Abstract
In several species, including rodents and fish, it has been shown that the Major Histocompatibility Complex (MHC) influences mating preferences and, in some cases, that this may be mediated by preferences based on body odour. In humans, the picture has been less clear. Several studies have reported a tendency for humans to prefer MHC-dissimilar mates, a sexual selection that would favour the production of MHC-heterozygous offspring, who would be more resistant to pathogens, but these results are unsupported by other studies. Here, we report analyses of genome-wide genotype data (from the HapMap II dataset) and HLA types in African and European American couples to test whether humans tend to choose MHC-dissimilar mates. In order to distinguish MHC-specific effects from genome-wide effects, the pattern of similarity in the MHC region is compared to the pattern in the rest of the genome. African spouses show no significant pattern of similarity/dissimilarity across the MHC region (relatedness coefficient, R = 0.015, p = 0.23), whereas across the genome, they are more similar than random pairs of individuals (genome-wide R = 0.00185, p<10−3). We discuss several explanations for these observations, including demographic effects. On the other hand, the sampled European American couples are significantly more MHC-dissimilar than random pairs of individuals (R = −0.043, p = 0.015), and this pattern of dissimilarity is extreme when compared to the rest of the genome, both globally (genome-wide R = −0.00016, p = 0.739) and when broken into windows having the same length and recombination rate as the MHC (only nine genomic regions exhibit a higher level of genetic dissimilarity between spouses than does the MHC). This study thus supports the hypothesis that the MHC influences mate choice in some human populations.
Author Summary
There has been a longstanding hypothesis that selection may have led to mating patterns that encourage heterozygosity at Major Histocompatibility Complex (MHC) loci because of improved immune response to pathogens in the offspring of such matings, and, indeed, this has been observed in several model systems. However, in humans, previous studies regarding the role of the MHC in mate choice or preference, both directly in couples and also indirectly in “sweaty T-shirts” experiments, have reported conflicting results. Here, by using genome-wide genotype data and HLA types in African and European American couples, we test whether humans tend to choose MHC-dissimilar mates. This approach allows us to distinguish MHC-specific effects from genome-wide effects. In the African sample, the patterns at MHC loci is confounded by genome-wide effects, possibly resulting from demographic processes relating to the social organization of this population, and no tendency to choose MHC-dissimilar mates is detected. On the other hand, the sampled European Americans appear to have favoured MHC-dissimilar mates, supporting the hypothesis that MHC influences mate choice in some human populations. Thus, this study suggests that, in some cases, humans may rely on biological factors, in addition to social factors, when choosing a mate.
Introduction
In vertebrates, several studies have revealed that highly polymorphic genes within the Major Histocompatibility Complex (MHC) may have a role in mate choice. In particular, it has been shown that MHC genes influence individual body odor in mice and rats [1]–[7] and that mice prefer MHC-dissimilar mates e.g. [8]–[11], and [12] for a review. Evidence for MHC-disassortative mating was also found in sand lizards [13]. Studies in fish (and in particular Arctic charr) have shown their ability to discriminate the odors of similar and dissimilar MHC siblings [14], and shown that salmon prefer MHC dissimilar mates [15] while female sticklebacks choose a mate in order to complement their own set of MHC genes and to optimize the number of different alleles in their offspring [16]. Complex MHC-based mate choice was also observed in birds [17],[18]. The MHC is the most important part of the genome with respect to immunity [19] and such MHC-based mate choice could increase or optimize the number of non-self antigens that future offspring can recognize and thus increase their resistance to pathogens [12],[20],[21]. It could also have contributed to the extraordinary polymorphism observed at MHC loci [20].
