Abstract
We have proposed that reactive oxygen species (ROS) play essential roles in cell differentiation. Enzymes belonging to the NADPH oxidase (NOX) family produce superoxide in a regulated manner. We have identified three distinct NOX subfamilies in the fungal kingdom and have shown that NoxA is required for sexual cell differentiation in Aspergillus nidulans. Here we show that Neurospora crassa NOX-1 elimination results in complete female sterility, decreased asexual development, and reduction of hyphal growth. The lack of NOX-2 did not affect any of these processes but led instead to the production of sexual spores that failed to germinate, even in the presence of exogenous oxidants. The elimination of NOR-1, an ortholog of the mammalian Nox2 regulatory subunit gp67phox, also caused female sterility, the production of unviable sexual spores, and a decrease in asexual development and hyphal growth. These results indicate that NOR-1 is required for NOX-1 and NOX-2 functions at different developmental stages and establish a link between NOX-generated ROS and the regulation of growth. Indeed, NOX-1 was required for the increased asexual sporulation previously observed in mutants without catalase CAT-3. We also analyzed the function of the penta-EF calcium-binding domain protein PEF-1 in N. crassa. Deletion of pef-1 resulted in increased conidiation but, in contrast to what occurs in Dictyostelium discoideum, the mutation of this peflin did not suppress the phenotypes caused by the lack of NOX-1. Our results support the role of ROS as critical cell differentiation signals and highlight a novel role for ROS in regulation of fungal growth.
A significant amount of recent research has established that reactive oxygen species (ROS), long considered as harmful by-products, can play cell signaling roles (1, 7, 14, 18, 23). For many years, we have used the model organism Neurospora crassa to investigate the role of ROS in the regulation of asexual development (conidiation). In this fungus, synchronous conidiation is started when a liquid culture is filtered and exposed to the air. The hyphal cells in contact with the air aggregate and adhere to each other within 40 min and grow aerial hyphae after 2 h, and then asexual spores (conidia) are formed at the tips of branched aerial hyphae (aerial mycelium) after 8 to 9 h of air exposure (37, 43). The occurrence of a hyperoxidant state at the start of each of these morphogenetic transitions (hyphal adhesion, formation of aerial mycelium, and conidium formation) has been documented (1, 2, 19-21, 30, 44, 45). In addition, N. crassa develops multicellular fruiting bodies called perithecia, which contain the sexual spores or ascospores. Under nitrogen limitation conditions, a strain with either mating type (A or a) can differentiate a multicellular structure called a protoperithecium and function as a “female” or acceptor strain. A protoperithecium is fertilized through a specialized hypha called the “trichogyne” which fuses with a cell, usually a conidium, from the opposite mating type. Fertilized protoperithecia develop into mature perithecia (6, 34, 46).
We have proposed that ROS are key players in the regulation of cell differentiation in microbial eukaryotes (1, 19, 20). According to this hypothesis, the inactivation of antioxidant enzymes should increase cell differentiation processes whereas the inactivation of prooxidant enzymes should inhibit these processes. As NADPH oxidases (NOX) produce ROS in a regulated manner, we sought to examine the occurrence of NOX enzymes in fungi and their possible roles in cell differentiation (1, 24).
Superoxide generation by the phagocyte NOX involves the formation of an enzyme complex composed of the membrane-associated catalytic core gp91phox (Nox2) and p22phox subunits, as well as regulatory subunits p40phox, p47phox, p67phox, and the GTPase Rac2. Nox2 activation requires the phosphorylation of the p47phox “organizer subunit,” which then interacts with p22phox and the “activator subunit” p67phox, also recruiting p40phox to the complex. In addition, Rac2 interaction with Nox2 and p67phox is essential for the activity of this NOX (reviewed in references 4 and 31). Several Nox2 homologs (NOX1, -3, -4, and -5 and DUOXA1 and DUOXA2) have been identified in mammalian cells, while two organizer (p47phox and NOXO1) and two activator (p67phox and NOXA1) subunits have been reported. Some of these NOX have been involved in cell proliferation and apoptosis, clearly indicating the importance of ROS in cell signaling (see references 4 and 23 for recent reviews).
Functional NOX enzymes were not recognized in microbial eukaryotes until recently. NoxA, the only Nox2 ortholog present in the fungus Aspergillus nidulans, is involved in ROS production and is essential for the differentiation of sexual fruiting bodies (24). An exhaustive phylogenetic analysis of the NoxA orthologs indicated the presence of three NOX subfamilies (NoxA to -C) within the filamentous fungi (1, 24). Some members of these families have now been characterized and shown to regulate different aspects of fungal biology. In the saprophytic fungus Podospora anserina, disruption of the Panox1 gene drastically reduces but does not eliminate the development of sexual fruiting bodies, while inactivation of the Panox2 gene results in production of sexual spores that are nonviable or unable to germinate (29). Null noxA mutants from the fungus Epichloë festucae show unregulated growth in its plant host, changing the interaction from mutualistic to antagonistic, while deletion of the noxB gene does not produce a detectable phenotype (41). In the plant pathogen Magnaporthe grisea NOX1 and NOX2 mutants differentiate penetration structures called appressoria but are unable to penetrate the plant and therefore are nonpathogenic. In addition, NOX1 NOX2 double mutants show a drastic reduction in the production of asexual spores or conidia (12). NOX genes have also been characterized or identified in the slime mold Dictyostelium discoideum (25), as well as in pluricellular and unicellular algae (22). D. discoideum contains three nox genes, and the inactivation of noxA, noxB, noxC, or a p22phox ortholog results in the same phenotype: arrested development and lack of asexual spores (25), indicating that the three NOX play partially redundant functions.
The subunit composition and regulation of NOX activity in microbial eukaryotes are still poorly understood. A p67phox ortholog was initially identified in D. discoideum (25), but its function was not evaluated. More recently, Takemoto et al. (40) identified p67phox orthologs in several fungi and showed that the E. festucae p67phox ortholog NoxR regulates NoxA, but only during association with the plant host. In addition, these authors showed that RacA, a mammalian Rac2 ortholog, shows specific interaction with NoxR. However, no clear orthologs of p22phox, p40phox, or p47phox have been yet identified in fungi (40).
The fact that NOX regulate developmental processes in different microbial eukaryotes suggests that ROS regulate cell differentiation and that this is a ROS ancestral role conserved throughout the eukaryotes. How ROS exert their functions and the identity of their downstream effectors are still unclear. Ca2+ signaling has been linked to NOX function in plants (15). Notably, the elimination of the alg-2b gene restored normal development in noxA- and noxB- but not noxC-null mutants in D. discoideum (25). In this organism, alg-2b encodes one of two calcium-binding penta-EF hand proteins, members of the peflin family (28). This suggests that ALG-2B inhibits a downstream effector of NOX signaling and indicates cross talk between ROS and Ca2+ signaling. The function of peflins or their possible interactions with NOX function have not been evaluated before in filamentous fungi.
