Abstract
Mouse zona pellucida (ZP) proteins are synthesized in developing oocytes and assembled into ZP after their secretion. This study has investigated whether anti-ZP3 antibodies affect ZP assembly. Peptides CP2 and CP3 were used to elicit antibodies to two ZP3 B cell epitopes, ZP3(335–342) and ZP3(171–180). Ovulated eggs from mice immunized with a mixture of CP2/CP3 showed an abnormal ZP; importantly, the ZP completely dissolved both in vitro and in vivo 12 hours after ovulation. Although CP3 immunization resulted also in abnormal ZP, the ZP did not dissociate. Binding of antibodies to the ZP prior to oocyte maturation was requisite, as in vitro incubation of ovulated eggs in combination with the two antibodies failed to induce ZP dissolution. Electron microscopic observation further demonstrated a significant abnormality in ZP structure in CP2/CP3-immunized mice, especially in mature follicles, suggesting that B cell epitopes may be involved in ZP assembly. Though antibody elicited by CP2 has been shown to inhibit fertilization, we show now that antibody induced by CP3 had no effect on fertility. However, immunization with CP3/CP2 resulted in a significantly lower fertility rate than CP2 alone. This suggests that infertility in these mice may be due to an unstable ZP structure. Our model provides a useful tool to study ZP assembly and its structure beyond molecular biology method.
Keywords: Antibody, zona pellucida, oocyte, ovulation
1. Introduction
The zona pellucida (ZP) is the extracellular coat that surrounds the mammalian oocytes (Litscher and Wassarman, 2007). The ZP plays important roles in oocyte development, fertilization and early embryo development (Rankin et al., 2001). The ZP consists of long filaments. In mice, the ZP filaments are composed of ZP2 and ZP3 proteins, which are cross-linked by ZP1 (Wassarman, 1987). However, the presence of ZP2 and ZP3 is sufficient to form a biologically functional structure (Hoodbhoy et al., 2006). ZP proteins are synthesized in the developing oocytes and secreted through independent traffic pathways (Epifano et al., 1995; Hoodbhoy et al., 2006). The ZP assembly mechanism has been studied in detail (Qi et al., 2002; Jovine et al., 2004). A conserved duplicated motif [EHP (external hydrophobic patch)/IHP (internal hydrophobic patch)], which regulates their assembly, has been identified in ZP proteins. In mouse ZP3, the IHP is located between positions 165–174; mutation of the phenylalanine at position 171 (F171) abrogates ZP assembly (Jovine et al., 2004).
ZP proteins are highly immunogenic; immunization with ZP proteins or epitopes derived from these proteins induced autoantibodies or autoimmune ovarian disease in numerous animal models (Wood et al., 1981; Sacco, 1987; Naz et al., 2005; Rhim et al., 1992). In humans, autoantibodies to ZP proteins have been detected in infertile women (Shiver and Dunbar, 1977). Using various antibodies, several unique B cell epitopes of ZP proteins have been discovered. For example, B cell epitope ZP3(335–342) overlaps the sperm binding site (Millar et al., 1989). Thus, antibody to ZP3(335–342) is highly effective in blocking fertilization. In an autoimmune ovarian disease model, a pathogenic T cell epitope of ZP3 triggers B cell epitope spreading. As a result, autoantibodies are elicited to multiple B cell epitopes of ZP proteins, including ZP3(171–180), which happens to overlap with the IHP (Lou et al., 1996).
We have designed two immunogenic peptides, CP2 and CP3, which link a universal helper T cell epitope with ZP3 B cell epitopes ZP3(335–342) and ZP3(171–180), respectively (Lou et al., 1996). We have shown that both peptides elicit high titer antibodies to native ZP without causing any ovarian pathology. As CP2 contains the B cell epitope overlapping the sperm binding site, immunization with this peptide resulted in significantly reduced fertility (Lou et al., 1995). In the present study, we demonstrate further that induction of antibodies to both ZP3 B cell epitopes results in rapid disassociation of the ZP after ovulation, suggesting that the two B cell epitopes may be involved in ZP assembly.
2. Materials and Methods
2.1. Immunization and antigenic peptide
BALB/c female mice, obtained from Jackson Laboratory (Bar Harbor, ME), were studied at 6 to 8 weeks of age. Peptide antigens, dissolved in Mill-Q water at 1mM and sterilized by ultrafiltration, were emulsified in an equal volume of complete Freund’s adjuvant (CFA). Each mouse received 0.1ml of mixture (containing 50 nmol of peptide) in one footpad and a subcutaneous site. Blood was sampled by tail bleeding and serum antibody titers determined by indirect immunofluorescence on normal frozen ovarian sections, and by ELISA using antigenic peptide (Lou et al., 1996). Mice with a high titer of circulating antibody were used for ovulation. In some cases, immunized mice were boosted with the same antigen mixed with ICFA. Sera with a titer higher than 6,400 were stored at −80°C for in vitro use.
