Abstract
Toxoplasma gondii is an intracellular parasite with a significant impact on human health, especially in cases where individuals are immunocompromised (e.g., due to human immunodeficiency virus/AIDS). In Europe and North America, only a few clonal genotypes appear to be responsible for the vast majority of Toxoplasma infections, and these clonotypes have been intensely studied to identify strain-specific phenotypes that may play a role in the manifestation of more-severe disease. To identify and genetically map strain-specific differences in gene expression, we have carried out expression quantitative trait locus analysis on Toxoplasma gene expression phenotypes by using spotted cDNA microarrays. This led to the identification of 16 Toxoplasma genes that had significant and mappable strain-specific variation in hybridization intensity. While the analysis should identify both cis- and trans-mapping hybridization profiles, we identified only loci with strain-specific hybridization differences that are most likely due to differences in the locus itself (i.e., cis mapping). Interestingly, a larger number of these cis-mapping genes than would be expected by chance encode either confirmed or predicted secreted proteins, many of which are known to localize to the specialized secretory organelles characteristic of members of the phylum Apicomplexa. For six of the cis-mapping loci, we determined if the strain-specific hybridization differences were due to true transcriptional differences or rather to strain-specific differences in hybridization efficiency because of extreme polymorphism and/or deletion, and we found examples of both scenarios.
In Europe and North America, a majority of described infections with Toxoplasma spp. appear to be due to only three clonal lineages (23), and a large body of work has been carried out to identify differences among these strains, especially in terms of disease outcome in both mice (33) and humans (22). Experimental genetic crosses between representatives of these lineages have been performed (24), allowing forward genetics to be used to identify the genetic bases for phenotypes of interest (see, e.g., references 30, 31, and 37). These phenotypic differences could be due to primary amino acid sequence differences and/or quantitative differences in gene expression.
Little is known about the exact mechanisms of transcriptional regulation in Toxoplasma, although many Toxoplasma genes are known to have transcript levels that vary among different Toxoplasma life stages (e.g., tachyzoites, bradyzoites, and sporozoites) (4, 13, 15, 29, 35). Recently, Behnke et al. (4) identified sequence elements that play a role in the transcription of two genes that are upregulated during the tachyzoite-to-bradyzoite transition (bradyzoite antigen 1 [BAG1] and bradyzoite nucleoside triphosphatase) using site-directed mutagenesis. Interestingly, these 6- to 8-bp motifs were distinct, suggesting that there are multiple mechanisms of transcriptional regulation in Toxoplasma. The fact that typical core palindromic sequences in Toxoplasma promoter regions or proteins with significant homology to known transcription factors have not been identified to date in the completed Toxoplasma genome sequence (www.toxodb.org) implies that transcriptional regulation via transcription factors either is rare or is mediated by factors that are phylogenetically distinct from those described for other eukaryotes. Recent work has also indicated that some of the developmentally regulated transcriptional differences are associated with changes in the interaction of histone-modifying enzymes with the relevant promoters (4, 32), suggesting that histone modifications play a significant role in transcriptional regulation in Toxoplasma gondii. The DNA sequence motifs responsible for these differences, however, are unknown.
One approach to identifying regulators of transcription and the DNA sequence motifs with which they interact is to identify and genetically map strain-specific differences in transcript abundance using expression quantitative trait locus (eQTL) mapping. For example, if a putative transcription factor differs either qualitatively or quantitatively between strains and this difference is heritable in F1 progeny, eQTL mapping should identify distinct loci with transcript levels that map to the putative transcription factor locus. These trans-mapping loci would be of great interest as potential transcription factors. On the other hand, transcript abundance differences between strains could also be due to cis elements. For example, polymorphisms in the promoter region could result in differences in the transcription of the locus itself. These cis-mapping loci are also of great interest for the identification of regulatory motifs.
To identify both cis- and trans-mapping loci in Toxoplasma gondii, we carried out eQTL mapping using 19 F1 progeny from a cross between a type II strain and a type III strain (24, 34). The results of these analyses identified 16 Toxoplasma genes with significant differences in hybridization intensity between the parents that segregated significantly among the F1 progeny. For those genes with a known chromosomal location, the eQTL was always found to cosegregate with the gene itself, arguing against a trans-factor being responsible for the observed hybridization differences. Instead, these differences seem likely to be due to (i) transcriptional differences, (ii) differences in transcript stability, and/or (iii) polymorphisms or deletions that affect the amount of hybridization between the cDNA sample and the probes on the array. For six of the identified genes, we determined which of these possibilities was most likely and found apparent examples of at least the first and third mechanisms.
MATERIALS AND METHODS
Toxoplasma cDNA microarrays.
Parasite microarrays were custom-printed cDNA arrays from either an RH (type I strain) tachyzoite cDNA library (“tachyzoite” array [1]) or a cDNA library from in vivo bradyzoites of the Prugniaud strain (type II; “bradyzoite” array [13]). For the tachyzoite array, 11,609 cDNA clones were PCR amplified and spotted onto the array as described elsewhere (30). For 7,488 of the cDNAs on the array, end sequence data were obtained previously and are available in GenBank (1). The Prugniaud bradyzoite cDNA array has been described previously (13) and consists of 4,402 cDNAs that were spotted three times each, 2,449 of which were end sequenced (13).
Microarray hybridization and data analysis.
Human foreskin fibroblast (HFF) monolayers were prepared as described previously (31) and infected with tachyzoites at a multiplicity of infection of 10, and total RNA was harvested 24 h postinfection using the Trizol reagent (Invitrogen, Carlsbad, CA). Poly(A)-tailed RNA was purified from total RNA using the Qiagen (Valencia, CA) Oligotex mRNA isolation kit. The tachyzoite array was hybridized with labeled cDNA from ME49 (n = 2), CTg (n = 3), and 19 F1 progeny (S and CL clones) from crosses between type II and III parasites (24), and the bradyzoite array was hybridized with labeled cDNA from ME49 and CTg (n = 3 for each strain) and from 18 of the same 19 type II × type III F1 progeny (for F1 progeny clone S26 [24], no data were obtained for the bradyzoite array). Labeled cDNA derived from each RNA sample was hybridized to the two Toxoplasma arrays, and a single biological replicate was performed for each F1 progeny clone. Single replicates of each F1 progeny clone are sufficient in QTL mapping, because at each marker locus, multiple F1 progeny have the same genotype (the expectation being that half of the F1 progeny have the type II genotype and the other half have the type III genotype), and therefore each marker-genotype combination has multiple replicates.
Labeling and array hybridization were carried out as described previously (13). Briefly, cDNA samples were labeled with Cy5-dUTP (Amersham Biosciences) using random primed labeling and were cohybridized with a universal common reference consisting of Cy3-dUTP-labeled cRNA produced by T7 RNA polymerase-mediated transcription of the empty cloning vector. For each spot, the log2 ratios of the normalized data were calculated. For the two-strain comparison (ME49 versus CTg), transcripts with significantly different abundances were determined using a t test implemented within MeViewer software, and transcript abundance was deemed significantly different at a P value of <0.05. Using the most recent Toxoplasma genetic map (24), microarray hybridization data from the 19 F1 progeny were tested for significant association with each genetic marker using R/QTL (10). Genome-wide significance for each spot (P < 0.05) was assessed using 1,000 permutations of the genotype data (10). Expressed sequence tag (EST) data derived from each spot on the array were used to associate the array spot with its corresponding Toxoplasma draft 3 gene model (www.toxodb.org) if it had a significant BLASTX hit (Expect < 10−10) against a member of the Toxoplasma protein set. For the remaining sequences, their membership in a particular Apidots EST assembly (2, 6) was used if that EST assembly had a significant (Expect < 10−10) BLASTX hit against a particular draft 3 gene model.
While the eQTL mapping strategy described above produces genome-wide significance levels for the association of a particular genetic marker with the abundance of each transcript, it does not control for the increase in type I errors (i.e., false positives) associated with analyzing thousands of phenotypes (microarray spots). For those cDNA spots with hybridization intensities that were significantly (P < 0.05) associated with a particular genetic marker (328 total microarray spots, representing 236 unique genes), a number of criteria were used to reduce the data set to what were more likely to be “true” positives. When genes showed multiple cDNA spots on the microarray (since the arrays were constructed from cDNA libraries), if more than one spot mapped to the same genetic marker and the rest of the spots for that gene did not map significantly to any other genetic marker (i.e., they did not pass the genome-wide significance threshold [P < 0.05]), they were considered to be putative expression QTLs. Among genes with only one cDNA spot on the array or with only one spot mapping to a particular locus, only those with evidence of differential expression between the parents (as determined by the t test analysis mentioned above [P < 0.05]) were considered to be putative expression QTLs. Genes in the resulting data set were then classified as mapping in either cis or trans based on the location of the gene itself and the QTL.