Here we used a genetic approach to examine the role of NOX-encoding genes nox-1 and nox-2 in N. crassa growth and cell differentiation. We provide evidence showing that, although each NOX is involved in different aspects of growth and development, a single regulatory subunit, NOR-1, is required for the function of both NOX. In addition, we show that NOX-1 is required for the increased sporulation due to inactivation of the antioxidant enzyme CAT-3 (30). Furthermore, we characterize mutants lacking the only penta-EF calcium-binding protein, PEF-1, and show that this protein does not appear to be related to NOX signaling.
MATERIALS AND METHODS
Neurospora crassa strains and growth conditions.
Strains used in this work are indicated in Table 1. General methodologies for growth and crossing have been reported previously (11). All strains were grown in Vogel's minimal medium supplemented with 1.5% sucrose. When needed, l-histidine (200 μg/ml) and myoinositol (50 μg/ml) were added. For sexual crosses, synthetic crossing medium was inoculated with 5 × 106 conidia and incubated for 6 days at 25°C in the light. Cultures were fertilized with 10 10-μl drops of a conidial suspension (2.5 × 106 conidia/drop) from the opposite mating type, and incubation was continued. Between 12 and 14 days, the ascospores expelled from perithecia were harvested in sterile water and incubated overnight at room temperature. Ascospores were activated at 60°C for 30 min; plated on Vogel's solid medium containing 2% l-sorbose, 0.05% fructose, and 0.05% glucose; and incubated for 24 to 48 h at 30°C. Colonies were isolated and transferred to tubes containing Vogel's medium for propagation. For 2-furfuraldehyde treatment, ascospore suspensions were incubated at 30°C for 6 h to induce swelling of contaminating conidia and later incubated at 46°C for 1 h for partial ascospore activation and killing of swollen conidia (38). After this treatment ascospores were incubated with 1 mM 2-furfuraldehyde (Sigma-Aldrich Corporation, St. Louis, MO) for 15 min at room temperature and plated on solid medium with l-sorbose. For germination experiments in the presence of H2O2, ascospores were activated by heat shock at 60°C for 30 min and then incubated with 0, 1, 5, 10, and 100 mM H2O2 for 20 min. Alternatively, H2O2 was added to ascospores before heat shock activation or ascospores were treated only with H2O2.
TABLE 1.
Neurospora crassa strains used in this study
| Strain | Genotype | Source or reference |
|---|---|---|
| 74-OR23-1A | mat A | FGSC#987 |
| 74-OR8-1a | mat a | FGSC#988 |
| Δmus-51 strain | mus-51Δ::bar+mat a | FGSC#9718 |
| RPNCR3A | mepRhis-3 InlΔ(SacI-BglII)::λA | 35 |
| TKA1b | mepRhis-3 InlΔ(SacI-BglII)::λA nox-2Δ::hph+ | This work; RPNCR3A transformed with pKAD9 |
| TCNB2 | mepRhis-3 InlΔ(SacI-BglII)::λA nox-1Δ::hph+mat A | This work; RPNCR3A transformed with pNCKA8 |
| TCNB10 | mepRhis-3 InlΔ(SacI-BglII)::λAΔnox-1::hph+mat A | This work, RPNCR3A transformed with pNCKA8 |
| H10.1 | nox-1Δ::hph+mat a | This work; progeny from TCNB10 × 74-OR8-1a |
| H10.17 | nox-1Δ::hph+mat A | This work; progeny from TCNB10 × 74-OR8-1a |
| CNCKA-Z | his-3::λA nox-2Δ::hph+mat A | This work; progeny from TKA1b × 74-OR8-1a |
| CNCKA-AN1 | InlΔ(SacI-BglII)::λa nox-2Δ::hph+mat a | This work; progeny from TKA1b × 74-OR8-1a |
| Nc28nor-1 | nor-1Δ::hph+mus-51Δ::bar+mat a | This work; Δmus-51 strain transformed with nor-1 deletion PCR product |
| Δpef-1.9a | pef-1Δ::hph+mus-51Δ::bar+mat a | This work; Δmus-51 strain transformed with pef-1 deletion PCR product |
| A9N1.10 | pef-1Δ::hph+nox-1Δ::hph+mat a | This work; progeny from Δpef-1.9a × H10.17 (nox-1Δ) |
| cat-3RIP | cat-3RIPmat a | 30 |
| cat-3RIP-A | cat-3RIPmat A | This work; progeny from cat-3RIP × 74-OR23-1A |
| C3H10.A10 | cat-3RIPnox-1Δ::hph+mat a | This work; progeny from cat-3RIP-A × H10.1 |
Synchronous development of ascogonia and protoperithecia was induced according to the method of Bistis (5). Briefly, water-agar plates were inoculated in the center with 1 × 103 conidia and incubated for 4 to 5 days at 25°C in the light. Next, four circles of solid crossing medium (4 mm in diameter) were transferred to the water-agar plate and placed equidistantly and near the limits of the colony. Ascogonia and protoperithecia were observed 24 and 48 h later, respectively.
Plasmid constructions.
nox-1 and nox-2 deletion plasmids were constructed based on the strategy published by Pratt and Aramayo (35). A nox-1 deletion construct was generated as follows: first, a 5′ nox-1 region was amplified by PCR, using N. crassa genomic DNA as template and primers 5-NX-1-Up (5′-CCT TTT GCT GAG TTG TCG) and 5-NX1-Lo (5′-TGA ATC TAA TCT TGG). The resulting 2,075-bp fragment was cloned into TOPO2.1 vector (Invitrogen, Carlsbad, CA) to generate plasmid pTOKNC1-7. Second, a portion of the nox-1 3′ region was amplified with primers 3-NotNx1 (5′-GTT TAG CGG CCG CAA TTT TAG GTT CTG GAA GG) and 3-NxNot (5′-GTT TAG CGG CCG CTC GAA GAT GAT AAC CTG G), which contained a NotI restriction site. The 1,814-bp PCR product cloned into TOPO2.1 vector was named pTOKNC-1. The nox-1 5′ region derived from pTOKNC1-7 was cloned into pKAD-10Nc, and the resulting plasmid was named pNCKA-6. Next, the nox-1 3′ region derived from pTOKNC-1 was subcloned into pNCKA-6 to generate pNCKA-8, which was used to transform N. crassa and delete nox-1.
For nox-2 deletion, the nox-2 3′region was amplified using primers NotP3394 (5′-TTT AGC GGC CGC AAC GCC GAT AAG GAT ACC) and NOX-2Nt (5′-TTT AGC GGC CGC TTC ATC CAT TCC ACC ACC). The PCR product (2,075 bp) was cloned into pDLAM89d to obtain plasmid pKAD28. The mat-a1 gene obtained from pRATT25d was cloned into pKAD28 to generate pKAD-23. A nox-2 3.5-kb fragment containing the 5′ region and open reading frame (ORF) was amplified with primers KpNOX-2 (5′-CGG GGT ACC TGA ACT GAG GCG ATA ACG) and nox2-2 (5′-TTA CCC AGG CTC ACT ATA C), using genomic DNA as template. This fragment was cloned into TOPO 2.1 vector to obtain pTOKAD-3.5. A 1.5-kb EcoRI fragment from pTOKAD-3.5 containing the nox-2 5′ region was subcloned into pKAD23 to generate pKAD9, which was used to delete nox-2.