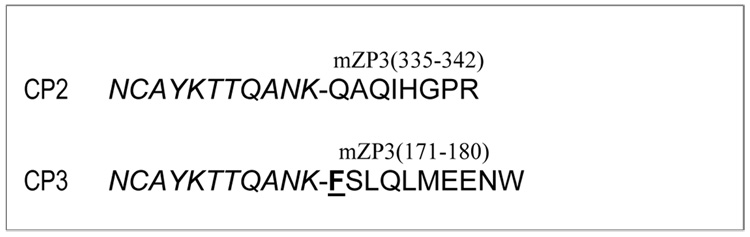
The sequence and structure of peptides CP2 and CP3 have been published (Lou et al., 1996) (Fig. 1). The peptides were synthesized by an automatic peptide synthesizer (Gilson, Middleton, IW) and purified by HPLC on a C18 reverse phase column (Waters, Millford, MA). All peptides exceeded 95% in purity. Amino acid sequence was verified by tandem mass spectrometry.
Figure 1.
Amino acid sequences for antigenic peptides CP2 and CP3. Phenylalanine at position 171 (F171), located within the internal hydrophobic patch (IHP), is underlined.
2.2. Superovulation induction and fertility trials
The mice were allowed to acclimate for a minimum of one week. A well established method was used for induvction of super-ovulation in young females (Zhou et al., 2004). Briefly, animals were injected with eCG (Sigma, St. Louis, MO) at 5IU/mouse intraperitoneally (i.p.). were injected i.p. with hCG (5IU/mouse, Sigma, St. Louis, MO) after 48hrs. Oviducts were removed for isolation of ovulated eggs. Cumulus-oocyte complexes were collected from oviducts of super-ovulated BALB/c females in medium-199 (M199, Gibco-BRL (Invitrogen), Carlsbad, CA). Unless indicated, three mice were used for each group. Cumulus cells were removed by treating eggs for 3 min with 1mg/ml hyaluronidase (Sigma, St. Louis, MO) in M199; eggs were washed through four 50ml drops of M199 medium covered with mineral oil using a pulled, heat-polished, Pasteur pipette (employed in all experiments). In some cases, ovaries were collected for the electron microscope (EM) or snap-frozen for immunofluorescence.
Fertility trials were performed following an established method (Lou et al., 1995). Immunized female mice were mated with male mice at a 1:1 ratio for 10 days. Successful mating was confirmed by the presence of a vaginal plug, and blood was immediately sampled from the tail vein by puncture. Antibody titer of this blood sample, measured by indirect immunofluorescence, was designated as ‘plug titer’. Female mice were sacrificed 18 days after confirmed mating, and the number of fetuses was counted.
2.3. In vitro fertilization
Sperm were collected from retired male breeders. Briefly, the caudae epididymis were removed and placed into 1ml drops of potassium simplex optimized medium (KSOM) supplemented with 0.4% (w/v) BSA (Sigma) under mineral oil in petri dishes. Epididymal contents were carefully squeezed out and incubated in a 6% CO2 incubator for 20 min to allow the sperm to disperse. After capacitation for 45–60 min at 37°C in the incubator, oocyte-cumulus complexes, isolated from immunized mice or mice that had received 40µg of IE-10 antibody (Millar et al., 1989), were transferred to the 100µl fertilization droplets (10µl sperm suspension with 90µl KSOM plus BSA). Incubation was allowed to proceed for 4h at 37°C in a 6% CO2 atmosphere. At the end of this period, the cumulus cells and attached sperm were removed from the oocytes by drawing the oocytes in and out of a fine-drawn pipette. The fertilized eggs (two-cell stage) were counted the next day.
2.4. Electron microscopy (EM)
A well established method was followed for EM (Lou and Takahashi, 1989). The ovaries were fixed in Karnovsky's fixative and treated with osmium tetroxide at 22°C. Specimens were dehydrated through a graded series of acetone and embedded in Epon resin (Electron Microscopy Sciences; Fort Washington, PA) according to the manufacturer's recommendations. Serial sections 90nm thick were cut on a Sorvall microtome, stained for 20 min with 5% uranyl acetate and observed in a JEOL 100CX electron microscope.
3. Results
3.1. Induction of antibodies to both B cell epitopes ZP3(335–342) and ZP3(171–180) results in rapid ZP dissolution in the ovulated oocytes
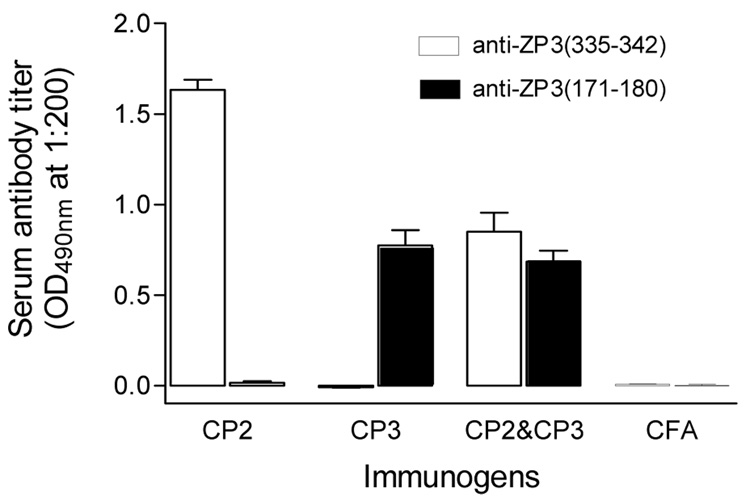
Mice were immunized with CP2, CP3 or a mixture of CP2/CP3. Mice immunized with CFA alone served as a negative control. Circulating antibodies to ZP3 B cell epitopes were measured by ELISA in the immunized mice (Fig. 2). CP2 or CP3 immunized mice produced high titers of antibody against ZP3(335–342) and ZP3(171–180), respectively. Mice immunized with CP2/CP3 showed circulating antibodies to both B cell epitopes. However, the titer to each epitope was lower than those elicited by CP2 or CP3 alone.
