Abstract
Cells slow down cell cycle progression in order to adapt to unfavorable stress conditions. Yeast (Saccharomyces cerevisiae) responds to osmotic stress by triggering G1 and G2 checkpoint delays that are dependent on the mitogen-activated protein kinase (MAPK) Hog1. The high-osmolarity glycerol (HOG) pathway is also activated by arsenite, and the hog1Δ mutant is highly sensitive to arsenite, partly due to increased arsenite influx into hog1Δ cells. Yeast cell cycle regulation in response to arsenite and the role of Hog1 in this process have not yet been analyzed. Here, we found that long-term exposure to arsenite led to transient G1 and G2 delays in wild-type cells, whereas cells that lack the HOG1 gene or are defective in Hog1 kinase activity displayed persistent G1 cell cycle arrest. Elevated levels of intracellular arsenite and “cross talk” between the HOG and pheromone response pathways, observed in arsenite-treated hog1Δ cells, prolonged the G1 delay but did not cause a persistent G1 arrest. In contrast, deletion of the SIC1 gene encoding a cyclin-dependent kinase inhibitor fully suppressed the observed block of G1 exit in hog1Δ cells. Moreover, the Sic1 protein was stabilized in arsenite-treated hog1Δ cells. Interestingly, Sic1-dependent persistent G1 arrest was also observed in hog1Δ cells during hyperosmotic stress. Taken together, our data point to an important role of the Hog1 kinase in adaptation to stress-induced G1 cell cycle arrest.
Arsenic is a toxic and carcinogenic agent but is also part of Trisenox, a drug routinely used in the treatment of acute promyelocytic leukemia and clinically evaluated for other hematological malignancies and solid tumors (23). Several studies have established the mechanisms of arsenic action in cancer cells that include cell cycle arrest at G1 and G2/M phases, induction of differentiation, and apoptosis (5). Despite the remarkable success of arsenic in curing acute promyelocytic leukemia, its efficacy in the treatment of other types of cancer is disappointing, probably due to the simultaneous activation of prosurvival pathways that requires the use of toxic levels of this metalloid to induce apoptosis (5, 10). The anticancer activity of arsenic could be potentiated in combinatorial therapy with drugs targeting prosurvival signaling pathways. However, in contrast to the well-studied mechanisms of arsenic-induced apoptosis, the pathways that antagonize the properties of arsenic that eliminate malignant cells are not fully elucidated.
It has been shown that leukemic cells respond to arsenic by activation of the mitogen-activated protein kinase (MAPK) p38 (22), a member of a family of signal-transducing serine/threonine kinases that respond to various stimuli and stress conditions to regulate several processes, including cell proliferation and apoptosis (4, 11). The stimulation of p38 in response to arsenic requires activation of its two upstream activators, the MAPK kinases Mkk3 and Mkk6 (8). p38 transduces the arsenic signal to the serine kinase Msk1 that phosphorylates histone H3 at Ser10, which probably leads to changes in the gene expression profile of leukemic cells (10). Interestingly, pharmacological inhibition of p38 activity enhances the ability of arsenic to induce apoptosis in malignant cells (8, 10, 22, 25). The results of these studies suggest that the p38 MAPK pathway is a negative regulatory feedback mechanism to control arsenite-induced apoptosis in leukemic cells and that the resistance of many types of cancer cells may be due to the activation of p38-dependent prosurvival responses.
The budding yeast Saccharomyces cerevisiae has proved to be an excellent eukaryotic model to study the mechanisms of arsenic tolerance and also a tool to identify and characterize mammalian tolerance factors (12, 13, 19, 20, 24). Although detoxification mechanisms are well studied, little is known about how yeast detects the presence of arsenic-induced stress signals, how this information is transduced, and what the downstream targets of such responses are. Interestingly, the p38 homologues Spc1/Sty1 in the fission yeast Schizosaccharomyces pombe and Hog1 in S. cerevisiae are phosphorylated and activated in the presence of trivalent arsenite (17, 18, 21). We have shown that arsenite sensitivity of the hog1Δ mutant is largely caused by increased influx of this metalloid via the Fps1 aquaglyceroporin because Hog1-dependent phosphorylation reduces transport through Fps1 (21). However, Hog1 regulates the basal activity of Fps1, even in the absence of arsenite. In addition, genetic evidence suggested that arsenite-activated Hog1 has additional downstream effectors beside Fps1 (21). Based on the known functions of p38 in controlling cell proliferation, we hypothesized that these new Hog1 targets might be cell cycle related (4).
Indeed, it has recently been reported that Hog1 plays an important role in the regulation of the cell cycle (3, 6). Hog1 promotes G1 and G2 checkpoint arrest upon osmotic stress by negatively targeting the activity of the cyclin-dependent kinase (Cdk) Cdc28 and downregulating the abundance of cyclins that associate with Cdc28. In G1, Hog1 phosphorylates Sic1, a Cdk inhibitor of S phase cyclin-Cdk complexes, to prevent the ubiquitylation and subsequent degradation of Sic1. In addition, Hog1-dependent reduction of the expression of the G1 cyclins CLN1, CLN2, and CLB5 seems to contribute to G1 arrest in response to osmotic stress (6). On the other hand, Hog1 activation in G2 leads to Swe1 kinase stabilization to maintain inhibitory phosphorylation of M phase-specific Clb2-Cdc28 complexes and simultaneous decrease of Clb2 mRNA and protein levels (3). Premature entry of hog1Δ cells into the cell cycle is believed to decrease survival of the mutant upon osmotic stress. Here, we asked whether Hog1 would play a similar role in regulating cell cycle progression during arsenite exposure.
