suzuki, M. Simonson, R. C. Smith, and K. Walsh. 1998. GATA-6 induces p21(Cip1) expression and G1 cell cycle arrest. J. Biol. Chem. 27313713-13718. [DOI] [PubMed] [Google Scholar]
- 56.Peterkin, T., A. Gibson, M. Loose, and R. Patient. 2005. The roles of GATA-4, -5 and -6 in vertebrate heart development. Semin. Cell Dev. Biol. 1683-94. [DOI] [PubMed] [Google Scholar]
- 57.Pikkarainen, S., H. Tokola, R. Kerkela, and H. Ruskoaho. 2004. GATA transcription factors in the developing and adult heart. Cardiovasc. Res. 63196-207. [DOI] [PubMed] [Google Scholar]
- 58.Plageman, T. F., Jr., and K. E. Yutzey. 2004. Differential expression and function of Tbx5 and Tbx20 in cardiac development. J. Biol. Chem. 27919026-19034. [DOI] [PubMed] [Google Scholar]
- 59.Pu, W. T., T. Ishiwata, A. L. Juraszek, Q. Ma, and S. Izumo. 2004. GATA4 is a dosage-sensitive regulator of cardiac morphogenesis. Dev. Biol. 275235-244. [DOI] [PubMed] [Google Scholar]
- 60.Rane, S. G., P. Dubus, R. V. Mettus, E. J. Galbreath, G. Boden, E. P. Reddy, and M. Barbacid. 1999. Loss of Cdk4 expression causes insulin-deficient diabetes and Cdk4 activation results in beta-islet cell hyperplasia. Nat. Genet. 2244-52. [DOI] [PubMed] [Google Scholar]
- 61.Reed, S. I. 1997. Control of the G1/S transition. Cancer Surv. 297-23. [PubMed] [Google Scholar]
- 62.Rivera-Feliciano, J., K. H. Lee, S. W. Kong, S. Rajagopal, Q. Ma, Z. Springer, S. Izumo, C. J. Tabin, and W. T. Pu. 2006. Development of heart valves requires Gata4 expression in endothelial-derived cells. Development 1333607-3618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Roberts, J. M., A. Koff, K. Polyak, E. Firpo, S. Collins, M. Ohtsubo, and J. Massague. 1994. Cyclins, Cdks, and cyclin kinase inhibitors. Cold Spring Harbor Symp. Quant. Biol. 5931-38. [DOI] [PubMed] [Google Scholar]
- 64.Rojas, A., S. De Val, A. B. Heidt, S. M. Xu, J. Bristow, and B. L. Black. 2005. Gata4 expression in lateral mesoderm is downstream of BMP4 and is activated directly by Forkhead and GATA transcription factors through a distal enhancer element. Development 1323405-3417. [DOI] [PubMed] [Google Scholar]
- 65.Searcy, R. D., E. B. Vincent, C. M. Liberatore, and K. E. Yutzey. 1998. A GATA-dependent nkx-2.5 regulatory element activates early cardiac gene expression in transgenic mice. Development 1254461-4470. [DOI] [PubMed] [Google Scholar]
- 66.Sedmera, D., T. Pexieder, M. Vuillemin, R. P. Thompson, and R. H. Anderson. 2000. Developmental patterning of the myocardium. Anat. Rec. 258319-337. [DOI] [PubMed] [Google Scholar]
- 67.Sedmera, D., M. Reckova, A. DeAlmeida, S. R. Coppen, S. W. Kubalak, R. G. Gourdie, and R. P. Thompson. 2003. Spatiotemporal pattern of commitment to slowed proliferation in the embryonic mouse heart indicates progressive differentiation of the cardiac conduction system. Anat. Rec. Part A 274773-777. [DOI] [PubMed] [Google Scholar]
- 68.Sherr, C. J., J. Kato, D. E. Quelle, M. Matsuoka, and M. F. Roussel. 1994. D-type cyclins and their cyclin-dependent kinases: G1 phase integrators of the mitogenic response. Cold Spring Harbor Symp. Quant. Biol. 5911-19. [DOI] [PubMed] [Google Scholar]
- 69.Sicinska, E., Y. M. Lee, J. Gits, H. Shigematsu, Q. Yu, V. I. Rebel, Y. Geng, C. J. Marshall, K. Akashi, D. M. Dorfman, I. P. Touw, and P. Sicinski. 2006. Essential role for cyclin D3 in granulocyte colony-stimulating factor-driven expansion of neutrophil granulocytes. Mol. Cell. Biol. 268052-8060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sicinski, P., J. L. Donaher, Y. Geng, S. B. Parker, H. Gardner, M. Y. Park, R. L. Robker, J. S. Richards, L. K. McGinnis, J. D. Biggers, J. J. Eppig, R. T. Bronson, S. J. Elledge, and R. A. Weinberg. 1996. Cyclin D2 is an FSH-responsive gene involved in gonadal cell proliferation and oncogenesis. Nature 384470-474. [DOI] [PubMed] [Google Scholar]
- 71.Stennard, F. A., M. W. Costa, D. A. Elliott, S. Rankin, S. J. Haast, D. Lai, L. P. McDonald, K. Niederreither, P. Dolle, B. G. Bruneau, A. M. Zorn, and R. P. Harvey. 2003. Cardiac T-box factor Tbx20 directly interacts with Nkx2-5, GATA4, and GATA5 in regulation of gene expression in the developing heart. Dev. Biol. 262206-224. [DOI] [PubMed] [Google Scholar]