Figure 2.
Circulating antibodies in mice immunized with different ZP3 peptides. Sera were sampled at day 20 post-immunization, and antibody titer determined by ELISA at 1:200 dilution. Ten mice were used in each group.
Three immunized mice with the highest titers were selected from each group and used for induction of superovulation. Ovulated egg-cumulus complex was isolated 8 hours after hCG injection from the fallopian tube. There were no significant differences in ovulated egg numbers among those groups. Binding of antibodies to the zona pellucida (ZP) of developing oocytes and the ovulated eggs was confirmed by direct immunofluorescence (Figs. 3A&B). After a brief digestion with hyaluronidase, all released eggs had ZP. However, it was notable that more than half of eggs from the CP2/CP3 or CP3 immunized groups showed a more transparent, loose, and slightly swollen ZP compared with the other groups (Table 1) (Figs. 3C&D). Surprisingly, the ZP completely dissolved in about 50% of the eggs from CP2/CP3 immunized mice after 4–6 hours (Table 1) (Fig. 3E). Despite the ZP being similar in appearance to that of the CP2/CP3 group, the ZP of the CP3 group did not dissolve until the end of the experiment when degradation of eggs occurred (56 hours after hCG injection). CP2 and CFA control groups showed intact normal ZP until the end of the experiment. Because of the possibility that the hyaluronidase might have contributed to rapid dissolution of the ZP, we isolated the ovulated eggs from fallopian tubes at 20 hours after hCG injection when cumuli had been naturally digested. Eggs from CFA control mice had intact ZP (Fig. 3G). Again the eggs from CP2/CP3 immunized mice showed a fragile, thin and swollen ZP, or nude eggs without ZP (Figs. 3H&I) (Table 1). These experiments were repeated two more times, and similar results were obtained. Thus, we concluded that ZP dissolution was caused by CP2/CP3 binding.
Figure 3.
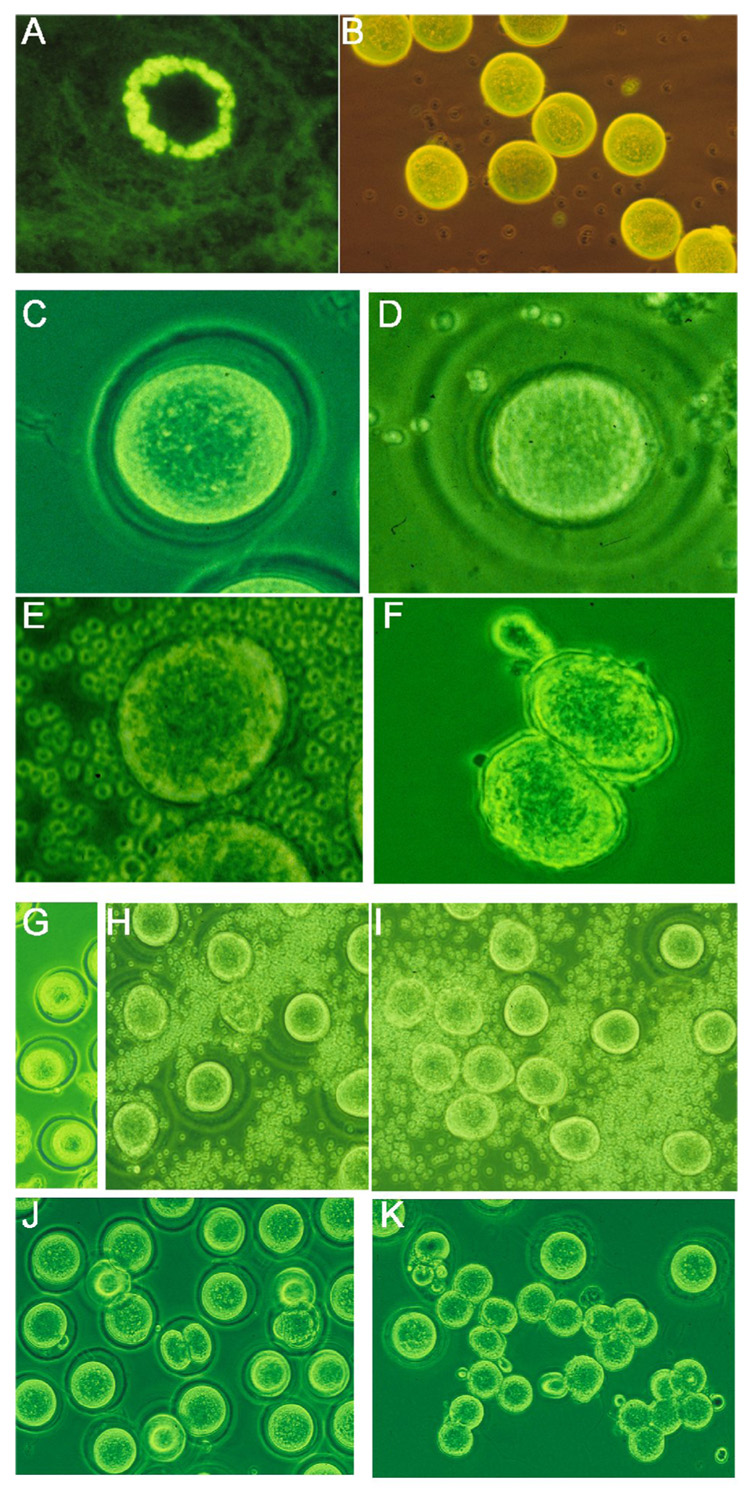
