Abstract
Small LDL and HDL particle size are characteristic of a proatherogenic lipoprotein profile. Aerobic exercise increases these particle sizes. Although visceral adipose tissue (VAT) has been strongly linked with dyslipidemia, the importance of intermuscular adipose tissue (IMAT) to dyslipidemia and exercise responses is less well understood. We measured exercise-associated changes in thigh IMAT and VAT and examined their relationships with changes in LDL and HDL particle size. Sedentary, dyslipidemic, overweight subjects (n = 73) completed 8–9 mo of aerobic training. Linear regression models were used to compare the power of IMAT change and VAT change to predict lipoprotein size changes. In men alone (n = 40), IMAT change correlated inversely with both HDL size change (r = −0.42, P = 0.007) and LDL size change (r = −0.52, P < 0.001). That is, reduction of IMAT was associated with a shift toward larger, less atherogenic lipoprotein particles. No significant correlations were observed in women. After adding VAT change to the model, IMAT change was the only significant predictor of either HDL size change (P = 0.034 for IMAT vs. 0.162 for VAT) or LDL size change (P = 0.004 for IMAT vs. 0.189 for VAT) in men. In conclusion, in overweight dyslipidemic men, exercise-associated change in thigh IMAT was inversely correlated with both HDL and LDL size change and was more predictive of these lipoprotein changes than was change in VAT. Reducing IMAT through aerobic exercise may be functionally related to some improvements in atherogenic dyslipidemia in men.
Keywords: aerobic training, visceral fat, adipose tissue, high-density lipoprotein size, low-density lipoprotein size
small ldl and hdl particle size are components of atherogenic dyslipidemia (2, 17, 22, 34), which is an established risk factor for cardiovascular disease (CVD). Aerobic exercise increases the average diameter of both of these particles (10, 15), resulting in a less atherogenic lipoprotein profile. Less is known, however, about the mechanisms by which exercise mediates these improvements.
The effects of regional fat distribution on atherogenic dyslipidemia have been well characterized, with increased visceral adiposity being associated with less favorable lipoprotein profiles (3, 18, 19, 22, 25). Visceral adipose tissue (VAT) has been described as an “ectopic” adipose depot associated with impaired fat oxidation and loss of the normal ability to store excess energy as subcutaneous fat (11). In contrast, relationships between lipoprotein profiles and thigh intermuscular adipose tissue (IMAT), which might be viewed as a peripheral ectopic fat depot, have not been widely studied. IMAT lies within the fascia lata surrounding the leg musculature, thus distinguishing it from subcutaneous adipose tissue (SAT) in the leg, and surrounds and infiltrates muscle groups, with which it shares a direct vascular connection. This anatomic relationship is analogous to that of visceral fat and the liver in the abdomen, suggesting that IMAT might have a functional influence on skeletal muscle metabolism analogous to that of VAT on liver metabolism.
The Studies of a Targeted Risk Reduction Intervention through Defined Exercise (STRRIDE) trial (16) was designed to compare the effects of differing amounts and intensities of aerobic exercise on plasma lipoproteins and other metabolic risk factors. The primary analysis of lipoprotein changes has been reported previously (15) and demonstrated graded improvements in LDL and HDL particle size with increasing exercise volume. Given the higher requirement of skeletal muscle for oxidative fuel at higher amounts of exercise and the close anatomic connection between IMAT and skeletal muscle, we hypothesized that IMAT is a readily mobilized source of lipid fuel and, thus, that exercise-induced change in IMAT would be associated with concurrent changes in particle sizes reflective of a more favorable lipoprotein profile. The objective of the present analysis was to determine the association between changes in thigh IMAT and in HDL and LDL particle size in the context of an aerobic exercise intervention and to compare the power of IMAT and VAT changes to predict these lipoprotein changes.
METHODS
A detailed description of the STRRIDE study design has been published elsewhere (16). The Institutional Review Board at Duke University reviewed and approved the study protocol, and informed consent was obtained from all subjects.
Subjects.
Subjects were 40–65 yr old, previously sedentary, overweight or class I obese (BMI 25–35 kg/m2), and met at least one of two criteria for dyslipidemia: 1) fasting LDL of 130–190 mg/dl and/or 2) fasting HDL less than 40 mg/dl for men or 45 mg/dl for women. The study included subjects self-classified as Caucasian, African-American, and other (e.g., Hispanic and Asian) races. Subjects were randomly assigned to one of three exercise training groups or an inactive control group that did not change physical activity habits. Subjects were recruited continuously from Durham, NC, and surrounding areas between January 1999 and June 2002. All exercise training was completed by April 2003. Since the objective was to study exercise-induced changes in IMAT, all exercise-exposed subjects with complete lipid data and midthigh CT scans obtained both before and after the training period (n = 73) were included in the present analysis, with control subjects excluded.
Exercise training.