- 72.Subramanian, A., P. Tamayo, V. K. Mootha, S. Mukherjee, B. L. Ebert, M. A. Gillette, A. Paulovich, S. L. Pomeroy, T. R. Golub, E. S. Lander, and J. P. Mesirov. 2005. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 10215545-15550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Suzuki, Y. J., R. M. Day, C. C. Tan, T. H. Sandven, Q. Liang, J. D. Molkentin, and B. L. Fanburg. 2003. Activation of GATA-4 by serotonin in pulmonary artery smooth muscle cells. J. Biol. Chem. 27817525-17531. [DOI] [PubMed] [Google Scholar]
- 74.Suzuki, Y. J., and T. Evans. 2004. Regulation of cardiac myocyte apoptosis by the GATA-4 transcription factor. Life Sci. 741829-1838. [DOI] [PubMed] [Google Scholar]
- 75.Takahashi, S., T. Komeno, N. Suwabe, K. Yoh, O. Nakajima, S. Nishimura, T. Kuroha, T. Nagasawa, and M. Yamamoto. 1998. Role of GATA-1 in proliferation and differentiation of definitive erythroid and megakaryocytic cells in vivo. Blood 92434-442. [PubMed] [Google Scholar]
- 76.Tamamori-Adachi, M., K. Hayashida, K. Nobori, C. Omizu, K. Yamada, N. Sakamoto, T. Kamura, K. Fukuda, S. Ogawa, K. I. Nakayama, and S. Kitajima. 2004. Down-regulation of p27Kip1 promotes cell proliferation of rat neonatal cardiomyocytes induced by nuclear expression of cyclin D1 and CDK4. Evidence for impaired Skp2-dependent degradation of p27 in terminal differentiation. J. Biol. Chem. 27950429-50436. [DOI] [PubMed] [Google Scholar]
- 77.Tanaka, H., I. Matsumura, K. Nakajima, H. Daino, J. Sonoyama, H. Yoshida, K. Oritani, T. Machii, M. Yamamoto, T. Hirano, and Y. Kanakura. 2000. GATA-1 blocks IL-6-induced macrophage differentiation and apoptosis through the sustained expression of cyclin D1 and bcl-2 in a murine myeloid cell line M1. Blood 951264-1273. [PubMed] [Google Scholar]
- 78.Tsai, F. Y., and S. H. Orkin. 1997. Transcription factor GATA-2 is required for proliferation/survival of early hematopoietic cells and mast cell formation, but not for erythroid and myeloid terminal differentiation. Blood 893636-3643. [PubMed] [Google Scholar]
- 79.Tsutsui, T., B. Hesabi, D. S. Moons, P. P. Pandolfi, K. S. Hansel, A. Koff, and H. Kiyokawa. 1999. Targeted disruption of CDK4 delays cell cycle entry with enhanced p27Kip1 activity. Mol. Cell. Biol. 197011-7019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Uchida, S., S. Fuke, and T. Tsukahara. 2007. Upregulations of Gata4 and oxytocin receptor are important in cardiomyocyte differentiation processes of P19CL6 cells. J. Cell. Biochem. 100629-641. [DOI] [PubMed] [Google Scholar]
- 81.Verzi, M. P., D. J. McCulley, S. De Val, E. Dodou, and B. L. Black. 2005. The right ventricle, outflow tract, and ventricular septum comprise a restricted expression domain within the secondary/anterior heart field. Dev. Biol. 287134-145. [DOI] [PubMed] [Google Scholar]
- 82.Watt, A. J., M. A. Battle, J. Li, and S. A. Duncan. 2004. GATA4 is essential for formation of the proepicardium and regulates cardiogenesis. Proc. Natl. Acad. Sci. USA 10112573-12578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wen, J., Q. Xia, C. Lu, L. Yin, J. Hu, Y. Gong, B. Yin, K. Monzen, J. Yuan, B. Qiang, X. Zhang, and X. Peng. 2007. Proteomic analysis of cardiomyocytes differentiation in mouse embryonic carcinoma P19CL6 cells. J. Cell. Biochem. 102149-160. [DOI] [PubMed] [Google Scholar]
- 84.Wessels, A., and D. Sedmera. 2003. Developmental anatomy of the heart: a tale of mice and man. Physiol. Genomics 15165-176. [DOI] [PubMed] [Google Scholar]
- 85.Xin, M., C. A. Davis, J. D. Molkentin, C. L. Lien, S. A. Duncan, J. A. Richardson, and E. N. Olson. 2006. A threshold of GATA4 and GATA6 expression is required for cardiovascular development. Proc. Natl. Acad. Sci. USA 10311189-11194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zeisberg, E. M., Q. Ma, A. L. Juraszek, K. Moses, R. J. Schwartz, S. Izumo, and W. T. Pu. 2005. Morphogenesis of the right ventricle requires myocardial expression of Gata4. J. Clin. Investig. 1151522-1531. [DOI] [PMC free article] [PubMed] [Google Scholar]