Abstract
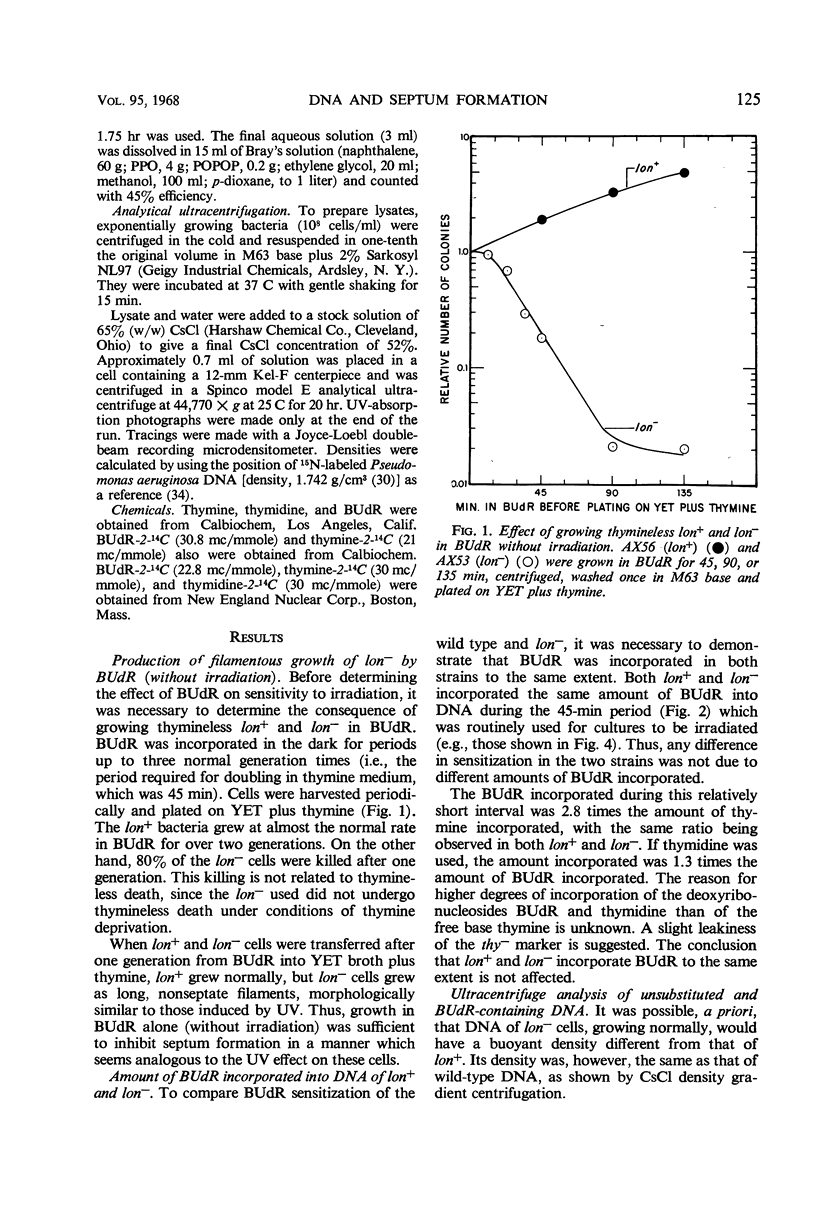
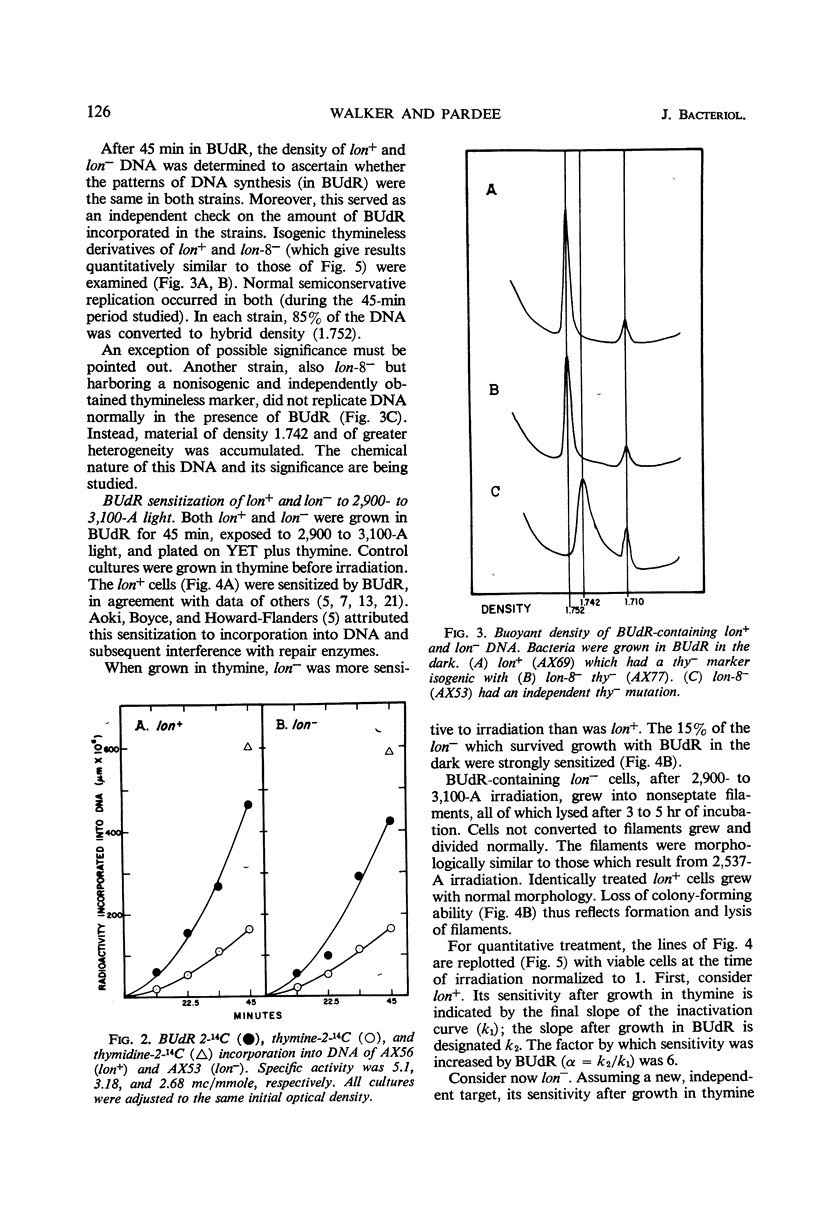
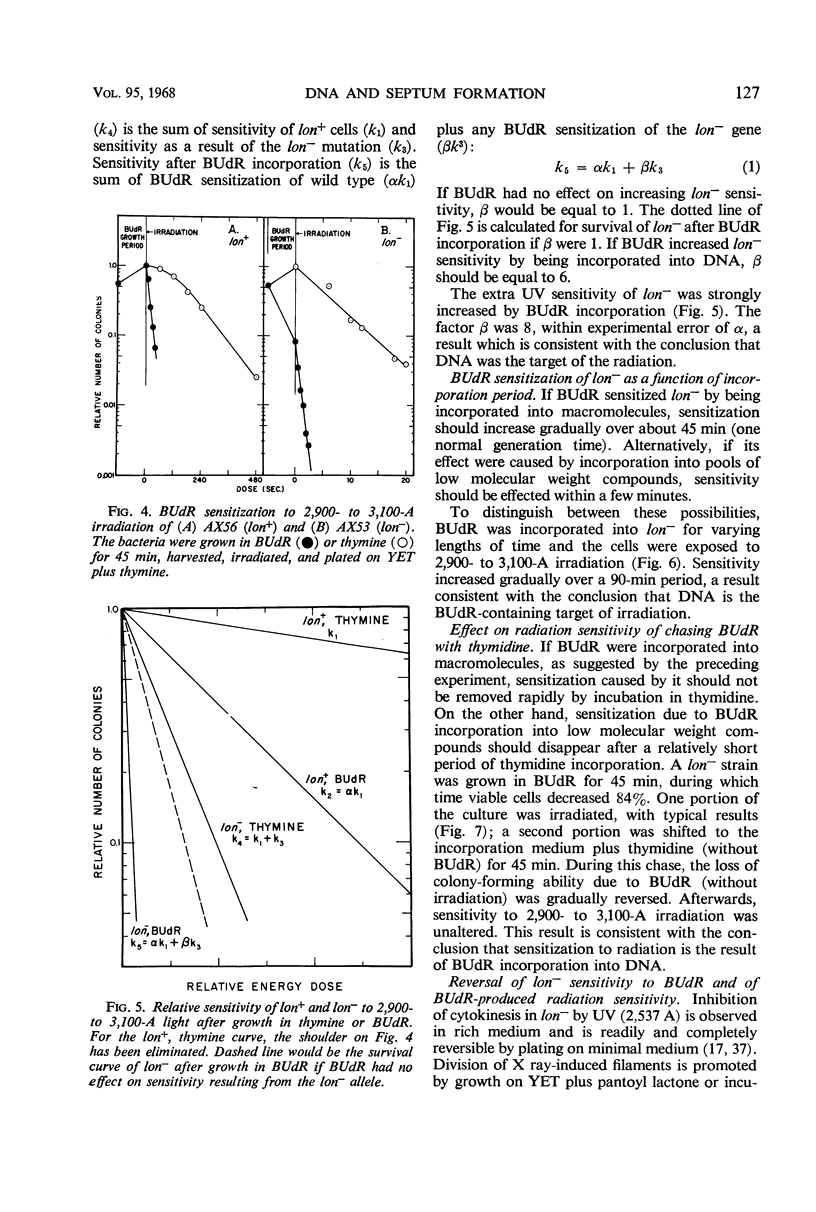
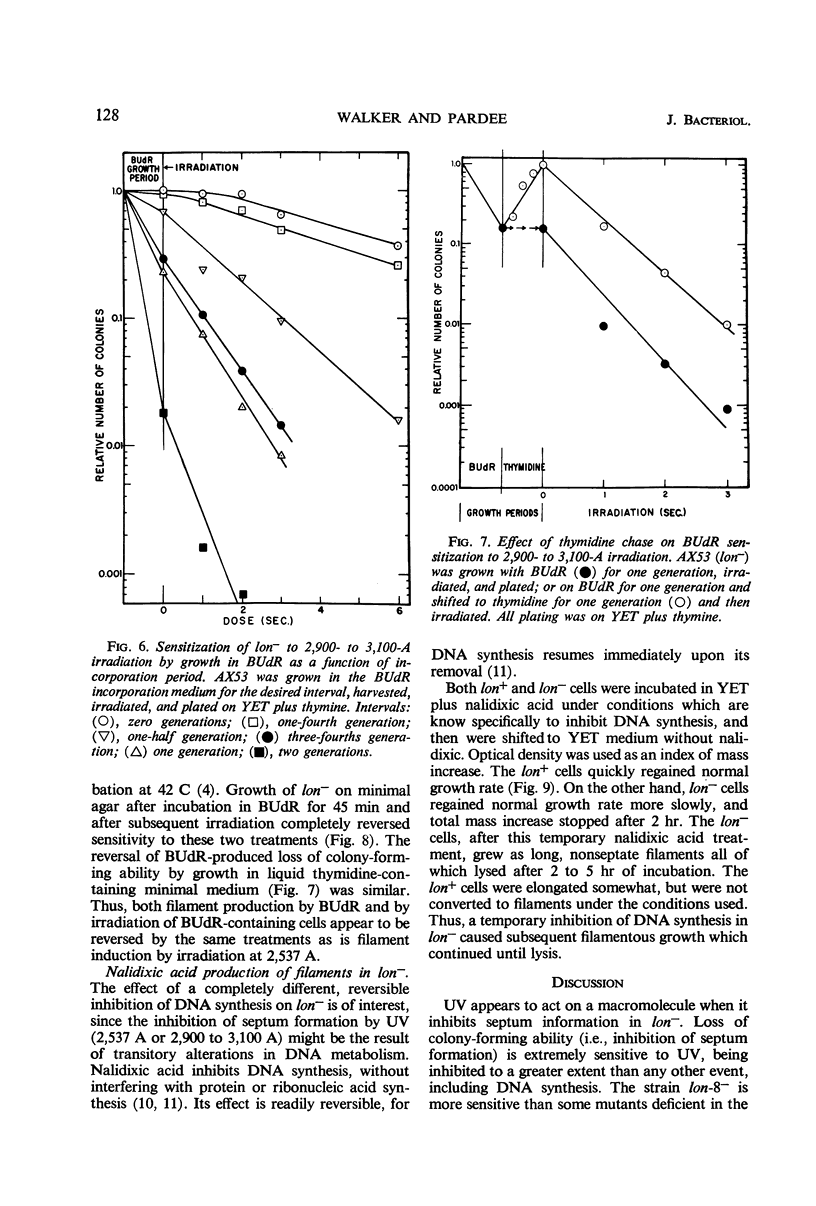
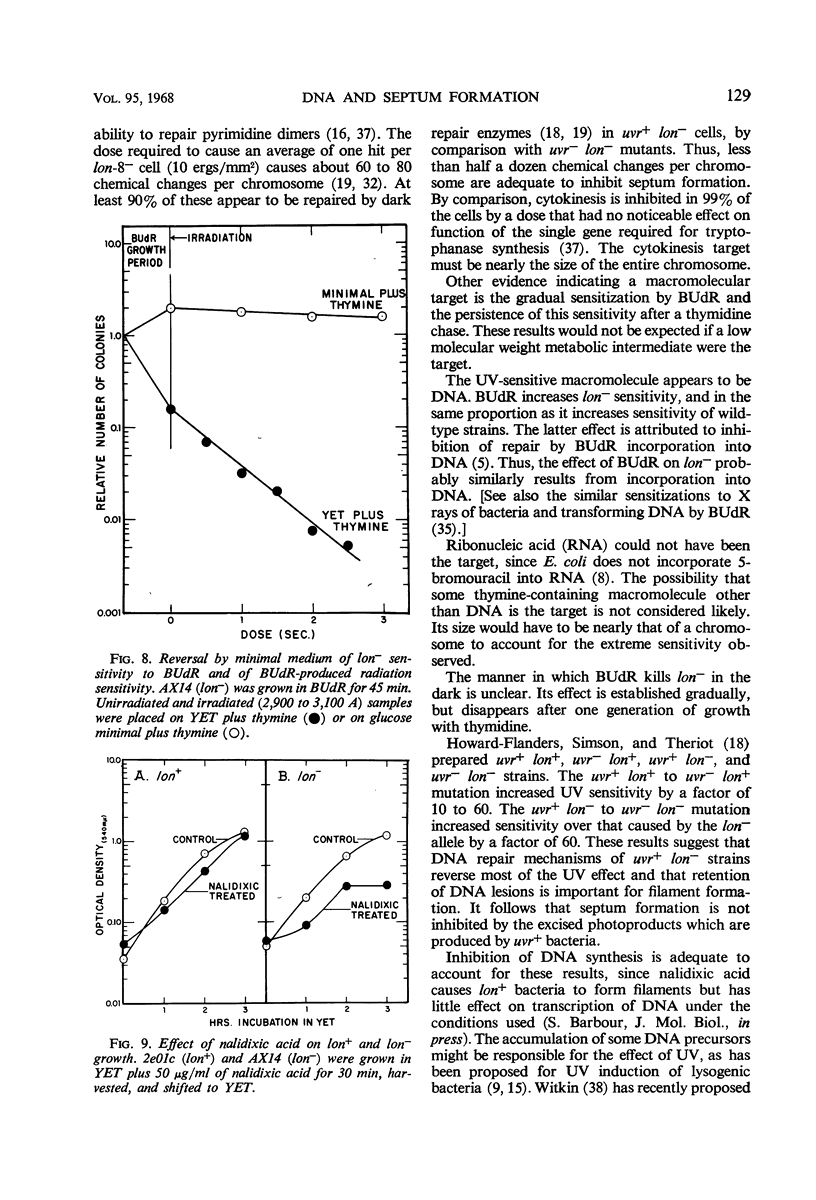
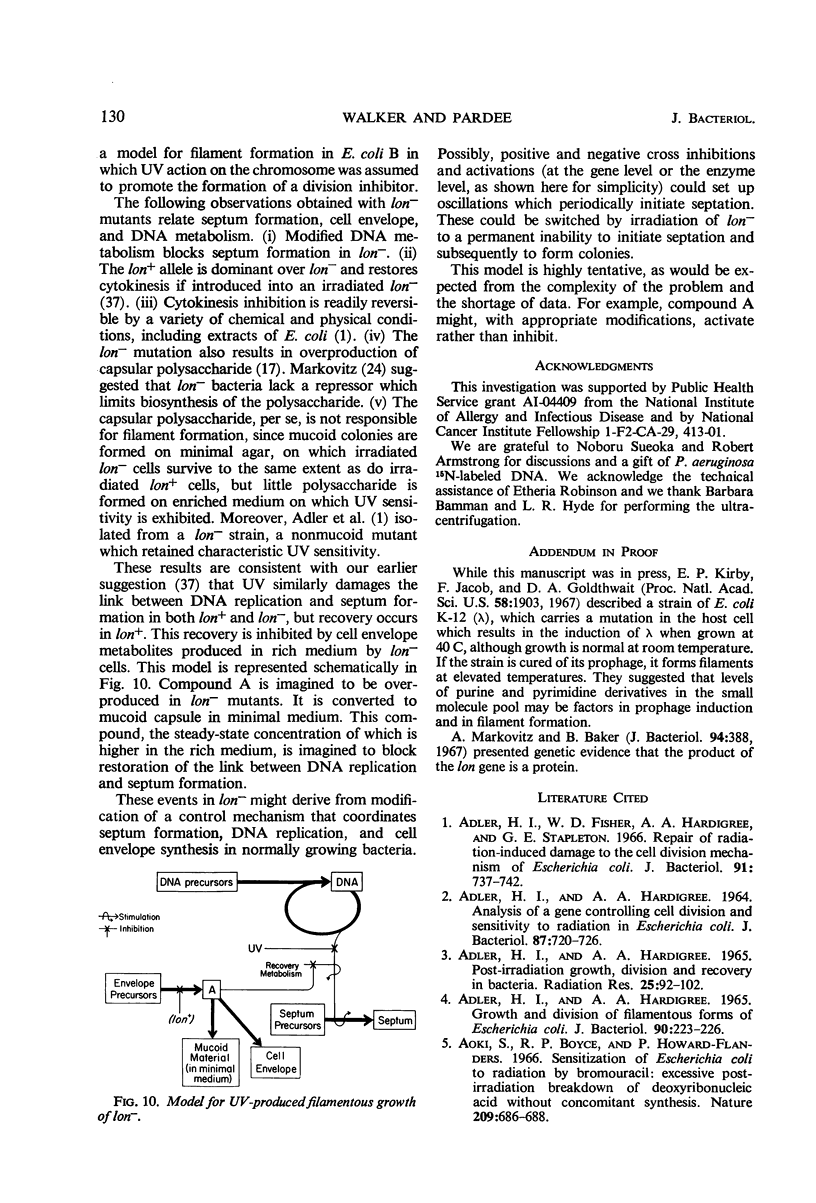
Septum formation is a key step in bacterial division, but the mechanism which controls periodic septum formation is unknown. In an attempt to understand this mechanism, lon− mutants, in which septum formation is blocked by very doses of ultraviolet light (UV), were investigated. UV must act on some part of the apparatus of cytokinesis; thus, identification of the UV target would identify part of this apparatus. As likely possibilities, UV might damage the septum-forming site or it might damage deoxyribonucleic acid (DNA), since DNA replication is normally coordinated with septum formation. To distinguish between these possibilities, DNA was specifically sensitized by incorporating bromodeoxyuridine into lon− bacteria. These bacteria were strongly sensitized to longer wavelength UV (2,900 to 3,100 A) so that they failed to form septa, grew into filaments which lysed, and did not form colonies. Various control experiments supported the conclusion that UV inhibits septum formation