The three exercise training groups were defined as follows: 1) High amount/vigorous intensity, 23 kcal·kg−1·wk−1 at 65–80% peak V̇o2; 2) Low amount/vigorous intensity, 14 kcal·kg−1·wk−1 at 65–80% peak V̇o2; 3) Low amount/moderate intensity, 14 kcal·kg−1·wk−1 at 40–55% peak V̇o2. These regimens are calorically equivalent to ∼20 miles/wk of jogging, 12 miles/wk of jogging, and 12 miles/wk of brisk walking, respectively, for a 90-kg person. Subjects exercised for a 2- to 3-mo ramp period followed by 6 mo at one of the above prescriptions for a total of 8–9 mo of exercise training. All exercise sessions were verified by recorded data from heart rate monitors (Polar Electro, Woodbury, NY).
Lipoprotein measurements.
Fasting plasma samples were analyzed by LipoScience (Raleigh, NC), using nuclear magnetic resonance spectroscopy as previously described (15, 21). The concentrations of four LDL and five HDL subclasses were measured and the weighted average sizes of LDL and HDL calculated.
Adipose tissue measurements.
Thigh and abdominal CT scans were obtained before and after the training period, using a General Electric CT/I (GE Medical Systems, Milwaukee, WI). With subjects in the supine position, a single 10-mm axial section was obtained from the midpoint of the left thigh (midway between the acetabulum and the patella) and from the abdomen at the level of the L4 pedicle. Exact locations for these sections were determined from digital frontal scout radiographs. Images were analyzed using Slice-O-Matic 4.3 software (Tomovision, Montreal, QC, Canada). For the thigh, the fascia lata was traced manually to distinguish SAT from IMAT. IMAT area was defined as all tissue within the attenuation range −190 to −30 Hounsfield units (9) lying deep to the fascia lata (5, 36). VAT area was defined as all tissue in the same attenuation range lying within the abdominal cavity.
Data analysis.
Change scores for IMAT area, VAT area, and lipoprotein particle sizes were calculated by subtracting the pre- from the posttraining value. Univariable relationships were tested with Pearson correlations. Multivariable relationships were tested using general linear models without selection; that is, we forced all of our explanatory variables of interest into the models. To address the possibility that associations differed by sex, our initial models included a sex × IMAT change interaction term. When this interaction term was significant, subsequent analyses were performed separately by sex. Models were validated on 1,000 bootstrap replicates of the sample to assess the stability of the observations. These replicates were generated with SAS 9.1 (Cary, NC); all other statistical analysis was performed with SAS Enterprise Guide 4.1 (Cary, NC). The threshold for statistical significance was set a priori at P < 0.05. Variables that were not normally distributed were log-transformed prior to statistical analysis. Except where noted, descriptive data are presented as means ± SD for continuous variables and frequency (%) for categorical variables.
RESULTS
Subject characteristics.
Subject characteristics are presented in Table 1. There were no sex differences in baseline BMI serum triglycerides, fasting glucose, or fasting insulin. There were similar proportions of Caucasians, African-Americans, and subjects of other races among men and women. Exercise group allocation was similar between sexes, and men and women were similarly adherent to their exercise prescriptions (all P > 0.05). Men were slightly younger, had higher body mass and higher peak V̇o2 at baseline, whereas women were more insulin sensitive. Men had lower HDL and LDL levels and smaller HDL and LDL sizes. Men also had more VAT but less IMAT and SAT in the thigh than women (all P < 0.05).
Table 1.
Subject characteristics
| Variable |
Women (n = 33) |
Men (n = 40)
|
||
|---|---|---|---|---|
| Baseline | Change | Baseline | Change | |
| Age, yr | 54.0±5.6 | 50.9±6.4* | ||
| Race | ||||
| Caucasian | 27 (82) | 33 (83) | ||
| African-American | 5 (15) | 5 (13) | ||
| Other | 1 (3) | 2 (5) | ||
| Body mass, kg | 77.2±10.6 | −0.8±2.5 | 94.4±10.8* | −2.4±2.5*† |
| BMI, kg/m2 | 29.8±2.8 | −0.3±1.0† | 30.4±2.5 | −0.8±0.8*† |
| Exercise group | ||||
| Low amount/moderate intensity | 10 (30) | 8 (20) | ||
| Low amount/vigorous intensity | 13 (39) | 16 (40) | ||
