Abstract
Obesity and elevated cytokine secretion result in a chronic inflammatory state and may cause the insulin resistance observed in type 2 diabetes. Recent studies suggest a key role for endoplasmic reticulum stress in hepatocytes and adipocytes from obese mice, resulting in reduced insulin sensitivity. To address the hypothesis that thiazolidinediones, which improve peripheral insulin sensitivity, act in part by reducing the endoplasmic reticulum stress response, we tested subcutaneous adipose tissue from 20 obese volunteers treated with pioglitazone for 10 wk. We also experimentally induced endoplasmic reticulum stress using palmitate, tunicamycin, and thapsigargin in the human HepG2 liver cell line with or without pioglitazone pretreatment. We quantified endoplasmic reticulum stress response by measuring both gene expression and phosphorylation. Pioglitazone significantly improved insulin sensitivity in human volunteers (P = 0.002) but did not alter markers of endoplasmic reticulum stress. Differences in pre- and posttreatment endoplasmic reticulum stress levels were not correlated with changes in insulin sensitivity or body mass index. In vitro, palmitate, thapsigargin, and tunicamycin but not oleate induced endoplasmic reticulum stress in HepG2 cells, including increased transcripts CHOP, ERN1, GADD34, and PERK, and increased XBP1 splicing along with phosphorylation of eukaryotic initiation factor eIF2α, JNK1, and c-jun. Although patterns of endoplasmic reticulum stress response differed among palmitate, tunicamycin, and thapsigargin, pioglitazone pretreatment had no significant effect on any measure of endoplasmic reticulum stress, regardless of the inducer. Together, our data suggest that improved insulin sensitivity with pioglitazone is not mediated by a reduction in endoplasmic reticulum stress.
Keywords: insulin sensitivity, fatty acid, thiazolidinediones, type 2 diabetes, obesity
the early pathophysiology of type 2 diabetes remains uncertain. Three key defects mark the onset of hyperglycemia in type 2 diabetes mellitus: increased hepatic glucose production, diminished insulin secretion, and impaired insulin action (7). Obesity generally accompanies type 2 diabetes and is the key modifiable risk factor. Recent data suggest that obesity leads to a state of chronic inflammation marked by elevated cytokine levels, which in turn may reduce insulin action. However, the proximal cause and molecular mechanisms of this inflammatory response are uncertain.
Recently, Ozcan et al. (25) and others (15, 22, 24) proposed endoplasmic reticulum (ER) stress as the proximal cause of chronic inflammation and reduced insulin action in adipocytes and hepatocytes. In mice, both high fat diet and genetic obesity (ob/ob mice) activated proximal sensors of ER stress, including increased transcription of GRP78 (also known as BiP, encoded by human gene HSPA5) and increased phosphorylation of proximal ER stress proteins PERK and IRE1 (encoded by human gene ERN1) and downstream factors including eukaryotic initiation factor eIF2α, JNK1, c-jun, and ultimately IRS1 (25). Manipulation of chaperones further downstream such as ORP150 (encoded by human gene HYOU1) also altered insulin sensitivity in mice (22, 24). Finally, this same mechanism may account for altered insulin secretion (14, 33), thus linking the three key defects in the pathogenesis of type 2 diabetes.
Thiazolidinediones (TZDs), which include the presently available drugs rosiglitazone and pioglitazone, are well established insulin sensitizing agents that act, at least in part, as agonists of the peroxisome proliferator-activated receptor (PPAR)γ. These drugs appear to act as primarily peripheral insulin sensitizers, but hepatic effects have been suggested also (1). Although the mechanism of action remains controversial, expansion of adipocyte mass may reduce ectopic fat and hence improve hepatic insulin sensitivity and β-cell function (37). TZDs reduce inflammatory cytokines and increase adiponectin (30, 31). Hence, TZDs affect the same pathways and genes that characterize diabetes pathogenesis and might be expected to act on the proximal cause, such as ER stress. Indeed, Loffler et al. (20) found significant downregulation of ER stress genes in livers from diabetic db/db mice treated with rosiglitazone, including HSPA5, DNAJC3, and X-box protein-1 (XBP1). Similarly, Han et al. (12) showed reduced liver and white adipose ER stress in diabetic db/db mice treated with the dual PPARα/γ agonist macelignan. Macelignan also suppressed thapsigargin-induced ER stress in mouse hepatocytes and adipocyte cell lines (12). These studies suggest that the beneficial effects of TZDs on insulin sensitivity may result in part from a reduction of ER stress, but no human data are available to address this hypothesis.
We tested the hypothesis that pioglitazone acts in part by reducing ER stress in two systems. First, we tested whether markers of adipocyte ER stress were reduced in human subjects with impaired glucose tolerance treated with pioglitazone for 10 wk. To extend these human studies, we next tested whether pioglitazone was able to protect the human hepatocyte HepG2 cell line or the human adipocyte Simpson-Golabi-Behmel syndrome (SGBS) cell line from experimentally induced acute ER stress. In both studies, we quantified the key indicators of the ER stress response.
MATERIALS AND METHODS
Human subjects.
The human studies have been described in detail previously (29, 38). To establish the presence of ER stress in obese humans, we examined subcutaneous adipose tissue from 86 no diabetic individuals (body mass index 19–40 kg/m2) who participated in several previous studies. The sample included 70 women and 16 men, aged 21–66 yr, of whom 32 individuals had impaired glucose tolerance. Pioglitazone effects on ER stress were tested in 20 individuals with impaired glucose tolerance (IGT) who were randomized to receive pioglitazone at 30 mg/day for 2 wk, then 45 mg/day for 8 wk (30). We report only the pioglitazone arm of the study, which randomized individuals to pioglitazone or metformin (36). Adipose and muscle biopsies and frequently sampled intravenous glucose tolerance tests were performed before and after treatment. All subjects provided written informed consent under protocols approved by University of Arkansas for Medical Sciences Institutional Review Board.
Experimental agents.
