Abstract
The twin-Cx9C motif protein Pet191 is essential for cytochrome c oxidase maturation. The motif Cys residues are functionally important and appear to be present in disulfide linkages within a large oligomeric complex associated with the mitochondrial inner membrane. The import of Pet191 differs from that of other twin-Cx9C motif class of proteins in being independent of the Mia40 pathway.
Cytochrome c oxidase (CcO), the terminal enzyme of the respiratory chain in mitochondria, consists of 12 or 13 subunits, with the 3 core enzyme subunits (Cox1 to Cox3) being encoded by the mitochondrial genome (3). The assembly of CcO requires a myriad of steps, including the insertion of heme a and copper cofactors. Copper insertion into newly synthesized Cox1 and Cox2 chains occurs on the intermembrane space (IMS) side of the inner membrane (IM), as the accessory molecules are localized within this compartment (10). Two proteins, Cox11 and Sco1, are associated with the IM and mediate the copper metallation of the CuB and CuA sites in Cox1 and Cox2, respectively (7, 18). Cu(I) ions transiently bound by Cox11 and Sco1 are provided by Cox17 within the IMS (12). Cox17 contains a twin-Cx9C structural motif that adopts a helical hairpin conformation, stabilized by two disulfide bonds with a single Cu(I) ion bound by vicinal Cys residues outside the twin-Cx9C motif (1, 2, 5). Two other IMS proteins, Cox19 and Cox23, are structurally related to Cox17 in containing a twin-Cx9C structural motif and function in an undefined step in CcO assembly (19, 21).
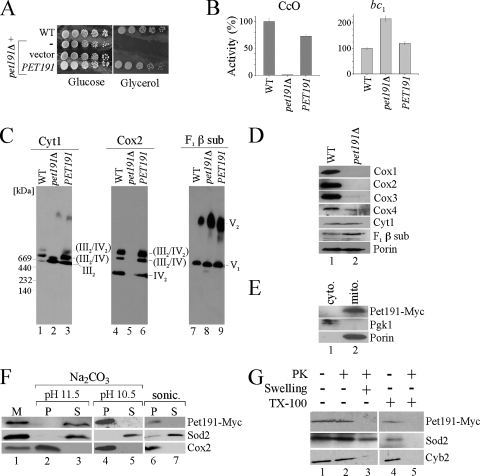
The uncharacterized Pet191 protein is a variant of the twin-Cx9C motif family. The conservation of the twin-Cx9C motif in Pet191 (see Fig. S1 in the supplemental material) and its importance in respiration motivated us to investigate the role of Pet191 in CcO assembly (15). Saccharomyces cerevisiae strains lacking Pet191 are known to be deficient on respiration and fail to propagate on growth medium containing glycerol as the sole carbon source (15) (Fig. 1A). The mutant cells are rho+, as a 3′ Myc-tagged PET191 gene can restore respiratory function. CcO activity was absent from cells cultured on glucose or raffinose, but succinate cytochrome c reductase activity was elevated above wild-type (WT) levels (Fig. 1B). Complex III activity is elevated in certain CcO assembly mutants (14). The specific defect in CcO was demonstrated by blue native polyacrylamide gel electrophoresis (BN-PAGE), as dimeric complex III and both monomeric and dimeric complex V species were observed in pet191Δ cells (Fig. 1C). Complex IV was absent, as visualized by Cox2 immunoblotting. Steady-state levels of Cox1, Cox2, and Cox3 were undetectable in pet191Δ cells (Fig. 1D). The diminution in Cox1 to Cox3 protein levels in pet191Δ cells arises from the impaired stability of CcO, since mitochondrial translation is comparable to what is seen for other CcO assembly mutants (4).
FIG. 1.