| High amount/high intensity | 10 (30) | 16 (40) | ||
| Adherence, % | 90.8±10.4 | 89.9±11.0 | ||
| Relative peak V̇o2, ml·kg−1·min−1 | 23.9±3.6 | 2.3±1.9† | 32.8±3.8* | 4.4±3.4*† |
| HDL, mg/dl | 60.7±15.5 | 0.4±6.4 | 40.8±9.4* | 1.9±5.2† |
| LDL, mg/dl | 129.5±22.2 | 0.0±14.1 | 119.4±20.2* | −0.2±18.9 |
| HDL size, nm | 9.14±0.37 | −0.01±0.11 | 8.74±0.22* | 0.02±0.19 |
| LDL size, nm | 21.25±0.63 | 0.00±0.37 | 20.45±0.70* | 0.13±0.42 |
| Triglyceride, mg/dl | 130.9±71.6 | −9.5±54.2 | 156.4±67.7 | −21.1±55.8† |
| Thigh IMAT, cm2 | 9.77±4.45 | −0.55±1.34† | 7.55±3.25* | −0.55±1.28† |
| Thigh SAT, cm2 | 124.71±34.84 | −5.59±11.49† | 65.82±18.10* | −3.96±7.53† |
| VAT, cm2 | 141.60±60.12 | −2.71±27.93 | 204.89±60.93* | −23.3±34.68*† |
| Fasting glucose, mg/dl | 95.0±11.7 | 0.2±8.4 | 95.4±8.3 | −0.3±8.5 |
| Fasting insulin, μU/ml | 8.3±4.7 | −1.6±2.9† | 11.4±9.3 | −1.6±5.1 |
| SI, mU·L−1·min−1 | 3.8±1.8 | 0.9±2.8 | 2.5±1.9* | 1.2±1.4† |
Values are given as means ± SD and frequency (%). Baseline SI given as geometric mean ± geometric SD, as distribution was not normal. Percentages may not total 100% due to rounding. BMI, body mass index; IMAT, intermuscular adipose tissue; SAT, subcutaneous adipose tissue; VAT, visceral adipose tissue; SI, insulin sensitivity index derived from iv glucose tolerance test with minimal-model analysis (12). Adherence was calculated as the number of minutes of exercise at the appropriate intensity divided by the prescribed number of minutes. Proportions of self-identified race and exercise group by sex were compared using χ2 tests of association. Significance of within- and between-sex differences was otherwise assessed with two-tailed t-tests.
Significant sex difference at P < 0.05;
significant within-sex change with training at P < 0.05.
Changes in subject characteristics are also summarized in Table 1. Men had greater decreases in body mass, BMI, and VAT and a greater improvement in fitness with exercise training. Thigh IMAT and thigh SAT decreased significantly with exercise in both men and women, but these changes did not differ by sex. Men had within-sex improvements in insulin sensitivity and triglycerides, whereas women improved fasting insulin.
Sex effects.
We first tested the association between exercise-induced change in IMAT and change in LDL and HDL particle sizes. We hypothesized that loss of IMAT would be associated with increases in these particle sizes, implying an improvement in metabolic state. Given known sex differences in regional fat distribution, we included sex and IMAT in the linear regression model. Although sex itself did not affect particle size change with exercise training (Table 2, P = 0.87 and 0.70 for HDL and LDL, respectively), the interaction term sex × IMAT change was significant for both HDL and LDL size change (P = 0.02 and 0.01, respectively). This indicated that the association between IMAT change and particle size change differed between men and women.
Table 2.
Regression coefficients for sex-IMAT change model
|
ΔHDL Particle Size, nm |
ΔLDL Particle Size, nm
|
|||||
|---|---|---|---|---|---|---|
| β | P | Model R2 | β | P | Model R2 | |
| Full model | 0.0120 | 0.1458 | 0.0022 | 0.1892 | ||
| ΔIMAT, cm2 | −0.063 | 0.0310 | −0.171 | 0.0154 | ||
| (Female) sex | 0.007 | 0.8671 | 0.036 | 0.7012 | ||
| ΔIMAT × sex | 0.065 | 0.0200 | 0.175 | 0.0108 | ||
Generated by general linear modeling without selection; n = 73 (40 men, 33 women). Δ, change.
Univariable relationship between IMAT change and particle size change.
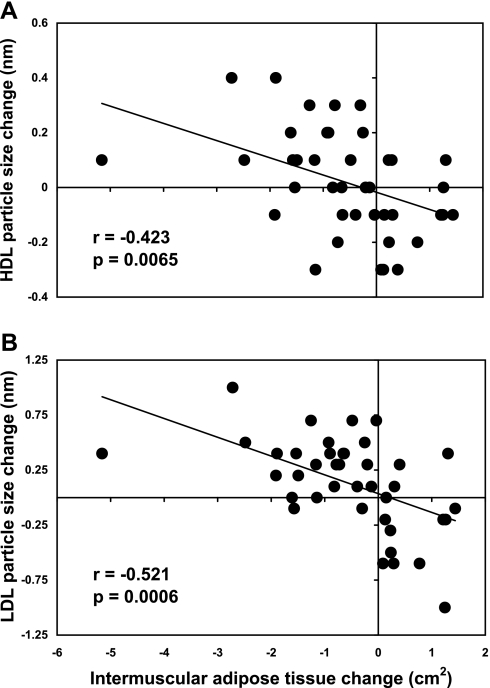
Given this finding, we evaluated the relationship between IMAT change and lipoprotein particle size change separately for each sex. In men, IMAT change was negatively correlated with change in both HDL size (r = −0.42, P = 0.0065) and LDL size (r = −0.52, P = 0.0006), as shown in Fig. 1. That is, loss of IMAT was associated with increases in size for both HDL and LDL particles in men. In contrast, there was no relationship between IMAT change and change in either HDL size (P = 0.87) or LDL size (P = 0.92) in women. Given the close functional relationship among VLDL, HDL, and LDL particle sizes, VLDL size change might be expected to have the converse relationship with IMAT change in men, and this indeed was observed in our study (r = 0.33, P = 0.0359).