Dual-luciferase assays.
For two genes that mapped in cis, the putative promoter regions from ME49 and CTg were compared based on their abilities to drive firefly luciferase expression. Specifically, PCR-amplified sequences were directionally cloned into the Gateway entry vector pENTR-d-Topo (Invitrogen, Carlsbad, CA), and plasmids with properly oriented sequences were used in Gateway cloning reactions (“LR reactions”) with a destination vector containing firefly luciferase and a 3′ untranslated region (UTR) from Toxoplasma DHFR (provided by Michael Behnke and Michael White, Department of Veterinary Molecular Biology, Montana State University) (38). For gene model 46.m01601, 1,004 bp upstream of the predicted start codon from either ME49 or CTg was cloned in frame with firefly luciferase using forward primer 5′-CACCGGATACAGGGATTCCCACAA-3′ (CACC is the sequence used for directional cloning into the pENTR-D-Topo vector) (20) and reverse primer 5′-ATCCATGCTGTTATTCGAGGGAAACTAAG-3′ (where ATCCAT encodes, in antisense, the start codon and an aspartic acid). For ROP18 (gene model 20.m03896 [www.toxodb.org]), 633 bp of sequence upstream of the presumed start codon was used as the putative promoter for the type II strain. This was generated by PCR amplification using forward primer 5′-CACCCTCGTCGACCACACAGCTAA-3′ and reverse primer 5′-ATCCATCACAACTTTCACACAAACTGGAC-3′. For the type III strain, the region upstream of ROP18 contains a deletion of 210 bp and an insertion of 2,242 bp relative to type II. This entire 2,617-bp region was cloned into the firefly luciferase vector using the same forward primer as that for the type II construct and reverse primer 5′-ATCCATTACAACATTCACACAAACTGTAC-3′ due to polymorphisms present in the ROP18 gene. For luciferase assays, equimolar amounts of each construct (typically between 50 and 100 μg) were cotransfected into strain CTg along with 20 to 100 μg of a vector containing Renilla luciferase driven by the TUB1 promoter (20). Parasites were harvested 24 to 48 h posttransfection, and firefly and Renilla luciferase levels were determined using the Dual-Luciferase assay kit (Promega, Madison, WI).
qPCR.
For toxofilin (33.m02185), TgDT.544460, and ROP8 (33.m00005), primers were designed from nonpolymorphic regions of the predicted transcript using Primer3 (http://fokker.wi.mit.edu/primer3/input.htm), followed by selection for use in quantitative PCR (qPCR) as described previously (7). Primers were as follows: for toxofilin, forward primer 5′-ATACAAGTCACGCCCTTTGG-3′ and reverse primer 5′-GGAGGGCGACATTGTAGATG-3′; for TgDT.544460, forward primer 5′-GGCATCTCCCCTCTCAACT-3′ and reverse primer 5′-GTGGTGCAAGAACCATCAGA-3′; for ROP8, forward primer 5′AGCCAGACGAGCAACCATA and reverse primer 5′GCGCACCAAATCCAGTAGA. Primers for the control gene, AMA1 (forward, 5′-ACGGTTTCTACTACGTGG-3′; reverse, 5′-CCAGCGATCAACGCAG-3′), have been described previously (30), and this gene was used to normalize the qPCR data because (i) there are few polymorphisms in the entire transcript from a type II (ME49) and a type III (VEG) strain (www.toxodb.org) and (ii) AMA1 has similar hybridization intensities in the type II and the type III strain used in this study (type II versus type III, 0.97 ± 0.10-fold difference across 5 microarray spots; P = 0.85). Total RNA was isolated from at least two different cultures of either ME49 or CTg tachyzoites grown for 24 h on HFF monolayers (multiplicity of infection, 10), and qPCR on the resulting cDNA was carried out on a Bio-Rad iCycler using Sybr green detection. Significant differences in transcript levels were determined using the ΔΔCT method as described previously (7, 26).
PCR amplification of 79.m00015.
To determine if the 79.m00015 locus was present in the type III genome, we designed primers that would distinguish 79.m00015 from one of its paralogous sequences (50.m05602) (see Results). These were forward primer 5′-TCCGCTCGGAAAACAAATAC-3′ and reverse primer 5′-ATTTCGCGTTCAGAAGCATT-3′. As a control we also constructed a primer set to amplify from homologous regions of both 79.m00015 and 50.m05602 (forward, 5′-ATGCACAACTTAGTCCTGGCGTTGC-3′; reverse, 5′-GCGGGTTTCCTACGGCCACGGTGCGGATTCGTCCTTTC-3′). Genomic DNA was harvested from strain ME49 (type II) and CTg (Type III) parasites and was used in PCR amplification reactions using standard methods.
Microarray data accession number.
The microarray data obtained in this study have been deposited in the Gene Expression Omnibus Database under accession number GSE11515. They can be downloaded at http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE11515.
RESULTS
Sixteen Toxoplasma genes have mappable expression phenotypes.
Gene expression profiling was carried out on the parents and F1 progeny from a cross between a type II (ME49) and a type III (CTg) strain, and expression phenotypes were genetically mapped using eQTL analysis. Based on BLASTX of the sequence data derived from the spots on the microarrays against the current draft of the predicted Toxoplasma protein set (www.toxodb.org), we estimate that we assayed 2,226 predicted gene models (out of a total of 7,793). Out of this data set, we identified 16 genes associated with cDNA spots that had hybridization intensities significantly associated with the genotype of a particular marker, and these data are summarized in Table 1. Interestingly, in our data set, there are 60 other gene models with strong evidence for differential hybridization between type II and type III strains (as confirmed by multiple spots for the same gene that show significant differences), but the hybridization profiles for these genes did not significantly map to any locus in the genome. This could be due to a number of factors, including multiple interacting loci or trans-acting loci that have more subtle effects that may be refractory to our analysis given the number of progeny examined (multilocus interactions require a much larger sample size for reliable identification).
TABLE 1.
Toxoplasma genes with hybridization profiles that significantly mapped to a marker in the genomea
| Identifier | Gene name/description | Chrom | Gene chrom pos (Mb) | Signal peptide? | Fold difference in transcript level, type II vs type IIIb
|
Max LOD score | Max LOD marker | Marker chrom | QTL range | QTL chrom range (Mb) | cis mapping? | Type II vs type III polymorphism (%)
|
|||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| This study | ToxoDB | Promoter | Coding | UTR | |||||||||||
| 113.m00783 | Conserved hypothetical | II | 1.8 | No | −2.22 | −1.83 | 2.47 | G11L-T7 | II | Whole chrom | 0.00-2.47 | Yes | 0.6 | 1.32 | 1.3 |
| 41.m01274 | Conserved hypothetical | II | 0.07 | No | −1.94 | −2.41 | 4.35 | G11L-T7 | II | Whole chrom | 0.00-2.47 | Yes | 3.24 | 2.58 | 1.62 |
| 41.m01339 | Hypothetical | II | 0.88 | No | 2.15 | 3.40 | 3.22 | G11L-T7 | II | Whole chrom | 0.00-2.47 | Yes | 1.65 | 0.5 | 2.28 |
| 20.m00005 | GRA7 | VIIa | 2.57 | Yes | 1.87 | 1.32 | 2.83 | CS2 or AK29 | VIIa | CS9-end | 1.81-4.50 | Yes | 0.7 | 2.25 | 1.3 |
| 20.m03784 | Hypothetical | VIIa | 2.58 | Yes | 1.86 | 14.28 | 4.7 | TgSUB1 | VIIa | CS9-M102 | 1.81-3.09 | Yes | 1.6 | 0.4 | 1 |
| 20.m03896 | ROP18 | VIIa | 1.48 | Yes | 3.57 | 239.97 | 4.61 | CS2 | VIIa | M95-TgSUB1 | 0.60-2.00 | Yes | 2.83 | 5.8 | 4.91 |
| 59.m03370 | Hypothetical | VIII | 6.62 | No | −2.98 | −6.55 | 5.18 | AK114 | VIII | MAG1-end | 5.31-6.92 | Yes | 1.4 | 1.14 | 1.68 |
| 57.m00004 | Protein kinase homologue | IX | 1.01 | No | 10.06 | 3.09 | 2.76 | L375 or AK123 | IX | AK87-AK59 or KT-62A-SAG5C | 0.36-3.38 or 3.56-4.75 | Yes | 0.9 | 1.1 | 0.5 |
| 57.m03117 | Phosphodiesterase homologue | IX | 0.99 | No | −1.3 | −1.37 | 3.96 | L375 or AK123 | IX | AK87-AK59 or KT-62A-SAG5C | 0.36-3.38 or 3.56-4.75 | Yes | 0.3 | 0.3 | 0.75 |
| 57.m03124 | Acetyl coenzyme A synthetase | IX | 1.16 | No | −1.49 | −4.35 | 3.43 | L375 or AK123 | IX | AK87-AK59 or KT-62A-SAG5C | 0.36-3.38 or 3.56-4.75 | Yes | 0.2 | 0 | 1.36 |
| 33.m00005 | ROP8 | X | 7.09 | Yes | 2.37c | −2.19 | 4.38 | AK148 | X | AK157-end | 6.93-7.42 | Yes | 2.03 | 3.11 | 1.1 |
| 33.m02185 | Toxofilin | X | 5.95 | Yes | 3.2 | 7.06 | 9.94 | AK153 | X | AK34-MIC2AP | 4.12-6.54 | Yes | 2.81 | 8.37 | 9.31 |
| 42.m05839 | JMCJ domain containing | X | 2.61 | No | 3.54 | 1.47 | 2.81 | AK65 | X | AK63-c19-TA3 | 1.60-4.05 | Yes | 1 | 0.5 | 0.75 |
| 46.m01601 | Hypothetical | X | 4.28 | Yes | −1.75 | −4.35 | 7.1 | AK66 | X | SRS4-AK153 | 2.78-4.39 | Yes | 1.7 | 1 | 1.05 |
| 79.m00015 | Hypothetical | Unkd | Unkd | Yes | 3.48 | 12.25 | 3.16 | AK65 | X | AK63-c19-TA3 | 1.60-4.05 | Unkd | Unke | Unke | Unke |
| TgDT.544460 | Hypothetical | Unkd | Unkd | No | 3.22c | N/A | 7.39 | AK128 | X | Start-Gra3 | 0.00-0.99 | Unkd | 5.89 | 9.71 | Unkf |
Chrom, chromosome; pos, position; max, maximum; Unk, unknown; N/A, not applicable.