Pet191 is required for CcO biogenesis. (A) Serial dilutions of pet191Δ cells transformed with an empty vector or PET191 were plated onto complete medium containing 2% glucose or 2% lactate/glycerol as a carbon source. The WT and pet191Δ diploid strains of Saccharomyces cerevisiae (BY4743) were used. (B) CcO and SDH/bc1 activities. Activities of the WT mitochondria were set as 100%. Mitochondria were isolated from cells cultured on raffinose medium. (C) Mitochondria were solubilized in a lysis buffer containing 1.5% digitonin and protein complexes were separated by BN-PAGE. The respiratory chain complexes (III, IV, and V) were analyzed by immunoblotting with respective antibodies. sub, subunit. (D) Steady-state levels of mitochondrial proteins were analyzed by sodium dodecyl sulfate (SDS)-PAGE followed by Western blotting. (E) Cells expressing Pet191-Myc were fractionated, and cytosolic (cyto.) and mitochondrial (mito.) fractions were collected, resolved by SDS-PAGE, and analyzed by immunoblotting with the anti-Myc, anti-Pgk1, and anti-porin antibodies. The Pet191-Myc was expressed on a pRS426 vector under the MET25 promoter and the CYC1 terminator (17). (F) Mitochondria of a strain expressing Pet191-Myc (M; lane 1) were treated with either 0.1 M Na2CO3 (pH 10.5 or 11.5) or sonicated (sonic.) and fractionated by high-speed centrifugation. Obtained pellet (P; lanes 2, 4, and 6) and supernatant (S; lanes 3, 5, and 7) fractions were analyzed by immunoblotting using antibodies against the Myc epitope, matrix protein Sod2, and IM-anchored protein Cox2. (G) Intact (lanes 1, 2, and 4) or swollen (lane 3) mitochondria were incubated with (lanes 2, 3, and 5) or without (lanes 1 and 4) proteinase K (PK). In lanes 4 and 5, mitochondria were lysed with 1% Triton X-100 (TX-100) prior to incubation with PK. Western blot analysis was performed using antibodies specific for the Myc tag, matrix protein Sod2, and IMS protein Cyb2.
Whereas the twin-Cx9C protein Cox17 has a role in copper metallation of CcO during biogenesis, Pet191 does not appear to have a prominent role in this process. The addition of supplemented copper salts to the growth medium of pet191Δ cells does not reverse the respiratory function-deficient phenotype, as occurs with cox17Δ cells. Copper ions used in the metallation of CcO and Sod1 in the IMS derive from the matrix copper-ligand complex (9). Cells lacking Pet191 have normal mitochondrial copper levels and normal Sod1 activity in mitochondria, suggesting that Pet191 does not perturb mitochondrial copper metallation processes or Sod1 activation within the IMS.
Immunoblotting of Myc-tagged Pet191 revealed that it localizes to the mitochondria (Fig. 1E) and was tightly associated with a membrane (Fig. 1F). Pet191 was not solubilized by sonication of the mitoplasts and was not released from the IM by sodium carbonate extraction at pH 10.5. However, at pH 11.5, sodium carbonate buffer was sufficient to solubilize Pet191. Pet191 remained associated with mitoplasts after hypotonic swelling but was degraded with the addition of proteinase K (Fig. 1G). The release of the IMS Cyb2, but not Pet191, upon hypotonic swelling suggested that Pet191 is not a soluble IMS protein. Thus, Pet191 is tightly associated with the IM facing the IMS side of the membrane.
Chromosomally HA-tagged Pet191 solubilized in digitonin migrated on BN-PAGE gels as a complex of approximately 500 kDa (Fig. 2A). Deoxycholate (DOC)-solubilized Pet191-Myc eluted upon size permeation chromatography at a volume corresponding to approximately 530 kDa (Fig. 2C). However, extraction of Pet191 with 0.1% DOC in the presence of 100 mM dithiothreitol (DTT) resulted in elution of Pet191 at a volume closer to the predicted monomeric mass (Fig. 2C). These results are consistent with Pet191 existing in an oxidized conformer in mitochondria. CcO-deficient cells contain a more reducing IMS, as assessed by the Mia40 redox state (6). To determine whether the Pet191 oligomer was sensitive to perturbations in the redox state of the IMS, BN-PAGE analysis was carried out on Pet191-HA in respiratory function-deficient cox11Δ cells cultured in raffinose. The Pet191 oligomer persisted, albeit at lower levels in cox11Δ cells (Fig. 2B).
FIG. 2.
Molecular size of Pet191. (A) Mitochondria isolated from WT cells and the strain containing genomically tagged Pet191 (carrying PET191::3HA) were solubilized and subjected to BN-PAGE followed by immunoblot analysis with an antibody against the HA epitope. A genomically HA-tagged variant of PET191 was generated by homologous recombination, inserting the triple HA tag 3′ to the open reading frame. (B) Mitochondria prepared from WT and cox11Δ cells carrying PET191::3HA were analyzed by BN-PAGE as described previously (25) using anti-HA and porin antibodies. (C) Cells expressing Pet191-Myc or its mutant form (C5A) were used for mitochondrial preparation. Isolated mitochondria were lysed in a buffer containing 0.1% DOC in the presence (+) or absence (−) of 100 mM DTT, and clarified lysates were loaded onto the size exclusion column (10/30 G-200; Superdex) equilibrated with buffer containing 0.05% DOC ± 5 mM DTT. Fractions were directly assayed by slot immunoblotting with anti-Myc antibodies. Column void (VE) and internal (VI) volumes are marked by arrows.
