Abstract
Our objective was to determine whether defects underlying impaired fasting glucose (IFG) are maintained and additive when combined with impaired glucose tolerance (IGT) (representing a progressive form of prediabetes) or are distinct in IFG/IGT (reflecting a parallel form of prediabetes). Volunteers with IFG (n = 10), IFG/IGT (n = 14), or normal glucose tolerance (NGT; n = 15) were matched for demographics and anthropometry. Insulin secretion was assessed using the glucose step-up protocol and insulin action through the use of a two-stage hyperinsulinemic euglycemic clamp with infusion of [6,6-2H2]glucose. Modeling of insulin secretory parameters revealed similar basal (Φb) but diminished dynamic (Φd) components in both IFG and IFG/IGT (P = 0.05 vs. NGT for both). Basal glucose rate of appearance (Ra) was higher in IFG compared with NGT (P < 0.01) and also, surprisingly, with IFG/IGT (P < 0.04). Moreover, glucose Ra suppressed more during the low-dose insulin clamp in IFG (P < 0.01 vs. NGT, P = 0.08 vs. IFG/IGT). Insulin-stimulated glucose uptake [glucose rate of disappearance (Rd)] was similar in IFG, IFG/IGT, and NGT throughout the clamp. We conclude that nuances of β-cell dysfunction observed in IFG were also noted in IFG/IGT. A trend for additional insulin secretory defects was observed in IFG/IGT, possibly suggesting progression in β-cell failure in this group. In contrast, basal glucose Ra and its suppressability with insulin were higher in IFG, but not IFG/IGT, compared with NGT. Together, these data indicate that IFG/IGT may be a distinct prediabetic syndrome rather than progression from IFG.
Keywords: insulin resistance, isotopes, clamp
prevalence of type 2 diabetes increased an alarming 61% between 1990 and 2001 (31) and currently affects 21 million Americans (9). Perhaps even more concerning are the 54 million Americans with prediabetes, up to 70% of whom will develop diabetes in their lifetime (26, 28, 38, 43). Although numerous trials have been undertaken to prevent diabetes in people with prediabetes (8, 19, 26, 43), surprisingly little is known about the pathophysiological differences between the types of prediabetes.
Prediabetes is not a singular entity but rather a heterogeneous group of metabolic defects that precede type 2 diabetes (28). “Impaired fasting glucose” (IFG) was added to “impaired glucose tolerance” (IGT) in the American Diabetes Association's definitions of prediabetes in 1997 (2), as repeated observations show little concordance between the two (28, 42). The vast majority of literature to date has used indexes of fasting glucose and insulin to differentiate IFG from IGT in terms of their basic physiological defects (i.e., insulin secretion vs. insulin resistance using homeostasis model assessment) (11, 21, 37). The use of such indexes in small physiological studies has sparked considerable debate because the conclusions are inconsistent with more robust methods (16).
A small number of investigations have been undertaken to delineate the underlying defects in IFG from those in IGT a priori (3, 6, 16, 29, 46). Together, the evidence supports elements of β-cell dysfunction and insulin resistance in IFG and IGT that are somewhat distinct from one another (32). Concurrence of IFG with IGT is a presumed obligatory convergence point from isolated IFG or IGT in the pathway to diabetes. Interestingly, however, when IFG and IGT are combined, the result is not the simple addition of defects seen when IFG and IGT occur in isolation (6, 46). Whether IFG/IGT represents a third pathway (in addition to isolated IFG and IGT) vs. a final common pathway to diabetes is not clear. Elucidating defects in IFG/IGT is clinically relevant because progression to diabetes is three times higher than in isolated IFG or IGT (26, 28). Therefore, the aim of the current investigation was to determine whether defects underlying IFG in humans are maintained and additive when combined with IGT (representing a progressive form of prediabetes) or are distinct in IFG/IGT (reflecting a parallel form of prediabetes). We hypothesized that the defects seen in IFG would be unique vs. combined IFG/IGT, providing evidence that IFG/IGT may be a discreet prediabetic state.
MATERIALS AND METHODS
Subjects
Thirty-nine men and women between the ages of 45 and 70 were studied. Subjects were placed into one of the three groups on the basis of two 2-h 75-g oral glucose tolerance tests (2-h OGTT) separated by 1 wk: a control group with normal glucose tolerance (NGT; n = 15; fasting glucose <100 mg/dl and 2-h OGTT <140 mg/dl), IFG (n = 10; fasting glucose 100–125 mg/dl and 2-h OGTT <140 mg/dl), or combined IFG/IGT (n = 14; fasting glucose 100–125 mg/dl and 2-h OGTT 140–200 mg/dl) (18). All subjects were deemed healthy by a medical history, physical examination, and screening blood tests. Subjects were excluded for <0.5 or >5.0 U/ml thyroid-stimulating hormone, >250 mg/dl fasting triglycerides, >1.5 mg/dl creatinine, elevated liver function tests (>2× normal), <38% hematocrit, or <3.0 × 103 white blood cells. Use of medications for lipid and/or glucose lowering also excluded enrollees. Women must not have used hormone replacement therapy in the past year. In addition, subjects were sedentary (<1.5 h of planned physical activity/wk) and nonsmokers, and women were postmenopausal (defined as the cessation of menses >1 yr prior to enrollment or FSH >30 mIU/ml). Body composition was estimated from dual-energy X-ray absorptiometry (0.03 mRem), as described previously (35). Approval for this study was obtained from the Colorado Multiple Institutional Review Board prior to its commencement. Informed, written consent for participation was obtained from the subjects before they entered the study. All research-related activity conformed to the principles outlined in the Declaration of Helsinki (updated 2004).
Prestudy Diet Control
Subjects were fed a control diet for 3 days prior to admission to the General Clinical Research Center for study. The control diet was isocaloric {calculated as 1.4 × [372 + (23.9 × fat-free mass)]}, using the fat-free mass (FFM) measured by dual-energy X-ray absorptiometry. The diet composition was standardized as 30% fat (saturated, polyunsaturated, and monounsaturated fats in a 1:1:1 ratio), 15% protein, and 55% carbohydrate.
Testing Protocol
Subjects were asked to fast overnight (∼12 h) and were admitted to the General Clinical Research Center at 0730 on the morning of each study day (glucose step-up protocol and insulin clamp protocol, separated by ∼2 wk). Upon admission, an intravenous catheter was placed in an antecubital vein for infusion of glucose, and sampling catheter was placed in a dorsal hand vein of the contralateral arm. For all blood samples, the heated hand technique was used to arterialize the blood (27). Background sampling began 30 min after sampling catheters had been placed.