Fig. 1.
Relationships between intermuscular adipose tissue (IMAT) change and lipoprotein particle size changes in men. A: HDL size change. B: LDL size change. Pearson correlations were performed for men (n = 40) and women (n = 33, not shown). Note: y-axis scales differ between panels.
In 1,000 bootstrap replicates of the sample, performed to assess the stability of the finding across repeated assessments, the mean ± SD Pearson correlation coefficient was −0.53 ± 0.10 for LDL size change in men, and the model was significant at P < 0.05 in 987 (98.7%) of the 1,000 replicates. For HDL size change in men, the mean correlation coefficient was −0.43 ± 0.10, and the model was significant in 892 (89.2%) of the 1,000 replicates.
Comparing effects of IMAT change and VAT change.
We compared the power of exercise-induced changes in IMAT and VAT to predict lipoprotein size change in men. When VAT change and IMAT change were both included in the multivariable model, IMAT change remained independently associated with HDL size change (P = 0.0343, Table 3), whereas VAT change did not (P = 0.16). IMAT change likewise remained independently associated with LDL size change in a multivariable model (P = 0.0042), whereas VAT change did not (P = 0.19). We then repeated the full fitted models on 1,000 bootstrap replicates of the sample to assess the stability of the observations. IMAT change was independently associated with change in HDL size in 593 (59.3%) of 1,000 bootstrap replicates compared with 245 (24.5%) for VAT change. IMAT change was independently associated with change in LDL size in 921 (92.1%) of 1,000 bootstrap replicates compared with 282 (28.2%) for VAT change.
Table 3.
Regression coefficients and type III sums of squares for IMAT change-VAT change model: men only
|
ΔHDL Particle Size, nm |
ΔLDL Particle Size, nm
|
|||||||
|---|---|---|---|---|---|---|---|---|
| β | P | Sum of Squares | Model R2 | β | P | Sum of Squares | Model R2 | |
| Full model | 0.0096 | 0.310 | 0.2220 | 0.0012 | 2.079 | 0.3055 | ||
| ΔIMAT, cm2 | −0.0508 | 0.0343 | 0.142 | −0.1472 | 0.0042 | 1.192 | ||
| ΔVAT, cm2 | −0.0012 | 0.1618 | 0.060 | 0.0024 | 0.1889 | 0.229 | ||
Generated by general linear modeling without selection; n = 40.
DISCUSSION
Atherogenic dyslipidemia is related to a range of genetic, metabolic, and body compositional characteristics. Although weight loss and reductions in adiposity are well known to improve the lipoprotein profile, the primary objective of the current study was to investigate the relationship between improvements in lipoprotein particle size and exercise-induced changes in thigh IMAT, which has recently attracted increasing interest as an ectopic fat depot. We also aimed to compare IMAT to VAT, a more established ectopic fat depot, in this context.
We observed that exercise-induced reductions in IMAT were associated with favorable increases in lipoprotein particle size in sedentary, overweight to mildly obese dyslipidemic men. We also observed that IMAT change remained independently associated with both HDL and LDL size change in men after controlling for the effects of VAT change. VAT change was not independently predictive in these models. No associations between IMAT change and particle size changes were observed in women.
There have been relatively few reports of relationships between changes in regional adipose depots and concurrent changes in CVD risk factors in the context of aerobic exercise. Our group has previously reported on decreases in VAT in the STRRIDE trial (29) and found that these decreases were associated with decreases in LDL particle number, whereas negative correlations between VAT change and both LDL and HDL size approached statistical significance (P = 0.052 and 0.057, respectively). Janssen et al. (13) failed to observe associations in obese women between reductions in VAT, by diet or diet plus exercise, and any metabolic variable, including serum triglyceride, LDL, and HDL concentrations.
In cross-sectional studies, high intermuscular adiposity in the thigh has been associated with elevated total cholesterol and fasting glucose (36), impaired glucose tolerance and type 2 diabetes (8), and metabolic syndrome (7). However, it does not necessarily follow that modifying fat distribution will, in turn, modify these metabolic factors. Cross-sectional body composition relationships are likely to be multifactorial in origin and, while providing descriptive information on high-risk metabolic states, may not inform our view of how those risks can be modified.