To delete nor-1, the 5′ and 3′ regions were amplified by PCR using genomic DNA as a template. The nor-1 5′ region was amplified with primers 5′GAPNOXR-UP (5′-GTA ACG CCA GGG TTT TCC CAG TCA CGA CGG CCT ATG TGA ACT CAC AAC C) and 3′GAPNOXR-LOW (5′-ATC CAC TTA ACG TTA CTG AAA TCT CCA ACG ACG ACT CGA GAT TAA CAG C). The 3′ primers were 5′SIZENOXR-UP (5′-CTC CTT CAA TAT CAT CTT CTG TCT CCG ACC GAC AGA CCT GTA CTT TTG G) and 3′SIZENOXR-LOW (5′-GCG GAT AAC AAT TTC ACA CAG GAA ACA GCG CTG TCA TAG CAT AGC ATC C). The hygromycin resistance gene was amplified by PCR using plasmid pCSN43 as a template and primers hph F and hph R (10). The three PCR products were mixed and used for fusion PCR with primers 5′GAPNOXR-UP and 3′SIZENOXR-LOW.
A pef-1 replacement construct was generated by double-joint PCR, using genomic DNA as template. First, a 5′ pef-1 fragment was amplified with primers 5′GAPALG-B (5′-GTA ACG CCA GGG TTT TCC CAG TCA CGA CGG GAT ACT GTC CAT ACC TAC G) and 3′GAPALG-B (5′-ATC CAC TTA ACG TTA CTG AAA TCT CCA ACG GAA AGG AGT AAA GGA GTC G). Second, a 3′ pef-1 fragment was amplified with primers 5′SIZE ALG-B (5′-CTC CTT CAA TAT CAT CTT CTG TCT CCG ACT CCA CAG TAT GCT GTC TAC G) and 3′SIZEALG-B (5′-GCG GAT AAC AAT TTC ACA CAG GAA ACA GCG AGT AGC AAT GCA TGG AAG C). Third, the hygromycin resistance gene was amplified by PCR using plasmid pCSN43 as a template. The three PCR fragments were purified, mixed, and used in a fusion PCR with primers 5′GAPALG-B and 3′SIZEALG-B.
Preparation of conidial spheroplasts and transformation of N. crassa.
Conidia were harvested from culture slants grown for 3 days at 30°C in the dark and 2 days at room temperature in the light. For spheroplast preparation, conidia were harvested and treated as reported elsewhere (35). For transformation, we used 150 μl of spheroplasts and 7.42 μg of linear plasmid pNCKA-8 or 2 to 10 μg of linear plasmid pKAD9. The transformation mixture was mixed with warm selective top agar (Vogel's salts with 1 M sorbitol, 0.05% glucose, 0.05% fructose, 1.0% agar, and supplements) and plated onto a petri dish containing the same medium plus 250 μg/ml of hygromycin B (Sigma-Aldrich, St. Louis, MO) and 2% agar. Original transformants were purified three times on selective medium with 150 μg/ml of hygromycin B.
The Δmus-51 strain (32) was also used for transformation. Fifty microliters of a suspension of conidia (1.25 × 108) prepared in 1 M sorbitol was mixed with 40 μl of a solution containing 10 μg of the DNA fusion PCR product and incubated on ice for 5 min. Forty microliters of this mixture was transferred to 0.2-cm electroporation cuvettes and electroporated using 1.5 kV, 600 Ω, and 2.5 μF in Gene Pulser II and Pulse Controller II (Bio-Rad, Hercules, CA) instruments. After electroporation, 960 μl of 1 M cold sorbitol was added, mixed with 25 ml of recovery solution (Vogel's medium with 2% yeast extract), and incubated at 30°C for 2 h. This solution was mixed with 25 ml of regeneration agar (Vogel's medium with 1 M sorbitol, 2% yeast extract, and 1% agar), and plated immediately on solid Vogel medium with l-sorbose, fructose, and glucose plus 200 μg/ml of hygromycin B. Plates were incubated at 30°C for 5 days, and the resulting transformants were purified four times in selective medium with 200 μg/ml hygromycin B.
Southern blot analysis.
For DNA genomic extraction, 1 × 107 conidia were inoculated in liquid-supplemented Vogel's medium and grown for 48 h at 30°C without shaking. Mycelium was frozen in liquid nitrogen, lyophilized, and ground with a mortar and pestle under liquid nitrogen. Genomic DNA was extracted according to the method of Timberlake (42). Seven to 10 μg of DNA was digested with different restriction enzymes, fractionated in an agarose gel, transferred to Hybond N membranes (Amersham Biosciences, Piscataway, NJ), and hybridized with different radioactive probes.
RNA extraction and Northern blot analysis.
Mycelial samples, frozen in liquid nitrogen and stored at −70°C until used, were ground with a mortar and pestle under liquid nitrogen. Total RNA was isolated with Trizol (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. Ten to 12 μg of RNA was separated in a 1% agarose gel containing formaldehyde, transferred to Hybond N membranes, and hybridized with nox-1 ORF- and nox-2 ORF-specific probes.
RESULTS
N. crassa nox-1 and nox-2 genes, encoding members of the fungal NoxA and NoxB subfamilies, are differentially expressed.
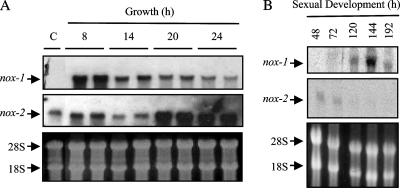
The phylogenetic analysis of the NOX family indicates the presence of three subfamilies (NoxA to NoxC) within the fungi (1, 24). The analysis of the N. crassa genome sequence (16) indicated that this fungus contained two different nox genes, which we designated nox-1 and nox-2. These genes predicted hypothetical proteins NCU02110.3 (553 amino acids) and NCU10775.3 (581 amino acids), respectively. The alignment of NOX-1, NOX-2, and other NOX shows that these proteins contain all NOX family signature regions (see Fig. S1 in the supplemental material). Sharing an identity of only 36%, NOX-1 and NOX-2 belong to the NoxA and NoxB subfamilies, respectively. Like other NoxB members, NOX-2 contains an N-terminal extension of unknown function (see Fig. S1 in the supplemental material). To characterize NOX functions in N. crassa, we first analyzed the pattern of nox mRNA accumulation at different developmental stages. As shown in Fig. 1A, nox-1 mRNA was not detected in conidia, accumulated between 8 and 14 h of growth in liquid culture, and decreased between 20 and 24 h. In contrast, nox-2 mRNA was present in conidia, maintained similar levels between 8 and 14 h, and showed high accumulation levels between 20 and 24 h. During sexual development (Fig. 1B), nox-1 started to accumulate around 120 h, peaked at 144 h, and decreased at 192 h. Under these conditions, the highest nox-1 mRNA levels coincided with the formation of the female sexual structures or protoperithecia (shown in Fig. 2B; see also Fig. 6C), while nox-2 mRNA was detected only before protoperithecium development. These results suggest that NOX-1 functions might be important during growth and sexual differentiation, while NOX-2 functions could be associated with conidiospore and/or stationary-phase physiology.
