Abstract
Diabetic patients frequently encounter ketosis that is characterized by the breakdown of lipids with the consequent accumulation of ketone bodies. Several studies have demonstrated that reactive species are likely to induce tissue damage in diabetes, but the role of the ketone bodies in the process has not been fully investigated. In this study, electron paramagnetic resonance (EPR) spectroscopy combined with novel spin-trapping and immunological techniques has been used to investigate in vivo free radical formation in a murine model of acetone-induced ketosis. A six-line EPR spectrum consistent with the α-(4-pyridyl-1-oxide)-N-t-butylnitrone radical adduct of a carbon-centered lipid-derived radical was detected in the liver extracts. To investigate the possible enzymatic source of these radicals, inducible nitric oxide synthase (iNOS) and NADPH oxidase knockout mice were used. Free radical production was unchanged in the NADPH oxidase knockout but much decreased in the iNOS knockout mice, suggesting a role for iNOS in free radical production. Longer-term exposure to acetone revealed iNOS overexpression in the liver together with protein radical formation, which was detected by confocal microscopy and a novel immunospin-trapping method. Immunohistochemical analysis revealed enhanced lipid peroxidation and protein oxidation as a consequence of persistent free radical generation after 21 days of acetone treatment in control and NADPH oxidase knockout but not in iNOS knockout mice. Taken together, our data demonstrate that acetone administration, a model of ketosis, can lead to protein oxidation and lipid peroxidation through a free radical-dependent mechanism driven mainly by iNOS overexpression.
Keywords: inducible nitric oxide synthase; acetone; free radicals; electron paramagnetic resonance
the overproduction of ketone bodies such as acetone and β-hydroxybutyrate is frequently a consequence of impaired glucose catabolism in patients with diabetes mellitus. Ketone bodies are produced in the liver mainly from the oxidation of fatty acids and are exposed to peripheral tissues for use as an energy source. Biochemically, pathophysiological changes of ketone body metabolism can present in three ways: ketosis, hypoketotic hypoglycemia, and abnormalities of the hydroxybutyrate and acetoacetate ratio (18). The plasma concentration of ketone bodies in cases of severe ketosis can be 50 times the concentration (∼25 mM) found in healthy individuals (1, 16, 25). The metabolism of ketone bodies has been widely studied in different experimental systems both in vitro and in vivo, and different metabolic pathways have been implicated (3, 15, 17). There is evidence for three major metabolic pathways for the metabolism of acetone. One is where acetone is converted to acetate and formate through 1,2-propanediol (22); the other pathways are to methylglyoxal and l-lactate (3), where methylglyoxal is known to trigger diabetic complications, for example, neuropathy and retinopathy (26, 27).
Several studies have demonstrated that reactive species are likely to induce tissue damage in diabetes, but the role of the ketone bodies or their metabolites in this process has not been identified. Based on literature data, it has been proposed that, during ketosis, acetoacetate but not hydroxybutyrate promotes oxidative stress although the mechanism by which radicals might be formed is unclear (9, 10, 12). The same authors showed that acetoacetate generates superoxide anion in vitro in human endothelial cells; therefore, ketosis can facilitate lipid peroxidation and even growth inhibition in these cells. In addition to these studies, aminoacetone, a threonine metabolite that accumulates in diabetes and contributes to the formation of the toxic methylglyoxal, was shown to induce iron-mediated oxidative damage in the mitochondria (7).
In addition to hyperglycemia, chronic inflammation, characterized by elevated levels of transcription factors such as nuclear factor-κB and circulating proinflammatory cytokines including tumor necrosis factor-α, and overexpression of inducible nitric oxide synthase (iNOS) in tissues also have been implicated as crucial mechanisms responsible for the progression and pathogenesis of diabetes and its complications (11). Recently, it has been demonstrated in streptozotocin-induced diabetes that iNOS in its uncoupled form drives a free radical mechanism through a transition metal-independent mechanism, leading to hydroxyl radical production and lipid peroxidation in the liver and kidney of diabetic rats (Stadler K, Bonini MG, Dallas S, Jiang J, Mason RP, Kadiiska MB, unpublished observation). In a persistant hyperglycemia, constantly high glucose levels upregulate the expression of transcription factors that ultimately lead to iNOS overexpression, thereby enhancing the possibility of vascular and systemic inflammation in diabetes (24, 28). Nevertheless, the effect of elevated ketone levels on protein expression associated with inflammatory conditions in the disease is not known.
To date, in vivo free radical generation by ketones and their possible participation in initiating a proinflammatory process as a complex scenario behind the mechanism of diabetic complications have not been demonstrated. The aim of our study was to show in vivo evidence for the generation of free radical species by acetone and to correlate them with subsequent pathophysiological damage. Electron paramagnetic resonance (EPR) spectroscopy, combined with in vivo spin trapping, and a novel immunospin-trapping technique together with confocal microscopy and immunohistochemistry have been used to investigate the mechanism of in vivo free radical formation, the appearance of lipid and protein oxidation, and consequent tissue damage in a murine model of acute or longer-term ketosis.
