Abstract
The adaptor protein-1 (AP-1) complex is involved in membrane transport between the Golgi apparatus and endosomes. In the protozoan parasite Leishmania mexicana mexicana, the AP-1 μ1 and σ1 subunits are not required for growth at 27°C but are essential for infectivity in the mammalian host. In this study, we have investigated the function of these AP-1 subunits in order to understand the molecular basis for this loss of virulence. The μ1 and σ1 subunits were localized to late Golgi and endosome membranes of the major parasite stages. Parasite mutants lacking either AP-1 subunit lacked obvious defects in Golgi structure, endocytosis, or exocytic transport. However, these mutants displayed reduced rates of endosome-to-lysosome transport and accumulated fragmented, sterol-rich lysosomes. Defects in flagellum biogenesis were also evident in nondividing promastigote stages, and this phenotype was exacerbated by inhibitors of sterol and sphingolipid biosynthesis. Furthermore, both AP-1 mutants were hypersensitive to elevated temperature and perturbations in membrane lipid composition. The pleiotropic requirements for AP-1 in membrane trafficking and temperature stress responses explain the loss of virulence of these mutants in the mammalian host.
Members of the genus Leishmania are sand fly-transmitted parasitic protozoa that can cause localized cutaneous lesions or disseminating mucocutaneous and lethal visceral disease in humans. There have been an estimated 12 million cases of leishmaniasis worldwide, resulting in more than 60,000 deaths (11). There are currently no vaccines against leishmaniasis, and current drug therapies (pentavalent antimonials, miltefosine, amphotericin B, and pentamidine) are inadequate because of high toxicity, lack of efficacy, expense, and the emergence of antimonial-resistant parasites (11). Infection of the mammalian host is initiated by flagellated Leishmania promastigotes that develop within the midgut of the female sand fly vector. Infective promastigotes are phagocytosed by macrophages and differentiate to small aflagellate amastigotes in an acidified phagolysosome compartment. Amastigotes are proliferative-stage organisms and can spread to other macrophages during host cell division or following host cell lysis, thus perpetuating the diseased state. For many species of Leishmania, promastigote differentiation to amastigotes can be induced by exposure to the elevated temperatures (33°C to 37°C) and low pH encountered in the macrophage phagolysosome (61). Leishmania adaptation to elevated temperature and differentiation is clearly associated with a robust heat shock response (58). However, there is increasing evidence that other metabolic processes may also contribute to the thermotolerance of these parasites and their capacity to adapt to conditions in the mammalian host (10, 34, 46).
A number of recent studies have highlighted the importance of endosome-lysosome function in parasite differentiation and virulence in macrophages and susceptible strains of mice (5). Leishmania mutants lacking lysosomal cysteine proteases (59) or ATG4, a component of the autophagosome assembly pathway (6), display defects in promastigote-amastigote differentiation and attenuated virulence in animal models. Similarly, overexpression of a dominant negative form of VPS4, an ATPase involved in endosomal transport, also prevented normal parasite differentiation (6). Endosome trafficking and lysosome function may be required for autophagic protein turnover during promastigote-amastigote differentiation (5), as well as the degradation of exogenous macromolecules and nutrient scavenging in the macrophage phagolysosome (40).
Another group of proteins that play key roles in regulating endocytic membrane transport in eukaryotic cells are the heterotetrameric adaptor protein (AP) complexes. Eukaryotic cells may contain up to four AP complexes that each comprises two large subunits (α, β, γ), a medium μ-subunit, and a small σ-subunit. AP-1 (comprising β1/γ1/μ1/σ1 subunits) is expressed in all eukaryotic cells and may have pleiotropic functions (8). In animals, the AP-1 adaptor complex is required for the assembly of clathrin-coated vesicles on the trans-Golgi network (TGN) (9, 48) and the bidirectional transport of the mannose-6-phosphate receptor and lysosomal membrane proteins between the TGN and endosomes (14, 36, 41). Specific isoforms of AP-1 may also target proteins from TGN/endosomes to the basolateral plasma membranes of polarized epithelial cells (16). In Saccharomyces cerevisiae, AP-1 is thought to regulate endosome-to-TGN protein transport. However, trafficking defects are observed only when the function of either clathrin or other coat proteins is also disrupted (17, 22). In contrast, deletion of the AP-1 μ1 adaptin subunit in Schizosaccharomyces pombe has pleiotropic effects on protein secretion, vacuole fusion, and cytokinesis (28). In the slime mold Dictyostelium discoideum, AP-1 is required for normal growth and protein targeting to conventional lysosomes (31), while in several other protists, this complex is involved in targeting proteins to specialized lysosome compartments (42, 54). These studies suggest that AP-1 complexes can fulfill a variety of functions in the eukaryotic endocytic pathway and that in some cases redundancy with other vesicle coat complexes can occur, leading to a wide variety of phenotypes in various AP-1 knockout organisms.
Recent studies have shown that AP-1 is essential for the growth of the major developmental stages of Trypanosoma brucei (1). RNA interference knockdown of AP-1 subunits leads to pleiotropic effects in endosomal membrane traffic and cell division, although protein transport to the lysosome is apparently not affected (1, 44). In contrast, genetic deletion of AP-1 μ1 or σ1 subunits in Leishmania mexicana mexicana has little effect on the growth of promastigote stages in rich medium (19). However, these mutants are highly attenuated in ex vivo macrophage infection studies and in vivo mice infections (19). The function of this AP-1 complex in Leishmania has not yet been defined, and the basis for the loss of infectivity remains unclear. In this study, we have localized the Leishmania AP-1 complex subunits to the endosome and trans-Golgi apparatus. We show that the Leishmania AP-1 mutants have no apparent defect in endocytosis, exocytosis, or Golgi structure. However, they display clear defects in lysosome transport, flagellar biogenesis, and lipid homeostasis. Significantly, these mutants are highly sensitive to elevated temperatures encountered in the mammalian host and are unable to differentiate to mammal-infective amastigote stages. This study highlights the important role endocytic traffic plays in regulating Leishmania lipid homeostasis, thermotolerance, and infectivity in the mammalian host.
MATERIALS AND METHODS
Cell culture and growth curves.
L. m. mexicana wild-type (MNYC/BZ/62/M379), Δμ1, and Δσ1 promastigotes were cultivated at 27°C in RPMI-1640 containing 10% fetal bovine serum (iFBS). Axenic amastigotes were obtained by suspending stationary-phase promastigotes (1 × 106 to 2 × 106 cells/ml) in RPMI-1640 supplemented with 20% iFBS, pH 5.5, and incubating them at 33 to 34°C (3). Cellular growth was measured by the protein bicinchoninic acid assay (Pierce). Partial inhibition of sphingolipid or sterol biosynthesis was achieved by the addition of 1 μg/ml myriocin (Sigma) or 2 μg/ml ketoconazole (Sigma), respectively (45, 55).
Cloning and episomal gene expression.
For episomal complementation of L. m. mexicana Δμ1 and Δσ1 promastigotes, L. m. mexicana genes encoding μ1 and σ1 were cloned into the pX vector, which had been modified with three copies of the influenza hemagglutinin peptide epitope (HA) within the multiple cloning site (39). Amplification of the μ1 and σ1 genes from genomic DNA was performed with primers 1 (GTACCCGGGATGGCGTCGGTGCTGTACATC) and 2 (GTACGGATCCGTCCGTTCGTATCTCGTATAC) for μ1 and 3 (GTACCCCGGGATGATTCAGTTCCTGCTGCTG) and 4 (GTACGGATCCTACACCCTTGATGGCGTTG) for σ1; these primers contained unique BamHI and SmaI restriction sites (underlined) to allow in-frame placement of the genes upstream of the triple HA epitope. The gene encoding the L. donovani myo-inositol transporter(MIT) was amplified from L. donovani genomic DNA by use of primers 5 (GACTGGATCCATGCGAGCATCTGTCATGCTATGTG) and 6 (ATCCTTGGGCTCGTGCGGAGCTGCTTTG) and cloned into pXG'-GFP to generate a C-terminal green fluorescent protein (GFP) fusion protein. The T. brucei GRIP domain GFP fusion protein was created previously by our laboratory (25, 35). All constructs were sequenced and then transfected into Δμ1, Δσ1, or wild-type L. m. mexicana promastigotes by electroporation (47). Parasites were cultured in drug-free media for 24 h, and positive transfectants were subsequently selected by the addition of 10 μg/ml of G418 sulfate. Following adaptation, parasites were maintained in medium containing 100 μg/ml of G418 sulfate.
FM4-64 and BODIPY-C5-ceramide staining.
