Abstract
Autosomal recessive hypophosphatemic rickets (ARHR), which is characterized by renal phosphate wasting, aberrant regulation of 1α-hydroxylase activity, and rickets/osteomalacia, is caused by inactivating mutations of dentin matrix protein 1 (DMP1). ARHR resembles autosomal dominant hypophosphatemic rickets (ADHR) and X-linked hypophosphatemia (XLH), hereditary disorders respectively caused by cleavage-resistant mutations of the phosphaturic factor FGF23 and inactivating mutations of PHEX that lead to increased production of FGF23 by osteocytes in bone. Circulating levels of FGF23 are increased in ARHR and its Dmp1-null mouse homologue. To determine the causal role of FGF23 in ARHR, we transferred Fgf23 deficient/enhanced green fluorescent protein (eGFP) reporter mice onto Dmp1-null mice to create mice lacking both Fgf23 and Dmp1. Dmp1−/− mice displayed decreased serum phosphate concentrations, inappropriately normal 1,25(OH)2D levels, severe rickets, and a diffuse form of osteomalacia in association with elevated Fgf23 serum levels and expression in osteocytes. In contrast, Fgf23−/− mice had undetectable serum Fgf23 and elevated serum phosphate and 1,25(OH)2D levels along with severe growth retardation and focal form of osteomalacia. In combined Dmp1−/−/Fgf23−/−, circulating Fgf23 levels were also undetectable, and the serum levels of phosphate and 1,25(OH)2D levels were identical to Fgf23−/− mice. Rickets and diffuse osteomalacia in Dmp1-null mice were transformed to severe growth retardation and focal osteomalacia characteristic of Fgf23-null mice. These data suggest that the regulation of extracellular matrix mineralization by DMP1 is coupled to renal phosphate handling and vitamin D metabolism through a DMP1-dependent regulation of FGF23 production by osteocytes.
Keywords: fibroblastic growth factor 23, dentin matrix protein 1, autosomal recessive hypophosphatemic rickets, hypophosphatemia, osteomalacia, phosphate homeostasis
x-linked hypophosphatemia (XLH), autosomal recessive hypophosphatemic rickets (ARHR), and autosomal dominant hypophosphatemic rickets (ADHR) are hereditary hypophosphatemic disorders respectively caused by mutations of PHEX (phosphate-regulating gene with homologies to endopeptidases on the X chromosome) (1, 37), dentin matrix protein 1 (DMP1), an extracellular matrix phosphoprotein belonging to the small integrin-binding ligand N-linked glycoprotein protein family (9, 24), and fibroblastic growth factor 23 (FGF23) (1a, 5, 39). These disorders have similar skeletal abnormalities, including defective calcification of cartilage and bone, leading to rickets and osteomalacia, as well as impaired renal tubular reabsorption of phosphate and aberrant regulation of 1,25(OH)2D3 production, leading to hypophosphatemia that is resistant to phosphorus and vitamin D therapy (1). In mice, inactivating mutations of Phex, deletion of the Dmp1 gene, and degradation-preventing mutations of Fgf23 result in phenotypic abnormalities homologous to XLH (6, 32), ARHR (9), and ADHR (1a, 5, 39).
It has been proposed that all three of these hereditary disorders share a common pathogenesis mediated by increased circulating FGF23 levels (21). Several findings support this possibility. First, FGF23 is produced predominantly by osteocytes in bone along with PHEX and DMP1. Second, FGF23 plays an essential role in regulating serum phosphate and 1,25(OH)2D3 levels, as evidenced by the fact that overexpression of FGF23 in mice causes hypophosphatemia and suppression of 1,25(OH)2D3 production by the kidney (4, 18, 29), whereas FGF23 deficiency (5, 28) or mutations increasing FGF23 degradation (17) result in hyperphosphatemia, increased serum 1,25(OH)2D3 levels, and soft tissue calcifications. Third, circulating FGF23 levels correlate with hypophosphatemia in ADHR, XLH, and ARHR (9, 38, 39). Mutations of a furin-like enzyme cleavage site, RXXR in FGF23, is the cause of ADHR (13). Recent data confirm that XLH is also caused by increased circulating levels of FGF23 (1b, 20, 40), since hypophosphatemia in Hyp mice is corrected by either the genetic ablation of Fgf23 (23, 30) or the administration of blocking antibodies for FGF23 (3). Interestingly, PHEX does not cleave FGF23 (7, 11, 20) but instead regulates FGF23 expression in osteocytes through unknown mechanisms involving intrinsic bone abnormalities (7, 20, 22, 23).
It remains to be established whether increased circulating FGF23 levels in ARHR and Dmp1-null mice are responsible for the hypophosphatemia and abnormalities in 1,25(OH)2D3 and bone mineralization (9). DMP1 is produced by osteoblasts/osteocytes and then localizes to the mineralization front and functions in bone as a nucleator for mineralization of extracellular matrix (10, 12, 33), suggesting that the rickets and osteomalacia in ARHR are directly mediated by the loss of DMP1. On the other hand, the colocalization of PHEX and DMP1 in osteocytes and the association of DMP1 mutations with increased FGF23 expression, hypophosphatemia, and reduced 1,25(OH)2D3 levels suggest that FGF23 may also contribute to the phenotype of ARHR.
In the current investigations, we examined the contribution of elevated Fgf23 levels to the systemic and skeletal abnormalities observed in Dmp1-null mice. To accomplish this, we crossed the Fgf23-null-eGFP reporter mouse (23) onto the Dmp1-null background. We found that Fgf23 expression in osteocytes was increased by Dmp1 deficiency and that the Fgf23-null phenotype was dominant to that of Dmp1 deficiency, resulting in conversion of hypophosphatemia and inappropriately low 1,25(OH)2D3 levels in Dmp1-null mice to hyperphosphatemia and elevated 1,25(OH)2D3 levels. With regard to the skeleton, superimposed Fgf23 deficiency and consequent increases in serum phosphate and 1,25(OH)2D3 levels corrected the rickets and improved the defective mineralization in Dmp1-null mice.
METHODS
Mouse models.
