Abstract
The hypothalamus plays a key role in the regulation of both energy homeostasis and reproduction. Evidence suggests that relaxin-3, a recently discovered member of the insulin superfamily, is an orexigenic hypothalamic neuropeptide. Relaxin-3 is thought to act in the brain via the RXFP3 receptor, although the RXFP1 receptor may also play a role. Relaxin-3, RXFP3, and RXFP1 are present in the hypothalamic paraventricular nucleus, an area with a well-characterized role in the regulation of energy balance that also modulates reproductive function by providing inputs to hypothalamic gonadotropin-releasing hormone (GnRH) neurons. Other members of the relaxin family are known to play a role in the regulation of reproduction. However, the effects of relaxin-3 on reproductive function are unknown. We studied the role of relaxin-3 in the regulation of the hypothalamo-pituitary-gonadal (HPG) axis. Intracerebroventricular (5 nmol) and intraparaventricular (540–1,620 pmol) administration of human relaxin-3 (H3) in adult male Wistar rats significantly increased plasma luteinizing hormone (LH) 30 min postinjection. This effect was blocked by pretreatment with a peripheral GnRH antagonist. Central administration of human relaxin-2 showed no significant effect on plasma LH. H3 dose-dependently stimulated the release of GnRH from hypothalamic explants and GT1-7 cells, which express RXFP1 and RXFP3, but did not influence LH or follicle-stimulating hormone release from pituitary fragments in vitro. We have demonstrated a novel role for relaxin-3 in the stimulation of the HPG axis, putatively via hypothalamic GnRH neurons. Relaxin-3 may act as a central signal linking nutritional status and reproductive function.
Keywords: RXFP3, RXFP1, paraventricular nucleus, gonadotropin-releasing hormone, gonadotropins
the link between energy balance and fertility relies on peripheral signals of nutritional status modulating central effectors that in turn influence hypothalamic gonadotropin-releasing hormone (GnRH) release. GnRH neurones are situated in the rostral hypothalamus and medial preoptic area (MPOA), as well as the basal forebrain (40, 55). Primary afferents to GnRH neurons are located predominantly in periventricular areas of the hypothalamus, including the arcuate nucleus (ARC) and paraventricular (PVN) nucleus (56). Several hypothalamic neuropeptides and their receptors are present in these nuclei, including the most recently characterized member of the insulin superfamily, relaxin-3 (1).
Before the discovery of the relaxin (RLN)-3 gene and its peptide, only one other relaxin gene, RLN1, had been characterized in most species. However, in humans and higher primates, two separate genes, RLN1 and RLN2, had been identified encoding for relaxin (13, 14, 43). [The human gene product of RLN2, human relaxin-2 (H2), is the functional ortholog of the RLN1 gene in other mammals]. Relaxin has been classically associated with female reproductive physiology, although it is also known to have marked effects on water intake (41, 50). In contrast, the physiological role of relaxin-3 remains unclear. However, evidence suggests it may play a role in appetite control (28–30). Unlike relaxin, relaxin-3 is highly homologous across species. It is expressed at greatest levels in the central nervous system, and expression is localized to a distinct area called the nucleus incertus (NI), situated in the caudoventral region of the pontine periventricular gray (6, 48). Anatomical studies suggest that this nucleus is involved in a midbrain behavior control network that influences circuits regulating locomotion, attention, and learning processes and that responds to stress-related neuroendocrine signals (10). Tracing studies have shown that the neurons of the NI project extensively to hypothalamic areas, including the lateral hypothalamic area (LHA), the posterior hypothalamic nucleus, and the medial and periventricular zones (10). Relaxin-3 immunoreactivity has been described in hypothalamic areas important in the regulation of the hypothalamic-pituitary-gonadal (HPG) axis, including the PVN, ARC, MPOA, and lateral preoptic area (LPOA) (48). Thus, although the NI itself has not been implicated in reproduction or feeding, relaxin-3 immunoreactive fibers project from this area to hypothalamic areas of the brain with well-characterized roles in the regulation of appetite and reproduction. Interestingly, relaxin-3 has also been shown to be expressed peripherally in the mouse by RT-PCR, in tissues including the testis, ovary, thymus, lung, and kidney (1). Peripheral expression of relaxin-3 mRNA has been detected by RT-PCR in human and rat testis (8, 22), reinforcing a potential peripheral role in reproduction. However, Northern blotting has indicated that the primary site of expression in the mouse is the brain (1).
Unlike insulin, which signals via tyrosine kinase receptors, relaxin peptides signal via G protein-coupled receptors to modulate intracellular cAMP. Relaxin binds with high affinity to two leucine-rich repeat-containing receptors, RXFP1 (formerly known as LGR7) and RXFP2 (formerly known as LGR8) (12). Both of these receptors are expressed in the brain, in addition to the testis and other peripheral tissues (7, 12, 39). Relaxin-3 binds to RXFP3 (8, 22, 46) and inhibits cAMP production, but it also binds and activates RXFP1 (45) and RXFP4 (21, 22), a receptor that is not expressed in the rat (8) but does contribute to relaxin-3 binding in the mouse brain (8, 21, 46). RXFP1 and RXFP3 are both expressed within the rat hypothalamus, particularly in the PVN and supraoptic nucleus (7, 22, 46, 47).
Because relaxin-3 immunoreactive fibers and relaxin-3 receptors are present in areas where GnRH neurons are present and/or primary afferents to GnRH neurons are located (10, 22, 40, 47, 48, 55), we hypothesized that relaxin-3 may play a role in the control of the HPG axis. The aims of these current studies were threefold. First, we sought to investigate the effects of central administration of relaxin-3 on circulating gonadotropins and total testosterone in male rats following administration of relaxin-3 in the third cerebral ventricle and injection in the PVN, an area rich in relaxin-3 immunoreactivity, relaxin-3 receptors, and afferents to GnRH neurons. Second, we sought to test the hypothesis that these actions are mediated via GnRH by examining 1) the effects of pretreatment with a peripheral GnRH antagonist on relaxin-3-mediated gonadotropin and total testosterone release in vivo, 2) the effects of relaxin-3 on gonadotropin secretion from rat anterior pituitary quarters, and 3) GnRH release from hypothalamic explants in vitro. Finally, we sought to investigate the possibility of a direct effect of relaxin-3 on GnRH neurons in vitro by examining relaxin-3 receptor expression and the actions of relaxin-3 on GT1-7 cells.
METHODS
Materials
Human relaxin-3 (H3; Phoenix Pharmaceuticals, Belmont, CA, and the Howard Florey Institute, Melbourne, Australia) was synthesized using solid-phase synthesis. H2 was purchased from Dr. A. Parlow, National Hormone and Peptide Program (Torrance, CA). The GnRH antagonist cetrorelix acetate (0.25-mg or 3-mg preparations; Asta Medica) was used in our in vivo studies (36), since it has been found to exert no systemic side effects in both pharmacological and long-term toxicological studies in rats and dogs (36). Reagents for basal hypothalamic explant studies and pituitary fragment experiments were supplied by BDH (Poole, Dorset, UK).
Animal Studies
Male Wistar rats (Specific pathogen free; Charles River) weighing 250–300 g were maintained in individual cages for all in vivo studies. Male Wistar rats weighing 200–220 g were caged in groups of five for use in hypothalamic and pituitary explant experiments. All animals were kept under controlled temperature (21–23°C) and light (12:12-h light-dark cycle, lights on at 0700) with ad libitum access to food (pelleted RM1 chow diet; Special Diet Services) and water unless otherwise stated. All procedures undertaken were approved under the British Home Office Animals (Scientific Procedures) Act of 1986 (project license 70/5516).