as a result of alterations in DNA metabolism. A relationship thus exists between DNA metabolism and septum formation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADLER H. I., HARDIGREE A. A. ANALYSIS OF A GENE CONTROLLING CELL DIVISION AND SENSITIVITY TO RADIATION IN ESCHERICHIA COLI. J Bacteriol. 1964 Mar;87:720–726. doi: 10.1128/jb.87.3.720-726.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ADLER H. I., HARDIGREE A. A. POSTIRRADIATION GROWTH, DIVISION, AND RECOVERY IN BACTERIA. Radiat Res. 1965 May;25:92–102. [PubMed] [Google Scholar]
- Adler H. I., Fisher W. D., Hardigree A. A., Stapleton G. E. Repair of radiation-induced damage to the cell division mechanism of Escherichia coli. J Bacteriol. 1966 Feb;91(2):737–742. doi: 10.1128/jb.91.2.737-742.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler H. I., Hardigree A. A. Growth and Division of Filamentous Forms of Escherichia coli. J Bacteriol. 1965 Jul;90(1):223–226. doi: 10.1128/jb.90.1.223-226.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki S., Boyce R. P., Howard-Flanders P. Sensitization of Escherichia coli to radiation by bromouracil: excessive post-irradiation breakdown of deoxyribonucleic acid without concomitant synthesis. Nature. 1966 Feb 12;209(5024):686–688. doi: 10.1038/209686a0. [DOI] [PubMed] [Google Scholar]