Antibodies to ZP3 B cell epitopes affect zona pellucida (ZP) structure. A and B. Immunofluorescence shows binding of IgG antibody to ZP of a developing oocyte (A) and ovulated eggs (B) in a CP2/CP3-immunized mouse. C to F, comparison between normal (C) and antibody-induced abnormal ZP (D-F); ZP is absent in an ovulated egg (E) or fertilized egg which has entered the two-cell stage (F). G. Normal eggs collected from fallopian tube after superovulation; H and I, eggs from fallopian tube of CP2/CP3-immunized mice show their abnormal ZP just after their harvest (H), which completely dissolved 6 hours later (I). J. eggs with intact ZP from mice pre-injected with IE-10 after fertilization; note that fertilization is blocked in most eggs. K. Eggs from CP2/CP3-immunized mice after fertilization; note that ZP is absent in fertilized eggs. x200
Table 1.
Structural changes in zona pellucida (ZP) of mice immunized with ZP3 peptides
| In vitro | In vivo | |||||
|---|---|---|---|---|---|---|
| Total oocytes | Oocytes with abnormal ZP | Oocytes without ZP | Total oocytes | Oocytes with abnormal ZP | Oocyte without ZP | |
| CFA | 51 | 0 | 0 | 43 | 0 | 0 |
| CP2 | 53 | 0 | 0 | - | - | - |
| CP3 | 47 | 34 | 0 | - | - | - |
| CP2/CP3 | 55 | 17 | 27 | 49 | 29 | 17 |
3.2. Binding of antibodies to the ZP after ovulation does not affect ZP solubility
We tested next whether in vitro incubation of the ovulated eggs with antibodies would lead to dissolution of the ZP. Sera were isolated from the CP2/CP3 immunized mice, which were confirmed to have disrupted ZP, and from CFA-immunized controls. Eggs collected from normal mice after super-ovulation induction were incubated with undiluted antisera. Binding of the antibodies from the CP2/CP3 immunized mice to ZP of the ovulated eggs was demonstrated by immunofluorescence after incubation. However, ZP of all 30 eggs with antibody binding remained intact and showed no sign of dissolution until the experiment was completed (48 hours). Undiluted sera did not interfere with ZP dissolution, as incubation of 15 eggs from CP2/C3 immunized mice with CFA serum failed to inhibit ZP dissolution. These results suggest that binding of antibodies to ZP after ovulation has no effect on ZP’s solubility. Thus, ‘pre-binding’ of antibodies to ZP before ovulation may be required for ZP dissolution.
