Abstract
Cucurbitacins have been shown to inhibit proliferation in a variety of cancer cell lines. The aim of this study was to determine their biological activity in colon cancer cell lines that do not harbor activated STAT3, the key target of cucurbitacin. In order to establish the role of activated kRas in the responsiveness of cells to cucurbitacins, we performed experiments in isogenic colon cancer cell lines, HCT116 and Hke-3, which differ only by the presence of an activated kRas allele. We compared the activity of 23, 24-dihydrocucurbitacin B (DHCB) and cucurbitacin R (CCR), two cucurbitacins that we recently isolated, with cucurbitacin I (CCI), a cucurbitacin with established antitumorigenic activity. We showed that cucurbitacins induced dramatic changes in the cytoskeleton (collapse of actin and bundling of tubulin microfilaments), inhibited proliferation and finally induced apoptosis of both HCT116 and Hke3 cells. However, the presence of oncogenic k-Ras significantly decreased the sensitivity of cells to the three cucurbitacins tested, CCR, DHCB and CCI. We confirmed that mutational activation of kRas protects cells from cucurbitacin-induced apoptosis using nontransfromed intestinal epithelial cells with inducible expression of k-RasV12. Cucurbitacins induced the expression of p53 and p21 predominantly in HCT116 cells that harbor mutant Ras. Using HCT116 cells with targeted deletion of p53 or p21 we confirmed that p53 and p21 protect cells from apoptosis induced by cucurbitacins. These results demonstrated that sensitivity of human colon cancer cell lines to cucurbitacins depends on the kRas and p53/p21 status, and established that cucurbitacins can exert antitumorigenic activity in the absence of activated STAT3.
1. Introduction
Cucurbitacins are tetracyclic triterpens isolated from plant families such as the Cucurbitaceae and Cruciferae that have been used for centuries as folk medicines [1]. Indeed, cucurbitacins have been shown to have anti-inflammatory, analgesic as well as cytotoxic activity in vitro and in vivo [2, 3]. Cayaponia tayuya (Cucurbitaceae) is a climbing lignified plant with a large tuber that has long been used in the folk medicines of Brazil, Peru, and Colombia as an anti-inflammatory, antitumour and anti-rheumatic agent [4]. We recently isolated two cucurbitacins from the roots of Cayaponia tayuya, identified as 23, 24-dihydrocucurbitacin B (DHCB) and cucurbitacin R (CCR) [3]. We demonstrated the anti-inflammatory, anti-allergic and anti-arthritic activity of DHCB and CCR in vitro and in vivo, due to their ability to inhibit the expression of TNFα in lymphocytes and in macrophages, and to interfere with the activity of the nuclear factor NF-AT [2, 5, 6].
The ability of cucurbitacins to inhibit the activity of COX2 [7] and the production of proinflammatory mediators such as iNOS and TNF [5] underlie their potent anti-inflammatory activity. Although these characteristics are likely to contribute to the ability of cucuritacins to inhibit tumor progression, their anti-tumorigenic activity has been ascribed mainly to their propensity to inhibit JAK/STAT3 signaling, an important oncogenic pathway activated in a number of cancers [8–10]. Several cucurbitacins (such as cucurbitacin I, cucurbitacin Q and cucurbitacin B) were shown to inhibit phosphorylation of STAT3 and to induce apoptosis in v-src transformed NIH3T3 cells, but had limited biological activity in cells with no activated STAT3 [11], which led to the conclusion that cucurbitacins exert anti-tumorigenic activity selectively in cells with activated STAT3.
Although activation of STAT3 has been demonstrated in primary colon tumors, the majority of established colon cancer cell lines lack constitutively activated STAT3 [12], suggesting that factors from the tumor microenvironment maintain constitutive activation of STAT3 in colon cancer. STAT3 transcriptional activity has been shown to be negatively regulated by PI3K and ERK signaling in melanoma cells [13]. Whether the STAT3 and Ras signaling pathways functionally interact in colon cancer cells, as we have shown for the STAT1 and Ras pathways [14], remains to be determined.
kRas mutations are found in 30–50% of primary colorectal cancers as well as in established colon cancer cell lines [15]. Ras genes encode small GTP-binding proteins that affect gene expression by acting as major switches in signal transduction processes, coupling extracellular signals with transcription factors [16–18]. Oncogenic forms of Ras are locked in their active state and transduce signals essential for transformation, angiogenesis, invasion and metastasis via downstream pathways involving the RAF/MEK/ERK cascade of cytoplasmic kinases, the small GTP-binding proteins RAC and RHO, phosphatidylinositol 3-kinase and others [19]. In addition to its role in tumor initiation, tumor maintenance and tumor progression, oncogenic Ras has been shown to modulate the responsiveness of cancer cells to a variety of chemotherapeutic and chemopreventive agents. We have shown that colon cancer cells that harbor mutant Ras are sensitized to apoptosis induced by butyrate [20], HDAC inhibitors [21] and 5-FU [22]. However, several clinical studies that have been conducted to examine the prognostic significance of Ras mutations in colorectal cancer have yielded inconclusive results [23, 24].
