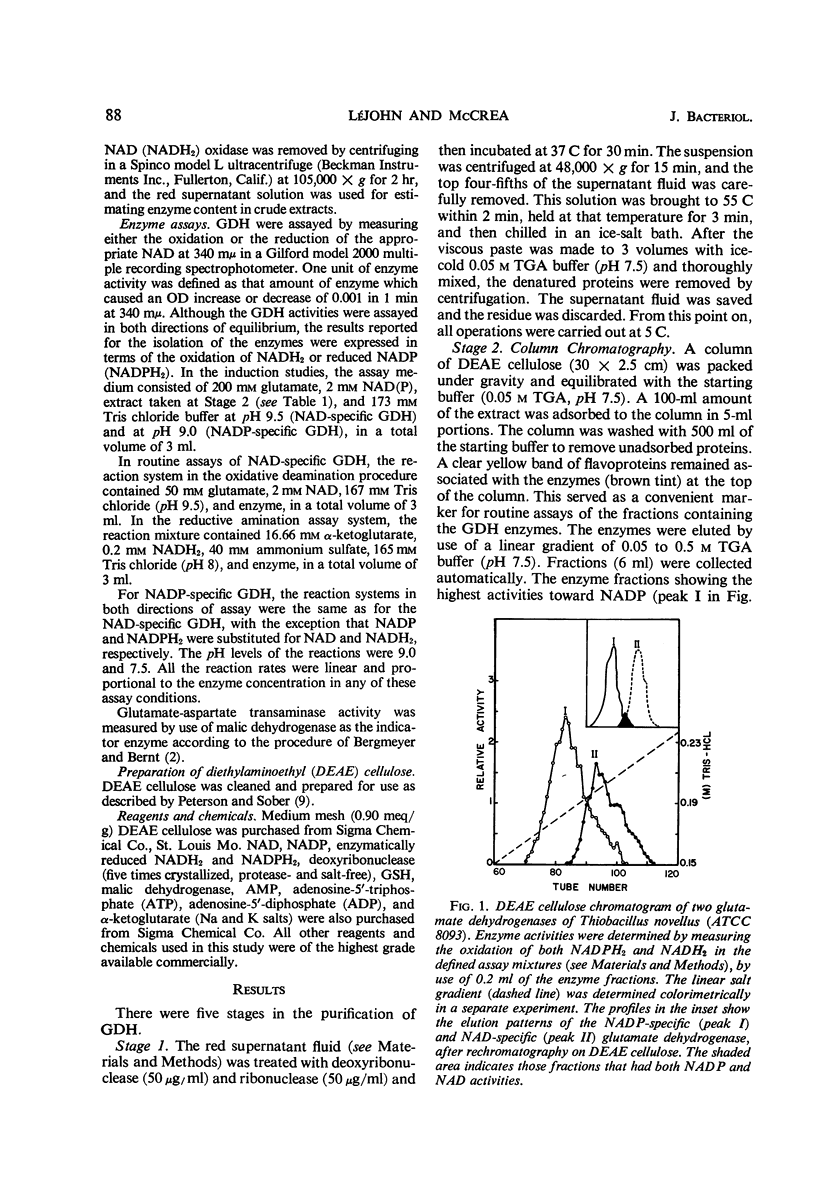
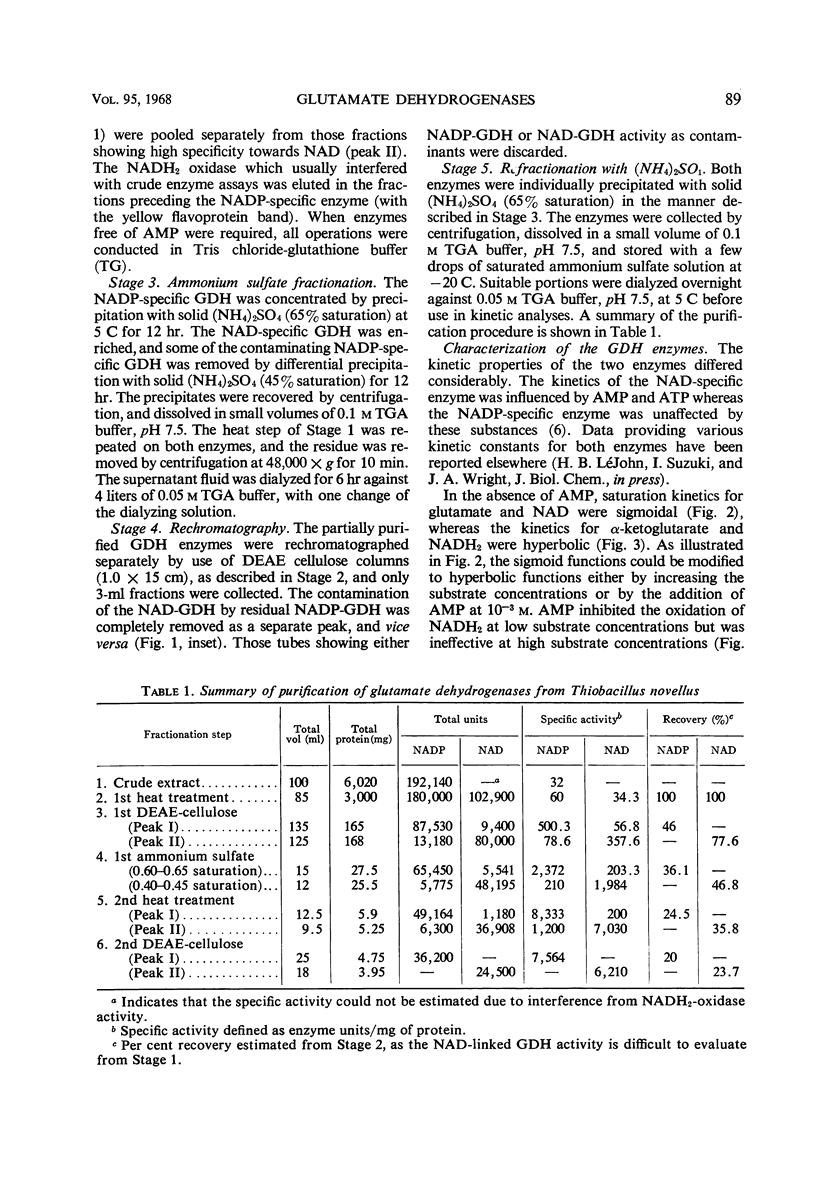
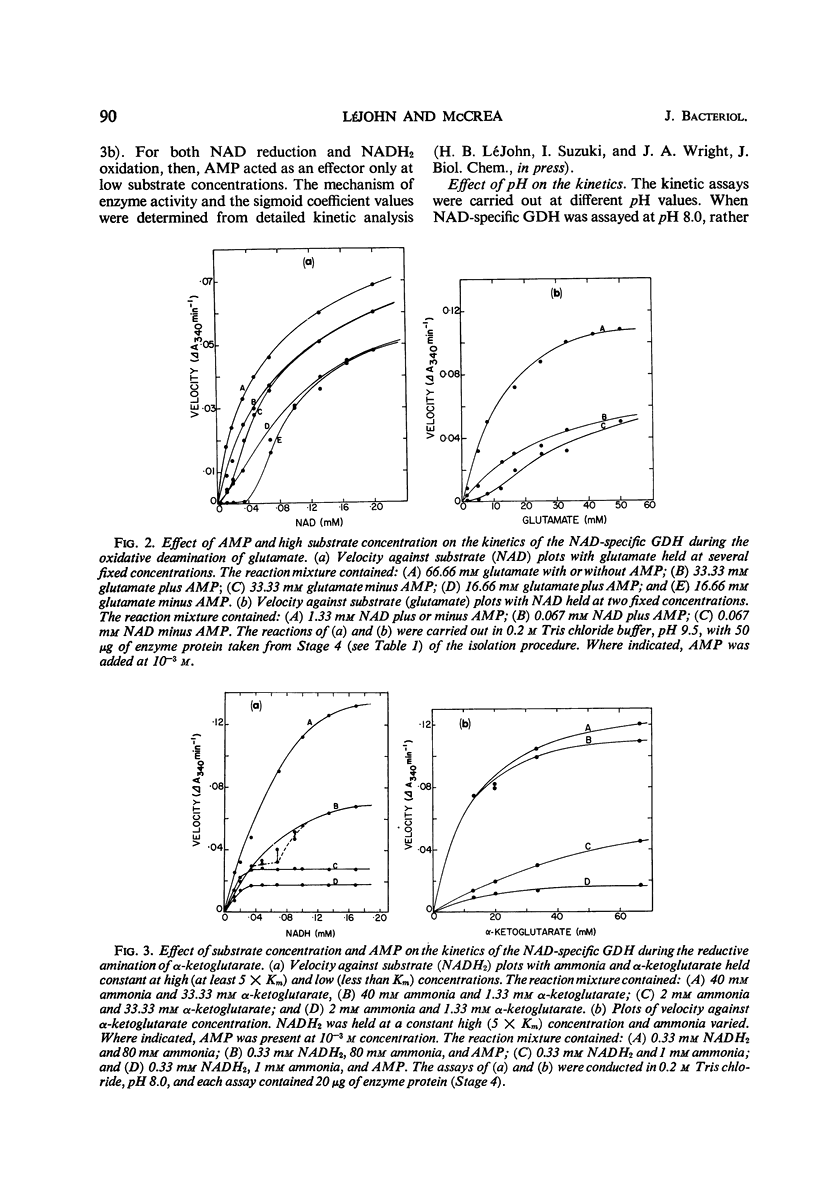
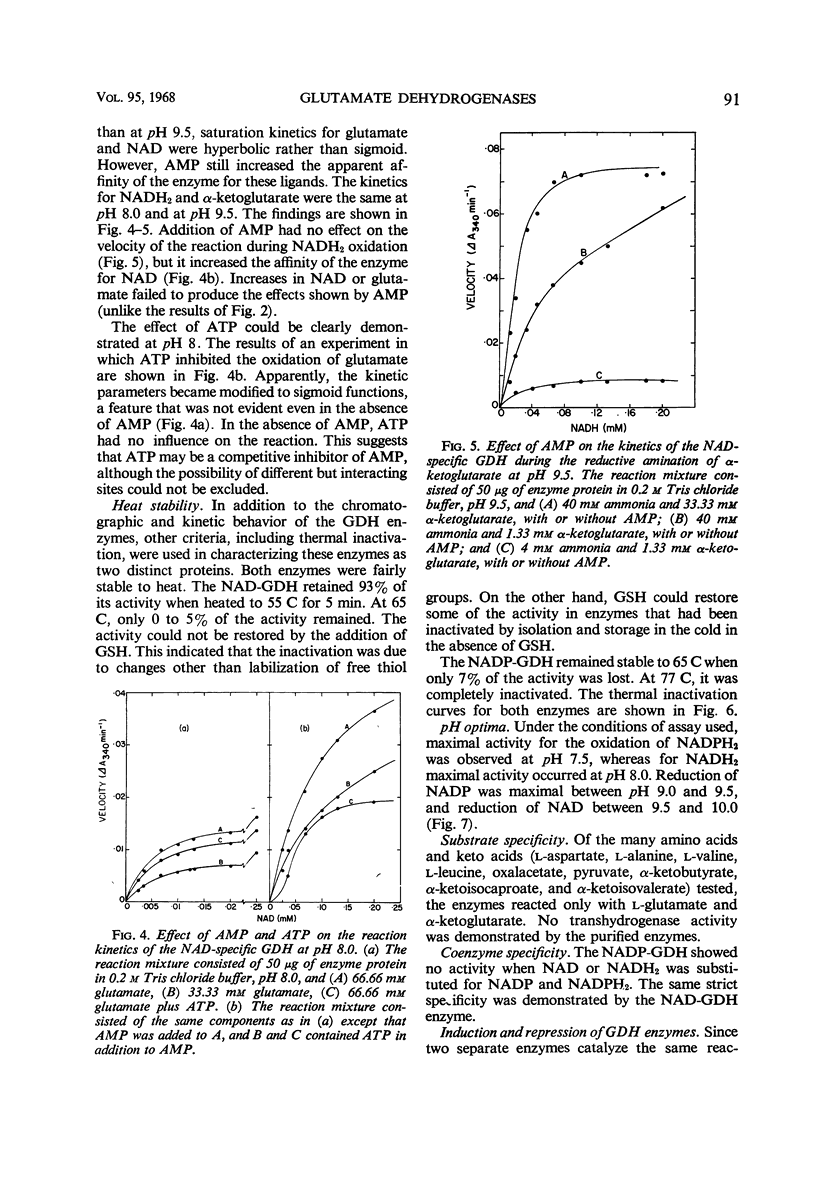
Abstract
When grown autotrophically in a thiosulfate-mineral salts medium, cells of the facultative chemoautotrophic bacterium, Thiobacillus novellus, produced two distinct glutamate dehydrogenases, one specific for nicotinamide adenine dinucleotide phosphate (NADP) and the other specific for nicotinamide adenine dinucleotide (NAD). When glutamate was supplied exogenously as the sole carbon source, the NAD-specific glutamate dehydrogenase was fully induced. Lower levels of the