Abstract
Menopause and premature gonadal steroid deficiency are associated with increases in fat mass and body weight. Ovariectomized (OVX) mice also show reduced locomotor activity. Glucose-dependent-insulinotropic-polypeptide (GIP) is known to play an important role both in fat metabolism and locomotor activity. Therefore, we hypothesized that the effects of estrogen on the regulation of body weight, fat mass, and spontaneous physical activity could be mediated in part by GIP signaling. To test this hypothesis, C57BL/6 mice and GIP-receptor knockout mice (Gipr−/−) were exposed to OVX or sham operation (n = 10 per group). The effects on body composition, markers of insulin resistance, energy expenditure, locomotor activity, and expression of hypothalamic anorexigenic and orexigenic factors were investigated over 26 wk in all four groups of mice. OVX wild-type mice developed obesity, increased fat mass, and elevated markers of insulin resistance as expected. This was completely prevented in OVX Gipr−/− animals, even though their energy expenditure and spontaneous locomotor activity levels did not significantly differ from those of OVX wild-type mice. Cumulative food intake in OVX Gipr−/− animals was significantly reduced and associated with significantly lower hypothalamic mRNA expression of the orexigenic neuropeptide Y (NPY) but not of cocaine-amphetamine-related transcript (CART), melanocortin receptors (MCR-3 and MCR-4), or thyrotropin-releasing hormone (TRH). GIP receptors thus interact with estrogens in the hypothalamic regulation of food intake in mice, and their blockade may carry promising potential for the prevention of obesity in gonadal steroid deficiency.
Keywords: estrogen, energy expenditure, body fat
gonadal steroid deficiency in the menopausal state is associated with changes in body composition, which include a loss of lean body mass and an increase in total and abdominal fat mass (15, 24, 30). Estrogen receptors are expressed in various metabolically relevant tissues and locations including areas of the brain that are known to be involved in the regulation of food intake, body weight, and energy expenditure (18, 27). Loss of circulating estrogen also increases fat mass and body weight in ovariectomized (OVX) rodents, and this is reversed by estrogen replacement (16). Changes in food intake have been further reported for states of gonadal steroid deficiency (10), as well as alterations in the expression of certain genes (12) and circulating levels of gut hormones (23) related to energy expenditure and lipid metabolism. The gut hormone glucose-dependent insulinotropic polypeptide (GIP) is released from the duodenum and upper jejunal endocrine K cells and modulates insulin secretion after carbohydrate or fat consumption in humans and rodents. Receptors for GIP (Gipr) are expressed in pancreatic β-cells but are also found in other tissues such as the brain and in adipose tissue (33). Consequently, recent research (17, 33) indicates that GIP might play an important role in fat metabolism. GIP has been shown to direct glucose uptake and stimulate the synthesis of triacylglycerols in adipocytes. Principally consistent with that observation, Gipr−/− mice fed a high-fat diet are protected from obesity (19) and might have an enhanced fat oxidation under conditions of reduced insulin action (34). Moreover, the novel data of transgenic mice overexpressing GIP suggest a role for GIP in the regulation of locomotor activity (6). It has been suggested that individuals in a condition of increased GIP levels are more prone to obesity and hyperinsulinemia (33). On the other hand, increased GIP levels in postmenopausal women might reflect differences in gonadal hormonal status (23), which is supported by the finding that circulating concentrations of GIP are modulated in postmenopausal women receiving estrogen replacement therapy (29).
These studies indicate that the effects of GIP and estrogen on the regulation of body weight and fat mass could be connected. To test this hypothesis, we investigated in Gipr−/− mice and wild-type controls which of the effects of ovariectomy (OVX) on body composition, energy metabolism, and hypothalamic neurocircuitry depended on the presence or absence of GIP.
MATERIALS AND METHODS
Animals.
The protocol for all animal experiments was approved by the Governmental Animal Ethic Review Board (State of Brandenburg, Germany). The generation of Gipr−/− mice in the C57BL/6 background used in these experiments has been previously described (22). Animals were housed individually at a temperature of 22°C with a 12:12-h light-dark cycle in cages with soft wood bedding. Animals were kept on a standard diet (Altromin 1324 fortified; Altromin, Lage, Germany) with unlimited access to chow and liquids. Six-week-old female Gipr−/− and C57BL/6 control mice were divided into four groups (n = 10 per group) and received either OVX or sham operation. Food intake rate was recorded for a total of 8 wk, beginning from week 8 after surgery and ending at week 19. This period had to be interrupted between weeks 14 to 17 because of interference with other examinations. Body weight was measured at baseline and after 4, 6, 10, 14, 18, 22, and 26 wk.
OVX procedures.