To our knowledge, previous comparisons of the relative importance of IMAT and VAT to metabolic risk variables have been exclusively cross-sectional. Yim et al. (36) reported that IMAT was more strongly associated than VAT with total cholesterol and fasting glucose. In nondiabetic women, higher IMAT content is associated with low insulin sensitivity index, independent of visceral adiposity and body weight (1).
In contrast to these studies, a randomized controlled trial of aerobic exercise interventions enabled us to investigate the relative importance of exercise-induced changes in IMAT and VAT to the mechanism of concurrent improvement in components of the lipoprotein profile. Reductions in IMAT were more strongly associated with improved lipoprotein particle sizes than were reductions in VAT, suggesting that IMAT changes rival or even predominate over VAT changes as possible mediators of exercise-induced improvements in some components of atherogenic dyslipidemia. Thus the observed association between IMAT change and changes in lipoprotein particle size may advance our understanding of the mechanisms by which aerobic exercise mitigates atherogenic dyslipidemia.
The definitive mechanistic link between IMAT and lipoprotein particle size remains to be determined. IMAT in the thigh is closely apposed to the skeletal muscle, which is directly targeted by aerobic exercise training. As the energy demands of the muscle increase with training, it is reasonable to expect that this adipose depot would serve as a ready source of free fatty acids for oxidation. Indeed, our group has previously shown that increases in lipoprotein particle size are associated in a dose-response manner with the volume (weekly caloric expenditure) of aerobic training regimens (15). It is possible that IMAT, as a putative ectopic fat depot, is preferentially mobilized as fuel and thus serves as a quantifiable marker of this relationship. The anatomic position of IMAT, within the myofascial compartment and with direct vascular connection to more metabolically active tissues, is analogous to that of VAT in the abdomen, with its direct vascular link to the liver. Although this characteristic of IMAT does not differ by sex, and thus does not explain why we observed relationships between IMAT and metabolic state in men but not in women, it should be noted that such sexually dimorphic relationships are not without precedent: in the STRRIDE cohort, associations between VAT and lipoprotein particle size and other metabolic variables were stronger in women (35), in direct opposition to the current observation in the peripheral limb.
Exercise-induced changes in lipoprotein lipase (LPL) activity may underlie the observed sex difference in the strength of the relationship between changes in IMAT and metabolic state. LPL acts locally at the vascular endothelium to cleave triglyceride from lipoproteins, thus allowing its incorporation into the nearby tissue for use as fuel (in skeletal muscle) or for storage (in adipose tissue). This results in larger lipoprotein particles, as the triglyceride is replaced by cholesterol esters. Indeed, serum LPL levels are positively correlated with both HDL and LDL size in cross-sectional analyses (26). One recent study reported that the oral hypoglycemic agent metformin increased both LPL activity and LDL size and that these changes were significantly correlated (20). The peroxisome proliferator-activated receptor (PPAR) activator troglitazone similarly increases both LDL size and LPL activity (30). Sex differences in LPL action have been inconsistently observed. In some cross-sectional analyses, overall LPL activity is higher in women (4, 6), whereas another report did not observe sex differences in LPL protein levels (32). Muscle-specific LPL activity does not differ by sex in either untrained or endurance-trained subjects (14). Following 5–13 days of exercise in men, LPL mRNA expression and enzyme activity increase in skeletal muscle but not in adipose tissue (27), although the mRNA increase is transient (28). At least one study (23) suggests that muscle-associated LPL activity increases acutely in men, but not in women, following aerobic exercise. Adipose tissue-associated LPL activity also increases in men, albeit to a lesser extent than in muscle, and decreases slightly in women under the same conditions. The HERITAGE family study showed a significantly greater increase in LPL activity in white men compared with women following 20 wk of exercise (6), while others have found no change in LPL protein level for either sex following 6 mo of training (32). LPL activity decreases to a greater degree in IMAT compared with other adipose depots and in male compared with female guinea pigs exposed to exercise training (24). Thus the effects of exercise on regulation of LPL activity differ by tissue and perhaps sex. If muscle LPL activity increased, and IMAT LPL activity decreased, in the context of increased fatty acid oxidation with aerobic training, we might expect mobilization of thigh IMAT as fuel to increase. If these LPL activity changes were more prominent in men than in women, IMAT loss would therefore be correlated with lipoprotein particle size increases in the sex-specific manner observed in this study.
Exercise likely affects lipoprotein particle size via hepatic mechanisms in addition to peripheral lipid oxidation (31). Specifically, higher hepatic lipase (HL) activity is associated with a higher ratio of (small) HDL3 to (large) HDL2 (4), and aerobic training decreases HL activity (33). Thompson et al. (32) observed that, in men but not in women, HL levels decrease and HDL2 concentrations increase following 6 mo of training, whereas HDL3 levels do not change significantly. Nonetheless, our data suggest that exercise-induced improvements in HDL and LDL size are partially driven by changes in peripheral skeletal muscle and fat metabolism.