The aim of the present study was to test whether DHCB and CCR, two cucurbitacins that we recently isolated from Cayaponia Tayuya, inhibit proliferation and/or induce apoptosis in colon cancer cell lines, despite the fact that these cells do not express activated STAT3. Finally, we determined whether the presence of oncogenic k-Ras, a frequent genetic alteration in colon cancer, alters the responsiveness of cells to cucurbitacins.
2. Materials and Methods
2.1. Cell lines and antibodies
The HCT116 human colon carcinoma cell line harbors an activating mutation in codon 13 of the k-Ras proto-oncogene and is wild type for p53. Isogenic cell clones with a disrupted mutant k-Ras allele [25], p21−/− HCT116 cells [26] and p53−/− HCT116 cells [27] were generated from the parental HCT116 cells by homologous recombination.
Cells were maintained in MEM medium supplemented with 10% FCS and antibiotics and their viability was assessed by the MTT assay (Boeringher, Mannheim). Rat intestinal epithelial cells (IEC-6), transfected with inducible k-Ras [28] were maintained in DMEM containing 400 µg/ml G418 and 150 µg/ml of hygromycin B. Expression of oncogenic Ras in these cells was induced by 5 mM IPTG. Immunoblotting was performed using standard procedures [14]. Antibodies specific for gelsolin were obtained from Sigma, and antibodies recognizing PARP, cleaved PARP, caspase-9, cleaved caspase-9, and cleaved caspase-3, were purchased from Cell Signaling Technology (Beverly, MA, USA). Antibodies recognizing p53, PUMA, and p21 were purchased from Santa Cruz Biotechnologies (Santa Cruz, CA, USA). Dihydrocucurbitacin B and cucurbitacin R were isolated from the roots of Cayaponia tayuya as we described before [3], and cucurbitacin I was purchased from EMD Biosciences (Darmstadt, Germany).
2.2. Transfection and Reporter Assays
To examine the effect of DHCB on the transcriptional activity of p21, we transiently transfected cells with 1 µg of plasmid containing a 2.4 kb genomic fragment of the p21 promoter cloned upstream of the LUC reporter gene [29], using the calcium phosphate transfection kit from Promega Biosciences (St. Louis, MO, USA). Twenty four hours after transfection, cells were treated with DHCB at increasing concentrations for 12 hours. Luciferase activity was normalized to TK-Renilla activity to control for transfection efficiency.
2.3. Clonogenic and proliferation assay
To assess the effect of cucurbitacins on the colony forming ability of cancer cells, we performed clonogenic assay. Cells were seeded at a density of 200 cells per 6 well plate, and were treated with increasing concentrations of CCR or DHCB for 24 hours. Cells were then washed and grown in complete media for seven days. Colonies were washed with PBS, fixed and stained with 6% glutaraldehyde and 0.5% crystal violet for 30 minutes at room temperature. Individual colonies with more than 50 cells were scored with Total Lab 1.1 software (Nonlinear Dynamics, Durham, NC, USA) and the surviving fraction was calculated.
Cell proliferation was assayed using a modified colorimetric Thiazoyl Blue Tetrazolium (MTT) assay. Briefly, 104 cells were plated in 96 well plates and treated with CCR (10–100 µM), DHCB (100 nM-10 µM), and CCI (10 nM-1 µM), and MTT activity was determined 24, 48, and 72 hours after treatment.
2.4. Apoptosis
Cells were treated with cucurbitacins, and both adherent and floating cells were collected and resuspended in hypotonic buffer (0.1% Triton X-100, 0.1% sodium citrate), and stained with propidium iodide (50 µg/ml) for 4h at 4°C. Samples were filtered through a nylon mesh (40 µm pore size) and analyzed by flow cytometry. Cell cycle distribution, and the extent of apoptosis (cells with a subG1 DNA content) were analyzed using Modfit software (Topshman, ME, USA).
2.5. Immunofluorescence
Cells were grown on chamber slides, and were either left untreated or were treated with DHCB as indicated. Cells were fixed in 4% paraformaldehyde for 20 min at 20°C. Incubation with antibodies that recognize activated caspase-3, or tubulin, was performed for 1h at 37°C. Slides were then washed with PBS and incubated with a secondary anti-rabbit antibody, conjugated to FITC, for 45 min at 37°C. Staining with phalloidin for detection of F-actin was performed for 30 minutes at room temperature. Samples were examined with a fluorescent microscope and images were acquired with a SPOT CCD camera.