Intracerebroventricular and Intraparaventricular Cannulation and Injection
Male Wistar rats underwent intracerebroventricular or unilateral intraparaventricular cannulation 7–10 days before the studies and were habituated to regular handling and injection, as previously described (54). Central injections [5 μl (icv) or 1 μl (iPVN)] were administered over 1 min via stainless steel injectors [27-gauge (icv) or 31-gauge (iPVN)], placed in and projecting 1 mm below the end of the cannula. Spread of a 1-μl injection in the PVN is reported to be limited to 1 mm3 (52). H3 (obtained from the Howard Florey Institute) and H2 for study 1 were dissolved in 0.9% saline. H3 (obtained from Phoenix Pharmaceuticals) for studies 2 and 3 and H2 for study 3 were dissolved in 10% acetonitrile in 0.9% saline (29). Thus vehicle for experiment 1 was 0.9% saline, and vehicle for experiments 2 and 3 was 10% acetonitrile in 0.9% saline. Studies were performed in satiated rats (n = 10–12) in the early light phase (0900–1000) unless otherwise stated.
Intracerebroventricular cannula position was verified by a positive dipsogenic response to ANG II (150 ng/rat). Only those animals with correct cannula placement were included in the data analysis. For the PVN-cannulated animals, cannula position was verified histologically at the end of the study (54). Immediately following decapitation, 1 μl India ink was injected in the cannula. The brains were removed and fixed in 4% paraformaldehyde, dehydrated in 40% sucrose, frozen, and stored at −70°C. Brains were sliced on a cryostat (Bright, Huntingdon, UK) in 15 μm coronal sections, and correct PVN-placement was determined by microscopy according to the position of the India ink.
In Vivo Effects of Relaxin-3 on the HPG Axis
Study 1: Effect of intracerebroventricular relaxin-3 on the HPG axis and effect of GnRH antagonist on relaxin-3-mediated luteinizing hormone release.
Male Wistar rats were preinjected subcutaneously with the GnRH antagonist Cetrorelix acetate (200 nmol/0.2 ml) (25) or vehicle. Animals received a single intracerebroventricular injection (5 μl) of vehicle, H3 (5 nmol), or H2 (5 nmol) 30 min later (n = 10/group). This intracerebroventricular dose is of the same order of magnitude as the effective intracerebroventricular dose of kisspeptin-10 used to stimulate the HPG axis in male Wistar rats (0.1–3 nmol) (49). After intracerebroventricular administration (30 min), animals were killed by decapitation, and plasma was collected in plastic lithium heparin tubes containing 4,200 kallikrein inhibitor units (KIU) aprotinin (Bayer; Haywards, Heath, UK). Plasma was separated by centrifugation, frozen, and stored at −20°C until RIA for measurement of luteinizing hormone (LH), follicle-stimulating hormone (FSH), and total testosterone.
Study 2: Time course effect of intraparaventricular relaxin-3 on the HPG axis.
Male Wistar rats (n = 10–12/group) received a single intraparaventricular injection (1 μl) of vehicle or H3 (540 pmol). After administration (15 or 30 min), animals were killed by decapitation, and plasma was collected in plastic lithium heparin tubes containing 4,200 KIU aprotinin (Bayer). Plasma was separated by centrifugation, frozen, and stored at −20°C until RIA for measurement of LH, FSH, and total testosterone.
Study 3: Dose response of intraparaventricular relaxin-3 on the HPG axis and effect of GnRH antagonist on relaxin-3-mediated LH release.
Male Wistar rats (n = 8–10/group) received a single intraparaventricular injection (1 μl) of vehicle, H3 at 1.8, 18, 180, 540, and 1,620 pmol, or H2 (540 pmol). A further group of animals (n = 4) received a subcutaneous injection of the GnRH antagonist Cetrorelix acetate (60 nmol/0.2 ml saline) 1 h before intraparaventricular injection of relaxin-3 (540 pmol). After intraparaventricular administration (30 min), animals were killed by decapitation, and plasma was collected in plastic lithium heparin tubes containing 4,200 KIU aprotinin (Bayer). Plasma was separated by centrifugation, frozen, and stored at −20°C until RIA for measurement of LH, FSH, and total testosterone.
In Vitro Effects of Relaxin-3 on the HPG Axis
Study 4: Effect of relaxin-3 on gonadotropin release from anterior pituitary quarters.
The effects of relaxin-3 on pituitary LH and FSH release were determined using anterior pituitary quarters. The method was a modification of that previously described (5). Rats were decapitated, and anterior pituitary glands were harvested immediately then divided into four pieces of approximately equal size. The segments were randomly placed (1 segment/well) in the wells of a 48-well tissue culture plate (Nunc International) and incubated in 500 μl of artificial cerebrospinal fluid (aCSF) (20 mM NaHCO3, 126 mM NaCl, 0.09 mM Na2HPO4, 6 mM KCl, 1.4 mM CaCl2, 0.09 mM MgSO4, 5 mM glucose, 0.18 mg/ml ascorbic acid, and 100 μg/ml aprotinin). The quarters were maintained at 37°C in a humidified environment saturated with 95% O2 and 5% CO2 for 2 h with the medium changed every hour. The segments were then incubated in aCSF alone (control), H3 at 100 nM, or 1,000 or 100 nM GnRH as a positive control for 4 h (n = 24/group). At the end of this period, the aCSF was collected and stored at −20°C until RIA for measurement of LH and FSH.
Study 5: Effect of relaxin-3 on hypothalamic GnRH release.
The static incubation system was used as previously described (44). Briefly, male Wistar rats were killed by decapitation, and the brain was removed immediately. The brain was mounted with the ventral surface uppermost and placed in a vibrating microtome (Microfield Scientific, Dartmouth, UK). A 1.7-mm slice to include the MPOA was taken from the basal hypothalamus and incubated in an individual chamber containing 1 ml aCSF (as described above) equilibrated with 95% O2 and 5% CO2. The tubes were placed on a shaking platform in a water bath maintained at 37°C. After an initial 2-h equilibration period, the hypothalami were incubated for 45 min in 600 μl aCSF (basal period) before being challenged with H3 (10, 100, or 1,000 nM) for 45 min (n = 15–16/group). Finally, the viability of the tissue was verified by a 45-min exposure to 56 mM KCl; isotonicity was maintained by substituting K+ for Na+. At the end of each period, the aCSF was removed and frozen at −20°C until RIA for measurement of GnRH.
Study 6: Effect of relaxin-3 on GnRH release in GT1-7 cells.
The effects of relaxin-3 on GnRH release from the immortalized GnRH-producing neuronal GT1-7 cell line were determined. The GT1-7 cells were maintained at 37°C in 6% CO2 in DMEM with 4.5 g/l glucose, 10% FBS, 100 IU/ml penicillin, and 100 μg/ml streptomycin. Before the experiments (3 days), cells were plated on poly-l-lysine-coated 24-well plates. The cell concentration was 3 × 106 cells/ml at the time of plating. After 3 days, the medium was discarded, and cells were preincubated for 2 h in serum-free medium. Thereafter, the serum-free medium was discarded, and the cells were incubated in 500 μl of serum-free medium or H3 (10, 100, or 1,000 nM) for 1 h (n = 9–10/group). Glucagon-like peptide-1 (GLP-1; 100 nM) was used as a positive control (2). At the end of each incubation period, medium was removed and frozen at −20°C until measurement of GnRH levels by RIA.
Study 7: Examination of RXFP1, RXFP3, and RXFP4 expression in GT1-7 cells.
RNA from GT1-7 cells was extracted using Trizol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer's protocol. Two micrograms were reverse-transcribed using an Omniscript RT kit (Qiagen, Valencia, CA) in a reaction primed using oligo(dT) (15-mer). One microliter of this reaction was subjected to PCR using primers obtained from the published sequences (mouse RXFP1: NM212452; mouse RXFP3: NM178717; and mouse RXFP4: NM181817). Primers were synthesized by Integrated DNA technologies (Coralville, IA). The following primers were used: RXFP1 coding region, 3′-GTG-CCA-GAG-TTG-ATG-GAT-CA and 5′-AAG-ACA-CGG-GAA-GTA-CTG-GA; RXFP3 coding region, 3′-CTT-GCG-GAA-CTC-GCG-GCG-CAC-TAA-GCA-GTA-GAG-GAT and 5′-TGC-TGG-GCT-TCC-TGC-TGC-CGC-TGA-GCA-TCA-TCA; and RXFP4 coding region, 3′-AGG-GCA-CGA-TGA-AGG-CCA-GAA-CTA-CCC-TCT and 5′-GTG-TGC-CCG-GCC-TGT-CTT-GTG-ATA-CCT-TTG. The concentrations used in the PCR mixture were as follows: RT mixture 1 μl, AmpliTaq DNA polymerase buffer 3 μl (Promega), MgCl2 2.4 μl, dNTP 0.6 μl, 5′-primer 0.72 μl, 3′-primer 0.72 μl, AmpliTaq DNA polymerase 0.15 μl (Promega), and glass-distilled water 21.41 μl. The conditions used for the PCR were 95°C for 45 s, 60°C (RXFP1) or 65°C (RXFP3 and -4) for 30 s, and 72°C for 45 s for 30 cycles. With the use of these primers, amplified fragments of 385 bp for RXFP1, 385 bp for RXFP3, and 440 bp for RXFP4 would be expected. Controls treated in an identical way, but with no RT added to the reverse transcription, were conducted at the same time. Mouse genomic DNA was used as a positive control in all PCR reactions, and all reactions were analyzed on a 1% agarose gel.