MATERIALS AND METHODS
Materials.
Analytical grade acetone, chloroform, and methanol were purchased from MG Chemicals. α-(4-Pyridyl-1-oxide)-N-t-butylnitrone (POBN) and 5,5-dimethyl-1-pyrroline-N-oxide (DMPO) (both high purity) were obtained from Alexis Biochemicals. Paraformaldehyde and sucrose were from ICN. 1400W was from Calbiochem. For the confocal and Western blot studies, monoclonal iNOS primary antibody (Sigma) and anti-DMPO antibody (4) were applied. Alexafluor 488 goat anti-mouse and 566 goat anti-rabbit fluorophore secondaries were from Invitrogen.
Animals.
C57BL/B6 male mice served as control animals throughout the study and were treated with acetone. Mice containing the disrupted gp91phox gene (gp91phox−/−) and iNOS gene (iNOS−/−) were used in knockout studies. All mice weighing 20–25 g obtained from Jackson's Laboratories (Bar Harbor, ME) were used in all experiments. Animals were housed in a room with air conditioning and a 12:12-h light-dark cycle, fed a standard chow (NIH open formula; Ziegler Brothers, Gardner, PA), and had access to water ad libitum. In the acute experiments, acetone (2,500 mg/kg) was given to control or knockout mice intragastrically for 1 h after opening the abdomen under pentobarbital sodium anesthesia; next, the spin trap POBN (dissolved in saline) was administered at 20 mg/mouse ip. Another group received the inhibitor 1400W (15 mg/kg) 30 min before acetone administration. In a longer exposure to the ketone body, mice were given 2% acetone dissolved in their drinking water for 5 days or 3 wk ad libitum. Another group of animals started receiving DMPO from the onset of the acetone treatment: 20 μl of the commercially available compound three times a day (every 6 h) intraperitoneally until the end of experiments. Each group contained at least five animals. All studies were approved by the institutional review board and adhered to National Institutes of Health guidelines for the care and handling of experimental animals.
In vivo spin-trapping studies.
In the acute experiments (described under Animals), 1 h after POBN injection, animals were killed, and livers were excised, washed in PBS, and put in a chloroform-methanol (2:1) solution to extract the lipids containing POBN adducts (23). Briefly, livers were homogenized with a Polytron homogenizer (Fisher Scientific PowerGen 125) in 2.5 ml chloroform-methanol (2:1) plus 4.5 ml Chelex-treated water containing 30 mM 2,2′-dipyridyl to inhibit ex vivo Fenton chemistry (8, 14). Homogenates were diluted with 16 ml chloroform-methanol mixture, shaken for 5 min to complete the extraction, and centrifuged at 2,500 revolutions/min (rpm) for 10 min. The water phase supernatant was discarded, and the organic phase was passed through a sodium sulfate column to eliminate water residue and evaporated under N2 atmosphere down to 0.5 ml (TurboVap II; Caliper LifeSciences).
EPR studies.
EPR spectra were recorded at room temperature on a Bruker EMX EPR spectrometer equipped with a super high-Q cavity using a quartz flat cell (Bruker, Billerica, MA). Each sample extract was bubbled with nitrogen for 2 min before measurement. Spectra were recorded on an IBM-compatible computer interfaced with the spectrometer with the following instrument settings and conditions: 20.2 mW microwave power, 100 kHz modulation frequency, 1 G modulation amplitude, 1.3 ms time constant, 655 ms conversion time, and a single scan of 80 G. The simulations of EPR spectra and determination of hyperfine coupling constants were performed by using the WINSIM program developed in our laboratory (6).
Confocal microscopy.
For confocal studies, C57BL/B6 wild-type, gp91phox−/−, and iNOS−/− mice in the longer-term studies (with or without receiving daily DMPO injections) were anesthetized by intraperitoneal injection of Nembutal (50 mg/kg), and liver perfusion was carried out through the heart using a peristaltic pump. First, saline was perfused through the liver until the organ was free of blood; next, the system was switched to 3.5% paraformaldehyde solution in PBS (pH = 7.4) for fixation. After fixation, livers were removed and placed in 30% sucrose for 24 h. Tissues were then sliced on a microtome into 70-μm sections, placed in PBS, and then permeabilized with 0.1% Surfact-Amps-X-100 for 1 h. After blocking with 0.1% BSA in PBS, staining of iNOS was performed using monoclonal anti-iNOS antibody as the primary antibody and Alexafluor 488 anti-mouse conjugated with fluorescein as the secondary antibody. Staining for protein radicals in the DMPO-treated group was made by a polyclonal anti-DMPO antibody and Alexafluor 568 anti-rabbit secondary antibody. Secondary controls were made to determine background fluorescence by applying the secondary antibody only. Slices were mounted on microscope cover glasses (22 × 22 mm, 1½ mm thickness) (Erie Scientific, Portsmouth, NH), and sections were analyzed under a confocal laser microscope (Zeiss). To give a quantitative evaluation of the protein oxidation detected by anti-DMPO, all of the images were converted into grayscale and quantified by the Scion Image program to obtain a mean fluorescence intensity for each group.