Endocytic compartments were labeled with the vital dye FM4-64 as previously described (39). Briefly, FM4-64 (final concentration of 10 μM) was mixed with parasites growing in normal culture media, and cells were examined by fluorescence microscopy at predesignated time intervals, typically 5 to 10 min for labeling of endosomal compartments and 30 min for labeling of the multivesicular tubule (MVT) lysosome. The promastigote MVT lysosome was also delineated with BODIPY-C5-ceramide [N-(4,4-difluoro-5,7-dimethyl-4-bora-3a,4a-diaza-s-indacene-3-pentanoyl)sphingosine; Molecular Probes Inc.] as previously described (25).
Microscopy.
For immunofluorescence microscopy, L. m. mexicana promastigotes were fixed in phosphate-buffered saline (PBS) containing 4% paraformaldehyde (0°C, 15 min) and then immobilized on poly-l-lysine-coated coverslips. The coverslips were immersed in methanol (0°C, 5 min), washed three times with PBS, immersed in 50 mM ammonium chloride (0°C, 10 min), and then incubated in PBS containing 1% bovine serum albumin (BSA) (25°C, 30 min). Coverslips containing fixed parasites were probed with the following primary antibodies: anti-α-tubulin (1:500 dilution, clone DM1A; Sigma), L3.8 anti-gp63 (1:100 dilution; kindly provided by Thomas Ilg), monoclonal anti-HA (1:80 dilution; Roche), polyclonal anti-SMP-1 (1:400 dilution [55]), and L8C4 anti-paraflagellar rod protein (1:4 dilution; kindly provided by Keith Gull). Antibodies to the Leishmania GRIP protein were generated by immunizing New Zealand White rabbits with a glutathione-S-transferase protein fused to the C-terminal 173 residues of the L. major GRIP protein (100 μg). Polyclonal antibodies from immunized rabbits reacted with a specific 120-kDa protein (data not shown) and were used at a dilution of 1:500. Sections were subsequently incubated with Alexa Fluor 594 or 488 goat anti-rabbit, anti-mouse, or anti-rat secondary antibodies. All antibodies were diluted with 1% BSA in PBS. The coverslips were mounted in Mowiol 4-88 (Calbiochem) containing Hoechst dye and cells viewed with a Zeiss Axioplan2 microscope equipped with an AxioCam Mrm digital camera. Transmission electron microscopy was carried out as described previously (39).
Triton X-100 extractions and immunoblotting.
L. m. mexicana promastigotes (2 × 107) were harvested by centrifugation (2,000 × g, 10 min), washed with ice-cold PBS, and resuspended in 200 μl Triton X-100 lysis buffer (1% Triton X-100, 25 mM HEPES, pH 7.4, 1 mM EDTA, and complete protease inhibitor cocktail [Roche]) at 0°C or 25°C for 30 min. The extract was centrifuged (14,000 × g, 20 min, 4°C) and the pellet washed with ice-cold PBS. The protein in the supernatant was precipitated by the addition of 9 volumes of acetone (−20°C, 16 h). Precipitated protein pellets, or cells that had been harvested and washed thoroughly in PBS, were solubilized in 1% sodium dodecyl sulfate (100°C, 3 min) and protein levels determined using the bicinchoninic acid assay with BSA as the standard (Pierce). Protein (40 μg) was separated by 10% or 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes. Nitrocellulose membranes were blocked with 5% powdered skim milk in Tris-buffered saline containing 0.05% Tween 20 (TTBS). The blots were probed with the following antibodies: anti-GFP (1:1,500 dilution; Roche), L3.8 anti-gp63 (1:10,000 dilution; kindly provided by T. Ilg), monoclonal anti-AP3 (1:5,000 dilution), and monoclonal anti-LT15 (6:1,000 dilution). AP3 and LT15 bind to epitopes in L. m. mexicana secreted acid phosphatase and proteophosphoglycan, respectively (24), and were kindly provided by Thomas Ilg. All antibodies were diluted in 5% powdered skim milk-TTBS. Bound antibody was detected using enhanced chemiluminescence (Amersham) and exposure to X-ray film (Biomax MR; Kodak).
Lysosome transport assay.
A new assay was developed for measuring endosome-to-lysosome transport. We have recently shown that several polytopic membrane proteins are targeted to the lysosomes of trypanosomatid parasites for degradation and that GFP-tagged versions of these proteins can be used as markers for the mature lysosome in live parasites (33). A GFP-tagged version of the L. donovani MIT (MIT-GFP) is constitutively transported to the MVT lysosome of L. m. mexicana promastigotes when these parasites are cultivated in inositol-replete medium, providing a stable marker for this organelle (reference 29 and unpublished data). L. m. mexicana wild-type and μ1 and σ1 mutant promastigotes were transfected with pX-NEO plasmid encoding MIT-GFP and parasites were cultivated in RPMI medium containing myo-inositol and iFCS. Transfected parasites were incubated with FM4-64 as described above and harvested at indicated time points for fluorescence microscopy. Lysosomal transport was assessed by quantitating the number of live parasites that contained detectable overlap in FM4-64 and GFP fluorescence (as depicted by yellow staining). Images of 70 to 100 cells were examined at each time point for quantitation.
LME assay.
Promastigotes were incubated in RPMI medium, 10% iFCS containing l-leucine methyl ester (LME) (10 to 60 mM) at 27°C for 2 h (23). Viability was measured by the MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-dephenyltetrazolium bromide] assay (37). Briefly, cells containing LME were incubated in triplicate with 900 μg/ml of MTT for 2 h at 27°C. The reaction was stopped and formazan crystals were solubilized by adding 0.04 M hydrochloric acid in isopropanol for 30 min at 25°C. The absorbance was then measured at 592 nm.
RESULTS
Leishmania AP-1 μ1 and σ1 subunits localize to the Golgi and endosomes.
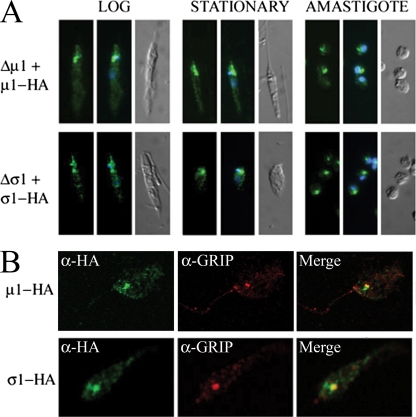
We have previously shown that the L. m. mexicana AP-1 μ1 and σ1 subunits are not required for growth at 27°C but are essential for virulence in the mammalian host. In order to define which processes mediated by AP-1 are essential for virulence, we investigated the function of these subunits in more detail. Their subcellular localization was investigated by expressing HA-tagged versions of the μ1 and σ1 subunits in L. m. mexicana Δμ1 and Δσ1 promastigotes, respectively. Epitope tagging was necessary, as antibodies raised against the L. m. mexicana AP-1 proteins failed to detect native proteins in immunofluorescence studies, although both proteins were detected in immunoblotting experiments and shown to be constitutively expressed in all life cycle stages (19). The epitope-tagged μ1-HA3 and σ1-ΗΑ3 were functional, as the complemented mutant parasites were capable of differentiating to amastigotes when incubated at 33°C, a temperature that kills the mutant strains (see below). The epitope-tagged proteins were localized to a cluster of punctate structures near the anterior ends of both promastigote and in vitro-differentiated amastigote stages (Fig. 1A). These structures were invariably juxtaposed to the kinetoplast (mitochondrial DNA) and the flagellar pocket, the sole site of exo- and endocytosis in these parasites, consistent with an endosomal and/or Golgi apparatus localization (35, 39). We have previously shown that ectopically expressed proteins containing a C-terminal GRIP domain are targeted to the trans-Golgi cisternae of Leishmania (35). Antibodies to the endogenous Leishmania GRIP protein were generated and Leishmania promastigotes expressing the HA-tagged AP-1 subunits fixed and labeled with anti-HA and anti-GRIP antibodies prior to immunofluorescence microscopy. As shown in Fig. 1B, the two AP-1 subunits colocalized with the endogenous trans-Golgi marker, as well as a more heterogeneous population of vesicles (Fig. 1A and B) that are indistinguishable from early endosomes (35, 39). When expression of μ1-ΗΑ3 and σ1-HA3 was increased with higher drug selection, a strong cytoplasmic staining was detected, indicating that membrane recruitment is saturable (data not shown). These results suggest that the AP-1 σ1 and μ1 subunits associate with both the anterior endosomes and late Golgi membranes, analogous to the situation found for other eukaryotes (9).
FIG. 1.