FIG. 1.
The NADPH oxidase genes nox-1 and nox-2 are differentially expressed during growth and sexual development. RNA was extracted from conidia (C) or mycelia from wild-type strain 74-ORS23-1A grown in liquid culture (A) or mycelia induced to undergo sexual development for the indicated time (hours) (B) and was hybridized with nox-1- or nox-2-specific probes. Bottom panels show rRNA as loading reference. To induce sexual development, conidia were grown on plates with solid crossing medium covered with a cellophane membrane, and samples were harvested at the indicated times.
FIG. 2.
NOX-1 is essential for protoperithecia but not for ascogonium development. (A) Conidia from wild-type (WT) strains 74-OR23-1A or 74-OR8-1a and Δnox-1 strains H10.1 (mat a) or H10.17 (mat A) were inoculated on crossing medium and grown for 6 days to be used as female partners, which were fertilized with wild-type or Δnox-1 strains from the opposite mating type. Pictures were taken 13 days after fertilization. The round black structures correspond to mature perithecia. (B) The same strains were grown under identical conditions for 10 days, and protoperithecium (arrowheads) development was observed under a stereoscopic microscope. (C) Development of ascogonia (arrows) and protoperithecia (arrowheads) was induced according to the method of Bistis (5). Briefly, conidia from strains 74-OR23-1A and H10.1 were inoculated onto water-agar medium, and after 4 days, four pieces of solid crossing medium were placed close to growing mycelia and incubation was continued for 24 h. NBT staining was carried out by flooding the plates with a water solution of 2.5 mM NBT for 30 min. Pictures were taken under a stereoscopic microscope.
FIG. 6.
Inactivation of peflin PEF-1 does not suppress the developmental and growth defects of Δnox-1 mutants. (A and B) Strains 74-OR8-1a (wild type [WT]), H10.1 (Δnox-1), Δpef-1.9a (Δpef-1), and A9N1.10 (Δpef-1 Δnox-1) were inoculated (A) and conidia were counted (B) as indicated for Fig. 5. (C) Protoperithecium development was induced as indicated for Fig. 2, and samples were processed for scanning electron microscopy. (D) Race tube cultures were treated as described for Fig. 5.
NOX-1 is essential for sexual development.
To determine nox-1 function, we deleted most of its ORF by gene replacement, using the hygromycin resistance gene as a genetic marker (see Fig. S2 in the supplemental material). Although the resulting mutants showed several phenotypes (see below), we first analyzed them for sexual development defects. Results in Fig. 2 show that Δnox-1 mutants, whether mating type A or a, were unable to cross with a wild-type strain and differentiate mature fruiting bodies or perithecia. However, this phenotype was observed only when Δnox-1 mutants were used as the recipient or female sexual partner. Indeed, Δnox-1 conidia were able to function as the donor or “male” partner and fertilize a wild-type strain, giving rise to perithecia (Fig. 2A, bottom) and viable ascospores (not shown). These results led us to ask whether Δnox-1 mutants were able to differentiate the female sexual structures or protoperithecia. Consistent with their female sterility, we found that Δnox-1 mutants were incapable of forming any protoperithecia (Fig. 2B). However, Δnox-1 mutants were able to develop ascogonia (Fig. 2C, left), which is the first recognizable stage in protoperithecium differentiation (46). As the NOX are involved in superoxide (O2·−) production, we used a nitroblue tetrazolium (NBT) reduction assay (3) to detect superoxide production in intact ascogonia and protoperithecia. While ascogonia were not stained by NBT (not shown), a dark formazan precipitate, indicative of NBT reduction, was readily formed around developing protoperithecia (Fig. 2C, right). These results indicate that, while not required for initial formation of female sexual structures (ascogonia), NOX-1-generated ROS are essential for subsequent development and formation of mature and viable protoperithecia.
NOX-2 is required for sexual spore viability.
We used a similar gene replacement strategy to delete most of the nox-2 ORF (see Fig. S3 in the supplemental material). Δnox-2 mutants did not show any obvious defect in cell growth or asexual or sexual development. However, when ascospores from heterozygous crosses were plated, Δnox-2 colonies (hygromycin resistant) were recovered at a very low frequency (less than 5%). Nevertheless, we isolated Δnox-2 strains from opposite mating types and performed different Δnox-2 homozygous crosses. All the ascospores isolated from these crosses failed to germinate whether activated or not by heat shock, despite the fact that under the microscope, Δnox-2 ascospores showed a wild-type appearance (Fig. 3). Incubation of Δnox-2 ascospores with 1, 5, 10, or 100 mM H2O2 before, during, or after heat shock activation did not have any positive effects on ascospore germination (not shown). Likewise, the presence of 1 mM furfural, which promotes heat shock-independent germination of N. crassa ascospores (13), did not restore Δnox-2 ascospore germination (not shown). Whether this phenotype is due to defective ascospore development or failure to germinate is not known. Nevertheless, our results show that NOX-2 is essential for sexual spore function in N. crassa.
FIG. 3.
The ascospores from Δnox-2 homozygous crosses are nonviable. Strains CNCKA-Z (Δnox-2 A) and CNCKA-AN1 (Δnox-2 a) were crossed and allowed to produce ascospores. Shot ascospores were collected as water suspensions, heat activated, plated on sorbose-containing medium, and incubated for 3 days (top panels) or incubated in liquid minimal medium and observed under the microscope (bottom panels; magnification, ×1,077). Only ascospores generated by the wild-type strains (WT) were able to germinate and generate colonies.
NOR-1 is required for NOX-1 and NOX-2 function.
Among microbial eukaryotes, one p67phox ortholog was identified in D. discoideum (25). Takemoto et al. (40) identified p67phox orthologs in several fungi and showed that E. festucae p67phox ortholog NoxR regulates NoxA but only during association of the fungus with its plant host. We asked whether the only NoxR ortholog present in N. crassa (NCU07850.3), which we have designated NOR-1 (NADPH oxidase regulator), was required for NOX-1 and/or NOX-2 functions. NCU07850.3 is incorrectly annotated as a protein of 471, instead of 571, amino acids (see Fig. S4 in the supplemental material). We deleted the nor-1 gene by transforming a Δmus-51 mutant strain (32) with a PCR construct generated by double-joint PCR (48). In mus mutants most DNA integration events occur by homologous recombination, resulting in high gene targeting frequencies. Several Δnor-1 mutants were identified after Southern blot analysis (see Fig. S5 in the supplemental material), all of which showed defects in asexual sporulation and radial growth, resembling those observed in Δnox-1 mutants (see below). When Δnor-1 mutants were analyzed for sexual development, we found a phenotype that was indistinguishable from that observed in Δnox-1 mutants. Indeed, Δnor-1 mutants failed to differentiate protoperithecia but were able to develop ascogonia (Fig. 4A). These results support the role of NOR-1 as a regulator of NOX-1 function during sexual development.