We report that under continuous exposure to arsenite, the hog1Δ mutant permanently arrests in the G1 phase of the cell cycle. This effect is not explained by aberrant activation of the pheromone response pathway (cross talk) or increased intracellular accumulation of arsenite; first, suppression of cross talk signaling does not abolish G1 checkpoint arrest in hog1Δ cells, and second, the acr3Δ mutant defective in arsenite efflux shows extended but not persistent G1 arrest. These data suggest a novel function(s) of Hog1 in adaptation to stress-induced G1 checkpoint arrest. Here, we found that during arsenite and hyperosmotic stress, the hog1Δ mutant showed a lack of Sic1 degradation, leading to failure to restart the cell cycle and to enter S phase. Moreover, persistent G1 cell cycle arrest in hog1Δ cells is relieved by deletion of the SIC1 gene. We conclude that Hog1 promotes Sic1 degradation to terminate stress-induced G1 delay.
MATERIALS AND METHODS
Yeast strains and growth conditions.
The yeast strains used in this study are listed in Table 1. Yeast strains were grown in standard rich medium (yeast extract-peptone-dextrose [YPD]) or in selective synthetic minimal medium at 26°C. Gene deletions were performed by PCR-based gene modification (14), and transformations were done by the lithium acetate procedure (9). Arsenite sensitivity assays were carried out as previously described (28).
TABLE 1.
Yeast strains used in this study
| Strain | Genotype | Source |
|---|---|---|
| W303-1A | MATaura3 leu2 trp1 his3 ade2 can1 | S. Hohmann |
| YSH818 | W303-1A hog1Δ::LEU2 | S. Hohmann |
| RW104 | W303-1A acr3Δ::kanMX | R. Wysocki |
| RW146 | W303-1A sic1Δ::kanMX | This work |
| RW147 | W303-1A SIC1::3HA-kanMX | This work |
| RW148 | W303-1A hog1Δ::TRP1 sic1Δ::kanMX | This work |
| YYI1 | W303-1A hog1Δ::TRP1 SIC1::3HA-kanMX | This work |
| YMT20 | W303-1A hog1Δ::LEU2 sho1Δ::TRP1 | M. Tamás |
| YIM2 | W303-1A hog1Δ::LEU2 msb2Δ::kanMX sho1Δ::TRP1 | This work |
| YAL51 | W303-1A hog1Δ::kanMX Yip-URA3-HOG1-as | F. Posas |
Cell cycle experiments.
Cell cycle synchronization and flow cytometry analysis of DNA content were performed as previously described (29, 30). The fraction of cells remaining arrested in G1 in the presence of arsenite was determined by means of an α-factor-nocodazole trap assay (7, 29, 30). Briefly, at 20-min intervals, 0.5-ml samples were collected, washed to remove arsenite from the medium, and resuspended in YPD medium. Next, samples were combined with 0.5 ml trapping YPD medium (10 μM α-factor, 30 μg/ml nocodazole) and incubated for 90 min at 26°C, fixed, and examined by phase microscopy to count cells displaying mating projections (G1 cells) or buds (post-G1 cells).
To analyze the G2/M cell cycle checkpoint, log-phase cultures were arrested with 15 μg/ml nocodazole for 180 min, washed, and released in fresh medium in the absence or presence of 0.5 mM sodium arsenite. Aliquots were removed every 30 min, fixed, treated with RNase, stained with Sytox green, and then examined by epifluorescence microscopy (Zeiss AxioImager.M1, fluorescein isothiocyanate filter set, 40/0.75× numerical aperture objective) to score the percentage of binucleated large-budded cells. All cell cycle experiments were repeated a minimum of three times, and representative results are presented.
Cell extracts and immunoblotting.
Exponentially growing cells (in YPD at 26°C) were treated with α-factor to induce G1 arrest and then washed with 5 volumes of YP (1% yeast extract, 2% peptone) to get rid of α-factor and resuspended in the initial volume of YPD. Cell samples were collected before and after the addition of 0.5 mM sodium arsenite or 0.6 M NaCl, and proteins were extracted in buffer A (100 mM Tris-HCl (pH 6.8), 20% glycerol, 200 mM dithiothreitol, 4% sodium dodecyl sulfate, 10 mM NaF, 0.1 mM Na3VO4, 20 mM β-mercaptoethanol, and a protease inhibitor cocktail) by boiling. Extracted proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and blotted onto nitrocellulose filters. Sic1 tagged with hemagglutinin (Sic1-3HA) and phosphorylated Fus3 were detected by using an anti-HA antibody (Sigma) and an anti-phospho-p44/42 MAPK antibody (Cell Signaling Technology), respectively. An anti-PSTAIRE antibody (sc-53; Santa Cruz Biotechnology) was used to detect total Cdc28 as a loading control. Alternatively, the blotted membranes were stained for total protein with Ponceau red (Sigma) before immunodetection.
DIC microscopy.
To analyze the morphology of yeast strains, mid-log phase or G1-synchronized cells were released in fresh YPD medium, in medium containing 5 μM α-factor, or medium containing 0.5 mM sodium arsenite. After 5 h of incubation, cells were harvested, fixed in 70% (vol/vol) ethanol, and examined by differential interference contrast (DIC) microscopy (Zeiss AxioImager.M1, 100/1.3× numerical aperture objective).
RESULTS AND DISCUSSION
It has been shown that Hog1 regulates cell cycle progression in response to osmotic stress (3, 6). Hog1 is also activated by arsenite and antimonite, and the hog1Δ mutant is highly sensitive to these metalloids (18, 21). We hypothesized that defects in cell cycle regulation may contribute to the metalloid sensitivity of hog1Δ cells. To test this, we monitored cell cycle progression in wild-type and hog1Δ cells in the presence of 0.5 mM arsenite, a concentration that induces Hog1 phosphorylation and inhibits the growth of hog1Δ cells (21). First, we used flow cytometry to test the effect of arsenite on the kinetics of cell cycle progression in asynchronously growing yeast cells (Fig. 1A). Within the first hour of arsenite exposure, wild-type and hog1Δ cells arrested in both G1 and G2 phases of the cell cycle. After 2 h, G1-arrested wild-type cells initiated slow S phase and accumulated in G2, while hog1Δ cells showed strong accumulation in G1 and remained in this phase for the duration of the experiment. These results strongly suggest that the hog1Δ mutant cannot recover from arsenite-induced G1 checkpoint arrest.