Glucose step-up protocol.
Baseline blood samples were drawn for determination of insulin, glucose, C-peptide, free fatty acids (FFA), glycerol, and lactate concentrations. Twenty percent dextrose was then infused in a sequential stepwise fashion at 2, 4, 6, 8, and 10 mg·kg−1·min−1 for 40 min each. Blood was drawn every 10 min throughout the 240-min protocol for the determination of glucose, insulin, and C-peptide concentrations.
Insulin clamp protocol.
Baseline blood samples were drawn for determination of background isotope enrichment as well as hormone and substrate (insulin, glucose, C-peptide, FFA, glycerol, and lactate) concentrations. For the measurement of glucose turnover, a primed (3.5 mg/kg) constant (0.04 mg·kg−1·min−1) infusion of [6,6-2H2]glucose was initiated and continued through the end of the clamp. Resting blood measurements for tracer, hormone, and substrate concentrations (see above) were made over the final 30 min of the 120-min infusion to allow for equilibration of the tracer in the glucose pool. Indirect calorimetry was performed before resting blood sampling was started with a respiratory canopy (Sensormedics 2900; Sensormedics, Yorba Linda, CA).
A two-stage hyperinsulinemic euglycemic clamp was then initiated and continued for the next 3 h using the method of DeFronzo et al. (13). Briefly, a primed continuous infusion of insulin was infused at 4 mU·m2·min−1 for 1.5 h and then increased to 40 mU·m2·min−1 for the final 1.5 h. A variable infusion of 20% dextrose was infused to maintain blood glucose at ∼90 mg/dl. Blood was sampled every 5 min to determine glucose concentration, and the dextrose infusion adjusted as necessary. The dextrose was “spiked” with 15 mg/ml [6,6-2H2]glucose to minimize changes in isotope enrichment. Blood samples were taken over the final 30 min of both stages of the clamp for measurement of glucose kinetics as well as hormone and substrate concentrations (see above). Immediately prior to blood sampling during each stage, measurement of respiratory gas exchange was made.
Analytical Procedures
Models of C-peptide secretion and kinetics.
Blood measurements of glucose and C-peptide concentrations taken during the glucose step-up protocol were input into the minimal-model insulin secretion (39). The model assumes that whole body C-peptide kinetics is described by the two-compartment model, originally proposed by Eaton et al. (15), also incorporating age associated changes in C-peptide kinetics as measured by Jiang et al. (24). The model assumes that insulin secretion is made up of two components, a dynamic component and a static one. The dynamic component is likely to represent secretion of promptly releasable insulin and is proportional to the rate of increase of glucose concentration through a parameter, dynamic β-cell responsivity index (Φd; dimensionless), that defines the response to a given increment in glucose. The static component is believed to represent the provision of new insulin into a releasable pool and is characterized by a static responsivity index (Φs; min−1) and by a delay time constant (min). The global β-cell response to glucose (Φg; min−1) is a composite of Φd and Φs. Finally, basal sensitivity index (Φb; min−1) measures basal insulin secretion rate over basal glucose concentration.
Posthepatic insulin appearance was predicted using insulin and glucose measurements taken during the step-up protocol in conjunction with the above-mentioned models. Hepatic insulin extraction was then calculated as the difference between from pre- and posthepatic insulin secretion (23).
Parameters of the model were estimated with their precision by weighted nonlinear least squares using the SAAM II software (4).
Circulating hormone and substrate concentrations.
All samples were stored at −80°C until analysis. Radioimmunoassay was used to determine insulin (Linco Research, St. Louis, MO) and C-peptide (γ-counter; Diagnostic Products, Los Angeles, CA) concentrations. Standard enzymatic assays were used to measure glucose (COBA-Mira Plus; Roche Diagnostics, Mannheim, Germany), lactate (Sigma Kit no. 826; Sigma, St. Louis, MO), glycerol (Boehringer Mannheim Diagnostics, Mannheim, Germany), and FFA (NEFA kit; Wako Chemicals).
Whole body substrate oxidation.
Whole body substrate oxidation was measured using indirect calorimetry. Oxygen consumption and carbon dioxide production are used to calculate metabolic rate as well as the oxidation of carbohydrate and fat using standard equations (45).
Gas chromatography-mass spectroscopy methods.
Glucose isotopic enrichment was measured with gas chromatography-mass spectrometry (gas chromatography model 6890 series II and mass spectrometry model 5973A; Hewlett-Packard), using standard methods as described previously (33).
Calculations
Rates of glucose appearance (Ra), disappearance (Rd), and metabolic clearance rate (MCR) before the clamp were calculated using a modified Steele equation, as described by Wolfe (47), for stable isotopes. Equations described by Finegood et al. (17) were used to account for the tracer in the “spiked” dextrose solution during the insulin clamp. Nonoxidative glucose disposal was calculated by subtracting carbohydrate oxidation from glucose Rd.
Statistical Analysis
Comparisons between groups with IFG, IFG/IGT, and NGT were made using a one-way ANOVA, with least significant difference post hoc analyses to determine which means differed (SPSS, Chicago, IL). Differences within groups during the insulin clamp were made using repeated-measures ANOVA. Due to close matching of the groups, data are presented unadjusted. Rd are expressed as milligram per kilogram FFM per minute. All data are presented as means ± SE. Overall significance was set at P ≤ 0.05.
RESULTS
Demographics
The IFG/IGT group was older than the IFG group (P < 0.05) and contained more people with a first-degree relative who had type 2 diabetes (P < 0.05 vs. IFG and NGT). Sex, body mass index, percent body fat, and waist-to-hip ratio were similar among the groups (Table 1). All subjects were sedentary, engaging in <90 min of planned physical activity/wk.
Table 1.