On the other hand, in humans, the role of the MHC in mate choice is very controversial. Ober et al studied classical HLA types for 400 couples from the Hutterite community and found significantly fewer HLA matches between husbands and wives than expected when taking into account the social structure of Hutterites [22]. On the other hand, no evidence of MHC-based mate choice was found in a study of 200 couples from South Amerindian tribes [23]. In a less direct way, other studies have focused on odor preferences: in “sweaty T-shirts experiments”, in which females were asked to smell T-shirts worn by different males, it was shown that females significantly prefer the odor of T-shirts worn by MHC-dissimilar males, although such preference was not found among females taking the contraceptive pill [24],[25]. However, in another sweaty T-shirts experiment, in which males where chosen from a different ethnicity from the females and females were not aware of the nature of the smell (contrary to the two previous studies), females significantly preferred the odor of males having a small number of HLA alleles matching their paternal inherited alleles than the odor of males having fewer matches [26]. Although it has not been established that odor preference is a key factor in mate choice, such studies support the hypothesis that humans are able to discriminate MHC types of potential mates through odor cues and that humans may use such information when choosing a mate. However, the lack of congruence between these studies means that there is still uncertainty as to whether MHC variation influences mate choice in humans, and to what extent. The availability of genetic variation data at genomic scales now allows careful assessment of this question. Crucially, it allows us to distinguish MHC-specific effects from genome-wide effects.
In this study, we tested the existence of MHC-disassortative mating in humans by directly measuring the genetic similarity at the MHC level between spouses. These data were extracted from the HapMap II dataset, which includes 30 European American couples from Utah and 30 African couples from the Yoruba population in Nigeria [27]. Our analyses are based on HLA types and on 9,010 Single Nucleotide Polymorphisms (SNPs) densely distributed across the MHC. Moreover, in order to control for genome-wide effects, we compared the pattern observed in the MHC region to patterns assessed in the rest of the genome, using 3,214,339 SNPs.
Results
The genetic similarity at a given genetic variant for a given couple c was measured using a relatedness coefficient R, defined as a ratio of probabilities of identity in state R = (Qc−Qm)/(1−Qm), where Qc is the proportion of identical variants between the two spouses and Qm is the mean proportion of identical variants in the sample (that is, averaged over all possible pairs of individuals). This coefficient, combined over the genetic variants in a region or across the genome, allows an assessment of whether spouses are more genetically similar or dissimilar than random pairs of individuals. Significance was assessed by permuting individuals between couples. All p-values below are two-sided. Positive values of R indicate genetic similarity between spouses and negative values indicate genetic dissimilarity between spouses, relative to random mating in the sample.
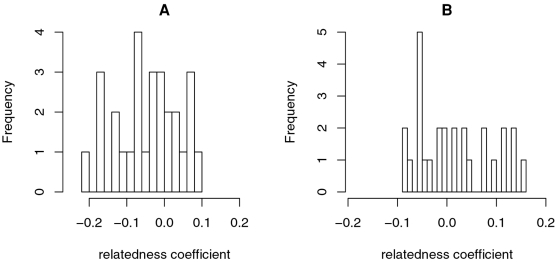
Using molecular markers (average relatedness coefficients across 9,010 SNPs), we observed that on average European American spouses were significantly more MHC-dissimilar from each other than random pairs of individuals (R = −0.043, p = 0.015). Moreover, the distribution of genetic relatedness coefficients across couples shows no outliers (Figure 1), thus excluding the possibility that this significantly negative coefficient could result from only a few couples having extremely low genetic relatedness. On the other hand, the MHC relatedness coefficient was positive but not significantly so in African couples (R = 0.015, p = 0.23). In addition, our analyses based on HLA types for 6 genes confirmed this broad pattern: the multilocus relatedness coefficient was marginally significantly negative in European American couples (R = −0.062, p = 0.084) and not significantly positive in Yoruba couples (R = 0.023, p = 0.412). (These analyses refer to the 4 digit classification. Similar patterns were seen with 2 digit classification; data not shown.) Using SNP data, we observed a higher mean SNP diversity in the MHC region in the African sample (0.366) than in the European American sample (0.349).
Figure 1. Distribution of relatedness coefficients across the MHC region among couples in the two samples.
A) European American sample. B) African sample.
To control for genome-wide effects, we compared these observations to the pattern of genetic similarity across the genome (3,214,339 markers). Genome-wide, European American spouses were not significantly more or less similar than random pairs of individuals (genome-wide R = −0.00016, p = 0.739). On the other hand, African spouses were more similar genome-wide than random pairs of individuals (genome-wide R = 0.00185, p<10−3).