Mutational analysis of Pet191 was carried out to assess whether the cysteine residues are functionally important. Cysteinyl residues within the twin-Cx9C motif as well as the linker motif were singly substituted with alanine residues (Fig. 3B). Mutant alleles of PET191 were transformed into pet191Δ cells and tested for their ability to support growth on glycerol-containing medium. Cells harboring C5A and C56A mutant alleles were respiratory function deficient, whereas three additional alleles, the C15A, C32A, and C46A alleles, were partially compromised in growth at 30°C or 37°C (Fig. 3A). If Pet191 folds in a helical hairpin in a manner analogous to that seen for Cox17 or Cox12, then Cys5 and Cys56 may be an aligned pair existing as a disulfide bridge (Fig. 3B). All mutant proteins were equivalently expressed, as shown by immunoblot analysis (Fig. 3C).
FIG. 3.
Conserved cysteine residues are important for Pet191 function. (A) Serial dilutions of pet191Δ cells transformed with an empty vector, either PET191 or one of its mutant forms, were plated onto complete medium containing 2% glucose or 2% lactate/glycerol as a carbon source and grown for 4 days at 30°C or 37°C. Mutant forms of Pet191 (the C5A, C15A, C21A, C32A, C46A, C56A, and C86A mutants) were generated by site-directed mutagenesis using pRS426-PET191-Myc as a template. (B). Schematic demonstrating possible conformation of the Pet191 molecule where two Cx9C motifs face each other. Positions of the most critical cysteine residues are depicted by light gray circles. Other Cys residues are shown in black. (C) Mitochondria isolated from pet191Δ cells carrying an empty vector, PET191, or its mutant forms were subjected to SDS-PAGE followed by Western blot analysis with the antibodies against the Myc epitope and porin. (D) WT cells overexpressing PET191 or its C5A, C15A, or C56A mutant versions were serially diluted, plated as described for panel A, and grown for 3 days at 30°C. (E) Mitochondria from the cells coexpressing Pet191-HA and Pet191-Myc or its C5A mutant form were solubilized and analyzed as described above, except that DTT treatment was not included. Collected fractions were analyzed using anti-Myc and anti-HA antibodies. (F) Mitochondria isolated from the WT PET191::3HA strain with (lanes 1 to 3) or without (lanes 4 to 6) Pet191-Myc were lysed, and clarified extracts were immunoprecipitated with agarose-coupled anti-Myc antibodies. Co-IP of Pet191-Myc and Pet191-HA was performed as described previously (20), using anti-Myc-agarose-coupled beads (Santa Cruz Biotechnology). The load (representing 5% of the extracts; lanes 1 and 4), entire-wash (lanes 2 and 5), and eluate (lanes 3 and 6) fractions were analyzed by immunoblotting.
Transformation of WT cells with the mutant PET191 alleles revealed that the presence of either C5A or C56A Pet191 had a slight dominant negative effect on respiratory growth (Fig. 3D). In contrast, the C15A mutant, which was only weakly compromised in supporting glycerol growth of pet191Δ cells, lacked any negative effects on the growth of WT cells on glycerol medium. The C5A mutant protein existed in a complex that was small relative to that for the WT protein, as determined by gel filtration (Fig. 3E). Thus, the nonfunctionality of the C5A protein may result from an abnormal Pet191 complex. To determine whether the dominant negative effect of C5A Pet191 influenced the endogenous Pet191, we carried out gel filtration studies on WT cells harboring the C5A Pet191 mutant. The presence of C5A Pet191 in WT cells led to an attenuation in the size of the solubilized WT protein (Fig. 3E).
The dominant negative effect of the mutant Pet191 on the WT protein suggested that the two proteins interact. This was confirmed by immunoprecipitation (IP) studies. Mitochondria isolated from cells harboring a vector encoded Pet191-Myc and chromosomal Pet191-HA were used for IP with anti-Myc beads. Pet191-HA exhibited co-IP with Pet191-Myc (Fig. 3F). Thus, Pet191 complex is a homo-oligomer, but the large size of the complex, ∼500 kDa, may suggest that additional proteins are present.
