Abstract
Exenatide is a long-acting glucagon-like peptide-1 (GLP-1) mimetic used in the treatment of type 2 diabetes. There is increasing evidence that GLP-1 can influence glycemia not only via pancreatic (insulinotropic and glucagon suppression) and gastric-emptying effects, but also via an independent mechanism mediated by portal vein receptors. The aim of our study was to investigate whether exenatide has an islet- and gastric-independent glycemia-reducing effect, similar to GLP-1. First, we administered mixed meals, with or without exenatide (20 μg sc) to dogs. Second, to determine whether exenatide-induced reduction in glycemia is independent of slower gastric emptying, in the same animals we infused glucose intraportally (to simulate meal test glucose appearance) with exenatide, exenatide + the intraportal GLP-1 receptor antagonist exendin-(9-39), or saline. Exenatide markedly decreased postprandial glucose: net 0- to 135-min area under the curve = +526 ± 315 and −536 ± 197 mg·dl−1·min−1 with saline and exenatide, respectively (P < 0.05). Importantly, the decrease in plasma glucose occurred without a corresponding increase in postprandial insulin but was accompanied by delayed gastric emptying and lower glucagon. Significantly lower glycemia was induced by intraportal glucose infusion with exenatide than with saline (92 ± 1 vs. 97 ± 1 mg/dl, P < 0.001) in the absence of hyperinsulinemia or glucagon suppression. The exenatide-induced lower glycemia was partly reversed by intraportal exendin-(9-39): 95 ± 3 and 92 ± 3 mg/dl with exenatide + antagonist and exenatide, respectively (P < 0.01). Our results suggest that, similar to GLP-1, exenatide lowers glycemia via a novel mechanism independent of islet hormones and slowing of gastric emptying. We hypothesize that receptors in the portal vein, via a neural mechanism, increase glucose clearance independent of islet hormones.
Keywords: glucagon-like peptide-1, insulin-independent, glucose regulation
exendin-4 is a natural 39-amino acid peptide present in the salivary gland of the Gila monster lizard (Heloderma suspectum). The peptide has powerful effects on glucose homeostasis in mammals (15). The effects of exendin-4 are attributable to ∼50% amino acid sequence identity with the natural hormone glucagon-like peptide-1 (GLP-1), which is released by intestinal L cells in response to meal ingestion (14). GLP-1 has a multitude of peripheral and central actions that contribute to glucose tolerance acutely and chronically. The systemic physiological roles for GLP-1 are effects on pancreatic islets (stimulation of insulin secretion and suppression of glucagon release, stimulation of β-cell proliferation, and inhibition of apoptosis) and on the digestive tract (inhibition of gastric emptying and intestinal motility) (for review see Refs. 8 and 18). However, recent evidence suggests a role for GLP-1 in glucose homeostasis beyond the above-mentioned effects. Reports from several laboratories, including our own, suggest that GLP-1 might contribute to increased postprandial glucose disposition via increased muscle glucose utilization and/or inhibition of hepatic glucose output (19, 27). We have hypothesized that the latter effects are mediated via a central mechanism involving receptors in the portohepatic area.
Exenatide, the synthetic form of exendin-4, is used in the treatment of diabetes. Exenatide has been shown to decrease fasting and postprandial glucose in healthy humans and patients with type 2 diabetes in acute and chronic settings (11, 23, 24), as well as patients with type 1 diabetes (10). The effects on postprandial glycemia have been attributed to several mechanisms, including glucagon suppression, delay of gastric emptying, and insulinotropic effects (23, 26, 30). In addition, it is possible that, similar to GLP-1, exendin-4 may have an effect on glucose homeostasis, independent of changes in pancreatic hormones and gastrointestinal motility. In contrast to GLP-1, which has a very short half-life (1.5–2 min) (7), exendin-4 has a longer half-life: 26 min in intravenous administration (11) and up to 4 h after subcutaneous administration (24). Thus, although a potential GLP-1 effect mediated by portal receptors might be evident only with portal increases of GLP-1 (e.g., after increased endogenous release or intraportal administration), because of its longer half-life, exendin-4 might exert an islet-independent effect on glucose homeostasis, even in systemic administration, as it is used in diabetes therapy. This effect could provide an additional benefit for treatment of patients with type 1 or type 2 diabetes.
The aim of the present study was to investigate whether exenatide, administered subcutaneously, can produce a reduction in postprandial glycemia beyond that mediated by islet hormones and gastrointestinal motility. If such an independent effect can be identified, it could provide a new pathway for therapy.
METHODS
Animals
Experiments were performed on mature mongrel dogs (27.7 ± 0.8 kg body wt, n = 8) in the conscious relaxed state. The animals were housed under controlled kennel conditions in the University of Southern California Medical School Vivarium and fed a standard diet (27% protein, 32% fat, and 41% carbohydrate; Labdiet, PMI Nutrition, Richmond, IN) once per day. Dogs were used for experiments only if judged to be in good health as determined by body temperature, hematocrit, regularity of food intake, and direct observation. The National Institutes of Health (NIH) Principles of Laboratory Animal Care (NIH Publication No. 86-23, revised 1985) was followed, and all surgical and experimental procedures were approved by the University of Southern California Institutional Animal Care and Use Committee.
Surgical Procedures
At least 1 wk before the first experiment, chronic catheters (Tygon, Norton Plastics, Akron, OH) were implanted under general anesthesia in the portal vein, 4 cm upstream from the porta hepatis, and in the jugular vein, with the tip advanced into the right atrium for sampling of right atrial mixed venous blood.
Experimental Design
All experiments were performed in the morning after 12–16 h of fasting. In each of eight animals, we performed the following experiments: mixed-meal tests with or without subcutaneous exenatide (protocols 1a and 1b), intraportal glucose infusions to simulate portal glucose absorption, with or without exenatide (protocols 2a and 2c), and (in 5 of the animals) intraportal glucose + exenatide along with intraportal infusion of the exenatide antagonist exendin-(9-39) (protocol 2b).
For all experiments, at approximately 7 AM the animals were brought to the laboratory and placed in a Pavlov sling. After a period of rest, sampling was started at −45 min and continued until 240 min.