Localization of AP-1 subunits to the Golgi and endosomal membranes. (A) L. m. mexicana Δσ1 and Δμ1 parasites expressing HA-tagged σ1 or μ1 proteins were fixed and analyzed with anti-HA antibodies in log-phase or stationary-phase promastigote or axenic amastigote growth. The distribution of anti-HA antibody (green fluorescence), nucleus and kinetoplast (mitochondrial DNA) distribution (blue fluorescence), and differential interference contrast (DIC) images are shown in the left, middle, and right, respectively. Representative images of the entire cell population are shown. (B) L. m. mexicana Δσ1 or Δμ1 promastigotes expressing HA-tagged σ1 or μ1 proteins were fixed and stained with anti-HA (green) and antibodies directed to the endogenous TGN GRIP protein (red). The merged image shows the partial overlap of HA and GRIP staining (yellow).
Loss of μ1 or σ1 results in an altered MVT lysosome morphology.
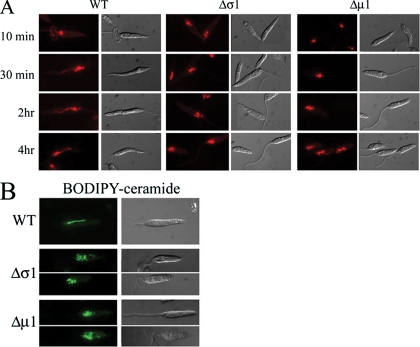
To investigate whether loss of the AP-1 μ1 and σ1 subunits resulted in altered endocytic and lysosome transport, wild-type and mutant parasites were incubated with the endocytic tracer FM4-64. FM4-64 rapidly labels the anteriorly localized endosomes, prior to being transported to the distinctive MVT lysosome, which extends from near the flagellar pocket to the posterior end of wild-type promastigotes (Fig. 2A) (18, 39, 56). In Δμ1 or Δσ1 promastigotes, FM4-64 was internalized into early endosomal compartments following a 10-min incubation, indicating that the μ1 and σ1 subunits are not required for endocytosis. However, subsequent transport of FM4-64 from anterior endosomes to the extended MVT lysosome was not observed for the mutant promastigotes, even when the time of FM4-64 labeling was extended up to 4 h. Instead, FM4-64 accumulated in a series of vacuoles at the anterior ends of these parasites, between the nucleus and the flagellar pocket (Fig. 2A). As these vacuoles appear to be the terminal compartment labeled by FM4-64, they may reflect the fragmentation of the MVT lysosome.
FIG. 2.
Loss of AP-1 subunits induces fragmentation of tubular lysosomes. (A) L. m. mexicana wild-type (WT), Δσ1, or Δμ1 promastigotes were incubated with the endocytic marker FM4-64 for the indicated times and the distribution of dye was determined by fluorescence microscopy. Representative images of the entire cell population are shown. (B) Wild-type and AP-1 mutant parasites were incubated with the vital dye BODIPY-C5-ceramide, and the distribution of dye was monitored by fluorescence and DIC microscopy. Representative images of the entire cell population are shown.
BODIPY-C5-ceramide is another vital stain for the MVT lysosome (25). Unlike FM4-64, this fluorescent lipid marker selectively labels the MVT lysosome and not the early endosomes of wild-type parasites (Fig. 2B) (39). BODIPY-C5-ceramide accumulated in the unusual anterior vacuoles of the AP-1 mutant parasites (Fig. 2B), providing a second line of evidence that these vacuoles correspond to a fragmented MVT lysosome.
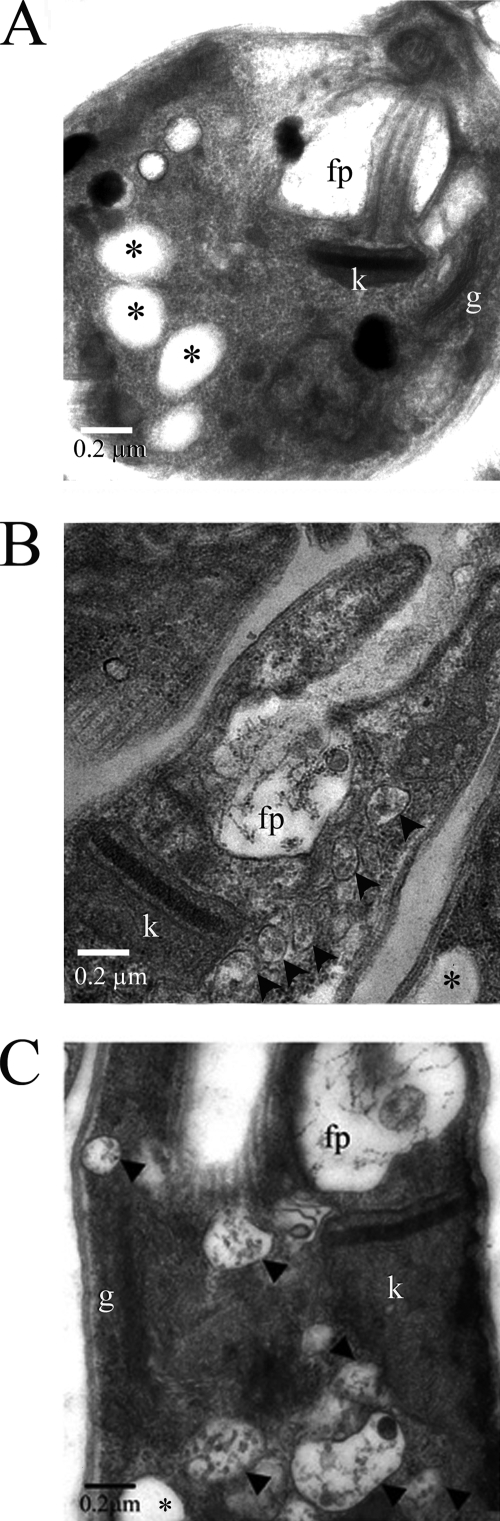
To further investigate the nature of the anterior vacuoles that accumulate in the AP-1 mutants, wild-type and mutant promastigotes were fixed and analyzed by transmission electron microscopy. Analysis of serial sections of >20 cells of each cell line revealed no change in the ultrastructure of the flagellar pocket, the single Golgi apparatus, the acidocalcisomes, or the tubular endosomes of the AP-1 mutants (Fig. 3; also see Fig. S1 in the supplemental material). The subcellular localization of the GFP-GRIP chimera, which is selectively targeted to the Golgi, was also unaltered in the Δσ1 and Δμ1 mutants (see Fig. S1B in the supplemental material). However, both mutants contained a population of large (>50-nm) multivesicular bodies (MVBs) at the anterior ends of the cells that were absent from wild-type promastigotes (Fig. 3). Each of the MVBs contained a limiting membrane and a heterogeneous population of internal membrane vesicles (Fig. 3B and C), reminiscent of the structure of the MVT lysosome (39). Quantitative analysis of serial sections revealed that L. m. mexicana Δμ1 promastigotes contained 8.15 ± 3.26 large MVBs per cell, while none were detected in wild-type promastigotes. The MVB structures were largely restricted to the anterior end of mutant promastigotes and are likely to correspond to the structures labeled in live parasites with FM4-64 and BODIPY-C5-ceramide. Collectively, these results suggest that the deletion of the AP-1 adaptor subunits results in the fragmentation of the MVT lysosome but has little effect on the organization of the Golgi and early endosome membranes.
FIG. 3.
Ultrastructures of L. m. mexicana Δσ1 and Δμ1 promastigotes. Wild-type (A), Δμ1 (B), and Δσ1 (C) promastigotes were harvested in log-phase growth, fixed, and analyzed by transmission electron microscopy. fp, flagellar pocket; k, kinetoplast; g, Golgi; *, acidocalcisome; arrowheads, large MVBs.
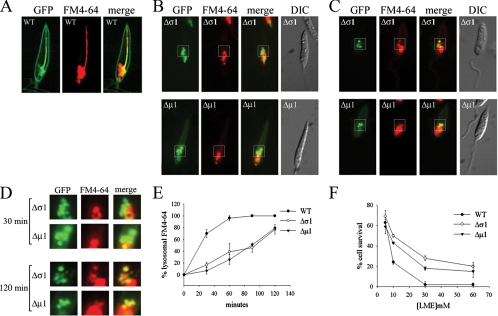
Endosome-to-lysosome transport is decreased in Δμ1 and Δσ1 parasites.