Pioglitazone (a gift from Takada Pharmaceutical, Deerfield, IL) was dissolved in dimethyl sulfoxide (Sigma, St. Louis, MO). ER stress was induced as described below by tunicamycin (Sigma), thapsigargin (Sigma), oleic acid (MP Biomedical, Solon, OH), or palmitic acid (Calbiochem, La Jolla, CA). Tunicamycin was dissolved in DMSO, and thapsigargin was dissolved in ethanol. Palmitic and oleic acids were conjugated to fatty acid-free bovine serum albumin (BSA, MP Biomedical) at a 2.5:1 molar ratio by dissolving them in ethanol and mixing with an aqueous BSA solution (BSA in MEM culture medium) at 45°C until homogeneous, after which they were passed through a 0.2 μM filter (18). All references to palmitic and oleic acids refer to the BSA conjugate. Antibodies for Western blot experiments included phospho-eIF2α (BioSource Division of Invitrogen, Carlsbad, CA), total eIF2α (Santa Cruz Biotechnology, Santa Cruz, CA), PERK (Santa Cruz Biotechnology), phospho-PERK (Santa Cruz Biotechnology), phospo-JNK1 (Santa Cruz Biotechnology), total JNK1 (Santa Cruz Biotechnology), phospho-c-jun (Cell Signaling, Danvers, MA), and β-actin (Chemicon Division of Millipore, Billerica, MA). Mouse and rabbit horseradish peroxidase-conjugated secondary antibodies were purchased from Amersham Biosciences (Pittsburgh, PA).
Cell culture.
Human hepatocyte HepG2 (American Type Culture Collection, Manassas, VA) cells were cultured in minimum essential medium (MEM) with Earle's balanced salt solution supplemented with 2 mM l-glutamine, penicillin, streptomycin, and 10% fetal bovine serum (all from Omega Scientific, Tarzana, CA). At 80–90% confluence, cells were transferred to medium containing 2% fetal bovine serum (FBS). Cells were preincubated for 16 h in pioglitazone (10 μM) or control (DMSO), after which tunicamycin (1 μg/ml), thapsigargin (25 nM), or vehicle (DMSO or ethanol) was added to the culture plates in the presence or absence of pioglitazone (10 μM). Palmitic acid and oleic acid experiments were conducted similarly, except that after the preincubation period, cells were washed twice in serum-free MEM and transferred to serum-free medium with or without pioglitazone (10 μM) and palmitic acid-BSA conjugate (1 mM), oleic acid-BSA conjugate (1 mM), or BSA. Cells were harvested at 6 and 12 h posttreatment for RNA or protein extraction.
Human SGBS preadipocytes were cultured as described previously (3). Briefly, SGBS cells were morphologically differentiated (70%) into mature adipocytes and conditioned in serum-free basal medium (DMEM-F12) for 24 h, followed by 16 h of pretreatment with pioglitazone. ER stress was induced by treatment with palmitic acid (0.5 mM) for 6–12 h in serum-free basal medium, after which cells were harvested for RNA extraction.
RNA isolation and gene expression.
Total RNA from human subcutaneous adipose tissue was isolated using an RNeasy Lipid Tissue Mini Kit (Qiagen, Valencia, CA). Total RNA from HepG2 cells was isolated using the RNeasy Mini Kit (Qiagen) and from SGBS cells by using the RNAqueous kit (Ambion, Austin, TX) following the manufacturer's instruction. The quantity of isolated RNA was determined spectrophotometrically and the quality by electrophoresis using an Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA). Total RNA (0.4–1 μg) was reverse transcribed using random hexamer primers with TaqMan Reverse Transcription Reagents (Applied Biosystems, Foster City, CA). Reverse-transcribed RNA (cDNA) was amplified with 1× Sybr Green PCR Master Mix (Applied Biosystems) and 0.3 μmol/l gene-specific forward and reverse primers on an ABI 7500 Fast RT-PCR system (Applied Biosystems) or a Rotorgene 2000 Real-Time PCR system (Corbett Life Science, Sydney, Australia). Samples were normalized to 18S ribosomal RNA. Standard curves were generated using pooled cDNA from the samples assayed. Primer sequences (Supplemental Table S1; supplemental data are available at the online version of this article) were designed to capture all major known splice forms, to span an intron, and to give a single band of the expected size. Experiments in HepG2 cells were conducted with three technical replicates for each of two biological replicates, yielding six readings for each experiment. XBP1 splicing was examined by gel electrophoresis and quantified by densitometry for cell culture experiments. Additionally, spliced XBP1 was specifically assayed from human adipose tissue cDNA using real-time PCR.
Immunoblot analysis.
HepG2 Cells were washed with cold PBS and harvested using 1× RIPA buffer (Santa Cruz Biotechnology; 1× TBS, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS, 0.004% sodium azide) supplemented with Protease Inhibitor Cocktail (10 μl/ml; Santa Cruz Biotechnology), phenylmethylsulfonyl fluoride (10 μl/ml), and Phosphatase Inhibitor Cocktail 1 (10 μl/ml, Sigma) and Cocktail 2 (10 μl/ml, Sigma). Cell lysates were homogenized, and protein content of clear lysates was estimated by the Bradford method (6). Equal amounts (20–30 μg) of total protein were dissolved in Lammeli buffer (50 mM Tris, pH 6.8, 100 mM β-mercaptoethanol, 2% SDS, 10% glycerol), separated on 8% SDS-polyacrylamide gel, and transferred to Trans-Blot Nitrocellulose membrane (Bio-Rad Laboratories, Hercules, CA). Proteins were detected by immunoblot according to the manufacturer's protocols, developed with SuperSignal Electro-Chemiluminescent reagent (Pierce Biotechnology Division of Thermo-Fisher Scientific, Waltham, MA) and scanned in ChemiDox Image Analyzer (Bio-Rad). Western blots were quantified using Quantity One Image Analysis software (v4.6.3; Bio-Rad). All total proteins were normalized to β-actin, and phosphoproteins were normalized to total protein and/or to β-actin. Data represent samples from three independent experiments with technical replicates.
Data analysis and statistics.