Protocols 1a and 1b: mixed meal with or without exenatide.
After basal sampling, at −15 min, an injection of exenatide (20 μg of Byetta; Amylin, San Diego, CA) or saline was administered subcutaneously. An additional sample was taken at −1 min. At time 0, a mixed meal (471 kcal; 9 ml/kg mixture of equal parts of sour cream and 1 M sucrose solution containing 640 mg of acetaminophen) was administered by oral gavage, and sampling was carried out for 240 min. Although the exenatide dose was at the upper limit of the dose used in humans, it was well tolerated; i.e., there were no signs of discomfort or vomiting.
Protocol 2a: intraportal glucose infusion with exenatide injection.
After basal sampling, at −15 min, a subcutaneous injection of 20 μg of exenatide was administered, and another sample was obtained at −1 min. At time 0, an intraportal infusion of glucose (20% hydrated dextrose; B Braun, Irvine, CA) was started and continued for 240 min. The intraportal glucose infusion rate was variable: it was designed to result in glycemia matching the systemic glucose seen during the meal test in protocol 1. This glucose infusion rate was determined in a previous study in which an identical meal test was administered and matching intraportal infusions were performed (19). The glucose infusion rate (mg·kg−1·min−1) was the same for all dogs. Mixed venous blood samples were taken for 240 min.
Protocol 2b: intraportal glucose infusion with exenatide injection and infusion of GLP-1 receptor antagonist exendin-(9-39).
After basal sampling at −15 min, a subcutaneous injection of 20 μg of exenatide was administered. Concomitantly, an intraportal infusion of 75 pmol·kg−1·min−1 exendin-(9-39) (Bachem; dissolved in saline with 0.5% dog albumin) was started at −15 min and infused for 75 min, until t 60 min, when exendin-(9-39) infusion was stopped. At time 0, an intraportal infusion of glucose, at infusion rates identical to protocol 2a, was started and continued for 240 min.
Protocol 2c: intraportal glucose infusion with saline.
This control protocol was identical to protocols 2a and 2b, except intraportal saline was used to replace exenatide and exendin-(9-39).
Blood Sampling and Assays
Samples for determination of glucose, insulin, C-peptide, glucagon, nonesterified fatty acid (NEFA), GLP-1, and acetaminophen were collected into tubes coated with LiF and heparin containing EDTA, EDTA and Trasylol, or EDTA and dipeptidyl peptidase IV inhibitor, as previously described (20). Plasma glucose concentrations were determined using an autoanalyzer (model YSI 2300, Yellow Springs Instruments, Yellow Springs, OH). Insulin was measured using a human ELISA kit (Linco Research, St. Charles, MO) adapted in our laboratory for dog plasma (35). C-peptide and glucagon were measured using RIA (Linco Research). Active GLP-1 was measured by ELISA (Linco Research). NEFA levels were measured with an NEFA C kit (Wako Chemicals, Neuss, Germany). Acetaminophen was measured using ELISA (Immunalysis, Pomona, CA).
Calculations and Statistical Analysis
Values are means ± SE. Two-way repeated-measures ANOVA for comparison of time course data was followed by the Student-Newman-Keuls test for multiple comparisons. Increases from basal levels were analyzed with one-way ANOVA followed by Dunnett's post test. Area under the curve (AUC) was calculated as net AUC above basal (positive peaks − negative peaks). Differences were considered statistically significant when P < 0.05.
RESULTS
Mixed Meal
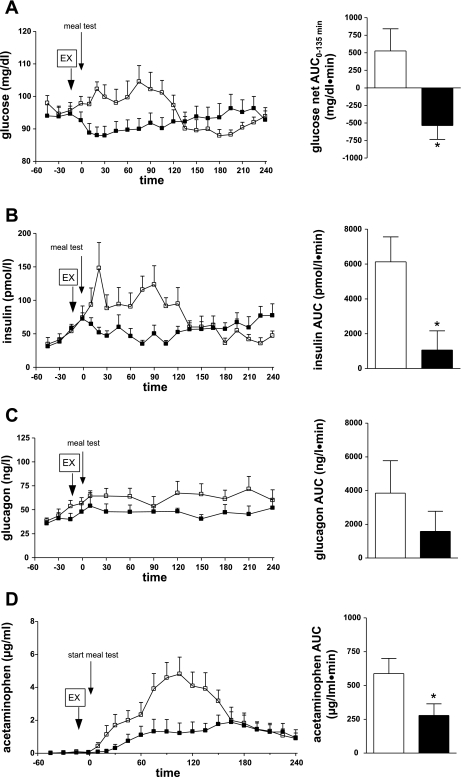
Subcutaneous injection of exenatide 15 min before the meal tended to reduce basal glucose at time 0 (93 ± 3 mg/dl) compared with 98 ± 2 mg/dl after saline (P = 0.07; Fig. 1A). There were no differences with or without exenatide in premeal insulin (73 ± 9 vs. 74 ± 17 pmol/l, P = 0.93; Fig. 1B) or glucagon (57 ± 6 vs. 48 ± 8 ng/l, P = 0.12; Fig. 1C). Neither GLP-1 nor NEFA was changed by exenatide administration in the fasted state (Table 1).
Fig. 1.
Glucose (A), insulin (B), glucagon (C), and acetaminophen (D) plasma concentrations (left) and area under the curve (AUC, right) during meal test with exenatide (EX; ▪, solid bars) or without exenatide (□, open bars). *P < 0.05.
Table 1.