OVX or sham operations were performed in anesthetized animals by bilateral dorsal abdominal incisions so that the ovary and the oviduct could be rapidly removed. In the sham surgery group, the ovary and oviduct were visualized before incisions were sutured. Because estrogen influences the uterine weight, atrophy of the uterus was used as an indicator of reduced estrogen levels as described previously (4). The success of OVX procedures was confirmed at the end of the study by measuring uterine weights.
Total energy expenditure.
Total energy expenditure (TEE) was measured by indirect calorimetry in individual mice, using an open respirometric system (gas analyzers: Magnos 16 and Uras 14, Hartmann & Braun) at week 22 after surgery. Mice were unrestrained and had free access to chow and water during analysis. Oxygen consumption and CO2 production were determined every 6 min over a 23-h period. Recorded energy expenditure was normalized for metabolic body weight (body weight in kg0.75).
Body composition.
Body composition (fat mass, lean mass, and free fluid) was measured once a month using nuclear magnetic resonance spectroscopy (Mini Spect MQ 10 NMR Analyser; Bruker, Karlsruhe, Germany). In addition, body weight was measured using an electronic scale.
Spontaneous locomotor activity analysis.
Mouse locomotor activity within home cage environment was measured using an infrared light system (TSE, Bad Homburg, Germany). The sensors registered the activity of the animal by sensing the body-heat image and its spatial displacement over time. Activity was recorded in week 21 over a 5-day period and expressed as counts per hour.
Plasma analyses.
Animals were investigated in the overnight fasted state. Mouse plasma insulin levels were determined by ELISA for rat insulin using a mouse insulin standard (both from Crystal Chem, Chicago, IL) as described previously (25). Blood was obtained from the retro-orbital sinus during anesthesia using Isoflurane (Baxter, Unterschleissheim, Germany). Plasma glucose was measured using a commercial kit following manufacturer's instructions (Glucose HK CP; ABX Pentra, Montpellier, France). Leptin was measured in plasma of fed mice using a commercial ELISA (R&D Systems, Minneapolis, MN).
RNA extraction and real-time RT-PCR.
Total RNA was extracted from hypothalamus of animals in nonfasted state by Trizol reagent (Invitrogen, Carlsbad, CA). Mouse hypothalamic expression of the mRNA encoding neuropeptide Y (NPY), agouti-related peptide (AgRP), cocaine-amphetamine-related transcript (CART), thyrotropin-releasing hormone (TRH), leptin receptor (db), estrogen receptor-α (ER-α), and melanocortin receptor-3 and -4 (MCR-3 and MCR-4) were measured by Biosystems 7300 real-time RT-PCR system as described previously (20). Cycle threshold values from each experimental sample were used to calculate the amount of each gene and hypoxanthine-guanine phosphoribosyltransferase (HPRT) mRNA compared with the standard. For all samples, the results in terms of gene expression levels were normalized to those of the internal control HPRT. The same procedure was used to measure gene expression of fatty acid synthase (FAS), stearoyl-CoA-desaturase-1 (SCD-1), and l-carnitine-palmitoyl-transferase-1 (CPT-1) in abdominal white adipose tissue. The oligonucleotide-specific primers were as follows: HPRT: up5′-CAGTCCCAGCGTCGTGATTA-3′, lo5′-AGCAAGTCTTTCAGTCCTGTC-3′; ER: up5′-TGTCCAGCTACAAACCAATGCA-3′, lo5′-TCCGTATGCCGCCTTTCAT-3′; db: up5′-GATTTCTTGGGACAGCCAAA-3′, lo5′-CTCTTGCTCCTCACCTGGAC-3′; NPY: up5′-AGGCTTGAAGACCCTTCCAT-3′, lo5′-ACAGGCAGACTGGTTTCAGG-3′; AgRP: up5′-TGTGTAAGGCTGCACGAGTC-3′, lo5′-GGCAGTAGCAAAAGGCATTG-3′; TRH: up5′-GTGCCAACCAAGACAAGGAT-3′, lo5′-TTCTTCCCAGCTTCTTTGGA-3′; CART: up5′-CATGATGCAAGAGGAGCTGA-3′, lo5′-CTTCCCCTGTGATGCTTTGT-3′; MCR-3: up5-AGCAGCCCTGAGTGTCATCT-3′, lo5′-CTTGCCGGAAGTCTAAGCAC-3′; MCR-4: up5′-TCATCTGTAGCCTGGCTG-3′, lo5′-GGTACTGGAGCGCGTAAAAG-3′; FAS: up5′-AGGAGGTGGTGATAGCCGGTAT-3′, lo5′-GGTAATCCATAGAGCCCAGCCT-3′; SCD-1: up5′-GCCCACATGCTCCAAGAGATCT-3′, lo5′-AGGACGGATGTCTTCTTCCAGG-3′; and CPT-1: up5′-CCTGCATTCCTTCCCATTTG-3′, lo5′-CCCATGTCCTTGTAATGTGCG-3′.