Experimental conditions in in vitro experiments were compared using the nonparametric Mann-Whitney U-test for unpaired samples or the Kruskal-Wallis test for three or more. Human adipose samples obtained before and after pioglitazone were compared using the Wilcoxon signed rank test. Correlations between the change in insulin sensitivity (ΔSI), change in body mass index (ΔBMI), and change in gene expression were calculated using the Spearman correlation coefficient or partial regression controlling for either ΔBMI or ΔSI. All analyses were performed in SPSS for Windows v12.0 (SPSS, Chicago, IL).
RESULTS
ER stress is increased with obesity in human subcutaneous adipose tissue.
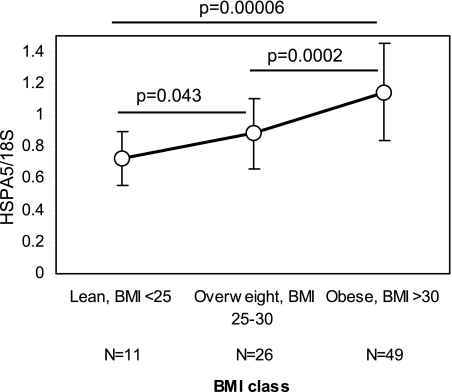
ER stress is activated in adipose tissue from obese mice, but no data are available in humans. To address the role of ER stress in humans, we examined subcutaneous adipose tissue from 86 individuals over a range of BMI (19–40 kg/m2) for the ER stress marker HSPA5, which is generally viewed as the best single marker of ER stress (25). HSPA5 transcript levels rose from lean to overweight and obese individuals (mean and SD: 0.73 ± 0.17 for lean; 0.89 ± 0.22 overweight; 1.15 ± 0.31 obese; Fig. 1), with a 57% increase in obese when compared with lean individuals (P = 0.00006). Additionally, in 83 subjects for whom data were available, HSPA5 levels correlated significantly with insulin sensitivity (SI; r = −0.43, P = 0.00006). Age did not differ significantly among groups and did not contribute to ER stress. Thus, as in mice, ER stress is activated in human subcutaneous adipose tissue with obesity.
Fig. 1.
Endoplasmic reticulum (ER) stress is activated in subcutaneous adipose tissue of obese human subjects. Subcutaneous adipose tissue expression of the ER chaperone HSPA5 from 86 individuals over a range of body mass indexes (BMIs) from 19 to 40 kg/m2 shows significant increase from lean to overweight and obese individuals. Raw expression values of HSPA5 were normalized to 18S RNA. Data are presented as means ± SD of normalized HSPA5 expression for 3 BMI classes: lean (BMI <25 kg/m2), overweight (BMI ≥25 kg/m2 and BMI ≤30 kg/m2), or obese (BMI >30 kg/m2). P = statistical significance determined from Mann-Whitney U-test.
Pioglitazone treatment does not reduce ER stress in human adipose tissue.
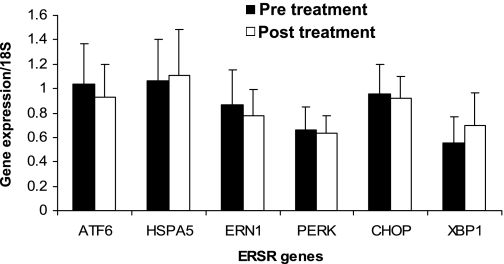
PPARγ agonists significantly reduced ER stress response in liver and white adipose in diabetic db/db mice (12, 20). To determine whether TZDs reduce ER stress in humans with IGT, we examined subcutaneous adipose from 20 obese individuals with IGT (30) before and after 10 wk of pioglitazone. We measured six key transcriptional markers of ER stress: HSPA5, CHOP, ATF6, ERN1, PERK, and XBP1. Despite a significant improvement in SI (Table 1; P = 0.002), levels of ER stress gene transcripts were unchanged (P > 0.12; Fig. 2; Supplemental Fig. S1A). Splicing of XBP1 is a marker of acute ER stress. We did not observe spliced XBP1 before or after pioglitazone therapy using a gel-based assay (Supplemental Fig. S1B), and real-time quantification of spliced XBP1 transcript was not changed with pioglitazone therapy (Fig. 2). Because pioglitazone increased BMI, and BMI is closely associated with ER stress markers in mice and humans (see data above), we examined the correlation of increase in BMI with the change in gene expression. No significant correlation was noted (P > 0.1); similarly, we found no significant correlation between the change in SI and the change in gene expression before and after pioglitazone, even when controlling for the change in BMI (P > 0.3).
Table 1.
Baseline characteristics of subjects and changes in response to pioglitazone
| Baseline | Post-Tx | P Value | |
|---|---|---|---|
| Age, yr | 47.85±7.64 | ||
| Sex, M/F | 3/17 | ||
| Ethnicity, Caucasian/Non-Caucasian | 16/4 | ||
| BMI, kg/m2 | 32.40±3.78 | 33.42±4.22 | 0.001 |
| Body fat, % | 40.85±6.18 | 41.46±5.70 | 0.048 |
| Fasting glucose, mg/dl | 92.81±13.04 | 86.20±8.95 | 0.003 |
| 2-h Glucose, mg/dl | 165.90±20.90 | 122.30±26.88 | 0.0003 |
| SI, 10−4 min−1·μU−1·ml−1 | 1.82±0.62 | 2.56±1.15 | 0.002 |
| HbA1c, % | 5.56±0.53 | 5.47±0.41 | NS |
| Total cholesterol, mg/dl | 206.60±52.74 | 190.10±40.48 | NS |
| Triglyceride, mg/dl | 185.90±101.62 | 138.50±60.57 | 0.001 |
| HDL, mg/dl | 51.30±8.08 | 53.20±11.45 | NS |
| LDL, mg/dl | 115.84±42.75 | 109.10±39.24 | NS |
Data are presented as means ± SD in their original scales. BMI, body mass index; HbA1c, glycosylated hemoglobin; SI, insulin sensitivity; LDL and HDL, low- and high-density lipoprotein, respectively; M, male; F, female; NS, not significant. P value: statistical significance was determined from Wilcoxon's signed rank test.
Fig. 2.