3. Results
3.1. Cucurbitacins induce profound cytoskeleton changes and induce growth arrest in colon cancer cells
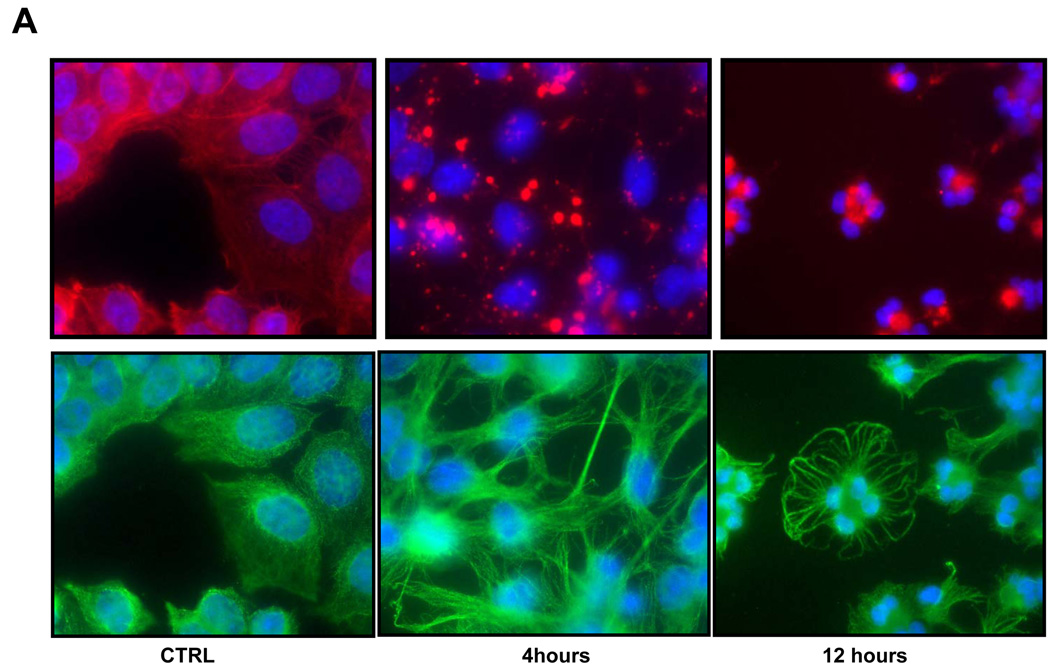
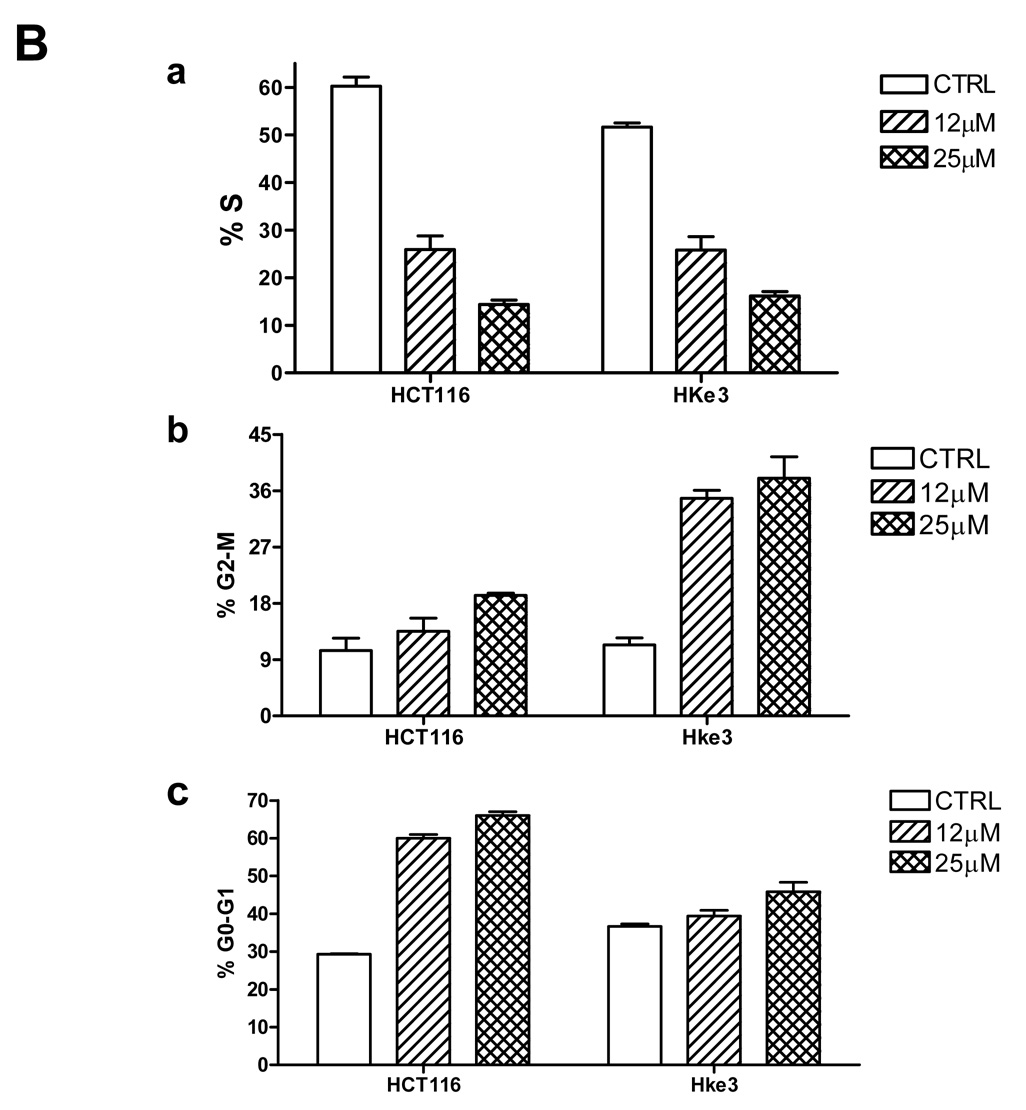
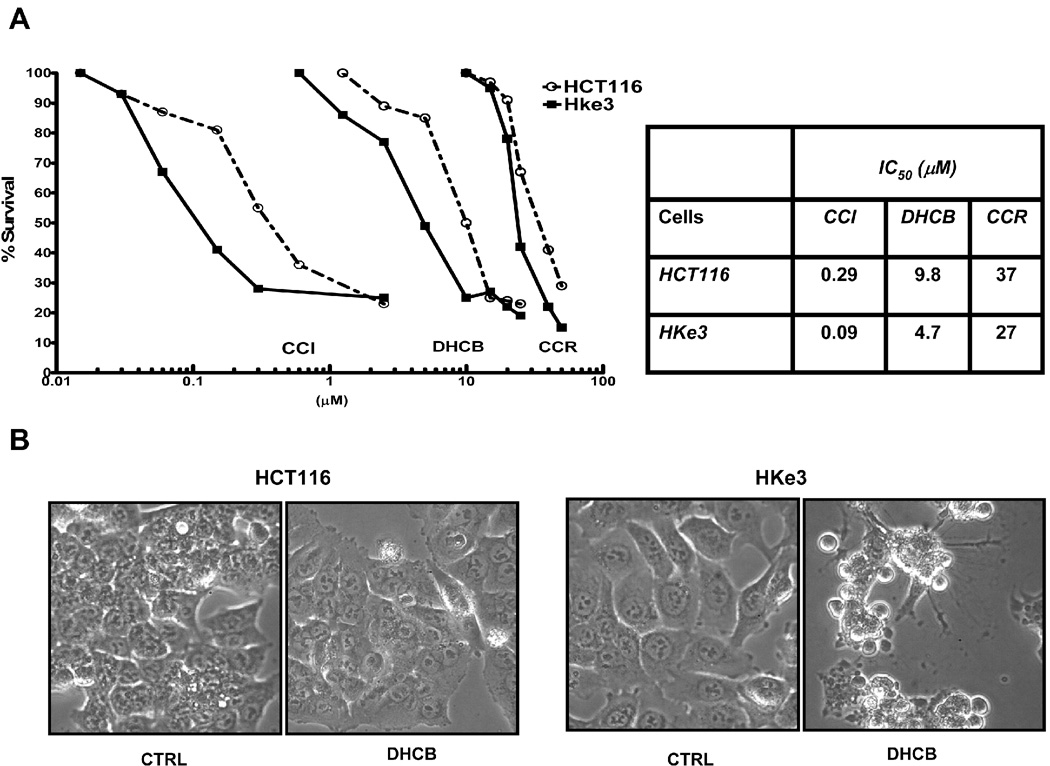
We evaluated the activity of cucurbitacins in HCT116 cells and Hke3 cells, two isogenic colon cancer cell lines that differ only by the presence of a mutant kRas allele [25]. HCT116 cells harbor an activated and a WT kRas allele, while Hke-3 cells have only the wild type kRas allele. We compared the activities of dihydrocucurbitacin B (DHCB) and cucurbitacin R (CCR) to cucurbitacin I (CCI), a cucurbitacin with established antitumorigenic activity (structures shown in Supplemental Fig. 1). Treatment of HCT116 and Hke-3 cells with DHCB, CCR and CCI induced dramatic morphological changes, such as rounding of the cells (not shown, Fig. 1, Fig. 2), pointing to a disruption of the cytoskeleton in treated cells. Indeed, as shown in Fig. 1A, treatment of HKe-3 cells with DHCB triggered a rapid collapse of the actin cytoskeleton. DHCB completely disrupted the organization of actin filaments, and resulted in the formation of patchy, disorganized and clumpy actin structures. In addition, DHCB treatment induced a marked bundling of tubulin filaments, suggesting that cucurbitacins also affect the dynamics of microtubule stabilization (Fig.1A). Similar changes were seen in cells treated with CCR and CCI, but not with taxol, a tubulin binding drug (data not shown). The changes in cytoskeleton were rapid, taking place as early as 30 minutes after treatment, they were progressive, and occurred in both HCT116 and Hke-3 cells. There were no signs of apoptosis in cells early after treatment (data not shown, see below). However, cell cycle analysis revealed strong inhibition of S phase and G0/G1 and G2/M arrest of cells that were treated with DHCB (Fig. 1B) or CCR (not shown) for 12 hours. Although inhibition of S phase was similar in both HCT116 and Hke-3 cells (Fig. 1B), the G2/M arrest appeared to be more pronounced in HKe-3 cells, with G0/G1 arrest predominating in HCT116 cells, suggesting that oncogenic kRas controls the nature of the cell cycle arrest in response to cucurbitacins.
Fig. 1.