C-peptide, NEFA, and GLP-1 during meal test with exenatide or saline
| Basal |
Meal Test |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| −1 min | 10 min | 20 min | 30 min | 60 min | 90 min | 120 min | 240 min | ||
| C-peptide, nmol/l | |||||||||
| Sal | 0.11±0.02 | 0.15±0.04 | 0.17±0.05 | 0.24±0.04 | 0.18±0.03 | 0.22±0.04 | 0.21±0.04 | 0.17±0.04 | 0.12±0.03 |
| Ex | 0.13±0.01 | 0.17±0.01 | 0.15±0.02 | 0.14±0.02 | 0.14±0.01 | 0.13±0.01 | 0.13±0.01 | 0.14±0.02 | 0.14±0.03 |
| NEFA, mmol/l | |||||||||
| Sal | 0.65±0.05 | 0.57±0.09 | 0.42±0.09* | 0.36±0.10† | 0.26±0.09† | 0.52±0.08† | 0.19±0.06† | 0.18±0.05† | 0.41±0.07† |
| Ex | 0.67±0.04 | 0.56±0.07 | 0.55±0.06 | 0.45±0.04† | 0.46±0.05† | 0.45±0.06† | 0.48±0.07†‡ | 0.48±0.07†‡ | 0.26±0.07† |
| GLP-1, pmol/l | |||||||||
| Sal | 15±5 | 13±8 | 14±8 | 21±10 | 23±12* | 19±10 | 16±9 | 15±9 | 15±9 |
| Ex | 11±3 | 11±6 | 11±6 | 11±6 | 11±6 | 10±6 | 12±7 | 11±6 | 11±6 |
Values are means ± SE. NEFA, nonesterified fatty acid; GLP-1, glucagon-like peptide-1 Ex, exenatide (20 μg, 15 min before test meal); Sal, saline. Significantly different from basal:
P < 0.05;
P < 0.01.
Significantly different from saline: P < 0.05.
Meal administration resulted in significantly different glycemia during experiments with or without exenatide [P < 0.001 (drug-time interaction); Fig. 1A]. In the first half of the postprandial period (0–135 min), we measured moderate hyperglycemia in the saline control, while glucose remained below basal levels in the exenatide experiment (net prandial 0- to 135-min AUC = −536 ± 197 and +526 ± 315 mg·dl−1·min−1 with and without exenatide, respectively, P < 0.05). In the saline control group, glucose increased immediately after the meal. However, with exenatide administration, glucose appearance in blood was significantly delayed. Postprandial glucose level was lower (96 ± 4 and 105 ± 5 mg/dl with exenatide and saline, respectively) and peaked much later (195 vs. 75 min) in the exenatide group. The exenatide-induced reduction in postprandial glycemia was not due to an insulinotropic effect of the peptide (Fig. 1B). As expected, insulin concentration increased in response to the meal test in saline and exenatide groups (P < 0.05). However, insulin levels were 83% lower in the exenatide group than in the saline group, and there was a significant difference between the two experiments [P < 0.001 (drug-time interaction), AUC = 1,064 ± 1,103 and 6,129 ± 1,434 pmol·l−1·min−1 with exenatide and saline, respectively, P < 0.05]. There was a significant direct correlation between all glucose and insulin concentrations in the saline (r2 = 0.77, P < 0.0001) and exenatide (r2 = 0.32, P < 0.05) experiments, suggesting that the lower insulin in the exenatide experiment is due to a lower glucose signal. C-peptide measurements paralleled the insulin data, showing less increase during the exenatide experiment than during the control experiment (Table 1) and, thus, indicating an exenatide-induced decrease in insulin secretion.
During the mixed meal, glucagon did not significantly change from basal in either group (from 46 ± 3 to 66 ± 11 ng/l with saline and from 39 ± 3 to 54 ± 14 ng/l with exenatide, P = 0.2; Fig. 1C). However, glucagon tended to be lower with than without exenatide (AUC = 3,848 ± 1,927 and 1,581 ± 1,192 ng·l−1·min−1 with saline and exenatide, respectively, P = 0.06). Thus administration of exenatide before a mixed meal resulted in glycemic reduction, without a corresponding increase in insulin secretion and with a smaller rise in glucagon, suggesting that other mechanisms, such as delayed gastric emptying, accounted for the hypoglycemic effect.
To investigate the role of gastric emptying in exenatide-induced reduction of postprandial glycemia, we measured plasma appearance of acetaminophen. There was a significant difference between saline and exenatide plasma acetaminophen [P < 0.001 (drug-time interaction); Fig. 1D]. During the control meal, acetaminophen concentration increased immediately. In contrast, exenatide induced a delay in the plasma acetaminophen rise. Additionally, maximum acetaminophen concentration was lower with exenatide (1.9 ± 0.6 and 4.8 ± 1.0 μg/ml with exenatide and saline, respectively, P < 0.05). AUC was reduced 53% with exenatide (278 ± 87 and 588 ± 112 μg·ml−1·min−1 with exenatide and saline, respectively, P < 0.05), indicating a significant exenatide-induced delay in gastric emptying (Fig. 1D).
NEFA levels were significantly suppressed during the meal with or without exenatide; however, there was less suppression of NEFA in the exenatide than in the control experiment, possibly due to lower insulin levels (Table 1).
Plasma active GLP-1 levels increased with meal administration, from a basal level of 15 ± 5 pmol/l to a peak of 23 ± 12 pmol/l at 30 min (P < 0.05; Table 1). In contrast, with exenatide, GLP-1 did not significantly increase from basal level (from 11 ± 3 to 12 ± 7 pmol/l, P = 0.7).
Intraportal Glucose Infusions
Mixed-meal results showed a major effect of exenatide to decrease postprandial glycemia, with no corresponding increase in insulin secretion but with a tendency to lower glucagon, and delay of gastric emptying. To examine whether the hypoglycemic effect of exenatide is maintained in the absence of gastric motility changes, we performed paired experiments (with or without exenatide) in which glucose was infused intraportally at the same rate in both experiments, simulating identical appearance of glucose from the intestinal bed.