Subject demographics
| n | Sex (M/F) | Age, yr | Nonwhite Ethnicity (n) | FDR T2DM (n) | BMI, kg/m2 | %Body Fat | W/H, % | |
|---|---|---|---|---|---|---|---|---|
| NGT | 15 | 7/8 | 58±1.6 | 2 | 9 | 31±1.2 | 37±1.2 | 0.92±0.03 |
| IFG | 10 | 5/5 | 55±1.9 | 1 | 6‡ | 31±1.3 | 36±2.9 | 0.88±0.04 |
| IFG/IGT | 14 | 6/8 | 61±1.2† | 3 | 11* | 30±1.1 | 36±2.3 | 0.92±0.03 |
Values are means ± SE. NGT, normal glucose tolerance; IFG, impaired fasting glucose; IGT, impaired glucose tolerance; M, males; F, females; FDR T2DM, first-degree relatives with type 2 diabetes; BMI, body mass index; W/H, waist-to-hip ratio. All subjects engaged in <90 min of planned physical activity/wk.
P < 0.05 vs. NGT;
P < 0.05 vs. IFG;
P < 0.05 vs. IFG/IGT.
Parameters of Insulin Secretion and Clearance
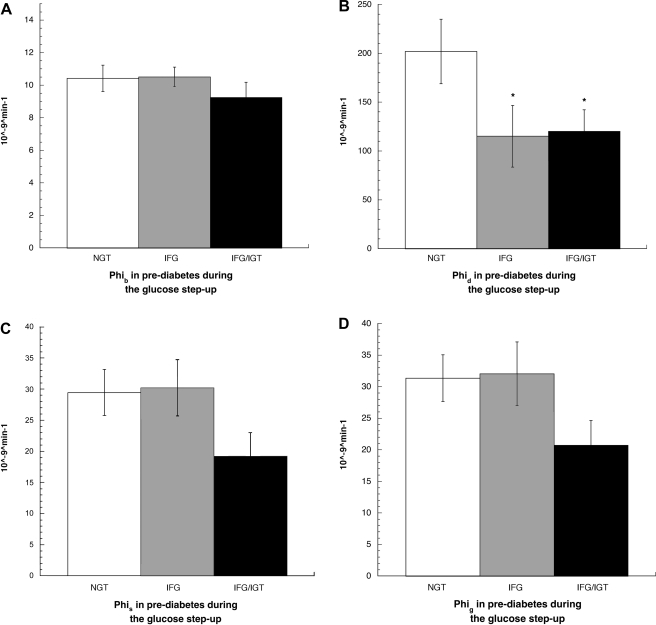
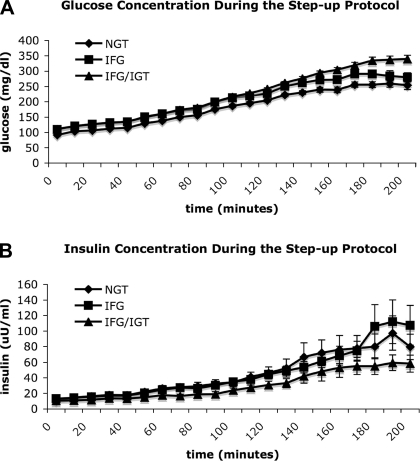
Mathematical modeling of the data collected during the glucose step-up protocol allowed examination of insulin secretory dynamics in humans in vivo. Basal β-cell responsivity (Φb) was similar between the groups, whereas the stimulatory effect of the rate of change in glucose on insulin secretion (Φd) was diminished in both IFG and IFG/IGT (P = 0.05 for both vs. NGT; Fig. 1, A and B). A nonsignificant trend for lower static (Φs; P = 0.07 vs. IFG and NGT) and global β-cell responsivity indexes (Φg; P = 0.07 vs. NGT and P = 0.08 vs. IFG) in IFG/IGT was noted (Fig. 1, C and D). Overall, there was a linear correlation between 2-h glucose concentration and Φb (r2 = −0.35, P = 0.03), Φs (r2 = −0.45, P = 0.004), and Φg (r2 = −0.46, P = 0.003) but no such relationship between fasting glucose concentration and any parameter of insulin secretion. Further examination of β-cell function via calculation of the disposition index (Φg × glucose Rd during the high-dose clamp) did not reveal any additional differences (data not shown). No correlations were noted between any parameter of insulin secretion and insulin action. No significant differences between the groups, with respect to hepatic insulin extraction, were appreciated at baseline or during the glucose step-up protocol (data not shown). Circulating concentrations of glucose and insulin during the step-up protocol are depicted in Fig. 2, A and B.
Fig. 1.
A: basal insulin secretion rate over basal glucose concentration [basal sensitivity index (Φb)] in normal glucose tolerance (NGT), impaired fasting glucose (IFG), and IFG/impaired glucose tolerance (IGT) during the glucose step-up procedure. Values are means ± SE. B: the stimulatory effect of the rate of change in glucose on insulin secretion [dynamic β-cell responsivity index (Φd)] in NGT, IFG, and IFG/IGT during the glucose step-up procedure. Values are means ± SE. *P = 0.05 vs. NGT. C: the stimulatory effect of glucose on insulin secretion at a steady-state level above basal [static responsivity index (Φs)] in NGT, IFG, and IFG/IGT during the glucose step-up procedure. Values are means ± SE. P = 0.07, NGT vs. IFG/IGT. D: the global effect of glucose on insulin secretion [global β-cell response to glucose (Φg) = Φs × Φd] in NGT, IFG, and IFG/IGT during the glucose step-up procedure. Values are means ± SE. P = 0.07, NGT vs. IFG/IGT.
Fig. 2.
A: blood glucose concentration (mg/dl) during the 200-min glucose step-up protocol in NGT, IFG, and IFG/IGT. B: blood insulin concentration (uU/ml) during the 200-min glucose step-up protocol in NGT, IFG, and IFG/IGT.
Hormone and Substrate Concentrations at Baseline and During the Clamp
Baseline.
Fasting glucose concentration was higher in subjects with IFG and IFG/IGT vs. NGT (P < 0.05 for both, but not different between IFG and IFG/IGT), and 2-h glucose concentration was higher in subjects with IFG/IGT vs. NGT and IFG (P < 0.05 for both, but not different between NGT and IFG) by study design. Baseline C-peptide concentration was higher in IFG (P < 0.05 vs. NGT), and lactate concentration was higher in IFG/IGT (P < 0.05 vs. IFG). No difference in insulin, glycerol, or FFA levels between the groups was observed (Table 2).
Table 2.