Hormone Assays
GnRH was measured by a sensitive and specific RIA (reagents and methods provided by Dr. H. M. Fraser, Medical Research Council Reproductive Biology Unit, Edinburgh, Scotland). The intra- and interassay coefficients of variation were 8.3 and 8.5%, respectively. LH levels for all studies and FSH levels for study 2 were assayed using reagents and methods provided by the National Institute of Diabetes and Digestive and Kidney Diseases and the National Hormone and Pituitary Program (Dr. A. Parlow, Harbor University of California, Los Angeles Medical Center) as previously described (3). The intra- and interassay coefficients of variation were 8.2 and 8.6%, respectively, for LH and 8.3 and 12.4% for FSH. For studies 1 and 4, plasma FSH was measured by commercial immunoradiometric assay (IDS, Boldon, UK), and for all studies total plasma testosterone was measured by commercial Coat-a-Count assay kit (EURO/DPC Limited, Caernarfon, UK).
Statistical Analysis
Results are shown as means ± SE. All in vivo studies (studies 1–3) and in vitro studies 4 and 6 were analyzed by ANOVA with post hoc Tukey's test (Systat, Evanston, IL). Hypothalamic explant data (study 5) were compared by paired Student's t-test between control and treated groups. In all cases, P < 0.05 was considered to be statistically significant.
RESULTS
In Vivo Effects of Relaxin-3 on the HPG Axis
Study 1: Effect of intracerebroventricular relaxin-3 on the HPG axis and effect of a GnRH antagonist on relaxin-3-mediated LH release.
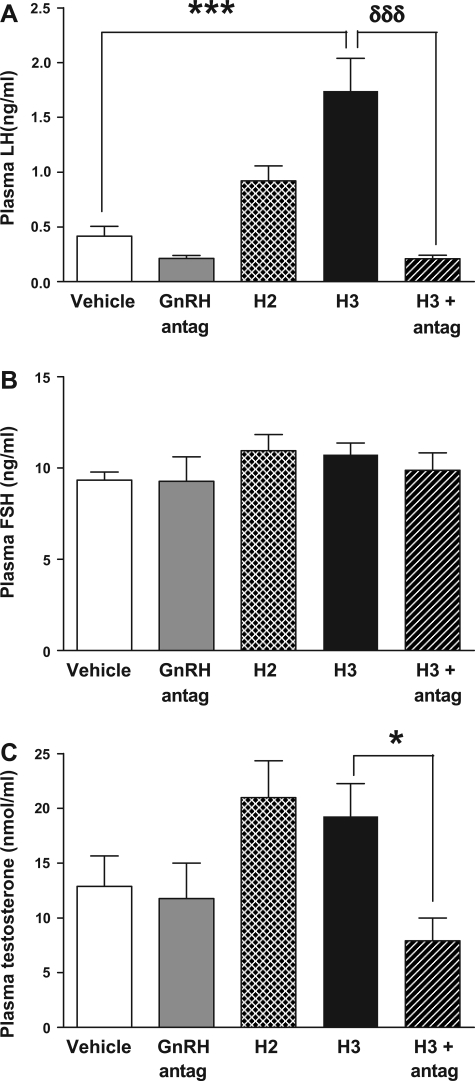
A single intracerebroventricular injection of H3 (5 nmol) to male Wistar rats significantly increased plasma LH 30 min postinjection [0.42 ± 0.09 ng/ml (vehicle) vs. 1.74 ± 0.30 ng/ml (H3), P < 0.001 vs. vehicle] (Fig. 1A). This effect was blocked by preadministration of a peripheral GnRH antagonist [1.74 ± 0.30 (H3) vs. 0.21 ± 0.03 ng/ml (H3 and GnRH antagonist), P < 0.001 vs. H3] (Fig. 1A). Administration of peripheral GnRH antagonist alone had no significant effect on LH release [0.42 ± 0.09 ng/ml (vehicle) vs. 0.32 ± 0.04 ng/ml (GnRH antagonist)] (Fig. 1A). A single intracerebroventricular injection of H2 to male Wistar rats showed a trend toward increased plasma LH 30 min postinjection, but this did not achieve statistical significance (Fig. 1A). Total plasma testosterone was not significantly increased at 30 min postinjection of H3 or H2 (Fig. 1C). However, testosterone levels in the H3 group pretreated with peripheral GnRH antagonist were significantly lower compared with testosterone levels in the group treated with H3 alone [19.27 ± 3.00 nmol/l (H3) vs. 7.91 ± 2.08 nmol/l (H3 and GnRH antagonist), P < 0.05 vs. H3] (Fig. 1C). There was no change in plasma FSH levels 30 min postintracerebroventricular administration of H2 and H3 (Fig. 1B).
Fig. 1.
Effect of icv administration of human relaxin-2 (H2, 5 nmol), human relaxin-3 (H3, 5 nmol), and H3 (5 nmol) with gonadotropin-releasing hormone (GnRH) antagonist (200 nmol) on plasma luteinizing hormone (LH, ng/ml; A), plasma follicle-stimulating hormone (FSH, ng/ml; B), and total plasma testosterone (nmol/l; C) at 30 min postinjection in male adult rats. *P < 0.05, ***P < 0.001, and δδδP < 0.001 by ANOVA with post hoc Tukey's test, n = 10/group.
Study 2: Time course effect of intraparaventricular relaxin-3 on the HPG axis.
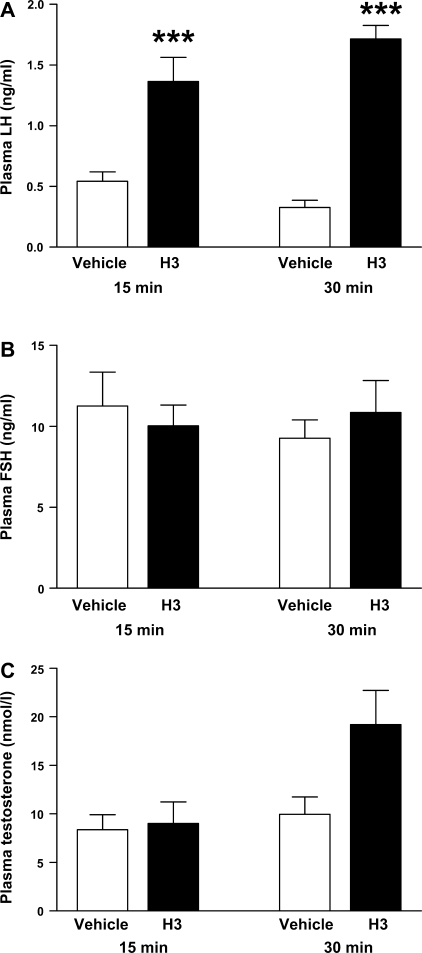
The time course effect of intraparaventricular H3 administration on the HPG axis was examined. A single intraparaventricular injection of H3 (540 pmol) to male Wistar rats significantly increased plasma LH at 15 min postadministration of H3 [0.54 ± 0.08 ng/ml (vehicle) vs. 1.37 ± 0.20 ng/ml (H3), P < 0.001], and this effect was maintained at 30 min postinjection [0.33 ± 0.07 ng/ml (vehicle) vs. 1.72 ± 0.11 ng/ml (H3), P < 0.001] (Fig. 2A). Plasma FSH levels were unchanged at both time points post-H3 administration. There was no change in total plasma testosterone 15 min postinjection. Although testosterone was increased 30 min following injection, this effect failed to reach statistical significance [9.97 ± 1.79 nmol/l (vehicle) vs. 19.19 ± 3.53 nmol/l (H3), P = 0.056], (Fig. 2B).