Positive numbers indicate that the type II strain had higher hybridization intensity, while negative numbers indicate that the type III strain had higher hybridization intensity. ToxoDB data were downloaded from www.toxodb.org.
Validated in this study using real-time qPCR. See the text.
The chromosomal location for this gene is unknown.
This locus is apparently missing from the type III genome (VEG genomic sequence [www.toxodb.org]).
The 3′ UTR is either deleted or highly divergent in the type III genome (VEG genomic sequence [www.toxodb.org]).
For the 14 genes with known genomic locations, the phenotype mapped within the genomic region containing the gene itself (i.e., the array hybridization phenotype putatively mapped in cis). Given the low recombination rate for Toxoplasma (1 centimorgan per ∼100 kb [24]) and the number of progeny used in this study (18 progeny), the eQTL span a genomic region that is typically 1 to 3 Mb. Therefore, on smaller chromosomes for which there were no intrachromosomal recombination events in any of the F1 progeny (such as chromosome II [24]), the QTL for the expression phenotype spans the entire length of the chromosome. The designation of “cis,” therefore, is clearly tentative (a trans-acting factor that maps 1 Mb away would be missed), but the fact that all 14 genes mapped in this way and the results described below suggest that most, if not all, of this set of genes have differential hybridization as a result of differences in the genes themselves. Overall, the 16 genes presented in Table 1 represent a curated data set of transcripts with significant eQTL based on differential hybridization intensity between type II and type III strains and among their F1 progeny.
Recently, an Affymetrix oligonucleotide array containing probes for all predicted Toxoplasma genes was constructed, and data quantifying hybridization intensity in a type II strain (Prugniaud) and a type III strain (VEG) were made available through ToxoDB (www.toxodb.org). When we compared our hybridization data to this public data set, we found that for 14 of the 15 genes listed in Table 1 that were present on the Affymetrix chip, the differences in hybridization intensity between ME49 and VEG were qualitatively similar to our observations (Table 1). For the one transcript that was not assigned to a gene model and therefore is not present in the data from ToxoDB (TgDT.544460), as well as for the one gene (ROP8) where our spotted cDNA data differed significantly from the ToxoDB data, we validated the observed hybridization differences using real-time qPCR. In our spotted cDNA array data, levels of both the TgDT.544460 and the ROP8 transcript were higher in the type II parent than in the type III parent (3.22- and 2.37-fold, respectively), and using real-time qPCR we found qualitatively similar transcript abundance differences between strains for these two genes: the TgDT.544460 level was 1,600-fold higher in ME49 than in CEP (n = 5; P = 0.008), and the ROP8 transcript level was 92-fold higher in ME49 than in CEP (n = 5; P = 0.02). Spotted cDNA microarrays are well known to sometimes significantly underestimate differences (13), and this was clearly the case here. The key observation, however, that these two genes show markedly more transcript abundance in ME49 than in CEP, was confirmed.
Genes that encode proteins with predicted signal peptides are overrepresented in the eQTL group.
The 14 cis-mapping loci identified in this study are predicted to have a wide variety of functions. Interestingly, 6 of the 14 (42%) contain a signal peptide as predicted by SignalP (18) and are therefore likely to enter the secretory pathway. This is a larger percentage than would be expected by chance (hypergeometric distribution, P = 0.004), since only 281 of the 2,226 predicted genes represented on the microarray (13%) contain predicted signal peptides (www.toxodb.org). In T. gondii, many of the proteins that enter the secretory pathway are destined for a set of secretory organelles unique to the phylum. These include the rhoptries, the dense granules, and the micronemes, all of which can secrete their contents directly into the host cell (19), into the parasitophorous vacuole (14), or onto the parasite surface (11). In fact, five of the six cis-mapping genes with signal peptides were already known to localize to this group of secretory organelles. GRA7 is found in the dense granules (14); ROP18 (17, 30), toxofilin (9, 28), and ROP8 (16) are found in the rhoptries; and 46.m01601 was found in stimulated secretion products containing primarily dense granule and microneme proteins (formerly called TgTwinsan_2661 [41]). Moreover, GRA7, ROP18, and ROP8 can all be found in the host cell at different times postinvasion (14, 16, 30). These proteins have been intensely studied because they appear crucial to Toxoplasma biology. The subcellular location for the remaining cis-mapping gene with a predicted signal peptide (gene model 20.m03784) is unknown.
46.m01601 and ROP18: upstream sequences from type II and type III strains have different activities in luciferase reporter assays.
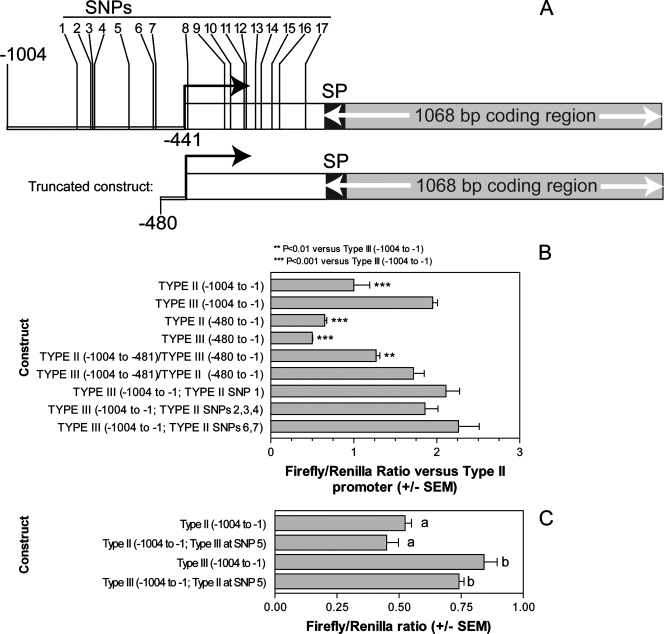
For eQTL that map in cis, multiple scenarios could produce the observed differences in hybridization: either (i) the transcript is highly polymorphic between the parental lines, and the corresponding cDNA from one of the predicted alleles hybridizes inefficiently to the probe spotted on the array (which is derived from only a single strain), (ii) the locus is deleted in one of the parental strains, or (iii) the transcript exhibits different abundances in the parental lines and F1 progeny due to polymorphisms, insertions, or deletions in the gene's promoter or stability control regions (typically 5′ and 3′ UTRs). To investigate which of these explanations applies to specific loci, several additional experiments were necessary. For the 46.m01601 locus, all three of the microarray spots had hybridization profiles that were significantly associated with a region on chromosome X (logarithm of odds [LOD] scores ranged from 5.1 to 7.1) delimited by markers SRS4 and AK153, a 1.61-Mb region that contains the 46.m01601 locus itself. In this case, the type III strain (CTg) had a 1.8-fold greater signal for 46.m01601 than the type II strain (ME49) (Table 1), and in the F1 progeny the type III allele at this locus was also strongly associated with higher signals for the 46.m01601 spots. Sequence analysis of the 46.m01601 locus in type II and type III strains (using direct sequencing as well as publicly available genomic sequences from a type II [ME49] and a type III [VEG] strain [http://www.toxodb.org]) revealed a relatively high level of sequence similarity in the predicted transcript (99.2% over the 1,503-bp transcript), suggesting that the differences in hybridization signal were not due to differences in hybridization efficiency between the cDNAs derived from the type II and type III strains. Based on this finding, we hypothesized that polymorphisms present in the region upstream (the promoter and 5′ UTR) of the 46.m01601 coding region could be responsible for this difference. Using the position of the 5′-most EST (2, 6) that mapped to this locus, we estimated the putative transcriptional start site to be at position −441 relative to the start codon (Fig. 1A). Direct sequence analysis of the type III strain used in this study (CTg) and comparison to the type II genomic sequence revealed that the 1,004 bp upstream of the start codon contained 17 single nucleotide polymorphisms (SNPs) between the type II and type III strains. All 17 of these differences are also present in the complete genome sequence from a type III strain (VEG) (http://www.toxodb.org).