To investigate whether the altered lysosome morphology in the AP-1 mutants reflected changes in endosome-to-lysosome membrane transport, we developed an in vivo assay to measure this step. Wild-type and AP-1 mutant strains were transfected with a plasmid encoding a polytopic membrane protein MIT-GFP. This protein is localized to the cell surface but is also constitutively transported to the MVT lysosome under myo-inositol-replete conditions, providing a stable marker for this compartment (Fig. 4A). In the AP-1 mutant strains, this construct is targeted to anterior vacuoles that are indistinguishable from those labeled with BODIPY-C5-ceramide and FM4-64 (Fig. 4B to D). MIT-GFP-expressing wild-type and mutant parasites were pulse-labeled with FM4-64, and the colocalization of GFP and FM4-64 fluorescence (yellow staining) was used as a measure of endosome-to-lysosome transport. As expected, FM4-64 was transported to the GFP-positive MVT lysosome within 30 min in wild-type promastigotes (Fig. 4A). In contrast, FM4-64 transport to the GFP-positive vacuoles of the AP-1 mutant parasites was detected only after 1 to 2 h (Fig. 4B to D). Quantitative analyses indicated that membrane transport from the endosomes to the fragmented lysosomal vacuoles of AP-1 mutants is approximately fourfold slower than endosome-to-MVT lysosome transport in wild-type parasites (Fig. 4E).
FIG. 4.
Endosome-to-lysosome trafficking is perturbed in Δσ1 and Δμ1 cells. (A) Wild-type (WT) L. m. mexicana cells expressing MIT-GFP were incubated with FM4-64 for 30 min and examined by fluorescence microscopy. MIT-GFP is both expressed at the cell surface and internalized into the MVT lysosome when the medium contains myo-inositol. (B to D) Δσ1 and Δμ1 promastigotes expressing MIT-GFP were incubated for 30 (B) or 120 (C) minutes with FM4-64, and the localizations of GFP (green signal) and FM4-64 (red signal) were determined by fluorescence microscopy. The boxed regions shown in panels B and C are magnified and shown in panel D. Representative images of the entire cell population are shown throughout. (E) L. m. mexicana wild-type, Δσ1, and Δμ1 promastigotes expressing MIT-GFP were analyzed for overlap of lysosomal GFP fluorescence and FM4-64 fluorescence at the indicated time intervals. Eighty to 100 cells were examined for each time point and the percentage of cells showing overlap in GFP/FM4-64 fluorescence was measured. The standard deviation was calculated from three separate samples (error bars). (F) L. m. mexicana wild-type, Δσ1, and Δμ1 promastigotes were incubated with LME for 2 h and cellular viability was measured against control (nontreated) cells by use of the MTT assay. Error bars represent the standard deviations for three samples.
Lysosomal transport can also be assessed indirectly by measuring sensitivity to the lysomotropic amino acid ester LME (23). LME uptake results in lysosome swelling and disruption, leading to cell death. Wild-type parasites were found to be markedly more sensitive to LME than were either of the AP-1 mutants (Fig. 4F), supporting the conclusion that lysosomal transport is reduced in the mutant cell lines.
In contrast, we could find no significant defect in the localization, targeting, or secretion of soluble proteins, glycosylphosphatidylinositol (GPI)-anchored proteins, or transmembrane proteins in the Δμ1 and Δσ1 parasites (data not shown), suggesting that AP-1 does not play a significant role in the surface transport or secretion of soluble or membrane-bound proteins in Leishmania parasites.
Sterols accumulate in the fragmented lysosomes of the L. m. mexicana AP-1 mutants.
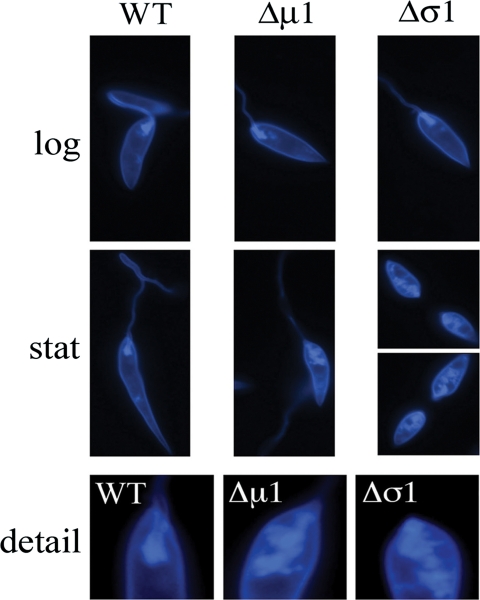
Defects in lysosomal transport are known to lead to changes in sterol trafficking and homeostasis in other eukaryotes (32). To determine if the loss of the AP-1 subunits affects the distribution of sterols in Leishmania, wild-type and mutant promastigotes were labeled with a highly fluorescent polyene macrolide, filipin. Filipin specifically intercalates into sterol-rich membranes and can be used to visualize these membranes in live and fixed cells (27). Similar patterns of filipin staining were observed for log-phase wild-type and AP-1 mutant promastigotes. Specifically, filipin staining was almost entirely restricted to the plasma membrane surrounding the cell body, the flagellum, and the flagellum pocket (Fig. 5, top). In contrast, marked differences were observed in the filipin staining patterns of wild-type and AP-1 mutant stationary-phase promastigotes. While filipin staining of stationary-phase wild-type promastigotes was essentially the same as that seen for the log phase, a distinct population of intracellular membranes were strongly labeled in the stationary-phase AP-1 mutant promastigotes (Fig. 5). These intracellular membranes were largely concentrated at the anterior end of the parasites and were indistinguishable from the fragmented lysosomes of these parasites. Total levels of ergosterol, as determined by gas chromatography-mass spectrometry, were unchanged for both wild-type and mutant parasites (data not shown). These results show that ergosterol preferentially accumulates in the fragmented lysosomes of the AP-1 mutants in stationary-phase growth.
FIG. 5.
L. m. mexicana AP-1 mutants accumulate sterols in intracellular compartments. L. m. mexicana wild-type, Δσ1, and Δμ1 parasites were harvested in log - and stationary-phase (stat) culture, and fixed cells were stained with filipin. Labeled parasites were viewed by fluorescence microscopy. (Bottom) Detail of anterior region of stationary-phase promastigotes. Images represent the entire cell population.
Promastigote Δμ1 and Δσ1 parasites have defects in flagellum biogenesis.
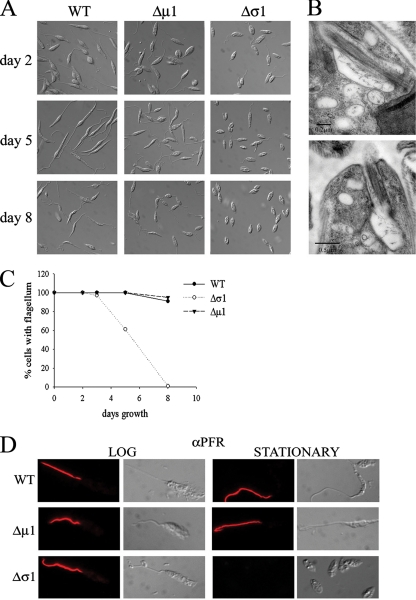
In the course of our analysis of the L. m. mexicana AP-1 mutants, we noted that the flagella of Δσ1 promastigotes shortened dramatically when this mutant entered the stationary growth phase (Fig. 6A to C). The L. m. mexicana Δμ1 mutant promastigotes also exhibited shorter flagella in stationary phase, although to an extent lesser than that seen for the Δσ1 mutant (Fig. 6A). The short flagella of Δσ1 stationary-phase promastigotes retained the normal axoneme with nine plus two microtubule doublets (Fig. 6B) but lacked the paraflagellar rod proteins that are present only in emergent portions of the flagella in wild-type parasites (Fig. 6D).
FIG. 6.
The L. m.mexicana Δσ1 mutant displays a distinct flagellum morphology in stationary-phase growth. (A) Wild-type(WT), Δσ1, and Δμ1 parasites were harvested while in early log growth (day 2), early stationary growth (day 5), and late stationary growth (day 8), and cell morpholog ies were examined by DIC microscopy. Images shown are representative of the entire cell population. (B) Δσ1 promastigotes were harvested in stationary phase and fixed cells were examined by transmission electron microscopy. Serial sectioning of the same cell (approximately 180 nm of separation between top and bottom images) demonstrated that the flagellum extended to just beyond the mouth of the flagellum pocket and retained a highly truncated microtubule axoneme. The flagellar pocket is surrounded by a number of MVB s and translucent vesicles. (C) Wild-type, Δσ1, and Δμ1 promastigotes were examined for the presence or absence of a flagellum in different growth phases. At least 100 cells were examined for each time point. The results are representative of six independent experiments. (D) Wild-type, Δσ1, and Δμ1 promastigotes were harvested in log or stationary growth phase, and fixed parasites were labeled with antibody directed to the paraflagellar rod protein. Fluorescence and corresponding DIC images are shown.