Fig. 2.
Effect of iPVN administration of H3 (540 pmol) on plasma LH (ng/ml; A), plasma FSH (ng/ml; B), and total plasma testosterone (nmol/l; C) at 15 and 30 min postinjection in male adult rats. ***P < 0.001 vs. vehicle by ANOVA with post hoc Tukey's test (n = 10–12/group).
Study 3: Dose response of intraparaventricular relaxin-3 on the HPG axis and effect of GnRH antagonist on relaxin-3-mediated LH release.
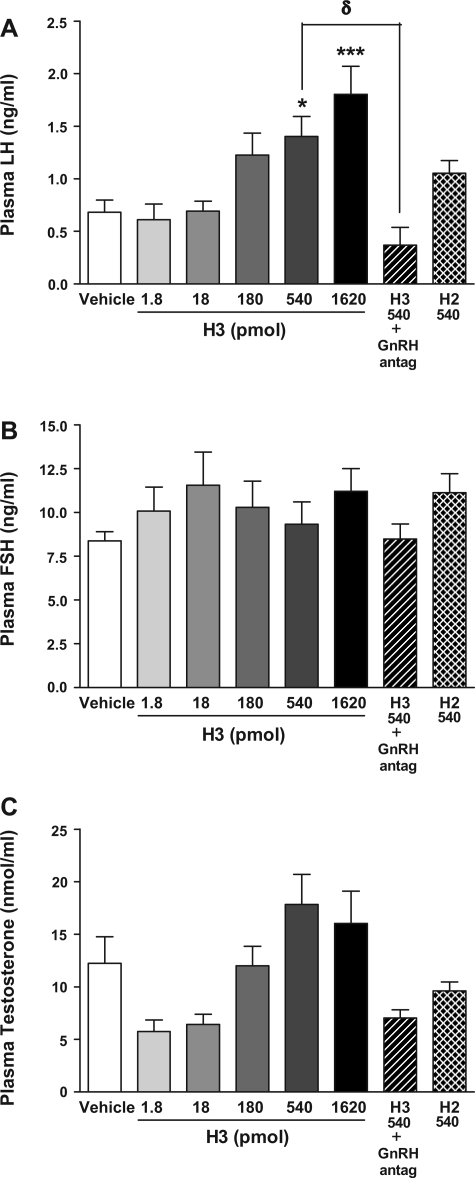
To investigate the effects of increasing doses of intraparaventricular H3 on LH release, a single intraparaventricular injection of H3 was given to male Wistar rats. Intraparaventricular H3 was found to dose dependently increase plasma LH 30 min postinjection. Plasma LH was significantly increased following intraparaventricular injection of 540 and 1,620 pmol H3 [0.68 ± 0.12 ng/ml (vehicle) vs. 1.40 ± 0.19 ng/ml (540 pmol H3), P < 0.05, and 1.80 ± 0.27 ng/ml (1,620 pmol H3), P < 0.001] (Fig. 3A). There was no statistically significant effect on plasma LH at lower doses of H3 (1.8–180 pmol) (Fig. 3A). The rise in LH stimulated by H3 (540 pmol) at 30 min postinjection was abolished in rats pretreated with a peripheral GnRH antagonist [0.68 ± 0.12 ng/ml (vehicle) vs. 1.40 ± 0.19 ng/ml H3 (540 pmol), P < 0.05 vs. vehicle, vs. 0.37 ± 0.1.7 ng/ml (GnRH antagonist and 540 pmol H3), P < 0.05 vs. H3 (540 pmol)] (Fig. 3A). A single intraparaventricular injection of H2 (540 pmol) to male Wistar rats increased plasma LH 30 min postinjection, but this did not achieve statistical significance (Fig. 3A). Plasma FSH (Fig. 3B) and total plasma testosterone (Fig. 3C) were not significantly increased at 30 min postinjection at all doses of H3, although rises in LH were associated with nonsignificant increases in total plasma testosterone at equivalent time points and doses (Fig. 3, A and C). Total plasma testosterone levels in the H3 group pretreated with a peripheral GnRH antagonist showed a nonsignificant reduction compared with the group treated with H3 alone [17.84 ± 5. 64 nmol/l (540 pmol H3) vs. 7.03 ± 3.52 nmol/l (540 pmol H3 and GnRH antagonist), P = 0.12 vs. H3 by ANOVA with post hoc Tukey test] (Fig. 3C).
Fig. 3.
Effect of iPVN administration of H2 (540 pmol), H3 (1.8–1,620 pmol) (n = 10–12/group), and GnRH antagonist (60 nmol) and H3 (540 pmol) (n = 4) on plasma LH (ng/ml; A), plasma FSH (ng/ml; B), and total plasma testosterone (nmol/l; C) at 30 min postinjection in male adult rats. *P < 0.05 vs. vehicle, ***P < 0.001 vs. vehicle, and δP < 0.05 vs. relaxin-3 (540 pmol) by ANOVA with post hoc Tukey's test.
In Vitro Effects of Relaxin-3 on the HPG Axis
Study 4: Effect of relaxin-3 on gonadotropin release from anterior pituitary fragments.
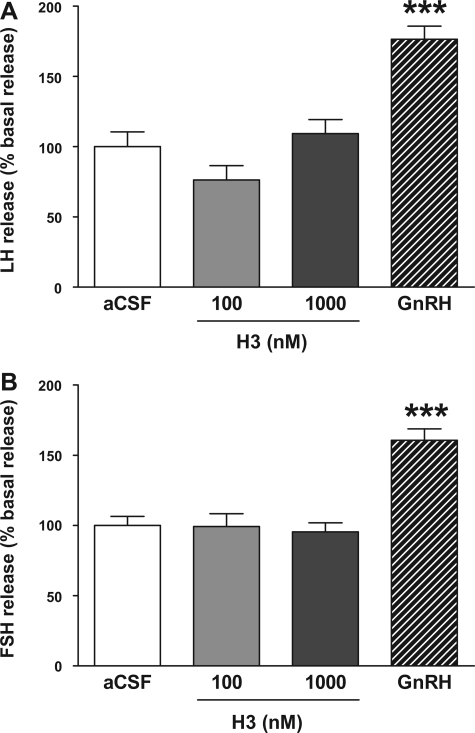
Administration of H3 (100 and 1,000 nM) had no effect on the release of LH or FSH from in vitro pituitary fragments (Fig. 4, A and B), suggesting that relaxin-3 alone is not acting at the level of the pituitary to influence the HPG axis. The positive control GnRH significantly increased LH release [100 ± 10.5 basal vs. 176.5 ± 9.3 (GnRH 100 nM), P < 0.001] and FSH release [100 ± 6.4 basal vs. 160.6 ± 8.2 (GnRH 100 nM), P < 0.001], with data shown as a percentage of basal release and with basal aCSF indicated as 100% (Fig. 4, A and B).
Fig. 4.
Effect of H3 (100 and 1,000 nM) on the release of gonadotropins from in vitro anterior pituitary fragments [data shown as %basal release with basal artificial cerebrospinal fluid (aCSF) indicated as 100%]. A: LH; B: FSH. GnRH was used as a positive control. ***P < 0.001 vs. basal aCSF by ANOVA with post hoc Tukey's test, n = 24/group.
Study 5: Effect of relaxin-3 on hypothalamic GnRH release.
