Abstract
A new decoupler for on-column electrochemical detection in capillary electrophoresis is presented. The decoupler is constructed by etching a series of holes through the side of the separation capillary with a CO2 laser and then coating the holes with cellulose acetate. The decoupler shows isolation of the detection circuit for separation currents up to 30 μA. Detection limits below 1 nM were achieved for four model compounds, including anions, neutrals, and cations, using the laser-etched decoupler. This decoupler design combines excellent mechanical stability, effective shunting of high separation currents, and ease of manufacture.
Capillary electrophoresis (CE) is a powerful separation technique with excellent mass detection limits owing to the small volume of sample typically injected. Unfortunately, with common detectors, such as UV absorbance, the limited sample volume and detector path length result in compromised concentration detection limits. This has fueled the implementation of highly sensitive detection schemes such as laser-induced fluorescence (LIF) and electrochemical detection (EC). LIF is a highly effective detection scheme resulting in the lowest concentration detection limits;1,2 however, the implementation of LIF can be quite difficult. On the other hand, EC is relatively simple, inexpensive, applicable to a wide range of analytes, and easily miniaturized. Subnanomolar detection limits have been reported with CE-EC, rivaling those achieved by CE-LIF3.
There are two primary strategies for coupling EC to CE, end-column detection and on-column detection. In end-column detection, the electrode is positioned outside the capillary. Reproducible alignment of the electrode with the capillary effluent can be difficult, requiring careful manipulation of the electrode with an x–y–z positioner. When end-column detection is used, the effect of the separation field on the working electrode is minimized by use of extremely low separation currents. To maintain low separation currents, the analysis is limited to small-inner diameter capillaries, low concentration buffers or nonaqueous buffers, and low separation voltages.
To prevent the loss of analyte in the detection cell, on-column detection can be employed. This also improves the ease of electrode positioning since only the depth of electrode insertion within the capillary needs to be controlled. In this arrangement, the separation potential must be grounded prior to the capillary outlet. Several methods for accomplishing this have been described. Initially, Wallingford and Ewing4 reported a porous glass decoupler. In this design, the capillary is fractured ~5 cm from the detection cell, and a porous glass sleeve is glued over the fracture. This section of the capillary is then placed into a grounded reservoir. Ions can migrate through the fracture completing the electrical circuit, while the EOF pushes the bulk fluid and analytes past the fracture to the detection cell. Other decoupler designs have included bare fractures5 and covering the fracture with a porous graphite tube6 or a polymer tubing7 or casting a polymer (cellulose acetate, Nafion) over the fracture.8–10 Furthermore, direct electrical contact has been made with the capillary flow by employing palladium decouplers.11 Additionally, decouplers have been employed using HF etched capillaries, in which a section of the capillary wall is etched until it becomes porous or until a hole is etched through the capillary that is subsequently filled with epoxy.12 This design has been applied to on-column13 and end-column14 decouplers.
While the goal of the decoupler is to ground the separation voltage prior to the detection cell, it has been observed that the noise at the detector is proportional to the separation current.3 Most decouplers cannot shunt all of the current. The most effective designs maximize the surface area of the decoupler. Since the decoupler introduces an area of possible band broadening, larger decouplers can reduce separation efficiency. In the most effective decouplers reported, polymers have been cast into tubes and used as either on-column or end-column decouplers.3,15 While the cast tube decoupler can be as long as 2 mm, the casting process ensures that the tube maintains the same inner diameter as the separation capillary, therefore, not introducing dead volume. Unfortunately, decouplers in general, and especially the cast tubing design, are difficult to manufacture and are fragile. Specifically, only Nafion has been found to have adequate mechanical stability to use in cast tubing designs. However, Nafion is anionic at most pH values and acts as a cation exchange membrane. Although methods have been employed to reduce the loss of cations in the Nafion decoupler3, it cannot be completely suppressed.
While CE-EC is a highly sensitive technique requiring only very small sample volumes, its use has been limited by the difficulty in its implementation. Our goal was to develop a mechanically stable decoupler that will support a high surface area cellulose acetate membrane. This decoupler should be easy to implement and be applicable to the routine analysis of biological compounds, where the sample often dictates the use of separation conditions resulting in high separation currents.