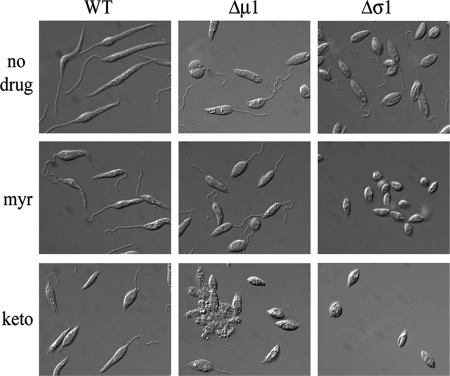
We have recently shown that partial inhibitions of either ergosterol (the major sterol of these parasites) or sphingolipid biosynthesis in Leishmania promastigotes have similar effects on flagellar morphology (55). Specifically, treatment of wild-type parasites with either ketoconazole (an inhibitor of sterol-14′-demethylase, a late step in ergosterol biosynthesis) or myriocin (an inhibitor of the serine:palmitoyl-coenzyme A transferase, the first step in de novo sphingolipid biosynthesis) resulted in flagellum retraction in stationary-phase promastigotes (55). A similar effect was observed following the genetic disruption of the gene encoding the serine:palmitoyl-coenzyme A transferase (12, 60). To test whether the flagellar phenotype of the AP-1 mutants reflects alterations in membrane lipid homeostasis, the AP-1 mutants were cultivated in the presence of low concentrations of ketoconazole and myriocin (55). At the concentrations used, neither inhibitor had any effect on flagellum length in wild-type parasites (Fig. 7). However, both inhibitors induced the complete retraction of the flagellum in log-phase Δσ1 mutant promastigotes (Fig. 7). Significantly, ketoconazole treatment, but not myriocin treatment, had a similar effect on the flagellar length of Δμ1 log-phase promastigotes (Fig. 7). These results indicate that AP-1 is required for normal flagellum biogenesis, possibly by regulating lipid homeostasis in the flagellum membrane.
FIG. 7.
Inhibitors of sterol and sphingolipid biosynthesis enhance the flagellum-shortening phenotype.Wild-type(WT), Δσ1, and Δμ1 promastigotes were grown in the absence or presence of low concentrations of myriocin (myr) (1 μg/ml) or ketoconazole (keto) (2 μg/ml) at 27°C for 3 days, and cell morphology was monitored by DIC microscopy. Images represent the entire cell population.
Leishmania AP-1 subunit mutants are highly sensitive to elevated temperature and sterol biosynthetic inhibitors.
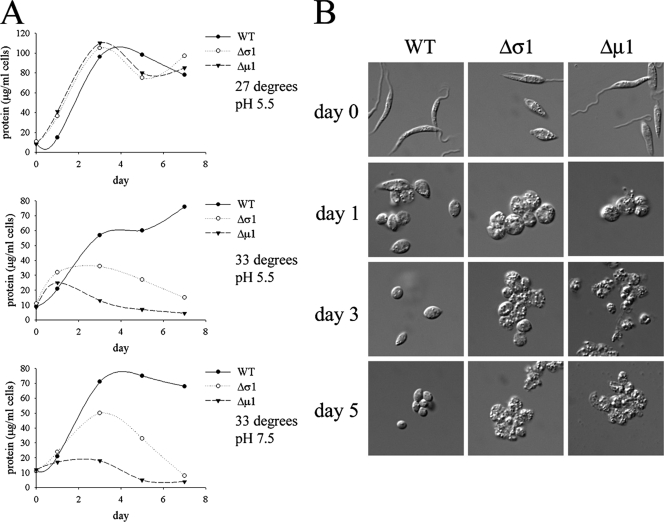
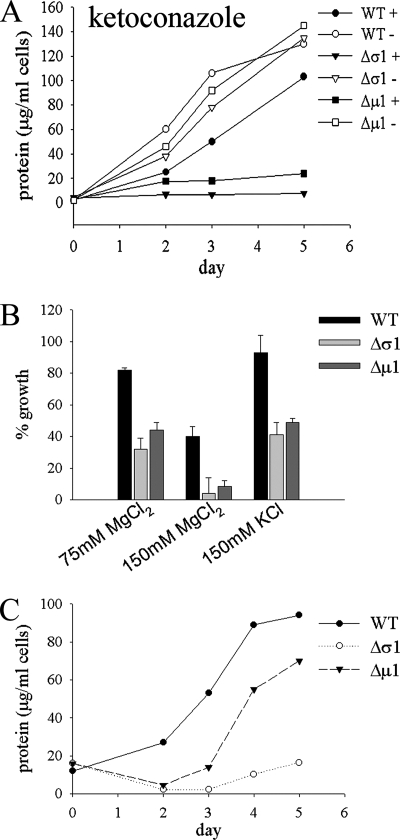
To further understand why the AP-1 mutant parasite lines are avirulent, we investigated the capacities of these mutants to grow at the elevated temperatures (27°C to 33°C) and/or low-pH conditions encountered in the mammalian host, which together stimulate the process of differentiation from promastigotes into amastigotes both in vivo and in vitro. Both strains exhibited growth rates essentially identical to those of wild-type parasites when subjected to neutral or low pH at 27°C but displayed greatly reduced growth and increased cell death when grown at 33°C, regardless of the pH (Fig. 8A). Interestingly, the L. m. mexicana Δμ1 promastigotes were consistently observed to be more sensitive to heat shock than were the L. m. mexicana Δσ1 promastigotes (Fig. 8A). Elevated temperature and reduced pH induced the wild-type and AP-1 mutant promastigotes to round up and form amastigote-like forms (Fig. 8B). While the wild-type parasites clearly remained viable and continued to grow under these conditions, the AP-1 mutant parasites became more granular and vacuolated and lost membrane integrity after 3 days (Fig. 8B). The AP-1 mutants were also more sensitive than wild-type parasites to a range of other stresses, including low concentrations of ketoconazole (2 μg/ml) (Fig. 9A), elevated levels of chloride ions (Fig. 9B), and starvation (Fig. 9C). The increased sensitivities of the AP-1 mutants to environmental stresses, particularly elevated temperature, are likely to account for the loss of the virulence phenotype of these mutants in susceptible animal models.
FIG. 8.
L. m. mexicana AP-1 mutant promastigotes are sensitive to elevated temperature. (A) L. m. mexicana wild-type (WT), Δμ1, and Δσ1 stationary-phase promastigotes were cultivated in RPMI medium, 20% iFBS that was adjusted to either pH 5.5 or pH 7.5, and the promastigotes were incubated at either 27°C or 33°C as indicated. Parasite growth was monitored by measuring increases in cellular protein levels. The results were verified on more than three separate occasions. (B) Differentiation of L. m. mexicana wild-type and AP-1 mutant parasites. Stationary-phase promastigotes were suspended in low-pH medium and incubated at 33°C, and differentiation to amastigotes was monitored by DIC microscopy.
FIG. 9.
L. m. mexicana AP-1 mutant promastigotes are sensitive to agents that perturb membrane function and nutrient starvation. (A) Wild-type (WT), Δσ1, and Δμ1 promastigotes were grown in the absence (open symbols) or presence (closed symbols) of ketoconazole (2 μg/ml) at 27°C, and cell growth was monitored. (B) Wild-type and AP-1 mutant promastigotes were cultivated in RPMI medium containing elevated chloride levels. Growth (measured as cell protein) is shown relative to that for promastigotes grown in the absence of added chloride ions. Triplicate samples were used to calculate the standard deviation (error bars). (C) Wild-type and AP-1 mutant promastigotes were suspended in PBS, pH 7.5, for 48 h at 27°C. Parasites were harvested by centrifugation and resuspended in full RPMI medium, 10% FCS, and recovery was monitored by following the increase in cell protein.
DISCUSSION
Leishmania parasites are predicted to express three AP complexes, AP-1, AP-2, and AP-3 (13). Recent studies have shown that the AP-3 complex is required for the transport of some polytopic membrane proteins to acidocalcisomes, specialized lysosome-like vacuoles rich in calcium and (poly)phosphates (7). In this study, we provide evidence that the AP-1 complex plays a role in lysosome biogenesis and trafficking, intracellular sterol homeostasis, and flagellum biogenesis. The AP-1 subunit-deficient mutants are also hypersensitive to a range of environmental stresses and membrane-perturbing agents. This study highlights the pleiotropic functions of AP-1 subunits in Leishmania membrane transport and provides an explanation for why the AP-1 mutants exhibit a severe loss-of-virulence phenotype in the mammalian host.