FIG. 1.
Analysis of strain-specific differences in 46.m01601 promoter activity. (A) Structure of the 46.m01601 locus, showing the upstream 1,004 bp used as the putative promoter and the 17 polymorphisms that distinguish the type II and type III sequences. Transcription starts at about position −441 relative to the start codon, based on alignment of EST sequences to the locus. The truncated version of the promoter used in luciferase assays is also shown. SP, signal peptide sequence. (B) Effects of truncation of the type II and type III promoters, engineering of chimeric promoter constructs, and effect of mutation of multiple SNPs in the type III promoter on luciferase production. All data were normalized to the level with the type II promoter. Data are shown for the type II and type III wild-type promoters, the type II and type III truncated promoters (type II and type III, −480 to −1), the chimeric promoter constructs [e.g., type II (−1004 to −481)/type III (−480 to −1)], and the type III promoter mutated to the type II sequence at either SNP1, SNPs 2, 3, and 4, or SNPs 6 and 7 [e.g., type III (−1004 to −1; type II SNP 1)]. Asterisks indicate significant differences from the results with the full type III promoter by one-way analysis of variance and Tukey's multiple-comparison posttest. **, P < 0.01; ***, P < 0.001. (C) Versions of the 46.m01601 promoter were constructed where SNP5 was mutated in the type II promoter to the type III nucleotide [type II (−1004 to −1; type III at SNP5)] or in the type III promoter to the type II nucleotide [type III (−1004 to −1; type II at SNP5) and fused to firefly luciferase. Luciferase production for each mutated construct was compared to that for its wild-type counterpart, and data are represented as ratios of the firefly luciferase signal to the Renilla luciferase signal. Means with the same letter are not significantly different (P > 0.05). SEM, standard error of the mean.
To test whether these differences are responsible for the observed transcript level differences, the type II and type III upstream sequences of 46.m01601 were fused to firefly luciferase and assayed for expression using transient transfection. The constructs contained the 1,004 bp of upstream sequence discussed above (referred to below as the 46.m01601 promoter). In these assays the type III promoter yielded 2.0 (±0.13)-fold higher luciferase production than the type II promoter (P < 0.001) (Fig. 1B). This is in close agreement with the microarray data for the parental strains, and it also confirms that one or more of the polymorphisms in the 46.m01601 promoter are almost certainly responsible for most, if not all, of the differences in transcript abundance.
To identify the specific region of the upstream sequence that was responsible for the differences in promoter activity, we created chimeric luciferase reporter constructs that contained either the first (5′-most) 524 bp of the type II promoter fused to the last 480 bp of the type III promoter (type II/III fusion) or vice versa (type III/II fusion) and compared them to the wild-type CTg (type III) promoter. The type II/III fusion yielded significantly less luciferase (about 1.5-fold [P < 0.01]) than the type III wild-type promoter (Fig. 1B), while the type III/II fusion yielded levels of luciferase similar to those for the type III promoter (P > 0.05) (Fig. 1B). These data suggest that the one or more of the seven polymorphisms within the 5′ half of the type III strain 46.m01601 promoter are important for the increased promoter activity, although contributions from the polymorphisms present in the 3′ half (in which there are 10 polymorphisms between the type II and type III sequences [Fig. 1A]) may also play a role. When the 5′-most 524 bp is removed from the type III promoter and fused to luciferase, this truncated construct (containing only 39 bp upstream of the putative transcriptional start site) results in a level of luciferase production that is above background but significantly lower than that seen for the 1,004-bp type III promoter. A similarly truncated promoter construct derived from the type II sequence also exhibited lower luciferase production than the 1,004-bp type II promoter (Fig. 1B). In aggregate, these data provide further support for the role that the first 524 bp of the putative promoter plays in the increased luciferase production typical of the type III strain sequence.
There are 128 different possible combinations of the seven SNPs in the 5′ half of the 46.m01601 promoter. Testing all possible combinations would be prohibitive in terms of the effort involved, so we focused on four sets of physically clustered SNPs (SNP1, SNPs 2, 3, and 4, SNP5, and SNPs 6 and 7 [Fig. 1A]). SNP5 was of particular interest because it was within a 7-bp sequence that is a good match to a consensus heptamer (GAGACGC) often found upstream of the transcriptional start site and has been implicated in promoter function for several Toxoplasma genes (27, 36). In the 46.m01601 upstream region, this motif (at position −180 with respect to the transcriptional start site) is GAGACGA in the type III strain and GACACGA, a weaker match to the consensus, in the type II strain. To test the hypothesis that this single G-to-C SNP was responsible for the differences in 46.m01601 transcription, we swapped just this residue in the type III and type II promoters and compared the abilities of these upstream regions to drive luciferase expression. Surprisingly, neither of these mutations resulted in any significant differences (P > 0.05) in luciferase production between the mutant promoter and its wild-type counterpart (Fig. 1C). To test the remaining six SNPs in the 46.m01601 upstream half, we made three different constructs in which we mutated just SNP 1, SNPs 2, 3, and 4, or SNPs 6 and 7 from the type III to the type II sequence, and we compared their luciferase production with that of constructs harboring the unaltered type III promoter. Again, these constructs were all as effective at driving luciferase production as the type III promoter (P > 0.05), resulting in 1.7- to 2.3-fold more luciferase production than the type II promoter (Fig. 1B). Therefore, while in aggregate the SNPs in the first 524 bp are at least partially responsible for the increased luciferase production of the type III promoter compared to the type II promoter, these data suggest that either all seven are necessary for the increased activity of this promoter or that particular, untested combinations of SNPs (e.g., SNP4 and SNP7) are necessary for the increased promoter activity of the type III allele. With respect to SNP5 and the heptamer motif, the data show either that the heptamer motif is not involved in the promoter activity, at least in this assay, or that it is not substantially affected by the G/C polymorphism at position 3.
Differences between types II and III in microarray signal strength for the transcripts encoding the rhoptry kinase ROP18 (ToxoDB gene model 20.m03896) were also observed and confirmed by qPCR to be true reflections of major differences in transcript abundance between the two strains (30). Analysis of the type II × type III F1 progeny showed that this difference was significantly associated with marker CS2 (LOD score, 4.6), which, based on the genetic map, falls between markers M95 and TgSUB1, a 1.4-Mb region encompassing the ROP18 locus itself (30). When we sequenced the upstream (putative promoter) region of a type III strain (CTg) and compared it to the ME49 genomic sequence, we found that ROP18 in the CTg strain contains an insertion/deletion just upstream of the predicted transcriptional start site (as determined by EST alignments to the genome [30]). Relative to ME49, the insertion was 2,242 bp long and the deletion was 210 bp long (type III strain; GenBank accession no. EF092842). This difference was also recently confirmed by near-complete genomic sequencing of another type III strain (www.toxodb.org). Interestingly, three nearly perfect 44-bp tandem repeats are present just upstream of the insertion/deletion site in the type II sequence, while in the type III promoter region this sequence is represented only once. This 44-bp repeat is not found in any recognizable form outside of the ROP18 locus in the Toxoplasma genomic sequence from type I, type II, or type III strains (www.toxodb.org).
To determine if this insertion/deletion is responsible for the transcript abundance differences for ROP18, the upstream regions from type II and type III were compared (using 633 and 2,668 bp 5′ of the start codon, respectively) by the dual-luciferase assay described above. The results showed that, as predicted by the array data, the type II promoter drove the production of substantially more luciferase (6.2 ± 1.4-fold) than the type III construct after transfection of equimolar amounts of each construct (data not shown). Hence, not surprisingly, the major insertion/deletion within its promoter region appears to have a substantial effect on ROP18 expression.
Toxofilin hybridization differences are due mostly to polymorphisms, not to transcript abundance.