The Fgf23 promoter-eGFP reporter mouse model was created by knocking in an eGFP construct following the ATG in exon 1 of the Fgf23 gene, as described previously (23). Dmp1-deficient mice were created by replacing exon 6 with the lacZ and neo cassette, as described previously (8). Hyp mice were originally purchased from the Jackson Laboratory (Bar Harbor, ME) and maintained in our vivarium. All mice were maintained and utilized in accordance with the recommendations in the Guide for Care and Use of Laboratory Animals prepared by the Institute on Laboratory Animal Resources, National Research Council (Department of Health and Human Services Publication, National Institutes of Health 86-23, National Academy Press, 1996) and the guidelines established by the University of Kansas Medical Center Institutional Animal Care and Use Committee. All experiments reported here were approved by the Institutional Animal Care and Use Committee. Mice were maintained under standard 5K67 diet (PMI Nutrition International, Brentwood, MO), which contains 1.15% calcium, 0.85% phosphorus, and 4 IU/g vitamin D3.
Transfer of Fgf23 deficiency onto the Dmp1-deficient background.
To generate double Fgf23- and Dmp1-null mice, we crossed male double-heterozygous Fgf23- and Dmp1-deficient mice (Fgf23+/−/Dmp1+/−) with female double-heterozygous Fgf23- and Dmp1-deficient mice (Fgf23+/−/Dmp1+/−). We examined male wild-type (WT) and mutant mice (Dmp1−/−, Fgf23−/−, and Dmp1−/−/Fgf23−/−) at 5 wk of age. To assess Fgf23 promoter activity in bones of WT and Dmp1-null mice, we also collected long bone from Dmp1-WT and Dmp1-null mice carrying one Fgf23-eGFG reporter allele (Dmp1+/+/Fgf+/− and Dmp1−/−/Fgf23+/−) at 5 wk of age.
Genotyping.
Genomic DNA tissue was extracted from the tail or ear biopsy of each mouse using REDExtract-N-Amp Tissue PCR kit (Sigma-Aldrich, St. Louis, MO). WT (Fgf23+/+), homozygous Fgf23-deficient (Fgf23−/−), and homozygous Dmp1-deficient (Dmp1−/−) and combined homozygous Fgf23- and Dmp1-deficient (Dmp1−/−/Fgf23−/−) mice were genotyped by PCR as, reported previously (Fig. 1) (8, 19). Mice with different genotypes were born at the expected frequencies.
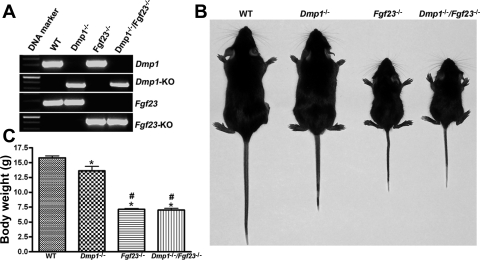
Fig. 1.
Generation of combined fibroblast growth factor 23 (Fgf23)- and dentin matrix protein 1 (Dmp1)-deficient mice. A: genotyping of mice by PCR. Representative PCR analysis of genomic DNA for the Dmp1 gene, specific DNA fragment in Dmp1-knockout (KO) allele, Fgf23 gene, and specific DNA fragment in Fgf23-knockout allele [enhanced green fluroescent protein (eGFP)] in wild-type (WT), Dmp1-null (Dmp1−/−), Fgf23-null (Fgf23−/−), and combined Dmp1−/−/Fgf23−/− mice. B: gross appearance of WT, Dmp1-null (Dmp1−/−), Fgf23-null (Fgf23−/−), and combined Dmp1−/−/ Fgf23−/− mice at 5 wk of age. C: body weight of WT, Dmp1−/−, Fgf23−/−, and combined Dmp1−/−/Fgf23−/− mice at 5 wk of age. * and #P < 0.05 by 1-way ANOVA compared with WT and Hyp mice, respectively.
Serum biochemical measurements.
Serum FGF23 levels were measured by using FGF23 ELISA kit (Kainos Laboratories, Tokyo, Japan), following the manufacturer's protocol. Serum calcium was measured using a Calcium CPC Liquicolor kit (Stanbio Laboratories, Boerne, TX), and serum phosphorus was measured using the phosphomolybdylate-ascorbic acid method as described previously (23). Serum parathyroid hormone (PTH) levels were measured using a Mouse Intact PTH ELISA kit (Immutopics, Carlsbad, CA). Serum 1,25(OH)2D levels were measured using a 1,25-Dihydroxy Vitamin D EIA kit (Immunodiagnostic Systems, Fountain Hills, AZ).
Histological analysis.
eGFP fluorescent imaging in tissues was performed using previously described methods (23). Briefly, mouse bone was quickly dissected and fixed in 4% paraformaldehyde in PBS (pH 7.4) and then embedded in frozen embedding medium. Cryosectioning was performed on a Leica CM1900 Cryostat (Leica, Nussloch, Germany) equipped with a CryoJane frozen sectioning kit (Instrumedics, Hackensack, NJ). Five-micrometer sections were obtained from embedded bone samples. eGFP was examined with a Leica DM IRB inverted microscope equipped with a Leica DM 500 digital camera.
For the histological analysis of nondecalcified bone, mice were prelabeled at 6 days and 1 day before they were killed for tissue collection with alizarin complexone (Acros Organics, Fair Lawn, NJ) and calcein (Sigma-Aldrich, St. Louis, MO) by intraperitoneal injection. Tibias were fixed in 70% ethanol and embedded in methyl methacrylate. Five-micrometer sections were stained with Goldner's stain and analyzed under transmitted light, and 10-μm unstained sections were evaluated under fluorescent light (23). Growth plate width was assessed with the Osteomeasure system (Osteometrics, Atlanta, GA).
Bone marrow harvest and stromal cell culture.
Bone marrow stromal cells (BMSCs) from long bones isolated from 6-wk-old male mice were cultured as described previously (23). Briefly, both femurs and tibias were isolated from Dmp1+/+/Fgf23+/− and Dmp1−/−/Fgf23+/− mice, and bone marrow cells from those mice were flushed out from long bone and cultured in growth medium (α-MEM containing 10%). Adherent cells were grown in the differentiation medium (growth medium supplemented with 5 mmol/l β-glycerophosphate and 25 μg/ml of ascorbic acid) to induce osteoblastic differentiation. During the culture period, we checked the cells under inverted microscope with both bright field and fluorescent every day. On day 16, the cells were taken picture under bright field and fluorescent light microscopy.