Statistical analysis.
Quantitative data are presented as means ± SE. Data were analyzed using one-way ANOVA with Bonferroni post hoc test or two-tailed Student's t-test for unpaired samples (SPSS 11.5, Chicago, IL). Not normally distributed data were calculated using nonparametric Mann-Whitney's U-test. P < 0.05 was considered significant.
RESULTS
OVX induces obesity in wild type but not in Gipr−/− mice.
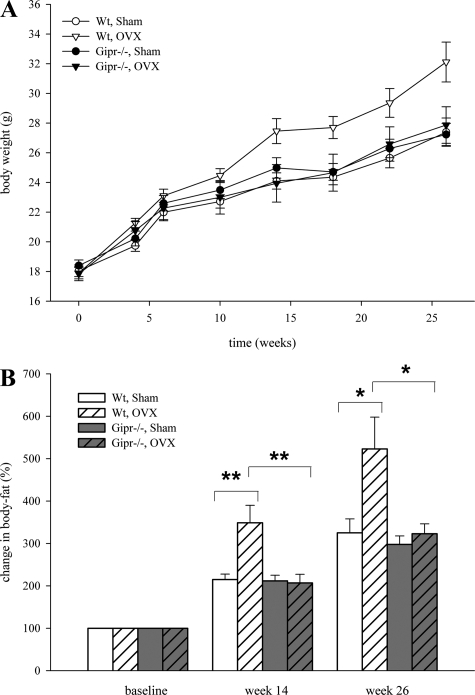
Figure 1A shows the changes in body weight during the experiment. The area under the curve (AUC) of incremental weight over the whole period was significantly increased in wild-type OVX mice (198.8 ± 11.6), compared with the sham group (131.9 ± 9.9; P < 0.01). No difference was detectable in both sham-operated and OVX knockout animals. OVX wild types gained more weight than mice in all other groups and significantly more than OVX Gipr−/− mice (147.2 ± 16.3; P < 0.05). This weight gain was consistent with increased body fat in NMR analysis. Fourteen weeks after surgery a significantly increased body fat was detected in OVX wild-type mice compared with both sham-treated controls (P < 0.01) and OVX knockouts (P < 0.01). Body fat gain remained significantly different until the end of the study in week 26 (P < 0.05; Fig. 1B).
Fig. 1.
Wild-type mice (Wt) and Gipr−/− mice were ovariectomized (OVX) or sham operated (Sham; n = 10 in each group). A: body weight alterations over 26 wk. AUC of body weight during the observation period was significantly increased in wild-type mice. B: NMR analysis of change in body fat; representative results from monthly measurements in weeks 14 and 26 after surgery. Body fat was increased only in wild-type mice after ovariectomy (*P < 0.05; **P < 0.01).
Twenty-four weeks after surgery OVX animals appeared to develop increased insulin resistance as indicated by the product of fasting plasma glucose and insulin concentration (Table 1).
Table 1.
Plasma glucose and insulin after over night fasting (24 wk after surgery)
| n | Plasma Glucose, mmol/l | Plasma Insulin, pg/ml | Glucose × Insulin, mmol/l × pg/ml | |
|---|---|---|---|---|
| WT, Sham | 10 | 7.34±0.27 | 182.47±41.28 | 1375.4±340.8 |
| WT, OVX | 10 | 8.76±0.70 | 327.25±46.83* | 2,840.9±406.9* |
| Gipr−/−, Sham | 9 | 6.96±0.23 | 175.16±51.36 | 1,216.2±353.2 |
| Gipr−/−, OVX | 10 | 7.16±0.30 | 251.56±33.29 | 1,825.1±194.0† |
WT, wild type; Gipr−/−, GIP receptor knockout; Sham, sham-treated group; OVX, ovariectomized group.
P < 0.05, WT OVX vs. WT Sham;
P < 0.05, Gipr−/− OVX vs. WT OVX.
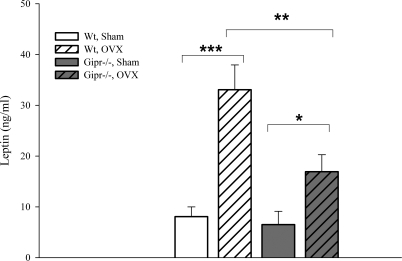
Plasma leptin significantly increased in both ovariectomized wild-type (33.1 ± 4.89 vs. 8.1 ± 1.92 ng/ml; P < 0.001) and Gipr−/− (16.9 ± 3.35 vs. 6.5 ± 2.62 ng/ml; P < 0.05) mice. Estrogen-lacking Gipr−/− animals showed significantly lower leptin levels compared with wild-type mice (P < 0.01; Fig. 2).
