Summary
Cells have the innate ability to sense and move towards a variety of chemoattractants. We investigate the pathways by which cells sense and respond to chemoattractant gradients. We focus on the model system Dictyostelium and compare our understanding of chemotaxis in this system with recent advances made using neutrophils and other mammalian cell types, which share many molecular components and signaling pathways with Dictyostelium. The review also examines models that have been proposed to explain how cells are able to respond to small differences in ligand concentrations between the anterior leading edge and posterior of the cell. In addition, we highlight the overlapping functions of many signaling components in diverse processes beyond chemotaxis, including random cell motility and cell division.
Keywords: Chemotaxis, polarization, Dictyostelium, neutrophils, PI3K, heterotrimeric G proteins
Introduction
Chemotaxis, the directed movement of cells along extracellular gradients, is a vital cellular response that plays a critical role during the life cycle of eukaryotic organisms. Many single-celled organisms use a chemosensory system to track down food, avoid predators, and find optimal environmental conditions. In higher organisms, this process regulates cellular movements during development and directs the many links between the cells in the nervous system in a growing embryo [1]. Numerous guidance molecules and chemoattractants steer cells to proper locations in the forming animal. In mature organisms, cell movements are required for tissue maintenance and restoration, as seen during the remodeling of the vascular system and in wound healing [2]. In addition, the cells of the immune and inflammatory systems shuttle between the vascular and lymphatic systems and migrate from circulation towards sites of infection [3,4]. Besides these roles in normal physiology, improper cell migration is the source for many pathological conditions, including cancer metastasis and various chronic inflammatory diseases [5-11].
Chemotaxis is a dynamic process that requires directional sensing, cell polarization, and cell adhesion and motility [12-14]. Cells must interact with the chemoattractant, transmit the signal across the plasma membrane, and localize the response. An underlying directional sensing system acts as a compass and thus favors pseudopodia formation towards or away from the source of chemoattractant or repellant and thereby orients cell movement in relation to the ligand gradient (Figure 1). This motility also generally requires the cell to rearrange the membrane and cytoskeleton to achieve a polarized morphology, in which the cells have a distinct front and rear [15]. This involves numerous feedback mechanisms so that actin polymerization in pseudopodia at the leading edge of the cell can be synchronized with contractile forces generated by myosin motor proteins at the rear [16,17]. These features of polarization play key roles in random cell motility and cytokinesis and will be discussed further below. This review will focus on one of the favored model systems for chemotaxis, the social amoeba Dictyostelium discoideum, with highlights of numerous mammalian cell types, including neutrophils. We will discuss the mechanisms and models that have been proposed to allow cells to navigate during gradient sensing downstream from G protein-coupled receptors. Because of space limitations, chemotaxis through receptor tyrosine kinases will not be explored.
Figure 1. Chemotaxis of Dictyostelium cells.

The DIC image shows Dictyostelium cells chemotaxing towards a micropipette emitting the chemoattractant cAMP.
Dictyostelium as a model system
The social amoeba Dictyostelium discoideum has emerged as a powerful model system for understanding the mechanisms underlying signaling during eukaryotic chemotaxis [18-20]. Many of the chemoattractant-induced mechanisms that mediate directional sensing and motility in eukaryotes have been determined using Dictyostelium as the experimental system, and many reporters for the spatial localization of proteins and signaling pathways during chemotaxis were first developed in Dictyostelium. Most of the molecular genetic techniques associated with other model organisms, such as homologous recombination, RNA interference, insertional mutagenesis, and multicopy suppression are available in Dictyostelium [21,22]. There is also a useful online database that greatly facilitates collaborations and provides a resource center for techniques and a web-based ordering system for vectors, strains, and reporters from a central stock [23]. The organism's genome has been sequenced, aligned, and annotated [24].
In chemotaxing Dictyostelium cells, the receptors and heterotrimeric G-proteins, the machinery of the signaling pathway that binds to and transmits the signaling to the downstream pathways, remain uniformly distributed over the cell surface [25,26] (Figure 2). Moreover, receptor occupancy and G-protein activation parallel the concentration of attractant, and there appears to be little amplification of the chemoattractant gradient in these initial steps of gradient sensing [27-29]. The first sign of an amplified, asymmetric response appears at the level of the proteins that regulate the synthesis and degradation of PI(3,4,5)P3 (Figure 2). Receptor/G-protein signaling promotes Ras activation, as shown by the localization of a GFP fusion of the Ras binding domain of the mammalian protein Raf1 (GFRP-RBDRaf1) to the leading edge of the cell [30]. Ras is important for PI3K activation in Dictyostelium [31] and abrogation of Ras signaling blocks directional sensing in Dictyostelium [30,32,33]. Localized activation of Ras at the leading edge of the cell likely activates membrane-recruited PI3Ks at this site. Although PI3Ks localize to the regions of the plasma membrane closest to the chemoattractant source (higher chemoattractant concentrations), PTEN, the phosphatase that removes the phosphate from the 3 position of the inositol ring, delocalizes from these sites and becomes restricted to the lateral sides and posterior (areas of lower chemoattractant concentration) [31,34,35]. This reciprocal regulation of PI3Ks and PTEN leads to the localized synthesis and accumulation of PI(3,4,5)P3 at the leading edge of the cell and subsequent activation of F-actin polymerization, probably through the activation of Dock180 family RacGEFs (Rac guanine nucleotide exchange factors) [36,37] (RAF, unpub. obser.). While details of the mechanism have not yet been worked out, evidence in mammalian cells suggests that this localization of PI(3,4,5)P3 also results in the polymerization of F-actin at these sites leading to pseudopod extension through the modulation of Rac and Cdc42 GTPases [38-42]. The strongest evidence for the role of PI(3,4,5)P3 in the generation of polymerized actin comes from Dictyostelium cells lacking PTEN. pten null cells have elevated levels of PI(3,4,5)P3 and correspondingly high levels of F-actin [43]. PI3K plays an important role in directional sensing, especially in shallow or weak chemoattractant gradients [44,45], although cells can chemotax to steep chemoattractant gradients reasonably well, albeit more slowly, in the absence of PI3K signaling, suggesting parallel directional sensing pathways must exist [44-47]. Recent studies have identified PLA2 as a component of such a pathway. A gene encoding a PLA2 homologue was identified in a screen for mutants that were more sensitive to chemotaxis perturbation in the presence of the PI3K inhibitor LY294002 [48]. The role of PLA2 in chemotaxis was supported by work using PLA2 inhibitors combined with mutant analysis [45]. In addition, the van Haastert group found that PLC and Ca++ signaling were necessary for the PI(3,4,5)P3 (through regulation of PTEN localization, see details below) and PLA2 pathways, respectively.
Figure 2. Directional sensing occurs downstream of G-protein activation and upstream of PI(3,4,5)P3 accumulation.
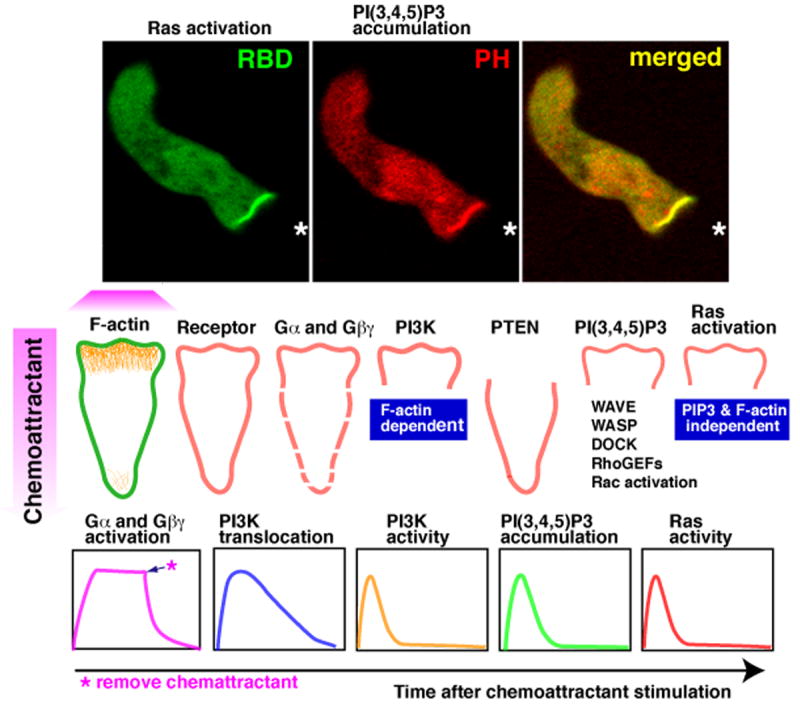
Upper panels show the spatial co-localization of PI(3,4,5)P3 (PI3K activity) and activated Ras (Ras-GTP) to the leading edge of a chemotaxing Dictyostelium cell coexpressing the reporters RFP-PHAtk and GFP-Ras binding domain (RBDRaf1), respectively. The central panels show schematic depictions of the distribution of components or reactions at different steps in the chemotactic signaling pathway. With the exception of receptor occupancy and G-protein activation, all of the distributions were determined by imaging GFP fusion proteins in chemotaxing cells. The distribution of F-actin was inferred from imaging of the actin binding proteins LimEΔcoil and coronin and was confirmed by phalloidin staining in fixed cells. Receptor occupancy was visualized by single molecule imaging of Cy3-cAMP. G-protein activation is inferred by a fluorescence resonance energy transfer assay that measures dissociation of the α- and β-subunits of the G-protein. The lower panels depict the activation kinetics of the components described above in response to a uniform (global) stimulation by chemoattractant (cAMP). All of the responses except heterotrimeric G protein activation are transient. For Ras, peak activation occurs ∼3-5 sec after stimulation, while PI3K activity peaks at ∼5-7 sec. The figure illustrates that heterotrimeric G protein activation rapidly ceases upon removal of the chemoattractant. This figure is reproduced from Sasaki et al [112].