3.3. Binding of antibodies to ZP in vivo affects ZP structure of antral follicles
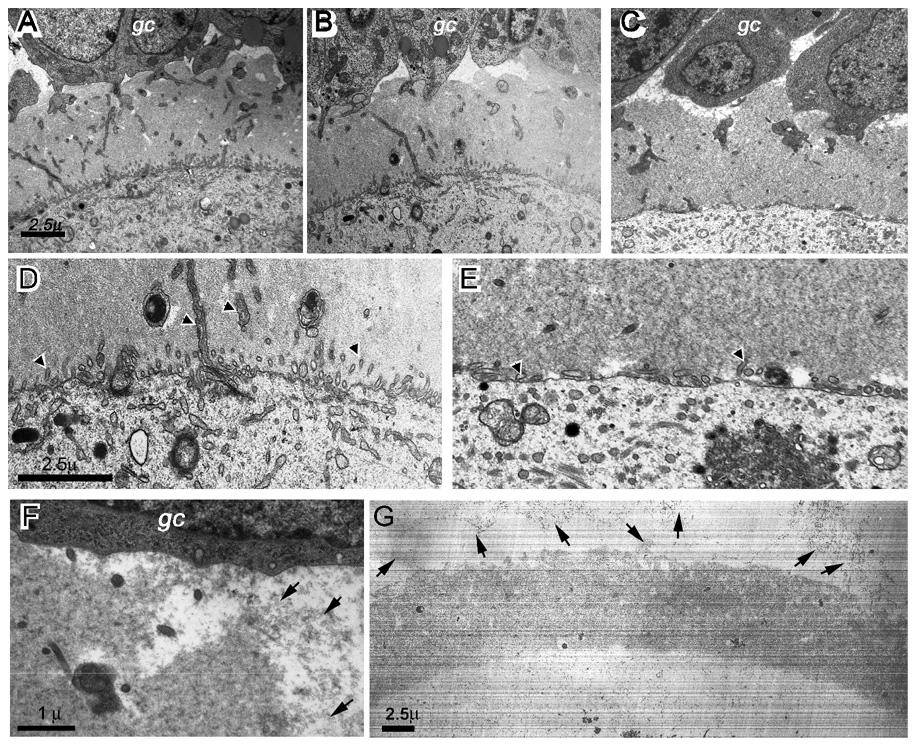
It still remained unclear how antibodies to the two ZP B cell epitopes induced ZP dissolution post-ovulation. However, it is clear that pre-binding of antibodies to ZP was necessary. Thus, ZP structural changes before ovulation were investigated in the immunized mice. We used electron microscopy (EM) to study the fine structure of ZP from normal, CP2 or CP2/3 immunized mice. Dissolution of ZP in the mice immunized with CP2/CP3 was first confirmed after super-ovulation induction. The ovaries were then sampled from two mice. In parallel, ovaries were sampled also from the other groups (2 mice/group). There were no significant differences in ZP structure in primary or early secondary follicles. However, early stage antral follicles from CP2/CP3-immunized mice showed a thinned ZP, and there were significant differences observed in the large antral follicles of the CP2/CP3 group compared to the other groups. First, trans-zona processes, which form gap junctions between oocyte and granulose cells, were significantly fewer in the CP2/CP3 group (Figs. 4A to E); some vesicle-like structures were also seen at the edge close to the oocyte. Second, the ZP filaments were distributed evenly throughout the ZP in the CP2/CP3 group, especially at a low magnification, while there were many ‘crack’-like spaces in other groups (Fig. 4A to C). Third, the edge of ZP was blurred in the CP2/CP3 group in contrast to a sharp edge of ZP in other groups (Fig. 4F). In addition, there were numerous filament-like particles present in the spaces between ZP and granulosa cells or between the granulosa cells. Such filaments were absent in other groups. Although the nature of these filaments needs to be determined, it is highly possible that they were disassociated from ZP. Our results suggest that antibodies must bind to ZP in the early stage of antral follicles before they can disrupt the ZP structure.
Figure 4.
Ultrastructural changes in zona pellcuida (ZP) of CP2/CP3-immunized mice. A to C, EM micrographs of ZP of antral follicles from a normal (A), CP2-immunized (B), or CP2/CP3-immunized mouse (C) show more homogeneous distribution of ZP filaments in CP2/CP3-immunized mice. D and E, EM micrographs at a higher magnification show significantly fewer transzonal processes (arrowheads) in a CP2/CP3-immunized mouse (E) than in a normal mouse (D). F. EM micrograph at a high magnification shows detached ZP filaments (arrowed) from ZP layer in an antral follicle of CP2/CP3-immunized mouse. G. EM micrograph of an ovulated egg from a CP2/CP3-immunized mouse shows detachment of ZP filaments from ZP (arrows). gc, granulosa cells.
We observed next the ovulated eggs in the fallopian tube. The eggs showed much lower densities of the ZP filaments. More importantly, an obvious gradient of filament density was seen toward the ZP edge. Numerous protein particles, which resembled ZP filaments, were present in the space between the ZP and oocyte. Some filaments were present in proximity of ZP while others were still associated with the ZP structure (Fig. 4G). These observations suggest that the ZP had undergone structural changes in the CP2/CP3-immunized mice before ovulation. Direct immunofluorescence showed that the antibodies were not present within the oocytes. Thus, it is unlikely that the antibodies would interfere with the ZP protein synthesis and secretion pathway within the oocytes. Rather, the antibodies may have interfered with ZP assembly.
3.4. Antibody to ZP3(171–180) does not inhibit fertilization
It has been shown that epitope ZP3(335–342) overlaps the sperm receptor (Millar et al, 1989). Monoclonal antibodies or serum antibody to this epitope inhibited fertilization (Millar et al, 1989; Lou et al, 1995). We tested first whether antibody to ZP3(171–180) could inhibit fertilization. Eggs harvested from various groups after superovulation were used for in vitro fertilization. A control group of mice, which were pre-injected with IE-10, a monoclonal antibody to ZP3, had a dramatically reduced fertility rate (Table 2). However, in the current studies, the CP3 group showed a high fertility rate comparable to the CFA group (Table 2). Thus, B cell epitope ZP3(171–180) may not be involved in the fertilization process. Fertilization rates in the CP2/CP3 group were lower than the CP3 or CFA group, probably due to activity of CP2 antibody, but were much higher than the IE-10 group (Table 2). Although most eggs from the IE-10 injection group failed to be fertilized, a few fertilized eggs entered the two-cell stage with intact ZP (Fig. 3J). As expected, either the fertilized or unfertilized eggs from the CP2/CP3 group lost their ZP 6–8 hours later (Fig. 3K). Without being constrained by the ZP, two-cell stage embryos of the CP2/CP3 group had a shape of ‘dumb bell’ (Figs. 3F&K).