Fig. 2.
Plasma leptin levels 24 wk after surgery in fed mice (*P < 0.05; **P < 0.01; ***P < 0.001).
Uterine weight was used as an indicator for successful OVX surgery. Both in OVX wild-type mice (38.2 ± 3.99 mg) and in OVX Gipr−/− (35.8 ± 2.55 mg) there was a significant atrophy of the uterus compared with the sham-operated controls (206.6 ± 11.22 mg and 201.1 ± 17.8 mg; P < 0.001).
Reduced TEE after OVX.
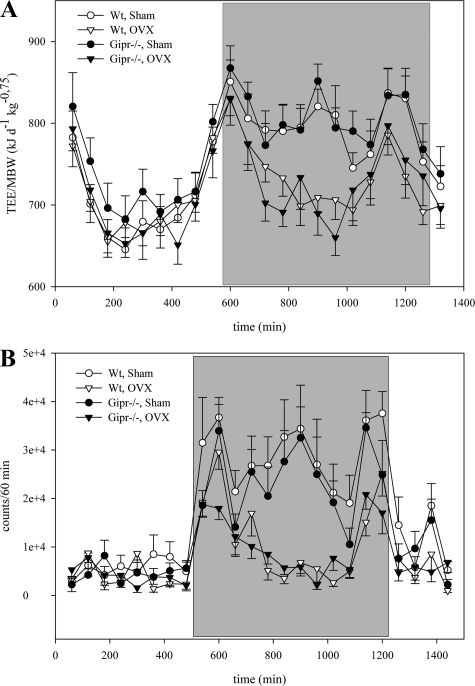
TEE was measured 22 wk after surgery. Because TEE is a function of body weight, data were normalized to metabolic body weight. Both wild-type mice (798.5 ± 19.9 vs. 735.9 ± 14.8 kJ·d−1·kg−0.75) and knockout animals (809.0 ± 21.5 vs. 735.3 ± 22.7 kJ·d−1·kg−0.75) showed a reduced TEE during the dark phase after OVX (P < 0.05; Figure 3 A). There was no difference in TEE during resting at the light phase, indicating a potential dependence of this energy expenditure phenomenon on activity induced thermogenesis.
Fig. 3.
A: total energy expenditure (TEE) 22 wk after surgery was decreased in both wild-type and Gipr−/− mice in the dark phase (shaded section). TEE was normalized for metabolic weight (MBW). B: representative analyses of spontaneous locomotor activity of 24-h section during 5-day measurement. During dark phase (shaded part), activity was decreased in ovariectomized wild-type and Gipr−/− compared with sham-treated animals.
Reduced spontaneous locomotor activity after OVX.
We therefore measured spontaneous physical activity in the home cage environment. OVX reduced locomotor activity during the dark phase in both wild-type mice (145,082 ± 16,078 vs. 351,306 ± 53,196 counts; P < 0.01) and knockout animals (132,167 ± 15,883 vs. 287,056 ± 25,966 counts; P < 0.001). Figure 3B presents the results of a 24-h section during the 5-day measurement. This difference remained significant over 24 h (P < 0.05), although there was no significant difference during the light phase.
Reduced food intake in OVX Gipr−/− mice.
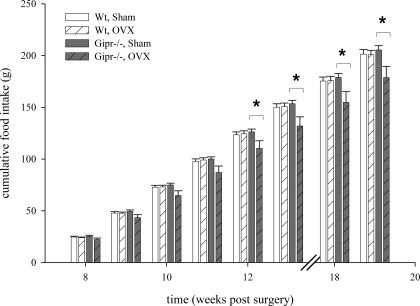
Cumulative food intake was recorded for a total period of 8 wk (Fig. 4) between weeks 8 and 19 after surgery. The amount of chow was assessed weekly. Cumulative food intake in OVX Gipr−/− mice was significantly lower compared with sham-treated animals (P < 0.05), beginning from week 12 after surgery, and remained significantly different throughout the recorded period. No difference in food intake was found between OVX and sham-operated wild types (P = 1.00).
Fig. 4.
Cumulative food intake rate was recorded for a total of 8 wk in the period between weeks 8 and 19 after surgery. Food consumption was reduced in ovarectomized Gipr−/− mice from week 12 after surgery (P < 0.05).
Differential expression pattern of orexigenic and anorexigenic neuropeptides.