Adipocyte ER stress markers in response to pioglitazone. Subcutaneous adipose tissue expression of 5 ER stress pathway genes before and after pioglitazone treatment in 20 human volunteers. Raw expression values of ER stress response (ERSR) genes were normalized to 18S RNA. Data are presented as means ± SD of 18S-normalized gene expression.
Pioglitazone does not protect HepG2 cells from tunicamycin- and thapsigargin-induced ER stress.
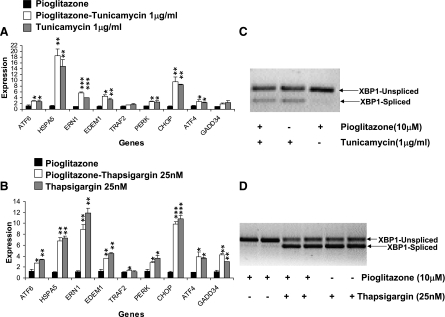
Hepatocytes may be a more important source of ER stress than subcutaneous fat (39), thus explaining the lack of reduction in adipocyte ER stress with pioglitazone in humans. Because we were unable to evaluate hepatocytes from pioglitazone-treated humans, we tested whether pioglitazone could protect against induced ER stress in HepG2 cells. We initially induced acute ER stress using either tunicamycin (1 μg/ml) or thapsigargin (25 nM). Both classic inducers of ER stress increased HSPA5 transcript levels and XBP1 splicing in a time-dependent fashion, and preincubation with pioglitazone failed to protect against markers of ER stress with either inducer (Fig. 3). These data thus support the results in human adipose tissue. Pioglitazone did have a pharmacological effect in HepG2 cells, as demonstrated by upregulation of known PPARγ target ApoA2 by 47% (1.08 ± 0.11 control, 1.59 ± 0.08 pioglitazone), as reported by others (28).
Fig. 3.
ER stress response to tunicamycin or thapsigargin with pioglitazone pretreatment. A: expression of ER stress transcripts after 16 h of pretreatment with pioglitazone or DMSO, followed by 12 h of treatment with tunicamycin (1 μg/ml) or control (DMSO). B: ER stress transcripts after 16 h of pretreatment of pioglitazone or DMSO, followed by 12 h of treatment with thapsigargin (25 nM) or control (ethanol). Raw expression values were normalized to 18S RNA. All data were normalized to the lowest value of the control condition as 1 and are presented as means ± SD for 3 independent experiments with 2 biological replicates. *P < 0.05, **P < 0.01, and ***P < 0.001 vs. control (pioglitazone alone). C: representative gels showing spliced and unspliced XBP1 transcripts for the pioglitazone/tunicamycin experiment described in A. D: representative gel showing XBP1 splicing for the pioglitazone/thapsigargin experiment described in B.
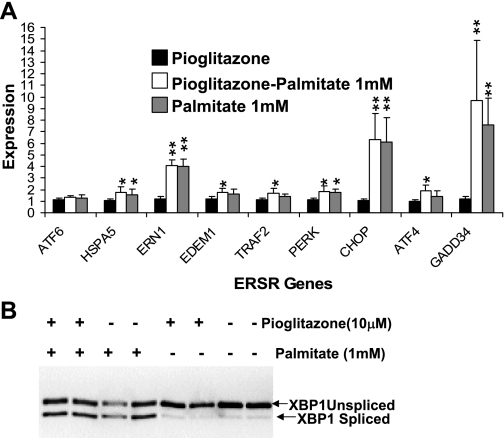
Palmitate but not oleate induces ER stress in HepG2 cells.
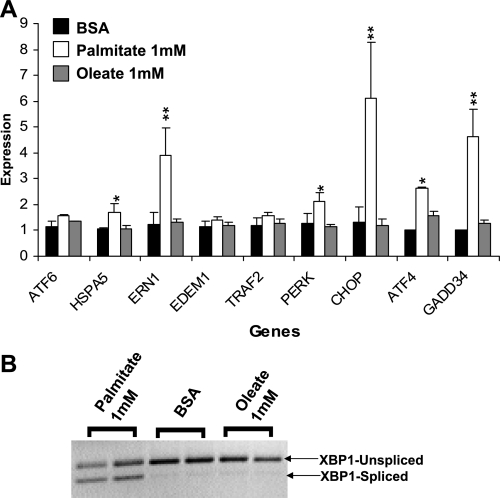
Obese individuals have elevated levels of circulating free fatty acids (16). Hence, we asked whether a more physiological stress might be ameliorated by pioglitazone in HepG2 cells. Palmitic (C16:0) acid (1 mM) induced ER stress with 12 h of incubation, marked by significant (P = 0.04–0.002) elevation of CHOP, ERN1, GADD34, and ATF4 and increased XBP1 splicing. In contrast, equimolar oleic acid was identical to control (Fig. 4). Notably, palmitate induction of ER stress differed from that observed with tunicamycin or thapsigargin, both of which strongly upregulated HSPA5, EDEM1, and ATF6 (Fig. 3 compared with Fig. 4 and Supplemental Table S2).
Fig. 4.
Palmitate induces ER stress. A: expression of ER stress transcripts after 12 h of treatment with 1 mM palmitate, 1 mM oleate, or BSA control. Raw expression values were normalized to 18S RNA. Values were expressed relative to the lowest control value as 1 and are presented as means ± SD for 3 independent experiments with 2 biological replicates. *P < 0.05 and **P < 0.01 vs. control (BSA). B: XBP1 splicing for the experiment in A from a representative gel.
Pioglitazone does not reduce palmitate-induced ER stress.