- DUNN D. B., SMITH J. D., ZAMENHOF S., GRIBOFF G. Incorporation of halogenated pyrimidines into the deoxyribonucleic acids of Bacterium coli and its bacteriophages. Nature. 1954 Aug 14;174(4424):305–307. [PubMed] [Google Scholar]
- GOLDTHWAIT D., JACOB F. SUR LE M'ECANISME DE L'INDUCTION DU D'EVELOPPEMENT DU PROPHAGE CHEZ LES BACT'ERIES LYSOG'ENES. C R Hebd Seances Acad Sci. 1964 Jul 20;259:661–664. [PubMed] [Google Scholar]
- GOSS W. A., DEITZ W. H., COOK T. M. MECHANISM OF ACTION OF NALIDIXIC ACID ON ESCHERICHIA COLI. J Bacteriol. 1964 Oct;88:1112–1118. doi: 10.1128/jb.88.4.1112-1118.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOSS W. A., DEITZ W. H., COOK T. M. MECHANISM OF ACTION OF NALIDIXIC ACID ON ESCHERICHIA COLI.II. INHIBITION OF DEOXYRIBONUCLEIC ACID SYNTHESIS. J Bacteriol. 1965 Apr;89:1068–1074. doi: 10.1128/jb.89.4.1068-1074.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREER S. Studies on ultraviolet irradiation of Escherichia coli containing 5-bromouracil in its DNA. J Gen Microbiol. 1960 Jun;22:618–634. doi: 10.1099/00221287-22-3-618. [DOI] [PubMed] [Google Scholar]