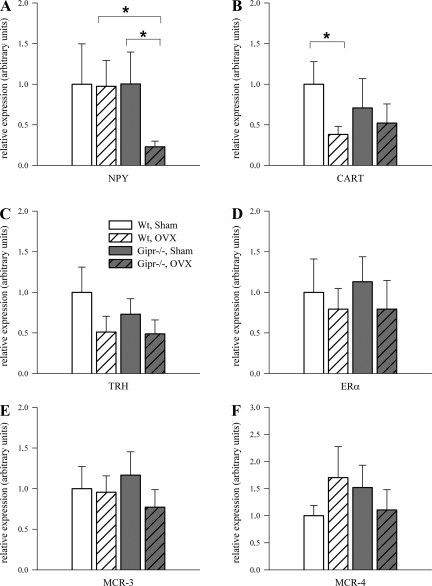
Quantitative PCR was performed to investigate whether differences in food intake were associated with altered expression of hypothalamic orexigenic and anorexigenic factors. The relative expression of orexigenic NPY was reduced by 77% in OVX Gipr−/− mice vs. OVX controls (P < 0.05; Fig. 5A).
Fig. 5.
Real-time RT-PCR analysis of orexigenic and anorexigenic factors in hypothalamus. Results were normalized to internal control hypoxanthine-guanine phosphoribosyltransferase (HPRT), and the wild-type sham-treated group was set to 100% (n = 9–10 each group). A: neuropeptide Y (NPY) mRNA expression was reduced in ovariectomized Gipr−/− vs. sham-treated controls and ovariectomized wild-type mice, P < 0.05. B: cocaine-amphetamine-related transcript (CART) expression level was reduced after ovariectomy in wild-type animals only (P < 0.05). C: in wild-type animals thyrotropin-releasing hormone (TRH) tended to decrease after ovariectomy (P = 0.07). Estrogen receptor (ER)-α (D) and melanocortin receptors (E: MCR-3; F: MCR-4) mRNA expression were not different between genotypes.
The mRNA level of CART was decreased by 62% in OVX wild types (P < 0.05) but was not significantly changed in knockout animals (Fig. 5B). Expression of TRH, which acts as another anorexigenic factor (14), was downregulated although not statistically significant in OVX wild types (1.00 ± 0.31 vs. 0.51 ± 0.19; P = 0.07) and was unchanged in OVX Gipr−/− animals (0.73 ± 0.19 vs. 0.49 ± 0.17; P = 0.19; Fig. 5C). No significant alteration in the hypothalamic expression levels of ER-α (Fig. 5D), MCR-3 (Fig. 5E), and MCR-4 (Fig. 5F) were detected, as well as in expression levels of AgRP and leptin receptor (data not shown).
We also found no significant differences in gene expression pattern of FAS, SCD-1, and CPT-1 in white adipose tissue (data not shown).
DISCUSSION
The health consequences of obesity include increased risk of heart disease, hypertension, diabetes, sleep apnea, cancer, osteoarthritis, and mental health problems. Postmenopausal women tend to gain body fat, placing them at a higher risk for the disorders described above. The increase in adiposity in that situation seems to be a consequence of the decline in endogenous estrogens and the reduced energy expenditure. A better understanding of the mechanisms involved in increased fat mass in the postmenopausal state might be very helpful to face this problem.
In the present study, we report novel data showing that GIP receptor knockout prevents OVX-induced obesity in mice. Reduced energy expenditure and spontaneous locomotor activity in OVX animals were in agreement with previous studies (5), and this was independent of the GIP genotype. However, compared with OVX wild-type animals, cumulative food intake in OVX Gipr−/− mice was significantly lower. Consequently, body weight and fat mass markedly increased in OVX wild types but not in OVX Gipr−/− animals, compared with sham-operated controls, and were associated with significantly increased markers of insulin resistance in OVX wild-type mice. The increased plasma leptin levels in OVX animals most likely reflected increased fat mass, which is in agreement with previous studies (9). The decreased food intake in OVX Gipr−/− mice is an unexpected and important novel result because previous studies (19) have shown that there was no significant difference in food intake between WT and Gipr−/− mice. Furthermore, it has been also reported that the intracerebroventricular administration of GIP does not affect food intake in non-OVX animals (11, 32), indicating that there is little effect of GIP on feeding behavior in estrogenized animals. The decreased food intake uncovered here in OVX Gipr−/− mice suggests that Gipr may be a relevant signal mediating feeding under specific physiological conditions. Interestingly, and in contrast to previous studies (13), Nyberg et al. (21) showed the presence of both Gipr and GIP protein in the rodent brain. GIP seems to play an important role in the brain, as it was found that GIPR−/− mice have decreased brain volumes. Among the several brain areas expressing GIP, the hypothalamus was one of them (21). The hypothalamus is the major brain center that regulates food intake and body weight. Many neuropeptides, some of the arguably most important of which are NPY, AgRP, CART, melanocortin receptors, and TRH have been shown to control energy homeostasis by modulating energy intake or energy expenditure (26). Gene expression analysis of hypothalamic orexigenic and anorexigenic factors in the present study exhibited changes that could contribute to explaining the observed differences. Hypothalamic NPY mRNA was markedly reduced in OVX Gipr−/− mice but that phenomenon was neither observed in sham-operated Gipr−/− animals nor in OVX wild-type controls. NPY is one of the most abundant neurotransmitters in the brain (2), which, when secreted or activated, potently increases food intake and reduces energy expenditure (28). Vice versa, in the absence of NPY, ob/ob mice are less obese due to reduced food intake and increased energy expenditure (7). Even though a causal relationship cannot be stated, reduced expression of hypothalamic NPY mRNA in the present study might have contributed to reduced food intake in OVX Gipr−/− mice. No difference was observed in the hypothalamic expression of the leptin receptor, which is in agreement with previous studies (1).