Bone densitometry and three-dimensional analysis of the femurs by microcomputed tomography.
The femurs from 5-wk-old mice were collected and fixed in 70% ethanol. Bone mineral densities of femurs were measured using a PIXImus bone densitometer (Lunar, Madison, WI). High-resolution microcomputed tomography, (μCT40; Scanco Medical, Basserdorf, Switzerland) was used to scan and evaluate bone volume fraction and microarchitecture of the metaphyseal region of the distal femurs (19). In addition, cortical thickness data were obtained at the midshaft. The entire femurs were scanned in a sample holder with 12.3 mm diameter at medium resolution, energy level of 55 kV, and intensity of 145 μA. The three-dimensional structure was constructed and 3-D morphometric analysis conducted with the built-in software of the μCT system. Trabecular bones from 50 cross-section slices (0.6 mm) underneath the growth plate and cortical bones from 20 cross-section slices at the midpoint of the femurs were analyzed using a threshold of 250.
RNA isolation and quantitative RT-PCR.
Total RNAs were extracted from long bone collected from WT and Dmp1-null mice at 5 wk of age using TRI Reagent (Molecular Research Center, Cincinnati, OH) and then treated with RNase-free DNase (Qiagen, Valencia, CA). First-strand cDNA was synthesized using iScript cDNA Synthesis kit (Bio-Rad, Hercules, CA). Total RNA (1 μg) was used in each 20-μl reverse transcriptase reaction. For real-time RT-PCR, 200 ng of total RNA was used in each PCR reaction. The iCycler iQ Real-Time PCR Detection System and iQ SYBR Green Supermix (Bio-Rad) were used for real-time quantitative PCR analysis. The relative gene expression was expressed as previously described using cycle threshold values of the gene of interest normalized with glyceraldehyde-3-phosphate dehydrogenase (GAPDH) in the same sample and then divided by the values from WT (23). Sequences of primers used for real-time quantitative RT-PCR were listed in table 1.
Table 1.
Primers used for RT-PCR
| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| Fgf23 | GTGTCAGATTTCAAACTCAG | GGATAGGCTCTAGCAGTG |
| Phex | TGATGGAAGCAGAAACAG | CTTGGAAACTTAGGAGACC |
| Mepe | TCAAGACAGCATTCACAAGGAC | GGAGGGCAGCACCATACC |
| Osteocalcin | GACCTCACAGATGCCAAG | TCACAAGCAGGGTTAAGC |
| Gapdh | GCAAGTTCAAGGGCACTGTAAAG | ACTCGGCTCCAGCATCTCC |
Fgf23, fibroblastic growth factor 23; Phex, phosphate-regulating gene with homologies to endopeptidases on the X chromosome; Mepe, matrix extracellular phosphoglycoprotein.
Statistics.
We evaluated differences among groups by one-way ANOVA followed by a Tukey posttest. The differences were considered to be statistically significant at P < 0.05. All values are expressed as means ± SE. Computations were performed using either GraphPad Prism (GraphPad Software, San Diego, CA) or Statgraphics statistical graphic system (STSC, Rockville, MD).
RESULTS
Gross appearance.
Compared with WT mice, 5-wk-old Dmp1−/− mice displayed evidence of mild growth retardation and skeletal dysplasia (e.g., reduced body weight and shorter limbs and tails; Fig. 1, B and C). In contrast, by 5 wk of age, Fgf23−/− mice were noticeably smaller and had significantly shorter body length and body weight compared with WT littermates (Fig. 1, B and C), consistent with the previously reported effect of FGF23 ablation to cause severe growth retardation (28, 30). Transfer of homozygous Fgf23−/− onto Dmp1−/− mice resulted in a gross appearance identical to that of Fgf23−/− mice (Fig. 1, B and C). Dmp1-null mice had survival rates indistinguishable from WT mice, whereas combined Fgf23−/−/Dmp1−/− mice had mortality rates comparable with Fgf23−/− mice (i.e., mortality beginning 6 wk after birth, with the majority of mice dying by 10 wk of age; data not shown). Thus, the phenotype of the complete loss of Fgf23 predominated over the manifestations of Dmp1 deficiency. In contrast, heterozygous Fgf23+/− and Dmp1+/− mice were indistinguishable from WT mice, and superimposing heterozygous Fgf23+/− onto the Dmp1-null background resulted in mice that grossly resembled Dmp1−/− mice (data not shown).
Effect of combined Fgf23 and Dmp1 deficiency on serum biochemical parameters.
The serum Fgf23 concentration in Dmp1-null mice was roughly 18-fold greater than in WT littermates, whereas serum Fgf23 levels in 5-wk-old homozygous Fgf23−/− and combined Fgf23−/−/Dmp1−/− mice were undetectable (Table 2). The increased Fgf23 levels in Dmp1-null mice were associated with a significant reduction in serum phosphate and inappropriately normal 1,25(OH)2D levels for the degree of hypophosphatemia. In contrast, the absence of circulating Fgf23 levels in Fgf23−/− mice resulted in significantly higher serum phosphate and 1,25(OH)2D levels (Table 2). In combined homozygous Fgf23−/−/Dmp1−/− mice, the serum phosphate and 1,25(OH)2D levels were increased to levels indistinguishable from the values in Fgf23−/− mice. In addition, serum calcium was increased and PTH decreased in both Fgf23−/− mice and combined Fgf23−/−/Dmp1−/− mice, consistent with the increase in 1,25(OH)2D.
Table 2.
Serum biochemistry of WT, Dmp1−/−, Fgf23−/−, and Dmp1−/−/Fgf23−/− mice
| Serum Markers | WT | Dmp1−/− | Fgf23−/− | Dmp1−/−/Fgf23−/− |
|---|---|---|---|---|
| Phosphorus, mg/dl | 9.3±0.4 | 5.1±0.3* | 14.3±0.5*† | 14.7±0.6*† |
| Calcium, mg/dl | 8.8±0.1 | 8.3±0.2 | 10.2±0.3*† | 10.1±0.4*† |
| 1,25(OH)2D, pM | 260±29 | 257±36 | 1,056±135*† | 1121±163*† |
| PTH, pg/ml | 83±25 | 103±27 | 28±4*† | 26±4*† |
| FGF23, pg/ml | 77±7 | 1404±108* | ND | ND |
Values are means ± SE from ≥9 mice at 5 wk of age. WT, wild type; Dmp1, dentin matrix protein 1; PTH, parathyroid hormone; ND, not detectable. One-way ANOVA was used for statistical analysis.