Despite the progress achieved, our understanding of the mechanisms regulating the chemotactic response such as that illustrated in Figure 1 still faces several intriguing and unique challenges. Cells possess an extremely sensitive ability to respond to shallow and rapidly changing external gradients. Faced with receptor occupancy differences of as little as a 5% between front and back, cells are able to generate the spatially restricted and localized internal responses depicted in Figure 2. This requires a means of comparing receptor occupancy levels at different points along the cell membrane as well as a mechanism for amplifying these differences. These measurements must occur continuously as a cell migrates up or down a chemoattractant gradient. The cells also possess a powerful mechanism for adaptation to a chemoattractant. Studies in Dictyostelium and mammalian neutrophils indicate that cells respond to changes in receptor occupancy and adapt when occupancy is held constant [49,50]. As outlined above, many of the signaling components activated in response to chemoattractants have been elucidated; however, it is still unclear how the cell compass manages to localize sharp responses at the leading edge and rear of a migrating cell.
Mechanisms of gradient sensing
Cells have the ability to sense asymmetric extracellular cues and generate amplified responses, as visualized by observing signaling and cytoskeletal proteins that act as biosensors when fused to the green fluorescent protein (GFP). This directional sensing response does not require a cell to be polarized and can also occur in cells that are immobilized through the inhibition of F-actin polymerization [51]. Since eukaryotic cells like Dictyostelium and neutrophils manage to localize signaling molecules in the absence of motility when placed in a chemoattractant gradient, they possess a spatial sensing mechanism for directional sensing [36,52,53]. On the other hand, the bacterium Escherichia coli have very localized receptors and rely entirely on a temporal mechanism to steer in a chemical gradient [54]. For eukaryotes, this spatial sensing mechanism usually requires the cell to constantly measure receptor occupancy across the entire perimeter of the cell. In some cases, the polarized morphology of a cell leads to polarized sensitivity, with the leading edge of a cell being more sensitive to chemoattractants than the rear, a process that helps stabilize signaling complexes at the leading edge [12,30]. In some cell types, the receptors localize to distinct regions of the cell, suggesting that eukaryotic cells can measure and respond to changes of chemoattractant over short distances across the cell membrane [55]. How do the cells accomplish such a remarkable feat in both steep and shallow gradients as well as chemical gradients with vastly different midpoint concentrations? Many models have been put forth over recent years to explain the phenomena observed during gradient sensing. We will remark on a number of the schemes that have been suggested to regulate directional sensing.
Temporal models
The best understood signaling network for chemotaxis is that which has been worked out for E. coli. These bacteria use a half-dozen or so proteins that undergo various modifications in response to changing chemoattractant concentrations. This mechanism allows the cells to adapt their physiology so they can move continuously in a relatively straight line and “run” when going up the gradient or “tumble” into a new random direction when chemoattractant concentrations decline [56]. This temporal method of sensing using a few proteins allows bacterial cells to go in the desired direction. Eukaryotic cells, on the other hand, use signaling networks that are much more complex. Recent work has suggested that chemotaxis in shallow gradients by relatively unpolarized mammalian and Dictyostelium cells may be mediated by biased choices between random pseudopodia and use a mechanism similar to the “pilot pseudopod” scheme proposed over thirty years ago [57-59]. In this model, pseudopodia that extend up the gradient experience a positive change in chemoattractant concentration and are reinforced, whereas those which project down the gradient, on average, receive a negative signal and are extinguished. The generation of these pseudopodia across the cell appears to be quite random, suggesting that an underlying mechanism that generates these pseudopodia is uncoupled from the chemoattractant gradient. A similar mechanism appears to be at work during folic acid chemotaxis in Dictyostelium. Unpolarized, vegetative cells, which are sensitive to the chemoattractant folic acid when grown in the presence of bacteria, perform a biased random walk and meander in the right direction when exposed to a gradient of folic acid (CJ, unpub. obser.).
Positive feedback schemes
There are also models that rely on strong internal “positive feedback” loops. Signaling molecules are selectively amplified at the leading edge of the cell and sharpen the cell response [30,60-62]. Another scheme links a positive action at the front of the cell to an opposing action at the back. The “intermediate depletion” mechanism proposes that highly cooperative binding at the front limits the availability of free signaling molecules at the rear [63]. A similar mechanism, which contains no feedback amplification or inhibition, has been proposed for dermal fibroblasts, in which PI3K is depleted from a cytosolic pool [64]. Fibroblasts are less sensitive than Dictyostelium cells or neutrophils, and depend on both the relative PDGF gradient and the midpoint concentration. In neutrophils, alternatively, a Rac to PI3K positive feedback loop has been identified as a key component for the activation of PI3K and gradient sensing [40,65]. Other positive feedback models have incorporated further mechanisms to explain the localization of proteins in the rear, as well as those in the front. In one scheme, simulations were performed on a cell that was modeled as a reaction-diffusion system, in which coupled positive feedback and or cooperative interactions led to bifurcations. In this case, the activation of PI3K caused the inhibition of PTEN [66]. These positive feedback models provide considerable amplification and may be useful for cell polarization. However, such models do not explain the responses of cells to incremental increases that are proportional to the relative gradient. They also cannot explain responses in which there are rapid shifts in directional input, given that the response should be relatively independent from the input. Nevertheless, variations of these models have been used to explain polarized sensitivity as well as account for spontaneous polarization after uniform addition of chemoattractants [61,67-69].
Local excitation, global inhibition model
Other models have been developed that use an interplay between a local activator and a global inhibitor to control spatial sensing [60,61,70-72]. In the local excitation, global inhibition (LEGI) model, the adaptive properties of the chemotactic response system can be modeled in terms of a rapid “excitation” and a slower “inhibition” process (Figure 3) [51,73]. The difference between these two processes controls the cellular response. Although their kinetics differ, the final steady-state levels of excitation and inhibition are each linked to receptor occupancy. When occupancy is suddenly increased, excitation rises rapidly and for a time exceeds inhibition. As a result, the response rises. As the slower inhibitory process approaches the new steady-state level and reestablishes the balance, the response declines towards the pre-stimulus level. The adapted cells can respond further if receptor occupancy is increased again. The model in Figure 3 conveniently predicts the observed responses of cells to temporal and spatial stimuli. The LEGI mechanism is able to account for most of the observed responses of various signaling and cytoskeletal proteins seen in Dictyostelium cells and neutrophils in response to a uniform stimulus or when cells are in a gradient. A two-LEGI model has been proposed to regulate, in parallel, the binding sites for PI3K and PTEN at the front and rear, respectively, of a migrating cell [74]. This reciprocal regulation of these enzymes can account for the localized PI(3,4,5)P3 responses seen in Latrunculin-treated cells. However, it is likely that models involving positive feedback loops are needed to fully explain the responses observed in polarized cells. PI3K localization is severely inhibited in cells treated with Latrunculin, suggesting that the anterior cytoskeleton may stabilize the interaction with PI3K and thereby reinforce the initial symmetry [30,62]. Aspects of this model may also apply to random cell motility in which both positive feedback loops and negative regulatory components appear to control pseudopod formation in the absence of exogenous signals [75]. It will be intriguing to determine if a similar connection to the cytoskeleton occurs at the rear to help localize PTEN. Interestingly, cells that are highly polarized and have sharp localizations of PTEN in the rear do not exhibit a loss of PTEN when given a uniform chemoattractant stimulus, suggesting that the polarized morphology of the cytoskeleton may play a role in stabilizing PTEN localization. Another model that shares some of the components of the LEGI model has been proposed to help explain the switch-like behavior that cells display in a gradient, where they acquire a defined front and rear [76]. Levine and colleagues add a third element to their “balanced inactivation” model, a membrane-bound inactivator that is antagonistic to the response and allows a rapid switch-like response. Lastly, a more qualitative model, similar to that proposed by Bourne and colleagues, has been proposed by Onsum and Rao for neutrophil polarization. They suggest a model that does not require a global inhibitor [68,77]. This proposal involves the mutual antagonism between “frontness” and “backness” in cells as they develop a polarized morphology.
Figure 3. Local excitation, global inhibition model for temporal and spatial sensing.
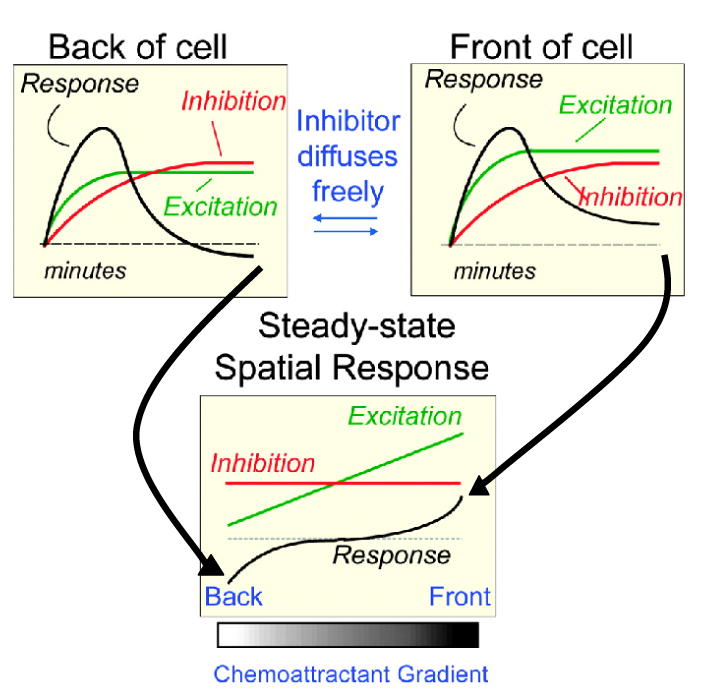
Receptor occupancy regulates two opposing processes, excitation and inhibition, which together regulate the response (green, red, and black lines, respectively). When a cell is initially exposed to a gradient, both ends respond. The fast local excitation processes increase proportionally to the local fraction of occupied receptors. The slow inhibitory response rises, driven by the global fraction of occupied receptors. When both processes reach a steady state (lower panel), the profile of excitation along the length of the cell is proportional to the local fraction, whereas the global inhibitor is proportional to the mean level of receptor occupancy, respectively. Thus, at the front, excitation exceeds inhibition, leading to a persistent response and vice versa at the rear.