EXPERIMENTAL SECTION
Materials
Dopamine, hydroquinone, gentisic acid, and 3,4-dihyroxybenzoic acid were obtained from Sigma (St. Louis, MO). Cellulose acetate was obtained from Aldrich (Milwaukee, WI). All other chemicals were reagent grade or better and used as received. All stock solutions were prepared at 10 mM in 0.1 M perchloric acid and diluted to the desired concentration in background electrolyte (BGE) just prior to analysis. Cellulose acetate solutions were prepared as 6 wt % in acetone. BGE was prepared from the free acid by adjusting the pH with solid lithium hydroxide.
CEEC Apparatus
Electrophoresis was driven by a high-voltage dc (0–30 kV) power supply (Spellman High Voltage, Hauppauge, NY). The anodic high-voltage end was isolated in a Plexiglas box fitted with an interlock for operator safety. Uncoated fused-silica capillaries (Polymicro Technologies, Phoenix, AZ) 70 cm in length and 75-μm i.d. were used as separation capillaries. The electrochemical cell also served as the cathodic buffer reservoir. Electrodes were prepared as described previously,3 with the exception that 25-μm platinum wire (Medwire, Mount Vernon, NY) was substituted for carbon fiber. Fresh platinum wire electrodes were placed in the detection cell and cleaned by flushing with 0.1 N HCl for 10 min before use. A platinum auxiliary electrode and a Ag/AgCl reference electrode were used. All potentials are reported versus the Ag/AgCl reference. The detector potentiostat was an LC-4CE from Bioanalytical Systems, Inc. (West Lafayette, IN). Data acquisition was through an PCI-MIO-16XE-50 A/D computer card, and programming was performed in-house using LabView software (National Instruments, Austin, TX).
Preparation of Electrical Decoupler
A common trophy engraving laser (35-W CO2 laser, M25 class, Universal Laser, Denver, CO) set at 8% power and 3.1% speed was used to etch the capillaries for decouplers. First, a 70-cm capillary with a 75-μm i.d. and 365-μm o.d. was taped to a glass plate and placed inside the laser housing. The laser was focused for the surface of the glass plate, and controls for the laser pattern were downloaded to the laser from Corel Draw (Corel Corp,, Ontario, Canada). The laser pattern was repeated 5 times in order to open holes through the capillary to the internal channel. The laser has a resolution of 25 μm and a minimum surface line width of 75 μm; however, the laser cuts were beveled such that the walls of the cuts converge at the bottom. There was no apparent error in the reproducible alignment of subsequent laser passes. After five passes with the laser, each line opened a circular hole through one side of the capillary measuring ~25 μm in diameter with 195 μm between holes. The laser-etched decoupler started 1.5 cm from the capillary outlet and extended a distance of 2 and 5 mm for the 10- and 24-hole decouplers, respectively. Once the laser-etched holes were constructed, the capillary was threaded through a septum and placed into a Plexiglas holder for ease of manipulation. Although the capillary does not need additional support, it is very fragile at this stage and care should be taken not to bump the detection end of the capillary. A 50-μm tungsten wire was threaded into the outlet of the capillary 5 cm past the decoupler. A drop of cellulose acetate was dragged across the surface of the decoupler to deposit cellulose acetate into the holes. This was repeated 4 times until the cellulose acetate could be seen to fill the laser-etched lines. Excess cellulose acetate was removed from the capillary, and the decoupler was placed under a 100-W light bulb for 5 min to increase the viscosity of the cellulose acetate solution before excessive amounts flowed into the separation capillary. The capillary was then heated in an oven at 90 °C for 1 h to completely cure the cellulose acetate, after which the tungsten wire was removed. Once completed, the capillary was flushed with 0.1 N NaOH and the decoupler was allowed to soak in 0.1 N NaOH for 1 h. The decoupler was left to soak in BGE continually until use. Occasionally the cellulose acetate would not adhere properly to the capillary. Removal of the cellulose acetate was achieved by soaking the decoupler in acetone. Reapplication of the polymer layer with a fresh cellulose acetate solution solved the problem. The lifetime of the decoupler is in excess of four months, and no difference in the performance of different decouplers of the same size was observed.