- Greenberg J. Radiation sensitivity in Escherichia coli: some properties of the radiation-sensitive Hfr K12 mutant, PAM 401. Mutat Res. 1965 Aug;2(4):304–311. doi: 10.1016/0027-5107(65)90064-3. [DOI] [PubMed] [Google Scholar]
- HOWARD-FLANDERS P., SIMSON E., THERIOT L. A LOCUS THAT CONTROLS FILAMENT FORMATION AND SENSITIVITY TO RADIATION IN ESCHERICHIA COLI K-12. Genetics. 1964 Feb;49:237–246. doi: 10.1093/genetics/49.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOWARD-FLANDERS P., SIMSON E., THERIOT L. THE EXCISION OF THYMINE DIMERS FROM DNA, FILAMENT FORMATION AND SENSITIVITY TO ULTRAVIOLET LIGHT IN ESCHERICHIA COLI K-12. Mutat Res. 1964 Oct;106:219–226. doi: 10.1016/0027-5107(64)90002-8. [DOI] [PubMed] [Google Scholar]
- Hertman I., Luria S. E. Transduction studies on the role of a rec+ gene in the ultraviolet induction of prophage lambda. J Mol Biol. 1967 Jan 28;23(2):117–133. doi: 10.1016/s0022-2836(67)80021-4. [DOI] [PubMed] [Google Scholar]
- Howard-Flanders P., Boyce R. P., Theriot L. Three loci in Escherichia coli K-12 that control the excision of pyrimidine dimers and certain other mutagen products from DNA. Genetics. 1966 Jun;53(6):1119–1136. doi: 10.1093/genetics/53.6.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard-Flanders P., Theriot L. Mutants of Escherichia coli K-12 defective in DNA repair and in genetic recombination. Genetics. 