A: Effect of DHCB on distribution of F-actin and tubulin. Hke-3 cells were left untreated (CTRL) or were left untreated or were treated with DHCB (10 mM) for 4 or 12 hours. Immunofluorescence was performed with antibodies specific for tubulin (green) or by staining with phalloidin for F-actin (red). B: Inhibition of S phase and induction of G0/G1 and G2/M arrest in cucurbitacin treated cells. HCT116 and Hke-3 cells were left untreated or were treated with 12 µM or 25 µM DHCB for 12 hours and the cell cycle profile was determined by PI staining.
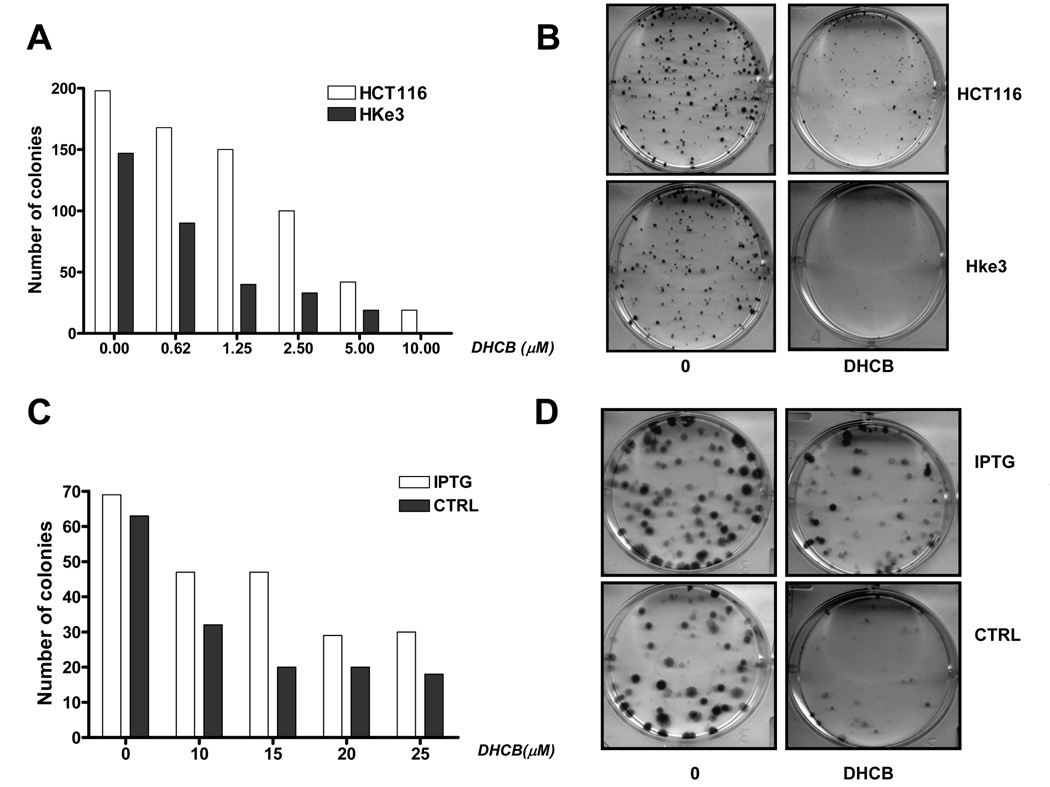
Figure. 2.
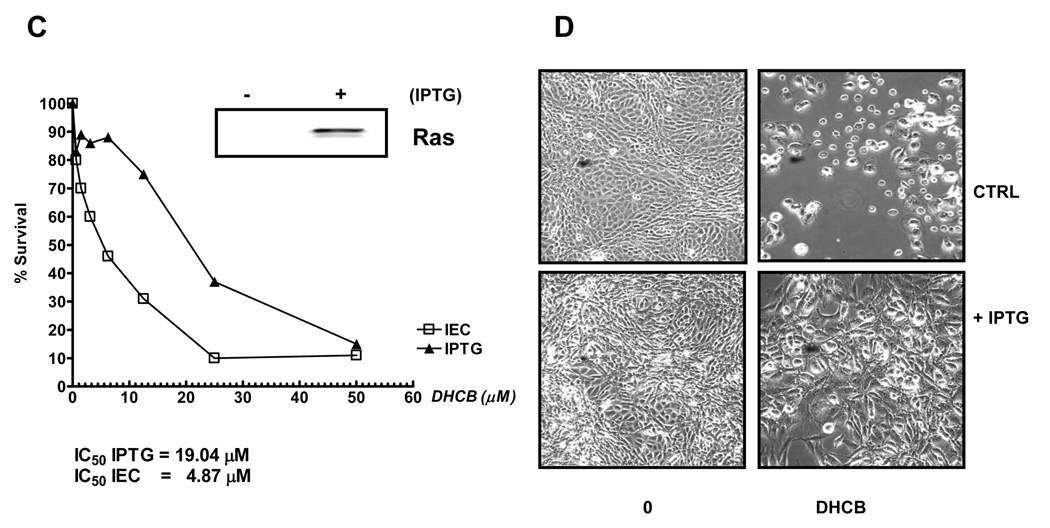
A: Survival of HCT116 cells and Hke3 cells upon treatment with cucurbitacins. Cells were treated with CCR (10–100 µM), DHCB (100 nM-10 µM), and CCI (10 nM-1µM) for 48 hours and cell survival was calculated from data obtained by the MTT assay. IC50 were calculated by linear regression. B: Photographs were taken 24 hours after treatment of cells with DHCB (12 µM). C: Inducible expression of an oncogenic KRas renders cells resistant to DHCB. IEC-iK-Ras cells were induced with 5 mM IPTG for 24 h and the induction of kRasV12 by IPTG was confirmed by Western blotting. Cells were treated with increasing concentrations of DHCB for 48 h in the absence or the presence of IPTG as indicated (C, D).