Western blotting.
Western blot analysis was applied to determine the time-course pattern of iNOS protein expression 1, 3, and 5 days after acetone exposure in murine liver, kidney, and lung samples. Tissue samples were homogenized with a Polytron homogenizer in RIPA buffer containing the protease inhibitor Complete Mini pill from Roche. After incubation at 4°C for 30 min, samples were centrifuged at 14,000 rpm for 10 min. Protein concentrations were quantitated with a protein assay kit (Bio-Rad), and an equal amount of protein (40 μg/lane) was separated on reducing NuPAGE 4–12% Bis-Tris gel (Invitrogen) and transferred to a nitrocellulose membrane. After being blocked for 1 h (1% BSA, 1% amicase in 0.1 M PBS, pH = 7.4), the membrane was probed with a monoclonal iNOS primary antibody (Sigma) followed by an anti-mouse secondary antibody and CDP-Star chemiluminescent substrate (Roche). Band intensities were evaluated using the Image J program.
Quantitative real-time RT-PCR.
Total RNA was extracted from livers with RNeasy kits (QIAGEN) according to the manufacturer's protocol. Real-time PCR was performed using the 7900HT sequence detection system predesigned primer/probe sets (mouse nitric oxide synthase-2) available from Applied Biosystems (Foster City, CA) and following the manufacturer's instructions. The signal obtained from each gene primer/probe set was normalized to that of the unregulated housekeeping gene cyclophilin B primer/probe set (also available from Applied Biosystems). Each primer/probe set was analyzed with at least three different sets of RNA.
Immunohistochemistry.
Murine livers from all experimental groups were excised and washed in cold PBS after chronic acetone exposure for 5 or 21 days, cut into sections, fixed in 10% neutral-buffered formalin for 24 h, and then transferred to 70% ethanol. Two- to three-millimeter-thick slices of liver tissue samples were embedded in paraffin. Sections with 3 μm thickness were probed with a monoclonal anti-4-hydroxynonenal antibody (Alexis Biochemicals) as the primary antibody and anti-rabbit as the secondary to detect the adduct in tissue samples. Hematoxylin-eosin stainings were also performed to evaluate any histological changes.
Statistical analysis.
Data were expressed as means ± SE except EPR hyperfine coupling data, which were given as means ± SD. Statistical significance between groups was determined by the analysis of variance and Student's t-test. P < 0.05 was considered to be statistically significant.
RESULTS
Lipid radical formation induced by acetone.
To determine free radical generation in an acute ketosis model, animal studies were performed using acetone given intragastrically. EPR signals of POBN radical adducts were evaluated in lipid extracts of liver tissues from animals exposed to the ketone for 1 h.
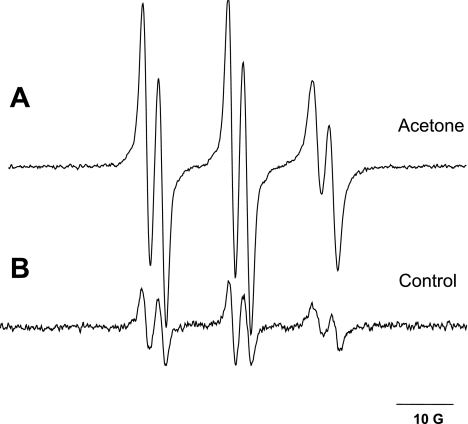
Spin-trapping experiments performed in mice after acute exposure to acetone led to the detection of a well-defined six-line EPR signal of a POBN radical adduct. Radical adducts were reproducibly observed in the lipid extract of liver samples 1 h after spin trap administration (Fig. 1A). In age- and weight-matched control mice, only minor residual signals of POBN radical adducts were detected (Fig. 1B), confirming increased in vivo free radical formation in an acute ketosis caused by acetone. The EPR spectrum of the free radicals trapped was simulated, yielding hyperfine splitting constants of aN = 15.75 ± 0.06 G and aβH = 2.77 ± 0.07 G. These coupling constants were very similar to POBN radical adducts identified and published previously as carbon-centered, polyunsaturated fatty acid-derived (23). Therefore, the POBN adducts detected by acute acetone treatment are identified as carbon-centered, lipid-derived radicals, apparently formed as a result of enhanced lipid peroxidation.
Fig. 1.
Free radical production in murine liver after 1 h of acute acetone treatment. C57BL/B6 mice were administered a single intragastric injection of acetone (2,500 mg/kg) to mimic ketosis, and spin trapping was performed by an ip injection of α-(4-pyridyl-1-oxide)-N-t-butylnitrone (POBN, 20 mg/mouse). Representative electron paramagnetic resonance (EPR) spectra of POBN radical adducts detected in the lipid extracts of livers from acetone-treated (A) or control (B) mice 1 h after POBN injection. Spectra are representatives of at least 5 independent experiments.
Knockout mice and inhibitor studies with acute acetone exposure.