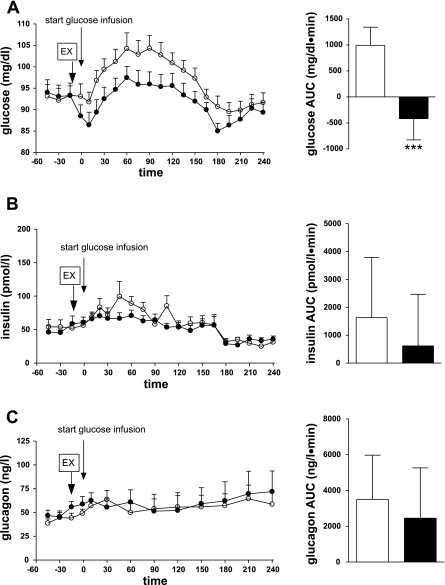
Glycemia decreased with exenatide in the fasting state, before the start of the glucose infusion (from 93 ± 2 to 89 ± 2 mg/dl, P < 0.05; Fig. 2A). Intraportal glucose infusion at identical rates resulted in significantly different glycemia during the saline or exenatide experiments [P < 0.01 (drug-time interaction); Fig. 2A]. In the saline experiment, we measured mild hyperglycemia very similar to the mixed meal: glucose increased from 93 ± 3 to 104 ± 4 mg/dl (P < 0.01 vs. basal) and returned to the basal level at the end of the experiment. Subcutaneous exenatide injection before the glucose infusion considerably reduced systemic glycemia: glucose levels further decreased 10 min after the start of glucose infusion (from 93 ± 2 mg/dl to a nadir of 86 ± 3 mg/dl, P < 0.001 vs. basal). For the duration of the experiment, glycemia was significantly lower with exenatide than with saline (AUC = 992 ± 349 vs. −419 ± 407 mg·dl−1·min−1, P < 0.001; Fig. 2A).
Fig. 2.
Glucose (A), insulin (B), and glucagon (C) plasma concentrations (left) and 0- to 240-min AUC (right) during intraportal glucose infusion with exenatide (•, solid bars) or without exenatide (○, open bars). ***P < 0.001.
The lower glycemia measured in the exenatide experiment was not a result of increased insulin secretion, since there was no significant difference in plasma insulin with or without exenatide [P = 0.23 (drug-time interaction); Fig. 2B]. Without exenatide, insulin significantly increased from a basal level of 53 ± 6 pmol/l to a maximum of 99 ± 23 pmol/l (P < 0.01); with exenatide, there was no significant insulin increase: from a basal level of 50 ± 5 pmol/l to a maximum of 71 ± 8 pmol/l (P = 0.09). There was no statistically significant difference between the insulin AUC with saline and exenatide: 1,631 ± 2,161 and 619 ± 1,853 pmol·l−1·min−1, respectively (P = 0.71; Fig. 2B). The insulin results were paralleled by C-peptide measurements, which showed significantly increased insulin secretion in the saline but not the exenatide group (Table 2) and no significant difference between saline and exenatide [P = 0.27 (drug-time interaction)]. Since glycemia was significantly lower during exenatide treatment, no difference in insulin secretion suggests an increased insulin response to glucose in the exenatide-treated group; however, the ratios of insulin to glucose (0.58 ± 0.04 and 0.61 ± 0.06 pM·mg−1·dl−1 with exenatide and saline, respectively, P = 0.26) and C-peptide to glucose (1.53 ± 0.10 and 1.45 ± 0.10 nM·mg−1·dl−1 with exenatide and saline, respectively, P = 0.28) were not significantly higher in the exenatide group.
Table 2.
C-peptide, NEFA, and GLP-1 during intraportal glucose infusion with exenatide or saline
| Basal |
Glucose Infusion |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| −1 min | 10 min | 20 min | 30 min | 60 min | 90 min | 120 min | 240 min | ||
| C-peptide, nmol/l | |||||||||
| Sal | 0.11±0.01 | 0.14±0.02 | 0.14±0.02 | 0.17±0.04* | 0.15±0.03 | 0.21±0.03† | 0.15±0.03 | 0.13±0.03 | 0.09±0.03 |
| Ex | 0.13±0.02 | 0.16±0.03 | 0.16±0.04 | 0.18±0.04 | 0.16±0.03 | 0.16±0.02 | 0.15±0.03 | 0.14±0.03 | 0.09±0.03 |
| NEFA, mmol/l | |||||||||
| Sal | 0.69±0.06 | 0.64±0.08 | 0.70±0.010 | 0.60±0.09 | 0.56±0.10 | 0.45±0.09† | 0.38±0.07† | 0.37±0.05† | 0.66±0.08 |
| Ex | 0.67±0.04 | 0.55±0.08 | 0.59±0.10 | 0.56±0.06 | 0.48±0.06* | 0.40±0.05† | 0.39±0.08† | 0.37±0.04† | 0.65±0.09 |
| GLP-1, pmol/l | |||||||||
| Sal | 13±5 | 14±9 | 13±8 | 13±8 | 13±8 | 12±8 | 13±9 | 12±8 | 12±8 |
| Ex | 13±4 | 13±8 | 12±8 | 12±8 | 12±8 | 12±8 | 13±8 | 13±9 | 12±8 |
Values are means ± SE. Significantly different from basal:
P < 0.05;
P < 0.01
Suppression of glucagon was not involved in the hypoglycemic effect of exenatide: glucagon levels were similar during experiments with or without exenatide (Fig. 2C). Glucagon concentration changed slightly and nonsignificantly from the basal level in both experiments (from 42 ± 3 to 64 ± 10 ng/l with saline and from 49 ± 3 to 72 ± 22 ng/l with exenatide, P = 0.69), and there was no significant glucagon difference between saline and exenatide [P = 0.67 (drug-time interaction); Fig. 2C].
There were no significant differences in NEFA or GLP-1 between matched intraportal glucose infusions with or without exenatide (Table 2). However, in the exenatide experiment, NEFA levels were suppressed earlier (30 and 60 min with exenatide and saline, respectively), suggesting increased insulin sensitivity.