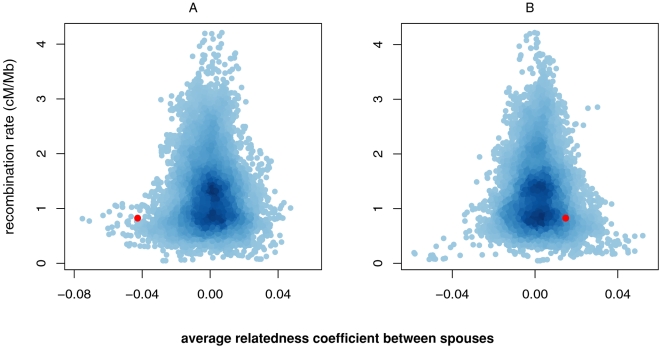
To further control for genome-wide effects, we asked whether the MHC region was unusual relative to similar regions across the genome with regard to its similarity/dissimilarity between spouses, by comparing the similarity between spouses at the MHC to that of all genomic windows having the same length as the MHC (3.6 Mb). Strikingly, in the European American couples, only 0.4% of the windows, concentrated in 9 genomic regions (listed in Table 1), exhibited a higher level of genetic dissimilarity between spouses than the MHC (Figure 2). To account for the particular linkage disequilibrium structure of the MHC and its low recombination rate [28], we compared the MHC region to a sub-set of windows having the same or lower recombination rate and still found that only 0.1% of these windows had less genetic similarity between spouses than did the MHC. In the African sample, 9% of the windows (and 17% when matching for the recombination rate) concentrated in 116 regions exhibited more genetic similarity between spouses than the MHC (Figure 2).
Table 1. Locations (in build 35 coordinates) of the nine regions exhibiting a higher level of genetic dissimilarity between spouses in the European American couples than the MHC.
| Chr | start | end |
| 1 | 168300000 | 171900000 |
| 3 | 78600000 | 83100000 |
| 4 | 27300000 | 30900000 |
| 4 | 149400000 | 154200000 |
| 6 | 116400000 | 120000000 |
| 10 | 100500000 | 106200000 |
| 12 | 46800000 | 51000000 |
| 15 | 40800000 | 47700000 |
| 17 | 53700000 | 57600000 |
Figure 2. Average relatedness coefficients between spouses across overlapping 3.6 Mb regions throughout the genome, plotted against their recombination rate.
The MHC is plotted in red. A) European American sample. B) African sample.
Discussion
At the molecular level, we found that the European American couples we studied are significantly more MHC-dissimilar than random pairs of individuals, and that this pattern of dissimilarity is extreme when compared to the rest of the genome, both globally and when broken into windows having the same length and recombination rate as the MHC. Our analyses based on HLA types also show a signature of dissimilarity between spouses. Such dissimilarity, observed from both molecular and serological data, cannot be explained by demographic processes, since such effects would affect the whole genome. On the other hand, this MHC dissimilarity could result from pressure for disassortative mating at the MHC level. Such a mechanism could be triggered by our olfactory capacity for discriminating MHC-mediated odour types [21],[29]. Alternatively, this genetic dissimilarity could result from selection of the spermatozoa by the female oocyte (post-copulatory sexual selection), a further safeguard favouring the production of MHC-heterozygous offspring more resistant to pathogens see [29]–[31] for reviews. Indeed, all studied couples were selected for having offspring, and the excess of dissimilarity observed could be restricted to fertile couples, rather than couples in general. However, further analysis showed that the offspring of these couples were not more MHC-diverse than expected by random selection of parental gametes (results not shown). Moreover, our results in European American couples reinforce previous evidence of MHC-disassortative mating among Hutterite couples [22], in which all couples were included, regardless of whether they had a child (C. Ober, personal communication). Like the Ober study, the sampled couples in our study are from a cultural isolate (in our case sampled from the Mormon community), so one might speculate that MHC-based mate choice is stronger or easier to detect in settings where there is less heterogeneity in other factors which influence mate choice, but the current absence of detailed molecular studies of mate choice in other human populations makes this impossible to assess. The two studies in Swiss males and females showing a significant preference of females for the odor of MHC-dissimilar (over MHC-similar) males [24],[25] implicate one possible mechanism by which couples may implement MHC-dependent mate choice. Taken together, these results strengthen the hypothesis that MHC genetic variation influences mate choice in some human populations.