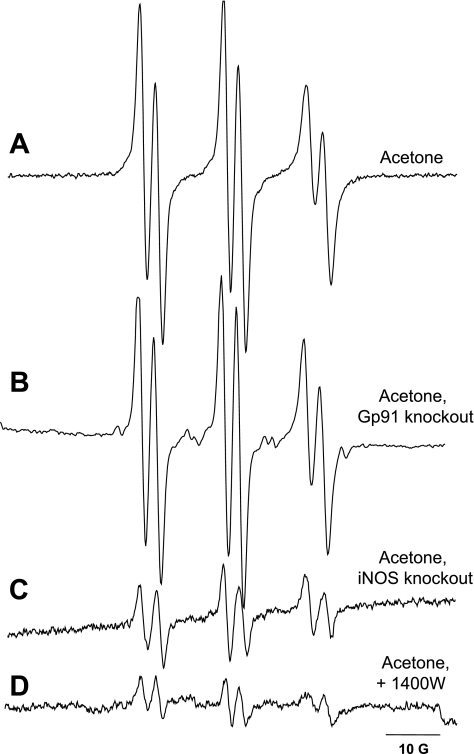
To further investigate the mechanism of free radical production and to gain details about the possible source of radical generation in acetone-treated mice, spin-trapping experiments in iNOS and NADPH knockout mice were performed (Fig. 2). These enzymes have been shown to be major sources of free radical intermediates in a variety of pathophysiological conditions. In these experiments, iNOS and NADPH oxidase knockout mice were given a single intragastric injection of acetone, and spin trapping was carried out with POBN similarly to those experiments performed in normal mice. NADPH oxidase knockout mice exhibited EPR signals as intense as the wild-type mice treated with acetone (Fig. 2B), whereas in iNOS knockout animals, only minor background signals comparable to untreated controls were identified (Fig. 2C). To further confirm this result, normal C57BL mice received the highly specific iNOS inhibitor 1400W 30 min before acetone administration. In this case, similarly to the iNOS knockout mice, only a residual signal was observed (Fig. 2D). These results indicate the involvement of iNOS but not NADPH oxidase in the process that ultimately leads to lipid radical generation in acetone exposure.
Fig. 2.
Lipid radical production in the liver of mice containing the disrupted gp91phox gene (gp91phox−/−) and inducible nitric oxide synthase (iNOS) gene (iNOS−/−) or mice pretreated with the inhibitor 1400W. Lipid radical adduct production was measured by EPR spectroscopy. Knockout mice were injected intragastrically with acetone (2,500 mg/kg) and with the spin trap POBN (20 mg/mouse ip) for 1 h. Lipid radical adducts were determined in the lipid extracts of liver samples. Each experiment was made in quadruplicate. Representative EPR spectra of lipid radicals detected in the liver of acetone-treated C57BL mice (A), acetone-treated NADPH oxidase knockout mice (B), acetone-treated iNOS knockout mice (C), and acetone-treated C57Bl mice pretreated with the iNOS inhibitor 1400W (15 mg/kg) (D).
iNOS overexpression and protein damage after 5 days of acetone treatment.
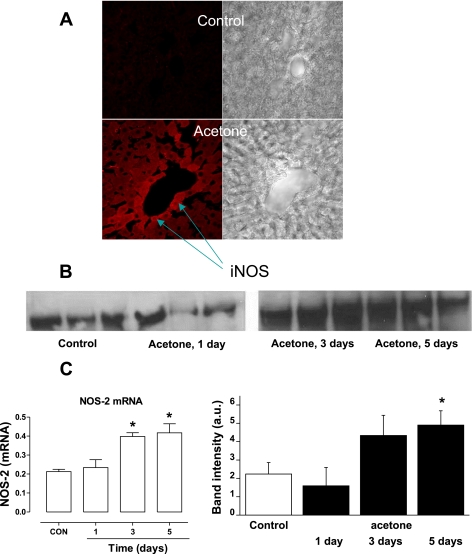
To further characterize iNOS as a mediator of free radical overproduction in acetone-related ketosis, mice received 2% acetone in drinking water for 5 days. Using confocal microscopy, iNOS expression was localized in liver tissue slices obtained from both control and acetone-treated mice. As shown in Fig. 3A, increased iNOS immunostaining was observed in the liver from acetone-exposed mice, and the enzyme was consistently localized around the centralobular vein. In addition, time-course Western blot and real-time PCR analysis of iNOS in liver homogenates further confirmed increased expression of the enzyme after 5 days of acetone treatment compared with the controls and an increase in iNOS mRNA after 3 days (Fig. 3, B and C). Interestingly, the expression of the enzyme was unchanged in other organs such as the kidney or the lung (data not shown).