Two of the six conserved Cys residues (Cys5 and Cys56) important for Pet191 function may participate in the disulfide stabilization of the complex, since the C5A Pet191 allele product fails to assemble into the WT complex. Structures of three twin-Cx9C motif proteins, Cox12, Cox17, and Qcr6, reveal disulfide-bonded helical hairpin conformations. Cox12 and Qcr6 are IMS-facing subunits of the CcO and bc1 complexes, respectively. In S. cerevisiae, only one of the two Cys pairs exists in Qcr6. If Pet191 adopts a related helical hairpin conformation, the functionally important Cys residues Cys5 and Cys56 may form a disulfide pair.
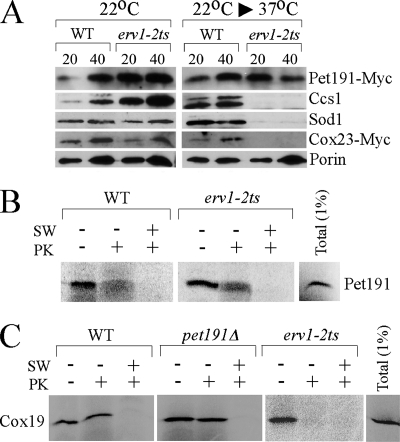
Twin-Cx9C motif proteins like Cox17 are imported into the IMS by the MIA import pathway through an oxidative folding mechanism involving Mia40 and Erv1 (8, 16, 22, 24). Since Pet191 has a related twin-Cx9C motif, we addressed if Pet191 was imported through the MIA pathway. Temperature-sensitive erv1-2ts mutant (13) cells cultured at 22°C import IMS proteins normally, but import is attenuated upon a shift of cells to the nonpermissive temperature (16, 22). We observed that erv1-2ts cells cultured at 22°C have normal levels of Sod1, Ccs1, Cox23, and Pet191 within the mitochondria (Fig. 4A). However, cells shifted to 37°C have attenuated levels of Sod1, Ccs1, and Cox23 but not Pet191-Myc, suggesting that Pet191 is imported in a MIA-independent pathway. The attenuated levels of Sod1, Ccs1, and Cox23 are consistent with their dependency on the MIA complex for IMS import. An independent assessment of the role of Erv1 in Pet191 uptake was conducted using in vitro mitochondrial import of Pet191 translated in a rabbit reticulocyte lysate. Mitochondria were isolated from WT or erv1-2ts cells and tested for 35S-labeled Pet191 import. After treatment with proteinase K, Pet191 was observed in both WT and erv1-2ts mitochondria incubated at the nonpermissive temperature, at which Erv1 is inactive (Fig. 4B). Whereas the import of Pet191 into erv1-2ts mitochondria was normal, the import of radiolabeled Cox19, a known MIA substrate, was impaired in the respective mutant. The import of Cox19 was also normal in mitochondria isolated from pet191Δ cells (Fig. 4C). Thus, we conclude that Pet191 is imported into the mitochondria independent of Mia40/Erv1. The actual mechanism of Pet191 import is unclear, as it lacks an N-terminal mitochondrial import motif, as deduced by either the MITOPROT or the PSORT algorithm.
FIG. 4.
erv1 mutant mitochondria have normal levels of Pet191-Myc. (A) WT and temperature-sensitive erv1-2ts strains expressing Pet191-Myc or Cox23-Myc were grown at 22°C. The temperature-sensitive strain erv1-2ts (13) and the corresponding WT strain were a kind gift from Roland Lill. For isolation of mitochondria from erv1-2ts cells, the cells were pregrown in supplemented synthetic medium containing 2% raffinose at 22°C until the early logarithmic phase. Cultures were split into two halves, one of which was subjected to a temperature shift (22°C to 37°C), while the other was left at 22°C. After 7 to 10 h of incubation, cells were harvested and mitochondria isolated as described above. Mitochondria were isolated, separated by SDS-PAGE, and analyzed by immunoblotting with antibodies recognizing Myc-tag, Ccs1, Sod1, and porin. (B) Pet191 was synthesized in rabbit reticulocyte lysate in the presence of [35S]methionine and incubated for 20 min at 30°C with mitochondria isolated from either WT or erv1-2ts cells. After incubation, mitochondria were reisolated, washed, and either swollen in hypotonic medium (SW) or incubated with proteinase K (PK). Samples were then subjected to SDS-PAGE followed by autoradiography. (C) Radiolabeled Cox19 was imported into the WT, pet191Δ, and erv1-2ts mitochondria as described above.