Table 2.
Summary of in vitro fertilization using eggs from BALB/c mice immunized with various ZP3 peptides.
| Mice number | Total eggs | Fertilized eggs | Two-cell stage without ZP | ||
|---|---|---|---|---|---|
| number | % | ||||
| IE-101 | 2 | 37 | 4 | 10.8 | 0 (0%) |
| CFA | 2 | 41 | 35 | 85.4 | 0 (0%) |
| CP32 | 3 | 56 | 47 | 83.9 | 0 (0%) |
| CP2/CP33 | 3 | 61 | 33 | 54.1 | 21 (66.7%) |
Mice were injected i.p. with 40mg of IE-10 antibody at the initial injection for super-ovulation
Antibody titers in all three were higher than 15,000
Antibody titers in all three were higher than 10,000
3.5. Antibodies to ZP3(335–342) and ZP3(171–180) have additive anti-fertility effect
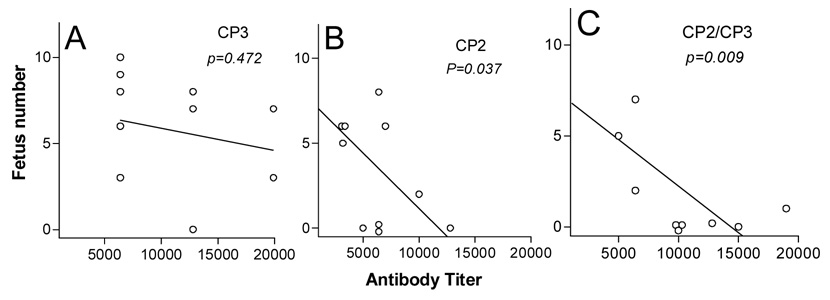
We asked next whether CP3 antibody could enhance the anti-fertility effects of CP2 antibody in vivo. We expected that, although CP3 elicited a high titer of antibody to native ZP, it would have an insignificant effect on fertility in vivo (Fig. 5A). Unexpectedly, the CP2/CP3 group had a much lower fertility than the CP2 group, despite much lower titers of antibody to ZP3(345–342) in this group compared with the CP2 group (Figs. 5B&C; for antibody titer, refer to Fig. 2). The infertility in each mouse was correlated with anti-ZP titers in both CP2 and CP2/CP3 groups. Reduced fertility in the CP2/CP3 group was probably not due to interrupted in vivo fertilization. It is highly possible that premature ZP dissolution may have a negative influence on early embryo development, which may be responsible for further reduction in fertility compared with CP2 alone.
Figure 5.
Correlation between fertility and anti-zona pellucida (ZP) antibody titer in mice immunized with CP3 (A), CP2 (B) or CP2/CP3 (C). Sera were sampled immediately after a successful mating; antibody titers were determined by indirect immunofluorescence on normal ovarian section. Female mice were sacrificed 18 days after confirmed mating, and the number of fetuses was counted.
4. Discussion
ZP protein trafficking and assembling mechanism has been studied in detail (Qi et al., 2002; Jovine et al., 2004; Hoodbhoy et al., 2006). The ZP proteins are secreted through independent traffic pathways (Qi et al., 2002; Hoodbhoy et al., 2006). After undergoing protease cleavage, ZP proteins are assembled finally to form a stable ZP layer through polymerization (Jovine et al., 2004). F171 of ZP3, which is located in the internal hydrophobic patch (IHP) region, has been identified as critical for ZP assembly (Jovine et al., 2004). Thus, a mutation in this position completely abrogates ZP assembly. Since F171 is part of the B cell epitope of CP3, it is likely that antibodies to CP3 may directly disrupt ZP assembly by blocking F171. This may explain also why binding of antibodies to ovulated eggs failed to induce ZP dissolution, as ZP assembly had completed.
Our experiments showed that the CP3 antibody alone was not enough to induce complete ZP disassociation, although it did cause ZP abnormality. On the other hand, the CP2 B cell epitope has been identified as a potential sperm receptor, and antibodies to it inhibited fertilization both in vitro and in vivo (Millar et al., 1989; Lou et al., 1995). Unlike CP3, CP2 B cell epitope ZP3(335–342) is not located within the hydrophobic patch and thus the antibody may not affect ZP assembly. Our observation that CP2 antibody is required to induce ZP dissolution raises interesting mechanistic questions, and we offer several possible explanations. First, B cell epitope ZP3(335–342) may be a special domain for ZP assembly beyond the identified EHP and IHP (since EHP and IHP are universal for extracellular structure assembly, ZP3(335–342) may be unique to ZP). Second, as ZP is composed of ZP proteins of filamentous structure, it is possible that ZP3(335–342) participates in connection of these macro-filaments instead of between the ZP proteins. Third, as antibody is a relatively large molecule, it’s binding to ZP3(335–342) may also shield EHP region. We believe this is unlikely because antibody binding did not show any ZP abnormality. Fourth, antibody binding to ZP may cause a minor structural change in ZP3; the change itself may not affect ZP assembly. However, it may greatly enhance antibody binding to ZP3(171–180) and/or its effect, which eventually leads to complete disassociation of ZP structure.