Significantly different compared with WT (P < 0.05);
significantly different compared with Dmp1−/− mice (P < 0.05).
Effects of Dmp1 ablation on Fgf23 promoter-eGFP reporter mice.
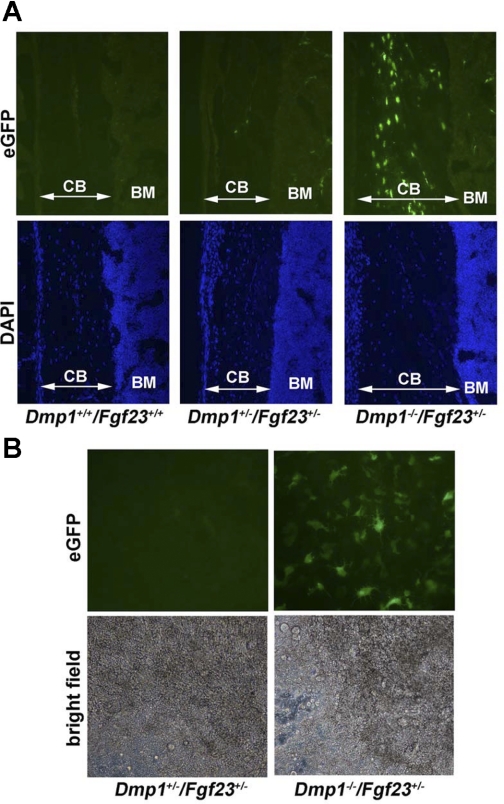
Heterozygous Fgf23 promoter-eGFP reporter mice with intact Dmp1 displayed rare eGFP expression in osteocytes embedded in bone but no eGFP expression in osteoblast cells on the bone surfaces. Superimposing Dmp1-null onto Fgf23 promoter-eGFP reporter mice resulted in marked increases in expression of eGFP in the majority of osteocytes embedded in bone, whereas surface osteoblasts remained negative for eGFP expression (Fig. 2A). The faint fluorescence on bone surfaces observed in Dmp1+/−/Fgf23+/− and Dmp1−/−/Fgf23+/− is likely due to background autofluorescence, since Dmp1+/+/Fgf23+/+ (WT) mice, which do not contain the eGFP cDNA cassette in their genome, had similar bone surface autofluorescence (Fig. 2A). We failed to detect increments in eGFP expression in combined Dmp1−/−/Fgf23+/− mice at any other site (data not shown). These findings are consistent with the published reports of increased expression of Fgf23 transcripts in bone of Dmp1-null mice by real-time PCR (9) and suggest that the increased circulating Fgf23 levels in Dmp1−/− mice is due to increased production by osteocytes. In addition, we examined the temporal pattern of eGFP expression in BMSCs from Dmp1+/+/Fgf23+/− and Dmp1−/−/Fgf23+/− mice grown for 16 days under conditions promoting osteoblast differentiation in vitro. We found that loss of Dmp1 resulted in a marked stimulation of eGFP expression only in cells embedded within mineralization nodules (Fig. 2B).
Fig. 2.
Expression of eGFP driven by endogenous Fgf23 promoter in bone and bone marrow stromal cell (BMSC) cultures. A: longitudinal frozen sections from femurs of WT (used as control for autofluorescent background), Dmp1+/+/Fgf23+/−, and Dmp1−/−/Fgf23+/− mice viewed under fluorescent microscope showing eGFP expression (top) and 4,6-diamidino-2-phenylindole (DAPI)-stained nuclei (bottom) (×200). Very few osteocytes express eGFP in Dmp1+/+/Fgf23+/− mice with intact Dmp1 expression; however, eGFP is expressed in the majority of osteocytes in Dmp1−/−/Fgf23+/− mice. The faint surface fluorescence observed on bone surfaces at top is due to background autofluorescence, which is not different from WT background control Dmp1+/+/Fgf23+/+. B: BMSCs were grown in ascorbic acid and β-glycerol phosphate for 16 days to induce osteoblast differentiation. Top: the high eGFP expression is shown only in the subset of cells embedded in mineralization nodules in BMSCs derived from Dmp1−/−/Fgf23+/− mice, but minimal eGFP expression in mineralization nodule BMSC cultures from Dmp1+/−/Fgf23+/−, consistent with Dmp1 deficiency stimulating Fgf23 promoter activity in osteocytes. Bottom: images from the same fields viewed under bright-field light microscope showing mineralization nodule formed by palisading and clustering cells (×200). CB, cortical bone; BM, bone marrow.
Effect of superimposed Fgf23 deficiency on rickets and osteomalacia in Dmp1-null mice.
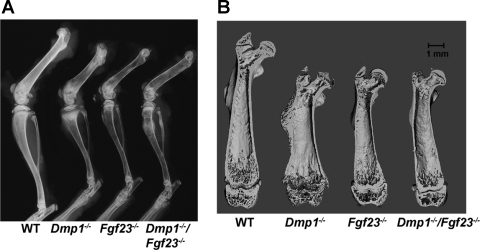
Dmp1-null mice displayed classical features of rickets, including splaying of the ends of the long bones and widening of the growth plate by radiographic and μCT analysis (Fig. 3, A and B). Homozygous Fgf23−/− mice were characterized by miniaturized bones of normal shape that lacked any features of rickets by radiographic and μCT analysis (Fig. 3). Histological analysis revealed that the growth plates were wider in Dmp1−/− mice and narrower in Fgf23−/− mice compared with WT littermates [growth plate width of 151.0 ± 13.10 μm in WT, 240.4 ± 21.84 μm in Dmp1−/−, and 94.8 ± 2.49 μm in Fgf23−/− mice (means ± SE; n = 4)]. The widened growth plate width in Dmp1−/−-null mice was due to an increase in the zone of hypertrophic chondrocytes (Fig. 4A). Interestingly, transfer of Dmp1-null onto Fgf23−/− mice resulted in correction of both the radiographic and histological manifestations of rickets, which were replaced with growth plate abnormalities and overall bone appearance resembling those of Fgf23−/− mice (Fig. 3 and Fig. 4A). In this regard, the growth plates in combined homozygous Dmp1−/−/Fgf23−/− mice were not significantly different from Fgf23−/− mice (growth plate width of 99.5 ± 10.73 μm in Dmp1−/−/Fgf23−/− and 94.8 ± 2.49 μm in Fgf23−/−).