To our knowledge, this is the first study quantifying hypothalamic CART mRNA levels in this model, and we observed a significant reduction of CART mRNA in OVX vs. sham-operated wild types. This could be expected to increase food intake. However, the mRNA expression of the anorexigenic hypothalamic neuropeptide CART was not significantly altered in both OVX and sham-operated knockout animals, and higher circulating insulin in OVX wild types might have prevented a further increase in food intake via central action (26). In addition to CART, we also measured TRH, another hypothalamic neuropeptide that has, apart from its role as a central nervous system releasing hormone controlling thyroid metabolism, known effects on food intake and locomotor activity (31). TRH and CART are closely neuronally connected between the brainstem and the hypothalamus (8). For instance, CART-immunoreactive axons densely innervate the majority of hypophysiotropic TRH-containing neurons in the hypothalamic paraventricular nucleus and establish asymmetric synaptic connections with the TRH neurons (8). The expression of other neuropeptides with a crucial role in food intake such as MCR-3, MCR-4, or ER-α was not significantly altered. Therefore, we hypothesize that the observed similar expression pattern of TRH and CART mRNA in the present study with a tendency to decreased mRNA levels in wild-type OVX animals but not in OVX Gipr−/− mice together with reduced NPY expression in OVX Gipr−/− mice might explain the different food intake between wild-type and Gipr−/− OVX groups.
Apart from food intake and energy expenditure, nutrient partitioning is another well-known key factor in the regulation of body weight and could have contributed to differences in body weight and fat mass between sham and OVX mice, with potential differences in fat being preferentially directed to either oxidative processes or to triglyceride storage in white adipose tissue. To test this hypothesis, we measured the expression of several key enzymes involved in lipid metabolism in white adipose tissue and found that the expression levels of FAS, SCD-1, and CPT-1 were not significantly different between groups. Thus our results indicate that the changes observed here in body weight and fat mass were a consequence of changes in food intake behavior, rather than of alterations in nutrient partitioning.
Taken together, we demonstrate that Gipr knockout prevents OVX-induced obesity in mice. The exact mechanisms of how estrogens, GIP, and the different neuropeptides functions might be linked at a molecular level need to be investigated in future studies. Challenging OVX Gipr−/− mice with other, i.e., high-fat, diets might be of further interest. Although our data do not necessarily apply to humans because of known differences in female cycle and different diurnal rhythms (3), the data presented here indicate that endogenous GIP signaling might exert unfavorable effects on body weight, fat mass, and insulin resistance in gonadal steroid deficiency.
GRANTS
This work was supported in part by Deutsche Forschungsgemeinschaft Grant PF 164/14-1 (to F. Isken, A. F. H. Pfeiffer, M. A. Osterhoff, and M. O. Weickert) and National Institute of Diabetes and Digestive and Kidney Diseases Grant 1RO1-DK-069987 (to M. H. Tschöp). R. Nogueiras is currently a fellow of the Marie Curie Foundation.