1966 Jun;53(6):1137–1150. doi: 10.1093/genetics/53.6.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAPLAN H. S., SMITH K. C., TOMLIN P. A. Effect of halogenated pyrimidines on radiosensitivity of E. coli. Radiat Res. 1962 Jan;16:98–113. [PubMed] [Google Scholar]
- KUEMPEL P. L., PARDEE A. B. THE CYCLE OF BACTERIAL DUPLICATION. J Cell Physiol. 1963 Oct;62:SUPPL1–SUPPL1:30. doi: 10.1002/jcp.1030620404. [DOI] [PubMed] [Google Scholar]
- Kirby E. P., Jacob F., Goldthwait D. A. Prophage induction and filament formation in a mutant strain of Escherichia coli. Proc Natl Acad Sci U S A. 1967 Nov;58(5):1903–1910. doi: 10.1073/pnas.58.5.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lark K. G. Regulation of chromosome replication and segregation in bacteria. Bacteriol Rev. 1966 Mar;30(1):3–32. doi: 10.1128/br.30.1.3-32.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARKOVITZ A. REGULATORY MECHANISMS FOR SYNTHESIS OF CAPSULAR POLYSACCHARIDE IN MUCOID MUTANTS OF ESCHERICHIA COLI K12. Proc Natl Acad Sci U S A. 1964 Feb;51:239–246. doi: 10.1073/pnas.51.2.