Cucurbitacins are known to exert antitumorigenic activity through inhibition of the constitutive STAT3 activity [11, 30]. Of note, neither HCT116 nor HKe-3 cells have detectable levels of phosphorylated STAT3, and the levels of total STAT3 are comparable in the two cell lines (Supplemental Figure 2).
3.2. Oncogenic ras modulates sensitivity of cells to cucurbitacins
We have demonstrated that activating mutations of Ras increase sensitivity of colon cancer cells to apoptosis induced by the dietary chemopreventive agent butyrate [20], structurally unrelated inhibitors of HDAC activity [21], and the chemotherapeutic agent 5-fluorouracil [22]. Because we found that the nature of cell cycle arrest in cucurbitacin-treated cells depends on the status of kRas (Fig. 1B) we sought to determine whether oncogenic RasV12 also alters the sensitivity of cells to cucurbitacins. HCT116 and Hke-3 cells were treated with increasing concentrations of CCR, DHCB and CCI (Fig. 2A) and were monitored for their viability by the MTT assay. Cells were most sensitive to CCI (IC50 value of 0.09–0.29 µM), followed by DHCB (IC50 value of 4.7–9.8 µM) and CCR (IC50 value of 27–37 µM). Importantly, this experiment revealed that all cucurbitacins tested were less effective in HCT116 cells (which harbor oncogenic kras) than in Hke-3 cells (Fig. 2A and 2B), suggesting that constitutive signaling by kRas blunts the biological activity of cucurbitacins. Among the cucurbitacins that we isolated, DHCB was more potent than CCR, therefore we performed most of our subsequent experiments with DHCB.
To confirm that oncogenic kRas alters the biological activity of cucurbitacins, we performed experiments in nontransformed rat intestinal epithelial cells with inducible oncogenic kRasV12, IECiKras cells [28], in which the expression of kRasV12 was induced with 5 mM IPTG (Fig. 2C and 2D). Twenty four hours after induction with IPTG we treated cells with increasing concentrations of cucurbitacins. The calculated IC50 for DHCB was 4.89 µM in the absence of IPTG and 19.79 µM in the presence of IPTG (Fig. 2C), confirming that activated kRas decreases the sensitivity of cells to cucurbitacins. The induction of kRasV12 by IPTG in IECiKras cells also protected cells from CCR and CCI (data not shown).
Finally, to corroborate the antitumorigenic activity of DHCB in colon cancer cells, we tested their activity performing clonogenic assays [31]. Briefly, we plated 200 cells in a 6 well plate and treated them with increasing concentrations of DHCB for 24 hours. Cells were then washed and the number of emerging colonies was scored 10 days after treatment. We performed the assay in both HCT116 and Hke-3 cells, and in IEC ikRas cells with inducbile oncogenic kRasV12. As shown in Fig. 3A and 3B, the ability of DHCB to inhibit clonogenicity was more pronounced in Hke-3 cells than in HCT116 cells. Similarly, induction of activated kRas by IPTG in iECiKRas cells increased the capacity of these cells to produce colonies upon cucurbitacin treatment (Fig. 3 C and D).
Fig. 3.
Clonogenic assay in HCT116 and Hke3 cells (A, B) and in IECiKRas cells with IPTG inducible expression of kRasV12 (C, D). Cells were treated with increasing concentrations of DHCB as indicated and the number of colonies was determined after 10 days.
Altogether, these results firmly established that the ability of DHCB to exert anti-tumorigenic activity is perturbed by the presence of oncogenic kRas.
3.3. Oncogenic kras protects cells from cucurbitacin-induced apoptosis
To define the nature of cell death induced by DHCB, we first performed propidium iodide staining and determined the percentage of cells with subG1 content of DNA, a hallmark of apoptotic cells. As shown in Fig. 1B, 12–24 hours after treatment with DHCB, we observed a strong G2/M or G0/G1 arrest in HCT116 and Hke-3 cells. Apoptosis was first detected 48h after treatment with DHCB in Hke-3 cells, but not in HCT116 cells (Fig. 4A). 72 hours after treatment, DHCB induced apoptosis in both cell lines, but the extent of apoptosis was significantly higher in Hke-3 cells, confirming that oncogenic Ras protects cells from cucurbitacin-induced apoptosis (Fig. 4A). Similar results were obtained with CCR (data not shown).