Further investigation is necessary to identify in greater detail the physiological mechanisms linking reduction of IMAT to increases in lipoprotein particle size. The results of the current analysis will help generate hypotheses for further study. At a minimum, lifestyle intervention studies of this type may help differentiate putative risk factors and markers that are modifiable and drive changes in CVD risk from those that are only cross-sectionally related.
Strengths.
Although regional fat distribution has been shown to correlate with many markers of cardiovascular risk in cross-sectional analyses, the present study design afforded the opportunity to determine the relationship between changes in fat stores and changes in these risk factors. Thus, although we do not yet comprehensively understand the mechanisms of exercise-induced improvements in lipoprotein profile, our data do help to generate hypotheses for investigating these causal pathways. In addition, our subjects maintained a constant diet and body weight throughout the study, allowing us to examine the effects of aerobic exercise in isolation.
Limitations.
The present analysis had a relatively low sample size, which did not confer sufficient power to determine the effect of different exercise training regimens on changes in thigh fat distribution. Such data would have been informative in light of previously reported differences in particle size improvements among exercise groups differing in amount and intensity in the STRRIDE trial (15). We also lacked a three-dimensional measure of regional adiposity, which might have proven more precise than single-slice CT images, particularly for VAT, where the abdominal viscera may cause a single slice to misrepresent true adipose tissue volume. We also lacked data on tissue-level LPL activity and circulating leptin and adiponectin levels and thus were unable to investigate their influence on our findings. Finally, all correlative observations should be interpreted with some caution and are not meant to imply causation. The current findings should be validated in larger, independent samples.
Conclusions.
In conclusion, we observed that, in men but not women, aerobic exercise-induced loss of thigh IMAT was associated with increases in LDL and HDL particle sizes, both of which represent improvements in atherogenic dyslipidemia. In multivariable models, IMAT change, but not VAT change, was associated with both HDL and LDL size change. These data suggest that, in overweight, dyslipidemic men, thigh IMAT may be mechanistically involved in exercise-induced improvements in atherogenic dyslipidemia. This provides evidence that aerobic exercise confers local, peripheral metabolic benefits that are likely to be additive with more centrally oriented dietary or pharmacological mechanisms of reducing cardiovascular disease risk.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute National Institutes of Health (NIH) Grant no. R01-HL-57354 (Kraus, PI). M. T. Durheim was supported by NIH Grant no. TL1-RR-024126.
Acknowledgments
We thank Dr. Kim Huffman for help with statistical analysis. Preliminary findings included in this analysis were first presented in abstract form at the 2008 American Diabetes Association Scientific Sessions, San Francisco, CA.
S. K. Mabe's current affiliation: Division of Neuro-Oncology, Department of Surgery, Duke University Medical Center, Durham, NC.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Albu JB, Kovera AJ, Allen L, Wainwright M, Berk E, Raja-Khan N, Janumala I, Burkey B, Heshka S, Gallagher D. Independent association of insulin resistance with larger amounts of intermuscular adipose tissue and a greater acute insulin response to glucose in African American than in white nondiabetic women. Am J Clin Nutr 82: 1210–1217, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheung MC, Brown BG, Wolf AC, Albers JJ. Altered particle size distribution of apolipoprotein A-I-containing lipoproteins in subjects with coronary artery disease. J Lipid Res 32: 383–394, 1991. [PubMed] [Google Scholar]
- 3.Despres JP, Couillard C, Gagnon J, Bergeron J, Leon AS, Rao DC, Skinner JS, Wilmore JH, Bouchard C. Race, visceral adipose tissue, plasma lipids, and lipoprotein lipase activity in men and women: the Health, Risk Factors, Exercise Training, and Genetics (HERITAGE) family study. Arterioscler Thromb Vasc Biol 20: 1932–1938, 2000. [DOI] [PubMed] [Google Scholar]