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markovitz A., Baker B. Suppression of radiation sensitivity and capsular polysaccharide synthesis in Escherichia coli K-12 by ochre suppressors. J Bacteriol. 1967 Aug;94(2):388–395. doi: 10.1128/jb.94.2.388-395.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markovitz A., Rosenbaum N. A regulator gene that is dominant on an episome and recessive on a chromosome. Proc Natl Acad Sci U S A. 1965 Oct;54(4):1084–1091. doi: 10.1073/pnas.54.4.1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROODYN D. B., MANDEL H. G. A simple membrane fractionation method for determining the distribution of radioactivity in chemical fractions of Bacillus cereus. Biochim Biophys Acta. 1960 Jun 17;41:80–88. doi: 10.1016/0006-3002(60)90371-1. [DOI] [PubMed] [Google Scholar]
- RORSCH A., EDELMAN A., COHEN J. A. The gene-controlled radiation sensitivity in Escherichia coli. Biochim Biophys Acta. 1963 Feb 26;68:263–270. doi: 10.1016/0006-3002(63)90141-0. [DOI] [PubMed] [Google Scholar]
- RORSCH A., EDELMAN A., van der KAMP, COHEN J. A. Phenotypic and genotypic characterization of radiation sensitivity in Escherichia coli B. Biochim Biophys Acta. 1962 Aug 20;61:278–289. doi: 10.1016/0926-6550(62)90090-7. [DOI] [PubMed] [Google Scholar]