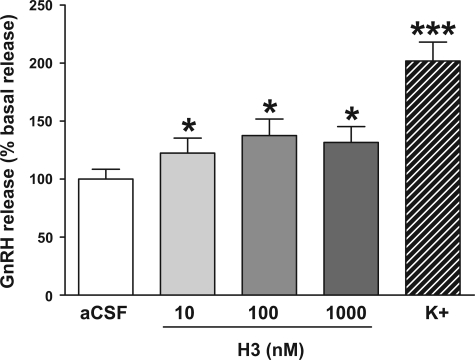
Administration of H3 (10–1,000 nM) to hypothalamic explants stimulated the release of GnRH [100 ± 8.5 basal vs. 122.4 ± 12.9 (10 nM H3), 137.5 ± 14.3 (100 nM H3), 131.6 ± 13.6 (1,000 nM H3), data shown as %basal release with basal aCSF indicated as 100%, P < 0.001 vs. basal aCSF] (Fig. 5). The stimulation of GnRH release appeared to reach a plateau at a dose of 100 nM H3, with no further increase at the higher dose of 1,000 nM H3 (Fig. 5). The positive control (aCSF with 56 nM K+) significantly increased GnRH release [100 ± 8.5 basal vs. 201.8 ± 16.3 (aCSF with 56 nM K+), P < 0.001 by paired t-test] (Fig. 5).
Fig. 5.
Effect of H3 (10, 100, and 1,000 nM) on stimulation of GnRH release from in vitro hypothalamic explants (data shown as %basal release with basal aCSF indicated as 100%). The positive control was aCSF containing 56 nM K+. *P < 0.001 vs. aCSF control by paired t-test, n = 15–16/group.
Study 6: Effect of relaxin-3 on GnRH release in GT1-7 cells.
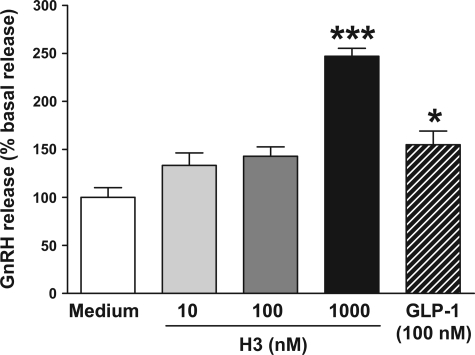
The effect of increasing doses of H3 (10–1,000 nM) on GnRH release from GT1-7 cells was examined at 1 h. Exposure of GT1-7 cells to H3 resulted in an increase in GnRH release at 1 h that became highly significant at a concentration of 1,000 nM H3 [100 ± 10.1 basal vs. 247.2 ± 8.3 (1,000 nM H3), data shown as %basal, P < 0.001 vs. serum-free medium] (Fig. 6). The positive control (100 nM GLP-1) significantly increased GnRH release at 1 h [100 ± 10.1 basal vs. 154.9 ± 14.2 (100 nM GLP-1), P < 0.05] (Fig. 6).
Fig. 6.
Effect of H3 (10, 100, and 1,000 nM) on stimulation of GnRH release from GT1-7 cells at 0–1 h (data shown as %basal release with basal serum-free medium indicated as 100%). *P < 0.05 and ***P < 0.001 vs. serum-free medium by ANOVA with post hoc Tukey's test, n = 10/group.
Study 7: Examination of RXFP1, RXFP3, and RXFP4 expression in GT1-7 cells.
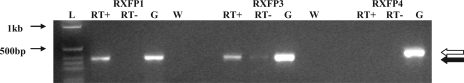
Reverse transcription of DNA extracted from GT1-7 cells followed by PCR demonstrated bands of the appropriate size for RXFP1 and RXFP3, but no band was identified following PCR for RXFP4. These results suggest that both RXFP1 and RXFP3 are expressed in GT1-7 cells and may be involved in mediating relaxin-3-stimulated GnRH release (Fig. 7).
Fig. 7.
Relaxin receptor expression in GT1-7 cells. Gel demonstrating PCR products following amplification with RXFP1-, RXFP3-, and RXFP4-specific primers of reverse transcribed GT1-7 cell RNA and murine genomic DNA as a control. L, ladder; RT+, reverse transcribed GT1-7 cell RNA; RT−, GT1-7 cell RNA without reverse transcriptase;, expected size of RXFP4 product;, expected size of RXFP1 and RXFP3 product; G, genomic DNA; W water.
DISCUSSION
Energy homeostasis and reproduction are intrinsically linked (18, 42). A large number of peripheral hormones that alter food intake, including leptin, ghrelin, and peptide Y 3–36 (PYY3–36), have been shown to play a role in the control of the reproductive axis (9). Central regulators of appetite, including neuropeptide Y (NPY), the orexins, and galanin-like peptide, can also modulate the HPG axis (16, 18, 34, 38). However, the regulation of, and interactions between, energy status and reproductive function are not yet fully understood.
We have previously shown that central administration of H3 increases food intake in rats and modulates the HPG axis (28, 29). These studies also demonstrated that central H3 injection increases plasma LH, an effect that is blocked by administration of a peripheral GnRH antagonist. Relaxin-3 immunoreactivity is found at high concentration in the PVN, and RXFP3 receptors are also present here. Although the PVN is considered important in the regulation of energy balance (53), it is not traditionally associated with a role in reproduction. However, recent studies have shown that intraparaventricular injections of the neuropeptide kisspeptin, a potent central and peripheral stimulator of the HPG axis, increase LH and testosterone levels in male adult rats (32). Primary afferents to GnRH neurons are located in the PVN (56), suggesting this nucleus may play a role in coordinating reproductive responses to altered energy balance. This evidence, together with the presence of relaxin-3 immunoreactivity and relaxin-3 receptors in the PVN, prompted us to investigate the role of intraparaventricular H3 on the HPG axis. A single intraparaventricular injection of H3 stimulated the HPG axis, reinforcing the suggestion that the PVN may be important in providing inputs to GnRH neurons. The effects of intraparaventricular H3 on LH release in adult male rats were dose dependent, with an intranuclear dose of 540 pmol of H3 being required to elicit a significant response at 30 min postinjection. Following intracerebroventricular H3 administration, total plasma testosterone rose, mirroring changes in circulating LH concentrations, although these changes did not reach statistical significance. Because changes in plasma testosterone levels are downstream from changes in plasma LH, a later time point, e.g., 60 min following central administration of relaxin-3, may have shown a significant increase in plasma testosterone (49). There was no effect on circulating FSH with intracerebroventricular or intraparaventricular administration of H3 at 30 min at all doses of peptide. Changes in the GnRH pulse pattern can independently affect the synthesis and release of LH and FSH (24). Many central stimulators of the HPG axis have a lesser effect on circulating FSH levels relative to changes in LH (15, 49). In addition, stimulatory effects on FSH release often occur later than those on LH release, and a later time point may have revealed an effect of relaxin-3 on FSH levels. Further studies are required to investigate whether the differential effects of H3 administration on LH and FSH release reflect a physiological or pharmacological effect.
We hypothesized that relaxin-3 stimulates the reproductive axis via modulation of hypothalamic GnRH pathways. In accord with this hypothesis, preadministration of a peripheral GnRH antagonist abolished the increase in circulating concentrations of LH induced by intracerebroventricular or intraparaventricular injection of H3 in adult male rats. Our in vitro studies also support this hypothesis. H3 dose dependently stimulated the release of GnRH from in vitro hypothalamic explants, appearing to reach a maximal effect at 100 nM relaxin-3. This effect may represent an indirect stimulation of GnRH release by relaxin-3 via afferent neurons in the PVN and ARC where relaxin-3 immunoreactivity is present. However, H3 also stimulated the release of GnRH from the hypothalamic cell line, GT1-7, in a dose-dependent manner, and the relaxin receptors, RXFP1 and RXFP3, are expressed on GT1-7 cells. It is therefore possible that the stimulatory effects of relaxin-3 on GnRH release are partly via a direct effect on GnRH neurons. This is in accord with reports of relaxin-3 immunoreactivity in the MPOA. However, further studies, such as dual immunohistochemistry (IHC)/in situ hybridization (ISH) for GnRH and relaxin receptors, are required to further investigate the possibility of a direct effect of relaxin-3 on GnRH neurons. Current commercially available RXFP3 antibodies have limited utility for IHC, suggesting that dual ISH may be the more useful technique.