- 4.Despres JP, Gagnon J, Bergeron J, Couillard C, Leon AS, Rao DC, Skinner JS, Wilmore JH, Bouchard C. Plasma post-heparin lipase activities in the HERITAGE Family Study: the reproducibility, gender differences, and associations with lipoprotein levels. HEalth, RIsk factors, exercise Training and GEnetics. Clin Biochem 32: 157–165, 1999. [DOI] [PubMed] [Google Scholar]
- 5.Gallagher D, Kuznia P, Heshka S, Albu J, Heymsfield SB, Goodpaster B, Visser M, Harris TB. Adipose tissue in muscle: a novel depot similar in size to visceral adipose tissue. Am J Clin Nutr 81: 903–910, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garenc C, Perusse L, Bergeron J, Gagnon J, Chagnon YC, Borecki IB, Leon AS, Skinner JS, Wilmore JH, Rao DC, Bouchard C. Evidence of LPL gene-exercise interaction for body fat and LPL activity: the HERITAGE Family Study. J Appl Physiol 91: 1334–1340, 2001. [DOI] [PubMed] [Google Scholar]
- 7.Goodpaster BH, Krishnaswami S, Harris TB, Katsiaras A, Kritchevsky SB, Simonsick EM, Nevitt M, Holvoet P, Newman AB. Obesity, regional body fat distribution, and the metabolic syndrome in older men and women. Arch Intern Med 165: 777–783, 2005. [DOI] [PubMed] [Google Scholar]
- 8.Goodpaster BH, Krishnaswami S, Resnick H, Kelley DE, Haggerty C, Harris TB, Schwartz AV, Kritchevsky S, Newman AB. Association between regional adipose tissue distribution and both type 2 diabetes and impaired glucose tolerance in elderly men and women. Diabetes Care 26: 372–379, 2003. [DOI] [PubMed] [Google Scholar]
- 9.Goodpaster BH, Thaete FL, Kelley DE. Thigh adipose tissue distribution is associated with insulin resistance in obesity and in type 2 diabetes mellitus. Am J Clin Nutr 71: 885–892, 2000. [DOI] [PubMed] [Google Scholar]
- 10.Halverstadt A, Phares DA, Wilund KR, Goldberg AP, Hagberg JM. Endurance exercise training raises high-density lipoprotein cholesterol and lowers small low-density lipoprotein and very low-density lipoprotein independent of body fat phenotypes in older men and women. Metabolism 56: 444–450, 2007. [DOI] [PubMed] [Google Scholar]
- 11.Heilbronn L, Smith SR, Ravussin E. Failure of fat cell proliferation, mitochondrial function and fat oxidation results in ectopic fat storage, insulin resistance and type II diabetes mellitus. Int J Obes Relat Metab Disord 28, Suppl 4: S12–S21, 2004. [DOI] [PubMed] [Google Scholar]
- 12.Houmard JA, Tanner CJ, Slentz CA, Duscha BD, McCartney JS, Kraus WE. Effect of the volume and intensity of exercise training on insulin sensitivity. J Appl Physiol 96: 101–106, 2004. [DOI] [PubMed] [Google Scholar]
- 13.Janssen I, Fortier A, Hudson R, Ross R. Effects of an energy-restrictive diet with or without exercise on abdominal fat, intermuscular fat, and metabolic risk factors in obese women. Diabetes Care 25: 431–438, 2002. [DOI] [PubMed] [Google Scholar]
- 14.Kiens B, Roepstorff C, Glatz JF, Bonen A, Schjerling P, Knudsen J, Nielsen JN. Lipid-binding proteins and lipoprotein lipase activity in human skeletal muscle: influence of physical activity and gender. J Appl Physiol 97: 1209–1218, 2004. [DOI] [PubMed] [Google Scholar]
- 15.Kraus WE, Houmard JA, Duscha BD, Knetzger KJ, Wharton MB, McCartney JS, Bales CW, Henes S, Samsa GP, Otvos JD, Kulkarni KR, Slentz CA. Effects of the amount and intensity of exercise on plasma lipoproteins. N Engl J Med 347: 1483–1492, 2002. [DOI] [PubMed] [Google Scholar]
- 16.Kraus WE, Torgan CE, Duscha BD, Norris J, Brown SA, Cobb FR, Bales CW, Annex BH, Samsa GP, Houmard JA, Slentz CA. Studies of a targeted risk reduction intervention through defined exercise (STRRIDE). Med Sci Sports Exerc 33: 1774–1784, 2001. [DOI] [PubMed] [Google Scholar]
- 17.Lamarche B, Tchernof A, Moorjani S, Cantin B, Dagenais GR, Lupien PJ, Despres JP. Small, dense low-density lipoprotein particles as a predictor of the risk of ischemic heart disease in men. Prospective results from the Quebec Cardiovascular Study. Circulation 95: 69–75, 1997. [DOI] [PubMed] [Google Scholar]
- 18.Nguyen-Duy TB, Nichaman MZ, Church TS, Blair SN, Ross R. Visceral fat and liver fat are independent predictors of metabolic risk factors in men. Am J Physiol Endocrinol Metab 284: E1065–E1071, 2003. [DOI] [PubMed] [Google Scholar]
- 19.Nieves DJ, Cnop M, Retzlaff B, Walden CE, Brunzell JD, Knopp RH, Kahn SE. The atherogenic lipoprotein profile associated with obesity and insulin resistance is largely attributable to intra-abdominal fat. Diabetes 52: 172–179, 2003. [DOI] [PubMed] [Google Scholar]
