Abstract
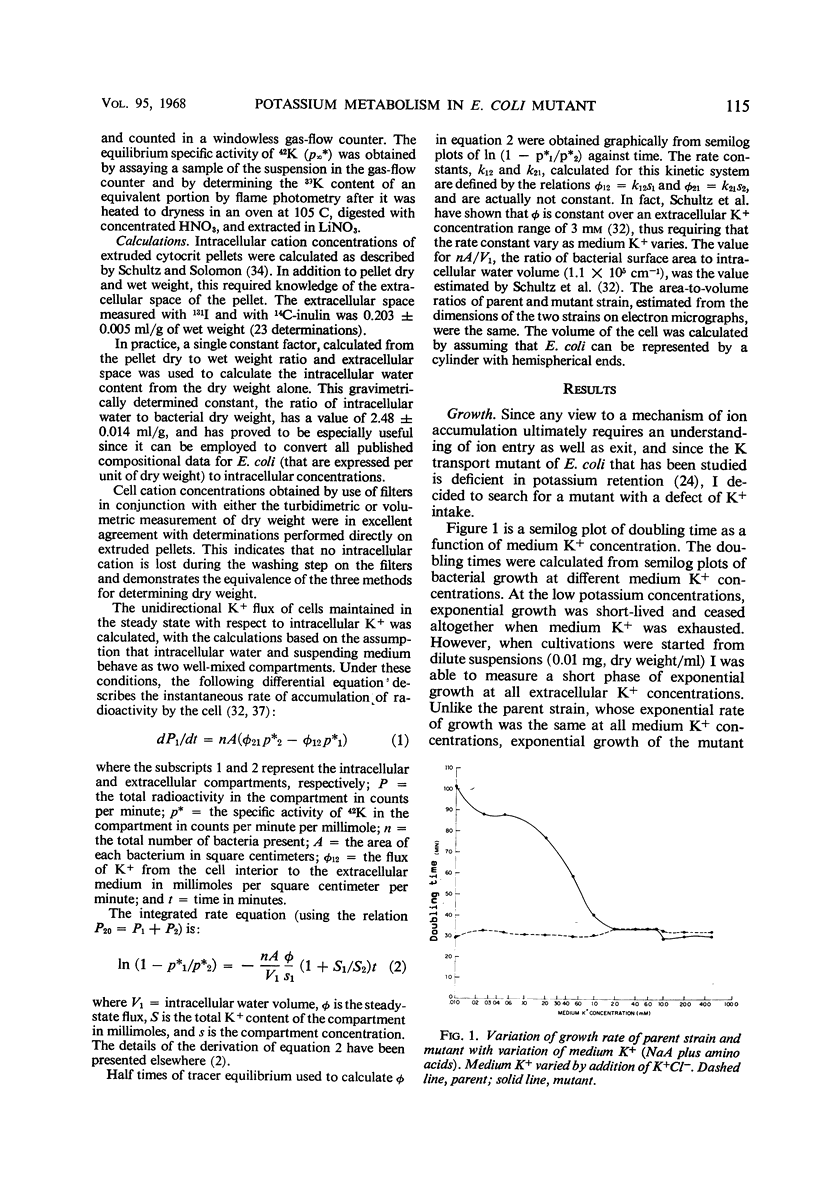
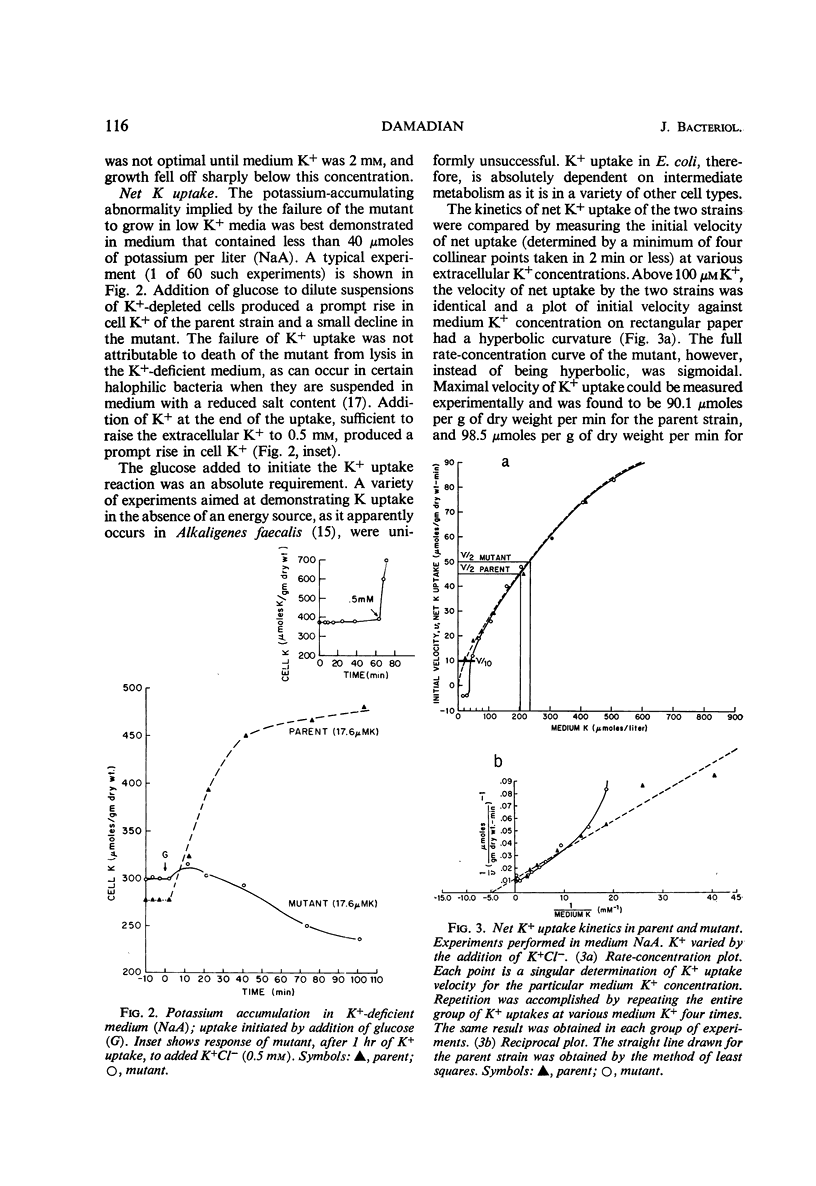
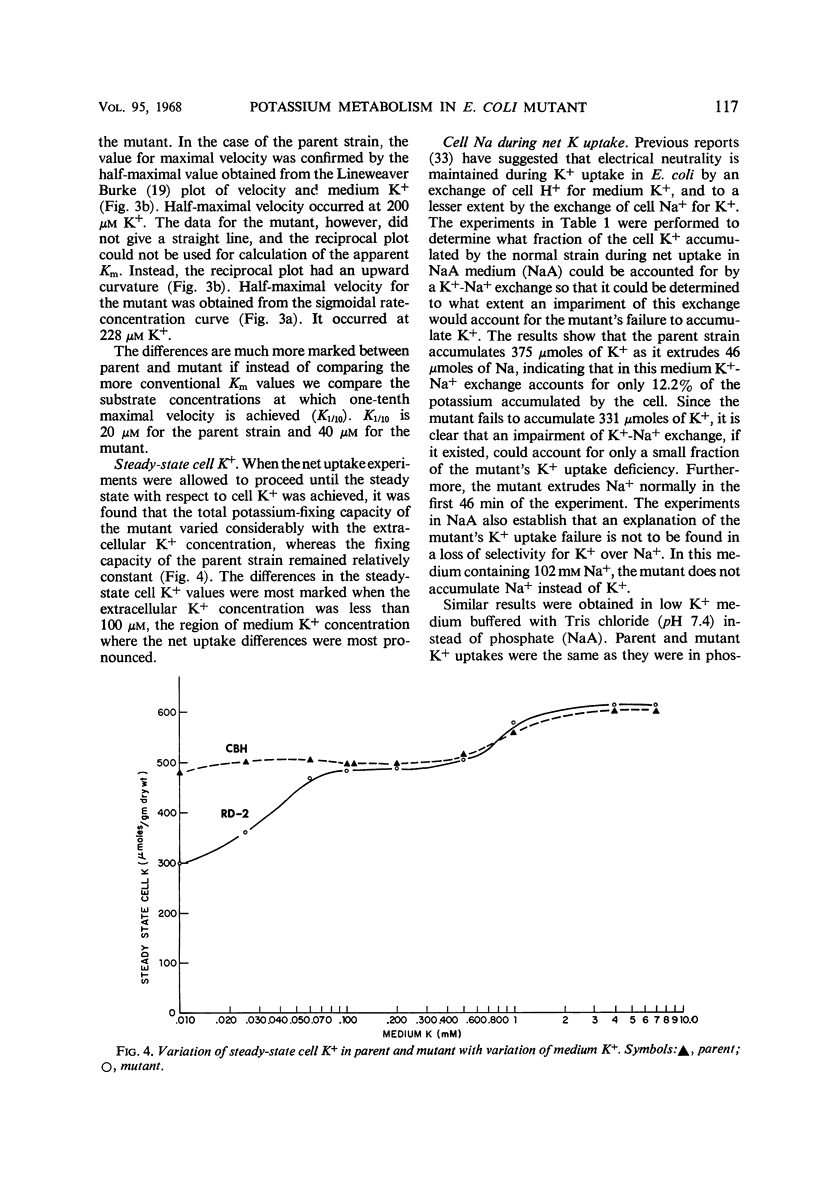
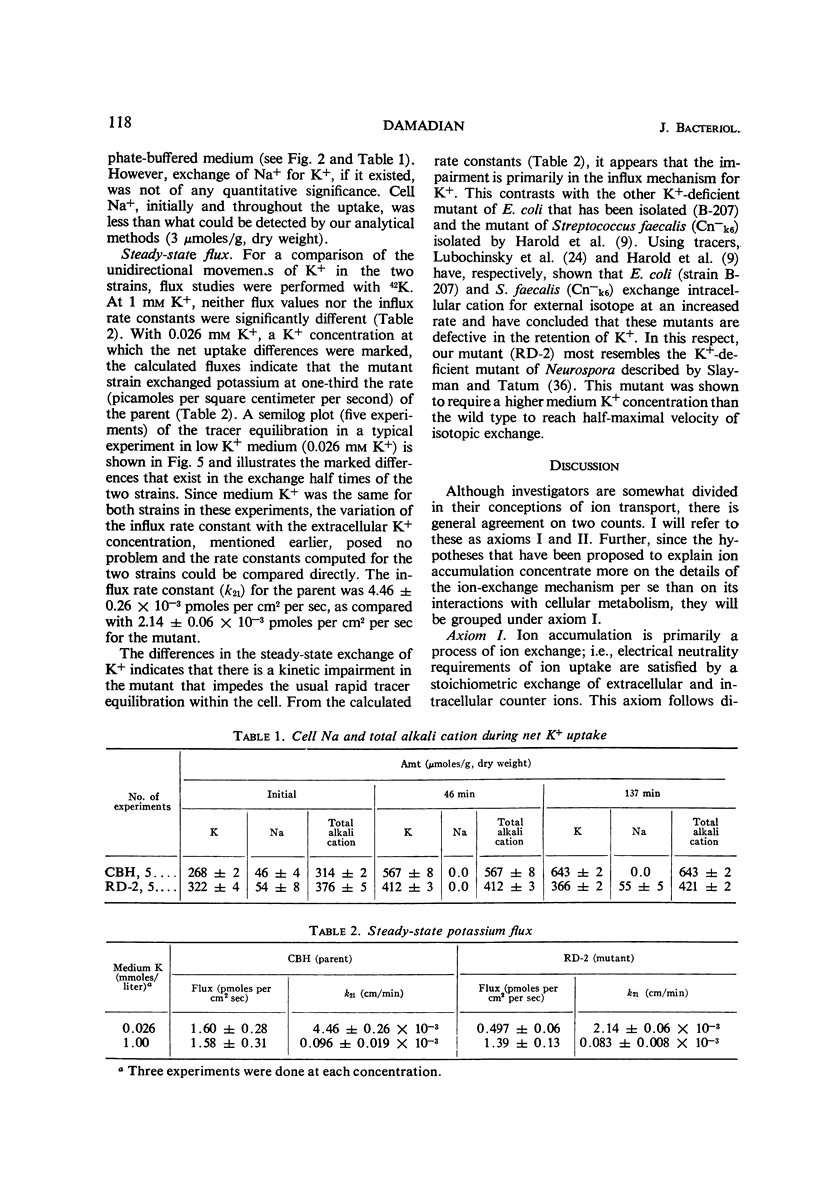
A potassium transport mutant of Escherichia coli is described which is deficient in the intake of potassium. The phenotype of this mutant is characterized by (i) failure to grow in K+-deficient medium, (ii) failure to accumulate K+ in K+-deficient medium, (iii) a steady-state intracellular K+ that varies sigmoidally with the medium K+ concentration, (iv) a signoidally shaped rate-concentration curve and a curved reciprocal plot for net K+ uptake kinetics, and (v) a low steady-state flux of potassium associated with a reduced influx rate constant. The data are discussed in terms of the present day models of cation transport. These models have led to four possible explanations of the mutant's phenotype: (i) a selectivity reversal such that intracellular cation binding sites bind another cation instead of K+; (ii) a structural alteration of cation binding cell proteins so that K+ is bound by “cooperative binding” (sigmoid isotherm) instead of by simple adsorption (hyperbolic isotherm); (iii) conversion of an enzyme in intermediate metabolism that rate-limits K+ uptake to an allosteric protein; (iv) conversion of the “carrier protein” for K+ to an allosteric protein.