Functionally active, epitope-tagged AP-1 μ1 and σ1 subunits were localized to endosome and the trans-Golgi membranes. These results suggest that, as in other eukaryotes (17, 22, 31), the leishmanial AP-1 complex is involved in regulating membrane traffic between these compartments, most likely by recruiting protein cargo and/or clathrin coats to either Golgi or endosomal membranes (13, 56). However, loss of the AP-1 μ1 and σ1 subunits had no discernible effect on of the uptake of FM4-64 or the exocytosis of a range of surface and secreted glycolipids and proteins. The ultrastructure of the Golgi apparatus and the localization of a trans-Golgi marker were also unchanged in the AP-1 mutants. These results suggest that the AP-1 complex is not required for endocytosis or post-Golgi exocytic pathways involving either Golgi-plasma membrane or Golgi-endosome-plasma membrane itineraries (20). In contrast, transport from the endosomes to the lysosome appeared to be partially disrupted in both AP-1 subunit mutants. Specifically, both mutants lacked the distinctive tubular lysosome of wild-type promastigotes and accumulated a population of MVBs that have the characteristics of fragmented lysosomes. Transport of both membrane (FM4-64) and soluble (LME) markers from the endosome compartment to the MVBs/fragmented lysosomes was reduced by nearly fourfold. However, proteins such as MIT-GFP and DPMS-GFP (39) that are delivered to these MVBs were proteolytically degraded (data not shown), indicating that endogenous proteases are still trafficked to this compartment despite the slower transport kinetics. These results suggest that endosome-lysosome transport dynamics are altered in the AP-1 mutant, possibly as a result of changes in the recycling kinetics of one or more proteins in the endosomes and/or TGN. However, the proteolytic capacities of the fragmented vacuoles do not appear to be altered, suggesting that the stress response phenotypes of the AP-1 mutant are not due to gross changes in proteolytic lysosomal degradation.
Interestingly, genetic deletion or knockdown of AP-1 subunits in other single-celled eukaryotes often leads to defects in lysosome trafficking. For example, a Schizosaccharomyces pombe mutant lacking the AP-1 μ1 subunit has multiple defects in membrane trafficking and accumulates fragmented vacuoles (28). Loss of the AP-1 μ1 subunit in Dictyostelium discoideum and Toxoplasma gondii also results in the accumulation of cytoplasmic vesicles and defective transport of a range of lysosomal hydrolases (31, 42). In contrast, knockdown of the AP-1 γ-subunit in the related trypanosomatid parasite T. brucei had little effect on lysosomal morphology or transport of the major lysosome membrane protein p67 (1). However, AP-1 was required for normal growth and architecture of the endosome system (1). All these studies are consistent with AP-1 having a primary role in regulating Golgi-endosome membrane dynamics, with potential secondary effects on lysosome structure and transport.
The fragmented lysosomes of the L. m. mexicana AP-1 mutants appeared to contain high levels of sterols, as shown by filipin staining. Interestingly, sublethal concentrations of ketoconazole and myriocin induce similar fragmentation s of the MVT lysosome (J. Heng, D. L. Tull, and M. J. McConville, unpublished data), indicating that alterations in membrane lipids may contribute directly to the altered morphology of this organelle in the AP-1 mutants. Sterols are required for vacuole transport in S. cerevisiae (21, 27, 30), and alterations in sterol levels may cause an imbalance in anterograde/retrograde membrane transport between the endosomes and the MVT lysosome, leading to fragmentation. Alternatively, alterations in sterol concentration may result in the destabilization of the extended tubular structure of the MVT lysosome (49). Changes in lipid composition could also conceivably lead to a loss of membrane proteins that tether the MVT lysosome to a band of two to four microtubules that extends from the anterior to the posterior end of Leishmania promastigotes (39), further contributing to the destabilization and fragmentation of this organelle.
The presence of high sterol levels in the lysosomes of the L. m. mexicana Δσ1 and Δμ1 mutants is reminiscent of the phenotype observed for some mammalian lysosomal storage diseases, such as Niemann-Pick type C (NPC) (32). NPC disease is associated with the accumulation of cholesterol and sphingolipids in late endosomes due to mutations in two proteins, NPC1 and NPC2, which are thought to be involved in recycling cholesterol from the lysosome to the plasma membrane (32, 51). Intriguingly, recent studies suggest that the localization and function of NPC proteins in yeast and mammalian cells requires the AP-1, GGA, or AP-3 complexes (4). Leishmania lacks obvious homologues of the NPC proteins but is likely to have retained some mechanism for sensing and sorting lipids in the endocytic pathway. Disruption of sterol sensing in the AP-1 mutants could thus underlie some of the observed defects in both lysosomal transport and flagellum biogenesis.
Unexpectedly, deletion of the AP-1 subunits had a profound effect on flagellum length in stationary-phase promastigotes. This phenotype was evident in both mutants but was most dramatic in the Δσ1 null mutant. There is increasing evidence that flagellar membrane proteins and lipids are transported from the Golgi apparatus to the base of the flagellum prior to incorporation into the flagellum membrane. In some vertebrate cell types, specialized AP-1 complexes are involved in either the selection of protein cargo or the movement of these transport vesicles to the flagellum base (2, 15). The difference in the severities of the flagellum phenotypes of the L. m. mexicana Δσ1 and Δμ1 mutants is consistent with the Leishmania AP-1 complex having a role in targeting flagellum proteins to post-Golgi vesicles, as each of these subunits bind different targeting motifs in vesicle cargo (26, 38). Intriguingly, treatment of stationary-phase promastigotes with ketoconazole or myriocin (55) or genetic disruption of sphingolipid biosynthesis (12, 60) also induces flagellum shortening, and the flagellar phenotype of the two AP-1 mutants was exacerbated when either sterol or sphingolipid biosynthesis was inhibited. These observations raise the possibility that disruption of AP-1-mediated vesicle transport has a direct effect on flagellum membrane lipid composition. Alternatively, changes in sterol levels in the endocytic pathway of AP-1 mutants may lead indirectly to changes in the lipid composition of the flagellum membrane. Global changes in endocytic trafficking also affect flagellum biogenesis in the related trypanosomatid, Trypanosoma brucei. Loss of the ADP ribosylation factor orthologue ARF1 in T. brucei bloodstream forms results in the enlargement of the flagellar pocket and the formation of intracellular flagella (43). The latter phenotype may reflect defects in the delivery of membrane proteins/lipids to the flagellar pocket (50). Targeted disruption of Leishmania genes encoding mitogen-activated protein kinases as well as thymidine kinase can also affect the length of promastigote flagellum (53, 57). Whether these cytoplasmic proteins directly or indirectly modulate lipid biosynthesis and/or Golgi-flagellum trafficking pathways has not been investigated.
During infection in the mammalian host, Leishmania parasites must be able to adapt to elevated temperatures (33°C to 37°C), which together with the decrease in pH, stimulate their differentiation into the amastigote life stage. The L. m. mexicana AP-1 mutants grow normally when cultured as promastigotes at 27°C but are unable to grow or survive at 33°C. The thermosensitivity of these mutants provides an explanation for their loss of virulence in the murine models (19). However, exposure to other stresses in the animal host may also contribute to the loss of virulence. For example, the growth of both AP-1 mutants was profoundly affected by nutrient starvation and agents that perturbed membrane function (ketoconazole and chloride ions). AP-1 function may therefore be required, either directly or indirectly, for Leishmania responses to general environmental stresses encountered in the mammalian host. Interestingly, loss of AP-1 μ1 expression is also associated with increased temperature sensitivity in the fission yeast S. pombe (28). As seen for Leishmania, the S. pombe AP-1 μ1 subunit is not required for growth at 27°C but is essential for growth at 36°C. Loss of the S. pombe AP-1 μ1 subunit leads to hypersensitivity to inhibitors of the calcineurin stress response pathway, while loss of both AP-1 μ1 and calcineurin is synthetically lethal (28, 52). We have found that the L. m. mexicana AP-1 mutants also display increased sensitivity to inhibitors of the calcineurin pathway when cultivated at 27°C (D. L. Tull, J. E. Vince, and M. J. McConville, unpublished data), raising the possibility that perturbations induced by loss of AP-1 are sensed by an equivalent stress response pathway in Leishmania. Whether this pathway is also required for the heat shock responses of Leishmania and infectivity in the mammalian host remains to be determined.
Supplementary Material
Acknowledgments
We thank Keith Gull (University of Oxford) for providing the anti-PRF antibodies.