Fig. 3.
iNOS overexpression in murine liver tissues upon chronic acetone treatment. Mice received 2% acetone for 5 days in their drinking water. A: immunofluorescence detection of iNOS using confocal microscopy. Incubation of liver slices with primary anti-iNOS and Alexafluor 488 secondary antibody shows significant increase of iNOS expression in acetone-treated animals. The enzyme expression was more pronounced around the centralobular vein. Omitting the primary antibody demonstrated only minor background fluorescence (data not shown). B: RT-PCR and Western blot analysis of iNOS protein expression in liver tissues of control mice and acetone-treated mice 1, 3, and 5 days after treatment. Control and acetone-treated liver homogenate samples containing equal amounts of protein (40 μg/lane) were resolved by SDS-PAGE, transferred to a nitrocellulose membrane, and probed with anti-iNOS monoclonal antibody. Total RNA was extracted from liver samples, and quantitative RT-PCR was performed. Data are representative of 3 independent experiments. C: statistical evaluation of Western blot band intensities and quantitative real-time PCR analysis of iNOS mRNA after a time course of acetone treatment. *P < .05 vs. control group.
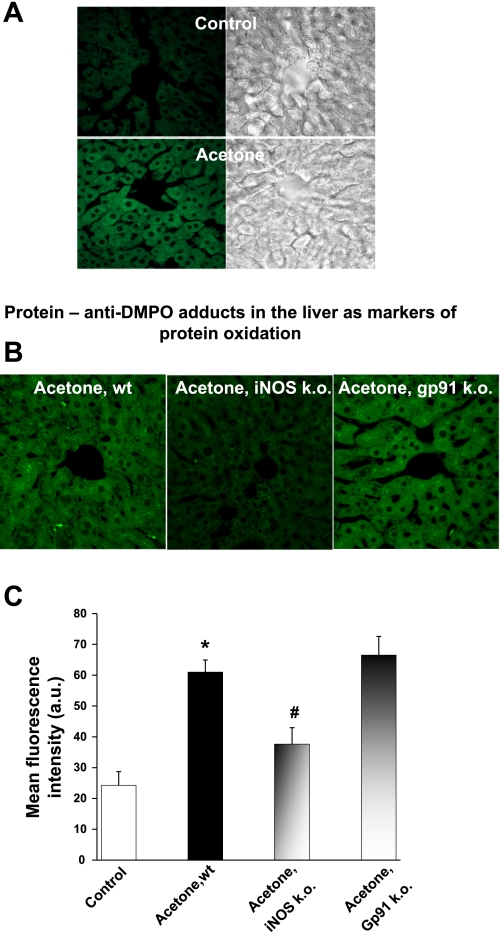
To further investigate whether longer exposure to acetone leads to more extensive damage in addition to lipid radical formation, animals in another set of experiments received DMPO on a regular schedule for 5 days (see materials and methods). DMPO reacts with protein radicals in situ and real time, producing a stable nitrone adduct that has DMPO covalently attached to the site of radical formation in proteins. The adduct can be localized with an anti-DMPO antiserum developed in our laboratory (4) and validated to image protein radicals in cell cultures (2). When the technique is combined with confocal microscopy, it is possible to localize and image protein free radicals in tissues. Through immunospin trapping, protein radicals were identified in liver tissue slices obtained from mice with 5 days of acetone treatment combined with DMPO injection. Figure 4A shows the localization of extensive protein free radical formation in acetone-treated livers, whereas there is no observable immunostaining in healthy animals. If DMPO was omitted and only the antibody was applied on control or treated liver slices, no immunostaining of protein radicals was observed (data not shown). The majority of the damage colocalizes with the location of iNOS overexpression in the liver around the centralobular region (Fig. 3A). According to Fig. 4B, the protein radical formation was markedly diminished in iNOS knockout mice but remained comparable to the wild-type-treated ones in the NADPH oxidase knockout mice. All of the images were quantified, and a mean fluorescence intensity was given for each group (Fig. 4C) where statistically significant differences were confirmed between control vs. acetone-treated, and acetone-treated vs. iNOS knockout acetone-treated groups.
Fig. 4.
Protein oxidation and immunofluorescence detection of protein radical formation after 5 days of acetone treatment. Mice were receiving 2% acetone in their drinking water for 5 days together with the ip administration of 20 μl 5,5-dimethyl-1-pyrroline-N-oxide (DMPO) 3 times/day. A: incubation of liver slices with primary anti-DMPO and Alexafluor 488 secondary rabbit antibody shows enhanced protein oxidation in acetone-treated liver (intense green staining). Omitting the primary antibody or applying the anti-DMPO antibody only without DMPO treatment demonstrated only minor background fluorescence (data not shown). B: compared with wild-type mice treated with acetone, iNOS knockout mice show a significant decrease in protein radical formation, whereas no changes were detected in the liver of NADPH oxidase knockout mice. C: quantitative comparison of the levels of protein oxidation. Confocal images were compared using the Scion Image program, and the degree of protein oxidation was expressed as mean fluorescence intensity in each group. P < 0.05 vs. control group (*) and vs. acetone-treated group (#).
Lipid peroxidation and histopathological changes after 5 and 21 days of acetone treatment.