Does an inhibitor exist?
Various models have postulated the need for an inhibitor or an inactivator during directional sensing. Is there evidence that an inhibitor exists? A number of experiments have attempted to answer this question. Latrunculin-treated Dictyostelium cells were treated with various spatial and temporal stimulus paradigms and their PI(3,4,5)P3 responses were monitored by the examination of PHcrac-GFP [30,36,73]. The experimental findings suggested that two types of responses were generated by the same internal mechanism. First, naïve cells were exposed to a cAMP gradient emitted from a micropipette being rapidly brought into close proximity. As predicted by the LEGI model, there was a rapid uniform increase in PI(3,4,5)P3 across the cell, which gradually lowered at the rear of the cell as the suspected inhibitor slowly elevated (Figure 4A) [73]. In a similar experiment, the transient disappearance of the PH-GFP crescent response in Figure 4B after the addition of a rapid, uniform stimulus also provides strong evidence for a chemoattractant-induced inhibitor that dissipates slowly when the stimulus is removed. Why does such a response suggest the presence of an inhibitor? In the absence of a chemoattractant, proteins like myosin II and PTEN are found on the plasma membrane (except for areas where pseudopodia are extending by a G-protein-independent mechanism) [43,46]. Since the cells are not stimulated, the default in the absence of external signaling is for these proteins to be localized to the membrane. This is similar to what happens when cells adapt to constant chemoattractant stimulation, and suggests that external signals are no longer eliciting a response and are inhibited. The mechanism has yet to be defined, although some default factor(s) or molecule(s) are present and carefully regulated. One such molecule appears to be PI(4,5)P2, the substrate for both PLC and PI3K, whose levels are highest in the absence of signaling and may directly or indirectly recruit these membrane-localized molecules.
Figure 4. Response of cells to combinations of stimuli.
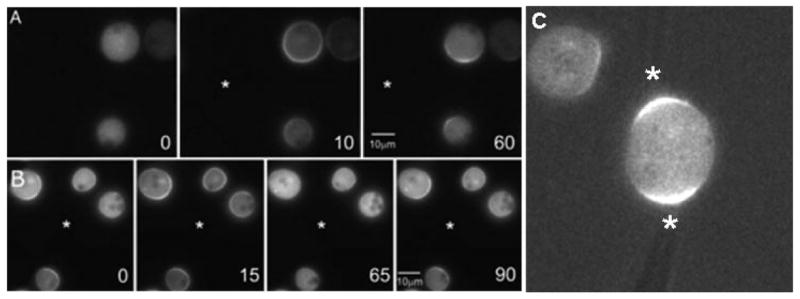
Latrunculin-treated cells were exposed to sequential temporal and spatial stimuli, and images were captured. (A) A micropipette (location denoted by the asterisk) producing a stable cAMP gradient was introduced to naïve cells after the first frame (0 sec). (B) Cells originally in a gradient (0 sec) were further stimulated by a transient bolus of cAMP generated by pumping the micropipette. Fluorescent images of the Cy3-cAMP used in these experiments demonstrated that the stimulus from the initial bolus dissipated in the 4-ml chamber, and the stable gradient was re-established within 15 sec. (C) Cells were exposed to competing gradients of cAMP and their PH-GFP responses were acquired. PH-GFP responses could be elicited or extinguished by gradually lowering or raising the micropipette pressures. Asterisks mark the locations of micropipettes.
This same membrane state appears to be transferred to the rear of the cell when sensing a gradient. In the experiment described in Figure 4B, the receptors on the high side of the gradient are always activated, yet the response subsides after the transient uniform stimulation. It remains off for a period of time even after the cAMP has diffused away and the original gradient re-forms. This demonstrated that the inhibition was not only slow to rise, but slow to fall. This same inhibitory mechanism may be at work when cells round up and localize PTEN and myosin II to the entire cell periphery at the onset of cytokinesis. However, this quiescent state is controlled by an internal regulator. Interestingly, these cells at this early metaphase stage appear unresponsive to the chemoattractant folic acid, even though the receptor/G-protein signaling apparatus is present (CJ, unpub. obser.). The gradient sensing experiments also demonstrated that an equivalent gradient will generate the same response whether it is formed by increasing the concentration at the front or by decreasing it at the rear of the cell (Figure 4B and C) [73]. Cells respond to changes in receptor occupancy and adapt when occupancy is held constant. The LEGI model accounts for such transient responses, directional responses to spatial gradients, and observed responses to combinations of temporal and spatial stimuli. This model is also consistent with the ability of the cell to respond to gradients with a wide range of midpoint concentrations. These multi-stimulus experiments demonstrated that the final steady-state response of the cell is completely independent of the stimulus history of the cell. Furthermore, models in which the signaling asymmetry is established by locally deactivating the rear of the cell cannot readily explain the responses to dual stimulation (Figure 4C) or the ability of a cell to accumulate PI(3,4,5)P3 locally in a gradient after a uniform stimulus [63,71] (Figure 4B). Although the LEGI mechanism can account for the responses seen in unpolarized cells in the absence of F-actin-mediated feedback loops, it is clear that additional amplification steps are needed for polarized cells. Components of the other models will likely be useful to explain the spontaneous polarization and hysteretic behaviors of cells. These features could be affected through actin-dependent, positive-feedback loops. Elements of directional sensing and polarization are most likely conserved among Dictyostelium, leukocytes, and many other cell types.
Role of PI(3,4,5)P3 and other lipid signaling pathways
A tremendous amount of effort in numerous systems has been exerted to understand the role of PI(3,4,5)P3, the product of PI3K, in gradient sensing and cell motility since the Pleckstrin Homology (PH) domain of the cytosolic regulator of adenylyl cyclase (PHcrac) and Akt/PKB (PHAkt) were found to localize to the leading edge of chemotaxing Dictyostelium cells and subsequently to the leading edge in neutrophils and fibroblasts [36,53,78,79]. The localized synthesis and accumulation of PI(3,4,5)P3 to the leading edges of pseudopodia, filopodia, and lamellopodia is highly suggestive of a role for PI(3,4,5)P3 in regulating these F-actin-mediated cell protrusions. However, the precise role of PI(3,4,5)P3 in chemotaxis has remained elusive. In vitro studies in the 1990s linked PI3K activity with the Rho GTPases, Rac, and Cdc42 and suggested an important role in actin dynamics [80,81]. In addition, a number of guanine nucleotide exchange factors (GEFS) as well as downstream Rac/Cdc42 effectors like WAVE (SCAR) and WASP, interact with and are activated by PI(3,4,5) P3 [82,83]. Several studies have reported positive-feedback loops between PI3K and Rac through F-actin polymerization [16,40,84]. The resulting activation of Rac1 at the leading edge of primary human neutrophils and neutrophils from mouse Rac1/Rac2 null cells has shown that Rac1 can inhibit local Rho and myosin activation at the uropod [85]. This finding follows the proposal that Rac-dependent leading edge “frontness” and rear Rho-reliant “backness” modules regulate cell polarity through mutual inhibition [77]. More recent work in neutrophils indicates that PI(3,4,5)P3 and Cdc42 maintain stable polarity at the leading edge by strengthening pseudopodia and by promoting RhoA-dependent actomyosin contraction at the rear [86]. Interestingly, a new study by Costa and colleagues suggests that PI3K may regulate Rac activity by contributing to GAP activation [41]. This, they postulate, may actually allow PI(3,4,5)P3 production to both activate and restrain positive feedback loops that amplify chemotactic gradient sensing, suggesting that PI(3,4,5) P3 effects may even be more complex.