Our analyses of the European American sample also show that the results based on molecular data were more significant than those based on HLA types. Although we cannot rule out power effects in explaining such a difference, it seems plausible, and consistent with our data, that the biological mechanisms involved in disassortative mating would depend on the MHC in ways that are not simply captured by HLA types. Such biological mechanisms could possibly result from a summation of effects over multiple genes, and not only from the six HLA genes studied here.
On the other hand, Yoruba couples exhibited a significant genome-wide signature of assortative mating, which is likely to result from socio-demographic processes specific to this population. The Yoruba are still organized in paternal lineages, which are exogamous units [32] and C. Adebamowo, personal communication. Although we do not have specific ethnological data collected with the Yoruba samples to explain our observations, a process in which matrimonial exchanges between genealogically related lineages are more frequent than matrimonial exchanges between genealogically unrelated lineages could have left such a genome-wide signature. On the contrary, for the MHC region, no significant pattern of similarity/dissimilarity was observed, at either the molecular level or the serological level. Several hypotheses can be proposed to explain this observation: firstly, it is possible either that the MHC is not involved in mate choice in this population, or that social factors are relatively more important than the MHC and that the sample size here does not allow detection of MHC effect on mate choice. Secondly, it is possible that MHC-based mate choice is aiming for an optimal, rather than maximal, number of MHC alleles previous theoretical and experimental evidence for this hypothesis are reviewed in [21]. Such a mechanism would explain why evidence of disassortative mating was found in the European Americans, all sampled in the Mormon community exhibiting a relatively low SNP diversity in the MHC (0.349), as well as in the genetically isolated Hutterite community [22], but not in Yoruba. Indeed, the Yoruba exhibit a relatively higher SNP diversity in the MHC (0.366) than the European American, and the optimization of the number of HLA alleles in Yoruba may involve mating with a not-so-MHC-dissimilar individual. This hypothesis is also consistent with the “sweaty T-shirts” experiment performed between females and males from different ethnicities (thus having a higher range of MHC dissimilarity than males and females coming from the same community) and showing that females prefer the odor of males with little MHC-dissimilarity than the odor of males with more extreme MHC-dissimilarity [26]. Finally, it is possible that in African populations, individuals carrying pathogen-resistant alleles are easier to identify than elsewhere, because of the higher pathogen pressure. In such conditions, it is possible that mating preferences for particular pathogen-resistant MHC alleles are stronger than mating preferences for MHC-dissimilarity per se [21].
In conclusion, our study, based on a large number of molecular markers which allow us to control for genome wide effects, indicates a clear-cut signature of MHC-disassortative mating in a sample of European American couples. This supports the existence of MHC-related biological factors contributing to mate choice in at least some human populations. On the other hand, the Yoruba exhibit a genome-wide tendency for enhanced similarity among couples but no significant pattern at the MHC level. This suggests that socio-demographic factors may be more important than biological factors for mate choice in this population, although the existence of MHC-dependent mate choice in Yoruba, aimed at optimizing (rather than maximizing) the number of HLA alleles in the offspring, cannot be excluded. Our study indicates that the relative importance of biological and social factors varies from one population to another. It also highlights the need for the exploration of further genome-wide data in larger sample sizes, including “just married” childless couples, sampled in several ethnically differentiated groups, in order to build a more robust view of the biological determinants acting on mate choice in humans.
Materials and Methods
Datasets
Two datasets were analysed in this study:
3,214,339 Single Nucleotide Polymorphism (SNPs) from the the Hapmap II dataset, typed in 30 European American couples (60 individuals) from Utah (Centre d'Etude du Polymorphisme Humain (CEPH) Collection) and 30 African couples (60 individuals) from the Yoruba population, Ibadan, Nigeria [27]. We used the phased data files and excluded SNPs with minor allele frequency below 5%. In this dataset, 9,010 SNPs were located in the MHC region, (positions 29,700,000–33,300,000 on chromosome 6 [19], in build 35 coordinates). Sex chromosomes were not included in the analyses.