FIG. 4.
Mutants lacking putative NOX regulatory subunit NOR-1 share Δnox-1 and Δnox-2 sexual phenotypes. (A) Strains 74-OR8-1a (wild type [WT]) and Nc28nor-1 (Δnor-1) were induced to develop ascogonia and protoperithecia (arrowheads). (B) Ascospores from crosses 74-OR23-1A × 74-OR8-1a, 74-OR8-1a × Nc28nor-1 (Δnor-1), and CNCKA-Z (Δnox-2) × Nc28nor-1 (Δnor-1) were collected and plated as reported for Fig. 3. The few ascospores from the Δnox-2 × Δnor-1 cross that were able to germinate and generate colonies (arrowheads) were sensitive to hygromycin and therefore carried nox-2 and nor-1 wild-type alleles.
To explore NOR-1 roles in NOX-2 activity, we used Δnor-1 conidia to fertilize protoperithecia from Δnox-2 mutants. The ascospores from this cross that grew on nonselective medium were unable to grow on hygromycin-containing medium and therefore corresponded to strains carrying nox-2 and nor-1 wild-type alleles. The fact that no Δnor-1 or Δnox-2 ascospores were recovered from these crosses indicates that mutation of either nor-1 or nox-2 results in the same phenotype: the production of defective sexual spores (Fig. 4B). Our results support a model in which NOR-1 regulates NOX-1 activity, required for protoperithecium development, as well as the activity of NOX-2, required at a later stage of sexual development to produce functional ascospores.
NOX-1 and NOR-1 regulate asexual development and hyphal growth.
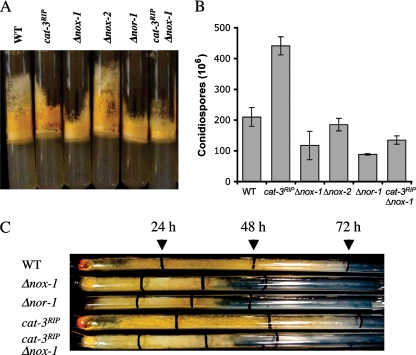
The lack of the ROS-producing enzyme NOX-1 not only affected sexual development but also resulted in a reduction in the amount of aerial mycelium compared to the wild type (Fig. 5A). In contrast, it has been reported that inactivation of the ROS-detoxifying enzyme CAT-3 leads to increased amounts of aerial mycelium and conidia (30). As these results support the notion that ROS levels regulate asexual development, we decided to examine the extent of asexual development in Δnox-1, Δnox-2, Δnor-1, and Δnox-1 cat-3RIP mutants. We found that, as reported, cat-3RIP mutants produced denser aerial mycelium and greater conidiation than did the wild-type strain (Fig. 5A and B). In contrast, Δnox-1 mutants not only formed shorter aerial mycelium but also produced conidiation yields lower than those of the wild type. Asexual development was not affected in the Δnox-2 mutant. The fact that a Δnor-1 mutant shared Δnox-1 phenotypes (Fig. 5A and B) further supports the essential role of NOR-1 in NOX-1 activation. Asexual development in the Δnox-1 cat-3RIP double mutant was similar to that observed for the Δnox-1 single mutant, indicating that NOX-1 activity is necessary for increased asexual development in cat-3RIP mutants.
FIG. 5.
CAT-3, NOX-1, and NOR-1 regulate asexual development and/or hyphal growth. (A and B) The lack of CAT-3 or NOX-1/NOR-1 has opposite effects on the formation of aerial mycelium (A) and conidiation (B). (C) Δnox-1 and Δnor-1 mutants show a drastic reduction in hyphal extension rate, which is not compensated by mutation of cat-3. Strains 74-OR8-1a (wild type [WT]), cat-3RIP, H10.1 (Δnox-1), CNCKA-AN1 (Δnox-2 a), Nc28nor-1 (Δnor-1), and C3H10.1-10 (cat-3RIP Δnox-1) were inoculated and grown at 30°C for 3 days in the dark, plus 2 days in the light. Conidia were harvested as water suspensions and counted. Conidium numbers are mean values from four independent samples. Race tube assays were started with mycelial plugs and conducted under constant darkness. Growth was marked (vertical black lines) after 24, 48, and 72 h at 30°C.
To determine whether ROS metabolism could have general effects on mycelial growth, we determined the growth rate of Δnox-1, Δnor-1, cat-3RIP, and Δnox-1 cat-3RIP mutants in race tubes. As shown in Fig. 5C, Δnox-1, Δnor-1, and Δnox-1 cat-3RIP mutants showed clear and similar reductions in mycelial extension rates. In contrast, the growth rate of cat-3RIP mutants was similar to that of the wild type. These results indicate that NOX-1 activity is necessary for proper hyphal growth and that lack of CAT-3 does not compensate for the lack of NOX-1.
Deletion of pef-1 does not suppress the phenotypes caused by a lack of NOX-1 or NOX-2.
NoxA and NoxB, the NOX-1 and NOX-2 orthologs in D. discoideum, respectively, are required for cell aggregation and asexual sporulation. Remarkably, the elimination of ALG-2B restored normal development in both noxA and noxB null mutants (25). ALG-2B is one of two calcium-binding penta-EF hand proteins or peflins (28) present in this organism. Recently, it has been shown that Pef1p, the ALG-2 ortholog in Saccharomyces cerevisiae, is involved in cell budding and polarization (47), but peflin function in filamentous fungi was unknown. In N. crassa, we identified hypothetical protein NCU02738 as the only ALG-2 ortholog in this fungus and designated it as PEF-1 (penta EF domain protein 1 or peflin 1). PEF-1 and its fungal orthologs are conserved at the C terminus, which includes the five putative calcium-binding EF domains, but show low conservation at the N terminus (see Fig. S6 in the supplemental material). We deleted the pef-1 gene to evaluate its function in N. crassa and possible interactions with nox-1. A pef-1 deletion construct, based on hygromycin resistance, was generated by PCR and used to transform the Δmus-51 strain (32). Purified transformants were analyzed by Southern blotting using restriction enzyme PvuI (see Fig. S7 in the supplemental material). Strain pef-1.9a was selected out of four transformants with the correct pef-1 deletion event and used in further experiments and sexual crosses. Δpef-1 mutants were able to develop fertile protoperithecia (Fig. 6C) and viable ascospores in homozygous crosses (not shown) and showed wild-type growth rates in race tubes (Fig. 6D). In contrast, a Δpef-1 mutant produced 65% more conidia than did the wild-type strain, despite the fact that the two strains formed similar amounts of aerial mycelia (Fig. 6A and B).