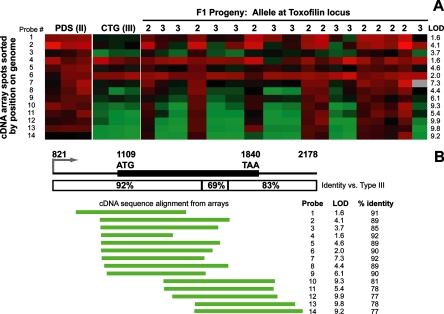
The LOD scores associated with the gene encoding the rhoptry protein toxofilin were among the highest that we observed (maximum LOD, 9.4), and the eQTL was found between genetic markers AK34 and MIC2AP, a 2.3-Mb region that encompassed the toxofilin locus. As shown in Fig. 2A, the hybridization intensity from cDNA derived from the type II strain was higher than that from the type III strain in nearly all of the toxofilin probes present on the array. On average, this difference was 3.2 ± 0.4-fold higher in strains with a type II allele at this locus than in those with a type III allele. It is possible that some of this difference is due to transcript abundance in these strains, but based on direct sequencing from type II (ME49) and type III (CTg) strains, the primary sequence of toxofilin is unusually divergent: in the 1.3-kb predicted transcript, there are 167 SNPs between the type II and type III sequences, plus 3 other sites where nucleotides are either deleted or inserted in the type III strain sequence with respect to the type II sequence. Furthermore, larger numbers of these polymorphisms are clustered at the 3′ end of the toxofilin transcript (121 of the 167 SNPs are in the last 707 bp of the transcript). This level of polymorphism (12.4% at the nucleic acid level) is dramatically higher than what is typical for type II and type III sequences (∼1 to 2% polymorphism [6]) and would be sufficient to cause differences in hybridization efficiency between type II- and type III-derived cDNAs and the cDNA fragments of toxofilin spotted on the array (which are from a type II strain for all toxofilin spots shown). This difference in hybridization to the probes on the array can be seen in Fig. 2, where the probes corresponding to the 5′ region of the transcript tend to hybridize more efficiently to cDNAs from type III strains than those corresponding to the more variable 3′ end of the transcript. Of particular note are probes 4 and 6, which have nearly equal hybridization with the type II and type III parents (0.8 ± 0.1-fold difference between type II and type III) and thus show no significant segregation with any genetic marker. In contrast, probes derived from the more divergent 3′ end of the transcript tend to have much higher LOD scores for marker association (as high as 9 for probes 10, 12, 13, and 15) and a corresponding difference in hybridization intensity between the type II and type III parents (4.0 ± 0.8-fold higher for type II than for type III). The nucleic acid identities between the end sequence data obtained from each spot and the corresponding region of the type III sequence were computed using ClustalW, and these identities were regressed against the maximum LOD score for the association between the hybridization intensity and the relevant region on chromosome X. As expected, there was a highly significant negative correlation (R2 = 0.56; P = 0.002) between the LOD score and the percentage of identity between the array spot sequences (from a type II strain) and the corresponding sequence in the type III strain. It should be noted that the probes on the array were sequenced only from the 5′ end, and therefore the exact length, and the exact percentage of identity in comparison to type III, of the entire sequence present on the array is unknown. Clearly, however, the probes that contain sequence from the 3′-most end of the toxofilin locus are much less efficient at hybridization to cDNAs from type III strains.
FIG. 2.
Microarray analysis of strain-specific differences at the toxofilin locus. (A) Microarray data from the 14 toxofilin probes from the bradyzoite microarray derived from a type II strain. Parasite cDNA was labeled with Cy5 (red) and cohybridized with a Cy3-labeled common reference. Data are shown from triplicate arrays for the type II and type III parents, and single arrays were done for each of the 18 F1 progeny. LOD scores as determined by R/QTL software are shown for each probe. (B) Structure of the toxofilin locus. The putative promoter and predicted N-terminal region of the protein are more conserved between type II and type III than the predicted C terminus and putative 3′ UTR. The locations of the 5′ ends of the cDNAs on the microarray are shown below the locus, sorted by the start position on the genome. The percentage of identity between the sequenced portion of the probe and the type III toxofilin sequence, along with the LOD score for each probe, is also shown, demonstrating the inverse correlation between the percentage of identity and the LOD score.
The above microarray results strongly suggested that the cis-mapping eQTL for toxofilin is the result of differences in hybridization efficiency between the alleles rather than of differences in transcript abundance. To confirm this, we used real-time qPCR with primers derived from nonpolymorphic regions of toxofilin. The results showed that the ratio of transcript levels in type III to those in type II was 1.02 (±0.29), a difference that was not statistically significant (P = 0.61) (data not shown). These data indicate that the strain-specific differences in signal intensity for toxofilin are due to the extreme polymorphism at the type III toxofilin locus relative to the type II locus rather than to actual differences in transcript abundance.
The 79.m00015 coding region is deleted in CTg and is a member of a small family of secreted proteins.
The chromosomal locations for two genes showing strain-specific differences in the microarray analysis are, surprisingly, unknown (Table 1). One of these proteins, 79.m00015, has a predicted signal peptide and showed an eQTL that mapped to a 3.4-Mb region of chromosome X. Based on the microarray data for this locus, the type II allele was associated with higher hybridization intensity, and in the parental lines we observed a 2.9-fold-higher signal for the type II strain than for the type III strain. The 79.m00015 gene model is predicted from genomic scaffold TGG_994719, an 8,740-bp scaffold that has not been assigned to a T. gondii chromosome. BlastP analysis of this predicted protein against the predicted Toxoplasma protein set (http://www.toxodb.org) reveals at least two paralogues that share homology to 79.m00015 along virtually its entire length. These are 50.m05602, which is found on chromosome XII, and 1.m00014 which, like 79.m00015, is predicted from a short genomic scaffold (TGG_995340) with an unknown chromosomal location. Over the first 180 amino acids of 79.m00015, 50.m05602 and 79.m00015 are identical, but they are then highly divergent over the rest of the predicted protein. Based on analysis of the existing type III strain genomic sequence (http://www.toxodb.org), the 50.m05602 locus appears to be present in the type III sequence, while the 79.m00015 and 1.m00014 loci appear to be deleted. We confirmed this for the 79.m00015 locus by using a PCR primer set designed to distinguish 79.m00015 from 50.m05602 and found that we could amplify 79.m00015 sequence only from ME49 genomic DNA. However, using primers designed to amplify both 79.m00015 and 50.m05602, we found that we could amplify 50.m05602 from both ME49 and CTg DNAs (J. P. Boyle and J. C. Boothroyd, unpublished data). The apparent absence of 79.m00015 in type III strains would certainly account for the dramatic difference in hybridization observed between type II and type III strains, and it will be important to determine if 79.m00015, and therefore genomic scaffold TGG_995340, are indeed found on chromosome X, as the genetic mapping data would suggest. The presence of multiple members of this gene family in type II strains suggests that this gene set may have expanded in this lineage. It will be interesting to express tagged versions of 79.m00015 and its paralogous proteins in order to determine their subcellular localization.
EST assembly TgDT.544460 is a short orphan sequence with unknown coding function but a clear difference in hybridization intensity.
Four cDNA probes that map to the same location on the 40-kbp T. gondii genomic scaffold TGG_995349 associate significantly with markers on chromosome X (AK128, three probes; AK154, one probe), with LOD scores ranging from 2.6 to 9.7 (P < 0.01 for all probes). These sequences do not correspond to any member of the current draft of the predicted Toxoplasma protein set, but they do correspond to EST assembly contig TgDT.544460 (www.toxodb.org) (25), which incorporates, among others, the sequences for all four cDNA spots that were on the microarrays (GenBank dbEST accession no. AA531996, AA519292, AA519751, and W96676). Genomic scaffold TGG_995349 has not been assigned to any T. gondii chromosome and does not contain any predicted gene models (www.toxodb.org). The fact that the hybridization intensity of this particular gene mapped significantly to genetic markers on chromosome X could indicate that the unmapped genomic scaffold TGG_995349 is also found on chromosome X, although a definitive determination of whether the hybridization intensity of this particular gene maps in cis or in trans is not possible without knowing the chromosomal location of the gene. Based on the array data, the type II allele is associated with higher levels of hybridization for this transcript (3.1-fold compared to type III [Table 1]), and this gene is also present in the fully sequenced genome from a type III strain (VEG [http://www.toxodb.org]). A 136-amino-acid open reading frame is predicted in the genomic region encompassing the TgDT.544460 EST assembly and encodes a protein of unknown function. However, the predicted open reading frame product has 91% identity with the first 136 (of 197) amino acids of another Toxoplasma protein, gene model 644.m00075 (found on chromosome III), and has high BLASTP similarity to eight other predicted T. gondii gene models (Expect < 10−10), suggesting that TgDT.544460 may also belong to a family of Toxoplasma proteins. It will be interesting to determine the subcellular localization of the protein encoded by TgDT.544460, as well as its Toxoplasma paralogues, and to determine the significance of the higher transcript abundance of TgDT.544460 in type II strains versus type III strains.