A set of HLA types for 6 of the main HLA genes (three class I genes: HLA-A, -B, -C, three class II genes: HLA-DQA, -DQB, DRB) in 44 European American couples and 30 African couples from the same collections as above [28]. 30 out of the 44 European American couples were shared with the HapMap II dataset, and all African couples were common to both datasets. This dataset is available on the following site: http://www.sanger.ac.uk/HGP/Chr6/.
Relatedness Analyses
We estimated the genetic relatedness between spouses using SNP data and HLA type data. In all cases, the relatedness coefficient for a given pair of spouses R was defined as R = (Qc−Qm)/(1−Qm), where Qc is the proportion of identical variants between the two spouses and Qm is the mean proportion of identical variants in the sample (that is, averaged over all possible pairs of individuals) [33]. The proportion of identical variants at a given SNP for a given pair of individuals was 0 if both individuals were homozygous and carrying a different allele (eg 00 and 11), 1 if both individuals were homozygous and carrying the same allele (eg 00 and 00), and 0.5 in all others cases. We also considered a variation of this definition, with pairs of heterozygous individuals (01 and 01) being attributed a proportion of identical variants of 1 (instead of 0.5). Both definitions gave similar results (we present here coefficients based on the first definition). We estimated the average genetic relatedness coefficient between spouses across the MHC and across the whole genome. We checked that our estimates were not affected by the heterogeneity of SNP density and linkage patterns across the genome, by redoing our analyses on reduced sets of approximately independent SNPs prepared using two different procedures implemented in the software PLINK (one based on pairwise SNP r2 values and the other on the variance inflation factor) [34]. We also computed the average genetic relatedness coefficient between spouses for sliding windows of 3.6 Mb across the genome (in increments of 300 Kb) having at least 1,000 SNPs and not overlapping a centromere. In the case of the HLA type data, we defined the proportion of identical variants as 0 if the two individuals carried different types, 0.5 if one of their two types was similar, and 1 in all other cases and we computed a multi-locus relatedness coefficient between spouses based on types for 6 HLA genes. R was summarized across the MHC region, the genome or the six HLA genes by averaging Qc and Qm over all SNPs or over all HLA loci (and then computing the ratio (Qc−Qm)/(1−Qm)). Using molecular data, we also computed the mean SNP diversity (probability that two randomly chosen chromosomes are different at a given SNP) in the MHC region for both samples.
We removed from both datasets two European American and three African couples, in which one of the spouses had previously been found to be closely related (relatedness coefficient equal or higher to 1/32, see supplementary table 15 from [35]) to another sample (in each case, we chose at random the couple to be excluded). The relatedness coefficients before and after these exclusions were very similar (we present in the paper the estimates without these couples). In the case of HLA type data, we considered both the 4 digit and the 2 digit classification, and found congruent relatedness coefficients (coefficients based on the 4 digit classification only are reported in this paper). The significance of the relatedness coefficient was assessed using a permutation approach: the two-sided p-value is the proportion of permutations (attributing a new wife randomly to each husband) in which the permuted couples had the same or more extreme mean relatedness coefficient than the real couples. 1000 permutations were performed.
Acknowledgments
We thank D. Davison, G. McVean, and C. Spencer, and several anonymous reviewers for helpful comments on a previous version of this manuscript, E. Heyer, P. Khaitovich and M. Somel for helpful discussion, and G. McVean for access to computational resource.
Footnotes
The authors have declared that no competing interests exist.
R. Chaix was funded by a postdoctoral fellowhip from EMBO. C. Cao was funded by the Chinese Academy of Sciences and Max Planck Society. P. Donnelly was supported by the Wellcome Trust and the Wolfson Foundation.