Acknowledgments
We thank Susann Richter, Kerstin Weinert, and Carola Plaue for excellent technical assistance.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Ainslie DA, Morris MJ, Wittert G, Turnbull H, Proietto J, Thorburn AW. Estrogen deficiency causes central leptin insensitivity and increased hypothalamic neuropeptide Y. Int J Obes Relat Metab Disord 25: 1680–1688, 2001. [DOI] [PubMed] [Google Scholar]
- 2.Allen YS, Adrian TE, Allen JM, Tatemoto K, Crow TJ, Bloom SR, Polak JM. Neuropeptide Y distribution in the rat brain. Science 221: 877–879, 1983. [DOI] [PubMed] [Google Scholar]
- 3.Bailey KJ Diurnal progesterone rhythms in the female mouse. J Endocrinol 112: 15–21, 1987. [DOI] [PubMed] [Google Scholar]
- 4.Clegg DJ, Brown LM, Woods SC, Benoit SC. Gonadal hormones determine sensitivity to central leptin and insulin. Diabetes 55: 978–987, 2006. [DOI] [PubMed] [Google Scholar]
- 5.Colvin GB, Sawyer CH. Induction of running activity by intracerebral implants of estrogen in overiectomized rats. Neuroendocrinology 4: 309–320, 1969. [DOI] [PubMed] [Google Scholar]
- 6.Ding KH, Zhong Q, Xie D, Chen HX, Della-Fera MA, Bollag RJ, Bollag WB, Gujral R, Kang B, Sridhar S, Baile C, Curl W, Isales CM. Effects of glucose-dependent insulinotropic peptide on behavior. Peptides 27: 2750–2755, 2006. [DOI] [PubMed] [Google Scholar]
- 7.Erickson JC, Hollopeter G, Palmiter RD. Attenuation of the obesity syndrome of ob/ob mice by the loss of neuropeptide Y. Science 274: 1704–1707, 1996. [DOI] [PubMed] [Google Scholar]
- 8.Fekete C, Lechan RM. Neuroendocrine implications for the association between cocaine- and amphetamine regulated transcript (CART) and hypophysiotropic thyrotropin-releasing hormone (TRH). Peptides 27: 2012–2018, 2006. [DOI] [PubMed] [Google Scholar]
- 9.Frederich RC, Hamann A, Anderson S, Lollmann B, Lowell BB, Flier JS. Leptin levels reflect body lipid content in mice: evidence for diet-induced resistance to leptin action. Nat Med 1: 1311–1314, 1995. [DOI] [PubMed] [Google Scholar]
- 10.Gale SK, Van Itallie TB. Genetic obestiy: estrogenic influences on the body weight and food intake of lean and obese adult Zucker (fa/fa) rats. Physiol Behav 23: 111–120, 1979. [DOI] [PubMed] [Google Scholar]
- 11.Irwin N, Hunter K, Flatt PR. Comparison of the metabolic effects of GIP receptor antagonism and PYY(3–36) receptor activation in high fat fed mice. Peptides 28: 2192–2198, 2007. [DOI] [PubMed] [Google Scholar]
- 12.Kamei Y, Suzuki M, Miyazaki H, Tsuboyama-Kasaoka N, Wu J, Ishimi Y, Ezaki O. Ovariectomy in mice decreases lipid metabolism-related gene expression in adipose tissue and skeletal muscle with increased body fat. J Nutr Sci Vitaminol (Tokyo) 51: 110–117, 2005. [DOI] [PubMed] [Google Scholar]
- 13.Kaplan AM, Vigna SR. Gastric inhibitory polypeptide (GIP) binding sites in rat brain. Peptides 15: 297–302, 1994. [DOI] [PubMed] [Google Scholar]
- 14.Lechan RM, Fekete C. The TRH neuron: a hypothalamic integrator of energy metabolism. Prog Brain Res 153: 209–235, 2006. [DOI] [PubMed] [Google Scholar]
- 15.Mayes JS, Watson GH. Direct effects of sex steroid hormones on adipose tissues and obesity. Obes Rev 5: 197–216, 2004. [DOI] [PubMed] [Google Scholar]
- 16.McElroy JF, Wade GN. Short- and long-term effects of ovariectomy on food intake, body weight, carcass composition, and brown adipose tissue in rats. Physiol Behav 39: 361–365, 1987. [DOI] [PubMed] [Google Scholar]
- 17.Meier JJ, Nauck MA. Clinical endocrinology and metabolism. Glucose-dependent insulinotropic polypeptide/gastric inhibitory polypeptide. Best Pract Res Clin Endocrinol Metab 18: 587–606, 2004. [DOI] [PubMed] [Google Scholar]
- 18.Mitra SW, Hoskin E, Yudkovitz J, Pear L, Wilkinson HA, Hayashi S, Pfaff DW, Ogawa S, Rohrer SP, Schaeffer JM, McEwen BS, Alves SE. Immunolocalization of estrogen receptor beta in the mouse brain: comparison with estrogen receptor alpha. Endocrinology 144: 2055–2067, 2003. [DOI] [PubMed] [Google Scholar]