enzyme were found in bacteria grown in l-arginine, l-alanine, glucose, glycerol, lactate, citrate, or succinate. Arginine, histidine, and aspartate, on the other hand, caused a marked repression of the NADP-specific glutamate dehydrogenase activity. The NAD-dependent glutamate dehydrogenase was allosteric. Adenosine-5′-monophosphate and adenosine-5′-diphosphate acted as positive effectors. Both glutamate dehydrogenases were purified about 250-fold and were shown to be distinct protein with different physical properties.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ATKINSON D. E., HATHAWAY J. A., SMITH E. C. KINETICS OF REGULATORY ENZYMES. KINETIC ORDER OF THE YEAST DIPHOSPHOPYRIDINE NUCLEOTIDE ISOCITRATE DEHYDROGENASE REACTION AND A MODEL FOR THE REACTION. J Biol Chem. 1965 Jun;240:2682–2690. [PubMed] [Google Scholar]
- FRIEDEN C. GLUTAMATE DEHYDROGENASE. VI. SURVEY OF PURINE NUCLEOTIDE AND OTHER EFFECTS ON THE ENZYME FROM VARIOUS SOURCES. J Biol Chem. 1965 May;240:2028–2035. [PubMed] [Google Scholar]
- KAPLAN N. O. Symposium on multiple forms of enzymes and control mechanisms. I. Multiple forms of enzymes. Bacteriol Rev. 1963 Jun;27:155–169. doi: 10.1128/br.27.2.155-169.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan N. O., Swartz M. N., Frech M. E., Ciotti M. M. PHOSPHORYLATIVE AND NONPHOSPHORYLATIVE PATHWAYS OF ELECTRON TRANSFER IN RAT LIVER MITOCHONDRIA. Proc Natl Acad Sci U S A. 1956 Aug;42(8):481–487. doi: 10.1073/pnas.42.8.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- LéJohn H. B. AMP-activation of an allosteric NAD -dependent glutamate dehydrogenase. Biochem Biophys Res Commun. 1967 Jul 10;28(1):96–102. doi: 10.1016/0006-291x(67)90412-3. [DOI] [PubMed] [Google Scholar]
- Starkey R. L. Cultivation of Organisms Concerned in the Oxidation of Thiosulfate. J Bacteriol. 1934 Oct;28(4):365–386. doi: 10.1128/jb.28.4.365-386.1934. [DOI] [PMC free article] [PubMed] [Google Scholar]