References
- 1.Brown RE, Roser B, Singh PB. Class I and class II regions of the major histocompatibility complex both contribute to individual odors in congenic inbred strains of rats. Behav Genet. 1989;19:659–674. doi: 10.1007/BF01066029. [DOI] [PubMed] [Google Scholar]
- 2.Lanyon CV, Rushton SP, O'Donnell AG, Goodfellow M, Ward AC, et al. Murine scent mark microbial communities are genetically determined. FEMS Microbiol Ecol. 2007;59:576–583. doi: 10.1111/j.1574-6941.2006.00252.x. [DOI] [PubMed] [Google Scholar]
- 3.Leinders-Zufall T, Brennan P, Widmayer PSPC, Maul-Pavicic A, et al. MHC class I peptides as chemosensory signals in the vomeronasal organ. Science. 2004;306:1033–1037. doi: 10.1126/science.1102818. [DOI] [PubMed] [Google Scholar]
- 4.Novotny MV, Soini HA, Koyama S, Wiesler D, Bruce KE, et al. Chemical identification of MHC-influenced volatile compounds in mouse urine. I: Quantitative Proportions of Major Chemosignals. J Chem Ecol. 2007;33:417–434. doi: 10.1007/s10886-006-9230-9. [DOI] [PubMed] [Google Scholar]
- 5.Singer AG, Beauchamp GK, Yamazaki K. Volatile signals of the major histocompatibility complex in male mouse urine. Proc Natl Acad Sci U S A. 1997;94:2210–2214. doi: 10.1073/pnas.94.6.2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singh PB, Brown RE, Roser B. MHC antigens in urine as olfactory recognition cues. Nature. 1987;327:161–164. doi: 10.1038/327161a0. [DOI] [PubMed] [Google Scholar]
- 7.Yamazaki K, Beauchamp GK, Singer A, Bard J, Boyse EA. Odortypes: their origin and composition. Proc Natl Acad Sci U S A. 1999;96:1522–1525. doi: 10.1073/pnas.96.4.1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Penn D, Potts WK. Untrained mice discriminate MHC-determined odors. Physiol Behav. 1998;64:235–243. doi: 10.1016/s0031-9384(98)00052-3. [DOI] [PubMed] [Google Scholar]
- 9.Potts WK, Manning CJ, Wakeland EK. Mating patterns in seminatural populations of mice influenced by MHC genotype. Nature. 1991;352:619–621. doi: 10.1038/352619a0. [DOI] [PubMed] [Google Scholar]
- 10.Yamazaki K, Beauchamp GK, Kupniewski D, Bard J, Thomas L, et al. Familial imprinting determines H-2 selective mating preferences. Science. 1988;240:1331–1332. doi: 10.1126/science.3375818. [DOI] [PubMed] [Google Scholar]
- 11.Yamazaki K, Boyse EA, Mike V, Thaler HT, Mathieson BJ, et al. Control of mating preferences in mice by genes in the major histocompatibility complex. J Exp Med. 1976;144:1324–1335. doi: 10.1084/jem.144.5.1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Penn DJ, Potts WK. The evolution of mating preferences and major histocompatibility complex genes. The American Naturalist. 1999;153:145–164. doi: 10.1086/303166. [DOI] [PubMed] [Google Scholar]
- 13.Olsson M, Madsen T, Nordby J, Wapstra E, Ujvari B, et al. Major histocompatibility complex and mate choice in sand lizards. Proc Biol Sci. 2003;270(Suppl 2):S254–256. doi: 10.1098/rsbl.2003.0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Olsen KH, Grahn M, Lohm J, Langefors A. MHC and kin discrimination in juvenile Arctic charr, Salvelinus alpinus (L.). Anim Behav. 1998;56:319–327. doi: 10.1006/anbe.1998.0837. [DOI] [PubMed] [Google Scholar]
- 15.Landry C, Garant D, Duchesne P, Bernatchez L. ‘Good genes as heterozygosity’: the major histocompatibility complex and mate choice in Atlantic salmon (Salmo salar). Proc Biol Sci. 2001;268:1279–1285. doi: 10.1098/rspb.2001.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aeschlimann PB, Häberli MA, Reusch TBH, Boehm T, Milinski M. Female sticklebacks Gasterosteus aculaetus use self-reference to optimize MHC allele number during mate selection. Behav Ecol Sociobiol. 2003;54:119–126. [Google Scholar]