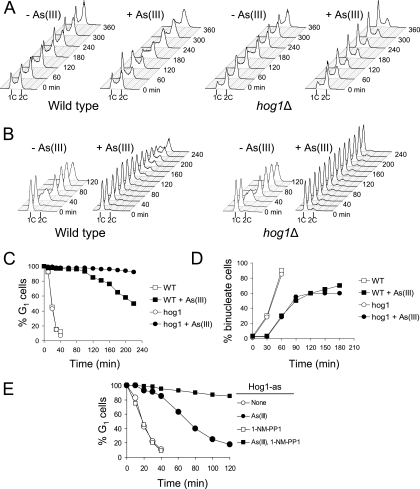
FIG. 1.
Persistent G1 cell cycle arrest in hog1Δ cells during continuous exposure to arsenite. (A) The results of flow cytometry reveal accumulation of G1 cells in asynchronously growing hog1Δ cells in the presence of 0.5 mM arsenite. (B) G1-synchronized hog1Δ cells fail to restart the cell cycle under permanent arsenite stress. (C) The α-factor-nocodazole trap assay results confirm persistent G1 arrest of hog1Δ cells. (D) Duration of G2/M checkpoint delay in hog1Δ cells is not affected during arsenite exposure. (E) Lack of Hog1 kinase activity results in a persistent G1 arrest, similar to that of hog1Δ cells. Cells with the analogue-sensitive HOG1-as allele were synchronized in G1 with α-factor, exposed to 12 μM of the inhibitor 1-NM-PP1 for 5 min, washed in the presence of inhibitor, and released in fresh medium containing 12 μM 1-NM-PP1 in the presence or absence of 0.25 mM arsenite. In a control experiment, no inhibitor was added. At 10-min or 20-min intervals, samples were collected for the α-factor-nocodazole trap assay. As(III), arsenite; +, present; −, absent; 1C and 2C, values of DNA content; WT, wild type.
To confirm this, wild-type and hog1Δ cells were synchronized in G1 with α-factor and released in fresh medium with or without arsenite. In the absence of arsenite, both strains initiated DNA replication within 20 min from the release as measured by an increase in DNA content (Fig. 1B). In response to arsenite, wild-type cells showed a 120-min G1 delay followed by slow S phase, while hog1Δ cells remained arrested in G1 with no indication of replication onset for the duration of the experiment. However, flow cytometry analysis does not distinguish G1 cells from early S-phase cells, which show only a slight increase in DNA content. To confirm a bona fide G1 arrest in hog1Δ cells during arsenite exposure, we performed an α-factor-nocodazole trap assay (7, 29, 30) using the same conditions as described above (Fig. 1C). At 20-min intervals after α-factor release, cells were collected, washed, and combined with medium containing α-factor and nocodazole. After 90 min of incubation in trapping medium, cells were examined by phase microscopy to determine the percentage of G1 cells that remained sensitive to α-factor and showed mating projections, in contrast to large-budded post-G1 cells trapped by nocodazole at G2/M phase. The results of the α-factor-nocodazole trap assay confirmed that long-term exposure to arsenite caused a persistent G1 arrest in the hog1Δ mutant (Fig. 1C). Interestingly, when hog1Δ cells were synchronized in G2/M phase by nocodazole and released in medium containing arsenite, we observed a normal G2/M delay, suggesting that the checkpoint adaptation defect of hog1Δ is restricted to the G1 checkpoint (Fig. 1D).
The observed cell cycle defect could be the result of adaptive changes in the physiology of hog1Δ cells chronically devoid of the Hog1 protein and/or its kinase activity. To demonstrate that the lack of Hog1 kinase activity, just at the time of arsenite-induced G1 checkpoint arrest, is responsible for the inability of hog1Δ cells to resume growth, we took advantage of an analogue-sensitive mutant of Hog1 (Hog1-as); Hog1-as retains wild-type function in the absence of inhibitor, but its activity is affected by the chemical inhibitor 4-amino-1-tert-butyl-3-(1-naphthylmethyl)phenylpyrazolo[3,4-d]pyrimidine (1-NM-PP1) (26). We found that the addition of 1-NM-PP1 to G1-synchronized Hog1-as cells during release from cell cycle arrest in the presence of 0.25 mM arsenite resulted in persistent G1 arrest, while in medium without inhibitor, Hog1-as cells restarted the cell cycle in 40 to 60 min (Fig. 1E). Taken together, our findings demonstrate that the catalytic function of Hog1 is required for the resumption of cell cycle progression following the transient G1 checkpoint delay in the presence of arsenite.