Effect of Antagonist Exendin-(9-39)
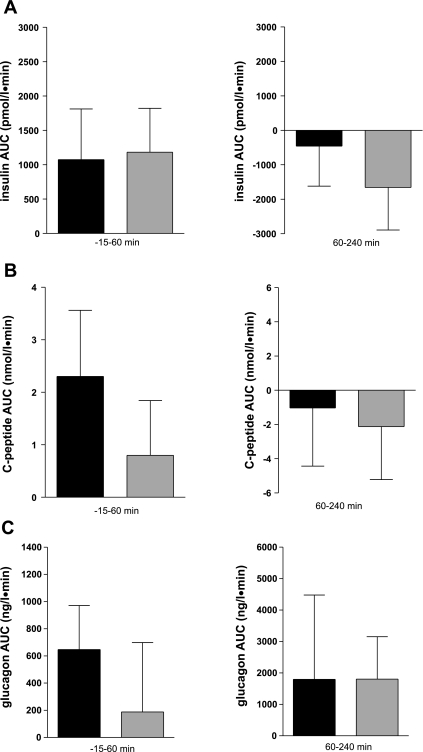
To investigate whether the exenatide-induced reduction of glycemia is mediated via a pancreatic-type portal GLP-1 receptor, in a separate experiment, after exenatide injection, we intraportally infused glucose (for 240 min) and exendin-(9-39) (from 0 to 60 min). Exendin-(9-39) partly reversed exenatide's hypoglycemic effect (Fig. 3A). Peripheral glycemia was significantly higher during antagonist administration than during exenatide experiments (95 ± 3 vs. 92 ± 3 mg/dl, P < 0.01), although it did not reach the levels of the saline control (99 ± 3 mg/dl; Fig. 3A). In contrast, after the antagonist infusion was stopped, glucose decreased to values significantly lower than the saline control (92 ± 3 vs. 96 ± 2 mg/dl, P < 0.05) and was not different from the level attained during the exenatide experiments (92 ± 3 vs. 91 ± 2 mg/dl, P = 0.43; Fig. 3A). The effects of antagonist administration are more evident in the AUC analysis during and after antagonist infusion (Fig. 3B). During antagonist administration, there was a statistically significant increase in the glucose AUC (−15- to 60-min AUC = −142 ± 63 and 51 ± 33 mg·dl−1·min−1 with exenatide and antagonist, respectively, P < 0.05); after antagonist infusion was stopped at 60 min, glucose reverted to levels that were not different from the exenatide experiment (60- to 240-min AUC = −98 ± 92 and −277 ± 362 mg·dl−1·min−1 with antagonist and exenatide, respectively, P = 0.71).
Fig. 3.
A: peripheral glycemia during exenatide (□), exenatide + antagonist (•), or saline (▴) infusion. For exenatide + antagonist, solid horizontal bar (−15 to 60 min) indicates antagonist exendin-(9-39) infusion, which was stopped at 60 min. B: glucose AUC during exenatide (solid bars) or exenatide + antagonist (gray bars) infusion from −15 to 60 min in the presence of antagonist (left) or from 60–240 min without antagonist (right). *P < 0.05.
Changes in glycemia during infusion of the antagonist exendin-(9-39) were independent of changes of pancreatic hormones (Fig. 4). There were no differences in plasma insulin concentration (Fig. 4A) or insulin secretion, as indicated by C-peptide concentrations between groups with or without the antagonist (Fig. 4B). Additionally, glucagon levels were similar in the presence and absence of exendin-(9-39) (Fig. 4C).
Fig. 4.
Insulin (A), C-peptide (B), and glucagon (C) AUC during exenatide (solid bars) or exenatide + antagonist (gray bars) infusion from −15 to 60 min in the presence of antagonist (left) or from 60 to 240 min without antagonist (right).
Active GLP-1 was not significantly increased from the basal level during exenatide treatment with (from 7 ± 3 to 7 ± 3 pmol/l, P = 0.49) or without (from 13 ± 8 to 12 ± 8 pmol/l, P = 0.23) antagonist. Exendin-(9-39) did not change NEFA levels, which were similar with and without antagonist (data not shown).
DISCUSSION
Exenatide is widely used in the treatment of type 2 diabetes, because it lowers glycemia and improves glucose tolerance, decreases HbA1c, induces weight loss, and stimulates β-cell proliferation and neogenesis (9). The mechanisms by which exenatide and its natural hormone counterpart GLP-1 exert these effects are not completely understood. Our present results show that exenatide lowers glycemia, in part, via a novel effect that is independent of changes in pancreatic hormones or gastrointestinal motility.
Administration of exenatide before a mixed meal resulted in lower glycemia than the saline control. Interestingly, this lower glycemia was evident without a corresponding increase in insulin secretion. Exendin-4- and GLP-1-induced reduction of postprandial glycemia has been noted in healthy humans (11) and in patients with type 1 (10) and type 2 (23) diabetes. The absence of a corresponding insulin increase in our study, as well as in those cited above, is presumably due to a lower glycemic signal. This conjecture is supported by the direct correlation between glucose and insulin levels and by the timing of glycemic increase and insulin increase in the mixed meals with and without exenatide (Fig. 1A). In the absence of a corresponding increase in insulin secretion, the postprandial hypoglycemic effect of exenatide has been explained by glucagon suppression and by delay of gastric emptying (11, 23). In our study, the exenatide-induced glucagon suppression was rather modest. However, we measured a significant slowing of gastric emptying, as demonstrated by acetaminophen data. To match portal glucose appearance in the control and exenatide experiments and rule out the delay of gastric emptying as the sole mechanism responsible for the exenatide-induced reduction of glycemia, we infused glucose intraportally with and without exenatide. The hypoglycemic effect of exenatide was still evident, thus demonstrating that the slowing of gastric emptying could not totally account for the exenatide-induced reduction of glycemia. There was no difference in glucagon concentration between the two experiments; therefore, other mechanisms, such as increased glucose disappearance independent of changes in pancreatic hormones and gut, account for the phenomenon. Moreover, in the exenatide experiment, NEFA levels were suppressed 30 min earlier than in the saline experiment, suggesting increased insulin sensitivity.