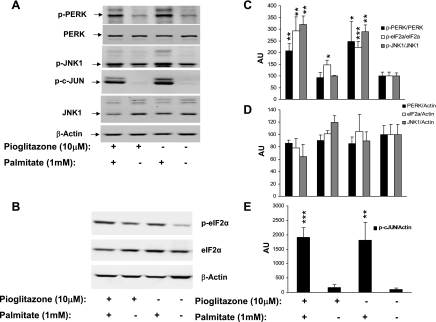
Pioglitazone pretreatment of HepG2 cells (16 h, 10 μM) and the presence of pioglitazone during palmitate treatment failed to reduce ER stress markers induced by 6 or 12 h of 1 mM palmitate treatment, measured by transcript levels (Fig. 5A), XBP1 splicing (Fig. 5B), phosphorylation of eukaryotic initiation factor eIF2α (see Fig. 6, B and C), or phosphorylation of factors downstream of ERN1, including PERK, JNK1, and c-jun (Fig. 6, A, C, and E). Previous studies showed induction of ER stress by a 30–50 μM concentration of TZD. In our study, 10 μM pioglitazone showed no induction of ER stress transcripts even after 28 h (16-h pretreatment followed by a 12-h treatment period; Supplemental Fig. S2). However, we observed a significant increase (P = 0.014) in eukaryotic initiation factor eIF2α phosphorylation (Fig. 6C). To examine another model of human adipose, we repeated this experiment in the human adipocyte SGBS cell line. Palmitate (0.5 mM) modestly increased apoptotic markers CHOP, GADD34, and XBP1 splicing by 6 h; pioglitazone pretreatment for 16 h did not reduce markers of ER stress (Supplemental Fig. S3).
Fig. 5.
Palmitate-induced ER stress after pioglitazone pretreatment. A: expression of ER stress transcripts after 16 h of pretreatment of pioglitazone or DMSO, followed by 12 h of treatment with palmitate (1 mM) or control (BSA). Values were normalized to 18S RNA, and then ratios were normalized to the lowest value of control condition as 1. Data are expressed as means ± SD of 3 independent experiments with 2 biological replicates. *P < 0.05 and **P < 0.01 vs. control (pioglitazone alone). B: XBP1 splicing for the experiment in A.
Fig. 6.
Palmitate-induced ER stress after pioglitazone pretreatment at protein level. A: Western blot showing total and phosphorylated forms of PERK, JNK1, and phosphorylated c-jun from a representative of 3 independent experiments. B: Western blot showing phosphorylated and unphosphorylated eukaryotic initiation factor eIF2α from a representative experiment performed as in Fig. 5A. β-Actin is shown as the loading control. C–E: mean and SD of expression of ERSR marker protein and phosphoproteins after normalization with β-actin or total protein from experiments described in A and B. Data are plotted as arbitrary unit (AU) relative to the mean of the control conditions (DMSO) as 100. *P < 0.05 and **P < 0.01 vs. control (DMSO alone).
DISCUSSION
TZDs are among the most effective drugs in humans at improving glucose homeostasis (32). Although TZDs appear to act primarily to improve peripheral SI, the precise mechanism by which these PPARγ agonists act is unknown. Many pathways have been proposed from stimulation of adipogenesis to activation of AMP kinase or increased adiponectin secretion (5). Animal studies have suggested that ER stress in adipose and liver is a key source of insulin resistance and impaired glucose homeostasis (25), and indeed reduction of ER stress using small molecule chaperones markedly improved glucose homeostasis in vivo in whole animals (26). If ER stress is central to the impaired insulin action and disordered glucose homeostasis of type 2 diabetes, drugs that effectively improve SI could be expected to reduce ER stress. Indeed, recent studies provide some support for that hypothesis in db/db mice (12, 20), whereas opposing data in cell lines suggest that at least some TZDs may induce ER stress by non-PPARγ-mediated mechanisms involving ER calcium depletion (9). Hence we sought evidence that the TZD pioglitazone acts in part to relieve adipocyte or hepatic ER stress in humans. The present study is to our knowledge the only one to examine human tissues and human-derived cell lines with a TZD in active clinical use.
In the present study, we examined each of the major branches of the ER unfolded protein response pathway downstream of the proximal sensors PERK, ATF6, and ERN1 in subcutaneous adipose tissue from humans treated with pioglitazone. The key findings of our study are that pioglitazone neither reduced nor increased adipocyte markers of ER stress in individuals treated for 10 wk with pioglitazone, although these individuals experienced a marked improvement in SI. The improved SI with pioglitazone in a similar population has been reported by our laboratory previously (30). Thus the mechanism of improved SI does not appear to be amelioration of ER stress in adipocytes. Furthermore, pioglitazone suppressed inflammatory adipokines and increased adiponectin levels (31). Although ER stress and inflammatory pathways are tightly connected (13), the failure of pioglitazone to reduce ER stress markers while improving SI and reducing inflammatory cytokines suggests that these pathways can be dissociated, and that a reduction of ER stress is not essential to improve SI and glucose homeostasis.
We recognized that induction of ER stress in hepatocytes might be of additional or larger physiological significance. Unfortunately liver biopsies pre- and post-pioglitazone treatment cannot be justified in humans. Hence we also sought to develop a model of ER stress using the human-derived HepG2 hepatocyte cell line to test whether pioglitazone might protect from ER stress in that system. To examine the role in hepatocytes, we tested the ability of pioglitazone to protect cells from ER stress induced by several mechanisms, including the classic stressors thapsigargin and tunicamycin. As in adipocytes, pioglitazone failed to protect HepG2 cells from ER stress induced by blocking of glycosylation (tunicamycin) or altering of ER calcium levels (thapsigargin). Because these chemical stressors might be considered nonphysiological, we searched for inducers of ER stress in hepatocytes that were closer to the human adipocyte model. Recent reports suggested that levels of circulating free fatty acids in humans are in the range of 0.7–0.8 mM in obese humans and 0.3–0.4 mM in lean individuals (16), thus suggesting that the 1 mM concentrations of palmitate and oleate are near physiological. These studies resulted in a second key finding in the present study: that palmitate but not oleate treatment induced markers of acute ER stress in HepG2 cells.
Similar results showing induction of ER stress with saturated but not unsaturated fatty acids have been reported in a rat liver hepatoma cell line (36), in mouse 3T3-L1 cells and cultured preadipocytes (11), and in a mouse pancreatic β-cell line (17). In contrast, Ota et al. (23) recently reported that prolonged exposure of another hepatocyte line to oleate induced steatosis and ER stress. The reason for this discrepancy is unclear. The mechanism by which saturated fatty acids induce ER stress is uncertain, although recent data suggest that ceramide accumulation is not part of the mechanism (35, 36). Borradaile et al. (4) provided evidence for both intracellular accumulation of reactive oxygen and impaired ER morphology resulting from saturated lipid accumulation and compromised ER membrane integrity.