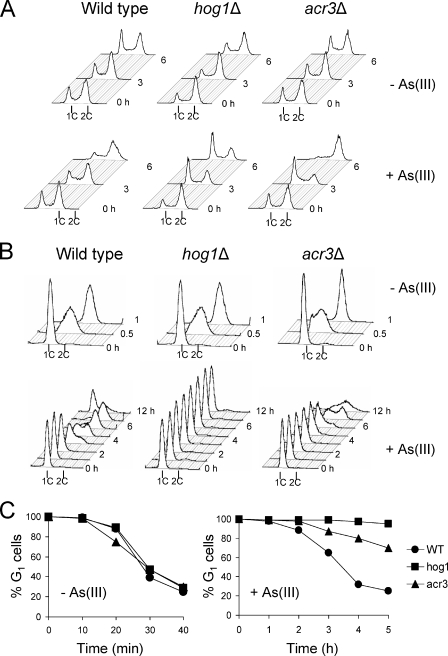
Having described the persistent G1 arrest phenotype of hog1Δ cells during arsenite exposure, we sought to determine a mechanism leading to this cell cycle defect. We recently showed that cells lacking Hog1 exhibit an increased influx of arsenite (21). To test whether persistent G1 arrest in hog1Δ cells is a result of elevated intracellular arsenite levels, we monitored cell cycle progression in asynchronous and G1-synchronized cells lacking the arsenite efflux transporter gene ACR3 (Fig. 2). Indeed, in asynchronous culture, both acr3Δ and hog1Δ cells arrested in G1 phase within 3 h after the addition of 0.5 mM arsenite (Fig. 2A). However, in contrast to hog1Δ cells, the acr3Δ mutant was able to recover from this G1 delay and accumulated at G2 phase. Similarly, the G1-synchronized culture of acr3Δ showed G1 arrest for 3 h, which is 1 h longer than for wild-type cells, but then initiated slow S phase as measured by an increase in DNA content (Fig. 2B) and loss of sensitivity toward mating pheromone (Fig. 2C). Thus, despite the fact that acr3Δ cells accumulate significantly more arsenite than hog1Δ cells (21, 27), prolonged but not persistent G1 arrest was observed in arsenite-exposed acr3Δ cells. These results indicate that the lack of exit from G1 arrest in hog1Δ cells is not merely a result of elevated cytosolic arsenite levels.
FIG. 2.
Defect in adaptation to arsenite-induced G1 checkpoint arrest is not solely caused by increased accumulation of arsenite. The acr3Δ mutant lacking the arsenite efflux transporter and exhibiting elevated levels of arsenite in the cytosol shows prolonged but not persistent G1 arrest, as shown by the results of flow cytometry in asynchronous (A) and G1-synchronized cells (B), as well as by the results of the α-factor-nocodazole trap assay (C). As(III), arsenite; +, present; −, absent; 1C and 2C, values of DNA content; WT, wild type.
Previous studies have shown “cross talk” between the high-osmolarity glycerol (HOG) and pheromone response MAPK pathways (15, 16). In wild-type cells, the pheromone response pathway is only stimulated by the presence of pheromones leading to the activation of mating-specific genes, G1 arrest, and changes in cell morphology (1). However, when the HOG pathway is activated by osmotic stress in cells lacking the MAPK Hog1, the stress signal is transduced to Fus3, the MAPK of the pheromone response pathway. Cross talk involves the MAPK kinase kinase Ste11 which is a common component of both the HOG and pheromone response pathways, as well as the upstream “sensors” of the HOG pathway Sho1 and Msb2. Hence, in wild-type cells, Hog1 prevents such cross talk to maintain signaling specificity (15, 16). Thus, we asked whether the persistent G1 delay observed in arsenite-exposed hog1Δ cells could be caused by inappropriate activation of the pheromone response pathway.
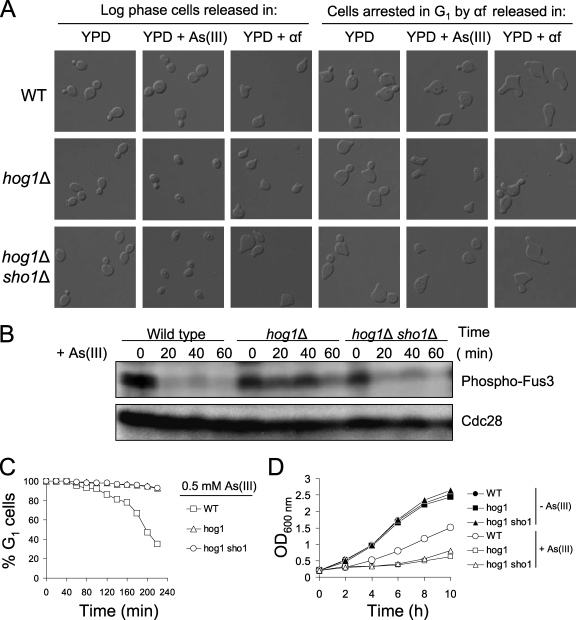
In response to mating factor and during cross talk in hog1Δ cells, the polarization of the cytoskeleton is changed, resulting in the formation of pear-shaped cells called shmoo (15). Therefore, we examined the cell morphology of hog1Δ and wild-type cells from exponentially growing cultures exposed to arsenite for 5 h (Fig. 3A). The results of DIC microscopy revealed that 90% of the arsenite-exposed hog1Δ cells were unbudded and ∼20% exhibited a shmoo-like morphology. Consistent with the flow cytometry data (see Fig. 1), arsenite induced large-budded morphology in wild-type cells, indicating G2/M arrest (Fig. 3A). We performed a similar experiment using G1-synchronized cells released in the presence of arsenite or α-factor. Extended exposure of G1-arrested wild-type and hog1Δ cells to α-factor resulted in the formation of additional mating projections which were also seen in G1-synchronized hog1Δ cells released in medium containing arsenite (Fig. 3A). By contrast, wild-type cells did not respond to arsenite by forming additional shmoos but instead started budding. These results indicate that an aberrant activation of the pheromone response pathway might be responsible for the arsenite-induced G1 arrest of hog1Δ cells.
FIG. 3.
Arsenite activates the pheromone response pathway in cells lacking Hog1. (A) Long-term incubation of hog1Δ cells in the presence of arsenite results in a shmoo-like morphology. (B) The pheromone response MAPK Fus3 is phosphorylated in the hog1Δ mutant in response to arsenite. (C) Suppressing cross talk signaling by deletion of SHO1 does not prevent persistent G1 cell cycle arrest in arsenite-treated cells lacking the Hog1 kinase. G1/S transition kinetics in wild-type and HOG pathway mutants was analyzed by the α-factor-nocodazole trap method. (D) Growth of hog1Δ cells in the presence of 0.5 mM arsenite is not improved by additional deletion of SHO1. As(III), arsenite; αf, α-factor; +, present; −, absent; WT, wild type; OD600 nm, optical density at 600 nm.