Chronic administration of exenatide or GLP-1 has been shown to improve glycemia, insulin sensitivity, and parameters of glucose tolerance, although, at least in part, the chronic effects on insulin sensitivity can be attributed to reduction of food intake and increased β-cell mass and function (13, 17, 40, 41). Data regarding the acute effect of GLP-1 or exendin-4 on insulin sensitivity are more controversial. A number of investigators reported increases in insulin sensitivity with acute administration of GLP-1 (3, 4, 25, 32), while others failed to find an effect (16, 29, 37, 38). It has been suggested that the delivery route (peripheral vs. the more natural, portal route) of GLP-1 could account for some of the differences in outcome between various studies. Indeed, when GLP-1 was delivered intraportally in studies performed in our laboratory and in others (6, 19, 27), an insulin secretion-independent effect of GLP-1 on glucose disappearance was measured. Since exenatide has a longer half-life than GLP-1 (24), even subcutaneous administration will considerably increase the peptide's levels in portal circulation and produce an effect, as reported in the present study. We found only one study in humans in which effects of exendin-4 on insulin sensitivity were investigated in an acute setting (38). In this study, infusion of GLP-1 or exendin-4 during a euglycemic-hyperinsulinemic clamp in healthy volunteers produced only a minimal, nonstatistically significant increase in glucose infusion rate and glucose disappearance (38). However, this finding conflicts with results of a previous study by the same authors indicating that, in type 1 diabetic patients, GLP-1 increases total body glucose uptake (39). Additionally, cortisol and endogenous glucose production were increased with GLP-1 and exendin-4, suggesting that a compensatory increase in glucose might have offset the sensitizing effects of exendin-4. Thus a difference in the methodology and the model could explain these discordant results.
Another argument supporting an exenatide-induced increase in glucose disappearance independent of pancreatic hormones is the lowering of glycemia in the fasted state, an effect found in our present study and previously reported by several investigators (11, 40). Edwards et al. (11) found that exendin-4 reduced fasting glycemia in healthy volunteers, without changes in insulin or glucagon, whereas Young et al. (40) showed that acute administration of exenatide decreased fasting glucose in obese diabetic (ob/ob and db/db) mice, diabetic fatty Zucker rats, and diabetic rhesus monkeys. Interestingly, the effect appeared to be glucose dependent, with the greatest effect obtained at the highest fasting glycemia. Similarly, in the present study, we measured lower glycemia, with a trend toward significance in the fasted state; after the glucose load, the difference in glycemia became more pronounced and statistically significant.
To investigate the mechanism of exenatide-induced increase in glucose disappearance, we intraportally infused a known antagonist of the GLP-1 receptor, exendin-(9-39). The antagonist infusion partly reversed the effect of exenatide on glycemia: the glucose AUC during antagonist administration significantly increased compared with that during exenatide administration; however, it did not completely revert to saline control values, indicating only a partial blocking effect at a dose of 75 pmol·kg−1·min−1. Selection of an appropriate dose of exendin-9 was affected by the lack of information regarding dosage and effects of exenatide and exendin-(9-39) in dogs, so human data were used. In humans studies, a ratio of GLP-1 to exendin-(9-39) of ≥1:100 but, more frequently, ≥1:1,000 (5, 12, 33) has been shown to be necessary to counteract the effects of GLP-1 when exendin-(9-39) is administered peripherally. Even though the dose of exendin-(9-39) used in the present study (75 pmol·kg−1·min−1) might not have been appropriate to completely counteract the effect of exenatide on glycemia, the dose was sufficient to affect glycemia to an extent that the resulting glucose concentration was statistically significantly different from that in the exenatide experiment. Importantly, this effect occurred at a ratio of exenatide to exendin-(9-39) of 1:30, strongly suggesting that local action in the portohepatic area might be involved in the effect. Alternatively, the partial blocking could be explained by the fact that exenatide has other peripheral effects, independent of the effects in the portal vein. In a previous study (19), we presented data supporting a portal mechanism of GLP-1 action, by showing that intraportal glucose and GLP-1, but not peripheral glucose and GLP-1, produce an increase in glucose disappearance, a result that is concordant with data from other investigators (2, 21, 27). The portohepatic area is richly innervated and is sensitive to GLP-1 (1, 28). Recent data suggest that circulating GLP-1 can bind to nerve receptors in the portal vein and initiate signals that are carried in vagal afferent nerves (22); administration of a GLP-1 antagonist locally to block receptor binding impaired glucose tolerance (36). Further studies are necessary to elucidate the precise location of the receptor that mediates exenatide's effect on glycemia and the exact mechanism of this effect. GLP-1 has been shown to have insulin-independent effects on suppressing hepatic glucose production and increasing liver glucose uptake in several studies (6, 31); in other studies, GLP-1 has been shown to primarily increase peripheral glucose utilization (25, 27, 32, 34).
An interesting aspect of GLP-1 physiology revealed by the present study is the potential impact of exenatide on endogenous GLP-1 secretion. Exenatide caused an 88% reduction of postprandial plasma active GLP-1 concentration. This result correlates with findings from other studies (11) in which administration of exedin-4 in healthy humans reduced endogenous GLP-1 secretion by a similar extent. Since during the meal test we measured a considerable slowing of gastric emptying after exenatide injection, it is possible that the delivery of nutrients to GLP-1-secreting L cells was delayed, thus explaining the lower GLP-1 levels. An alternative explanation that has been advanced for this effect is that the presence of exendin-4 results in downregulation of endogenous GLP-1 production (11).
In conclusion, our study presents, for the first time, clear evidence that exenatide, in subcutaneous administration similar to the one used in the treatment of type 2 diabetes, reduces peripheral glycemia via a mechanism independent of a delay of gastric emptying or corresponding changes in pancreatic hormone secretion. In addition, the present study suggests that this effect may be mediated by pancreatic-type GLP-1 receptors, perhaps located in the portohepatic area. This finding could provide an additional tool in the use of exenatide for the treatment of type 1 and type 2 diabetes.
GRANTS
This work was supported by an investigator-initiated research grant from Amylin. V. Ionut was supported by an American Diabetes Association Mentor Award and R. N. Bergman by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-27619 and DK-29867.