It has been shown that S. cerevisiae Pef1p binds calcium and zinc in vitro and that pef1 disruption causes defective growth in sodium dodecyl sulfate or cation-depleted medium (47). However, we plated Δpef-1 conidia on medium containing 0.005% sodium dodecyl sulfate or 0, 10, 20, and 30 mM EGTA and found no significant differences in colony size after 48 h from the wild-type strain (not shown). In summary, elimination of the only peflin present in N. crassa did not produce any clear phenotype, except for an increase in conidiation.
Next, we generated Δpef-1 Δnox-1 double mutants, which were confirmed by Southern blot analysis (not shown), to examine whether PEF-1 elimination could restore normal development in nox-1-null mutants. As shown in Fig. 6C, the Δpef-1 Δnox-1 mutant was not able to differentiate normal protoperithecia. Indeed, when this mutant was fertilized with wild-type spores, no mature perithecia or ascospores were formed (not shown). The mutation of pef-1 also failed to restore normal conidiation (Fig. 6B) or hyphal growth rate (Fig. 6C) in a Δnox-1 background. These results indicate that, in contrast to what occurs in D. discoideum, mutation of the N. crassa ALG-2 ortholog pef-1 did not restore any of the nox-1-null mutant phenotypes.
DISCUSSION
NOX-1 is required for sexual and asexual development, and normal hyphal growth, while NOX-2 seems specifically involved in sexual spore function.
Previous work indicates that NOX-1 orthologs play rather specific roles in fungal development. NoxA inactivation in A. nidulans results in complete arrest of sexual development, without notably affecting growth (24). Likewise, lack of PaNox-1 in P. anserina causes a major reduction in the number of protoperithecia and fruiting bodies but does not affect growth rate (29). noxA mutants of the symbiotic fungus E. festucae grow normally in culture but show unregulated and increased growth in its plant host (41). nox1 mutants of the plant pathogen M. grisea show a slight increase in radial growth and fail to penetrate its plant host (12), while Claviceps purpurea Cpnox1 mutants grow normally, despite showing decreased germination and defective colonization of plant ovarian tissue (17).
As we report here, N. crassa NOX-1 is not only essential for protoperithecium development but also required for normal development of aerial hyphae and conidiation, as well as for vegetative growth. This suggests that all these processes require the production of ROS derived from NOX-1 activity. We detected superoxide production during differentiation of female organs or protoperithecia (Fig. 2C, right), while Hansberg et al. detected ROS at the three morphogenetic events that are characteristic of asexual sporulation in N. crassa (21). Interestingly, the first peak of ROS detected during aggregation of hyphae, 30 min after the mycelium was exposed to air (21), coincided with increased nox-1 mRNA levels (not shown). ROS regulation of asexual development is further indicated because the elimination of ROS-decomposing (CAT-3) and ROS-generating (NOX-1) activities increases (30) and decreases (this work) aerial mycelium and conidiospore development, respectively. The fact that asexual development in the Δnox-1 cat-3RIP double mutant was similar to that observed for the Δnox-1 single mutant suggests that NOX-1-derived ROS are required for the increase in asexual development observed in cat-3 mutants. Although ROS are difficult to specifically detect at hyphal tips and have not yet been found to be involved in regulation of growth in N. crassa, our results indicate that NOX-1-derived ROS play a role in hyphal growth, perhaps regulating the rate of apical extension. ROS have also been detected during asexual spore germination (27). Sexual spores from mutants lacking NOX-2 were not viable or able to germinate, suggesting that ROS might be required for spore germination. However, mutants lacking NOX-1 or NOX-2 did not show detectable defects in the germination rate of conidia (not shown).
NOX redundancy and regulation.
NOX play partially redundant functions in some organisms. In D. discoideum, noxA, noxB, and noxC are sequentially expressed and elimination of any of these genes brings about the same phenotype (25). M. grisea Nox1 and Nox2 are independently required for pathogenicity, although inactivation of both NOX affects asexual development (12). In P. anserina, PaNox1 and PaNox2 play different roles during sexual development, but PaNox2 seems to partially replace PaNOX1 functions (29). Botrytis cinerea BcNoxA and BcNoxB are both required for sclerotium formation and pathogenicity, and double mutants show additive effects on these processes (36). In contrast, N. crassa NOX-1 and NOX-2 do not seem to play redundant functions. Although nox-2 is expressed during growth, its inactivation did not produce any of the phenotypes observed when nox-1 was eliminated. Furthermore, mutation of the p67phox ortholog NOR-1, required for NOX-1 and NOX-2 activity, did not enhance the phenotypes caused by the lack of NOX-1.
The fact that NOR-1 is required for NOX-1 and NOX-2 activity, despite the fact that these two NOX are required at different developmental stages, indicates that NOR-1 availability does not appear to be a limiting factor for NOX activity. In E. festucae the NOR-1 ortholog NoxR seems required for NoxA activity only during symbiosis, and it is not known if it is needed for NoxB activity (40). While this paper was in preparation, Segmüller et al. (36) reported that the NoxR ortholog BcNoxR is required for BcNoxA and BcNoxB functions in B. cinerea. However, these NOX do play partially redundant functions. Our results raise questions on what triggers NOX activation and suggest that the activity of all fungal A/B-type NOX is dependent on p67phox orthologs. As with many of the mammalian NOX, the Rac subunit seems essential for NOX activation in fungi (40). As occurs in plants, GDP dissociation inhibitors might in turn regulate Rac activity (8). Therefore, it is possible that events leading to Rac activation could be triggering NOX activation. In this context, it is interesting that GTPases Ras and Rac have been linked to ROS production in Colletotrichum trifolii (9).
As mitogen-activated protein kinase signaling has been involved in NOX regulation (24), it is interesting that N. crassa mutants lacking NOR-1 or the mitogen-activated protein kinase MAK-2 share several phenotypes, i.e., they show reduced growth rates, produce short aerial hyphae, fail to develop protoperithecia, and produce unviable ascospores (26). As mak-2 mutants are also defective for hyphal fusion (33), it will be interesting to assess this defect in the nox-1, nox-2, and nor-1 mutants, as well as to determine if MAK-2 is a positive upstream regulator of NOX function in this fungus.
Elimination of peflin PEF-1 results in increased conidiation but does not restore development in Δnox-1 mutants.
In mammalian cells peflins such as ALG-2 have been shown to bind Ca2+ and regulate processes such as apoptosis and vesicle trafficking (28). Peflin Pef1p functions in cation-dependent budding and cell polarization in S. cerevisiae. We found that the lack of peflin PEF-1 did not have any evident impact on N. crassa biology, except that Δpef-1 mutants showed higher conidiation, an effect that cannot be explained at this time.