Yeast responds to mating pheromones by phosphorylating the MAPK Fus3, which in turn activates the Cdc28 inhibitor Far1 to impose G1 cell cycle arrest, as well as transcription of genes involved in mating (1). To confirm cross talk signaling in arsenite-treated hog1Δ cells at the molecular level, we monitored the activation of the pheromone response pathway in these cells by assessing the phosphorylation of the MAPK Fus3 using an anti-phospho-p44/42 MAPK antibody (Fig. 3B). As expected, G1-arrested hog1Δ cells released in the presence of 0.5 mM arsenite maintained a high level of phosphorylated Fus3, suggesting ongoing activation of mating response in the absence of α-factor. In contrast, wild-type cells washed free of mating pheromone lost activation of the MAPK Fus3 shortly after release in the presence of arsenite (Fig. 3B). These results provide further evidence for the activation of the pheromone response pathway in hog1Δ cells exposed to arsenite.
Osmotic stress-induced cross talk between the HOG and the pheromone response pathways can be prevented by deleting the putative osmosensor Sho1 (15). Thus, we determined whether mutations in genes responsible for cross talk signaling can reverse persistent G1 arrest in hog1Δ cells. An hog1Δ sho1Δ double mutant was synchronized in G1 with α-factor, released in fresh medium containing 0.5 mM arsenite, and then examined under a microscope and assayed by α-factor-nocodazole trap analysis. Interestingly, although the hog1Δ sho1Δ double mutant did not show additional mating projections in the presence of arsenite, indicating loss of signaling cross talk, cells of this mutant did not initiate bud formation, suggesting that the persistent G1 arrest was not relieved (Fig. 3A). We confirmed that cross talk signaling in the hog1Δ sho1Δ strain was lost, since Fus3 phosphorylation was suppressed after release from α-factor arrest despite the presence of arsenite in the medium (Fig. 3B). Moreover, the α-factor-nocodazole trap analysis showed that the additional deletion of the SHO1 gene in hog1Δ cells did not abrogate the prolonged G1 delay and entry into S phase (Fig. 3C). In agreement with these results, the deletion of SHO1 in the hog1Δ background did not improve the growth of hog1Δ cells in the presence of arsenite (Fig. 3D). However, prolonged G1 arrest in the double hog1Δ sho1Δ mutant could potentially be maintained by residual cross talk signaling from the second osmosensor, Msb2 (15). Nevertheless, an hog1Δ msb2Δ sho1Δ triple mutant was also unable to restart the cell cycle after arsenite-induced G1 arrest (data not shown). In sum, during chronic arsenite exposure, cells lacking the MAPK Hog1 exhibited cross talk from the HOG to the pheromone response pathway. Such cross talk, together with increased influx of arsenite via the glycerol channel Fps1, may contribute to the prolonged G1 delay of hog1Δ cells. However, the deletion of genes responsible for cross talk signaling did not suppress the G1 phase exit defect of hog1Δ cells, strongly suggesting a role of arsenite-activated Hog1 in controlling specific target(s) to promote adaptation to G1 arrest during arsenite stress.
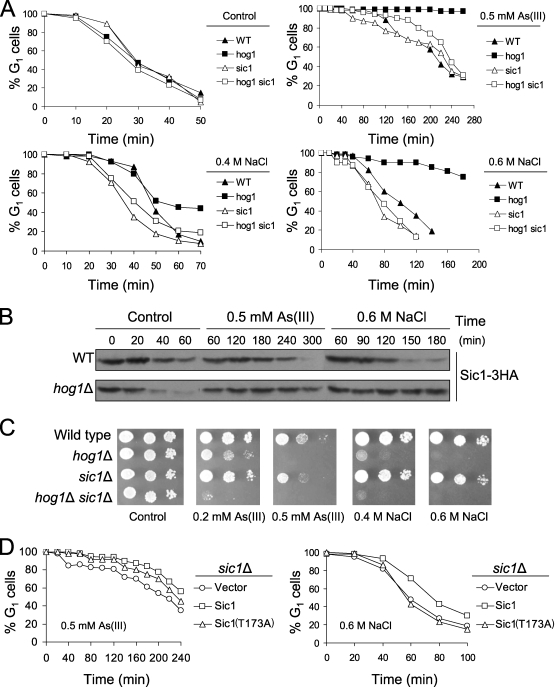
Recently, it was shown that the Cdk Pho85-dependent degradation of Cdk inhibitor Sic1 is required for adaptation to G1 checkpoint arrest after DNA damage (30). Thus, we examined the effect of arsenite on the kinetics of G1/S transition in the sic1Δ single and hog1Δ sic1Δ double mutants in the presence of 0.5 mM arsenite (Fig. 4A). We found that arsenite-treated sic1Δ cells exhibited only a 60-min G1 delay, followed by a premature entry into S phase, while wild-type cells restarted the cell cycle within 120 min after α-factor release. Furthermore, the deletion of SIC1 abolished persistent G1 arrest in hog1Δ cells. These data suggest that Sic1 is not required for the initial arsenite-induced G1 checkpoint arrest but is necessary for maintaining the cell cycle delay. Therefore, we hypothesized that Hog1 promotes timely entry into S phase by inducing Sic1 degradation. In order to test whether the length of G1 delay is linked to Sic1 stabilization, we assayed the abundance of HA-tagged Sic1 in arsenite-exposed wild-type and hog1Δ cells (Fig. 4B). In a control experiment, the level of Sic1 was significantly reduced after 20 min from α-factor release in both strains. Arsenite delayed the degradation of Sic1 by 180 min in wild-type cells, while Sic1 remained stable in hog1Δ cells for the whole course of the experiment.
FIG. 4.