The real excitement in the field linking PI(3,4,5)P3 to cell migration was generated when it was shown that the enzymes that control the spatial distribution of PI(3,4,5)P3, PI3K and PTEN, are restricted to the front and rear of migrating cells, respectively [31,43]. Mutations in Dictyostelium PI3K lower the amount of PI(3,4,5) P3 while mutations in PTEN cause elevated PI(3,4,5) P3 levels, loss of polarity, an increase in F-actin, and directionality defects. The expression of a lipid-tagged PI3K that is uniformly distributed along the plasma membrane leads to a similar loss of cell polarity and an up-regulation of F-actin synthesis and pseudopodia formation along the cortex [31]. These observations are consistent with a model in which PI(3,4,5)P3 production can drive F-actin synthesis. Observations by Soll and colleagues that migrating cells lacking PTEN, which do not polarize, have an increase in lateral pseudopod formation are consistent with this model [87]. Myosin II co-localizes with PTEN during cell motility and cytokinesis and likely plays a role in actomyosin-based contractions [88-90]. The study by Janetopoulos and colleagues determined that a cell rounds up at metaphase during the entry to cytokinesis. At this stage the cell is quiescent and lacks pseudopodia, and PTEN and myosin II are localized along the cell's cortex, suggesting that these pathways may generally control the cortical cytoskeleton during a number of dynamic reorganizations. Loss of PTEN therefore leads to loss of “backness” and cells are unable to stabilize their rear and PI3K-mediated F-actin-based projections are more likely to occur [also see the role of PI(4,5)P2 in polarity below]. This “dominant” phenotype in pten null and PI3K over-expressing cells would also be observed in cells in which redundant pathways control leading edge formation and directionality, as overproduction of PI(3,4,5)P3 can lead to F-actin polymerization. Knocking out a negative regulator, in this case PTEN, is more likely to produce a dramatic effect than reducing a component that may be sufficient but not pivotal on its own. In Dictyostelium, PI3K1 and PI3K2 account for ∼90% of the chemoattractant-induced PI(3,4,5)P3 activity [31,37]. Studies performed using the pi3k1-/pi3k2- double knockout or the pi3k1-/pi3k2-/pi3k3- triple knockout reveal an important role for PI(3,4,5)P3 generation when cells are in shallow gradients [44]; however, these and other studies indicate that well-developed cells lacking all PI3K function are capable of chemotaxis in steep gradients generated by micropipettes [44,46,47]. Cells in which the genes encoding all five class I PI3Ks that contain a Ras binding domain have been deleted, as well as another cell line that, in addition, lacks PTEN, were still able to undergo chemotax, but exhibited reduced velocities in micropipette assays [47]. It is not surprising that the initial phase of actin polymerization is unaffected in cells lacking PI3K activity, as has been shown in cells lacking PI3K1 and PI3K2 as well as those treated with the PI3K inhibitor LY294002 [46,91]. These studies suggest that the mechanisms controlling this first phase of actin polymerization may also help stabilize the actin cytoskeleton and the polarity of a cell in the absence of PI(3,4,5)P3. This initial peak in actin polymerization may act as a reset mechanism in which existing cell polarity is disrupted, allowing cells to respond to the gradient. Other studies uncovered a positive feedback mechanism for PI(3,4,5)P3 and the polymerization of F-actin [30,62,92], yet the Hoellar et al study showed that PI3K localizes in the absence of PI(3,4,5)P3, suggesting that positive feedback through PI(3,4,5,)P3 is not required for PI3K localization. This is consistent with previous studies indicating that F-actin is required for PI3K recruitment to the leading edge [30]. Future experiments should test whether Ras activity is affected in these mutant cells, as localization does not necessarily mean a functional PI3K would be activated. To further analyze the role of PI(3,4,5)P3 in gradient detection, it will also be interesting to monitor the ability of cells lacking all PI3Ks with a Ras binding domain and PTEN to migrate appropriately when exposed to gradients that change their frequency and direction. In neutrophils, cells lacking PI3Kγ were found to be impaired during chemotaxis [78]; however, other studies showing that these cells have velocity and motility defects makes interpretation of the exact role of PI(3,4,5)P3 in chemotaxis more difficult [93-95]. The genetic studies in which the genes encoding PI3K are disrupted do not consider the possibility that cells often compensate for pathway loss by up-regulating parallel pathways or other pathway components. For example, cells lacking PI3K1 and PI3K2 up-regulate RacB expression (RNA and protein), which might offset the loss of PI3 function [84]. Interestingly, cells treated with the PI3K inhibitor LY294002 gradually start to chemotax better after 40-45 min of treatment [44]. One possible explanation is that cells compensate for the loss of PI3K activity by up-regulating parallel pathways.
More recent studies have suggested that cells may use different pathways depending on the type of chemoattractant they use. Work from the Kubes group demonstrated a role for PI3K in mediating chemotaxis to IL8, whereas PI3Kγ null cells responding to the chemoattractant fMLP were unaffected [96]. They found that p38-MAPK-inhibited cells or cells lacking MK2, a protein kinase down stream of p38 MAPK, fail to polarize and respond to fMLP. This group observed that neutrophils prioritize and favor end target chemoattractants (e.g., fMLP and C5a) emanating from a site of infection over intermediary endogenous chemoattractants (e.g., IL-8 and LTB4) that might be encountered en route to sites of infection [97]. In addition to there being two alternate signaling pathways, Kubes and colleagues suggest that these data provide evidence for an intracellular signaling hierarchy wherein the end target chemoattractants activate p38 MAPK, which can, in turn, inhibit the intermediary chemoattractant-induced PI3K/Akt pathway. Similar findings were reported by Wu et al [98], who showed that neutrophils lacking MK2 also fail to respond to fMLP. Neutrophils lacking MK2 and neutrophils treated with a p38 MAPK inhibitor exhibit a disruption of PTEN localization at the rear of the cell, with some PTEN localizing to the leading edge. Wu and colleagues previously used antibody labeling to determine that PTEN localizes to the uropod of polarized neutrophils [99]. Recent parallel studies using mouse CD8 T cells have shown that while PI3Kγ is not essential for constitutive migration of these cells, it plays an important role in the migration of these cells to sites of inflammation [100]. These studies from the Kubes and Shimizu laboratories suggest that, as in Dictyostelium, the role that PI3K plays in directional migration is context dependent and suggests that cells have evolved a series of interdependent signaling pathways that mediate this essential cellular function to provide the needed flexibility to respond under different physiological conditions.
PI3K and PTEN in Dicytostelium are reciprocally regulated to control PI(3,4,5)P3 levels both temporally and spatially in response to a uniform stimulus and during gradient sensing [31,43]. Two studies in neutrophils have examined the effects on PI(3,4,5)P3 levels and chemotaxis in neutrophils lacking PTEN [93,101]. Subramanian et al found that these cells exhibited increased PI(3,4,5)P3 levels and actin polymerization but found limited effects on chemotaxis directionality. Nishio and colleagues, however, found no change in PI(3,4,5)P3 levels in comparison to wild-type neutrophils and no impact on chemotaxis. They observed that the Src homology 2 (SH2) domain-containing inositol-5-phosphatase 1 (SHIP1) can regulate PI(3,4,5)P3 levels and that cells lacking SHIP1 have severe polarity and motility defects, and are unable to assemble actin properly, but can still chemotax, albeit very slowly. Similar results were found for neutrophils lacking PI3Kγ or treated with the PI3K inhibitor wortmannin. Interestingly, ship1 null cells treated with wortmannin showed an increase in speed, likely due to focusing the membrane protrusions to the leading edge of the cell [93].
The data demonstrating that PI(3,4,5)P3 can drive actin polymerization in a number of systems are compelling and are therefore unlikely to be misleading [102,103]. Findings in both Dictyostelium [88] and in mammalian adherent cells [104] have demonstrated a role for PI(3,4,5)P3 at the poles of cells undergoing cytokinesis. Many of the molecular components localized at the leading or trailing edges of chemotaxing cells are also found at the poles or furrow, respectively, of dividing cells. Janetopoulos and colleagues found that PI3Ks and PTEN localize at the poles and furrow of dividing cells and PI(3,4,5)P3 accumulation is spatially correlated with and influences the polarity of the actin cytoskeleton during cytokinesis [88]. More recently, our laboratories determined that the regulation of PI3K and PTEN play an important role in random motility and that that the regulation of these enzymes during random motility and cytokinesis is completely independent of heterotrimeric G proteins [75]. In addition, Ras activity mirrors the localization of PI3K activity in both cell division and random migration, suggesting that a Ras/PI3K/F-actin network controls both pathways.
Sasaki et al (2007) suggested that the cell's chemosensory system (i.e., receptors and heterotrimeric G proteins) has tapped into the machinery used for random motility through the course of evolution (Figure 5) [75]. The internal signaling mechanism and the receptor/G protein signaling network appear to converge at the level of the small G-proteins and activation of PI3K and PTEN. Thus, the cell is likely measuring the gradient against the endogenous production of pseudopodia in a shallow gradient and may or may not move efficiently. In a steep gradient, the chemosensory system overrides the random pseudopodia [105]. The degree of polarity of a cell also plays an important role in this process. The more polarized the cell, the less steep the gradient required to get a cell to move in the correct direction. Once in the correct orientation, the cells will move quickly, even in a shallow gradient. Sasaki et al speculates that polarity in Dictyostelium, in which cells are elongated and display polarized sensitivity, evolved to keep the proper heading during aggregation (even in the absence of a gradient) since cells need not stray when migrating towards an aggregation center. Undifferentiated vegetative cells, on the other hand, must constantly change directions while looking for their next meal and remain sensitive to chemoattractants across their entire periphery. The addition of LY294002 to cells in the absence of a chemoattractant results in decreased motility. In a gradient, however, cells treated with PI3K inhibitors have their lateral pseudopodia suppressed and increase their directionality [91].
Figure 5. Model for the Ras/PI3K circuit during random movement and chemotaxis.