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CONWAY E. J., BRADY T. G. Biological production of acid and alkali; quantitative relations of succinic and carbonic acids to the potassium and hydrogen ion exchange in fermenting yeast. Biochem J. 1950 Sep;47(3):360–369. doi: 10.1042/bj0470360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAMADIAN R., SOLOMON A. K. BACTERIAL MUTANT WITH IMPAIRED POTASSIUM TRANSPORT AND METHIONINE BIOSYNTHESIS. Science. 1964 Sep 18;145(3638):1327–1328. doi: 10.1126/science.145.3638.1327. [DOI] [PubMed] [Google Scholar]
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damadian R. Abnormal phosphorus metabolism in a potassium transport mutant of Escherichia coli. Biochim Biophys Acta. 1967 May 2;135(2):378–380. doi: 10.1016/0005-2736(67)90137-x. [DOI] [PubMed] [Google Scholar]
- GERHART J. C., PARDEE A. B. The enzymology of control by feedback inhibition. J Biol Chem. 1962 Mar;237:891–896. [PubMed] [Google Scholar]
- Harold F. M., Harold R. L., Baarda J. R., Abrams A. A genetic defect in retention of potassium by Streptococcus faecalis. Biochemistry. 1967 Jun;6(6):1777–1784. doi: 10.1021/bi00858a028. [DOI] [PubMed] [Google Scholar]
- JUDAH J. D., AHMED K. THE BIOCHEMISTRY OF SODIUM TRANSPORT. Biol Rev Camb Philos Soc. 1964 May;39:160–193. doi: 10.1111/j.1469-185x.1964.tb00953.x. [DOI] [PubMed] [Google Scholar]
- Jardetzky O. Simple allosteric model for membrane pumps. Nature. 1966 Aug 27;211(5052):969–970. doi: 10.1038/211969a0. [DOI] [PubMed] [Google Scholar]
- KACHMAR J. F., BOYER P. D. Kinetic analysis of enzyme reactions. II. The potassium activation and calcium inhibition of pyruvic phosphoferase. J Biol Chem. 1953 Feb;200(2):669–682. [PubMed] [Google Scholar]
- KREBS H. A., WHITTAM R., HEMS R. Potassium uptake by Alcaligenes faecalis. Biochem J. 1957 May;66(1):53–60. doi: 10.1042/bj0660053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUBIN M. Enrichment of auxotrophic mutant populations by recycling. J Bacteriol. 1962 Mar;83:696–697. doi: 10.1128/jb.83.3.696-697.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUBIN M. INTRACELLULAR POTASSIUM AND CONTROL OF PROTEIN SYNTHESIS. Fed Proc. 1964 Sep-Oct;23:994–1001. [PubMed] [Google Scholar]