Hog1 triggers Sic1 degradation to promote adaptation to stress-induced G1 checkpoint arrest. (A) Deletion of SIC1 suppresses prolonged G1 delay of hog1Δ mutant in the presence of arsenite and sodium chloride. G1-synchronized cells were released in the presence or absence of 0.5 mM arsenite or 0.4 M or 0.6 M NaCl and analyzed by using an α-factor-nocodazole trap assay. (B) Sic1 remains stable in hog1Δ cells during arsenite and hyperosmotic stress. Cells were treated as described above, and Sic1 levels were monitored by Western blot analysis using an anti-HA antibody. (C) Bypass of persistent G1 arrest by deletion of SIC1 does not increase tolerance of hog1Δ cells for arsenite and sodium chloride. (D) Expression of Sic1(T173A) mutant lacking the Hog1 phosphorylation site does not phenocopy the cell cycle defect of hog1Δ mutation. Cells with plasmid pCM189 (vector [control]), pCM189-SIC1-4HA, or pCM189-SIC1T173A-4HA were synchronized in G1 with α-factor, exposed to 2 μg/ml doxycycline for 30 min to turn off expression of SIC1, washed, released in fresh medium containing 2 μg/ml doxycycline in the presence or absence of 0.5 mM arsenite or 0.6 M NaCl, and assayed by α-factor-nocodazole trapping. As(III), arsenite; WT, wild type.
It was reported that hyperosmotic-stress-induced cell cycle arrest in G1 involves Hog1-dependent stabilization of Sic1 and that the deletion of SIC1 suppressed the G1 cell cycle arrest observed in wild-type cells during hyperosmotic stress induced by 0.4 M NaCl (6). However, how HOG1 deletion would affect the kinetics of G1/S transition was not tested. Given the role of Hog1 in promoting G1 arrest by preventing Sic1 degradation after osmotic stress, we decided to monitor the kinetics of G1/S transition and the stability of Sic1 in the presence of 0.4 M and 0.6 M NaCl (Fig. 4A and B). The rationale behind using two concentrations of salt was that 0.4 M NaCl only reduces the growth of hog1Δ cells, while 0.6 M NaCl completely inhibits growth, suggesting a cell cycle defect (Fig. 4C). The results of α-factor-nocodazole trap analysis revealed that wild-type and hog1Δ cells showed a similar transitory G1 arrest in the presence of moderate osmotic stress in the form of 0.4 M NaCl (Fig. 4A). In contrast, G1 arrest was prolonged in the hog1Δ mutant exposed to 0.6 M NaCl. Moreover, the persistence of G1 arrest in hog1Δ cells in the presence of acute osmotic stress was suppressed by deleting SIC1. Consistently, Sic1 was stabilized in hog1Δ cells exposed to 0.6 M NaCl (Fig. 4B). Notably, our data are consistent with the results obtained for the hog1Δ mutant in earlier work of Bellí and coworkers, whose results also suggest the involvement of Hog1 in recovery from G1 arrest after exposure to 0.6 M NaCl (2). Finally, we compared the growth of the tested mutants in the presence of arsenite and NaCl in order to assay the impact of cell cycle defects on survival after stress (Fig. 4C). The sic1Δ single mutant showed slight sensitivity to arsenite and osmotic stress, while the hog1Δ sic1Δ double mutant was more sensitive to both agents than either single mutant. This suggests that premature entry into S phase in sic1Δ or bypass of persistent G1 arrest in hog1Δ cells had little influence on cell survival after stress.
Next, we asked whether Sic1 degradation during adaptation to G1 arrest is the result of direct phosphorylation of Sic1 by Hog1. Hog1 targets a single phosphorylation site in Sic1 at threonine 173 in response to 0.4 M NaCl (6). Thus, we tested the cell cycle progression of sic1Δ cells transformed with the plasmids bearing the wild-type Sic1 or the mutant Sic1(T173A) version under the control of a tetracycline-repressible promoter (31). We found that cells expressing Sic1(T173A) did not exhibit persistent G1 arrest but entered S phase earlier than cells containing wild-type Sic1 in the presence of 0.5 mM arsenite or 0.6 M NaCl (Fig. 4D). In addition, Sic1(T173A) complemented the arsenite sensitivity of sic1Δ (data not shown). Thus, we infer that Hog1-mediated phosphorylation of Sic1 at threonine 173 is not required for adaptation to G1 checkpoint arrest and tolerance to arsenite. Our results are in agreement with previous findings showing that during 0.4 M NaCl (moderate osmotic stress) or rapamycin (mimetic of nutrient starvation) treatment, phosphorylation of Sic1 at T173 interferes with Sic1 binding to the ubiquitin-protein ligase complex, resulting in Sic1 stabilization (6, 31). However, Sic1 possesses nine putative MAPK phosphorylation sites and we cannot exclude that Hog1 phosphorylates Sic1 at additional sites to promote Sic1 binding to the ubiquitin-protein ligase complex SCFCdc4 at the time of resumption of cell cycle during arsenite stress. On the other hand, Hog1 may also induce degradation of Sic1 indirectly by upregulating the expression of G1 cyclins and/or components of the SCFCdc4 complex.
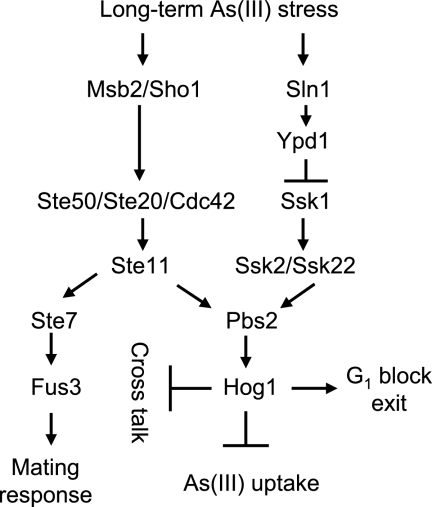
Altogether, our results and data in the literature suggest that Hog1 plays multiple roles during arsenite stress, including downregulation of arsenite influx (21), suppressing cross talk signaling to the pheromone pathway, and adaptation to G1 checkpoint arrest by promoting degradation of the Cdk inhibitor Sic1 (Fig. 5). Regarding exit from the G1 block, the mechanisms of Hog1-mediated degradation of Sic1 remain to be defined.