- 20.Ohira M, Miyashita Y, Ebisuno M, Saiki A, Endo K, Koide N, Oyama T, Murano T, Watanabe H, Shirai K. Effect of metformin on serum lipoprotein lipase mass levels and LDL particle size in type 2 diabetes mellitus patients. Diabetes Res Clin Pract 78: 34–41, 2007. [DOI] [PubMed] [Google Scholar]
- 21.Otvos JD, Jeyarajah EJ, Bennett DW. Quantification of plasma lipoproteins by proton nuclear magnetic resonance spectroscopy. Clin Chem 37: 377–386, 1991. [PubMed] [Google Scholar]
- 22.Pascot A, Lemieux I, Prud'homme D, Tremblay A, Nadeau A, Couillard C, Bergeron J, Lamarche B, Despres JP. Reduced HDL particle size as an additional feature of the atherogenic dyslipidemia of abdominal obesity. J Lipid Res 42: 2007–2014, 2001. [PubMed] [Google Scholar]
- 23.Perreault L, Lavely JM, Kittelson JM, Horton TJ. Gender differences in lipoprotein lipase activity after acute exercise. Obes Res 12: 241–249, 2004. [DOI] [PubMed] [Google Scholar]
- 24.Pond CM, Mattacks CA, Sadler D. The effects of exercise and feeding on the activity of lipoprotein lipase in nine different adipose depots of guinea pigs. Int J Biochem 24: 1825–1831, 1992. [DOI] [PubMed] [Google Scholar]
- 25.Rainwater DL, Mitchell BD, Comuzzie AG, Haffner SM. Relationship of low-density lipoprotein particle size and measures of adiposity. Int J Obes Relat Metab Disord 23: 180–189, 1999. [DOI] [PubMed] [Google Scholar]
- 26.Rip J, Nierman MC, Wareham NJ, Luben R, Bingham SA, Day NE, van Miert JN, Hutten BA, Kastelein JJ, Kuivenhoven JA, Khaw KT, Boekholdt SM. Serum lipoprotein lipase concentration and risk for future coronary artery disease: the EPIC-Norfolk prospective population study. Arterioscler Thromb Vasc Biol 26: 637–642, 2006. [DOI] [PubMed] [Google Scholar]
- 27.Seip RL, Angelopoulos TJ, Semenkovich CF. Exercise induces human lipoprotein lipase gene expression in skeletal muscle but not adipose tissue. Am J Physiol Endocrinol Metab 268: E229–E236, 1995. [DOI] [PubMed] [Google Scholar]
- 28.Seip RL, Mair K, Cole TG, Semenkovich CF. Induction of human skeletal muscle lipoprotein lipase gene expression by short-term exercise is transient. Am J Physiol Endocrinol Metab 272: E255–E261, 1997. [DOI] [PubMed] [Google Scholar]
- 29.Slentz CA, Aiken LB, Houmard JA, Bales CW, Johnson JL, Tanner CJ, Duscha BD, Kraus WE. Inactivity, exercise, and visceral fat. STRRIDE: a randomized, controlled study of exercise intensity and amount. J Appl Physiol 99: 1613–1618, 2005. [DOI] [PubMed] [Google Scholar]
- 30.Sunayama S, Watanabe Y, Ohmura H, Sawano M, Shimada K, Mokuno H, Daida H, Yamaguchi H. Effects of troglitazone on atherogenic lipoprotein phenotype in coronary patients with insulin resistance. Atherosclerosis 146: 187–193, 1999. [DOI] [PubMed] [Google Scholar]
- 31.Tall AR Exercise to reduce cardiovascular risk–how much is enough? N Engl J Med 347: 1522–1524, 2002. [DOI] [PubMed] [Google Scholar]
- 32.Thompson PD, Tsongalis GJ, Seip RL, Bilbie C, Miles M, Zoeller R, Visich P, Gordon P, Angelopoulos TJ, Pescatello L, Bausserman L, Moyna N. Apolipoprotein E genotype and changes in serum lipids and maximal oxygen uptake with exercise training. Metabolism 53: 193–202, 2004. [DOI] [PubMed] [Google Scholar]
- 33.Thompson PD, Yurgalevitch SM, Flynn MM, Zmuda JM, Spannaus-Martin D, Saritelli A, Bausserman L, Herbert PN. Effect of prolonged exercise training without weight loss on high-density lipoprotein metabolism in overweight men. Metabolism 46: 217–223, 1997. [DOI] [PubMed] [Google Scholar]
- 34.Vakkilainen J, Makimattila S, Seppala-Lindroos A, Vehkavaara S, Lahdenpera S, Groop PH, Taskinen MR, Yki-Jarvinen H. Endothelial dysfunction in men with small LDL particles. Circulation 102: 716–721, 2000. [DOI] [PubMed] [Google Scholar]
- 35.Willis LH, Slentz CA, Houmard JA, Johnson JL, Duscha BD, Aiken LB, Kraus WE. Minimal versus umbilical waist circumference measures as indicators of cardiovascular disease risk. Obesity (Silver Spring) 15: 753–759, 2007. [DOI] [PubMed] [Google Scholar]
- 36.Yim JE, Heshka S, Albu J, Heymsfield S, Kuznia P, Harris T, Gallagher D. Intermuscular adipose tissue rivals visceral adipose tissue in independent associations with cardiovascular risk. Int J Obes (Lond) 31: 1400–1405, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]