- LUBOCHINSKY B., MEURY J., STOLKOWSKI J. CIN'ETIQUE DES 'ECHANGES DE POTASSIUM CHEZ L'ESCHERICHIA COLI, SOUCHE B 207, QUI NE PEUT CRO ITRE NORMALEMENT QU'EN PR'ESENCE DE CONCENTRATIONS 'ELEV'EES EN POTASSIUM. C R Hebd Seances Acad Sci. 1964 May 20;258:5106–5109. [PubMed] [Google Scholar]
- MONOD J., CHANGEUX J. P., JACOB F. Allosteric proteins and cellular control systems. J Mol Biol. 1963 Apr;6:306–329. doi: 10.1016/s0022-2836(63)80091-1. [DOI] [PubMed] [Google Scholar]
- MUDD S. H., CANTONI G. L. Activation of methionine for transmethylation. III. The methionine-activating enzyme of Bakers' yeast. J Biol Chem. 1958 Mar;231(1):481–492. [PubMed] [Google Scholar]
- NISMAN B. Incorporation and activation of amino acids by disrupted protoplasts of Escherichia coli. Biochim Biophys Acta. 1959 Mar;32(1):18–31. doi: 10.1016/0006-3002(59)90549-9. [DOI] [PubMed] [Google Scholar]
- Pardee A. B., Prestidge L. S., Whipple M. B., Dreyfuss J. A binding site for sulfate and its relation to sulfate transport into Salmonella typhimurium. J Biol Chem. 1966 Sep 10;241(17):3962–3969. [PubMed] [Google Scholar]