GnRH neurones are not located in a single hypothalamic nucleus but are dispersed throughout the anterior hypothalamic area. In rodents, the vast majority of GnRH is produced by a small and diffuse population of neurons located in the MPOA, medial septum, and diagonal band of Broca (40). Within the hypothalamus, primary afferents to GnRH neurons are located in areas that include the PVN, ARC, LHA, dorsomedial hypothalamus, ventromedial hypothalamus, LPOA, and MPOA (56), areas where relaxin-3 immunoreactivity is also present (23, 48). In addition, RXFP1 and RXFP3 receptors are both present in several hypothalamic nuclei, including the supraoptic nucleus and the PVN, and RXFP1 receptors are also expressed in the ARC (7, 22, 46, 47). Relaxin-3 neurons and receptors may be strategically placed as part of the complex local intrahypothalamic circuitry providing information to GnRH neurons about nutritional and reproductive status. Further research is required to define the roles of specific populations of relaxin-3 immunoreactive and relaxin-3 responsive neurons. It would be interesting to assess in future whether hypothalamic nuclei other than the PVN may also be involved in stimulation of the HPG axis. For example, the anterior preoptic area is rich in both GnRH neurons and relaxin-3 immunoreactivity.
Expression of RXFP3 in the pituitary has been demonstrated by RT-PCR (26). RXFP1 has been shown to be expressed in the pituitary glands of pregnant mice, but not in those of male or nonpregnant female mice (19). In our studies, relaxin-3 had no effect on the release of LH or FSH from anterior pituitary fragments harvested from male rats, suggesting that relaxin-3 is unlikely to mediate its effect on the HPG axis via the anterior pituitary.
In contrast to H3, equimolar doses of H2 (5 nmol icv and 540 pmol iPVN), the human ortholog of relaxin, did not result in a statistically significant rise in LH at 30 min in adult male rats following intracerebroventricular or intraparaventricular administration. H2 and H3 bind to the RXFP1 receptor with high affinity, but only H3 binds to RXFP3, which suggests that the effects of relaxin-3 on the HPG axis may be mediated via RXFP3. However, in all experiments, H2 resulted in a nonsignificant increase in LH and testosterone levels. Actions at the RXFP1 receptor therefore cannot be excluded, and it is possible that higher doses of H2 may significantly stimulate the HPG axis. In addition, GT1-7 cells express both RXFP1 and RXPF3, making it difficult to determine which receptor is responsible for mediating the relaxin-3-induced release of GnRH. Further studies requiring as yet unavailable specific relaxin receptor agonists and antagonists will be necessary to determine the receptor system or systems that mediate the effects of the relaxins on the HPG axis.
It has long been recognized that regulation of appetite and reproduction is tightly linked, but the neuroendocrine networks responsible for this are only now being defined. Together with important peripheral factors such as leptin, ghrelin, and PYY3–36 (9), there are a growing number of central neuropeptides that have been found to exert an effect on both reproduction and energy balance (37). Classically, neuropeptides that stimulate appetite suppress the HPG axis and vice versa, although the pathways mediating the interaction between energy stores and fertility have yet to be fully delineated (11). Intracerebroventricular administration of a RXFP3 receptor antagonist to satiated rats decreases food intake induced by a RXFP3 agonist (20), but further experiments are required to show that relaxin-3 plays a physiological role in the regulation of food intake. To date, no changes in the expression of relaxin-3 mRNA or its receptors in satiated and starved animals have been reported. If relaxin-3 plays a physiological role as an anorexigenic peptide in controlling appetite, one might expect increased relaxin-3 expression in the brain stem during food restriction and decreased expression in the satiated state. Our previous studies have shown that exogenous H3 treatment is effective at increasing food intake in both satiated and nonsatiated rats (28), and one might postulate that relaxin-3 would also be effective at increasing LH in both states. However, food restriction might increase endogenous relaxin-3, and, in addition, the GnRH pulse generator is extremely sensitive to energetic stress. GnRH pulses are readily suppressed by starvation, extreme temperatures, or excessive exercise, and GnRH pulsatility returns rapidly when the energetic challenge is alleviated (4, 31). It is possible that exogenous relaxin-3 might be ineffective at increasing LH in food-restricted animals, and it would be interesting to study this effect in starved animals.
In addition, a number of neuropeptides have been shown to exert differential effects on the HPG axis depending on the steroid milieu. For example, intracerebroventricular administration of the potent orexigenic peptide NPY can stimulate LH release in sex-steroid primed ovariectomized (OVX) rats (17) but produces a striking inhibition of LH release in OVX rats (27). Chronic intracerebroventricular infusions of NPY also lead to a profound inhibition of the gonadotrophic axis in intact male rats (33). Orexins, which increase food intake and energy expenditure, have been shown to have a dual response similar to that of NPY by stimulating LH secretion in OVX steroid-primed rats and inhibiting LH secretion in unprimed OVX rats (34). This ovarian steroid-dependent bimodal LH response is also shown in response to the anorexigenic peptide neuromedin S, which increases LH in cycling rat females but decreases LH levels in OVX rats (51) (this latter response also being demonstrated by the structurally related peptide, neuromedin U) (35). It would be interesting to determine if the actions of relaxin-3 on LH are dependent on the ovarian/testicular steroid environment and whether relaxin-3 displays a dual action in the regulation of the HPG axis. The data presented here suggest that relaxin-3 may belong to a growing group of hypothalamic peptides that neurochemically bridge the regulatory networks controlling reproduction and energy balance.
In summary, we have investigated the effects of relaxin-3 on the HPG axis. Both intracerebroventricular and intraparaventricular administration of relaxin-3 increases plasma LH, and this effect is blocked by administration of a GnRH antagonist, suggesting that it is mediated via an effect on hypothalamic GnRH neurons. In accord with these results, relaxin-3 stimulates the release of GnRH from hypothalamic explants but has no effect on LH or FSH release from pituitary fragments. Stimulation of GnRH release from GT1-7 cells suggests that relaxin-3 may be able to stimulate GnRH neurons directly, and both RXFP1 and RXFP3 receptors are detectable from GT1-7 cell RNA by RT-PCR. The effects of relaxin-3 on the HPG axis seem likely to be mediated via RXFP3, but actions via RXFP1 cannot be excluded. We have therefore demonstrated a novel role for relaxin-3 in the regulation of the HPG axis in the adult male rat. Relaxin-3 may play a role in coordinating feeding and reproductive responses to alterations in energy balance. Further studies are required to define the physiological importance of relaxin-3 in the regulation of reproduction.