Hormone and substrate concentrations at baseline and during the clamp
| Fasting Glucose, mg/dl | 2-h Glucose, mg/dl | Insulin, μU/ml | C-Peptide, ng/ml | FFA, μmol/l | Glycerol, μmol/l | Lactate, mmol/l | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | ||||||||||||||
| NGT | 91±1.2 | 102±6.7‡ | 10.0±1.8 | 2.6±0.2 | 585±37 | 88±9 | 0.7±0.09 | |||||||
| IFG | 112±3.0* | 93±6.2‡ | 11.4±1.3 | 3.2±0.2* | 543±43 | 101±19 | 0.5±0.07 | |||||||
| IGT/IFG | 111±2.4* | 163±4.0 | 10.0±1.6 | 2.8±0.2 | 668±58 | 105±13 | 0.9±0.15† | |||||||
| Low-dose (4 mU·m2·min−1) clamp | ||||||||||||||
| GIR, mg glucose·kg−1·min−1 | ||||||||||||||
| NGT | 0.31±0.12 | 93±1.0 | 11.1±1.8 | 2.2±0.2 | 498±40 | 71±6 | 0.4±0.04 | |||||||
| IFG | 0.00±0.00* | 100±2.0* | 12.6±0.7 | 2.6±0.2 | 491±50 | 82±10 | 0.5±0.07 | |||||||
| IGT/IFG | 0.00±0.00* | 102±1.6* | 12.5±1.5 | 2.3±0.2 | 496±45 | 85±9 | 0.6±0.08 | |||||||
| High-dose (40 mU·m2·min−1) clamp | ||||||||||||||
| GIR, mg glucose·kg−1·min−1 | ||||||||||||||
| NGT | 3.8±0.5 | 89±1.0 | 76±8 | 1.3±0.2 | 59±18 | 39±6 | 0.7±0.05 | |||||||
| IFG | 4.0±0.6 | 90±1.3 | 70±10 | 1.3±0.2 | 97±41 | 45±8 | 0.6±0.07 | |||||||
| IGT/IFG | 3.1±0.5 | 89±2.7 | 69±5.4 | 1.1±0.1 | 65±19 | 56±14 | 0.8±0.10 | |||||||
Values are means ± SE. GIR, glucose infusion rate.
P < 0.05 vs. NGT;
P < 0.05 vs. IFG;
P < 0.05 vs. IFG/IGT.
Low-dose clamp.
Glucose infusion rate (GIR) during the low-dose clamp was lower in the two groups with prediabetes compared with those with NGT (P < 0.05) but comparable to one another (Table 2). GIR appeared related to baseline fasting (r2 = −0.366, P = 0.02), but not 2-h (r2 = −0.031, P = 0.85), glucose concentration. Glucose concentrations remained higher in IFG and IFG/IGT during the low-dose insulin infusion (P < 0.05 vs. NGT for both). Neither FFA concentrations nor the percent suppression (10–24%) were different between the groups. Insulin, C-peptide, lactate, and glycerol values were also not different during the low-dose clamp.
High-dose clamp.
GIR during the high-dose clamp was not different between the groups (Table 2). Circulating concentrations of glucose, insulin, C-peptide, FFA, glycerol, and lactate, as well as percent suppression of FFA (82–90%) by insulin, were all similar between the groups.
Glucose Oxidation and Kinetics
Baseline.
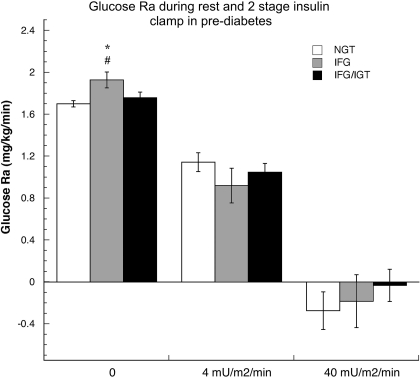
Respiratory exchange ratio (RER) was higher in IFG vs. NGT and IFG/IGT (P < 0.05 for both; Table 3). Consistent with the higher RER in IFG, carbohydrate (CHO) oxidation was higher and nonoxidative glucose disposal (NOGD) lower (P < 0.05 vs. NGT and IFG/IGT for all). Glucose Ra (Fig. 3) was higher in IFG at baseline (P = 0.003 vs. NGT and P = 0.03 vs. IFG/IGT), with no difference noted in baseline Rd normalized to FFM (Table 3). No difference between glucose Ra and Rd was observed between NGT and IFG/IGT. The MCR of glucose (Table 3) was similar between the groups at baseline. Baseline glucose Ra was strongly correlated to fasting glucose concentration (r2 = 0.460, P = 0.004) but not to 2-h glucose (r2 = −0.105, P = 0.53).
Table 3.
Glucose oxidation and kinetics
| RER | CHO Oxidation, mg·kg−1·min−1 | NOGD, mg·kg−1·min−1 | Rd, mg·kg FFM−1·min−1 | MCR, ml·kg−1·min−1 | |
|---|---|---|---|---|---|
| Baseline | |||||
| NGT | 0.77±0.01 | 0.44±0.13 | 1.28±0.11 | 1.70±0.04 | 1.92±0.06 |
| IFG | 0.82±0.02* | 1.12±0.25* | 0.82±0.24* | 1.93±0.07* | 1.95±0.10 |
| IGT/IFG | 0.76±0.02† | 0.51±0.18† | 1.23±0.20† | 1.77±0.06 | 1.76±0.14 |
| Low-dose (4 mU·m2·min−1) clamp | |||||
| NGT | 0.81±0.04 | 0.69±0.27 | 0.70±0.29 | 1.35±0.08 | 1.62±0.11 |
| IFG | 0.80±0.02 | 0.78±0.22 | 0.34±0.25 | 1.41±0.08 | 1.50±0.08 |
| IGT/IFG | 0.76±0.01 | 0.34±0.12† | 1.12±0.17† | 1.40±0.11 | 1.49±0.10 |
| High-dose (40 mU·m2·min−1) clamp | |||||
| NGT | 0.82±0.02 | 0.94±0.16 | 2.07±0.33 | 3.25±0.37 | 4.28±0.51 |
| IFG | 0.84±0.03 | 1.43±0.44 | 1.79±0.41 | 3.04±0.75 | 4.31±0.62 |
| IGT/IFG | 0.84±0.01 | 1.30±0.14 | 1.37±0.29 | 2.53±0.31 | 3.08±0.37 |
Values are means ± SE. RER, respiratory exchange ratio; CHO, carbohydrate; NOGD, nonoxidative glucose disposal; Rd, glucose rate of disappearance; MCR, metabolic clearance rate of glucose.