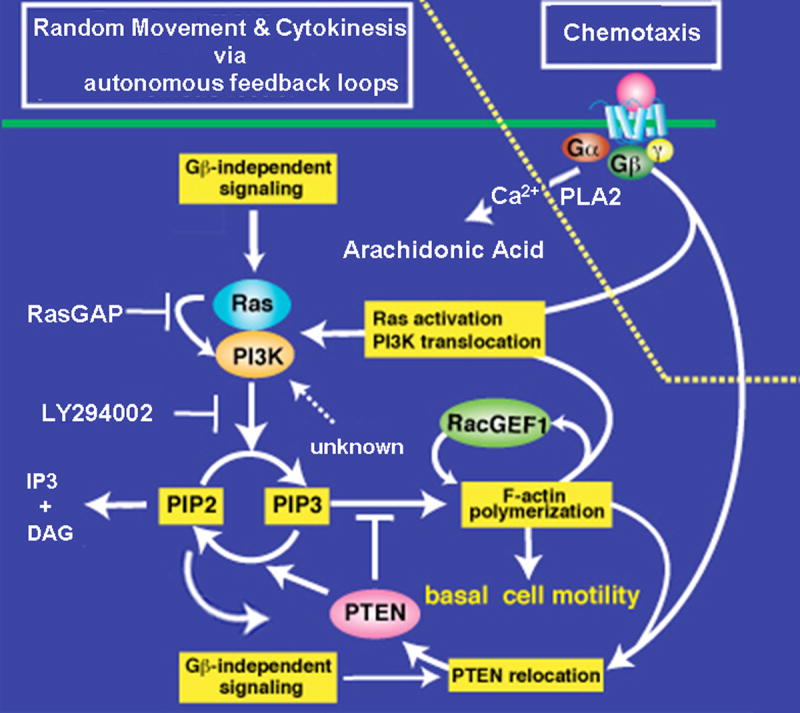
We propose that Ras/PI3K/PTEN/F-actin components form a feedback loop that is activated autonomously and without extracellular stimuli. The feedback loop we describe requires cytoskeletal regulators that simultaneously recruit PI3K and de-localize PTEN from the protrusion site. We assume that the regulators for Ras/PI3K/PTEN and F-actin polymerization/disassembly can influence the initiation and decay of the circuit. As the process is stochastic, we hypothesize that an increase in the level of any of the responses over a threshold level may be sufficient to trigger the feedback loops and pseudopod formation, while components such as GAPs and phosphatases regulate the threshold and level/time of activation.
The model illustrates the proposed intracellular signaling pathways leading to a positive feedback amplification of the pathways controlling pseudopod extension. PI3K, which is translocated to the membrane and activated by Ras-GTP together with PTEN released from the membrane and possibly other regulators, induces PI(3,4,5)P3 synthesis, which elicits F-actin polymerization. PLC activation leads to a loss of PI(4,5)P2, and thus may limit the number of binding sites for PTEN, which contains a putative PI(4,5)P2-binding motif. PI3K and RacGEF1 are recruited to the F-actin polymerization site, by a mechanism that is dependent on F-actin and possibly other cellular factors, and induce further PI(3,4,5)P3 synthesis and Rac activation, respectively. F-actin polymerization and PI(3,4,5)P3 signaling provoke additional Ras activation, possibly through the recruitment of RasGEFs. Each of these enzymatic processes is modulated by negative regulators of the cytoskeleton and signaling modules, such as RasGAPs, RacGAPs, PTEN, and PLC. Those negative regulators, or inhibitory events, determine the turnover and threshold for autonomous activation of the Ras/PI3K/F-actin feedback loop. Chemoattractants induce Ras/PI3K activation and reciprocal PI3K and PTEN localization through heterotrimeric G-proteins (right), as well as the activation of PLC. We expect that there are additional upstream regulators and factors that mediate PI3K's cortical localization, which is dependent on F-actin-polymerization (sensitive to Lattrunculin A/B). Activation of Ras/PI3K and inhibition of PTEN are integrated into a similar positive feedback loop that amplifies the initial response and is required for pseudopod formation and the formation of a robust, stable leading edge [30]. Chemotaxing wild-type cells and mutant strains such as pi3k1/2 null cells have a higher threshold for the autonomous Ras/PI3K/F-actin activation than unstimulated (“naïve”) vegetative cells. In chemotaxing cells, the threshold is higher at the back than at the front because PTEN, and possibly other negative regulators, localize at the back, as is graphically illustrated. PTEN activity and synthesis of PI(4,5)P2 likely forms a positive feedback loop with its own recruitment to the plasma membrane. Ligand-induced Ras/PI3K activation at the front, but not at the back, reaches a threshold level, which activates downstream responses. In polarized, chemotaxing cells, the threshold for pathway activation is significantly higher at the back than at the front. This differential threshold depends on both the stabilization of signaling complexes at the existing leading edge by the F-actin cytoskeleton and the localization of negative regulators at the cell's posterior.
A role for microtubules
With much focus on determining the mechanisms regulating actin polymerization, other components that make up the cytoskeleton have been overlooked. While most cells have decreased motility, previous studies indicate that treatment of cells with colchicine, which disassembles microtubules, causes neutrophil granulocytes to activate Rho kinase, polarize, and migrate [106]. Xu et al recently obtained similar results with neutrophils treated with the microtubule inhibitor nocodazole [29]. These studies determined that loss of microtubules stimulates backness by increasing Rho- and actomyosin-dependent contractility. Also, treatment of NIH 3T3 fibroblasts with PI3K inhibitors or expression of a dominant-negative form of Akt decreased the amount of stabilized microtubules after stimulation with platelet-derived growth factor (PDGF), suggesting a role for both PI(3,4,5)P3 and Akt in the maintenance of polarity [107]. These results are consistent with studies correlating the loss of microtubules in cells entering cytokinesis (where cells round up, localize PTEN to the membrane, and are essentially all “back”) [88]. As the spindle elongates, astral microtubules interact with the poles of the cell, which are analogous to the leading edge of the cell, and have PI3K activity, actin polymerization, and membrane ruffling. Feedback loops between microtubules, PI(3,4,5)P3 and the actin cytoskeleton may help promote “frontness.” Dictyostelium cells with aberrant PI(3,4,5)P3 levels or which are treated with PI3K inhibitors have cytokinesis defects whereas inhibition of PI3K in adherent cells results in spindle misalignment [88,104]. This new role that PI(3,4,5)P3 plays, in addition to its many other roles, further brings into perspective the importance of this secondary messenger in helping regulate the cytoskeleton during a variety of cell shape changes. In cells lacking proper PI(3,4,5)P3 regulation, it is possible that the cells adjust to get through important processes like cell division, and these redundant pathways compensate for the loss of PI(3,4,5)P3, as described above for up-regulation of RacB expression.
The regulation of PI(4,5)P2
Although much attention has been placed on the local amassing of PI(3,4,5)P3 during these processes, evidence suggests that there may also be a reciprocal accumulation of PI(4,5)P2. This temporal and spatial regulation of both PI(3,4,5)P3 and PI(4,5)P2 may recruit specific cytoskeletal regulators and act as an important switch in defining where a cell makes protrusions and where a cell contracts, respectively. Evolution has apparently built a lot of redundancy into the system regulating polarized morphology. A recent report from the Huttenlocher laboratory implicates a PI5 kinase (PIPKIγ661) in the rear of migrating neutrophils [108]. It will be intriguing to see if this PI5 kinase localizes to the furrow of dividing cells. Arguments against the lowering of PI(4,5)P2 along the periphery of the cell stem from the high levels of PI(4,5)P2 thought to be in the cell [109].
However, there is plenty of support; chemoattractants applied as a gradient, such as cAMP with Dictyostelium or fMLP with neutrophils, induce the activation of phospholipase C (PLC) and PI3Ks at the front of the cell. van Haastert et al recently reported that the loss of PLC, which normally hydrolyzes PI(4,5)P2 and yields inositol 1,4,5-trisphosphate (IP3) and diacylglycerol (DAG), resulted in increased membrane-bound PTEN [110]. They reason that PLC null cells have higher plasma membrane PI(4,5)P2 levels which in turn recruit more PTEN through its N-terminal PI(4,5)P2 binding motif [45,110,111]. They found that there is no loss of PTEN from the plasma membrane in response to uniform cAMP in the PLC null cells. This discovery suggests that PLC is normally activated in response to a chemoattractant, hydrolyzes PI(4,5)P2, and PTEN moves to the cytosol. In the PLC null cells, this recruitment of PTEN, in turn, acts on the PI3K pathway by negatively regulating PI(3,4,5)P3 levels. They also determined that PI(3,4,5)P3 levels do not dramatically change in response to a uniform cAMP stimulus [45]. The small changes in PI(3,4,5)P3 levels would be expected to lower the levels of PI(4,5)P2 to some degree, and thus negatively regulate PTEN levels on the membrane, but these changes may be below the limit of sensitivity using fluorescence microscopy. Interestingly, they observed that cells over expressing PLC, in turn, deplete levels of PTEN on the membrane, and these cells resemble pten null cells in that they follow a less direct path towards a micropipette and have broad pseudopodia and polarity defects because of their inability to regulate PI(3,4,5)P3 levels. These studies and those on PI3K demonstrate that lipid metabolism is a key regulator of cell motility and chemotaxis. It will be exciting to uncover the inter-relationship between the pathways at genetic and biochemical levels in mediating the spatial responses of cells.