Separation and Detection Conditions
Samples containing dopamine and hydroquinone or gentisic acid and 3,4-dihydroxy-benzoic acid (DHBA) were used to evaluate the detector arrangement. Sample introduction was performed hydrodynamically for 0.5 s at 4 psi. The BGE consisted of 10 mM lithium phosphate at pH 6.1. Noise measurements are reported as the average of the maximum baseline noise from three replicate injections of hydroquinone with detection performed at 500 mV. The signal was filtered through a four-pole Bessel filter set at 0.5 Hz. All other conditions are reported in the text.
RESULTS AND DISCUSSION
Design Considerations
The goal of the laser-etched decoupler was to use the capillary as a support for the polymer film. This would allow the creation of a high surface area decoupler and still lend enough mechanical support for use of brittle polymers such as cellulose acetate. The initial design used the laser to etch along the length of the capillary. Unfortunately, problems arose due to the performance of the laser. The first problem was the difficult alignment of the laser cuts with the capillary. If the alignment was off, even by a small amount, the laser cuts would not pierce the capillary wall in contact with the flow channel. Instead, a majority of the cut would remove the capillary wall on the side of the flow channel, making the capillary more fragile without the benefit of adding surface area to the decoupler. The second problem was that, rather than one continuous cut, the laser cuts in a series of pulses. In practice, heat transfer along the laser cut created inconsistent depth and shape on the laser cuts. This resulted in a limited number of small, irregularly shaped holes being opened to the capillary flow channel. As additional passes with the laser were applied to open an acceptable surface area for the decoupler, the laser pulses traveled across the capillary channel etching divots in the opposite wall of the channel.
The final solution to these issues was to arrange the laser cut perpendicular to the capillary length as illustrated in Figure 1. This resulted in a series of short and highly reproducible cuts. Each cut resulted in one hole at the base of the cut that opened to the capillary lumen without etching the opposite wall of the capillary. Additionally, if the alignment was slightly off, there was no effect on the performance of the decoupler.
Figure 1.
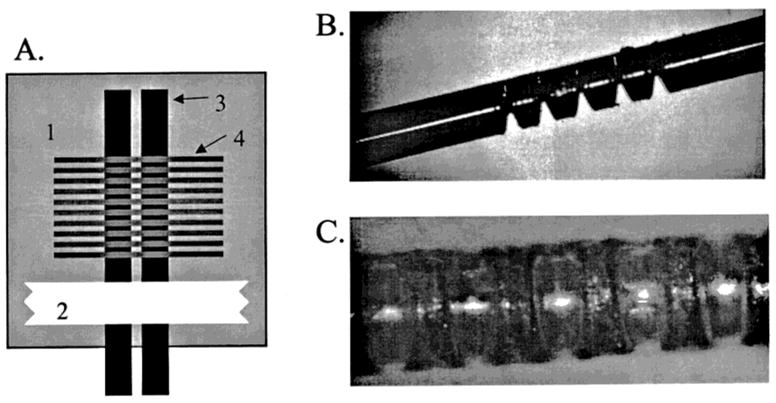
Schematic of the laser-etched decoupler. (A) Layout of the capillary to be etched: (1) glass plate, (2) tape, (3) capillary, and (4) pattern used to control the laser. (B) Laser-etched capillary. (C) Finished decoupler.
Evaluation of the Isolation of the Separation Circuit
As previously reported, the noise at the detector is a function of the separation current.3 The laser-etched decoupler was evaluated to determine its ability to effectively isolate the detection circuit from the separation circuit. The noise at the working electrode was evaluated for two different decouplers as a function of separation current. The first decoupler consisted of 10 laser-etched holes, providing a surface area of 4900 μm2. The noise at the detector showed a dramatic increase with the application of any electrophoretic current, indicating too high of an electrical resistance through the decoupler. By increasing the surface area to almost 11 800 μm2 with a 24-hole decoupler, there was no increase in the detector noise above 1 pA until the electrophoretic current reached 30 μA. When a separation current of 30 μA was applied, the noise at the detector was 1.4 pA, indicating that for low noise applications this decoupler is limited to separations resulting in 30 μA of current or less if the surface area of the decoupler is not increased.