Acknowledgments
We are especially grateful to Dr. Joyce Richey for insightful comments; we thank Elza Demirchyan and Rita Thomas for technical assistance and Dr. Erlinda Kirkman and Ed Zuniga for animal care and assistance with the experiment.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Berthoud HR, Kressel M, Neuhuber WL. An anterograde tracing study of the vagal innervation of rat liver, portal vein and biliary system. Anat Embryol (Berl) 186: 431–442, 1992. [DOI] [PubMed] [Google Scholar]
- 2.Burcelin R, Da Costa A, Drucker D, Thorens B. Glucose competence of the hepatoportal vein sensor requires the presence of an activated glucagon-like peptide-1 receptor. Diabetes 50: 1720–1728, 2001. [DOI] [PubMed] [Google Scholar]
- 3.D'Alessio DA, Kahn SE, Leusner CR, Ensinck JW. Glucagon-like peptide 1 enhances glucose tolerance both by stimulation of insulin release and by increasing insulin-independent glucose disposal. J Clin Invest 93: 2263–2266, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.D'Alessio DA, Prigeon RL, Ensinck JW. Enteral enhancement of glucose disposition by both insulin-dependent and insulin-independent processes. A physiological role of glucagon-like peptide I. Diabetes 44: 1433–1437, 1995. [DOI] [PubMed] [Google Scholar]
- 5.D'Alessio DA, Vogel R, Prigeon R, Laschansky E, Koerker D, Eng J, Ensinck JW. Elimination of the action of glucagon-like peptide 1 causes an impairment of glucose tolerance after nutrient ingestion by healthy baboons. J Clin Invest 97: 133–138, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dardevet D, Moore MC, Neal D, DiCostanzo CA, Snead W, Cherrington AD. Insulin-independent effects of GLP-1 on canine liver glucose metabolism: duration of infusion and involvement of hepatoportal region. Am J Physiol Endocrinol Metab 287: E75–E81, 2004. [DOI] [PubMed] [Google Scholar]
- 7.Deacon CF, Pridal L, Klarskov L, Olesen M, Holst JJ. Glucagon-like peptide 1 undergoes differential tissue-specific metabolism in the anesthetized pig. Am J Physiol Endocrinol Metab 271: E458–E464, 1996. [DOI] [PubMed] [Google Scholar]
- 8.Drucker DJ The biology of incretin hormones. Cell Metab 3: 153–165, 2006. [DOI] [PubMed] [Google Scholar]
- 9.Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet 368: 1696–1705, 2006. [DOI] [PubMed] [Google Scholar]
- 10.Dupre J, Behme MT, McDonald TJ. Exendin-4 normalized postcibal glycemic excursions in type 1 diabetes. J Clin Endocrinol Metab 89: 3469–3473, 2004. [DOI] [PubMed] [Google Scholar]
- 11.Edwards CM, Stanley SA, Davis R, Brynes AE, Frost GS, Seal LJ, Ghatei MA, Bloom SR. Exendin-4 reduces fasting and postprandial glucose and decreases energy intake in healthy volunteers. Am J Physiol Endocrinol Metab 281: E155–E161, 2001. [DOI] [PubMed] [Google Scholar]
- 12.Edwards CM, Todd JF, Mahmoudi M, Wang Z, Wang RM, Ghatei MA, Bloom SR. Glucagon-like peptide 1 has a physiological role in the control of postprandial glucose in humans: studies with the antagonist exendin 9-39. Diabetes 48: 86–93, 1999. [DOI] [PubMed] [Google Scholar]
- 13.Egan JM, Meneilly GS, Elahi D. Effects of 1-mo bolus subcutaneous administration of exendin-4 in type 2 diabetes. Am J Physiol Endocrinol Metab 284: E1072–E1079, 2003. [DOI] [PubMed] [Google Scholar]
- 14.Eng J, Andrews PC, Kleinman WA, Singh L, Raufman JP. Purification and structure of exendin-3, a new pancreatic secretagogue isolated from Heloderma horridum venom. J Biol Chem 265: 20259–20262, 1990. [PubMed] [Google Scholar]
- 15.Eng J, Kleinman WA, Singh L, Singh G, Raufman JP. Isolation and characterization of exendin-4, an exendin-3 analogue, from Heloderma suspectum venom. Further evidence for an exendin receptor on dispersed acini from guinea pig pancreas. J Biol Chem 267: 7402–7405, 1992. [PubMed] [Google Scholar]
- 16.Freyse EJ, Knospe S, Becher T, El Hag O, Goke B, Fischer U. Glucagon-like peptide-1 has no insulin-like effects in insulin-dependent diabetic dogs maintained normoglycemic and normoinsulinemic. Metabolism 48: 134–137, 1999. [DOI] [PubMed] [Google Scholar]
- 17.Gedulin BR, Nikoulina SE, Smith PA, Gedulin G, Nielsen LL, Baron AD, Parkes DG, Young AA. Exenatide (exendin-4) improves insulin sensitivity and β-cell mass in insulin-resistant obese fa/fa Zucker rats independent of glycemia and body weight. Endocrinology 146: 2069–2076, 2005. [DOI] [PubMed] [Google Scholar]
- 18.Holst JJ On the physiology of GIP and GLP-1. Horm Metab Res 36: 747–754, 2004. [DOI] [PubMed] [Google Scholar]
- 19.Ionut V, Hucking K, Liberty I, Bergman RN. Synergistic effect of portal glucose and glucagon-like peptide-1 to lower systemic glucose and stimulate counter-regulatory hormones. Diabetologia 48: 967–975, 2005. [DOI] [PubMed] [Google Scholar]
- 20.Ionut V, Liberty IF, Hucking K, Lottati M, Stefanovski D, Zheng D, Bergman RN. Exogenously imposed postprandial-like rises in systemic glucose and GLP-1 do not produce an incretin effect, suggesting an indirect mechanism of GLP-1 action. Am J Physiol Endocrinol Metab 291: E779–E785, 2006. [DOI] [PubMed] [Google Scholar]
- 21.Johnson KMS, Edgerton DS, Rodewald T, Scott M, Farmer B, Neal D, Cherrington AD. Intraportally delivered GLP-1, in the presence of hyperglycemia induced via peripheral glucose infusion, does not change whole body glucose utilization. Am J Physiol Endocrinol Metab 294: E380–E384, 2008. [DOI] [PubMed] [Google Scholar]