Previous studies with GN4 rat liver epithelial cells, RINm5F rat pancreatic β-cells, and HepG2 cells (9, 21, 34) showed induction of ER stress with TZD treatment as evidenced by increased phosphorylation of PERK and eIF2α, as well as increased CHOP transcript levels. The doses of pioglitazone used in the present study (10 μM) were well above levels effective for transcriptional activation of human PPARγ (0.28 μM), but well below levels previously shown to be cytotoxic (10). In contrast, ciglitazone and troglitazone were cytotoxic at much lower levels (10), and treatment levels that induced ER stress were much closer to levels demonstrated to have cytotoxicity. Thus we observed no induction ER stress transcripts or splicing of XBP1 in pioglitazone-treated HepG2 cells in the present study, and neither Maniratanachote et al. (21) nor Weber et al. (34) observed increased levels of HSPA5 transcripts at rosiglitazone or troglitazone concentrations below 25–30 μM. Like those previous studies, we did observe a significant increase in eIF2α phosphorylation, suggesting possible selective induction of ER stress by low doses of TZDs.
Recent work from Lin et al. (19) suggested that the ER stress responses differ over the time course of the stress and in different tissues. Such observations suggest the importance of studying multiple tissues and cell lines. For example, in our experiments, ERN1 was threefold increased with palmitate, whereas that increase was not reported in rat hepatoma cell lines (36). Similarly, different inducers of ER stress gave very different patterns of response, both in our studies and in other reports, suggesting that different stresses activate the three proximal ER response pathways to different degrees (8, 27). Regardless of the mechanism and in both liver and adipose, we found that pioglitazone failed to protect against induction of ER stress.
Surprisingly, few other studies have addressed the role of TZDs or insulin sensitizers on ER stress despite a strong rationale for TZD effects on the ER stress response in the improvement of SI. Han et al. (12) recently reported that the relatively weak dual α/γ-agonist macelignan significantly improved glucose homeostasis and insulin action, reduced inflammation, and reduced both hepatic and adipocyte indicators of ER stress in db/db mice including JNK phosphorylation. In contrast, the TZD troglitazone had no significant effect on ER stress markers (12). Additionally, macelignan but not troglitazone was protective in both the 3T3-L1 mouse adipocyte and SK-HEP1 mouse hepatic cell lines treated with either tunicamycin or thapsigargin (12). These data suggest that effects of macelignan on ER stress are not acting through PPARγ and thus are similar to our findings with pioglitazone in human tissues.
If, as suggested by our studies and those of Han et al. (12), TZDs do not act to reduce ER stress, small molecule chaperones (26) that improve insulin action and glucose homeostasis could be synergistic with TZDs. Although our study is the first to address this question in human tissues, our study also has limitations that are inevitable in a human-based study. First, the liver may be a more important source of ER stress-induced insulin resistance than adipocytes but could not be studied in humans. We have used cell lines to address the role of TZDs in the liver, although they are an imperfect surrogate for human hepatocytes. Furthermore, given the ability of palmitate to induce ER stress in HepG2 cells, we cannot exclude the possibility that TZDs act indirectly by reducing plasma free fatty acids to decrease hepatic ER stress and thus hepatic insulin resistance (1, 2). Free fatty acid levels before and after pioglitazone were not available in our study. Likewise, we cannot exclude the possibility that TZDs have an effect on ER stress in visceral adipose, which to our knowledge has not been addressed in mice either. The individuals studied before and after pioglitazone were otherwise healthy, obese individuals with impaired glucose tolerance. Both data presented here for ER stress transcript HSPA5 in 86 individuals and extensive studies of transcription and phosphorylation in subcutaneous adipose tissue from an independent population strongly support the activation of ER stress with obesity (S. C. Elbein, unpublished data). Whether individuals with diabetes or poor glucose control have a different pattern of ER stress is presently under study, and we cannot exclude the possibility that TZDs might reduce ER stress in the setting of hyperglycemia. Finally, muscle is thought to be the primary organ involved in postprandial glucose uptake and thus peripheral SI. We did not include this tissue because ER stress is not activated in muscle from obese db/db mice (25) or in humans (S. C. Elbein, unpublished data).
In summary, despite improved SI and reduced inflammatory cytokines and increased adiponectin in obese, glucose-intolerant subjects treated with pioglitazone, our data suggest no improvement in adipocyte ER stress. These findings are supported by the failure of pioglitazone to protect either the HepG2 hepatocyte or SGBS adipocyte cell lines from ER stress induced by a variety of mechanisms. We suggest that, in humans, one can dissociate reduced inflammation and improved SI from reduced ER stress. Thus, although inflammation, ER stress, and SI are interconnected, reduced adipocyte ER stress is not necessary to reduce inflammation and improve SI. Whether reductions in ER stress using small molecule chaperones could also improve SI in humans remains to be demonstrated.
DISCLOSURES
P. A. Kern has received speaking honoraria from Takeda Pharmaceuticals. N. Rasouli has received investigator-initiated grants in addition to honoraria for speaking engagements from Takeda and Abbott Pharmaceuticals.
GRANTS
This work was supported by the Research Service of the Department of Veterans Affairs (merit funds to S. C. Elbein, P. A. Kern, and N. Rasouli; Research Enhancement Award Program funds) and in part by the General Clinical Research Center (grant no. M01-RR-14288 from the National Center for Research Resources, National Institutes of Health, to the University of Arkansas for Medical Sciences).