FIG. 5.
Model depicting the role of Hog1 during arsenite exposure. Long-term exposure to arsenite results in Hog1-independent cell cycle arrest in G1 and G2/M. After a transient delay, wild-type cells adapt and resume growth despite the presence of arsenite. In contrast, cells lacking Hog1 are not able to exit the G1 arrest to restart the cell cycle. When Hog1 is absent, stimulation of the Sho1/Mbs2 branch of the HOG pathway results in cross talk signaling to the pheromone pathway, phosphorylation of the MAPK Fus3, and activation of mating response, including G1 cell cycle arrest. However, suppressing cross talk signaling in the hog1Δ mutant by deleting SHO1 and MSB2 does not relieve arsenite-induced persistent G1 delay. This suggests that Hog1 itself may regulate specific targets to promote adaptation to G1 cell cycle arrest. Indeed, we found that arsenite-induced G1 arrest is associated with stabilization of the Cdk inhibitor Sic1 and that Hog1 is required to induce Sic1 degradation and promote exit from G1 phase. In addition, Hog1 downregulates the activity of the glycerol channel Fps1 to reduce arsenite influx (21). As(III), arsenite.
Notably, our data provide evidence that it is possible to achieve permanent cell cycle arrest by combining drug action with manipulations of signaling pathways. Interestingly, it has recently been shown that pharmacological inhibition of the p38 MAPK pathway or targeted disruption of p38α, a homolog of Hog1, increased the antileukemic properties of arsenic trioxide, including both growth inhibition and the induction of apoptosis (8, 25). In addition, it was shown that elevated activation of p38 MAPK associates with the appearance of arsenic trioxide resistance in myeloma cells (25). Thus, pharmacological downregulation of the p38 MAPK pathway might be a means to overcome resistance of cancer cells to arsenic trioxide therapy. In summary, our results show that studies of responses to arsenite treatment in yeast cells may contribute to the understanding of arsenic's action in human cells and of how to enhance the anticancer activity of arsenic.
Acknowledgments
We thank Lilia Alberghina, Paola Coccetti, Stefan Hohmann, and Francesc Posas for sharing yeast strains and plasmids, Morten Grøtli for the 1-NM-PP1 inhibitor, and Steve Kron for helpful discussions.
This study was supported by grants PBZ-Min-015/P05/2004 and N30105931/1785 from the Ministry of Science and Higher Education research funds in the years 2006 to 2009 (to R.W.) and by the Swedish Research Council (VR), the Carl Trygger Foundation, and the Chemical Biology Platform at the University of Gothenburg (to M.J.T.).
Footnotes
Published ahead of print on 13 June 2008.
REFERENCES
- 1.Bardwell, L. 2005. A walk-through of the yeast mating pheromone response pathway. Peptides 26339-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bellí, G., E. Garí, M. Aldea, and E. Herrero. 2001. Osmotic stress causes a G1 cell cycle delay and downregulation of Cln3/Cdc28 activity in Saccharomyces cerevisiae. Mol. Microbiol. 391022-1035. [DOI] [PubMed] [Google Scholar]
- 3.Clotet, J., X. Escote, M. A. Adrover, G. Yaakov, E. Gari, M. Aldea, E. de Nadal, and F. Posas. 2006. Phosphorylation of Hsl1 by Hog1 leads to a G2 arrest essential for cell survival at high osmolarity. EMBO J. 252338-2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cuenda, A., and S. Rousseau. 2007. p38 MAP-kinases pathway regulation, function and role in human diseases. Biochim. Biophys. Acta 17731358-1375. [DOI] [PubMed] [Google Scholar]
- 5.Dilda, P. J., and P. J. Hogg. 2007. Arsenical-based cancer drugs. Cancer Treat. Rev. 33542-564. [DOI] [PubMed] [Google Scholar]
- 6.Escote, X., M. Zapater, J. Clotet, and F. Posas. 2004. Hog1 mediates cell-cycle arrest in G1 phase by the dual targeting of Sic1. Nat. Cell Biol. 6997-1002. [DOI] [PubMed] [Google Scholar]
- 7.FitzGerald, J. N., J. M. Benjamin, and S. J. Kron. 2002. Robust G1 checkpoint arrest in budding yeast: dependence on DNA damage signaling and repair. J. Cell Sci. 1151749-1757. [DOI] [PubMed] [Google Scholar]
- 8.Giafis, N., E. Katsoulidis, A. Sassano, M. S. Tallman, L. S. Higgins, A. R. Nebreda, R. J. Davis, and L. C. Platanias. 2006. Role of the p38 mitogen-activated protein kinase pathway in the generation of arsenic trioxide-dependent cellular responses. Cancer Res. 666763-6771. [DOI] [PubMed] [Google Scholar]
- 9.Gietz, R. D., and R. H. Shiestl. 2007. High-efficiency yeast transformation using the LiAc/SS carrier DNA/PEG method. Nat. Protoc. 231-34. [DOI] [PubMed] [Google Scholar]
- 10.Kannan-Thulasiraman, P., E. Katsoulidis, M. S. Tallman, J. S. Arthur, and L. C. Platanias. 2006. Activation of the mitogen- and stress-activated kinase 1 by arsenic trioxide. J. Biol. Chem. 28122446-22452. [DOI] [PubMed] [Google Scholar]