Another significant change in CP2/CP3-immunized mice was reduced trans-ZP processes in the pre-ovulatory follicles. Since those ovaries were sampled just after ovulation, it is unlikely that those follicles in CP2/CP3-immunized mice were preovulatory. ZP2 or ZP3 knockout mice display defects in early antral and preovulatory follicle development, cumulus-oocyte complex formation and ovulation (Matzuk et al., 2002), suggesting that ZP is important in mediating granulosa cell signals and connections to oocytes that optimize their later developmental potential (Zhao and Dean, 2002). Reduction in trans-ZP processes suggests that antibody binding to ZP may interfere with formation of the processes. However, the anti-ZP3 antibody was obviously not able to completely block such communication, as follicle development and ovulation were normal in the immunized mice.
The reasons that premature dissolution of ZP leads to infertility remain uncertain. There are a few possibilities. Clinically, premature dissolution of ZP and delayed hatching of the embryo, both associated with ZP abnormality, may lead to infertility (Lopata and Hay, 1989). A recent study showed that the blastocyst axis aligns with the ZP shape during embryo development, suggesting a critical role of an intact stable ZP structure during embryo development (Kurotaki et al., 2007). An earlier study demonstrated that an intact zona pellucida is essential for normal oviductal embryo transport in mice (Bronson & McLaren, 1970). Thus, the antibody-mediated premature ZP dissolution in vivo observed in our study might lead to the impaired transport of preimplantation embryos and points to another opportunity to investigate infertility caused by anti-ZP antibody.
Abnormalities such as premature or delayed hatching may be treated through IVF techniques (Blake et al., 2001). Our finding may also provide new therapeutic strategies for infertility, especially for hatching of the embryo of fertilized eggs. Although combination of antibodies to ZP3(335–342) and ZP3(171–180) may accelerate ZP dissolution in vivo, it seems difficult to apply directly this method to such cases, unless the antibodies are delivered in vivo. Thus, additional studies are required to determine the latest stage of follicular development during which binding of antibodies would lead to disruption of the integrity of ZP. Also, the mechanism for premature dissolution or delayed hatching has not been addressed (Gordon and Dapunt, 1993). Our findings may provide a model to address this issue – it would seem relevant to investigate whether infertility-associated anti-ZP antibodies in humans are involved in premature ZP disassociation.
Antibodies to ZP proteins have been widely explored as a potential contraceptive vaccine for either humans or animals (Wood et al., 1981; Sacco, 1987; Millar et al., 1989; Lou et al., 1995; Naz et al., 2005). Despite intensive studies for several decades, an effective contraceptive vaccine has not been developed. In the past, studies have focused on blockage of fertilization by elicitation of specific antibodies. CP2, which is one among many others, was able to reduce fertility up to approximately 64% in the immunized mice without causing ovarian pathology (Lou et al., 1995). Our findings may lead to a new strategy for vaccine development by antibody-mediated ZP instability in that CP2/CP3-immunized mice had a more dramatic reduction in fertility than mice immunized with CP2.
Acknowledgments
This study was supported by NIH RO1 HD049613 (Y.H.L.) and partially by NIH RO1HD35596 (Y.H.L.) and RO1HD38353 (S.A.C). We thank Wick Kraemer and Ming Lei for valuable technical support. Monoclonal antibody IE-10 was a gift from Dr. J. Dead (NIH) through Dr. K. Tung (University of Virginia).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Blake DA, Forsberg AS, Johansson BR, Wikland M. Laser zona pellucida thinning--an alternative approach to assisted hatching. Hum. Reprod. 2001;16:1959–1964. doi: 10.1093/humrep/16.9.1959. [DOI] [PubMed] [Google Scholar]
- Bronson RA, McLaren A. Transfer to the mouse oviduct of mouse eggs with and without the zona pellucida. J. Reprod. Fertil. 1970;22:129–137. doi: 10.1530/jrf.0.0220129. [DOI] [PubMed] [Google Scholar]
- Epifano O, Liang LF, Familari M, Moos MC, Jr, Dean J. Coordinate expression of the three zona pellucida genes during mouse oogenesis. Development. 1995;121:1947–1956. doi: 10.1242/dev.121.7.1947. [DOI] [PubMed] [Google Scholar]
- Gordon JW, Dapunt U. A new mouse model for embryos with a hatching deficiency and its use to elucidate the mechanism of blastocyst hatching. Fertil. Steril. 1993;59:1296–1301. doi: 10.1016/s0015-0282(16)55993-7. [DOI] [PubMed] [Google Scholar]