Pet191 joins the list of twin-Cx9C motif proteins that are involved in CcO biogenesis, i.e., Cox17, Cox19, and Cox23. A series of other twin-Cx9C proteins whose functions are unknown exist within the IMS. These proteins include Mic14 and Mic17 (11). Although Mic14 contains a duplicated twin-Cx9C structural motif, it has no role in CcO biogenesis. Cells lacking Mic14 show no growth defect on glycerol/lactate medium, consume oxygen at WT levels, and have normal CcO activity. Thus, only a subset of soluble twin-Cx9C proteins in the IMS have roles in CcO biogenesis.
The conservation of Pet191 in mammalian cells suggests that Pet191 may have a significant role in mammalian mitochondria. Functional studies on the human Pet191 ortholog have not appeared, nor have human mutations in PET191 been identified for patients with CcO deficiency (23).
Supplementary Material
Acknowledgments
This work was supported by grant ES 03817 from the National Institutes of Environmental Health Sciences, NIH, to D.R.W.
We acknowledge the support of the CEMH core facility for fast-protein liquid chromatography (DK P30 072437). We acknowledge the assistance of Nataliya Zahayko. This paper is dedicated to the memory of Volodymyr P. Khalimonchuk.
Footnotes
Published ahead of print on 23 May 2008.
Supplemental material for this article may be found at http://ec.asm.org/.
REFERENCES
- 1.Abajian, C., L. A. Yatsunyk, B. E. Ramirez, and A. C. Rosenzweig. 2004. Yeast Cox17 solution structure and copper(I) binding. J. Biol. Chem. 27953584-53592. [DOI] [PubMed] [Google Scholar]
- 2.Arnesano, F., E. Balatri, L. Banci, I. Bertini, and D. R. Winge. 2005. Folding studies of Cox17 reveal an important interplay of cysteine oxidase and copper binding. Structure 13713-722. [DOI] [PubMed] [Google Scholar]
- 3.Barrientos, A., M. H. Barros, I. Valnot, A. Rotig, P. Rustin, and A. Tzagoloff. 2002. Cytochrome oxidase in health and disease. Gene 28653-63. [DOI] [PubMed] [Google Scholar]
- 4.Barrientos, A., A. Zambrano, and A. Tzagoloff. 2004. Mss51p and Cox14p jointly regulate mitochondrial Cox1p expression in Saccharomyces cerevisiae. EMBO J. 233472-3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barros, M. H., A. Johnson, and A. Tzagoloff. 2004. Cox23, a homologue of COX17, is required for cytochrome oxidase assembly. J. Biol. Chem. 27931943-31947. [DOI] [PubMed] [Google Scholar]
- 6.Bihlmaier, K., N. Mesecke, N. Terziyska, M. Bien, K. Hell, and J. M. Herrmann. 2007. The disulfide relay system of mitochondria is connected to the respiratory chain. J. Cell Biol. 179389-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carr, H. S., G. N. George, and D. R. Winge. 2002. Yeast Cox11, a protein essential for cytochrome c oxidase assembly, is a Cu(I) binding protein. J. Biol. Chem. 27731237-31242. [DOI] [PubMed] [Google Scholar]
- 8.Chacinska, A., S. Pfannschmidt, N. Wiedemann, V. Kozjak, L. K. Sanjuan Szklarz, A. Schulze-Specking, K. N. Truscott, B. Guiard, C. Meisinger, and N. Pfanner. 2004. Essential role of Mia40 in import and assembly of mitochondrial intermembrane space proteins. EMBO J. 233735-3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cobine, P. A., F. Pierrel, M. L. Bestwick, and D. R. Winge. 2006. Mitochondrial matrix copper complex used in metallation of cytochrome oxidase and superoxide dismutase. J. Biol. Chem. 28136552-36559. [DOI] [PubMed] [Google Scholar]
- 10.Cobine, P. A., F. Pierrel, and D. R. Winge. 2006. Copper trafficking to the mitochondrion and assembly of copper metalloenzymes. Biochim. Biophys. Acta 1763759-772. [DOI] [PubMed] [Google Scholar]