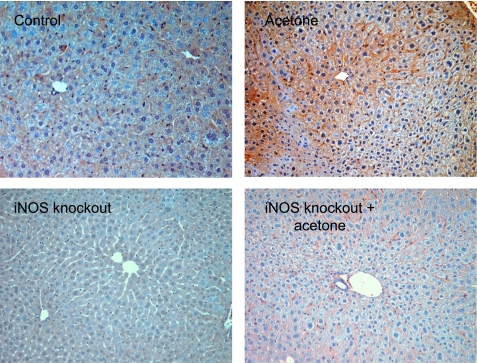
Based on the previous experiments, to evaluate the degree of tissue damage caused by free radical overproduction, liver tissues were examined after 5 and 21 days of acetone treatment, applying immunohistochemical staining for 4-hydroxynonenal, a specific marker of lipid peroxidation. Histopathological analysis showed no significant changes in the liver after 5 or 21 days of acetone treatment (data not shown). However, significant positive 4-hydroxynonenal staining was observed in the C57BL mouse liver (Fig. 5, top), showing a centralobular pattern. On the other hand, iNOS knockout animals with or without treatment showed only minor background staining for 4-hydroxynonenal (Fig. 5, bottom). Both results described above imply a close correlation between the overexpression of iNOS, increased free radical generation, and consequent lipid and protein damage.
Fig. 5.
Immunohistochemical detection of 4-hydroxynonenal in liver as a marker of lipid peroxidation and lipid-protein conjugation. Positive staining showing a centrolobular pattern was observed in acetone-treated livers after 21 days while no significant staining was present in control liver samples (top). Only minor background staining was detected in control or acetone-treated iNOS knockout animals (bottom).
DISCUSSION
It has been shown that diabetic patients frequently suffering from ketosis have an increased incidence of vascular disease and inflammation, morbidity, and mortality (13). However, very little is known about the role of ketosis in diabetes-related oxidative stress and the biochemical mechanisms by which ketone bodies accelerate cellular and tissue damage through free radical generation.
In the current study, we demonstrated that in vivo exposure to acetone leads to the generation of carbon-centered, lipid-derived free radicals in the liver. We unequivocally detected these radical species formed in the liver upon acute acetone treatment by EPR spin trapping. The EPR signals of POBN radical adducts were evaluated in lipid extracts of liver tissues from animals exposed to the ketone for 1 h. In this technique, the short-lived free radical intermediates react with the spin trap, producing more stable free radical adducts that are removed from the tissues with a lipid extraction procedure. These are then detected and characterized through their EPR spectra fingerprint. For a given radical, the fraction of the radical trapped is proportional to the total amount of free radical produced, thereby allowing comparative quantitation.
In these experiments, it is necessary to provide high concentrations of the spin-trapping agent at the site of radical formation to successfully compete with endogenous compounds for reaction with the free radicals. At a dose of 20 mg/mouse POBN administered by intraperitoneal injection, POBN radical adducts were reproducibly detected in the liver of acetone-intoxicated mice. Radicals were identified as carbon-centered lipid radicals, indicating that, indeed, acetone treatment caused increased radical formation as a result of enhanced lipid peroxidation (Fig. 1). Through the 1,2-propanediol pathway, the metabolism of acetone can provide formate and acetate. Consistent with our results, formate has previously been shown to generate free radical species during formate intoxication in rats (5). Another very similar compound, acetaldehyde, has also been shown to be metabolized to methyl and acetyl radicals as detected by EPR spin trapping (20); however, the implications of carbonyl-derived radical formation in tissue damage remain to be demonstrated.
To probe the source of free radical production, we used iNOS and NADPH oxidase knockout mice to identify the possible enzymatic mediator of acetone-derived radical formation. These knockouts were chosen based on the hypothesis that either iNOS or NADPH oxidase are likely candidates involved in increased free radical generation under inflammatory conditions. In other disease models where inflammation occurs, investigators have shown that both iNOS or NADPH oxidase can be mediators of free radical production (19, 23). Lipid radical generation was diminished in the iNOS knockout mice or when iNOS was inhibited chemically by the specific inhibitor 1400W, demonstrating the involvement of the enzyme in the process. Because nitric oxide itself (the product of iNOS-mediated oxidation of arginine) is not a strong enough oxidant to convert acetone to radicals, we hypothesize that peroxynitrite, a product of a diffusion- controlled reaction between nitric oxide and the ubiquitous superoxide radical, may be a key intermediate, mediating acetone oxidation to carbon-centered radicals. Peroxynitrite has been shown to be a powerful nucleophile where this oxidant in its deprotonated form attacks the carbonyl group (21).