This work was funded by an Australian National Health and Medical Research Council program grant. J.E.V. was supported by an Australian postgraduate award, M.J.M. is an NHMRC principal research fellow, and G.I.M. is an Australian Research Council Federation fellow.
Footnotes
Published ahead of print on 30 May 2008.
Supplemental material for this article may be found at http://ec.asm.org/.
REFERENCES
- 1.Allen, C. L., D. Liao, W. L. Chung, and M. C. Field. 2007. Dileucine signal-dependent and AP-1-independent targeting of a lysosomal glycoprotein in Trypanosoma brucei. Mol. Biochem. Parasitol. 156175-190. [DOI] [PubMed] [Google Scholar]
- 2.Bae, Y. K., H. Qin, K. M. Knobel, J. Hu, J. L. Rosenbaum, and M. M. Barr. 2006. General and cell-type specific mechanisms target TRPP2/PKD-2 to cilia. Development 1333859-3870. [DOI] [PubMed] [Google Scholar]
- 3.Bates, P. A. 1994. The developmental biology of Leishmania promastigotes. Exp. Parasitol. 79215-218. [DOI] [PubMed] [Google Scholar]
- 4.Berger, A. C., G. Salazar, M. L. Styers, K. A. Newell-Litwa, E. Werner, R. A. Maue, A. H. Corbett, and V. Faundez. 2007. The subcellular localization of the Niemann-Pick type C proteins depends on the adaptor complex AP-3. J. Cell Sci. 1203640-3652. [DOI] [PubMed] [Google Scholar]
- 5.Besteiro, S., R. A. Williams, G. H. Coombs, and J. C. Mottram. 2007. Protein turnover and differentiation in Leishmania. Int. J. Parasitol. 371063-1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Besteiro, S., R. A. Williams, L. S. Morrison, G. H. Coombs, and J. C. Mottram. 2006. Endosome sorting and autophagy are essential for differentiation and virulence of Leishmania major. J. Biol. Chem. 28111384-11396. [DOI] [PubMed] [Google Scholar]
- 7.Besteiro, S., D. Tonn, L. Tetley, G. H. Coombs, and J. C. Mottram. 2008. The AP3 adaptor is involved in the transport of membrane proteins to acidocalcisomes of Leishmania. J. Cell Sci. 121561-570. [DOI] [PubMed] [Google Scholar]
- 8.Boehm, M., and J. S. Bonifacino. 2001. Adaptins: the final recount. Mol. Biol. Cell 122907-2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boehm, M., and J. S. Bonifacino. 2002. Genetic analyses of adaptin function from yeast to mammals. Gene 286175-186. [DOI] [PubMed] [Google Scholar]
- 10.Burchmore, R. J., D. Rodriguez-Contreras, K. McBride, P. Merkel, M. P. Barrett, G. Modi, D. Sacks, and S. M. Landfear. 2003. Genetic characterization of glucose transporter function in Leishmania mexicana. Proc. Natl. Acad. Sci. USA 1003901-3906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Croft, S. L., S. Sundar, and A. H. Fairlamb. 2006. Drug resistance in leishmaniasis. Clin. Microbiol. Rev. 19111-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Denny, P. W., D. Goulding, M. A. Ferguson, and D. F. Smith. 2004. Sphingolipid-free Leishmania are defective in membrane trafficking, differentiation and infectivity. Mol. Microbiol. 52313-327. [DOI] [PubMed] [Google Scholar]
- 13.Denny, P. W., G. W. Morgan, M. C. Field, and D. F. Smith. 2005. Leishmania major: clathrin and adaptin complexes of an intra-cellular parasite. Exp. Parasitol. 10933-37. [DOI] [PubMed] [Google Scholar]
- 14.Doray, B., P. Ghosh, J. Griffith, H. J. Geuze, and S. Kornfeld. 2002. Cooperation of GGAs and AP-1 in packaging MPRs at the trans-Golgi network. Science 2971700-1703. [DOI] [PubMed] [Google Scholar]
- 15.Dwyer, N. D., C. E. Adler, J. G. Crump, N. D. L'Etoile, and C. I. Bargmann. 2001. Polarized dendritic transport and the AP-1 mu1 clathrin adaptor UNC-101 localize odorant receptors to olfactory cilia. Neuron 31277-287. [DOI] [PubMed] [Google Scholar]
- 16.Folsch, H., M. Pypaert, S. Maday, L. Pelletier, and I. Mellman. 2003. The AP-1A and AP-1B clathrin adaptor complexes define biochemically and functionally distinct membrane domains. J. Cell Biol. 163351-362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Foote, C., and S. F. Nothwehr. 2006. The clathrin adaptor complex 1 directly binds to a sorting signal in Ste13p to reduce the rate of its trafficking to the late endosome of yeast. J. Cell Biol. 173615-626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ghedin, E., A. Debrabant, J. C. Engel, and D. M. Dwyer. 2001. Secretory and endocytic pathways converge in a dynamic endosomal system in a primitive protozoan. Traffic 2175-188. [DOI] [PubMed] [Google Scholar]
- 19.Gokool, S. 2003. Sigma 1- and mu 1-adaptin homologues of Leishmania mexicana are required for parasite survival in the infected host. J. Biol. Chem. 27829400-29409. [DOI] [PubMed] [Google Scholar]
- 20.Grunfelder, C. G., M. Engstler, F. Weise, H. Schwarz, Y. D. Stierhof, G. W. Morgan, M. C. Field, and P. Overath. 2003. Endocytosis of a glycosylphosphatidylinositol-anchored protein via clathrin-coated vesicles, sorting by default in endosomes, and exocytosis via RAB11-positive carriers. Mol. Biol. Cell 142029-2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heese-Peck, A., H. Pichler, B. Zanolari, R. Watanabe, G. Daum, and H. Riezman. 2002. Multiple functions of sterols in yeast endocytosis. Mol. Biol. Cell 132664-2680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang, K. M., K. D'Hondt, H. Riezman, and S. K. Lemmon. 1999. Clathrin functions in the absence of heterotetrameric adaptors and AP180-related proteins in yeast. EMBO J. 183897-3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huynh, C., and N. W. Andrews. 2003. Leishmania amazonensis Rab7 promotes toxicity of the amino acid ester Leu-OMe in amastigote megasomes. Mol. Biochem. Parasitol. 132101-104. [DOI] [PubMed] [Google Scholar]
- 24.Ilg, T., D. Harbecke, M. Wiese, and P. Overath. 1993. Monoclonal antibodies directed against Leishmania secreted acid phosphatase and lipophosphoglycan. Partial characterization of private and public epitopes. Eur. J. Biochem. 217603-615. [DOI] [PubMed] [Google Scholar]
- 25.Ilgoutz, S. C., J. L. Zawadzki, J. E. Ralton, and M. J. McConville. 1999. Evidence that free GPI glycolipids are essential for growth of Leishmania mexicana. EMBO J. 182746-2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Janvier, K., Y. Kato, M. Boehm, J. R. Rose, J. A. Martina, B. Y. Kim, S. Venkatesan, and J. S. Bonifacino. 2003. Recognition of dileucine-based sorting signals from HIV-1 Nef and LIMP-II by the AP-1 gamma-sigma1 and AP-3 delta-sigma3 hemicomplexes. J. Cell Biol. 1631281-1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kato, M., and W. Wickner. 2001. Ergosterol is required for the Sec18/ATP-dependent priming step of homotypic vacuole fusion. EMBO J. 204035-4040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kita, A., R. Sugiura, H. Shoji, Y. He, L. Deng, Y. Lu, S. O. Sio, K. Takegawa, M. Sakaue, H. Shuntoh, and T. Kuno. 2004. Loss of Apm1, the micro1 subunit of the clathrin-associated adaptor-protein-1 complex, causes distinct phenotypes and synthetic lethality with calcineurin deletion in fission yeast. Mol. Biol. Cell 152920-2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klamo, E. M., M. E. Drew, S. M. Landfear, and M. P. Kavanaugh. 1996. Kinetics and stoichiometry of a proton/myo-inositol cotransporter. J. Biol. Chem. 27114937-14943. [DOI] [PubMed] [Google Scholar]
- 30.Lebrand, C., M. Corti, H. Goodson, P. Cosson, V. Cavalli, N. Mayran, J. Faure, and J. Gruenberg. 2002. Late endosome motility depends on lipids via the small GTPase Rab7. EMBO J. 211289-1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lefkir, Y., B. de Chassey, A. Dubois, A. Bogdanovic, R. J. Brady, O. Destaing, F. Bruckert, T. J. O'Halloran, P. Cosson, and F. Letourneur. 2003. The AP-1 clathrin-adaptor is required for lysosomal enzymes sorting and biogenesis of the contractile vacuole complex in Dictyostelium cells. Mol. Biol. Cell 141835-1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liscum, L. 2000. Niemann-Pick type C mutations cause lipid traffic jam. Traffic 1218-225. [DOI] [PubMed] [Google Scholar]