Other significantly mapping genes.
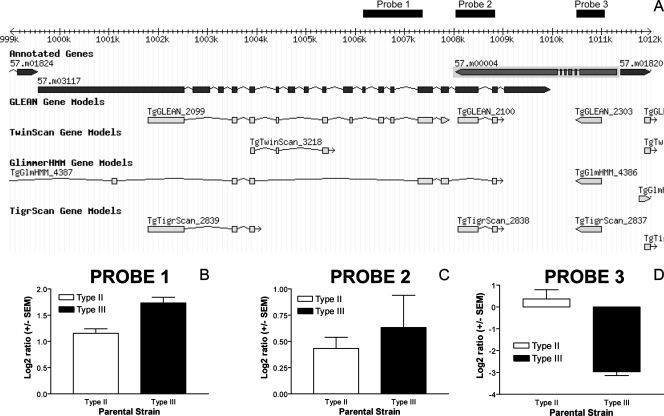
Besides TgDT.544460, the eight remaining genes with significant eQTL that did not include sequences encoding predicted signal peptides were distributed across four different chromosomes. They are listed, along with their genomic locations, in Table 1. Some of these proteins are predicted to have enzymatic activity, including one with a predicted kinase domain (57.m00004), one with predicted phosphodiesterase activity (57.m03117), and one with predicted acetyl coenzyme A synthetase activity (57.m03124), all on chromosome IX (Table 1). Remarkably, 57.m03117 and 57.m00004 are adjacent to each other in the genome in a tail-to-tail orientation (Fig. 3A), and based on the predicted protein sets, the 3′ ends of the transcripts are predicted to overlap. For 57.m03117, two cDNA probes (probes 1 and 2 [Fig. 3A]) had mappable hybridization intensity phenotypes, and in this case greater hybridization intensity was associated with the type III strain (on average, 1.30-fold higher for CTg than for ME49 [Table 1; Fig. 3B and C]). A single probe mapped for 57.m00004, and the type II strain had dramatically higher (10.3-fold) hybridization intensity for this spot than the type III strain (Fig. 3D). Interestingly, the second cDNA probe that we mapped to 57.m03117 via BLASTN also overlaps the predicted location of 57.m00004 (Fig. 3A), although it shows hybridization intensity differences between the type II and type III parents that are more consistent with the other 57.m03117 probe (probe 1 [Fig. 3A and B]). The 57.m00004 locus appears to be present in the type III genome (Table 1) and does not appear to have a significantly high level of polymorphism (1 to 2%) that would affect the hybridization of type III cDNAs to the array spot. Therefore, it is likely that both 57.m03117 and 57.m00004 exhibit different abundances in type II and type III strains. Given their tail-to-tail orientation (Fig. 3A), it is possible that the highly active transcription of 57.m00004 in type II strains compared to type III strains inhibits the efficient transcription of 57.m03117 in type II strains, and the converse may be true in type III strains.
FIG. 3.
Genomic locations of two cis-mapping transcripts corresponding to gene models 57.m03117 and 57.m00004. (A) Genome browser view (www.toxodb.org) encompassing bp 999000 to 1012000 on chromosome IX. The positions of the three cDNA probes with hybridization intensities that mapped to this locus are shown (probes 1 to 3). (B to D) Log2 ratios of probes 1 to 3, respectively, in the parental lines used in this study, showing the higher hybridization of type III strains to probe 1 and the higher hybridization intensity of type II strains with probe 3.
Interestingly, three proteins on chromosome II had cis-mapping hybridization intensity levels (although since there was no recombination on chromosome II in any of the F1 progeny, these are called cis mapping only on the basis of the fact that the loci themselves are on the same chromosome as the eQTL). For these genes we have not performed any further experiments and therefore cannot know if the differences in hybridization intensity are due to actual expression differences (as for 46.m01601 and ROP18) or divergent sequences between the strains (as for toxofilin). As shown in Table 1, however, all of these loci appear to be clearly present in both type II and type III strains (i.e., they are not deleted), and none have a level of sequence variation in either the promoter/5′ UTR, the coding region, or the 3′ UTR that exceeds 3 SNPs per 100 bp, suggesting that they may represent genes that are under differential transcriptional regulation and/or have different levels of stability.
DISCUSSION
Some of the phenotypic differences between Toxoplasma strains could be due to quantitative differences in expression rather than to amino acid-changing polymorphisms. To identify these loci and their genetic basis, we carried out eQTL mapping on Toxoplasma transcriptional profiles using 19 F1 type II × type III progeny (24). We have identified 16 loci in Toxoplasma that have hybridization intensity profiles that could be genetically mapped in this fashion, and in all cases these genes were found to map in cis, perhaps suggesting that sequence differences within the promoter and/or 3′ UTRs were responsible for the differences in transcript abundance. For two such genes, (46.m01601 and ROP18), based on luciferase assays, the promoter was indeed found to be at least partly responsible for the observed differences in hybridization intensity between strains. For ROP18, these are likely due to the insertion/deletion present in the putative promoter, which makes the type III upstream region less efficient at driving luciferase production. For 46.m01601, there were no such insertions or deletions in the putative promoter region, although the mutation of single or multiple SNPs in the type III sequence relative to their type II counterparts was not sufficient to confer type II-like promoter activity. These data suggest that the interaction of SNPs from different regions of the upstream sequence may be necessary for the increased activity of the type III promoter. Regardless, for 46.m01601 the DNA binding proteins that mediate these differences in transcription are unknown, and it will be of great interest to determine if parasite nuclear proteins would interact more or less strongly with the type III 46.m01601 promoter than with the type II version. The identification of these putative transcription factor proteins could yield great insights into possibly novel mechanisms of gene regulation in Toxoplasma.
One of the goals of this work was to identify genes that might be responsible for differences in virulence seen between strains. To this end, we factored these data into parallel studies mapping virulence loci in Toxoplasma (30). One virulence locus mapped to a region of the genome that included ROP18, one of the genes identified here as having major strain-specific differences in transcript abundance. This difference was one of the factors used to identify ROP18 as potentially responsible for the heightened virulence of type II strains relative to type III strains (30). A type III strain stably transformed with a type II allele of ROP18 is >10,000-fold more virulent than the starting type III strain, confirming the key role of ROP18 in virulence.
The predicted ROP18 protein is highly polymorphic among types I, II, and III (30, 37). Based on quantitative real-time PCR data for ROP18 (30), the difference in hybridization intensity appears to be due to actual differences in transcript abundance between type II and type III strains rather than to this extreme level of polymorphism. Based on the luciferase reporter data presented here, the large insertion/deletion in the type III promoter compared to the type II promoter is likely responsible for at least some of the expression differences observed. Note, however, that the observed difference in luciferase activity (6.3 ± 0.6-fold higher in type II) was much smaller than the difference in ROP18 transcript levels between a type II and a type III strain as determined by real-time quantitative PCR (∼15,000-fold [30]). This suggests that transient transfection is only an approximate assay for measuring promoter strength and/or that other factors, such as polymorphisms present in UTRs, may also play a role (12). Indeed, the type II and type III ROP18 alleles have a comparatively high number of polymorphisms in those portions of the promoter/5′ UTR that they have in common as well as in the shared 2,000 bp 3′ of the stop codon (2.8 and 4.9%, respectively [Table 1]). Epigenetic factors, such as chromatin modifications, which have been shown to be important in T. gondii stage-specific gene expression (32), could also be responsible for this observed disparity and in general may also play a role in modulating strain-specific transcript abundance.
Secreted proteins represented an unexpectedly large fraction of the genes identified in this analysis. This may not be entirely surprising, since secreted proteins that are destined for the specialized Toxoplasma secretory organelles often encode proteins that are crucial for interactions with the host and therefore may be under strong positive selection for change to fine tune interactions with a particular host or even to expand the host range. Since the loci identified in this study encode putatively secreted proteins more often than would be predicted by chance, it will be of great interest to determine the effects that their expression level differences have on interactions with host cells in vitro and in vivo. One of the loci identified here, ROP8, is within a region in the T. gondii genome that appears to contain multiple copies of genes encoding highly similar, ROP8-like proteins (including ROP2 [3, 5]). The level of similarity of these sequences is such that the cDNAs present on the array would most likely not distinguish between them. One intriguing possibility is that the differences in hybridization intensity between type II and type III strains for the ROP8 spots on the array are due to differences in copy number between strains rather than to actual transcriptional differences. Further work dissecting the locus containing ROP2, ROP8, and other closely related copies of this gene family will be necessary in order to determine if this is the case.