Since decouplers do not shunt all of the separation current, some small proportion of the current still affects the detector. The noise profile versus current is flat until the limiting separation current (LSC, the electrophoretic current at which the detector noise begins to increase rapidly) is reached. This is the point at which the current leakage through the detection capillary has reached a sufficient magnitude that the noise due to the separation current is no longer masked by the environmental noise of the system. The current densities at the LSC with both the 24-hole laser etched decoupler and the fracture type decoupler are ~0.2 A/cm2 (Table 1). The current density at the 10-hole decoupler is half of this value, presumably indicating that the electrical resistance is too high through the decoupler relative to that through the end of the separation capillary. The current densities for the cast Nafion tube decouplers are significantly lower than that for the other decouplers. This is likely due to the different mechanism of ion transport through the two polymers. Cellulose acetate created a porous barrier allowing migration of ions through the polymer. Transport through the Nafion should be quite slow in comparison, allowing fewer cations to cross the polymer per unit surface area.
Table 1.
Comparison of the Current Density at Different Decouplers as Increased Noise Is Apparent at the Working Electrode
| decoupler type | limiting separation current (μA) | noise (pA) | current density (A/cm2) |
|---|---|---|---|
| 24 hole, laser etched | 30 | 1.4 | 0.255 |
| 10 hole, laser etched | 5 | 10 | 0.102 |
| Fracture | 5 | 1 | 0.212 |
| 300 μm, cast Nafion tube | 20 | 0.5 | 0.0212 |
| 500 μm, cast Nafion tube | 25 | 0.5 | 0.0283 |
| 1000 μm, cast Nafion tube | 30 | 0.5 | 0.0127 |
Figure 2 shows the dependency of noise and peak height on the insertion depth of the electrode inside the capillary. The noise at the electrode showed no increase regardless of the electrode insertion depth. Simultaneously, the behavior of the detector response followed expectations. As the electrode was inserted further into the capillary, a larger proportion of the working electrode surface was maintained within the capillary where analyte was not lost to diffusion in the detection cell, resulting in a larger response at the working electrode.
Figure 2.
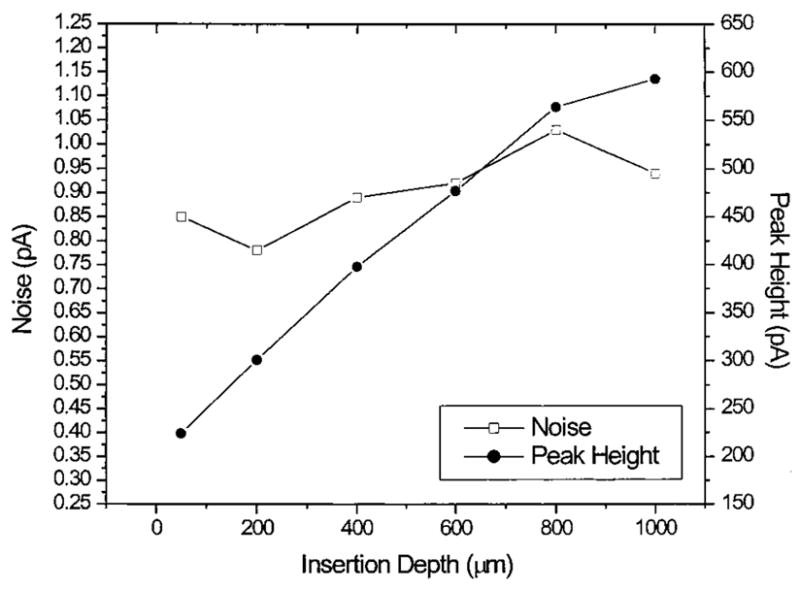
Evaluation of the detector noise and response as a function of the electrode insertion depth. For injections of 500 nM hydroquinone; working electrode potential, 500 mV; separation voltage, 20 kV; separation current, 18 μA.
Evaluation of the Precision and Limits of Detection of the Detector Arrangement
The precision of the detector was evaluated by the peak height of six replicate injections of 10 μM hydroquinone. The RSD of these injections was determined to be 2.4%. Additionally the linearity of the response was evaluated from 1 nM to 10 μM, with an r value of 0.9999, m = 0.945 pA/nM, and b = 0.020 pA. This indicates an analytical system with a high degree of reproducibility and an excellent linear range extending at least 4 orders of magnitude.