P < 0.05 vs. NGT;
P < 0.05 vs. IFG.
Fig. 3.
Glucose rate of appearance (Ra; mg·kg−1·min−1) in NGT, IFG, IGT, and IGT/IFG during rest, low-dose insulin infusion (4 mU m2/min), and high-dose (40 mU m2/min) insulin infusion. Values are means ± SE. *P = 0.003 vs. NGT; #P = 0.03 vs. IFG/IGT.
Low-dose clamp.
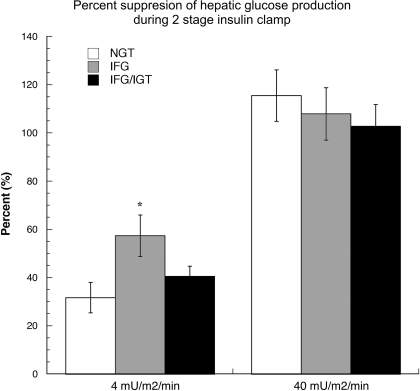
CHO oxidation was lower and NOGD higher, reflecting the lower RER, in IFG/IGT during the low-dose (4 mU·m2·min−1) insulin infusion (P < 0.05 vs. IFG for all; Table 3). Glucose Ra and Rd, as well as MCR, were comparable among all groups. Suppression of glucose Ra was greatest in IFG (57%, P = 0.008 vs. 31% in NGT and P = 0.08 vs. 41% in IFG/IGT; Fig. 4). Glucose Rd fell similarly in all groups during the low-dose insulin infusion, largely due to a decrease in glucose Ra. A nonsignificant trend toward suppression of glucose Ra during the low-dose clamp and fasting (r2 = 0.311, P = 0.057), but not 2-h (r2 = −0.032, P = 0.85), glucose concentration was noted.
Fig. 4.
Suppression of glucose Ra (%) during the low-dose (40 mU·m2·min−1) and high-dose (40 mU m2/min) insulin infusions. Values are means ± SE. *P = 0.08 vs. NGT.
High-dose clamp.
Glucose Ra was completely and similarly suppressed in all groups during the high-dose (40 mU·m2·min−1) insulin clamp (Fig. 4). Insulin-stimulated glucose uptake significantly, but similarly, increased in all groups during the high-dose clamp. No differences in glucose Ra, Rd, MCR, CHO oxidation, NOGD, or RER were observed (Table 3). Suppression of glucose Ra during the high-dose clamp was related neither to fasting (r2 = −0.080, P = 0.64) nor to 2-h (r2 = 0.066, P = 0.70) glucose concentrations.
DISCUSSION
Unraveling the complexities of prediabetes is absolutely essential in expanding the current knowledge and armamentarium for preventing type 2 diabetes. Although two-thirds of people with diabetes are overweight or obese (1), only 2–13% of those who are simply obese will ever acquire diabetes (22), whereas ≤70% of those with prediabetes will acquire the disease (12, 26, 43). Therefore, understanding and exploiting differences between simple obesity and the different types of prediabetes has enormous public health implications. The current study was undertaken to determine whether defects underlying IFG are maintained and additive when combined with IGT (representing a progressive form of prediabetes) or are distinct in IFG/IGT (reflecting a parallel form of prediabetes). Nuances of β-cell dysfunction observed in IFG were also noted in IFG/IGT. A trend for additional insulin secretory defects was observed in IFG/IGT, suggesting progression in β-cell failure in this group. In contrast, basal glucose Ra and its suppressability with insulin were higher in IFG, but not IFG/IGT, compared with NGT. Together, these data indicate that IFG/IGT could be a distinct prediabetic syndrome rather than simply a progression from IFG.
IFG
By definition, people with isolated IFG have mild fasting hyperglycemia but achieve euglycemia 2 h after an OGTT. Why insulin secretion is able to overcome postprandial, but not fasting, hyperglycemia is not clear. Fasting glucose concentration is purportedly proportional to endogenous glucose production (EGP) in type 2 diabetes (14) and thus would be expected to be higher in people with IFG in the progression to diabetes. Indeed, our study is in agreement with others where higher basal glucose Ra (a measure of EGP) in isolated IFG has been observed, but the difference is small (13%) and can either appear (6) or disappear (46) with adjustment of the data for confounding variables. Importantly, however, fasting glucose concentration correlated with basal glucose Ra, providing evidence that excess EGP plays an important role in IFG. Higher basal Ra in our study was associated with a higher RER, reflecting greater CHO oxidation in IFG. In contrast, nonoxidative glucose uptake, Rd, and MCR were not different in IFG vs. NGT. Thus, decreased peripheral insulin sensitivity, characteristic of type 2 diabetes, does not appear to be a major feature of isolated IFG in our study as it is in others (16, 29).
Why basal Ra is higher in IFG remains speculative. Hormonal and substrate mediators of EGP, such as insulin, lactate, glycerol, and FFA, were not different between IFG and NGT in the current study at baseline or at any other time point. Glucagon was not measured in this study but has been found to contribute more to EGP in IFG when IFG is combined with IGT or diabetes (6). To our knowledge, the role of cortisol or catecholamines in mediating EGP in IFG has not been studied. Consistent with the findings of Bock et al. (6), basal EGP is inappropriate given the fasting hyperglycemia in this group. An aspect of “glucose effectiveness” (40), the ability of glucose to regulate its own production, appears diminished. Others have observed fasting hyperinsulinemia in IFG, and thus higher EGP in this group may also be due, in part, to hepatic insulin resistance (5, 46). Taking our data with others, decreased glucose effectiveness and hepatic insulin resistance may be permissive for higher EGP in IFG.
Interestingly, our study did not observe fasting hyperinsulinemia in IFG but rather higher fasting C-peptide concentration. Disproportionate insulin-to-C-peptide ratio implicates a difference in whole body insulin clearance (7). Considerable data exist that insulin clearance normally decreases during the development of obesity (24, 44) and diabetes (25, 30) and is a presumed adaptation to preserve β-cell function. However, hepatic insulin extraction (by mathematical modeling) was not different in IFG vs. NGT. Dynamic insulin secretion (Φd; Fig. 1B), believed to represent the promptly releasable pool of stored insulin (1st phase), was impaired in IFG as in other studies (29, 32). It is possible that diminished dynamic insulin secretion reflects either defective glucose sensing, or insulin packaging and/or storage, within the β-cell. Whether altered dynamic insulin secretion is related to elevated fasting EGP in IFG remains to be determined but is unlikely given the close association between 2-h, not fasting glucose, concentration and insulin secretory parameters seen in the current study.