- Hoodbhoy T, Aviles M, Baibakov B, Epifano O, Jimenez-Movilla M, Gauthier L, Dean J. ZP2 and ZP3 traffic independently within oocytes prior to assembly into the extracellular zona pellucida. Mol. Cell. Biol. 2006;26:7991–7998. doi: 10.1128/MCB.00904-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovine L, Qi H, Williams Z, Litscher ES, Wassarman PM. A duplicated motif controls assembly of zona pellucida domain proteins. Proc. Natl. Acad. Sci. USA. 2004;101:5922–5927. doi: 10.1073/pnas.0401600101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurotaki Y, Hatta K, Nakao K, Nabeshima Y, Fujimori T. Blastocyst axis is specified independently of early cell lineage but aligns with the ZP shape. Science. 2007;316:719–723. doi: 10.1126/science.1138591. [DOI] [PubMed] [Google Scholar]
- Litscher ES, Wassarman PM. Egg extracellular coat proteins: from fish to mammals. Histol. Histopathol. 2007;22:337–347. doi: 10.14670/HH-22.337. [DOI] [PubMed] [Google Scholar]
- Lopata A, Hay DL. The potential of early human embryos to form blastocysts, hatch from their zona and secrete HCG in culture. Human Reprod. 1989;4(8 Suppl):87–94. doi: 10.1093/humrep/4.suppl_1.87. [DOI] [PubMed] [Google Scholar]
- Lou YH, Ang J, Thai H, McElveen F, Tung KS. A zona pellucida 3 peptide vaccine induces antibodies and reversible infertility without ovarian pathology. J. Immunol. 1995;155:2715–2720. [PubMed] [Google Scholar]
- Lou YH, McElveen MF, Garza KM, Tung KS. Rapid induction of autoantibodies by endogenous ovarian antigens and activated T cells: implication in autoimmune disease pathogenesis and B cell tolerance. J. Immunol. 1996;156:3535–3540. [PubMed] [Google Scholar]
- Lou YH, Takahashi H. Spermiogenesis in the Tilapia Oreochromis niloticus with notes on a unique pattern of unclear chromatin condensation. J. Morphol. 1989;200:321–320. doi: 10.1002/jmor.1052000307. [DOI] [PubMed] [Google Scholar]
- Matzuk MM, Burns KH, Viveiros MM, Eppig JJ. Intercellular communication in the mammalian ovary: oocytes carry the conversation. Science. 2002;296:2178–2180. doi: 10.1126/science.1071965. [DOI] [PubMed] [Google Scholar]
- Millar SE, Chamow SM, Baur AW, Oliver C, Robey F, Dean J. Vaccination with a synthetic zona pellucida peptide produces long-term contraception in female mice. Science. 1989;246:935–938. doi: 10.1126/science.2479101. [DOI] [PubMed] [Google Scholar]
- Naz RK, Gupta SK, Gupta JC, Vyas HK, Talwar AG. Recent advances in contraceptive vaccine development: a mini-review. Hum. Reprod. 2005;20:3271–3383. doi: 10.1093/humrep/dei256. [DOI] [PubMed] [Google Scholar]
- Qi H, William Z, Wassarman PM. Secretion and assembly of zona pellucida glycoproteins by growing mouse oocytes microinjected with epitope-tagged cDNAs from mZP2 and mZP3. Mol. Biol. Cell. 2002;13:530–541. doi: 10.1091/mbc.01-09-0440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin TL, O'Brien M, Lee E, Wigglesworth K, Eppig J, Dean J. Defective zonae pellucidae in Zp2-null mice disrupt folliculogenesis, fertility and development. Development. 2001;128:1119–1126. doi: 10.1242/dev.128.7.1119. [DOI] [PubMed] [Google Scholar]
- Rhim SH, Millar SE, Robey F, Luo AM, Lou YH, Yule T, Allen P, Dean J, Tung KS. Autoimmune disease of the ovary induced by a ZP3 peptide from the mouse zona pellucida. J. Clin. Invest. 1992;89:28–35. doi: 10.1172/JCI115572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacco AG. Zona pellucida: current status as a candidate antigen for contraceptive vaccine development. Am. J. Reprod. Immunol. 1987;15:122–130. [PubMed] [Google Scholar]
- Shivers CA, Dunbar BS. Autoantibodies to zona pellucida: a possible cause for infertility in women. Science. 1977;197:1082–1084. doi: 10.1126/science.70076. [DOI] [PubMed] [Google Scholar]
- Wassarman PM. The biology and chemistry of fertilization. Science. 1987;235:553–560. doi: 10.1126/science.3027891. [DOI] [PubMed] [Google Scholar]
- Wood DM, Liu C, Dunbar BS. Effect of alloimmunization and heteroimmunization with zonae pellucidae on fertility in rabbits. Biol. Reprod. 1981;25:439–450. doi: 10.1095/biolreprod25.2.439. [DOI] [PubMed] [Google Scholar]
- Zhao M, Dean J. The zona pellucida in folliculogenesis, fertilization and early development. Rev. Endocr. Metab. Disord. 2002;3:19–26. doi: 10.1023/a:1012744617241. [DOI] [PubMed] [Google Scholar]
- Zhou C, Borillo J, Wu J, Torres L, Lou YH. Ovarian expression of chemokines and their receptors. J. Reprod. Immunol. 2004;63:1–9. doi: 10.1016/j.jri.2004.03.002. [DOI] [PubMed] [Google Scholar]