The limits of detection were determined by preparing 1 nM solutions of dopamine and hydroquinone or gentisic acid and DHBA immediately prior to analysis. Samples were injected in triplicate, and limits of detection were determined by extrapolating to a S/N value of 3. Excellent LOD values of 0.2 nM for dopamine and hydroquinone, 0.4 nM for gentisic acid, and 0.6 nM for DHBA were obtained. These compare well with previously reported LOD values of 0.5 nM for hydroquinone and 1.4 nM for gentisic acid.3
The results for dopamine represent a 1 order of magnitude better performance as compared to previous reported values of 3.1 nM.3 Previous LOD values were obtained using a cast Nafion tube. Loss of cationic analytes with increasing surface area of the Nafion decoupler was found. The better performance of the current decoupler with respect to the detection of dopamine is attributed to the elimination of Nafion in the decoupler.
CONCLUSION
A new decoupler design has been shown to provide significant improvements over previous on-column detection strategies. The use of cellulose acetate rather than Nafion eliminates the loss of cationic species in the decoupler, allowing a 1 order of magnitude decrease in LOD, matching the results obtained for neutral and anionic analytes. The ability of the decoupler to isolate the detector from the separation current matches the best performance of published results and indicates that increasing the surface area of the decoupler will allow the limiting separation current to be extended for applications requiring higher electrophoretic currents. The manufacture of the decoupler is simplified as compared to other effective designs such as the cast Nafion tube or HF-etched capillaries. Additionally, the laser-etched decoupler is highly mechanically stable and extremely easy to manipulate.
Acknowledgments
This work was supported by a grant from the National Institutes of Health (GM-49900).
References
- 1.Gozel P, Gassman NE, Michelsen H, Zare RN. Anal Chem. 1987;59:44–49. [Google Scholar]
- 2.Wu S, Dovichi NJ. J Chromatogr. 1989;480:141–155. doi: 10.1016/s0021-9673(01)84284-9. [DOI] [PubMed] [Google Scholar]
- 3.Park S, Lunte SM, Lunte CE. Anal Chem. 1995;67:911–918. [Google Scholar]
- 4.Wallingford RA, Ewing AG. Anal Chem. 1987;59:1762–1766. doi: 10.1021/ac00141a005. [DOI] [PubMed] [Google Scholar]
- 5.Zhou J, Lunte SM. Electrophoresis. 1995;16:498–503. doi: 10.1002/elps.1150160182. [DOI] [PubMed] [Google Scholar]
- 6.Yik YF, Lee HK, Li SFY, Khoo SB. J Chromatogr. 1991;585:139–144. [Google Scholar]
- 7.O’Shea TJ, Greenhagen RD, Lunte SM, Lunte CE, Smyth MR, Radzik DM, Watanabe N. J Chromatogr. 1992;593:305–312. [Google Scholar]
- 8.Whang CW, Chen IC. Anal Chem. 1992;64:2461–2464. doi: 10.1021/ac00044a029. [DOI] [PubMed] [Google Scholar]
- 9.Chen IC, Whang CW. J Chromatogr. 1993;644:208–212. [Google Scholar]
- 10.O’Shea TJ, Lunte SM, LaCourse WR. Anal Chem. 1993;65:948–951. [Google Scholar]
- 11.Kok WT, Sahin Y. Anal Chem. 1993;65:2497–2501. [Google Scholar]
- 12.Zhang SS, Yuan ZB, Liu HX, Zou H, Wu YJ. J Chromatogr, A. 2000;872:259–268. doi: 10.1016/s0021-9673(99)01260-1. [DOI] [PubMed] [Google Scholar]
- 13.Hu S, Wang ZL, Li PB, Cheng JK. Anal Chem. 1997;69:264–267. [Google Scholar]
- 14.Qian J, Wu Y, Yang H, Michael AC. Anal Chem. 1999;71:4486–4492. doi: 10.1021/ac990338f. [DOI] [PubMed] [Google Scholar]
- 15.Park S, Lunte CE. Anal Chem. 1995;67:4366–4370. doi: 10.1021/ac00119a026. [DOI] [PubMed] [Google Scholar]