- 17.Bonneaud C, Chastel O, Federici P, Westerdahl H, Sorci G. Complex Mhc-based mate choice in a wild passerine. Proc Biol Sci. 2006;273:1111–1116. doi: 10.1098/rspb.2005.3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Richardson DS, Komdeur J, Burke T, von Schantz T. MHC-based patterns of social and extra-pair mate choice in the Seychelles warbler. Proc Biol Sci. 2005;272:759–767. doi: 10.1098/rspb.2004.3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Horton R, Wilming L, Rand V, Lovering RC, Bruford EA, et al. Gene map of the extended human MHC. Nat Rev Genet. 2004;5:889–899. doi: 10.1038/nrg1489. [DOI] [PubMed] [Google Scholar]
- 20.Parham P, Ohta T. Population biology of antigen presentation by MHC class I molecules. Science. 1996;272:67–74. doi: 10.1126/science.272.5258.67. [DOI] [PubMed] [Google Scholar]
- 21.Milinski M. The Major Histocompatibility Complex, Sexual Selection, and Mate Choice. Annu Rev Ecol Evol Syst. 2006;37:159–186. [Google Scholar]
- 22.Ober C, Weitkamp LR, Cox N, Dytch H, Kostyu D, et al. HLA and mate choice in humans. Am J Hum Genet. 1997;61:497–504. doi: 10.1086/515511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hedrick PW, Black FL. HLA and mate selection: no evidence in South Amerindians. Am J Hum Genet. 1997;61:505–511. doi: 10.1086/515519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wedekind C, Furi S. Body odour preferences in men and women: do they aim for specific MHC combinations or simply heterozygosity? Proc Biol Sci. 1997;264:1471–1479. doi: 10.1098/rspb.1997.0204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wedekind C, Seebeck T, Bettens F, Paepke AJ. MHC-dependent mate preferences in humans. Proc Biol Sci. 1995;260:245–249. doi: 10.1098/rspb.1995.0087. [DOI] [PubMed] [Google Scholar]
- 26.Jacob S, McClintock MK, Zelano B, Ober C. Paternally inherited HLA alleles are associated with women's choice of male odor. Nat Genet. 2002;30:175–179. doi: 10.1038/ng830. [DOI] [PubMed] [Google Scholar]
- 27.Frazer KA, Ballinger DG, Cox DR, Hinds DA, Stuve LL, et al. A second generation human haplotype map of over 3.1 million SNPs. Nature. 2007;449:851–861. doi: 10.1038/nature06258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Bakker PI, McVean G, Sabeti PC, Miretti MM, Green T, et al. A high-resolution HLA and SNP haplotype map for disease association studies in the extended human MHC. Nat Genet. 2006;38:1166–1172. doi: 10.1038/ng1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ziegler A, Kentenich H, Uchanska-Ziegler B. Female choice and the MHC. Trends Immunol. 2005;26:496–502. doi: 10.1016/j.it.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 30.Birkhead TR, Pizzari T. Postcopulatory sexual selection. Nat Rev Genet. 2002;3:262–273. doi: 10.1038/nrg774. [DOI] [PubMed] [Google Scholar]
- 31.Ziegler A, Dohr G, Uchanska-Ziegler B. Possible roles for products of polymorphic MHC and linked olfactory receptor genes during selection processes in reproduction. Am J Reprod Immunol. 2002;48:34–42. doi: 10.1034/j.1600-0897.2002.01097.x. [DOI] [PubMed] [Google Scholar]
- 32.Tamisier JC. Dictionnaire des Peuples, Sociétés d'Afrique, d'Amérique, d'Asie et d'Océanie. Paris: Larousse; 1998. pp. 351–354. [Google Scholar]
- 33.Rousset F. Inbreeding and relatedness coefficients: what do they measure? Heredity. 2002;88:371–380. doi: 10.1038/sj.hdy.6800065. [DOI] [PubMed] [Google Scholar]
- 34.Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.The International HapMap Consortium. A haplotype map of the human genome. Nature. 2005;437:1299–1320. doi: 10.1038/nature04226. [DOI] [PMC free article] [PubMed] [Google Scholar]