Supplementary Material
Acknowledgments
We thank the nursing and laboratory staff of the General Clinical Research Center at the University of Arkansas for Medical Sciences and study coordinators Leslie Miles and Regina Dennis for assisting with subject recruitment and support of the clinical studies. We thank Hua Wang for assistance with development of real-time PCR assays and Takeda Pharmaceuticals for providing pioglitazone for the in vitro experiments and partial support of the pioglitazone trial.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Basu R, Shah P, Basu A, Norby B, Dicke B, Chandramouli V, Cohen O, Landau BR, Rizza RA. Comparison of the effects of pioglitazone and metformin on hepatic and extra-hepatic insulin action in people with type 2 diabetes. Diabetes 57: 24–31, 2008. [DOI] [PubMed] [Google Scholar]
- 2.Boden G, Zhang M. Recent findings concerning thiazolidinediones in the treatment of diabetes. Expert Opin Investig Drugs 15: 243–250, 2006. [DOI] [PubMed] [Google Scholar]
- 3.Bodles AM, Banga A, Rasouli N, Ono F, Kern PA, Owens RJ. Pioglitazone increases secretion of high-molecular-weight adiponectin from adipocytes. Am J Physiol Endocrinol Metab 291: E1100–E1105, 2006. [DOI] [PubMed] [Google Scholar]
- 4.Borradaile NM, Han X, Harp JD, Gale SE, Ory DS, Schaffer JE. Disruption of endoplasmic reticulum structure and integrity in lipotoxic cell death. J Lipid Res 47: 2726–2737, 2006. [DOI] [PubMed] [Google Scholar]
- 5.Bouskila M, Pajvani UB, Scherer PE. Adiponectin: a relevant player in PPARgamma-agonist-mediated improvements in hepatic insulin sensitivity? Int J Obes (Lond) 29, Suppl 1: S17–S23, 2005. [DOI] [PubMed] [Google Scholar]
- 6.Bradford MM A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254, 1976. [DOI] [PubMed] [Google Scholar]
- 7.DeFronzo RA Pathogenesis of type 2 diabetes mellitus. Med Clin North Am 88: 787–835, ix, 2004. [DOI] [PubMed] [Google Scholar]
- 8.DuRose JB, Tam AB, Niwa M. Intrinsic capacities of molecular sensors of the unfolded protein response to sense alternate forms of endoplasmic reticulum stress. Mol Biol Cell 17: 3095–3107, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gardner OS, Shiau CW, Chen CS, Graves LM. Peroxisome proliferator-activated receptor gamma-independent activation of p38 MAPK by thiazolidinediones involves calcium/calmodulin-dependent protein kinase II and protein kinase R: correlation with endoplasmic reticulum stress. J Biol Chem 280: 10109–10118, 2005. [DOI] [PubMed] [Google Scholar]
- 10.Guo L, Zhang L, Sun Y, Muskhelishvili L, Blann E, Dial S, Shi L, Schroth G, Dragan YP. Differences in hepatotoxicity and gene expression profiles by anti-diabetic PPAR gamma agonists on rat primary hepatocytes and human HepG2 cells. Mol Divers 10: 349–360, 2006. [DOI] [PubMed] [Google Scholar]
- 11.Guo W, Wong S, Xie W, Lei T, Luo Z. Palmitate modulates intracellular signaling, induces endoplasmic reticulum stress, and causes apoptosis in mouse 3T3-L1 and rat primary preadipocytes. Am J Physiol Endocrinol Metab 293: E576–E586, 2007. [DOI] [PubMed] [Google Scholar]
- 12.Han KL, Choi JS, Lee JY, Song J, Joe MK, Jung MH, Hwang JK. Therapeutic potential of peroxisome proliferators–activated receptor-alpha/gamma dual agonist with alleviation of endoplasmic reticulum stress for the treatment of diabetes. Diabetes 57: 737–745, 2008. [DOI] [PubMed] [Google Scholar]
- 13.Hotamisligil GS Role of endoplasmic reticulum stress and c-Jun NH2-terminal kinase pathways in inflammation and origin of obesity and diabetes. Diabetes 54, Suppl 2: S73–S78, 2005. [DOI] [PubMed] [Google Scholar]
- 14.Kaneto H, Nakatani Y, Kawamori D, Miyatsuka T, Matsuoka TA, Matsuhisa M, Yamasaki Y. Role of oxidative stress, endoplasmic reticulum stress, and c-Jun N-terminal kinase in pancreatic beta-cell dysfunction and insulin resistance. Int J Biochem Cell Biol 37: 1595–1608, 2005. [DOI] [PubMed] [Google Scholar]
- 15.Kaneto H, Matsuoka TA, Nakatani Y, Kawamori D, Miyatsuka T, Matsuhisa M, Yamasaki Y. Oxidative stress, ER stress, and the JNK pathway in type 2 diabetes. J Mol Med 83: 429–439, 2005. [DOI] [PubMed] [Google Scholar]
- 16.Kankaanpaa M, Lehto HR, Parkka JP, Komu M, Viljanen A, Ferrannini E, Knuuti J, Nuutila P, Parkkola R, Iozzo P. Myocardial triglyceride content and epicardial fat mass in human obesity: relationship to left ventricular function and serum free fatty acid levels. J Clin Endocrinol Metab 91: 4689–4695, 2006. [DOI] [PubMed] [Google Scholar]
- 17.Karaskov E, Scott C, Zhang L, Teodoro T, Ravazzola M, Volchuk A. Chronic palmitate but not oleate exposure induces endoplasmic reticulum stress, which may contribute to INS-1 pancreatic beta-cell apoptosis. Endocrinology 147: 3398–3407, 2006. [DOI] [PubMed] [Google Scholar]
- 18.Li H, Ruan XZ, Powis SH, Fernando R, Mon WY, Wheeler DC, Moorhead JF, Varghese Z. EPA and DHA reduce LPS-induced inflammation responses in HK-2 cells: evidence for a PPAR-gamma-dependent mechanism. Kidney Int 67: 867–874, 2005. [DOI] [PubMed] [Google Scholar]