Fig. 3.
Effects of superimposed Dmp1 and Fgf23 deficiency on radiological features of bones. A: X-ray of hind legs from 5-wk-old WT and mutant mice. B: microcomputed tomography coronal section images of femurs from 5-wk-old WT and mutant mice.
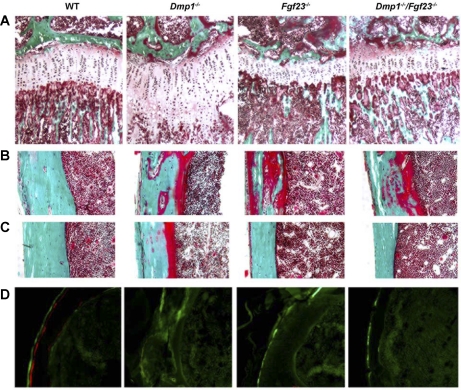
Fig. 4.
Effects of superimposed Dmp1 and Fgf23 deficiency on histological features of cartilage and bone. A: growth plate of proximal tibias of 5-wk-old mice (Goldner stain, ×200). B and C: CB of tibias of 5-wk-old mice (Goldner stain, ×200). Mineralized bone was stained in green, and unmineralized osteoid was stained in red. D: unstained cross-section of tibias viewed under fluorescent light (×200). Red fluorescent label was from alizarin complexone, and green fluorescent label was from calcein.
Dmp1−/− mice also exhibited by both bone densitometry and μCT analysis significant reductions in bone mineral density (Table 3), a surrogate marker for osteomalacia in this setting (19). Indeed, histological analysis revealed that Dmp1−/− mice displayed hyperosteoidosis and impaired mineralization (Fig. 4B), along with indistinct fluorescent labeling of bone, consistent with impaired mineralization (Fig. 4C). The absence of Fgf23 also results in impaired mineralization despite increased phosphate and 1,25(OH)2D3 levels, as reported previously (29). In this regard, Fgf23-null mice displayed a reduction in mineralized trabecular bone volume as measured by μCT (Table 3) and a reduced bone mineral density by bone densitometry and μCT (Table 3). Compared with WT mice, Fgf23−/− mice also displayed increased osteoid volume and impaired mineralization, as evidenced by the reduced intensity and the lack of double-fluorescent labeling of bone (Fig. 4), as reported previously (28). In contrast to the diffuse osteomalacia observed in Dmp1−/− mice, a patchy distribution of the widened osteoid seams (Fig. 4, B and C) and near absence of double-fluorescent labeling of bone in Fgf23-null mice (Fig. 4D) are consistent with a focal osteomalacia. Combined homozygous Dmp1−/−/Fgf23−/− mice resemble Fgf23−/− mice, as evidenced by a reduction of both the amount of osteoid and fluorochrome labeling, indicating that superimposed Fgf23 deficiency also corrects the diffuse hyperosteoidosis observed in Dmp1−/− mice. The focal osteomalacia consisting of bone surfaces with widened (Fig. 4B) and normal (Fig. 4C) osteoid seams and impaired fluorescent labeling (Fig. 4D) observed in Fgf23-null mice remained in Dmp1−/−/Fgf23−/− mice.
Table 3.
Bone densitometry and μCT analysis of femurs of WT and mutant mice
| WT | Dmp1−/− | Fgf23−/− | Dmp1−/−/Fgf23−/− | |
|---|---|---|---|---|
| Femural BMD, g/cm2 | 0.050±0.001 | 0.028±0.001* | 0.029±0.001* | 0.029±0.001* |
| BV/TV, % | 5.3±0.4 | 1.3±0.2* | 0.8±0.2* | 1.2±0.2* |
| Tb. density/BV, mg HA/ccm | 902.9±7.9 | 813.2±16.0* | 737.0±14.5*† | 807.0±7.1*‡ |
| Ct. Th, mm | 0.148±0.003 | 0.099±0.003* | 0.090±0.005* | 0.104±0.004*‡ |
| Ct. density/TV, mg HA/ccm | 1,087±16 | 797±21* | 839±17* | 810±34* |
| Ct. bone density/BV, mg HA/ccm | 1,256±15 | 1,054±18* | 978±26* | 988±32* |
Values means ± SE from ≥9 mice at 5 wk of age. μCT, microcomputed tomography; BMD, bone mineral density; BV, bone volume; TV, total volume; HA, hydroxyapatite; Tb., trabecular bone; Ct., cortical bone; Ct. Th, cortical thickness. One-way ANOVA was used for statistical analysis.
Significantly different compared with WT (P < 0.05);
significantly different compared with Dmp1−/− mice (P < 0.05);
significantly different compared with FGF23−/− mice (P < 0.05).
Gene expression analysis in bone from WT and Dmp1-null mice.
We compared Fgf23, Phex, matrix extracellular phosphoglycoprotein (Mepe), and osteocalcin expression in Dmp1-null mice and WT mice using quantitative real-time RT-PCR (Table 4). We observed an ∼150-fold increase in Fgf23 expression in Dmp1-null mice compared with WT mice, consistent with elevated promoter activity in Fgf23 promoter-eGFP reporter mice (Fig. 2). We also observed a minor increase in Phex and Mepe expression (1.6- and 1.5-fold, respectively) in Dmp1-null mice. However, the expression of the osteoblast marker osteocalcin was not changed in Dmp1-null mice.
Table 4.
Relative gene expression in bone measured by quantitative real-time RT-PCR
| Gene | WT | Dmp1−/− |
|---|---|---|
| Fgf23 | 1.0±0.40 | 154.4±27.17* |
| Phex | 1.0±0.09 | 1.6±0.21* |
| Mepe | 1.0±0.14 | 1.5±0.12* |
| Osteocalcin | 1.1±0.2 | 1.1±0.19 |
Values are means ± SE from ≥6 mice at 5 wk of age. Student's t-test was used for statistical analysis.
Significantly different compared with WT (P < 0.05).