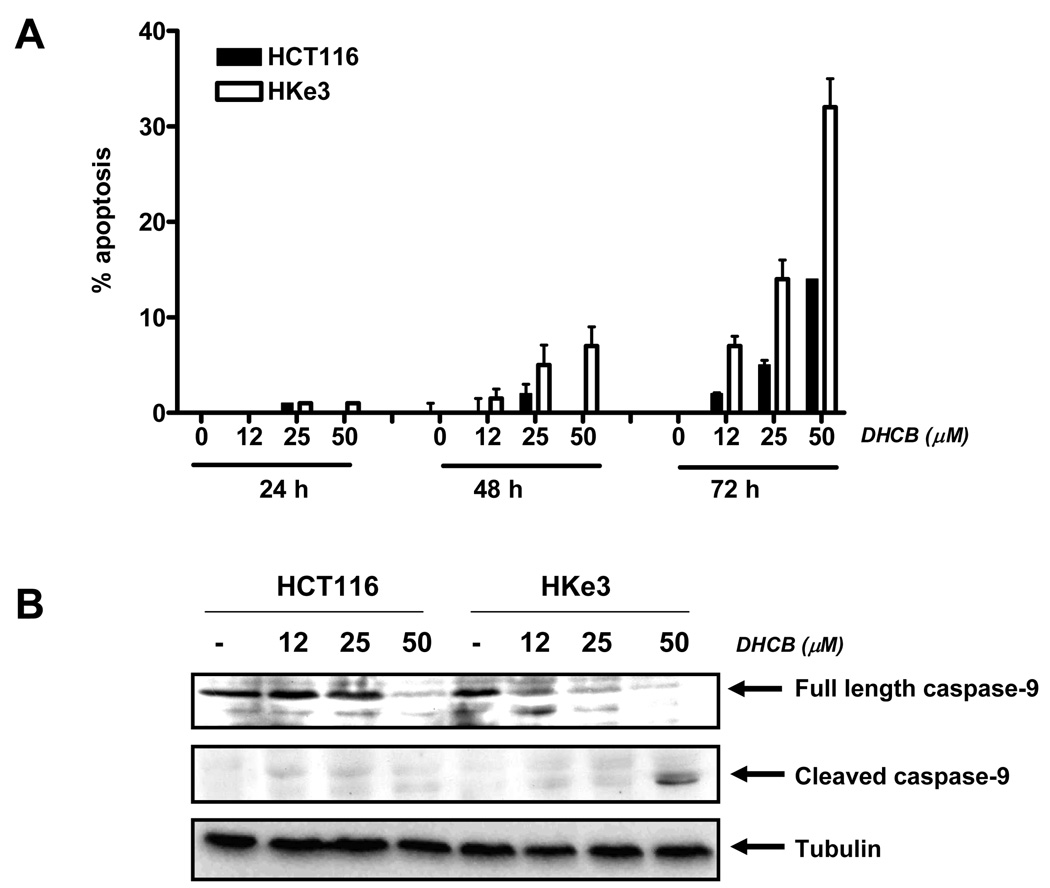
Fig. 4.
A: Signaling by oncogenic kRas interferes with apoptosis induced by DHCB. A:Apoptosis was determined in HCT116 and Hke-3 cells that were treated with increasing concentrations of DHCB for 24, 48 or 72 hours by PI staining. Error bars were derived from 3 experiments. B: The activation of caspase 9 was determined in cell lysates using antibody that recognizes both full length caspase 9 and cleaved caspase 9, as well as with antibody recognizing specifically cleaved caspase-9.
To confirm the apoptotic nature of cell death in DHCB treated cells, we first assessed the activation of caspase-9, the upstream caspase in the apoptotic cascade. We determined the activity of caspase 9 in HCT116 and Hke-3 cells by immunoblotting using antibodies that recognize full length caspase-9 and an antibody that specifically recognizes the cleaved product of caspase-9. We showed that DHCB induced cleavage of caspase 9 in Hke-3 cells at lower concentration than in HCT116 cells (see a rapid disappearance of the full length caspase-9 in HKe-3 cells upon treatment), consistent with enhanced apoptosis in DHCB-treated Hke-3 cells (Fig. 4B). Consistently, the cleaved product of caspase-9 was detected only in HKe-3 cells treated with 50 µM of DHCB (Fig. 4B) and the activation of caspase-3, an effector caspase, was more pronounced in HKe-3 cells (Supplemental Fig. 3). Similar results were obtained in cells treated with CCR (data not shown).
3.4. Induction of p53 and p21 by cucurbitacins is enhanced in cells harboring mutant Ras
Because p53 is a major regulator of growth arrest and apoptosis, we examined whether p53 mediates growth arrest and apoptosis induced by cucurbitacins. Treatment of cells with DHCB resulted in induction of p53 in both HCT116 and Hke-3 cells (Fig. 5A), however the induction of p53 by DHCB was consistently higher in HCT116 cells. Increased p53 induction was also observed in HCT116 cells treated with CCR and CCI (Supplemental Fig. 4).