Combined IFG and IGT
The current study illustrates some features of IFG that are maintained and additive in IFG/IGT and some that are truly distinct. Dynamic insulin secretion was impaired in both IFG and IFG/IGT and likely reflects a discriminating defect in groups with IFG. IFG/IGT also demonstrated a nonsignificant trend toward static and global β-cell dysfunction that was not seen in isolated IFG. The somewhat greater defects in insulin secretion seen in IFG/IGT vs. IFG are presumably secondary to those associated with IGT added to those maintained from IFG (3, 46). Although isolated IGT was not included in the current study, considerable previous work has closely examined β-cell function in IGT using a wide variety of techniques. Insulin secretion has been shown to be 21% greater in isolated IGT vs. IFG when using an intravenous glucose tolerance test (16) but 30% lower during a hyperglycemic clamp (29). Seemingly conflicting, insulin secretion during the intravenous glucose tolerance test reflects the first phase, whereas measurements taken during a hyperglycemic clamp reflect the second phase. Thus these findings may not be inconsistent. Certainly, convincing evidence exists that β-cell dysfunction is progressive when IFG and IGT are concurrent rather than in isolation (3, 46). Therefore, our data concur with others and may support the notion of IFG/IGT as a progressive state from IFG with respect to β-cell dysfunction.
In contrast to β-cell dysfunction, elevated basal glucose Ra was not appreciated in IGT/IFG as it was in IFG. Importantly, however, basal glucose Ra was likely inappropriate given fasting hyperglycemia in IGT/IFG, suggesting a mild decrease in hepatic glucose effectiveness in this group (6). Although noteworthy, diminished glucose effectiveness in both IFG and IFG/IGT is limited to the fasted state, as evidenced by greater suppression of glucose Ra in IFG and greater NOGD in IFG/IGT during the low-dose insulin infusion. Importantly, the low-dose insulin infusion decreased circulating glucose concentration, but did not change circulating insulin, in IFG and IFG/IGT. This observation supports the contention that diminished hepatic glucose effectiveness, rather than hepatic insulin resistance, may be as much, or more, contributory to increased glucose Ra in groups with IFG. Our study is consistent with some (5, 46), but not all (3), in this regard. More surprising was the lack of notable decrement in peripheral insulin action in either IFG or IFG/IGT vs. NGT in the current study and may relate to the close anthropometric matching between groups. Previous work has implicated obesity (10, 20), rather than glucose dysregulation (36, 46), in the decline of peripheral insulin action characteristic of diabetes. Alternatively, small numbers in the groups may have obscured differences, as Tripathy et al. (41) noted 19% lower peripheral insulin sensitivity comparing overweight people with IFG/IGT (n = 29) to those with NGT (n = 216). In summary, subtle impairment in hepatic glucose effectiveness coupled with no decrement noted in peripheral glucose uptake in the current study highlights the likely importance of global β-cell failure in the pathway from IFG/IGT to diabetes.
Whether the greater age, higher prevalence of first-degree relatives with type 2 diabetes, and/or higher baseline lactate concentration among those with IFG/IGT vs. IFG had additional unmeasured effects is unknown. However, lower insulin action observed with aging (23) or in first-degree relatives of those with type 2 diabetes (34) would have increased the likelihood of finding differences between groups; thus the lack of such a difference strengthens the conclusions. Close anthropometric matching between groups implies a central role for obesity, rather than glucose dysregulation, as dictating peripheral insulin action. Indeed, previous work has noted far greater differences in peripheral insulin action when obese NGT vs. lean NGT is compared (10, 20) than when obese NGT vs. prediabetes is compared (36, 46). In summary, no differences were noted in peripheral insulin action between IFG, IFG/IGT, and NGT in the current study. Somewhat greater β-cell dysfunction without the excess EGP seen in IFG suggests IFG/IGT as a parallel, rather than progressive, form of prediabetes.
Although subjects included in this analysis were well matched, there are several limitations in the current study worth noting. To focus on aspects of glucose dysregulation, and not obesity per se, subjects were matched for body mass index, percent body fat, and waist-to-hip ratio, and no lean control group was studied. Furthermore, the NGT group may not have been a true control due to the high prevalence of first-degree relatives with type 2 diabetes, a group known to have altered metabolism (34). Therefore, differences in insulin action and secretion were likely underestimated compared with “normal.” In addition, no group with isolated IGT was included. Several borderline significant trends and small absolute differences were noted, likely due to a small sample size. Small sample size increases the possibility that type II statistical errors will arise in data analysis. Despite this limitation, similar effect sizes in other studies with larger sample sizes have been observed (6, 46). Furthermore, the calculated probability that the lack of difference in basal glucose Ra between NGT and IFG/IGT was due to a type II statistical error was <5%. Together, this suggests that the differences between the types of prediabetes are subtle. Defects in insulin secretion are more easily observed, whereas impairments in insulin action are limited largely to the fasted state.
In conclusion, nuances of β-cell dysfunction observed in IFG were also noted in IFG/IGT. A nonsignificant trend for additional insulin secretory defects was observed in IFG/IGT, suggesting progression in β-cell failure in this group. In contrast, basal glucose Ra and its suppressability with insulin were higher in IFG, but not IFG/IGT, compared with NGT. Absolute differences between groups were small but important because they have implications for specific treatment or prevention strategies. Together, these data indicate that IFG/IGT may be a distinct prediabetic syndrome rather than a progression from IFG.
GRANTS
The National Institutes of Health funded this work (Grant Nos. DK-064811, DK-059739, and RR-0036).