Conclusions
Recent advances have made it clear that the regulation of cell morphology in a number of cellular processes uses the same overlapping signaling cascades and cytoskeletal components to perform similar cell shape changes. Since any given one of these processes may be critical depending on the type of cell, its environment, or its current status in the life cycle, it should not be surprising that cells have evolved redundant pathways to carry the cell forward when one pathway fails. Although much progress has been made in elucidating which components are involved, there is still much work to be done to understand how cells initiate a polarized morphology, whether it is during chemotaxis, random motility, or cell division. Many of the models proposed here for gradient sensing should be expanded to incorporate the mechanisms at work during the other processes, as they are likely to provide significant clues as to how a cell localizes responses in shallow chemical gradients and from internal cellular cues.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rubel EW, Cramer KS. Choosing axonal real estate: location, location, location. J Comp Neurol. 2002;448:1–5. doi: 10.1002/cne.10255. [DOI] [PubMed] [Google Scholar]
- 2.Martin P, Parkhurst SM. Parallels between tissue repair and embryo morphogenesis. Development. 2004;131:3021–34. doi: 10.1242/dev.01253. [DOI] [PubMed] [Google Scholar]
- 3.Moser B, Loetscher P. Lymphocyte traffic control by chemokines. Nat Immunol. 2001;2:123–8. doi: 10.1038/84219. [DOI] [PubMed] [Google Scholar]
- 4.Thelen M. Dancing to the tune of chemokines. Nat Immunol. 2001;2:129–34. doi: 10.1038/84224. [DOI] [PubMed] [Google Scholar]
- 5.Le Y, Zhou Y, Iribarren P, Wang J. Chemokines and chemokine receptors: their manifold roles in homeostasis and disease. Cell Mol Immunol. 2004;1:95–104. [PubMed] [Google Scholar]
- 6.Condeelis J, Jones J, Segall JE. Chemotaxis of metastatic tumor cells: clues to mechanisms from the Dictyostelium paradigm. Cancer Metastasis Rev. 1992;11:55–68. doi: 10.1007/BF00047603. [DOI] [PubMed] [Google Scholar]
- 7.Murphy PM. Chemokines and the molecular basis of cancer metastasis. N Engl J Med. 2001;345:833–5. doi: 10.1056/NEJM200109133451113. [DOI] [PubMed] [Google Scholar]
- 8.Wardlaw AJ, Brightling C, Green R, Woltmann G, Pavord I. Eosinophils in asthma and other allergic diseases. Br Med Bull. 2000;56:985–1003. doi: 10.1258/0007142001903490. [DOI] [PubMed] [Google Scholar]
- 9.Worthley SG, Osende JI, Helft G, Badimon JJ, Fuster V. Coronary artery disease: pathogenesis and acute coronary syndromes. Mt Sinai J Med. 2001;68:167–81. [PubMed] [Google Scholar]
- 10.Tarrant TK, Patel DD. Chemokines and leukocyte trafficking in rheumatoid arthritis. Pathophysiology. 2006;13:1–14. doi: 10.1016/j.pathophys.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 11.Condeelis J, Singer RH, Segall JE. The great escape: when cancer cells hijack the genes for chemotaxis and motility. Annu Rev Cell Dev Biol. 2005;21:695–718. doi: 10.1146/annurev.cellbio.21.122303.120306. [DOI] [PubMed] [Google Scholar]
- 12.Devreotes P, Janetopoulos C. Eukaryotic chemotaxis: distinctions between directional sensing and polarization. J Biol Chem. 2003;278:20445–8. doi: 10.1074/jbc.R300010200. [DOI] [PubMed] [Google Scholar]
- 13.Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, Parsons JT, Horwitz AR. Cell migration: integrating signals from front to back. Science. 2003;302:1704–9. doi: 10.1126/science.1092053. [DOI] [PubMed] [Google Scholar]
- 14.Chung CY, Funamoto S, Firtel RA. Signaling pathways controlling cell polarity and chemotaxis. Trends Biochem Sci. 2001;26:557–66. doi: 10.1016/s0968-0004(01)01934-x. [DOI] [PubMed] [Google Scholar]
- 15.Affolter M, Weijer CJ. Signaling to cytoskeletal dynamics during chemotaxis. Dev Cell. 2005;9:19–34. doi: 10.1016/j.devcel.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 16.Charest PG, Firtel RA. Feedback signaling controls leading-edge formation during chemotaxis. Curr Opin Genet Dev. 2006;16:339–47. doi: 10.1016/j.gde.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 17.Heid PJ, Wessels D, Daniels KJ, Gibson DP, Zhang H, Voss E, Soll DR. The role of myosin heavy chain phosphorylation in Dictyostelium motility, chemotaxis and F-actin localization. J Cell Sci. 2004;117:4819–35. doi: 10.1242/jcs.01358. [DOI] [PubMed] [Google Scholar]
- 18.Bagorda A, Mihaylov VA, Parent CA. Chemotaxis: moving forward and holding on to the past. Thromb Haemost. 2006;95:12–21. [PubMed] [Google Scholar]
- 19.Kimmel AR, Firtel RA. Breaking symmetries: regulation of Dictyostelium development through chemoattractant and morphogen signal-response. Curr Opin Genet Dev. 2004;14:540–9. doi: 10.1016/j.gde.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 20.Franca-Koh J, Kamimura Y, Devreotes P. Navigating signaling networks: chemotaxis in Dictyostelium discoideum. Curr Opin Genet Dev. 2006;16:333–8. doi: 10.1016/j.gde.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 21.Landree MA, Devreotes PN. Analyzing chemotaxis using Dictyostelium discoideum as a model system. Methods Mol Biol. 2004;239:91–104. doi: 10.1385/1-59259-435-2:91. [DOI] [PubMed] [Google Scholar]
- 22.Williams RS, et al. Towards a molecular understanding of human diseases using Dictyostelium discoideum. Trends Mol Med. 2006;12:415–24. doi: 10.1016/j.molmed.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 23.Fey P, Gaudet P, Pilcher KE, Franke J, Chisholm RL. dictyBase and the Dicty Stock Center. Methods Mol Biol. 2006;346:51–74. doi: 10.1385/1-59745-144-4:51. [DOI] [PubMed] [Google Scholar]
- 24.Eichinger L, et al. The genome of the social amoeba Dictyostelium discoideum. Nature. 2005;435:43–57. doi: 10.1038/nature03481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xiao Z, Zhang N, Murphy DB, Devreotes PN. Dynamic distribution of chemoattractant receptors in living cells during chemotaxis and persistent stimulation. J Cell Biol. 1997;139:365–74. doi: 10.1083/jcb.139.2.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jin T, Zhang N, Long Y, Parent CA, Devreotes PN. Localization of the G protein betagamma complex in living cells during chemotaxis. Science. 2000;287:1034–6. doi: 10.1126/science.287.5455.1034. [DOI] [PubMed] [Google Scholar]
- 27.Ueda M, Sako Y, Tanaka T, Devreotes P, Yanagida T. Single-molecule analysis of chemotactic signaling in Dictyostelium cells. Science. 2001;294:864–7. doi: 10.1126/science.1063951. [DOI] [PubMed] [Google Scholar]
- 28.Janetopoulos C, Jin T, Devreotes P. Receptor-mediated activation of heterotrimeric G-proteins in living cells. Science. 2001;291:2408–11. doi: 10.1126/science.1055835. [DOI] [PubMed] [Google Scholar]
- 29.Xu X, Meier-Schellersheim M, Jiao X, Nelson LE, Jin T. Quantitative imaging of single live cells reveals spatiotemporal dynamics of multistep signaling events of chemoattractant gradient sensing in Dictyostelium. Mol Biol Cell. 2005;16:676–88. doi: 10.1091/mbc.E04-07-0544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sasaki AT, Chun C, Takeda K, Firtel RA. Localized Ras signaling at the leading edge regulates PI3K, cell polarity, and directional cell movement. J Cell Biol. 2004;167:505–18. doi: 10.1083/jcb.200406177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Funamoto S, Meili R, Lee S, Parry L, Firtel RA. Spatial and temporal regulation of 3-phosphoinositides by PI 3-kinase and PTEN mediates chemotaxis. Cell. 2002;109:611–23. doi: 10.1016/s0092-8674(02)00755-9. [DOI] [PubMed] [Google Scholar]
- 32.Bolourani P, Spiegelman GB, Weeks G. Rap1 activation in response to cAMP occurs downstream of Ras activation during Dictyostelium aggregation. J Biol Chem. 2008;283:10232–40. doi: 10.1074/jbc.M707459200. [DOI] [PubMed] [Google Scholar]
- 33.Bolourani P, Spiegelman GB, Weeks G. Delineation of the roles played by RasG and RasC in cAMP-dependent signal transduction during the early development of Dictyostelium discoideum. Mol Biol Cell. 2006;17:4543–50. doi: 10.1091/mbc.E05-11-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Iijima M, Huang YE, Devreotes P. Temporal and spatial regulation of chemotaxis. Dev Cell. 2002;3:469–78. doi: 10.1016/s1534-5807(02)00292-7. [DOI] [PubMed] [Google Scholar]
- 35.Meili R, Firtel RA. Follow the leader. Dev Cell. 2003;4:291–3. doi: 10.1016/s1534-5807(03)00064-9. [DOI] [PubMed] [Google Scholar]
- 36.Parent CA, Blacklock BJ, Froehlich WM, Murphy DB, Devreotes PN. G protein signaling events are activated at the leading edge of chemotactic cells. Cell. 1998;95:81–91. doi: 10.1016/s0092-8674(00)81784-5. [DOI] [PubMed] [Google Scholar]
- 37.Huang YE, Iijima M, Parent CA, Funamoto S, Firtel RA, Devreotes P. Receptor-mediated regulation of PI3Ks confines PI(3,4,5)P3 to the leading edge of chemotaxing cells. Mol Biol Cell. 2003;14:1913–22. doi: 10.1091/mbc.E02-10-0703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ridley AJ. Rho proteins, PI 3-kinases, and monocyte/macrophage motility. FEBS Lett. 2001;498:168–71. doi: 10.1016/s0014-5793(01)02481-4. [DOI] [PubMed] [Google Scholar]