- 11.Gabriel, K., D. Milenkovic, A. Chacinska, J. Muller, B. Guiard, N. Pfanner, and C. Meisinger. 2007. Novel mitochondrial intermembrane space proteins as substrates of the MIA import pathway. J. Mol. Biol. 365612-620. [DOI] [PubMed] [Google Scholar]
- 12.Horng, Y. C., P. A. Cobine, A. B. Maxfield, H. S. Carr, and D. R. Winge. 2004. Specific copper transfer from the Cox17 metallochaperone to both Sco1 and Cox11 in the assembly of yeast cytochrome C oxidase. J. Biol. Chem. 27935334-35340. [DOI] [PubMed] [Google Scholar]
- 13.Lange, H., T. Lisowsky, J. Gerber, U. Muhlenhoff, G. Kispal, and R. Lill. 2001. An essential function of the mitochondrial sulfhydryl oxidase Erv1p/ALR in the maturation of cytosolic Fe/S proteins. EMBO Rep. 2715-720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mashkevich, G., B. Repetto, D. M. Glerum, C. Jin, and A. Tzagoloff. 1997. SHY1, the yeast homolog of the mammalian SURF-1 gene, encodes a mitochondrial protein required for respiration. J. Biol. Chem. 27214356-14364. [DOI] [PubMed] [Google Scholar]
- 15.McEwen, J. E., K. H. Hong, S. Park, and G. T. Preciado. 1993. Sequence and chromosomal localization of two PET genes required for cytochrome c oxidase assembly in Saccharomyces cerevisiae. Curr. Genet. 239-14. [DOI] [PubMed] [Google Scholar]
- 16.Mesecke, N., N. Terziyska, C. Kozany, F. Baumann, W. Neupert, K. Hell, and J. M. Herrmann. 2005. A disulfide relay system in the intermembrane space of mitochondria that mediates protein import. Cell 1211059-1069. [DOI] [PubMed] [Google Scholar]
- 17.Mumberg, D., R. Muller, and M. Funk. 1994. Regulatable promoters of Saccharomyces cerevisiae: comparison of transcriptional activity and their use of heterologous expression. Nucleic Acids Res. 225767-5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nittis, T., G. N. George, and D. R. Winge. 2001. Yeast Sco1, a protein essential for cytochrome c oxidase function is a Cu(I)-binding protein. J. Biol. Chem. 27642520-42526. [DOI] [PubMed] [Google Scholar]
- 19.Nobrega, M. P., S. C. B. Bandeira, J. Beers, and A. Tzagoloff. 2002. Characterization of COX19, a widely distributed gene required for expression of mitochondrial cytochrome c oxidase. J. Biol. Chem. 27740206-40211. [DOI] [PubMed] [Google Scholar]
- 20.Pierrel, F., M. L. Bestwick, P. A. Cobine, O. Khalimonchuk, J. A. Cricco, and D. R. Winge. 2007. Coa1 links the Mss51 post-translational function to Cox1 cofactor insertion in cytochrome c oxidase assembly. EMBO J. 264335-4346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rigby, K., L. Zhang, P. A. Cobine, G. N. George, and D. R. Winge. 2007. Characterization of the cytochrome c oxidase assembly factor Cox19 of Saccharomyces cerevisiae. J. Biol. Chem. 28210233-10242. [DOI] [PubMed] [Google Scholar]
- 22.Rissler, M., N. Wiedemann, S. Pfannschmidt, K. Gabriel, B. Guiard, N. Pfanner, and A. Chacinska. 2005. The essential mitochondrial protein Erv1 cooperates with Mia40 in biogenesis of intermembrane proteins. J. Mol. Biol. 353485-492. [DOI] [PubMed] [Google Scholar]
- 23.Tay, S. K. H., C. Nesti, M. Mancuso, E. A. Schon, S. Shanske, E. Bonilla, M. M. Davidson, and S. DiMauro. 2004. Studies of COX16, COX19 and PET191 in human cytochrome c oxidase deficiency. Arch. Neurol. 611935-1937. [DOI] [PubMed] [Google Scholar]
- 24.Terziyska, N., T. Lutz, C. Kozany, D. Mokranjac, N. Mesecke, W. Neupert, J. M. Herrmann, and K. Hell. 2005. Mia40, a novel factor for protein import into the intermembrane space of mitochondria is able to bind metal ions. FEBS Lett. 579179-184. [DOI] [PubMed] [Google Scholar]
- 25.Wittig, I., H. P. Braun, and H. Schagger. 2006. Blue native PAGE. Nat. Protoc. 1418-428. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.