As the mutation of PEF-1 ortholog ALG-2 bypassed the NoxA and NoxB requirement for asexual sporulation in D. discoideum (25), this supported the idea of cross talk between ROS and Ca2+ mobilization, as has been observed for plants (15). We found that the inactivation of PEF-1 did not restore the developmental defects of N. crassa nox-1-null mutants. However, this does not rule out a connection between ROS and Ca2+ signaling, and although we do not know how NOX-1 might regulate polar growth in N. crassa, it is interesting that polar growth in Arabidopsis thaliana root hair cells appears to be controlled by a positive feedback regulation between NOX-derived ROS and Ca2+ (39). Further research is needed to establish a connection between ROS and Ca2+ signaling in fungi.
ADDENDUM IN PROOF
In additional experiments, the elimination of pef-1 also failed to restore ascospore germination in Δnox-2 mutants.
Supplementary Material
Acknowledgments
This work was supported by grants 49667Q and 50716Q from CONACYT and grant IN228507-2 from PAPIIT-UNAM (México). N. Cano-Domínguez and K. Álvarez-Delfín were supported by scholarships from CONACYT and DGEP-UNAM.
We are grateful to Olivia Sánchez for experimental and technical support and to IFC-UNAM Molecular Biology and Microscopy units for DNA synthesis and electron scanning microscopy, respectively. We thank Rodolfo Aramayo (Texas A&M University) for helpful discussion, plasmids, and strains.
Footnotes
Published ahead of print on 20 June 2008.
Supplemental material for this article may be found at http://ec.asm.org/.
REFERENCES
- 1.Aguirre, J., M. Rios-Momberg, D. Hewitt, and W. Hansberg. 2005. Reactive oxygen species and development in microbial eukaryotes. Trends Microbiol. 13111-118. [DOI] [PubMed] [Google Scholar]
- 2.Aguirre, J., R. Rodriguez, and W. Hansberg. 1989. Oxidation of Neurospora crassa NADP-specific glutamate dehydrogenase by activated oxygen species. J. Bacteriol. 1716243-6250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baehner, R. L., and D. G. Nathan. 1967. Leukocyte oxidase: defective activity in chronic granulomatous disease. Science 155835-836. [DOI] [PubMed] [Google Scholar]
- 4.Bedard, K., and K. H. Krause. 2007. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol. Rev. 87245-313. [DOI] [PubMed] [Google Scholar]
- 5.Bistis, G. N. 1983. Synchronous induction and development of ascogonia. Neurospora Newsl. 3014. [Google Scholar]
- 6.Borkovich, K. A., L. A. Alex, O. Yarden, M. Freitag, G. E. Turner, N. D. Read, S. Seiler, D. Bell-Pedersen, J. Paietta, N. Plesofsky, M. Plamann, M. Goodrich-Tanrikulu, U. Schulte, G. Mannhaupt, F. E. Nargang, A. Radford, C. Selitrennikoff, J. E. Galagan, J. C. Dunlap, J. J. Loros, D. Catcheside, H. Inoue, R. Aramayo, M. Polymenis, E. U. Selker, M. S. Sachs, G. A. Marzluf, I. Paulsen, R. Davis, D. J. Ebbole, A. Zelter, E. R. Kalkman, R. O'Rourke, F. Bowring, J. Yeadon, C. Ishii, K. Suzuki, W. Sakai, and R. Pratt. 2004. Lessons from the genome sequence of Neurospora crassa: tracing the path from genomic blueprint to multicellular organism. Microbiol. Mol. Biol. Rev. 681-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carol, R. J., and L. Dolan. 2006. The role of reactive oxygen species in cell growth: lessons from root hairs. J. Exp. Bot. 571829-1834. [DOI] [PubMed] [Google Scholar]
- 8.Carol, R. J., S. Takeda, P. Linstead, M. C. Durrant, H. Kakesova, P. Derbyshire, S. Drea, V. Zarsky, and L. Dolan. 2005. A RhoGDP dissociation inhibitor spatially regulates growth in root hair cells. Nature 4381013-1016. [DOI] [PubMed] [Google Scholar]
- 9.Chen, C., and M. B. Dickman. 2004. Dominant active Rac and dominant negative Rac revert the dominant active Ras phenotype in Colletotrichum trifolii by distinct signalling pathways. Mol. Microbiol. 511493-1507. [DOI] [PubMed] [Google Scholar]
- 10.Colot, H. V., G. Park, G. E. Turner, C. Ringelberg, C. M. Crew, L. Litvinkova, R. L. Weiss, K. A. Borkovich, and J. C. Dunlap. 2006. A high-throughput gene knockout procedure for Neurospora reveals functions for multiple transcription factors. Proc. Natl. Acad. Sci. USA 10310352-10357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davies, R. H., and D. deSerres. 1970. Genetic and microbial research techniques for Neurospora crassa. Methods Enzymol. 27A79-143. [Google Scholar]
- 12.Egan, M. J., Z. Y. Wang, M. A. Jones, N. Smirnoff, and N. J. Talbot. 2007. Generation of reactive oxygen species by fungal NADPH oxidases is required for rice blast disease. Proc. Natl. Acad. Sci. USA 10411772-11777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Emerson, M. R. 1954. Some physiological characteristics of ascospore activation in Neurospora crassa. Plant Physiol. 29418-428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Finkel, T. 2003. Oxidant signals and oxidative stress. Curr. Opin. Cell Biol. 15247-254. [DOI] [PubMed] [Google Scholar]
- 15.Foreman, J., V. Demidchik, J. H. Bothwell, P. Mylona, H. Miedema, M. A. Torres, P. Linstead, S. Costa, C. Brownlee, J. D. Jones, J. M. Davies, and L. Dolan. 2003. Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature 422442-446. [DOI] [PubMed] [Google Scholar]
- 16.Galagan, J. E., et al. 2003. The genome sequence of the filamentous fungus Neurospora crassa. Nature 422859-868. [DOI] [PubMed] [Google Scholar]
- 17.Giesbert, S., T. Schürg, S. Scheele, and P. Tudzynski. 2008. The NADPH oxidase Cpnox1 is required for full pathogenicity of the ergot fungus Claviceps purpurea. Mol. Plant Pathol. 9317-327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Halliwell, B., and J. M. C. Gutteridge. 1989. Free radicals in biology and medicine, 2nd ed. Clarendon Press, Oxford University Press, Oxford, United Kingdom.