- 39.Wang F, Herzmark P, Weiner OD, Srinivasan S, Servant G, Bourne HR. Lipid products of PI(3)Ks maintain persistent cell polarity and directed motility in neutrophils. Nat Cell Biol. 2002;4:513–8. doi: 10.1038/ncb810. [DOI] [PubMed] [Google Scholar]
- 40.Srinivasan S, Wang F, Glavas S, Ott A, Hofmann F, Aktories K, Kalman D, Bourne HR. Rac and Cdc42 play distinct roles in regulating PI(3,4,5)P3 and polarity during neutrophil chemotaxis. J Cell Biol. 2003;160:375–85. doi: 10.1083/jcb.200208179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Costa C, et al. Negative feedback regulation of Rac in leukocytes from mice expressing a constitutively active phosphatidylinositol 3-kinase gamma. Proc Natl Acad Sci U S A. 2007;104:14354–9. doi: 10.1073/pnas.0703175104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Welch HC, et al. P-Rex1, a PtdIns(3,4,5)P3- and Gbetagamma-regulated guanine-nucleotide exchange factor for Rac. Cell. 2002;108:809–21. doi: 10.1016/s0092-8674(02)00663-3. [DOI] [PubMed] [Google Scholar]
- 43.Iijima M, Devreotes P. Tumor suppressor PTEN mediates sensing of chemoattractant gradients. Cell. 2002;109:599–610. doi: 10.1016/s0092-8674(02)00745-6. [DOI] [PubMed] [Google Scholar]
- 44.Takeda K, Sasaki AT, Ha H, Seung HA, Firtel RA. Role of phosphatidylinositol 3-kinases in chemotaxis in Dictyostelium. J Biol Chem. 2007;282:11874–84. doi: 10.1074/jbc.M610984200. [DOI] [PubMed] [Google Scholar]
- 45.van Haastert PJ, Keizer-Gunnink I, Kortholt A. Essential role of PI3-kinase and phospholipase A2 in Dictyostelium discoideum chemotaxis. J Cell Biol. 2007;177:809–16. doi: 10.1083/jcb.200701134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Loovers HM, Postma M, Keizer-Gunnink I, Huang YE, Devreotes PN, van Haastert PJ. Distinct roles of PI(3,4,5)P3 during chemoattractant signaling in Dictyostelium: a quantitative in vivo analysis by inhibition of PI3-kinase. Mol Biol Cell. 2006;17:1503–13. doi: 10.1091/mbc.E05-09-0825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hoeller O, Kay RR. Chemotaxis in the absence of PIP3 gradients. Curr Biol. 2007;17:813–7. doi: 10.1016/j.cub.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 48.Chen L, Iijima M, Tang M, Landree MA, Huang YE, Xiong Y, Iglesias PA, Devreotes PN. PLA2 and PI3K/PTEN pathways act in parallel to mediate chemotaxis. Dev Cell. 2007;12:603–14. doi: 10.1016/j.devcel.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dinauer MC, Steck TL, Devreotes PN. Cyclic 3′,5′-AMP relay in Dictyostelium discoideum V. Adaptation of the cAMP signaling response during cAMP stimulation. J Cell Biol. 1980;86:554–61. doi: 10.1083/jcb.86.2.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lauffenburger D, Rothman C, Zigmond SH. Measurement of leukocyte motility and chemotaxis parameters with a linear under-agarose migration assay. J Immunol. 1983;131:940–7. [PubMed] [Google Scholar]
- 51.Parent CA, Devreotes PN. A cell's sense of direction. Science. 1999;284:765–70. doi: 10.1126/science.284.5415.765. [DOI] [PubMed] [Google Scholar]
- 52.Servant G, Weiner OD, Herzmark P, Balla T, Sedat JW, Bourne HR. Polarization of chemoattractant receptor signaling during neutrophil chemotaxis. Science. 2000;287:1037–40. doi: 10.1126/science.287.5455.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Meili R, Ellsworth C, Lee S, Reddy TB, Ma H, Firtel RA. Chemoattractant-mediated transient activation and membrane localization of Akt/PKB is required for efficient chemotaxis to cAMP in Dictyostelium. EMBO J. 1999;18:2092–105. doi: 10.1093/emboj/18.8.2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brown DA, Berg HC. Temporal stimulation of chemotaxis in Escherichia coli. Proc Natl Acad Sci U S A. 1974;71:1388–92. doi: 10.1073/pnas.71.4.1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nieto M, Frade JM, Sancho D, Mellado M, Martinez AC, Sanchez-Madrid F. Polarization of chemokine receptors to the leading edge during lymphocyte chemotaxis. J Exp Med. 1997;186:153–8. doi: 10.1084/jem.186.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Baker MD, Wolanin PM, Stock JB. Signal transduction in bacterial chemotaxis. Bioessays. 2006;28:9–22. doi: 10.1002/bies.20343. [DOI] [PubMed] [Google Scholar]
- 57.Andrew N, Insall RH. Chemotaxis in shallow gradients is mediated independently of PtdIns 3-kinase by biased choices between random protrusions. Nat Cell Biol. 2007;9:193–200. doi: 10.1038/ncb1536. [DOI] [PubMed] [Google Scholar]
- 58.Gerisch G, Hulser D, Malchow D, Wick U. Cell communication by periodic cyclic-AMP pulses. Philos Trans R Soc Lond B Biol Sci. 1975;272:181–92. doi: 10.1098/rstb.1975.0080. [DOI] [PubMed] [Google Scholar]
- 59.Weber I. Is there a pilot in a pseudopod? Eur J Cell Biol. 2006;85:915–24. doi: 10.1016/j.ejcb.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 60.Meinhardt H. Orientation of chemotactic cells and growth cones: models and mechanisms. J Cell Sci. 1999;112:2867–74. doi: 10.1242/jcs.112.17.2867. [DOI] [PubMed] [Google Scholar]
- 61.Narang A, Subramanian KK, Lauffenburger DA. A mathematical model for chemoattractant gradient sensing based on receptor-regulated membrane phospholipid signaling dynamics. Ann Biomed Eng. 2001;29:677–91. doi: 10.1114/1.1385805. [DOI] [PubMed] [Google Scholar]
- 62.Weiner OD, Neilsen PO, Prestwich GD, Kirschner MW, Cantley LC, Bourne HR. A PtdInsP(3)- and Rho GTPase-mediated positive feedback loop regulates neutrophil polarity. Nat Cell Biol. 2002;4:509–13. doi: 10.1038/ncb811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Postma M, Van Haastert PJ. A diffusion-translocation model for gradient sensing by chemotactic cells. Biophys J. 2001;81:1314–23. doi: 10.1016/S0006-3495(01)75788-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schneider IC, Haugh JM. Quantitative elucidation of a distinct spatial gradient-sensing mechanism in fibroblasts. J Cell Biol. 2005;171:883–92. doi: 10.1083/jcb.200509028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Weiner OD. Regulation of cell polarity during eukaryotic chemotaxis: the chemotactic compass. Curr Opin Cell Biol. 2002;14:196–202. doi: 10.1016/s0955-0674(02)00310-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Skupsky R, Losert W, Nossal RJ. Distinguishing modes of eukaryotic gradient sensing. Biophys J. 2005;89:2806–23. doi: 10.1529/biophysj.105.061564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Subramanian KK, Narang A. A mechanistic model for eukaryotic gradient sensing: spontaneous and induced phosphoinositide polarization. J Theor Biol. 2004;231:49–67. doi: 10.1016/j.jtbi.2004.05.024. [DOI] [PubMed] [Google Scholar]
- 68.Onsum M, Rao CV. A mathematical model for neutrophil gradient sensing and polarization. PLoS Comput Biol. 2007;3:e36. doi: 10.1371/journal.pcbi.0030036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Narang A. Spontaneous polarization in eukaryotic gradient sensing: a mathematical model based on mutual inhibition of frontness and backness pathways. J Theor Biol. 2006;240:538–53. doi: 10.1016/j.jtbi.2005.10.022. [DOI] [PubMed] [Google Scholar]
- 70.Levchenko A, Iglesias PA. Models of eukaryotic gradient sensing: application to chemotaxis of amoebae and neutrophils. Biophys J. 2002;82:50–63. doi: 10.1016/S0006-3495(02)75373-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rappel WJ, Thomas PJ, Levine H, Loomis WF. Establishing direction during chemotaxis in eukaryotic cells. Biophys J. 2002;83:1361–7. doi: 10.1016/S0006-3495(02)73906-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Xu X, Meier-Schellersheim M, Yan J, Jin T. Locally controlled inhibitory mechanisms are involved in eukaryotic GPCR-mediated chemosensing. J Cell Biol. 2007;178:141–53. doi: 10.1083/jcb.200611096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Janetopoulos C, Ma L, Devreotes PN, Iglesias PA. Chemoattractant-induced phosphatidylinositol 3,4,5-trisphosphate accumulation is spatially amplified and adapts, independent of the actin cytoskeleton. Proc Natl Acad Sci U S A. 2004;101:8951–6. doi: 10.1073/pnas.0402152101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ma L, Janetopoulos C, Yang L, Devreotes PN, Iglesias PA. Two complementary, local excitation, global inhibition mechanisms acting in parallel can explain the chemoattractant-induced regulation of PI(3,4,5)P3 response in dictyostelium cells. Biophys J. 2004;87:3764–74. doi: 10.1529/biophysj.104.045484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sasaki AT, et al. G protein-independent Ras/PI3K/F-actin circuit regulates basic cell motility. J Cell Biol. 2007;178:185–91. doi: 10.1083/jcb.200611138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Levine H, Kessler DA, Rappel WJ. Directional sensing in eukaryotic chemotaxis: a balanced inactivation model. Proc Natl Acad Sci U S A. 2006;103:9761–6. doi: 10.1073/pnas.0601302103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Xu J, et al. Divergent signals and cytoskeletal assemblies regulate self-organizing polarity in neutrophils. Cell. 2003;114:201–14. doi: 10.1016/s0092-8674(03)00555-5. [DOI] [PubMed] [Google Scholar]