Acknowledgments
We owe the success of this work to the research subjects who volunteered their time to participate and the committed staff of the General Clinical Research Center.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.No authors listed. Overweight, obesity, and health risk. National Task Force on the Prevention and Treatment of Obesity. Arch Intern Med 160: 898–904, 2000. [DOI] [PubMed] [Google Scholar]
- 2.No authors listed. Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 20: 1183–1197, 1997. [DOI] [PubMed] [Google Scholar]
- 3.Abdul-Ghani MA, Jenkinson CP, Richardson DK, Tripathy D, DeFronzo RA. Insulin secretion and action in subjects with impaired fasting glucose and impaired glucose tolerance: results from the Veterans Administration Genetic Epidemiology Study. Diabetes 55: 1430–1435, 2006. [DOI] [PubMed] [Google Scholar]
- 4.Barrett PH, Bell BM, Cobelli C, Golde H, Schumitzky A, Vicini P, Foster DM. SAAM II: Simulation, Analysis, and Modeling Software for tracer and pharmacokinetic studies. Metabolism 47: 484–492, 1998. [DOI] [PubMed] [Google Scholar]
- 5.Bock G, Chittilapilly E, Basu R, Toffolo G, Cobelli C, Chandramouli V, Landau BR, Rizza RA. Contribution of hepatic and extrahepatic insulin resistance to the pathogenesis of impaired fasting glucose: role of increased rates of gluconeogenesis. Diabetes 56: 1703–1711, 2007. [DOI] [PubMed] [Google Scholar]
- 6.Bock G, Dalla Man C, Campioni M, Chittilapilly E, Basu R, Toffolo G, Cobelli C, Rizza R. Pathogenesis of pre-diabetes: mechanisms of fasting and postprandial hyperglycemia in people with impaired fasting glucose and/or impaired glucose tolerance. Diabetes 55: 3536–3549, 2006. [DOI] [PubMed] [Google Scholar]
- 7.Bonora E, Zavaroni I, Coscelli C, Butturini U. Decreased hepatic insulin extraction in subjects with mild glucose intolerance. Metabolism 32: 438–446, 1983. [DOI] [PubMed] [Google Scholar]
- 8.Buchanan TA, Xiang AH, Peters RK, Kjos SL, Marroquin A, Goico J, Ochoa C, Tan S, Berkowitz K, Hodis HN, Azen SP. Preservation of pancreatic beta-cell function and prevention of type 2 diabetes by pharmacological treatment of insulin resistance in high-risk hispanic women. Diabetes 51: 2796–2803, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. Diabetes Fact Sheet (Online). http://www.cdc.gov/diabetes/pubs/references05.htm [2005].
- 10.Davidson MB Effect of obesity on insulin sensitivity on human adipose tissue. Diabetes 21: 6–12, 1972. [DOI] [PubMed] [Google Scholar]
- 11.Davies MJ, Raymond NT, Day JL, Hales CN, Burden AC. Impaired glucose tolerance and fasting hyperglycaemia have different characteristics. Diabet Med 17: 433–440, 2000. [DOI] [PubMed] [Google Scholar]
- 12.de Vegt F, Dekker JM, Jager A, Hienkens E, Kostense PJ, Stehouwer CD, Nijpels G, Bouter LM, Heine RJ. Relation of impaired fasting and postload glucose with incident type 2 diabetes in a Dutch population: The Hoorn Study. JAMA 285: 2109–2113, 2001. [DOI] [PubMed] [Google Scholar]
- 13.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol Endocrinol Metab Gastrointest Physiol 237: E214–E223, 1979. [DOI] [PubMed] [Google Scholar]
- 14.Dinneen S, Gerich J, Rizza R. Carbohydrate metabolism in non-insulin-dependent diabetes mellitus. N Engl J Med 327: 707–713, 1992. [DOI] [PubMed] [Google Scholar]
- 15.Eaton RP, Allen RC, Schade DS, Erickson KM, Standefer J. Prehepatic insulin production in man: kinetic analysis using peripheral connecting peptide behavior. J Clin Endocrinol Metab 51: 520–528, 1980. [DOI] [PubMed] [Google Scholar]
- 16.Festa A, D'Agostino R Jr, Hanley AJ, Karter AJ, Saad MF, Haffner SM. Differences in insulin resistance in nondiabetic subjects with isolated impaired glucose tolerance or isolated impaired fasting glucose. Diabetes 53: 1549–1555, 2004. [DOI] [PubMed] [Google Scholar]
- 17.Finegood DT, Bergman RN, Vranic M. Estimation of endogenous glucose production during hyperinsulinemic-euglycemic glucose clamps. Comparison of unlabeled and labeled exogenous glucose infusates. Diabetes 36: 914–924, 1987. [DOI] [PubMed] [Google Scholar]
- 18.Genuth S, Alberti KG, Bennett P, Buse J, Defronzo R, Kahn R, Kitzmiller J, Knowler WC, Lebovitz H, Lernmark A, Nathan D, Palmer J, Rizza R, Saudek C, Shaw J, Steffes M, Stern M, Tuomilehto J, Zimmet P. Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care 26: 3160–3167, 2003. [DOI] [PubMed] [Google Scholar]
- 19.Gerstein HC, Yusuf S, Bosch J, Pogue J, Sheridan P, Dinccag N, Hanefeld M, Hoogwerf B, Laakso M, Mohan V, Shaw J, Zinman B, Holman RR. Effect of rosiglitazone on the frequency of diabetes in patients with impaired glucose tolerance or impaired fasting glucose: a randomised controlled trial. Lancet 368: 1096–1105, 2006. [DOI] [PubMed] [Google Scholar]
- 20.Goodpaster BH, Thaete FL, Simoneau JA, Kelley DE. Subcutaneous abdominal fat and thigh muscle composition predict insulin sensitivity independently of visceral fat. Diabetes 46: 1579–1585, 1997. [DOI] [PubMed] [Google Scholar]
- 21.Hanefeld M, Koehler C, Fuecker K, Henkel E, Schaper F, Temelkova-Kurktschiev T. Insulin secretion and insulin sensitivity pattern is different in isolated impaired glucose tolerance and impaired fasting glucose: the risk factor in Impaired Glucose Tolerance for Atherosclerosis and Diabetes study. Diabetes Care 26: 868–874, 2003. [DOI] [PubMed] [Google Scholar]
- 22.Harris MI, Flegal KM, Cowie CC, Eberhardt MS, Goldstein DE, Little RR, Wiedmeyer HM, Byrd-Holt DD. Prevalence of diabetes, impaired fasting glucose, and impaired glucose tolerance in U.S. adults. The Third National Health and Nutrition Examination Survey, 1988–1994. Diabetes Care 21: 518–524, 1998. [DOI] [PubMed] [Google Scholar]