- 22.Klustaitis KM, Elfers EE, D'Alessio DA. Sensory neurons in the portal vein can access small molecules in circulation: support for a neuro-endocrine mechanism of action of GLP-1 (Abstract). Diabetes 56, Suppl 1: A1475-P, 2007. [Google Scholar]
- 23.Kolterman OG, Buse JB, Fineman MS, Gaines E, Heintz S, Bicsak TA, Taylor K, Kim D, Aisporna M, Wang Y, Baron AD. Synthetic exendin-4 (exenatide) significantly reduces postprandial and fasting plasma glucose in subjects with type 2 diabetes. J Clin Endocrinol Metab 88: 3082–3089, 2003. [DOI] [PubMed] [Google Scholar]
- 24.Kolterman OG, Kim DD, Shen L, Ruggles JA, Nielsen LL, Fineman MS, Baron AD. Pharmacokinetics, pharmacodynamics, and safety of exenatide in patients with type 2 diabetes mellitus. Am J Health Syst Pharm 62: 173–181, 2005. [DOI] [PubMed] [Google Scholar]
- 25.Meneilly GS, Mcintosh CHS, Pederson RA, Habener JF, Gingerich R, Egan JM, Finegood DT, Elahi D. Effect of glucagon-like peptide 1 on non-insulin-mediated glucose uptake in the elderly patient with diabetes. Diabetes Care 24: 1951–1956, 2001. [DOI] [PubMed] [Google Scholar]
- 26.Nielsen LL, Young AA, Parkes DG. Pharmacology of exenatide (synthetic exendin-4): a potential therapeutic for improved glycemic control of type 2 diabetes. Regul Pept 117: 77–88, 2004. [DOI] [PubMed] [Google Scholar]
- 27.Nishizawa M, Moore MC, Shiota M, Gustavson SM, Snead WL, Neal DW, Cherrington AD. Effect of intraportal glucagon-like peptide-1 on glucose metabolism in conscious dogs. Am J Physiol Endocrinol Metab 284: E1027–E1036, 2003. [DOI] [PubMed] [Google Scholar]
- 28.Nishizawa M, Nakabayashi H, Kawai K, Ito T, Kawakami S, Nakagawa A, Niijima A, Uchida K. The hepatic vagal reception of intraportal GLP-1 is via receptor different from the pancreatic GLP-1 receptor. J Auton Nerv Syst 80: 14–21, 2000. [DOI] [PubMed] [Google Scholar]
- 29.Orskov L, Holst JJ, Moller J, Orskov C, Moller N, Alberti KG, Schmitz O. GLP-1 does not acutely affect insulin sensitivity in healthy man. Diabetologia 39: 1227–1232, 1996. [DOI] [PubMed] [Google Scholar]
- 30.Parkes DG, Pittner R, Jodka C, Smith P, Young A. Insulinotropic actions of exendin-4 and glucagon-like peptide-1 in vivo and in vitro. Metabolism 50: 583–589, 2001. [DOI] [PubMed] [Google Scholar]
- 31.Prigeon RL, Quddusi S, Paty B, D'Alessio DA. Suppression of glucose production by GLP-1 independent of islet hormones: a novel extrapancreatic effect. Am J Physiol Endocrinol Metab 285: E701–E707, 2003. [DOI] [PubMed] [Google Scholar]
- 32.Sandhu H, Wiesenthal SR, MacDonald PE, Mccall RH, Tchipashvili V, Rashid S, Satkunarajah M, Irwin DM, Shi ZQ, Brubaker PL, Wheeler MB, Vranic M, Efendic S, Giacca A. Glucagon-like peptide 1 increases insulin sensitivity in depancreatized dogs. Diabetes 48: 1045–1053, 1999. [DOI] [PubMed] [Google Scholar]
- 33.Schirra J, Sturm K, Leicht P, Arnold R, Goke B, Katschinski M. Exendin(9-39)amide is an antagonist of glucagon-like peptide-1(7-36)amide in humans. J Clin Invest 101: 1421–1430, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shalev A, Ninnis R, Keller U. Effects of glucagon-like peptide 1 (7-36 amide) on glucose kinetics during somatostatin-induced suppression of insulin secretion in healthy men. Horm Res 49: 221–225, 1998. [DOI] [PubMed] [Google Scholar]
- 35.Steil GM, Ader M, Moore DM, Rebrin K, Bergman RN. Transendothelial insulin transport is not saturable in vivo. No evidence for a receptor-mediated process. J Clin Invest 97: 1497–1503, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vahl TP, Tauchi M, Durler TS, Elfers EE, Fernandes TM, Bitner RD, Ellis KS, Woods SC, Seeley RJ, Herman JP, D'Alessio DA. Glucagon-like peptide-1 (GLP-1) receptors expressed on nerve terminals in the portal vein mediate the effects of endogenous GLP-1 on glucose tolerance in rats. Endocrinology 148: 4965–4973, 2007. [DOI] [PubMed] [Google Scholar]
- 37.Vella A, Shah P, Basu R, Basu A, Holst JJ, Rizza RA. Effect of glucagon-like peptide 1(7-36) amide on glucose effectiveness and insulin action in people with type 2 diabetes. Diabetes 49: 611–617, 2000. [DOI] [PubMed] [Google Scholar]
- 38.Vella A, Shah P, Reed AS, Adkins AS, Basu R, Rizza RA. Lack of effect of exendin-4 and glucagon-like peptide-1-(7,36)-amide on insulin action in non-diabetic humans. Diabetologia 45: 1410–1415, 2002. [DOI] [PubMed] [Google Scholar]
- 39.Vella A, Shah P, Basu R, Basu A, Camilleri M, Schwenk FW, Holst JJ, Rizza RA. Effect of glucagon-like peptide-1(7-36)-amide on initial splanchnic glucose uptake and insulin action in humans with type 1 diabetes. Diabetes 50: 565–572, 2001. [DOI] [PubMed] [Google Scholar]
- 40.Young AA, Gedulin BR, Bhavsar S, Bodkin N, Jodka C, Hansen B, Denaro M. Glucose-lowering and insulin-sensitizing actions of exendin-4: studies in obese diabetic (ob/ob, db/db) mice, diabetic fatty Zucker rats, and diabetic rhesus monkeys (Macaca mulatta). Diabetes 48: 1026–1034, 1999. [DOI] [PubMed] [Google Scholar]
- 41.Zander M, Madsbad S, Madsen JL, Holst JJ. Effect of 6-week course of glucagon-like peptide 1 on glycaemic control, insulin sensitivity, and β-cell function in type 2 diabetes: a parallel-group study. Lancet 359: 824–830, 2002. [DOI] [PubMed] [Google Scholar]