GRANTS
The department is funded by program grants from the Medical Research Council (MRC) (G7811974) and Wellcome Trust (072643/Z/03/Z) and by European Union FP6 Integrated Project Grant LSHM-CT-2003-503041. We are also grateful for support from the National Institute of Health Research Biomedical Research Center funding scheme and an Integrated Mammalian Biology Capacity building award. B. M. McGowan is a clinical lecturer at Imperial College. S. A. Stanley is an MRC clinician scientist. E. L. Thompson is supported by a Research Council UK Academic Fellowship. K. G. Murphy is supported by a Biotechnology and Biological Sciences Research Council new investigator award.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Bathgate RA, Samuel CS, Burazin TC, Layfield S, Claasz AA, Reytomas IG, Dawson NF, Zhao C, Bond C, Summers RJ, Parry LJ, Wade JD, Tregear GW. Human relaxin gene 3 (H3) and the equivalent mouse relaxin (M3) gene. Novel members of the relaxin peptide family. J Biol Chem 277: 1148–1157, 2002. [DOI] [PubMed] [Google Scholar]
- 2.Beak SA, Heath MM, Small CJ, Morgan DG, Ghatei MA, Taylor AD, Buckingham JC, Bloom SR, Smith DM. Glucagon-like peptide-1 stimulates luteinizing hormone-releasing hormone secretion in a rodent hypothalamic neuronal cell line. J Clin Invest 101: 1334–1341, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beak SA, Small CJ, Ilovaiskaia I, Hurley JD, Ghatei MA, Bloom SR, Smith DM. Glucagon-like peptide-1 (GLP-1) releases thyrotropin (TSH): characterization of binding sites for GLP-1 on alpha-TSH cells. Endocrinology 137: 4130–4138, 1996. [DOI] [PubMed] [Google Scholar]
- 4.Bronson FH, Heideman PD. Short-term hormonal responses to food intake in peripubertal female rats. Am J Physiol Regul Integr Comp Physiol 259: R25–R31, 1990. [DOI] [PubMed] [Google Scholar]
- 5.Buckingham JC, Hodges JR. The use of corticotrophin production by adenohypophysial tissue in vitro for the detection and estimation of potential corticotrophin releasing factors. J Endocrinol 72: 187–193, 1977. [DOI] [PubMed] [Google Scholar]
- 6.Burazin TC, Bathgate RA, Macris M, Layfield S, Gundlach AL, Tregear GW. Restricted, but abundant, expression of the novel rat gene-3 (R3) relaxin in the dorsal tegmental region of brain. J Neurochem 82: 1553–1557, 2002. [DOI] [PubMed] [Google Scholar]
- 7.Burazin TC, Johnson KJ, Ma S, Bathgate RA, Tregear GW, Gundlach AL. Localization of LGR7 (relaxin receptor) mRNA and protein in rat forebrain: correlation with relaxin binding site distribution. Ann NY Acad Sci 1041: 205–210, 2005. [DOI] [PubMed] [Google Scholar]
- 8.Chen J, Kuei C, Sutton SW, Bonaventure P, Nepomuceno D, Eriste E, Sillard R, Lovenberg TW, Liu C. Pharmacological characterization of relaxin-3/INSL7 receptors GPCR135 and GPCR142 from different mammalian species. J Pharmacol Exp Ther 312: 83–95, 2005. [DOI] [PubMed] [Google Scholar]
- 9.Fernandez-Fernandez R, Martini AC, Navarro VM, Castellano JM, Dieguez C, Aguilar E, Pinilla L, Tena-Sempere M. Novel signals for the integration of energy balance and reproduction. Mol Cell Endocrinol 254–255: 127–132, 2006. [DOI] [PubMed]
- 10.Goto M, Swanson LW, Canteras NS. Connections of the nucleus incertus. J Comp Neurol 438: 86–122, 2001. [DOI] [PubMed] [Google Scholar]
- 11.Hill J, Elmquist JK, Elias CF. Hypothalamic pathways linking energy balance and reproduction. Am J Physiol Endocrinol Metab 294: E827–E832, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hsu SY, Nakabayashi K, Nishi S, Kumagai J, Kudo M, Sherwood OD, Hsueh AJ. Activation of orphan receptors by the hormone relaxin. Science 295: 671–674, 2002. [DOI] [PubMed] [Google Scholar]
- 13.Hudson P, Haley J, John M, Cronk M, Crawford R, Haralambidis J, Tregear G, Shine J, Niall H. Structure of a genomic clone encoding biologically active human relaxin. Nature 301: 628–631, 1983. [DOI] [PubMed] [Google Scholar]
- 14.Hudson P, John M, Crawford R, Haralambidis J, Scanlon D, Gorman J, Tregear G, Shine J, Niall H. Relaxin gene expression in human ovaries and the predicted structure of a human preprorelaxin by analysis of cDNA clones. EMBO J 3: 2333–2339, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Irwig MS, Fraley GS, Smith JT, Acohido BV, Popa SM, Cunningham MJ, Gottsch ML, Clifton DK, Steiner RA. Kisspeptin activation of gonadotropin releasing hormone neurons and regulation of KiSS-1 mRNA in the male rat. Neuroendocrinology 80: 264–272, 2004. [DOI] [PubMed] [Google Scholar]
- 16.Kageyama H, Takenoya F, Kita T, Hori T, Guan JL, Shioda S. Galanin-like peptide in the brain: effects on feeding, energy metabolism and reproduction. Regul Pept 126: 21–26, 2005. [DOI] [PubMed] [Google Scholar]
- 17.Kalra SP, Crowley WR. Norepinephrine-like effects of neuropeptide Y on LH release in the rat. Life Sci 35: 1173–1176, 1984. [DOI] [PubMed] [Google Scholar]
- 18.Kalra SP, Kalra PS. NPY–an endearing journey in search of a neurochemical on/off switch for appetite, sex and reproduction. Peptides 25: 465–471, 2004. [DOI] [PubMed] [Google Scholar]
- 19.Krajnc-Franken MA, van Disseldorp AJ, Koenders JE, Mosselman S, van DM, Gossen JA. Impaired nipple development and parturition in LGR7 knockout mice. Mol Cell Biol 24: 687–696, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuei C, Sutton S, Bonaventure P, Pudiak C, Shelton J, Zhu J, Nepomuceno D, Wu J, Chen J, Kamme F, Seierstad M, Hack MD, Bathgate RA, Hossain MA, Wade JD, Atack J, Lovenberg TW, Liu C. R3(BDelta23 27)R/I5 chimeric peptide, a selective antagonist for GPCR135 and GPCR142 over relaxin receptor LGR7: in vitro and in vivo characterization. J Biol Chem 282: 25425–25435, 2007. [DOI] [PubMed] [Google Scholar]
- 21.Liu C, Chen J, Sutton S, Roland B, Kuei C, Farmer N, Sillard R, Lovenberg TW. Identification of relaxin-3/INSL7 as a ligand for GPCR142. J Biol Chem 278: 50765–50770, 2003. [DOI] [PubMed] [Google Scholar]
- 22.Liu C, Eriste E, Sutton S, Chen J, Roland B, Kuei C, Farmer N, Jornvall H, Sillard R, Lovenberg TW. Identification of relaxin-3/INSL7 as an endogenous ligand for the orphan G-protein-coupled receptor GPCR135. J Biol Chem 278: 50754–50764, 2003. [DOI] [PubMed] [Google Scholar]
- 23.Ma S, Bonaventure P, Ferraro T, Shen PJ, Burazin TC, Bathgate RA, Liu C, Tregear GW, Sutton SW, Gundlach AL. Relaxin-3 in GABA projection neurons of nucleus incertus suggests widespread influence on forebrain circuits via G-protein-coupled receptor-135 in the rat. Neuroscience 144: 165–190, 2007. [DOI] [PubMed] [Google Scholar]
- 24.Marshall JC, Dalkin AC, Haisenleder DJ, Paul SJ, Ortolano GA, Kelch RP. Gonadotropin-releasing hormone pulses: regulators of gonadotropin synthesis and ovulatory cycles. Recent Prog Horm Res 47: 155–187, 1991. [DOI] [PubMed] [Google Scholar]
- 25.Matsui H, Takatsu Y, Kumano S, Matsumoto H, Ohtaki T. Peripheral administration of metastin induces marked gonadotropin release and ovulation in the rat. Biochem Biophys Res Commun 320: 383–388, 2004. [DOI] [PubMed] [Google Scholar]
- 26.Matsumoto M, Kamohara M, Sugimoto T, Hidaka K, Takasaki J, Saito T, Okada M, Yamaguchi T, Furuichi K. The novel G-protein coupled receptor SALPR shares sequence similarity with somatostatin and angiotensin receptors. Gene 248: 183–189, 2000. [DOI] [PubMed] [Google Scholar]