For two of the genes identified in this study, we showed that hybridization differences between the parents and among the progeny were due to extreme polymorphism (as for toxofilin) or deletion of the locus itself (gene model 79.m00015). For most of the genes identified here, however, the reasons for differences in transcript levels are unknown. In future work this can be determined by using a combination of promoter and polymorphism analyses and qPCR with primers for sequences in common between the two strains. Based on the degree of DNA sequence variation between type II and type III strains at these loci, however, it is likely that at least some of these genes will have sequences in their promoters that result in differences in transcript abundance (as was observed for 46.m01601), since many of them do not have levels of DNA variation substantially above average (Table 1).
The spotted cDNA microarrays used in the present study do have some disadvantages compared to recently designed oligonucleotide arrays. These include less-extensive gene coverage (our arrays contain probes for approximately 25% of the predicted Toxoplasma genes, in contrast to nearly complete coverage for the Affymetrix Toxoplasma expression array), a lower range of hybridization intensities than can be measured by the Affymetrix platform, and lower precision (see, e.g., reference 40). However, there are some advantages to the spotted cDNA platform for our eQTL mapping approach. In situ-synthesized oligonucleotide arrays (such as those produced by Affymetrix and Nimblegen) are extremely sensitive to polymorphisms between the sample and the probe sequence and are capable of differentiating a single polymorphism if it is in the middle of the probe. In contrast, spotted cDNA probes are much less affected by polymorphisms. In the present study, for the toxofilin locus, two probes with polymorphism percentages of 8 and 10% between type II and type III strains hybridized equally well to type II and type III cDNAs (probes 4 and 6 [Fig. 2A]). In most cases, only those probes with type II versus type III polymorphism percentages greater than 20% exhibited dramatic differences in hybridization to type II and type III cDNAs, which, we determined by qPCR, were not due to differences in transcript abundance. Another advantage of the spotted array platform in general is that the arrays contain randomly selected cDNAs, and therefore their presence on the microarray is not dependent on gene prediction algorithms. In the present study, this resulted in the identification of a transcript (TgDT.544460) that does not correspond to a predicted Toxoplasma gene but is clearly has different abundances in type II versus type III strains and among their F1 progeny. This transcript is not present on the current version of the Toxoplasma Affymetrix microarray. In the case of ROP8, we found that our spotted cDNA data were in conflict with publicly released data using the Toxoplasma Affymetrix gene chip for comparing type II and type III strains. However when we assessed differences in ROP8 transcript abundance using real-time qPCR, we found strong agreement with our spotted cDNA array data. This discrepancy could be due to differences in the type II and type III strains used (ME49 versus CTg in our study compared to Prugniaud versus VEG in the publicly released data) and/or their passage histories. While the majority of strain-specific gene expression profiles are consistent between our data and the publicly available data, the differences in ROP8 levels observed in the different studies may indicate that the transcript abundances of certain genes, and possibly of rhoptry proteins in general, may be more responsive than other genes to minor differences in parasite cultivation conditions in different laboratories. In turn, they may also be more responsive to changes in the host environment in vivo, which would be reasonable given their prominent role in the interactions between Toxoplasma and its host (5, 8).
No trans-mapping eQTL were identified in this study. This observation cannot be used to propose that there are no transcription factors in Toxoplasma gondii, since only 20% of the transcriptome was present on the microarrays that we used. Moreover, in at least one study, trans-mapping eQTL were at least 10-fold less frequent than those mapping in cis and were subject to higher false-negative rates than cis-mapping eQTL (21), indicating that their identification may be much more difficult. With only 19 progeny, an expression phenotype due to a trans-acting locus would have to be extremely robust and strictly dichotomous to be mappable.
Overall, these results indicate that strain-specific differences in gene expression are substantial, as first reported more than 20 years ago based on protein analyses (39). The data presented here indicate that strain-specific differences in transcript abundance can have any of a number of different mechanisms as their root cause and that many of these differences may be driven by substantial evolutionary pressures.
Acknowledgments
We thank Michael Behnke and Michael White (Department of Veterinary Molecular Biology, Montana State University) for the firefly luciferase plasmid and the tubulin-driven Renilla plasmid. Preliminary genomic and/or cDNA sequence data and Affymetrix microarray expression data were accessed via ToxoDB.org and/or www.tigr.org/tdb/t_gondii/, and we thank the contributors who generated those data, especially David Roos and Amit Bahl.
Genomic data were provided by The Institute for Genomic Research (supported by NIH grant AI05093) and by the Sanger Center (Wellcome Trust). EST sequences were generated by Washington University (NIH grant 1R01AI045806-01A1). S.Y.H. was supported by the Stanford Summer Research Program. This work was also supported by grants to J.W.A. from the BBSRC and the Wellcome Trust, to J.P.J.S. from the University of California Universitywide AIDS Research Program (F04-ST-216), and to J.P.B. from the NIH (F32AI60306), and by an Ellison Foundation Senior Scholar Award and grants AI21423 and AI73756 from the NIH (to J.C.B.).
Footnotes
Published ahead of print on 13 June 2008.
REFERENCES
- 1.Ajioka, J. W., J. C. Boothroyd, B. P. Brunk, A. Hehl, L. Hillier, I. D. Manger, M. Marra, G. C. Overton, D. S. Roos, K. L. Wan, R. Waterston, and L. D. Sibley. 1998. Gene discovery by EST sequencing in Toxoplasma gondii reveals sequences restricted to the Apicomplexa. Genome Res. 818-28. [DOI] [PubMed] [Google Scholar]
- 2.Aurrecoechea, C., M. Heiges, H. Wang, Z. Wang, S. Fischer, P. Rhodes, J. Miller, E. Kraemer, C. J. Stoeckert, Jr., D. S. Roos, and J. C. Kissinger. 2007. ApiDB: integrated resources for the apicomplexan bioinformatics resource center. Nucleic Acids Res. 35D427-D430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beckers, C. J., T. Wakefield, and K. A. Joiner. 1997. The expression of Toxoplasma proteins in Neospora caninum and the identification of a gene encoding a novel rhoptry protein. Mol. Biochem. Parasitol. 89209-223. [DOI] [PubMed] [Google Scholar]
- 4.Behnke, M. S., J. B. Radke, A. T. Smith, W. J. Sullivan, Jr., and M. W. White. 2008. The transcription of bradyzoite genes in Toxoplasma gondii is controlled by autonomous promoter elements. Mol. Microbiol. 681502-1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boothroyd, J. C., and J. F. Dubremetz. 2008. Kiss and spit: the dual roles of Toxoplasma rhoptries. Nat. Rev. Microbiol. 679-88. [DOI] [PubMed] [Google Scholar]
- 6.Boyle, J. P., B. Rajasekar, J. P. Saeij, J. W. Ajioka, M. Berriman, I. Paulsen, D. S. Roos, L. D. Sibley, M. W. White, and J. C. Boothroyd. 2006. Just one cross appears capable of dramatically altering the population biology of a eukaryotic pathogen like Toxoplasma gondii. Proc. Natl. Acad. Sci. USA 10310514-10519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boyle, J. P., X. J. Wu, C. B. Shoemaker, and T. P. Yoshino. 2003. Using RNA interference to manipulate endogenous gene expression in Schistosoma mansoni sporocysts. Mol. Biochem. Parasitol. 128205-215. [DOI] [PubMed] [Google Scholar]