The analysis of the data from longer-term acetone exposure demonstrates that acetone triggers iNOS overexpression, which was paralleled by an increased formation of protein-DMPO nitrone adducts in the liver after 5 days of acetone treatment (Fig. 3, A and B, and Fig. 4A). The secondary protein radicals identified with immunospin trapping are markers of protein oxidation and damage. Protein radical formation can lead to the loss of function of the protein, protein carbonyl production, or protein aggregation. When a 5-day treatment was applied in iNOS knockout mice, the detectable protein radical formation was significantly decreased, further confirming a role for the enzyme in free radical generation (Fig. 4, B and C). On the other hand, in good agreement with the EPR results, 5 days of acetone treatment led to protein damage in NADPH oxidase knockout mice similar to the wild-type mice, showing that NADPH oxidase has no significant role in the mechanism in this model (Fig. 4, B and C). Based on these results, we sought evidence of lipid peroxidation and further confirmation of protein damage in a long-term acetone exposure (5 and 21 days of treatment). 4-Hydroxynonenal was chosen as a marker since it is a well-characterized aldehyde product of lipid peroxidation that reacts with protein amine groups, chemically modifying proteins and thus contributing to tissue damage. As a result of iNOS overexpression, persistent free radical generation led to lipid peroxidation, and protein damage in the liver after 21 days, revealed by the extensive staining. Mice lacking iNOS did not develop such protein modification or tissue damage, indicating the fundamental role of this enzyme in the pathophysiological mechanism.
Our study demonstrates that iNOS overexpression as a result of acetone challenge leads to cellular protein oxidation and protein radical formation (which were trapped by DMPO and characterized through immunospin trapping) in an in vivo model within hours and, subsequently, lipid peroxidation and damage after longer exposure. The study also gives an example of protein radical localization in tissues of living animals (Fig. 4). In addition, the localization of iNOS and protein radicals, as well as the necrosis and the positive hydroxynonenal staining showing the same centrolobular pattern in liver tissues, further suggests that the radicals produced by the overexpression of iNOS are oxidizing the proteins in the surrounding tissue environment and that free radical overproduction due to a persistent ketosis is intimately related to the concomitant lipid peroxidation and protein oxidation.
In conclusion, the present study demonstrates, through several lines of evidence, that iNOS mediates free radical generation in an animal model of ketosis, which initiates lipid peroxidation in vivo. Furthermore, longer acetone exposure leads to protein oxidation, which precedes any detectable histological changes in acetone-related ketosis. Our data provide novel pathophysiological evidence and give new insights into the hypothesis that, like hyperglycemia, hyperketonemia can lead to a proinflammatory stage where, eventually, iNOS is expressed, enhancing oxidative stress and facilitating free radical production which may, in turn, promote some of the late complications of Type 1 and Type 2 diabetes.
GRANTS
This research was supported by the Intramural Research Program of the National Institutes of Health, National Institute of Environmental Health Sciences.
Acknowledgments
We thank Jean B. Corbett for excellent technical assistance, Yvette Rebolloso and Natasha Clayton for the outstanding immunohistochemistry analysis, and Mary J. Mason and Dr. Ann Motten for editing the manuscript.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Adrogue HJ, Wilson H, Boyd AE 3rd, Suki WN, Eknoyan G. Plasma acid-base patterns in diabetic ketoacidosis. N Engl J Med 307: 1603–1610, 1982. [DOI] [PubMed] [Google Scholar]
- 2.Bonini MG, Siraki AG, Atanassov BS, Mason RP. Immunolocalization of hypochlorite-induced, catalase-bound free radical formation in mouse hepatocytes. Free Radic Biol Med 42: 530–540, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Casazza JP, Felver ME, Veech RL. The metabolism of acetone in rat. J Biol Chem 259: 231–236, 1984. [PubMed] [Google Scholar]
- 4.Detweiler CD, Deterding LJ, Tomer KB, Chignell CF, Germolec D, Mason RP. Immunological identification of the heart myoglobin radical formed by hydrogen peroxide. Free Radic Biol Med 33: 364–369, 2002. [DOI] [PubMed] [Google Scholar]
- 5.Dikalova AE, Kadiiska MB, Mason RP. An in vivo ESR spin-trapping study: free radical generation in rats from formate intoxication–role of the Fenton reaction. Proc Natl Acad Sci USA 98: 13549–13553, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duling DR Simulation of multiple isotropic spin-trap EPR spectra. J Magn Reson B 104: 105–110, 1994. [DOI] [PubMed] [Google Scholar]