- 23.Jackson RA, Hawa MI, Roshania RD, Sim BM, DiSilvio L, Jaspan JB. Influence of aging on hepatic and peripheral glucose metabolism in humans. Diabetes 37: 119–129, 1988. [DOI] [PubMed] [Google Scholar]
- 24.Jiang X, Srinivasan SR, Berenson GS. Relation of obesity to insulin secretion and clearance in adolescents: the Bogalusa Heart Study. Int J Obes Relat Metab Disord 20: 951–956, 1996. [PubMed] [Google Scholar]
- 25.Jones CN, Abbasi F, Carantoni M, Polonsky KS, Reaven GM. Roles of insulin resistance and obesity in regulation of plasma insulin concentrations. Am J Physiol Endocrinol Metab 278: E501–E508, 2000. [DOI] [PubMed] [Google Scholar]
- 26.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 346: 393–403, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McGuire EA, Helderman JH, Tobin JD, Andres R, Berman M. Effects of arterial versus venous sampling on analysis of glucose kinetics in man. J Appl Physiol 41: 565–573, 1976. [DOI] [PubMed] [Google Scholar]
- 28.Meigs JB, Muller DC, Nathan DM, Blake DR, Andres R. The natural history of progression from normal glucose tolerance to type 2 diabetes in the Baltimore Longitudinal Study of Aging. Diabetes 52: 1475–1484, 2003. [DOI] [PubMed] [Google Scholar]
- 29.Meyer C, Pimenta W, Woerle HJ, Van Haeften T, Szoke E, Mitrakou A, Gerich J. Different mechanisms for impaired fasting glucose and impaired postprandial glucose tolerance in humans. Diabetes Care 29: 1909–1914, 2006. [DOI] [PubMed] [Google Scholar]
- 30.Mittelman SD, Van Citters GW, Kim SP, Davis DA, Dea MK, Hamilton-Wessler M, Bergman RN. Longitudinal compensation for fat-induced insulin resistance includes reduced insulin clearance and enhanced beta cell response. Diabetes 49: 2116–2125, 2000. [DOI] [PubMed] [Google Scholar]
- 31.Mokdad AH, Ford ES, Bowman BA, Dietz WH, Vinicor F, Bales VS, Marks JS. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA 289: 76–79, 2003. [DOI] [PubMed] [Google Scholar]
- 32.Nathan DM, Davidson MB, DeFronzo RA, Heine RJ, Henry RR, Pratley R, Zinman B. Impaired fasting glucose and impaired glucose tolerance: implications for care. Diabetes Care 30: 753–759, 2007. [DOI] [PubMed] [Google Scholar]
- 33.Perreault L, Lavely JM, Bergman BC, Horton TJ. Gender differences in insulin action after a single bout of exercise. J Appl Physiol 97: 1013–1021, 2004. [DOI] [PubMed] [Google Scholar]
- 34.Petersen KF, Dufour S, Befroy D, Garcia R, Shulman GI. Impaired mitochondrial activity in the insulin-resistant offspring of patients with type 2 diabetes. N Engl J Med 350: 664–671, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pietrobelli A, Formica C, Wang Z, Heymsfield SB. Dual-energy X-ray absorptiometry body composition model: review of physical concepts. Am J Physiol Endocrinol Metab 271: E941–E951, 1996. [DOI] [PubMed] [Google Scholar]
- 36.Qiao Q, Jousilahti P, Eriksson J, Tuomilehto J. Predictive properties of impaired glucose tolerance for cardiovascular risk are not explained by the development of overt diabetes during follow-up. Diabetes Care 26: 2910–2914, 2003. [DOI] [PubMed] [Google Scholar]
- 37.Snehalatha C, Ramachandran A, Sivasankari S, Satyavani K, Vijay V. Insulin secretion and action show differences in impaired fasting glucose and in impaired glucose tolerance in Asian Indians. Diabetes Metab Res Rev 19: 329–332, 2003. [DOI] [PubMed] [Google Scholar]
- 38.Tirosh A, Shai I, Tekes-Manova D, Israeli E, Pereg D, Shochat T, Kochba I, Rudich A. Normal fasting plasma glucose levels and type 2 diabetes in young men. N Engl J Med 353: 1454–1462, 2005. [DOI] [PubMed] [Google Scholar]
- 39.Toffolo G, Breda E, Cavaghan MK, Ehrmann DA, Polonsky KS, Cobelli C. Quantitative indexes of β-cell function during graded up&down glucose infusion from C-peptide minimal models. Am J Physiol Endocrinol Metab 280: E2–E10, 2001. [DOI] [PubMed] [Google Scholar]
- 40.Tonelli J, Kishore P, Lee DE, Hawkins M. The regulation of glucose effectiveness: how glucose modulates its own production. Curr Opin Clin Nutr Metab Care 8: 450–456, 2005. [DOI] [PubMed] [Google Scholar]
- 41.Tripathy D, Almgren P, Tuomi T, Groop L. Contribution of insulin-stimulated glucose uptake and basal hepatic insulin sensitivity to surrogate measures of insulin sensitivity. Diabetes Care 27: 2204–2210, 2004. [DOI] [PubMed] [Google Scholar]
- 42.Tripathy D, Carlsson M, Almgren P, Isomaa B, Taskinen MR, Tuomi T, Groop LC. Insulin secretion and insulin sensitivity in relation to glucose tolerance: lessons from the Botnia Study. Diabetes 49: 975–980, 2000. [DOI] [PubMed] [Google Scholar]
- 43.Tuomilehto J, Lindstrom J, Eriksson JG, Valle TT, Hamalainen H, Ilanne-Parikka P, Keinanen-Kiukaanniemi S, Laakso M, Louheranta A, Rastas M, Salminen V, Uusitupa M. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 344: 1343–1350, 2001. [DOI] [PubMed] [Google Scholar]
- 44.Valera Mora ME, Scarfone A, Calvani M, Greco AV, Mingrone G. Insulin clearance in obesity. J Am Coll Nutr 22: 487–493, 2003. [DOI] [PubMed] [Google Scholar]
- 45.Weir JB New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol 109: 1–9, 1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weyer C, Bogardus C, Pratley RE. Metabolic characteristics of individuals with impaired fasting glucose and/or impaired glucose tolerance. Diabetes 48: 2197–2203, 1999. [DOI] [PubMed] [Google Scholar]
- 47.Wolfe RR Radioactive and Stable Isotope Tracers in Biomedicine. New York: Wiley-Liss, 1992.