- 8.Bradley, P. J., and L. D. Sibley. 2007. Rhoptries: an arsenal of secreted virulence factors. Curr. Opin. Microbiol. 10582-587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bradley, P. J., C. Ward, S. J. Cheng, D. L. Alexander, S. Coller, G. H. Coombs, J. D. Dunn, D. J. Ferguson, S. J. Sanderson, J. M. Wastling, and J. C. Boothroyd. 2005. Proteomic analysis of rhoptry organelles reveals many novel constituents for host-parasite interactions in Toxoplasma gondii. J. Biol. Chem. 28034245-34258. [DOI] [PubMed] [Google Scholar]
- 10.Broman, K. W., H. Wu, S. Sen, and G. A. Churchill. 2003. R/qtl: QTL mapping in experimental crosses. Bioinformatics 19889-890. [DOI] [PubMed] [Google Scholar]
- 11.Carruthers, V. B., O. K. Giddings, and L. D. Sibley. 1999. Secretion of micronemal proteins is associated with Toxoplasma invasion of host cells. Cell. Microbiol. 1225-235. [DOI] [PubMed] [Google Scholar]
- 12.Cleary, M. D., C. D. Meiering, E. Jan, R. Guymon, and J. C. Boothroyd. 2005. Biosynthetic labeling of RNA with uracil phosphoribosyltransferase allows cell-specific microarray analysis of mRNA synthesis and decay. Nat. Biotechnol. 23232-237. [DOI] [PubMed] [Google Scholar]
- 13.Cleary, M. D., U. Singh, I. J. Blader, J. L. Brewer, and J. C. Boothroyd. 2002. Toxoplasma gondii asexual development: identification of developmentally regulated genes and distinct patterns of gene expression. Eukaryot. Cell 1329-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coppens, I., J. D. Dunn, J. D. Romano, M. Pypaert, H. Zhang, J. C. Boothroyd, and K. A. Joiner. 2006. Toxoplasma gondii sequesters lysosomes from mammalian hosts in the vacuolar space. Cell 125261-274. [DOI] [PubMed] [Google Scholar]
- 15.Dzierszinski, F., M. Nishi, L. Ouko, and D. S. Roos. 2004. Dynamics of Toxoplasma gondii differentiation. Eukaryot. Cell 3992-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.El Hajj, H., E. Demey, J. Poncet, M. Lebrun, B. Wu, N. Galeotti, M. N. Fourmaux, O. Mercereau-Puijalon, H. Vial, G. Labesse, and J. F. Dubremetz. 2006. The ROP2 family of Toxoplasma gondii rhoptry proteins: proteomic and genomic characterization and molecular modeling. Proteomics 65773-5784. [DOI] [PubMed] [Google Scholar]
- 17.El Hajj, H., M. Lebrun, S. T. Arold, H. Vial, G. Labesse, and J. F. Dubremetz. 2007. ROP18 is a rhoptry kinase controlling the intracellular proliferation of Toxoplasma gondii. PLoS Pathog. 3e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Emanuelsson, O., S. Brunak, G. von Heijne, and H. Nielsen. 2007. Locating proteins in the cell using TargetP, SignalP and related tools. Nat. Protoc. 2953-971. [DOI] [PubMed] [Google Scholar]
- 19.Gilbert, L. A., S. Ravindran, J. M. Turetzky, J. C. Boothroyd, and P. J. Bradley. 2007. Toxoplasma gondii targets a protein phosphatase 2C to the nuclei of infected host cells. Eukaryot. Cell 673-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gissot, M., K. A. Kelly, J. W. Ajioka, J. M. Greally, and K. Kim. 2007. Epigenomic modifications predict active promoters and gene structure in Toxoplasma gondii. PLoS Pathog. 3e77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goring, H. H., J. E. Curran, M. P. Johnson, T. D. Dyer, J. Charlesworth, S. A. Cole, J. B. Jowett, L. J. Abraham, D. L. Rainwater, A. G. Comuzzie, M. C. Mahaney, L. Almasy, J. W. MacCluer, A. H. Kissebah, G. R. Collier, E. K. Moses, and J. Blangero. 2007. Discovery of expression QTLs using large-scale transcriptional profiling in human lymphocytes. Nat. Genet. 391208-1216. [DOI] [PubMed] [Google Scholar]
- 22.Grigg, M. E., J. Ganatra, J. C. Boothroyd, and T. P. Margolis. 2001. Unusual abundance of atypical strains associated with human ocular toxoplasmosis. J. Infect. Dis. 184633-639. [DOI] [PubMed] [Google Scholar]
- 23.Howe, D. K., and L. D. Sibley. 1995. Toxoplasma gondii comprises three clonal lineages: correlation of parasite genotype with human disease. J. Infect. Dis. 1721561-1566. [DOI] [PubMed] [Google Scholar]
- 24.Khan, A., S. Taylor, C. Su, A. J. Mackey, J. Boyle, R. Cole, D. Glover, K. Tang, I. T. Paulsen, M. Berriman, J. C. Boothroyd, E. R. Pfefferkorn, J. P. Dubey, J. W. Ajioka, D. S. Roos, J. C. Wootton, and L. D. Sibley. 2005. Composite genome map and recombination parameters derived from three archetypal lineages of Toxoplasma gondii. Nucleic Acids Res. 332980-2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li, L., J. Crabtree, S. Fischer, D. Pinney, C. J. Stoeckert, Jr., L. D. Sibley, and D. S. Roos. 2004. ApiEST-DB: analyzing clustered EST data of the apicomplexan parasites. Nucleic Acids Res. 32D326-D328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Livak, K. J., and T. D. Schmittgen. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods (Duluth) 25402-408. [DOI] [PubMed] [Google Scholar]
- 27.Mercier, C., S. Lefebvre-Van Hende, G. E. Garber, L. Lecordier, A. Capron, and M. F. Cesbron-Delauw. 1996. Common cis-acting elements critical for the expression of several genes of Toxoplasma gondii. Mol. Microbiol. 21421-428. [DOI] [PubMed] [Google Scholar]
- 28.Poupel, O., H. Boleti, S. Axisa, E. Couture-Tosi, and I. Tardieux. 2000. Toxofilin, a novel actin-binding protein from Toxoplasma gondii, sequesters actin monomers and caps actin filaments. Mol. Biol. Cell 11355-368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Radke, J. R., M. J. Gubbels, M. E. Jerome, J. B. Radke, B. Striepen, and M. W. White. 2004. Identification of a sporozoite-specific member of the Toxoplasma SAG superfamily via genetic complementation. Mol. Microbiol. 5293-105. [DOI] [PubMed] [Google Scholar]
- 30.Saeij, J. P., J. P. Boyle, S. Coller, S. Taylor, L. D. Sibley, E. T. Brooke-Powell, J. W. Ajioka, and J. C. Boothroyd. 2006. Polymorphic secreted kinases are key virulence factors in toxoplasmosis. Science 3141780-1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saeij, J. P., S. Coller, J. P. Boyle, M. E. Jerome, M. W. White, and J. C. Boothroyd. 2007. Toxoplasma co-opts host gene expression by injection of a polymorphic kinase homologue. Nature 445324-327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saksouk, N., M. M. Bhatti, S. Kieffer, A. T. Smith, K. Musset, J. Garin, W. J. Sullivan, Jr., M. F. Cesbron-Delauw, and M. A. Hakimi. 2005. Histone-modifying complexes regulate gene expression pertinent to the differentiation of the protozoan parasite Toxoplasma gondii. Mol. Cell. Biol. 2510301-10314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sibley, L. D., and J. C. Boothroyd. 1992. Virulent strains of Toxoplasma gondii comprise a single clonal lineage. Nature 35982-85. [DOI] [PubMed] [Google Scholar]
- 34.Sibley, L. D., A. J. LeBlanc, E. R. Pfefferkorn, and J. C. Boothroyd. 1992. Generation of a restriction fragment length polymorphism linkage map for Toxoplasma gondii. Genetics 1321003-1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Singh, U., J. L. Brewer, and J. C. Boothroyd. 2002. Genetic analysis of tachyzoite to bradyzoite differentiation mutants in Toxoplasma gondii reveals a hierarchy of gene induction. Mol. Microbiol. 44721-733. [DOI] [PubMed] [Google Scholar]
- 36.Soldati, D., and J. C. Boothroyd. 1995. A selector of transcription initiation in the protozoan parasite Toxoplasma gondii. Mol. Cell. Biol. 1587-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Taylor, S., A. Barragan, C. Su, B. Fux, S. J. Fentress, K. Tang, W. L. Beatty, H. E. Hajj, M. Jerome, M. S. Behnke, M. White, J. C. Wootton, and L. D. Sibley. 2006. A secreted serine-threonine kinase determines virulence in the eukaryotic pathogen Toxoplasma gondii. Science 3141776-1780. [DOI] [PubMed] [Google Scholar]
- 38.Trujillo, M., R. G. Donald, D. S. Roos, P. J. Greene, and D. V. Santi. 1996. Heterologous expression and characterization of the bifunctional dihydrofolate reductase-thymidylate synthase enzyme of Toxoplasma gondii. Biochemistry 356366-6374. [DOI] [PubMed] [Google Scholar]
- 39.Ware, P. L., and L. H. Kasper. 1987. Strain-specific antigens of Toxoplasma gondii. Infect. Immun. 55778-783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Woo, Y., J. Affourtit, S. Daigle, A. Viale, K. Johnson, J. Naggert, and G. Churchill. 2004. A comparison of cDNA, oligonucleotide, and Affymetrix GeneChip gene expression microarray platforms. J. Biomol. Tech. 15276-284. [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou, X. W., B. F. Kafsack, R. N. Cole, P. Beckett, R. F. Shen, and V. B. Carruthers. 2005. The opportunistic pathogen Toxoplasma gondii deploys a diverse legion of invasion and survival proteins. J. Biol. Chem. 28034233-34244. [DOI] [PMC free article] [PubMed] [Google Scholar]