- 19.Miyawaki K, Yamada Y, Ban N, Ihara Y, Tsukiyama K, Zhou H, Fujimoto S, Oku A, Tsuda K, Toyokuni S, Hiai H, Mizunoya W, Fushiki T, Holst JJ, Makino M, Tashita A, Kobara Y, Tsubamoto Y, Jinnouchi T, Jomori T, Seino Y. Inhibition of gastric inhibitory polypeptide signaling prevents obesity. Nat Med 8: 738–742, 2002. [DOI] [PubMed] [Google Scholar]
- 20.Nogueiras R, Barreiro ML, Caminos JE, Gaytan F, Suominen JS, Navarro VM, Casanueva FF, Aguilar E, Toppari J, Dieguez C, Tena-Sempere M. Novel expression of resistin in rat testis: functional role and regulation by nutritional status and hormonal factors. J Cell Sci 117: 3247–3257, 2004. [DOI] [PubMed] [Google Scholar]
- 21.Nyberg J, Anderson MF, Meister B, Alborn AM, Strom AK, Brederlau A, Illerskog AC, Nilsson O, Kieffer TJ, Hietala MA, Ricksten A, Eriksson PS. Glucose-dependent insulinotropic polypeptide is expressed in adult hippocampus and induces progenitor cell proliferation. J Neurosci 25: 1816–1825, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Preitner F, Ibberson M, Franklin I, Binnert C, Pende M, Gjinovci A, Hansotia T, Drucker DJ, Wollheim C, Burcelin R, Thorens B. Gluco-incretins control insulin secretion at multiple levels as revealed in mice lacking GLP-1 and GIP receptors. J Clin Invest 113: 635–645, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ranganath L, Sedgwick I, Morgan L, Wright J, Marks V. The ageing entero-insular axis. Diabetologia 41: 1309–1313, 1998. [DOI] [PubMed] [Google Scholar]
- 24.Regitz-Zagrosek V, Lehmkuhl E, Weickert MO. Gender differences in the metabolic syndrome and their role for cardiovascular disease. Clin Res Cardiol 95: 136–147, 2006. [DOI] [PubMed] [Google Scholar]
- 25.Ristow M, Mulder H, Pomplun D, Schulz TJ, Muller-Schmehl K, Krause A, Fex M, Puccio H, Muller J, Isken F, Spranger J, Muller-Wieland D, Magnuson MA, Mohlig M, Koenig M, Pfeiffer AF. Frataxin deficiency in pancreatic islets causes diabetes due to loss of beta cell mass. J Clin Invest 112: 527–534, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schwartz MW, Woods SC, Porte D Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature 404: 661–671, 2000. [DOI] [PubMed] [Google Scholar]
- 27.Shimomura Y, Shimizu H, Takahashi M, Sato N, Uehara Y, Fukatsu A, Negishi M, Kobayashi I, Kobayashi S. The significance of decreased ambulatory activity during the generation by long-term observation of obesity in ovariectomized rats. Physiol Behav 47: 155–159, 1990. [DOI] [PubMed] [Google Scholar]
- 28.Stanley BG, Willett VL 3rd, Donias HW, Ha LH, Spears LC. The lateral hypothalamus: a primary site mediating excitatory amino acid-elicited eating. Brain Res 630: 41–49, 1993. [DOI] [PubMed] [Google Scholar]
- 29.Sztefko K, Rogatko I, Milewicz T, Jozef K, Tomasik PJ, Szafran Z. Effect of hormone therapy on the enteroinsular axis. Menopause 12: 630–638, 2005. [DOI] [PubMed] [Google Scholar]
- 30.Tchernof A, Desmeules A, Richard C, Laberge P, Daris M, Mailloux J, Rheaume C, Dupont P. Ovarian hormone status and abdominal visceral adipose tissue metabolism. J Clin Endocrinol Metab 89: 3425–3430, 2004. [DOI] [PubMed] [Google Scholar]
- 31.Wei E, Sigel S, Loh H, Way EL. Thyrotrophin-releasing hormone and shaking behaviour in rat. Nature 253: 739–740, 1975. [DOI] [PubMed] [Google Scholar]
- 32.Woods SC, West DB, Stein LJ, McKay LD, Lotter EC, Porte SG, Kenney NJ, Porte D Jr. Peptides and the control of meal size. Diabetologia 20: 305–313, 1981. [PubMed] [Google Scholar]
- 33.Yip RG, Wolfe MM. GIP biology and fat metabolism. Life Sci 66: 91–103, 2000. [DOI] [PubMed] [Google Scholar]
- 34.Zhou H, Yamada Y, Tsukiyama K, Miyawaki K, Hosokawa M, Nagashima K, Toyoda K, Naitoh R, Mizunoya W, Fushiki T, Kadowaki T, Seino Y. Gastric inhibitory polypeptide modulates adiposity and fat oxidation under diminished insulin action. Biochem Biophys Res Commun 335: 937–942, 2005. [DOI] [PubMed] [Google Scholar]