- SCHILDKRAUT C. L., MARMUR J., DOTY P. Determination of the base composition of deoxyribonucleic acid from its buoyant density in CsCl. J Mol Biol. 1962 Jun;4:430–443. doi: 10.1016/s0022-2836(62)80100-4. [DOI] [PubMed] [Google Scholar]
- STACEY K. A., SIMSON E. IMPROVED METHOD FOR THE ISOLATION OF THYMINE-REQUIRING MUTANTS OF ESCHERICHIA COLI. J Bacteriol. 1965 Aug;90:554–555. doi: 10.1128/jb.90.2.554-555.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz M. Location of the maltose A and B loci on the genetic map of Escherichia coli. J Bacteriol. 1966 Oct;92(4):1083–1089. doi: 10.1128/jb.92.4.1083-1089.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow R. B. Cyclobutane-type pyrimidine dimers in polynucleotides. Science. 1966 Jul 22;153(3734):379–386. doi: 10.1126/science.153.3734.379. [DOI] [PubMed] [Google Scholar]
- Walker J. R., Pardee A. B. Conditional mutations involving septum formation in Escherichia coli. J Bacteriol. 1967 Jan;93(1):107–114. doi: 10.1128/jb.93.1.107-114.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkin E. M. The radiation sensitivity of Escherichia coli B: a hypothesis relating filament formation and prophage induction. Proc Natl Acad Sci U S A. 1967 May;57(5):1275–1279. doi: 10.1073/pnas.57.5.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]