- 78.Rickert P, Weiner OD, Wang F, Bourne HR, Servant G. Leukocytes navigate by compass: roles of PI3Kgamma and its lipid products. Trends Cell Biol. 2000;10:466–73. doi: 10.1016/s0962-8924(00)01841-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Haugh JM, Codazzi F, Teruel M, Meyer T. Spatial sensing in fibroblasts mediated by 3′ phosphoinositides. J Cell Biol. 2000;151:1269–80. doi: 10.1083/jcb.151.6.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zheng Y, Bagrodia S, Cerione RA. Activation of phosphoinositide 3-kinase activity by Cdc42Hs binding to p85. J Biol Chem. 1994;269:18727–30. [PubMed] [Google Scholar]
- 81.Bokoch GM, Vlahos CJ, Wang Y, Knaus UG, Traynor-Kaplan AE. Rac GTPase interacts specifically with phosphatidylinositol 3-kinase. Biochem J. 1996;315:775–9. doi: 10.1042/bj3150775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Suetsugu S, Kurisu S, Oikawa T, Yamazaki D, Oda A, Takenawa T. Optimization of WAVE2 complex-induced actin polymerization by membrane-bound IRSp53, PIP(3), and Rac. J Cell Biol. 2006;173:571–85. doi: 10.1083/jcb.200509067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Charest PG, Firtel RA. Big roles for small GTPases in the control of directed cell movement. Biochem J. 2007;401:377–90. doi: 10.1042/BJ20061432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Park KC, Rivero F, Meili R, Lee S, Apone F, Firtel RA. Rac regulation of chemotaxis and morphogenesis in Dictyostelium. EMBO J. 2004;23:4177–89. doi: 10.1038/sj.emboj.7600368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pestonjamasp KN, Forster C, Sun C, Gardiner EM, Bohl B, Weiner O, Bokoch GM, Glogauer M. Rac1 links leading edge and uropod events through Rho and myosin activation during chemotaxis. Blood. 2006;108:2814–20. doi: 10.1182/blood-2006-01-010363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Van Keymeulen A, Wong K, Knight ZA, Govaerts C, Hahn KM, Shokat KM, Bourne HR. To stabilize neutrophil polarity, PIP3 and Cdc42 augment RhoA activity at the back as well as signals at the front. J Cell Biol. 2006;174:437–45. doi: 10.1083/jcb.200604113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wessels D, Lusche DF, Kuhl S, Heid P, Soll DR. PTEN plays a role in the suppression of lateral pseudopod formation during Dictyostelium motility and chemotaxis. J Cell Sci. 2007;120:2517–31. doi: 10.1242/jcs.010876. [DOI] [PubMed] [Google Scholar]
- 88.Janetopoulos C, Borleis J, Vazquez F, Iijima M, Devreotes P. Temporal and spatial regulation of phosphoinositide signaling mediates cytokinesis. Dev Cell. 2005;8:467–77. doi: 10.1016/j.devcel.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 89.Neujahr R, Heizer C, Albrecht R, Ecke M, Schwartz JM, Weber I, Gerisch G. Three-dimensional patterns and redistribution of myosin II and actin in mitotic Dictyostelium cells. J Cell Biol. 1997;139:1793–804. doi: 10.1083/jcb.139.7.1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rubin H, Ravid S. Polarization of myosin II heavy chain-protein kinase C in chemotaxing dictyostelium cells. J Biol Chem. 2002;277:36005–8. doi: 10.1074/jbc.M205986200. [DOI] [PubMed] [Google Scholar]
- 91.Chen L, Janetopoulos C, Huang YE, Iijima M, Borleis J, Devreotes PN. Two phases of actin polymerization display different dependencies on PI(3,4,5)P3 accumulation and have unique roles during chemotaxis. Mol Biol Cell. 2003;14:5028–37. doi: 10.1091/mbc.E03-05-0339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Niggli V. A membrane-permeant ester of phosphatidylinositol 3,4, 5-trisphosphate (PIP(3)) is an activator of human neutrophil migration. FEBS Lett. 2000;473:217–21. doi: 10.1016/s0014-5793(00)01534-9. [DOI] [PubMed] [Google Scholar]
- 93.Nishio M, et al. Control of cell polarity and motility by the PtdIns(3,4,5)P3 phosphatase SHIP1. Nat Cell Biol. 2007;9:36–44. doi: 10.1038/ncb1515. [DOI] [PubMed] [Google Scholar]
- 94.Ferguson GJ, et al. PI(3)Kgamma has an important context-dependent role in neutrophil chemokinesis. Nat Cell Biol. 2007;9:86–91. doi: 10.1038/ncb1517. [DOI] [PubMed] [Google Scholar]
- 95.Ward SG. Do phosphoinositide 3-kinases direct lymphocyte navigation? Trends Immunol. 2004;25:67–74. doi: 10.1016/j.it.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 96.Heit B, Liu L, Colarusso P, Puri KD, Kubes P. PI3K accelerates, but is not required for, neutrophil chemotaxis to fMLP. J Cell Sci. 2008;121:205–14. doi: 10.1242/jcs.020412. [DOI] [PubMed] [Google Scholar]
- 97.Heit B, Tavener S, Raharjo E, Kubes P. An intracellular signaling hierarchy determines direction of migration in opposing chemotactic gradients. J Cell Biol. 2002;159:91–102. doi: 10.1083/jcb.200202114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wu Y, Hannigan MO, Kotlyarov A, Gaestel M, Wu D, Huang CK. A requirement of MAPKAPK2 in the uropod localization of PTEN during FMLP-induced neutrophil chemotaxis. Biochem Biophys Res Commun. 2004;316:666–72. doi: 10.1016/j.bbrc.2004.02.107. [DOI] [PubMed] [Google Scholar]
- 99.Li Z, et al. Regulation of PTEN by Rho small GTPases. Nat Cell Biol. 2005;7:399–404. doi: 10.1038/ncb1236. [DOI] [PubMed] [Google Scholar]
- 100.Martin AL, Schwartz MD, Jameson SC, Shimizu Y. Selective regulation of CD8 effector T cell migration by the p110gamma isoform of phosphatidylinositol 3-kinase. J Immunol. 2008;180:2081–8. doi: 10.4049/jimmunol.180.4.2081. [DOI] [PubMed] [Google Scholar]
- 101.Subramanian KK, et al. Tumor suppressor PTEN is a physiologic suppressor of chemoattractant-mediated neutrophil functions. Blood. 2007;109:4028–37. doi: 10.1182/blood-2006-10-055319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Insall RH, Weiner OD. PIP3, PIP2, and cell movement--similar messages, different meanings? Dev Cell. 2001;1:743–7. doi: 10.1016/s1534-5807(01)00086-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Insall R, Andrew N. Chemotaxis in Dictyostelium: how to walk straight using parallel pathways. Curr Opin Microbiol. 2007;10:578–81. doi: 10.1016/j.mib.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 104.Toyoshima F, Matsumura S, Morimoto H, Mitsushima M, Nishida E. PtdIns(3,4,5)P3 regulates spindle orientation in adherent cells. Dev Cell. 2007;13:796–811. doi: 10.1016/j.devcel.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 105.Soll DR, Wessels D, Heid PJ, Zhang H. A contextual framework for characterizing motility and chemotaxis mutants in Dictyostelium discoideum. J Muscle Res Cell Motil. 2002;23:659–72. doi: 10.1023/a:1024459124427. [DOI] [PubMed] [Google Scholar]
- 106.Niggli V. Microtubule-disruption-induced and chemotactic-peptide-induced migration of human neutrophils: implications for differential sets of signalling pathways. J Cell Sci. 2003;116:813–22. doi: 10.1242/jcs.00306. [DOI] [PubMed] [Google Scholar]
- 107.Onishi K, Higuchi M, Asakura T, Masuyama N, Gotoh Y. The PI3K-Akt pathway promotes microtubule stabilization in migrating fibroblasts. Genes Cells. 2007;12:535–46. doi: 10.1111/j.1365-2443.2007.01071.x. [DOI] [PubMed] [Google Scholar]
- 108.Lokuta MA, Senetar MA, Bennin DA, Nuzzi PA, Chan KT, Ott VL, Huttenlocher A. Type I gamma PIP kinase is a novel uropod component that regulates rear retraction during neutrophil chemotaxis. Mol Biol Cell. 2007;18:5069–5080. doi: 10.1091/mbc.E07-05-0428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Drayer AL, Van der Kaay J, Mayr GW, Van Haastert PJ. Role of phospholipase C in Dictyostelium: formation of inositol 1,4,5-trisphosphate and normal development in cells lacking phospholipase C activity. EMBO J. 1994;13:1601–9. doi: 10.1002/j.1460-2075.1994.tb06423.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Keizer-Gunnink I, Kortholt A, Van Haastert PJ. Chemoattractants and chemorepellents act by inducing opposite polarity in phospholipase C and PI3-kinase signaling. J Cell Biol. 2007;177:579–85. doi: 10.1083/jcb.200611046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Iijima M, Huang YE, Luo HR, Vazquez F, Devreotes PN. Novel mechanism of PTEN regulation by its phosphatidylinositol 4,5-bisphosphate binding motif is critical for chemotaxis. J Biol Chem. 2004;279:16606–13. doi: 10.1074/jbc.M312098200. [DOI] [PubMed] [Google Scholar]
- 112.Sasaki AT, Firtel RA. Regulation of chemotaxis by the orchestrated activation of Ras, PI3K, and TOR. Eur J Cell Biol. 2006;85:873–95. doi: 10.1016/j.ejcb.2006.04.007. [DOI] [PubMed] [Google Scholar]