DISCUSSION
ARHR, which is caused by inactivating mutations of DMP1 (9, 24), shares a common phenotype with two other hereditary hypophosphatemic disorders, ADHR and XLH, that are known to be caused by excess FGF23 (13, 38, 40). In this study, we confirm that Dmp1 deficiency in the mouse homologue of ARHR also results in the selective increase in osteocyte production of Fgf23 and elevated circulating levels of this phosphaturic hormone. In addition, we provide evidence that Fgf23 is the proximate cause of the hypophosphatemia and abnormal vitamin D metabolism in Dmp1-null mice by showing that ablation of Fgf23 results in increased serum phosphate and 1,25(OH)2D levels in Dmp1-null mice. In fact, the phenotype of combined Fgf23 and Dmp1-null mice was identical to Fgf23-null mice, indicating the dominant role of Fgf23 in the regulating phosphate and 1,25(OH)2D levels. The respective PTH and 1,25(OH)2D values in the current study (83 pg/ml and 260 pM in WT and 28 pg/ml and 1,056 pM in Fgf23-null mice; Table 2) and in our previous study (21 pg/ml and 241 pM in WT and 10 pg/ml and 535 pM in Fgf23-null mice) (23) are directionally the same, but the absolute values differ, likely due to differences in the diet (1.15% calcium, 0.85% phosphorus in current study vs. 1.36% calcium and 1.01% phosphorus in the previous study), differences in the assay kits, and/or differences in the age of the animals. Regardless, we found no evidence to support the possibilities that Dmp1 deficiency might stimulate the production of another phosphaturic factor or directly regulate phosphate transport in the kidney, since either of these alternative mechanisms would have lowered serum phosphate levels in combined Dmp1−/−/Fgf23−/− mice compared with Fgf23−/− mice.
The current studies do not address the mechanisms whereby Dmp1 deficiency stimulates transcription of Fgf23 in osteocytes. DMP1 is produced by osteoblasts/osteocytes and accumulates in the extracellular matrix, where it facilitates mineralization of collagen in bone and promotes osteoblast/odontoblast differentiation (1b, 26). In addition, DMP1 has the potential to regulate cell and extracellular matrix activity through its binding to integrin αvβ3 and matrix metalloproteinase-9 (15, 16). Phex deficiency was not the cause of the observed phenotype in Dmp1-null mice, since Phex mRNA expression was increased in these mice. Moreover, since Fgf23 is not expressed in osteoblasts, which are precursors to osteocytes, it is unlikely that increased Fgf23 expression is due to a maturational defect in the osteoblast-to-osteocyte transition. Moreover, osteocalcin message expression, a marker of mature osteoblasts, was similar in Dmp1-null and WT mice. It is known that extracellular matrix contains a multitude of growth factors capable of targeting osteoblasts and osteocytes in the bone microenvironment (14, 34). Therefore, Dmp1 deficiency may stimulate Fgf23 gene transcription via direct effects on osteocyte function or indirectly through alterations in extracellular matrix-related factors. Further studies will be needed to determine the mechanisms whereby DMP1 regulates FGF23 expression in osteocytes.
On the basis of the function of Dmp1 to act as a nucleator of mineralization, the a priori assumption is that defective bone mineralization in Dmp1-null mice would arise as a direct consequence of the lack of Dmp1 in bone; however, the current results indicate that hypophosphatemia and aberrant production of 1,25(OH)2D3, consequent to elevated Fgf23, were principally responsible for rickets and diffuse osteomalacia in Dmp1-null mice. The possibility that increased phosphate is responsible for the lack of rachitic changes in combined Dmp1/Fgf23-null mice is consistent with the observation that defective cartilage mineralization in vitamin D-deficient states can be corrected by restoring phosphate (2). The contributions of Fgf23 and hypophosphatemia to Dmp1-associated defective mineralization of bone are more complex because of the separate effects of Fgf23 deficiency to cause a focal osteomalacia (23, 28, 30). Indeed, superimposing Fgf23 deficiency onto Dmp1-null mice and consequent increments in serum phosphate and 1,25(OH)2D levels replaced the diffuse osteomalacia that characterized the Dmp1-null bone phenotype with the focal osteomalacia of Fgf23-null mice. The reduction in the extent of hyperosteoidosis in combined Dmp1- and Fgf23-null mice is likely related to the effect of increased phosphate to promote bone mineralization (25), but the mechanism underlying the focal mineralization defect in Fgf23-null mice is unknown. Finally, the presence of growth plate abnormalities leading to growth retardation in Fgf23-null mice and the expression of potential Fgf23 receptors in bone and cartilage (41) also raise the alternative possibility of a direct effect of Fgf23 on the skeleton. In addition, high levels of 1,25(OH)2D, as observed in Fgf23-null mice, are paradoxically associated with defective mineralization in 24-hydroxylase-null mice (31), suggesting that the excessive levels of 1,25(OH)2D can lead to impaired mineralization.
We originally proposed that PHEX and FGF23, which are coexpressed in osteocytes in bone, are part of a bone-kidney axis regulating phosphate homeostasis and mineralization (27). The existence of this enzyme-hormone cascade was supported by the association between increased FGF23 expression and inactivating PHEX mutations in osteocytes (20, 38) and by the observation that ablation of Fgf23 in Hyp mice rescued the hypophosphatemia (22). In the current study, we have added another component to this putative bone-kidney axis by demonstrating that lack of Dmp1, a protein regulating mineralization, stimulates Fgf23 expression in osteocytes and ablation of Fgf23 corrects the hypophosphatemia in Dmp1-null mice. Remarkably, the phenotype of Dmp1-null mice is very similar to Hyp mice, suggesting the functional importance of elevated Fgf23 in both models. Whether PHEX and DMP1 are regulating FGF23 production through common or distinct pathways remains to be determined. Regardless, PHEX, DMP1, and FGF23 produced by osteocytes appear to coordinate mineralization and systemic phosphate homeostasis. Further studies are needed to understand the potential interdependent regulation of these three factors.
GRANTS
This work was supported by National Institute of Arthritis and Musculoskeletal and Skin Diseases Grant No. RO1-AR-045955.