- 33.Lustig, Y., Y. Vagima, H. Goldshmidt, A. Erlanger, V. Ozeri, J. Vince, M. J. McConville, D. M. Dwyer, S. M. Landfear, and S. Michaeli. 2007. Down-regulation of the trypanosomatid signal recognition particle affects the biogenesis of polytopic membrane proteins but not of signal peptide-containing proteins. Eukaryot. Cell 61865-1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McConville, M. J., D. de Souza, E. Saunders, V. A. Likic, and T. Naderer. 2007. Living in a phagolysosome; metabolism of Leishmania amastigotes. Trends Parasitol. 23368-375. [DOI] [PubMed] [Google Scholar]
- 35.McConville, M. J., S. C. Ilgoutz, R. D. Teasdale, B. J. Foth, A. Matthews, K. A. Mullin, and P. A. Gleeson. 2002. Targeting of the GRIP domain to the trans-Golgi network is conserved from protists to animals. Eur. J. Cell Biol. 81485-495. [DOI] [PubMed] [Google Scholar]
- 36.Meyer, C., D. Zizioli, S. Lausmann, E. L. Eskelinen, J. Hamann, P. Saftig, K. von Figura, and P. Schu. 2000. mu1A-adaptin-deficient mice: lethality, loss of AP-1 binding and rerouting of mannose 6-phosphate receptors. EMBO J. 192193-2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miller, M. A., S. E. McGowan, K. R. Gantt, M. Champion, S. L. Novick, K. A. Andersen, C. J. Bacchi, N. Yarlett, B. E. Britigan, and M. E. Wilson. 2000. Inducible resistance to oxidant stress in the protozoan Leishmania chagasi. J. Biol. Chem. 27533883-33889. [DOI] [PubMed] [Google Scholar]
- 38.Motley, A. M., N. Berg, M. J. Taylor, D. A. Sahlender, J. Hirst, D. J. Owen, and M. S. Robinson. 2006. Functional analysis of AP-2 alpha and mu2 subunits. Mol. Biol. Cell 175298-5308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mullin, K. A., B. J. Foth, S. C. Ilgoutz, J. M. Callaghan, J. L. Zawadzki, G. I. McFadden, and M. J. McConville. 2001. Regulated degradation of an endoplasmic reticulum membrane protein in a tubular lysosome in Leishmania mexicana. Mol. Biol. Cell 122364-2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Naderer, T., M. A. Ellis, M. F. Sernee, D. P. De Souza, J. Curtis, E. Handman, and M. J. McConville. 2006. Virulence of Leishmania major in macrophages and mice requires the gluconeogenic enzyme fructose-1,6-bisphosphatase. Proc. Natl. Acad. Sci. USA 1035502-5507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nakayama, K., and S. Wakatsuki. 2003. The structure and function of GGAs, the traffic controllers at the TGN sorting crossroads. Cell Struct. Funct. 28431-442. [DOI] [PubMed] [Google Scholar]
- 42.Ngo, H. M., M. Yang, K. Paprotka, M. Pypaert, H. Hoppe, and K. A. Joiner. 2003. AP-1 in Toxoplasma gondii mediates biogenesis of the rhoptry secretory organelle from a post-Golgi compartment. J. Biol. Chem. 2785343-5352. [DOI] [PubMed] [Google Scholar]
- 43.Price, H. P., M. Stark, and D. F. Smith. 2007. Trypanosoma brucei ARF1 plays a central role in endocytosis and golgi-lysosome trafficking. Mol. Biol. Cell 18864-873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Qiao, X., B. F. Chuang, Y. Jin, M. Muranjan, C. H. Hung, P. T. Lee, and M. G. Lee. 2006. Sorting signals required for trafficking of the cysteine-rich acidic repetitive transmembrane protein in Trypanosoma brucei. Eukaryot. Cell 51229-1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ralton, J. E., K. A. Mullin, and M. J. McConville. 2002. Intracellular trafficking of glycosylphosphatidylinositol (GPI)-anchored proteins and free GPIs in Leishmania mexicana. Biochem. J. 363365-375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ralton, J. E., T. Naderer, H. L. Piraino, T. A. Bashtannyk, J. M. Callaghan, and M. J. McConville. 2003. Evidence that intracellular beta1-2 mannan is a virulence factor in Leishmania parasites. J. Biol. Chem. 27840757-40763. [DOI] [PubMed] [Google Scholar]
- 47.Robinson, K. A., and S. M. Beverley. 2003. Improvements in transfection efficiency and tests of RNA interference (RNAi) approaches in the protozoan parasite Leishmania. Mol. Biochem. Parasitol. 128217-228. [DOI] [PubMed] [Google Scholar]
- 48.Robinson, M. S. 2004. Adaptable adaptors for coated vesicles. Trends Cell Biol. 14167-174. [DOI] [PubMed] [Google Scholar]
- 49.Roux, A., D. Cuvelier, P. Nassoy, J. Prost, P. Bassereau, and B. Goud. 2005. Role of curvature and phase transition in lipid sorting and fission of membrane tubules. EMBO J. 241537-1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sheader, K., S. Vaughan, J. Minchin, K. Hughes, K. Gull, and G. Rudenko. 2005. Variant surface glycoprotein RNA interference triggers a precytokinesis cell cycle arrest in African trypanosomes. Proc. Natl. Acad. Sci. USA 1028716-8721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sobo, K., I. Le Blanc, P. P. Luyet, M. Fivaz, C. Ferguson, R. G. Parton, J. Gruenberg, and F. G. van der Goot. 2007. Late endosomal cholesterol accumulation leads to impaired intra-endosomal trafficking. PLoS ONE 2e851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Steinbach, W. J., J. L. Reedy, R. A. Cramer, Jr., J. R. Perfect, and J. Heitman. 2007. Harnessing calcineurin as a novel anti-infective agent against invasive fungal infections. Nat. Rev. Microbiol. 5418-430. [DOI] [PubMed] [Google Scholar]
- 53.Thiel, M., S. Harder, M. Wiese, M. Kroemer, and I. Bruchhaus. 2008. Involvement of a Leishmania thymidine kinase in flagellum formation, promastigote shape and growth as well as virulence. Mol. Biochem. Parasitol. 158152-162. [DOI] [PubMed] [Google Scholar]
- 54.Touz, M. C., L. Kulakova, and T. E. Nash. 2004. Adaptor protein complex 1 mediates the transport of lysosomal proteins from a Golgi-like organelle to peripheral vacuoles in the primitive eukaryote Giardia lamblia. Mol. Biol. Cell 153053-3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tull, D., J. E. Vince, J. M. Callaghan, T. Naderer, T. Spurck, G. I. McFadden, G. Currie, K. Ferguson, A. Bacic, and M. J. McConville. 2004. SMP-1, a member of a new family of small myristoylated proteins in kinetoplastid parasites, is targeted to the flagellar membrane in Leishmania. Mol. Biol. Cell 154775-4786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Weise, F., Y. D. Stierhof, C. Kuhn, M. Wiese, and P. Overath. 2000. Distribution of GPI-anchored proteins in the protozoan parasite Leishmania, based on an improved ultrastructural description using high-pressure frozen cells. J. Cell Sci. 1134587-4603. [DOI] [PubMed] [Google Scholar]
- 57.Wiese, M. 2007. Leishmania MAP kinases—familiar proteins in an unusual context. Int. J. Parasitol. 371053-1062. [DOI] [PubMed] [Google Scholar]
- 58.Wiesgigl, M., and J. Clos. 2001. Heat shock protein 90 homeostasis controls stage differentiation in Leishmania donovani. Mol. Biol. Cell 123307-3316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Williams, R. A., L. Tetley, J. C. Mottram, and G. H. Coombs. 2006. Cysteine peptidases CPA and CPB are vital for autophagy and differentiation in Leishmania mexicana. Mol. Microbiol. 61655-674. [DOI] [PubMed] [Google Scholar]
- 60.Zhang, K., M. Showalter, J. Revollo, F. F. Hsu, J. Turk, and S. M. Beverley. 2003. Sphingolipids are essential for differentiation but not growth in Leishmania. EMBO J. 226016-6026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zilberstein, D., and M. Shapira. 1994. The role of pH and temperature in the development of Leishmania parasites. Annu. Rev. Microbiol. 48449-470. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.