- 11.Katz, M., I. Amit, and Y. Yarden. 2007. Regulation of MAPKs by growth factors and receptor tyrosine kinases. Biochim. Biophys. Acta 17731161-1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu, Z., M. A. Sanchez, X. Jiang, E. Boles, S. M. Landfear, and B. P. Rosen. 2006. Mammalian glucose permease GLUT1 facilitates transport of arsenic trioxide and methylarsonous acid. Biochem. Biophys. Res. Commun. 351424-430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu, Z., J. Shen, J. M. Carbrey, R. Mukhopadhyay, P. Agre, and B. P. Rosen. 2002. Arsenite transport by mammalian aquaglyceroporins AQP7 and AQP9. Proc. Natl. Acad. Sci. USA 996053-6058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Longtine, M. S., A. McKenzie III, D. J. Demarini, N. G. Shah, A. Wach, A. Brachat, P. Philippsen, and J. R. Pringle. 1998. Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast 14953-961. [DOI] [PubMed] [Google Scholar]
- 15.O'Rourke, S. M., and I. Herskowitz. 1998. The Hog1 MAPK prevents cross talk between the HOG and pheromone response MAPK pathways in Saccharomyces cerevisiae. Genes Dev. 122874-2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O'Rourke, S. M., and I. Herskowitz. 2002. A third osmosensing branch in Saccharomyces cerevisiae requires the Msb2 protein and functions in parallel with the Sho1 branch. Mol. Cell. Biol. 224739-4749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rodriguez-Gabriel, M. A., and P. Russell. 2005. Distinct signaling pathways respond to arsenite and reactive oxygen species in Schizosaccharomyces pombe. Eukaryot. Cell 41396-1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sotelo, J., and M. A. Rodriguez-Gabriel. 2006. Mitogen-activated protein kinase Hog1 is essential for the response to arsenite in Saccharomyces cerevisiae. Eukaryot. Cell 51826-1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tamás, M. J., J. Labarre, M. B. Toledano, and R. Wysocki. 2006. Mechanisms of toxic metal tolerance in yeast, p. 395-454. In M. J. Tamás and E. Martinoia (ed.), Molecular biology of metal homeostasis and detoxification: from microbes to man. Springer, Heidelberg, Germany.
- 20.Tamás, M. J., and R. Wysocki. 2001. Mechanisms involved in metalloid transport and tolerance acquisition. Curr. Genet. 402-12. [DOI] [PubMed] [Google Scholar]
- 21.Thorsen, M., Y. Di, C. Tängemo, M. Morillas, D. Ahmadpour, C. Van der Does, A. Wagner, E. Johansson, J. Boman, F. Posas, R. Wysocki, and M. J. Tamás. 2006. The MAPK Hog1p modulates Fps1p-dependent arsenite uptake and tolerance in yeast. Mol. Biol. Cell 174400-4410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Verma, A., M. Mohindru, D. K. Deb, A. Sassano, S. Kambhampati, F. Ravandi, S. Minucci, D. V. Kalvakolanu, and L. C. Platanias. 2002. Activation of Rac1 and the p38 mitogen-activated protein kinase pathway in response to arsenic trioxide. J. Biol. Chem. 27744988-44995. [DOI] [PubMed] [Google Scholar]
- 23.Verstovsek, S., F. Giles, A. Quintás-Cardama, N. Perez, F. Ravandi-Kashani, M. Beran, E. Freireich, and H. Kantarjian. 2006. Arsenic derivatives in hematologic malignancies: a role beyond acute promyelocytic leukemia? Hematol. Oncol. 24181-188. [DOI] [PubMed] [Google Scholar]
- 24.Vujcic, M., M. Shroff, and K. K. Singh. 2007. Genetic determinants of mitochondrial response to arsenic in yeast Saccharomyces cerevisiae. Cancer Res. 679740-9749. [DOI] [PubMed] [Google Scholar]
- 25.Wen, J., H. Y. Cheng, Y. Feng, L. Rice, S. Liu, A. Mo, J. Huang, Y. Zu, D. J. Ballon, and C. C. Chang. 2008. P38 MAPK inhibition enhancing ATO-induced cytotoxicity against multiple myeloma cells. Br. J. Haematol. 140169-180. [DOI] [PubMed] [Google Scholar]
- 26.Westfall, P. J., and J. Thorner. 2006. Analysis of mitogen-activated protein kinase signaling specificity in response to hyperosmotic stress: use of an analog-sensitive HOG1 allele. Eukaryot. Cell 51215-1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wysocki, R., P. Bobrowicz, and S. Ulaszewski. 1997. The Saccharomyces cerevisiae ACR3 gene encodes a putative membrane protein involved in arsenite transport. J. Biol. Chem. 27230061-30066. [DOI] [PubMed] [Google Scholar]
- 28.Wysocki, R., C. C. Chéry, D. Wawrzycka, M. Van Hulle, R. Cornelis, J. M. Thevelein, and M. J. Tamás. 2001. The glycerol channel Fps1p mediates the uptake of arsenite and antimonite in Saccharomyces cerevisiae. Mol. Microbiol. 401391-1401. [DOI] [PubMed] [Google Scholar]
- 29.Wysocki, R., A. Javaheri, S. Allard, F. Sha, J. Côté, and S. J. Kron. 2005. Role of Dot1-dependent histone H3 methylation in G1 and S phase DNA damage checkpoint functions of Rad9. Mol. Cell. Biol. 258430-8443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wysocki, R., A. Javaheri, K. Kristjansdottir, F. Sha, and S. J. Kron. 2006. CDK Pho85 targets CDK inhibitor Sic1 to relieve yeast G1 checkpoint arrest after DNA damage. Nat. Struct. Mol. Biol. 13908-914. [DOI] [PubMed] [Google Scholar]
- 31.Zinzalla, V., M. Graziola, A. Mastriani, M. Vanoni, and L. Alberghina. 2007. Rapamycin-mediated G1 arrest involves regulation of the Cdk inhibitor Sic1 in Saccharomyces cerevisiae. Mol. Microbiol. 631482-1494. [DOI] [PubMed] [Google Scholar]