- 7.Dutra F, Bechara EJ. Aminoacetone induces iron-mediated oxidative damage to isolated rat liver mitochondria. Arch Biochem Biophys 430: 284–289, 2004. [DOI] [PubMed] [Google Scholar]
- 8.Ghio AJ, Kadiiska MB, Xiang QH, Mason RP. In vivo evidence of free radical formation after asbestos instillation: an ESR spin trapping investigation. Free Radic Biol Med 24: 11–17, 1998. [DOI] [PubMed] [Google Scholar]
- 9.Jain SK, Kannan K, Lim G. Ketosis (acetoacetate) can generate oxygen radicals and cause increased lipid peroxidation and growth inhibition in human endothelial cells. Free Radic Biol Med 25: 1083–1088, 1998. [DOI] [PubMed] [Google Scholar]
- 10.Jain SK, McVie R. Hyperketonemia can increase lipid peroxidation and lower glutathione levels in human erythrocytes in vitro and in type 1 diabetic patients. Diabetes 48: 1850–1855, 1999. [DOI] [PubMed] [Google Scholar]
- 11.Jain SK, McVie R, Bocchini JA Jr. Hyperketonemia (ketosis), oxidative stress and type 1 diabetes. Pathophysiology 13: 163–170, 2006. [DOI] [PubMed] [Google Scholar]
- 12.Jain SK, McVie R, Jackson R, Levine SN, Lim G. Effect of hyperketonemia on plasma lipid peroxidation levels in diabetic patients. Diabetes Care 22: 1171–1175, 1999. [DOI] [PubMed] [Google Scholar]
- 13.Jain SK, McVie R, Jaramillo JJ, Chen Y. Hyperketonemia (acetoacetate) increases the oxidizability of LDL + VLDL in type-I diabetic patients. Free Radic Biol Med 24: 175–181, 1998. [DOI] [PubMed] [Google Scholar]
- 14.Kadiiska MB, Mason RP, Dreher KL, Costa DL, Ghio AJ. In vivo evidence of free radical formation in the rat lung after exposure to an emission source air pollution particle. Chem Res Toxicol 10: 1104–1108, 1997. [DOI] [PubMed] [Google Scholar]
- 15.Kosugi K, Scofield RF, Chandramouli V, Kumaran K, Schumann WC, Landau BR. Pathways of acetone's metabolism in the rat. J Biol Chem 261: 3952–3957, 1986. [PubMed] [Google Scholar]
- 16.Laffel L Ketone bodies: a review of physiology, pathophysiology and application of monitoring to diabetes. Diabetes Metab Res Rev 15: 412–426, 1999. [DOI] [PubMed] [Google Scholar]
- 17.Lincoln BC, Des Rosiers C, Brunengraber H. Metabolism of S-3-hydroxybutyrate in the perfused rat liver. Arch Biochem Biophys 259: 149–156, 1987. [DOI] [PubMed] [Google Scholar]
- 18.Mitchell GA, Kassovska-Bratinova S, Boukaftane Y, Robert MF, Wang SP, Ashmarina L, Lambert M, Lapierre P, Potier E. Medical aspects of ketone body metabolism. Clin Invest Med 18: 193–216, 1995. [PubMed] [Google Scholar]
- 19.Nakai K, Kadiiska MB, Jiang JJ, Stadler K, Mason RP. Free radical production requires both inducible nitric oxide synthase and xanthine oxidase in LPS-treated skin. Proc Natl Acad Sci USA 103: 4616–4621, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakao LS, Kadiiska MB, Mason RP, Grijalba MT, Augusto O. Metabolism of acetaldehyde to methyl and acetyl radicals: in vitro and in vivo electron paramagnetic resonance spin-trapping studies. Free Radic Biol Med 29: 721–729, 2000. [DOI] [PubMed] [Google Scholar]
- 21.Nakao LS, Ouchi D, Augusto O. Oxidation of acetaldehyde by peroxynitrite and hydrogen peroxide/iron(II). Production of acetate, formate, and methyl radicals. Chem Res Toxicol 12: 1010–1018, 1999. [DOI] [PubMed] [Google Scholar]
- 22.Sakami W, Lafaye JM. Formation of formate and labile methyl groups from acetone in the intact rat. J Biol Chem 187: 369–378, 1950. [PubMed] [Google Scholar]
- 23.Sato K, Kadiiska MB, Ghio AJ, Corbett J, Fann YC, Holland SM, Thurman RG, Mason RP. In vivo lipid-derived free radical formation by NADPH oxidase in acute lung injury induced by lipopolysaccharide: a model for ARDS. FASEB J 16: 1713–1720, 2002. [DOI] [PubMed] [Google Scholar]
- 24.Stentz FB, Umpierrez GE, Cuervo R, Kitabchi AE. Proinflammatory cytokines, markers of cardiovascular risks, oxidative stress, and lipid peroxidation in patients with hyperglycemic crises. Diabetes 53: 2079–2086, 2004. [DOI] [PubMed] [Google Scholar]
- 25.Stephens JM, Sulway MJ, Watkins PJ. Relationship of blood acetoacetate and 3-hydroxybutyrate in diabetes. Diabetes 20: 485–489, 1971. [DOI] [PubMed] [Google Scholar]
- 26.Thornalley PJ Pharmacology of methylglyoxal: formation, modification of proteins and nucleic acids, and enzymatic detoxification–a role in pathogenesis and antiproliferative chemotherapy. Gen Pharmacol 27: 565–573, 1996. [DOI] [PubMed] [Google Scholar]
- 27.Vander Jagt DL, Hassebrook RK, Hunsaker LA, Brown WM, Royer RE. Metabolism of the 2-oxoaldehyde methylglyoxal by aldose reductase and by glyoxalase-I: roles for glutathione in both enzymes and implications for diabetic complications. Chem Biol Interact 130–132: 549–562, 2001. [DOI] [PubMed] [Google Scholar]
- 28.Yerneni KK, Bai W, Khan BV, Medford RM, Natarajan R. Hyperglycemia-induced activation of nuclear transcription factor kappaB in vascular smooth muscle cells. Diabetes 48: 855–864, 1999. [DOI] [PubMed] [Google Scholar]