Supplementary Material
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.No authors listed. A gene (PEX) with homologies to endopeptidases is mutated in patients with X-linked hypophosphatemic rickets. The HYP Consortium. Nat Genet 11: 130–136, 1995. [DOI] [PubMed] [Google Scholar]
- 1a.ADHR Consortium. Autosomal dominant hypophosphataemic rickets is associated with mutations in FGF23. Nat Genet 26: 345–348, 2000. [DOI] [PubMed] [Google Scholar]
- 1b.Almushayt A, Narayanan K, Zaki AE, George A. Dentin matrix protein 1 induces cytodifferentiation of dental pulp stem cells into odontoblasts. Gene Ther 13: 611–620, 2006. [DOI] [PubMed] [Google Scholar]
- 2.Amling M, Priemel M, Holzmann T, Chapin K, Rueger JM, Baron R, Demay MB. Rescue of the skeletal phenotype of vitamin D receptor-ablated mice in the setting of normal mineral ion homeostasis: formal histomorphometric and biomechanical analyses. Endocrinology 140: 4982–4987, 1999. [DOI] [PubMed] [Google Scholar]
- 3.Aono Y, Shimada T, Uamazaki Y, Hino R, Takeuchi Y, Fujita T, Fukumoto S, Nagano N, Wada M, Yamashita T. The neutralization of FGF-23 ameliorates hypophosphatemia and rickets in Hyp mice (Abstract). J Bone Miner Res 18: S16, 2003. [Google Scholar]
- 4.Bai X, Miao D, Li J, Goltzman D, Karaplis AC. Transgenic mice overexpressing human fibroblast growth factor 23 (R176Q) delineate a putative role for parathyroid hormone in renal phosphate wasting disorders. Endocrinology 145: 5269–5279, 2004. [DOI] [PubMed] [Google Scholar]
- 5.Bai XY, Miao D, Goltzman D, Karaplis AC. The autosomal dominant hypophosphatemic rickets R176Q mutation in fibroblast growth factor 23 resists proteolytic cleavage and enhances in vivo biological potency. J Biol Chem 278: 9843–9849, 2003. [DOI] [PubMed] [Google Scholar]
- 6.Beck L, Soumounou Y, Martel J, Krishnamurthy G, Gauthier C, Goodyer CG, Tenenhouse HS. Pex/PEX tissue distribution and evidence for a deletion in the 3′ region of the Pex gene in X-linked hypophosphatemic mice. J Clin Invest 99: 1200–1209, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benet-Pages A, Lorenz-Depiereux B, Zischka H, White KE, Econs MJ, Strom TM. FGF23 is processed by proprotein convertases but not by PHEX. Bone 35: 455–462, 2004. [DOI] [PubMed] [Google Scholar]
- 8.Feng JQ, Huang H, Lu Y, Ye L, Xie Y, Tsutsui TW, Kunieda T, Castranio T, Scott G, Bonewald LB, Mishina Y. The Dentin matrix protein 1 (Dmp1) is specifically expressed in mineralized, but not soft, tissues during development. J Dent Res 82: 776–780, 2003. [DOI] [PubMed] [Google Scholar]
- 9.Feng JQ, Ward LM, Liu S, Lu Y, Xie Y, Yuan B, Yu X, Rauch F, Davis SI, Zhang S, Rios H, Drezner MK, Quarles LD, Bonewald LF, White KE. Loss of DMP1 causes rickets and osteomalacia and identifies a role for osteocytes in mineral metabolism. Nat Genet 38: 1310–1315, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gajjeraman S, Narayanan K, Hao J, George A. Matrix macromolecules in hard tissues control the nucleation and hierarchical assembly of hydroxyapatite. J Biol Chem 282: 1193–1204, 2008. [DOI] [PubMed] [Google Scholar]
- 11.Guo R, Liu S, Spurney RF, Quarles LD. Analysis of recombinant Phex: an endopeptidase in search of a substrate. Am J Physiol Endocrinol Metab 281: E837–E847, 2001. [DOI] [PubMed] [Google Scholar]
- 12.He G, George A. Dentin matrix protein 1 immobilized on type I collagen fibrils facilitates apatite deposition in vitro. J Biol Chem 279: 11649–11656, 2004. [DOI] [PubMed] [Google Scholar]
- 13.Imel EA, Hui SL, Econs MJ. FGF23 concentrations vary with disease status in autosomal dominant hypophosphatemic rickets. J Bone Miner Res 22: 520–526, 2007. [DOI] [PubMed] [Google Scholar]
- 14.Jadlowiec JA, Zhang X, Li J, Campbell PG, Sfeir C. Extracellular matrix-mediated signaling by dentin phosphophoryn involves activation of the Smad pathway independent of bone morphogenetic protein. J Biol Chem 281: 5341–5347, 2006. [DOI] [PubMed] [Google Scholar]
- 15.Jain A, Karadag A, Fohr B, Fisher LW, Fedarko NS. Three SIBLINGs (small integrin-binding ligand, N-linked glycoproteins) enhance factor H's cofactor activity enabling MCP-like cellular evasion of complement-mediated attack. J Biol Chem 277: 13700–13708, 2002. [DOI] [PubMed] [Google Scholar]
- 16.Karadag A, Fedarko NS, Fisher LW. Dentin matrix protein 1 enhances invasion potential of colon cancer cells by bridging matrix metalloproteinase-9 to integrins and CD44. Cancer Res 65: 11545–11552, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Larsson T, Davis SI, Garringer HJ, Mooney SD, Draman MS, Cullen MJ, White KE. Fibroblast growth factor-23 mutants causing familial tumoral calcinosis are differentially processed. Endocrinology 146: 3883–3891, 2005. [DOI] [PubMed] [Google Scholar]
- 18.Larsson T, Marsell R, Schipani E, Ohlsson C, Ljunggren O, Tenenhouse HS, Juppner H, Jonsson KB. Transgenic mice expressing fibroblast growth factor 23 under the control of the alpha1(I) collagen promoter exhibit growth retardation, osteomalacia, and disturbed phosphate homeostasis. Endocrinology 145: 3087–3094, 2004. [DOI] [PubMed] [Google Scholar]