- 27.McDonald JK, Lumpkin MD, DePaolo LV. Neuropeptide-Y suppresses pulsatile secretion of luteinizing hormone in ovariectomized rats: possible site of action. Endocrinology 125: 186–191, 1989. [DOI] [PubMed] [Google Scholar]
- 28.McGowan BM, Stanley SA, Smith KL, Minnion JS, Donovan J, Thompson EL, Patterson M, Connolly MM, Abbott CR, Small CJ, Gardiner JV, Ghatei MA, Bloom SR. Effects of acute and chronic relaxin-3 on food intake and energy expenditure in rats. Regul Pept 136: 72–77, 2006. [DOI] [PubMed] [Google Scholar]
- 29.McGowan BM, Stanley SA, Smith KL, White NE, Connolly MM, Thompson EL, Gardiner JV, Murphy KG, Ghatei MA, Bloom SR. Central relaxin-3 administration causes hyperphagia in male Wistar rats. Endocrinology 146: 3295–3300, 2005. [DOI] [PubMed] [Google Scholar]
- 30.McGowan BM, Stanley SA, White NE, Spangeus A, Patterson M, Thompson EL, Smith KL, Donovan J, Gardiner JV, Ghatei MA, Bloom SR. Hypothalamic mapping of orexigenic action and fos-like immunoreactivity following relaxin-3 administration in male Wistar rats. Am J Physiol Endocrinol Metab 292: E913–E919, 2007. [DOI] [PubMed] [Google Scholar]
- 31.Parfitt DB, Church KR, Cameron JL. Restoration of pulsatile luteinizing hormone secretion after fasting in rhesus monkeys (Macaca mulatta): dependence on size of the refeed meal. Endocrinology 129: 749–756, 1991. [DOI] [PubMed] [Google Scholar]
- 32.Patterson M, Murphy KG, Thompson EL, Patel S, Ghatei MA, Bloom SR. Administration of kisspeptin-54 into discrete regions of the hypothalamus potently increases plasma luteinising hormone and testosterone in male adult rats. J Neuroendocrinol 18: 349–354, 2006. [DOI] [PubMed] [Google Scholar]
- 33.Pierroz DD, Catzeflis C, Aebi AC, Rivier JE, Aubert ML. Chronic administration of neuropeptide Y into the lateral ventricle inhibits both the pituitary-testicular axis and growth hormone and insulin-like growth factor I secretion in intact adult male rats. Endocrinology 137: 3–12, 1996. [DOI] [PubMed] [Google Scholar]
- 34.Pu S, Jain MR, Kalra PS, Kalra SP. Orexins, a novel family of hypothalamic neuropeptides, modulate pituitary luteinizing hormone secretion in an ovarian steroid-dependent manner. Regul Pept 78: 133–136, 1998. [DOI] [PubMed] [Google Scholar]
- 35.Quan H, Funabashi T, Furuta M, Kimura F. Effects of neuromedin U on the pulsatile LH secretion in ovariectomized rats in association with feeding conditions. Biochem Biophys Res Commun 311: 721–727, 2003. [DOI] [PubMed] [Google Scholar]
- 36.Reissmann T, Schally AV, Bouchard P, Riethmiiller H, Engel J. The LHRH antagonist cetrorelix: a review. Hum Reprod Update 6: 322–331, 2000. [DOI] [PubMed] [Google Scholar]
- 37.Schneider JE Energy balance and reproduction. Physiol Behav 81: 289–317, 2004. [DOI] [PubMed] [Google Scholar]
- 38.Seth A, Stanley S, Jethwa P, Gardiner J, Ghatei M, Bloom S. Galanin-like peptide stimulates the release of gonadotropin-releasing hormone in vitro and may mediate the effects of leptin on the hypothalamo-pituitary-gonadal axis. Endocrinology 145: 743–750, 2004. [DOI] [PubMed] [Google Scholar]
- 39.Shen PJ, Fu P, Phelan KD, Scott DJ, Layfield S, Tregear GW, Bathgate RA, Gundlach AL. Restricted expression of LGR8 in intralaminar thalamic nuclei of rat brain suggests a role in sensorimotor systems. Ann NY Acad Sci 1041: 510–515, 2005. [DOI] [PubMed] [Google Scholar]
- 40.Silverman AJ, Jhamandas J, Renaud LP. Localization of luteinizing hormone-releasing hormone (LHRH) neurons that project to the median eminence. J Neurosci 7: 2312–2319, 1987. [PMC free article] [PubMed] [Google Scholar]
- 41.Sinnayah P, Burns P, Wade JD, Weisinger RS, McKinley MJ. Water drinking in rats resulting from intravenous relaxin and its modification by other dipsogenic factors. Endocrinology 140: 5082–5086, 1999. [DOI] [PubMed] [Google Scholar]
- 42.Small CJ, Stanley SA, Bloom SR. Appetite control and reproduction: leptin and beyond. Semin Reprod Med 20: 389–398, 2002. [DOI] [PubMed] [Google Scholar]
- 43.Soloff MS, Gal S, Hoare S, Peters CA, Hunzicker-Dunn M, Anderson GD, Wood TG. Cloning, characterization, and expression of the rat relaxin gene. Gene 323: 149–155, 2003. [DOI] [PubMed] [Google Scholar]
- 44.Stanley SA, Small CJ, Kim MS, Heath MM, Seal LJ, Russell SH, Ghatei MA, Bloom SR. Agouti related peptide (Agrp) stimulates the hypothalamo pituitary gonadal axis in vivo & in vitro in male rats. Endocrinology 140: 5459–5462, 1999. [DOI] [PubMed] [Google Scholar]
- 45.Sudo S, Kumagai J, Nishi S, Layfield S, Ferraro T, Bathgate RA, Hsueh AJ. H3 relaxin is a specific ligand for LGR7 and activates the receptor by interacting with both the ectodomain and the exoloop 2. J Biol Chem 278: 7855–7862, 2003. [DOI] [PubMed] [Google Scholar]
- 46.Sutton SW, Bonaventure P, Kuei C, Nepomuceno D, Wu J, Zhu J, Lovenberg TW, Liu C. G-protein-coupled receptor (GPCR)-142 does not contribute to relaxin-3 binding in the mouse brain: further support that relaxin-3 is the physiological ligand for GPCR135. Neuroendocrinology 82: 139–150, 2005. [DOI] [PubMed] [Google Scholar]
- 47.Sutton SW, Bonaventure P, Kuei C, Roland B, Chen J, Nepomuceno D, Lovenberg TW, Liu C. Distribution of G-protein-coupled receptor (GPCR)135 binding sites and receptor mRNA in the rat brain suggests a role for relaxin-3 in neuroendocrine and sensory processing. Neuroendocrinology 80: 298–307, 2004. [DOI] [PubMed] [Google Scholar]
- 48.Tanaka M, Iijima N, Miyamoto Y, Fukusumi S, Itoh Y, Ozawa H, Ibata Y. Neurons expressing relaxin 3/INSL 7 in the nucleus incertus respond to stress. Eur J Neurosci 21: 1659–1670, 2005. [DOI] [PubMed] [Google Scholar]
- 49.Thompson EL, Patterson M, Murphy KG, Smith KL, Dhillo WS, Todd JF, Ghatei MA, Bloom SR. Central and peripheral administration of kisspeptin-10 stimulates the hypothalamic-pituitary-gonadal axis. J Neuroendocrinol 16: 850–858, 2004. [DOI] [PubMed] [Google Scholar]
- 50.Thornton SM, Fitzsimons JT. The effects of centrally administered porcine relaxin on drinking behaviour in male and female rats. J Neuroendocrinol 7: 165–169, 1995. [DOI] [PubMed] [Google Scholar]
- 51.Vigo E, Roa J, Lopez M, Castellano JM, Fernandez-Fernandez R, Navarro VM, Pineda R, Aguilar E, Dieguez C, Pinilla L, Tena-Sempere M. Neuromedin S as novel putative regulator of luteinizing hormone secretion. Endocrinology 148: 813–828, 2007. [DOI] [PubMed] [Google Scholar]
- 52.Ward H, Gardiner J, Kong WM, Murphy K, Bloom S. Distribution of fluorescence following injection of recombinant adeno-associated virus encoding green fluorescent protein into the paraventricular nucleus. Neuroendocrinology 77: 100–104, 2003. [DOI] [PubMed] [Google Scholar]
- 53.Williams G, Bing C, Cai XJ, Harrold JA, King PJ, Liu XH. The hypothalamus and the control of energy homeostasis: different circuits, different purposes. Physiol Behav 74: 683–701, 2001. [DOI] [PubMed] [Google Scholar]
- 54.Wren AM, Small CJ, Abbott CR, Dhillo WS, Seal LJ, Cohen MA, Batterham RL, Taheri S, Stanley SA, Ghatei MA, Bloom SR. Ghrelin causes hyperphagia and obesity in rats. Diabetes 50: 2540–2547, 2001. [DOI] [PubMed] [Google Scholar]
- 55.Wu TJ, Gibson MJ, Rogers MC, Silverman AJ. New observations on the development of the gonadotropin-releasing hormone system in the mouse. J Neurobiol 33: 983–998, 1997. [DOI] [PubMed] [Google Scholar]
- 56.Yoon H, Enquist LW, Dulac C. Olfactory inputs to hypothalamic neurons controlling reproduction and fertility. Cell 123: 669–682, 2005. [DOI] [PubMed] [Google Scholar]