- SCHULTZ S. G., EPSTEIN W., GOLDSTEIN D. A. Cation transport in Escherichia coli. III. Potassium fluxes in the steadystate. J Gen Physiol. 1962 Nov;46:343–353. doi: 10.1085/jgp.46.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHULTZ S. G., EPSTEIN W., SOLOMON A. K. CATION TRANSPORT IN ESCHERICHIA COLI. IV. KINETICS OF NET K UPTAKE. J Gen Physiol. 1963 Nov;47:329–346. doi: 10.1085/jgp.47.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHULTZ S. G., SOLOMON A. K. Cation transport in Escherichia coli. I. Intracellular Na and K concentrations and net cation movement. J Gen Physiol. 1961 Nov;45:355–369. doi: 10.1085/jgp.45.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SKOU J. C. ENZYMATIC BASIS FOR ACTIVE TRANSPORT OF NA+ AND K+ ACROSS CELL MEMBRANE. Physiol Rev. 1965 Jul;45:596–617. doi: 10.1152/physrev.1965.45.3.596. [DOI] [PubMed] [Google Scholar]
- Slayman C. W., Tatum E. L. Potassium transport in Neurospora. 3. Isolation of a transport mutant. Biochim Biophys Acta. 1965 Sep 27;109(1):184–193. doi: 10.1016/0926-6585(65)90102-0. [DOI] [PubMed] [Google Scholar]
- Zarlengo M. H., Schultz S. G. Cation transport and metabolism in Streptococcus fecalis. Biochim Biophys Acta. 1966 Oct 10;126(2):308–320. doi: 10.1016/0926-6585(66)90068-9. [DOI] [PubMed] [Google Scholar]