- 19.Hansberg, W., and J. Aguirre. 1990. Hyperoxidant states cause microbial cell differentiation by cell isolation from dioxygen. J. Theor. Biol. 142201-221. [DOI] [PubMed] [Google Scholar]
- 20.Hansberg, W., J. Aguirre, M. Rios-Momberg, P. Rangel, L. Peraza, Y. Montes de Oca, and N. Cano. 2008. Cell differentiation as a response to oxidative stress, p. 235-257. In S. V. Avery, M. Stratford, and P. Van West (ed.), Stress in yeasts and filamentous fungi, vol. 27. Academic Press, London, United Kingdom. [Google Scholar]
- 21.Hansberg, W., H. de Groot, and H. Sies. 1993. Reactive oxygen species associated with cell differentiation in Neurospora crassa. Free Radic. Biol. Med. 14287-293. [DOI] [PubMed] [Google Scholar]
- 22.Herve, C., T. Tonon, J. Collen, E. Corre, and C. Boyen. 2006. NADPH oxidases in eukaryotes: red algae provide new hints! Curr. Genet. 49190-204. [DOI] [PubMed] [Google Scholar]
- 23.Lambeth, J. D. 2004. NOX enzymes and the biology of reactive oxygen. Nat. Rev. Immunol. 4181-189. [DOI] [PubMed] [Google Scholar]
- 24.Lara-Ortiz, T., H. Riveros-Rosas, and J. Aguirre. 2003. Reactive oxygen species generated by microbial NADPH oxidase NoxA regulate sexual development in Aspergillus nidulans. Mol. Microbiol. 501241-1255. [DOI] [PubMed] [Google Scholar]
- 25.Lardy, B., M. Bof, L. Aubry, M. H. Paclet, F. Morel, M. Satre, and G. Klein. 2005. NADPH oxidase homologs are required for normal cell differentiation and morphogenesis in Dictyostelium discoideum. Biochim. Biophys. Acta 1744199-212. [DOI] [PubMed] [Google Scholar]
- 26.Li, D., P. Bobrowicz, H. H. Wilkinson, and D. J. Ebbole. 2005. A mitogen-activated protein kinase pathway essential for mating and contributing to vegetative growth in Neurospora crassa. Genetics 1701091-1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lledias, F., P. Rangel, and W. Hansberg. 1999. Singlet oxygen is part of a hyperoxidant state generated during spore germination. Free Radic. Biol. Med. 261396-1404. [DOI] [PubMed] [Google Scholar]
- 28.Maki, M., Y. Kitaura, H. Satoh, S. Ohkouchi, and H. Shibata. 2002. Structures, functions and molecular evolution of the penta-EF-hand Ca2+-binding proteins. Biochim. Biophys. Acta 160051-60. [DOI] [PubMed] [Google Scholar]
- 29.Malagnac, F., H. Lalucque, G. Lepere, and P. Silar. 2004. Two NADPH oxidase isoforms are required for sexual reproduction and ascospore germination in the filamentous fungus Podospora anserina. Fungal Genet. Biol. 41982-997. [DOI] [PubMed] [Google Scholar]
- 30.Michan, S., F. Lledias, and W. Hansberg. 2003. Asexual development is increased in Neurospora crassa cat-3-null mutant strains. Eukaryot. Cell 2798-808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nauseef, W. M. 2004. Assembly of the phagocyte NADPH oxidase. Histochem. Cell Biol. 122277-291. [DOI] [PubMed] [Google Scholar]
- 32.Ninomiya, Y., K. Suzuki, C. Ishii, and H. Inoue. 2004. Highly efficient gene replacements in Neurospora strains deficient for nonhomologous end-joining. Proc. Natl. Acad. Sci. USA 10112248-12253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pandey, A., M. G. Roca, N. D. Read, and N. L. Glass. 2004. Role of a mitogen-activated protein kinase pathway during conidial germination and hyphal fusion in Neurospora crassa. Eukaryot. Cell 3348-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pöggeler, S., M. Nowrousian, and U. Kück. 2006. Fruiting-body development in Ascomycetes, p. 325-355. In U. Kües and R. Fischer (ed.), Growth, differentiation and sexuality, 2nd ed., vol. I. Springer-Verlag, Berlin, Germany. [Google Scholar]
- 35.Pratt, R. J., and R. Aramayo. 2002. Improving the efficiency of gene replacements in Neurospora crassa: a first step towards a large-scale functional genomics project. Fungal Genet. Biol. 3756-71. [DOI] [PubMed] [Google Scholar]
- 36.Segmüller, N., L. Kokkelink, S. Giesbert, D. Odinius, J. van Kan, and P. Tudzynski. 2008. NADPH oxidases are involved in differentiation and pathogenesis in Botrytis cinerea. Mol. Plant-Microbe Interact. 21808-819. [DOI] [PubMed] [Google Scholar]
- 37.Springer, M. L., and C. Yanofsky. 1989. A morphological and genetic analysis of conidiophore development in Neurospora crassa. Genes Dev. 3559-571. [DOI] [PubMed] [Google Scholar]
- 38.Sussman, A. S. 1969. The dormancy and germination of fungus spores. Symp. Soc. Exp. Biol. 2399-121. [PubMed] [Google Scholar]
- 39.Takeda, S., C. Gapper, H. Kaya, E. Bell, K. Kuchitsu, and L. Dolan. 2008. Local positive feedback regulation determines cell shape in root hair cells. Science 3191241-1244. [DOI] [PubMed] [Google Scholar]
- 40.Takemoto, D., A. Tanaka, and B. Scott. 2006. A p67Phox-like regulator is recruited to control hyphal branching in a fungal-grass mutualistic symbiosis. Plant Cell 182807-2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tanaka, A., M. J. Christensen, D. Takemoto, P. Park, and B. Scott. 2006. Reactive oxygen species play a role in regulating a fungus-perennial ryegrass mutualistic interaction. Plant Cell 181052-1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Timberlake, W. E. 1980. Developmental gene regulation in Aspergillus nidulans. Dev. Biol. 78497-510. [DOI] [PubMed] [Google Scholar]
- 43.Toledo, I., J. Aguirre, and W. Hansberg. 1986. Aerial growth in Neurospora crassa: characterization of an experimental model system. Exp. Mycol. 14184-189. [Google Scholar]
- 44.Toledo, I., J. Aguirre, and W. Hansberg. 1994. Enzyme inactivation related to a hyperoxidant state during conidiation of Neurospora crassa. Microbiology 1402391-2397. [DOI] [PubMed] [Google Scholar]
- 45.Toledo, I., A. A. Noronha-Dutra, and W. Hansberg. 1991. Loss of NAD(P)-reducing power and glutathione disulfide excretion at the start of induction of aerial growth in Neurospora crassa. J. Bacteriol. 1733243-3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Turian, G. 1978. Sexual morphogenesis in the Ascomycetes, p. 315-333. In J. E. Smith and D. R. Berry (ed.), The filamentous fungi, vol. 3. John Wiley & Sons, Inc., New York, NY. [Google Scholar]
- 47.Vernarecci, S., G. Colotti, P. Ornaghi, E. Schiebel, E. Chiancone, and P. Filetici. 2007. The yeast penta-EF protein Pef1p is involved in cation-dependent budding and cell polarization. Mol. Microbiol. 651122-1138. [DOI] [PubMed] [Google Scholar]
- 48.Yu, J. H., Z. Hamari, K. H. Han, J. A. Seo, Y. Reyes-Dominguez, and C. Scazzocchio. 2004. Double-joint PCR: a PCR-based molecular tool for gene manipulations in filamentous fungi. Fungal Genet. Biol. 41973-981. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.