- 19.Liu S, Brown TA, Zhou J, Xiao ZS, Awad H, Guilak F, Quarles LD. Role of matrix extracellular phosphoglycoprotein in the pathogenesis of X-linked hypophosphatemia. J Am Soc Nephrol 16: 1645–1653, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu S, Guo R, Simpson LG, Xiao ZS, Burnham CE, Quarles LD. Regulation of fibroblastic growth factor 23 expression but not degradation by PHEX. J Biol Chem 278: 37419–37426, 2003. [DOI] [PubMed] [Google Scholar]
- 21.Liu S, Quarles LD. How fibroblast growth factor 23 works. J Am Soc Nephrol 18: 1637–1647, 2007. [DOI] [PubMed] [Google Scholar]
- 22.Liu S, Tang W, Zhou J, Vierthaler L, Quarles LD. Distinct roles for intrinsic osteocyte abnormalities and systemic factors in regulation of FGF23 and bone mineralization in Hyp mice. Am J Physiol Endocrinol Metab 293: E1636–E1644, 2007. [DOI] [PubMed] [Google Scholar]
- 23.Liu S, Zhou J, Tang W, Jiang X, Rowe DW, Quarles LD. Pathogenic role of Fgf23 in Hyp mice. Am J Physiol Endocrinol Metab 291: E38–E49, 2006. [DOI] [PubMed] [Google Scholar]
- 24.Lorenz-Depiereux B, Bastepe M, Benet-Pagès A, Amyere M, Wagenstaller J, Müller-Barth U, Badenhoop K, Kaiser SM, Rittmaster RS, Shlossberg AH, Olivares JL, Loris C, Ramos FJ, Glorieux F, Vikkula M, Jüppner H, Strom TM. DMP1 mutations in autosomal recessive hypophosphatemia implicate a bone matrix protein in the regulation of phosphate homeostasis. Nat Genet 38: 1248–1250, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murshed M, Harmey D, Millan JL, McKee MD, Karsenty G. Unique coexpression in osteoblasts of broadly expressed genes accounts for the spatial restriction of ECM mineralization to bone. Genes Dev 19: 1093–1104, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Narayanan K, Ramachandran A, Hao J, He G, Park KW, Cho M, George A. Dual functional roles of dentin matrix protein 1. Implications in biomineralization and gene transcription by activation of intracellular Ca2+ store. J Biol Chem 278: 17500–17508, 2003. [DOI] [PubMed] [Google Scholar]
- 27.Quarles LD Evidence for a bone-kidney axis regulating phosphate homeostasis. J Clin Invest 112: 642–646, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shimada T, Kakitani M, Yamazaki Y, Hasegawa H, Takeuchi Y, Fujita T, Fukumoto S, Tomizuka K, Yamashita T. Targeted ablation of Fgf23 demonstrates an essential physiological role of FGF23 in phosphate and vitamin D metabolism. J Clin Invest 113: 561–568, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shimada T, Urakawa I, Yamazaki Y, Hasegawa H, Hino R, Yoneya T, Takeuchi Y, Fujita T, Fukumoto S, Yamashita T. FGF-23 transgenic mice demonstrate hypophosphatemic rickets with reduced expression of sodium phosphate cotransporter type IIa. Biochem Biophys Res Commun 314: 409–414, 2004. [DOI] [PubMed] [Google Scholar]
- 30.Sitara D, Razzaque MS, Hesse M, Yoganathan S, Taguchi T, Erben RG, Jüppner H, Lanske B. Homozygous ablation of fibroblast growth factor-23 results in hyperphosphatemia and impaired skeletogenesis, and reverses hypophosphatemia in Phex-deficient mice. Matrix Biol 23: 421–432, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.St-Arnaud R, Arabian A, Travers R, Barletta F, Raval-Pandya M, Chapin K, Depovere J, Mathieu C, Christakos S, Demay MB, Glorieux FH. Deficient mineralization of intramembranous bone in vitamin D-24-hydroxylase-ablated mice is due to elevated 1,25-dihydroxyvitamin D and not to the absence of 24,25-dihydroxyvitamin D. Endocrinology 141: 2658–2666, 2000. [DOI] [PubMed] [Google Scholar]
- 32.Strom TM, Francis F, Lorenz B, Boddrich A, Econs MJ, Lehrach H, Meitinger T. Pex gene deletions in Gy and Hyp mice provide mouse models for X-linked hypophosphatemia. Hum Mol Genet 6: 165–171, 1997. [DOI] [PubMed] [Google Scholar]
- 33.Tartaix PH, Doulaverakis M, George A, Fisher LW, Butler WT, Qin C, Salih E, Tan M, Fujimoto Y, Spevak L, Boskey AL. In vitro effects of dentin matrix protein-1 on hydroxyapatite formation provide insights into in vivo functions. J Biol Chem 279: 18115–18120, 2004. [DOI] [PubMed] [Google Scholar]
- 34.Tatsuyama K, Maezawa Y, Baba H, Imamura Y, Fukuda M. Expression of various growth factors for cell proliferation and cytodifferentiation during fracture repair of bone. Eur J Histochem 44: 269–278, 2000. [PubMed] [Google Scholar]
- 37.Turner AJ, Brown CD, Carson JA, Barnes K. The neprilysin family in health and disease. Adv Exp Med Biol 477: 229–240, 2000. [DOI] [PubMed] [Google Scholar]
- 38.Weber TJ, Liu S, Indridason OS, Quarles LD. Serum FGF23 levels in normal and disordered phosphorus homeostasis. J Bone Miner Res 18: 1227–1234, 2003. [DOI] [PubMed] [Google Scholar]
- 39.White KE, Carn G, Lorenz-Depiereux B, Benet-Pages A, Strom TM, Econs MJ. Autosomal-dominant hypophosphatemic rickets (ADHR) mutations stabilize FGF-23. Kidney Int 60: 2079–2086, 2001. [DOI] [PubMed] [Google Scholar]
- 40.Yamazaki Y, Okazaki R, Shibata M, Hasegawa Y, Satoh K, Tajima T, Takeuchi Y, Fujita T, Nakahara K, Yamashita T, Fukumoto S. Increased circulatory level of biologically active full-length FGF-23 in patients with hypophosphatemic rickets/osteomalacia. J Clin Endocrinol Metab 87: 4957–4960, 2002. [DOI] [PubMed] [Google Scholar]
- 41.Yu X, Ibrahimi OA, Goetz R, Zhang F, Davis SI, Garringer HJ, Linhardt RJ, Ornitz DM, Mohammadi M, White KE. Analysis of the biochemical mechanisms for the endocrine actions of fibroblast growth factor-23. Endocrinology 146: 4647–4656, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.