- 19.Lin JH, Li H, Yasumura D, Cohen HR, Zhang C, Panning B, Shokat KM, Lavail MM, Walter P. IRE1 signaling affects cell fate during the unfolded protein response. Science 318: 944–949, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Loffler M, Bilban M, Reimers M, Waldhausl W, Stulnig TM. Blood glucose-lowering nuclear receptor agonists only partially normalize hepatic gene expression in db/db mice. J Pharmacol Exp Ther 316: 797–804, 2006. [DOI] [PubMed] [Google Scholar]
- 21.Maniratanachote R, Minami K, Katoh M, Nakajima M, Yokoi T. Chaperone proteins involved in troglitazone-induced toxicity in human hepatoma cell lines. Toxicol Sci 83: 293–302, 2005. [DOI] [PubMed] [Google Scholar]
- 22.Nakatani Y, Kaneto H, Kawamori D, Yoshiuchi K, Hatazaki M, Matsuoka TA, Ozawa K, Ogawa S, Hori M, Yamasaki Y, Matsuhisa M. Involvement of endoplasmic reticulum stress in insulin resistance and diabetes. J Biol Chem 280: 847–851, 2005. [DOI] [PubMed] [Google Scholar]
- 23.Ota T, Gayet C, Ginsberg HN. Inhibition of apolipoprotein B100 secretion by lipid-induced hepatic endoplasmic reticulum stress in rodents. J Clin Invest 118: 316–332, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ozawa K, Miyazaki M, Matsuhisa M, Takano K, Nakatani Y, Hatazaki M, Tamatani T, Yamagata K, Miyagawa J, Kitao Y, Hori O, Yamasaki Y, Ogawa S. The endoplasmic reticulum chaperone improves insulin resistance in type 2 diabetes. Diabetes 54: 657–663, 2005. [DOI] [PubMed] [Google Scholar]
- 25.Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi NN, Ozdelen E, Tuncman G, Gorgun C, Glimcher LH, Hotamisligil GS. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science 306: 457–461, 2004. [DOI] [PubMed] [Google Scholar]
- 26.Ozcan U, Yilmaz E, Ozcan L, Furuhashi M, Vaillancourt E, Smith RO, Gorgun CZ, Hotamisligil GS. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science 313: 1137–1140, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pirot P, Ortis F, Cnop M, Ma Y, Hendershot LM, Eizirik DL, Cardozo AK. Transcriptional regulation of the endoplasmic reticulum stress gene chop in pancreatic insulin-producing cells. Diabetes 56: 1069–1077, 2007. [DOI] [PubMed] [Google Scholar]
- 28.Qin S, Liu T, Kamanna VS, Kashyap ML. Pioglitazone stimulates apolipoprotein A-I production without affecting HDL removal in HepG2 cells: involvement of PPAR-alpha. Arterioscler Thromb Vasc Biol 27: 2428–2434, 2007. [DOI] [PubMed] [Google Scholar]
- 29.Rasouli N, Kern PA, Reece EA, Elbein SC. Effects of pioglitazone and metformin on beta-cell function in nondiabetic subjects at high risk for type 2 diabetes. Am J Physiol Endocrinol Metab 292: E359–E365, 2007. [DOI] [PubMed] [Google Scholar]
- 30.Rasouli N, Raue U, Miles LM, Lu T, Di Gregorio GB, Elbein SC, Kern PA. Pioglitazone improves insulin sensitivity through reduction in muscle lipid and redistribution of lipid into adipose tissue. Am J Physiol Endocrinol Metab 288: E930–E934, 2005. [DOI] [PubMed] [Google Scholar]
- 31.Rasouli N, Yao-Borengasser A, Miles LM, Elbein SC, Kern PA. Increased plasma adiponectin in response to pioglitazone does not result from increased gene expression. Am J Physiol Endocrinol Metab 290: E42–E46, 2006. [DOI] [PubMed] [Google Scholar]
- 32.Seufert J, Lubben G, Dietrich K, Bates PC. A comparison of the effects of thiazolidinediones and metformin on metabolic control in patients with type 2 diabetes mellitus. Clin Ther 26: 805–818, 2004. [DOI] [PubMed] [Google Scholar]
- 33.Wang H, Kouri G, Wollheim CB. ER stress and SREBP-1 activation are implicated in beta-cell glucolipotoxicity. J Cell Sci 118: 3905–3915, 2005. [DOI] [PubMed] [Google Scholar]
- 34.Weber SM, Chambers KT, Bensch KG, Scarim AL, Corbett JA. PPARγ ligands induce ER stress in pancreatic β-cells: ER stress activation results in attenuation of cytokine signaling. Am J Physiol Endocrinol Metab 287: E1171–E1177, 2004. [DOI] [PubMed] [Google Scholar]
- 35.Wei Y, Wang D, Pagliassotti MJ. Saturated fatty acid-mediated endoplasmic reticulum stress and apoptosis are augmented by trans-10, cis-12-conjugated linoleic acid in liver cells. Mol Cell Biochem 303: 105–113, 2007. [DOI] [PubMed] [Google Scholar]
- 36.Wei Y, Wang D, Topczewski F, Pagliassotti MJ. Saturated fatty acids induce endoplasmic reticulum stress and apoptosis independently of ceramide in liver cells. Am J Physiol Endocrinol Metab 291: E275–E281, 2006. [DOI] [PubMed] [Google Scholar]
- 37.Yang X, Smith U. Adipose tissue distribution and risk of metabolic disease: does thiazolidinedione-induced adipose tissue redistribution provide a clue to the answer? Diabetologia 50: 1127–1139, 2007. [DOI] [PubMed] [Google Scholar]
- 38.Yao-Borengasser A, Varma V, Bodles AM, Rasouli N, Phanavanh B, Lee MJ, Starks T, Kern LM, Spencer HJ 3rd, Rashidi AA, McGehee RE Jr, Fried SK, Kern PA. Retinol binding protein 4 expression in humans: relationship to insulin resistance, inflammation, and response to pioglitazone. J Clin Endocrinol Metab 92: 2590–2597, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yoshiuchi K, Kaneto H, Matsuoka TA, Kohno K, Iwawaki T, Nakatani Y, Yamasaki Y, Hori M, Matsuhisa M. Direct monitoring of in vivo ER stress during the development of insulin resistance with ER stress-activated indicator transgenic mice. Biochem Biophys Res Commun 366: 545–550, 2008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.