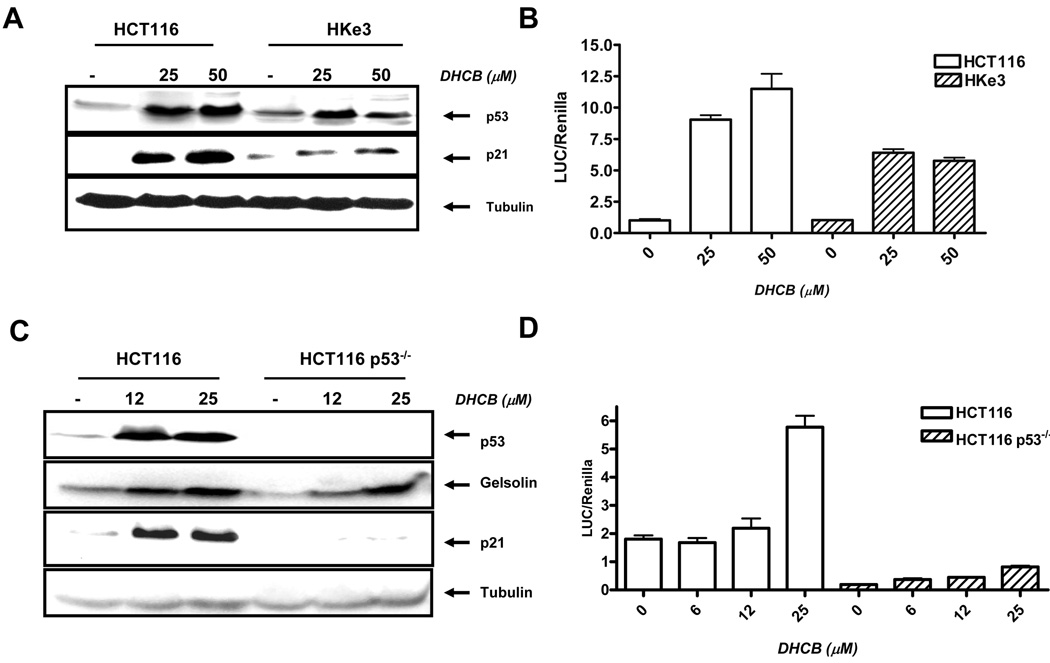
Fig. 5.
Treatment of cells with DHCB induced the expression of p53 and p21. A: Cells were treated with DHCB for 24 hours and the expression of p53 and p21 was determined by immunoblotting. B: p21 transcriptional analysis in HCT116 and Hke3 cells. Cells were transfected with the p21promoter reporter gene and were treated with DHCB as indicated for 18 hours. The luciferase and renilla activities were determined as described in Materials and Methods. C: HCT116 and HCT116 p53 −/− were treated with DHCB for 72 hours and immunoblotting was performed with antibodies specific for p53, gelsolin, p21 and tubulin. D: p21 transcriptional activity in HCT116 and in HCT116 p53−/− cells. Cells were transfected with p21 promoter reporter plasmid and were treated with DHCB as indicated for 18 hours.
Next we examined whether the expression of p53 target genes, such as p21, gelsolin, and puma, was also induced in cells treated with cucurbitacins. p21 has been suggested to have a crucial role in maintaining cell viability upon p53 induction and induction of p21 has been shown to impart cells with a survival advantage [32]. Indeed, we showed that the induction of p21 in response to cucurbitacins was reproducibly higher in HCT116 than in Hke-3 cells (Fig. 5A and Supplemental Fig. 4), following the pattern of p53 induction. In contrast, all cucurbitacins tested induced the expression of gelsolin to comparable levels in HCT116 and Hke-3 cells, suggesting that p53 does not mediate the induction of gelsolin in these cells (Supplemental Fig. 4). Puma, a target gene of p53 that mediates p53 induced apoptosis, was not induced by cucurbitacins in cell lines that we tested (data not shown).
Due to a critical role of p21 in growth arrest and apoptosis, we sought to determinate whether cucurbitacins induce p21 at the transcriptional level. HCT116 and Hke3 cells were transfected with a p21 promoter reporter plasmid, and treated with different concentrations of DHCB for 18 hours. We showed that DHCB activated transcription of p21 and that the induction of p21 transcriptional activity by DHCB was more pronounced in HCT116 than in Hke-3 cells (Fig. 5B), consistent with enhanced induction of p21 protein in HCT116 cells (Fig. 5A).
Is p53 required for p21 induction in response to DHCB? To answer this question, we examined the ability of DHCB to induce p21 in HCT116 cells in which p53 had been deleted (HCT116 p53−/−). HCT116 and HCT116 p53−/− cells were treated with DHCB and examined for the expression of p21 and gelsolin. We showed that in p53 deficient cells the induction of p21 was completely abrogated, demonstrating that the induction of p21 by DHCB is p53 dependent (Fig. 5C). Consistently, DHCB failed to induce p21 transcription in HCT116 p53−/− cells (Fig. 5D). In contrast, the expression of gelsolin was intact in p53 deficient HCT116 cells, confirming that cucurbitacins induce gelsolin expression in a p53 independent manner.
3.5. p53 and p21 protect colon cancer cells from cucurbitacin- induced apoptosis
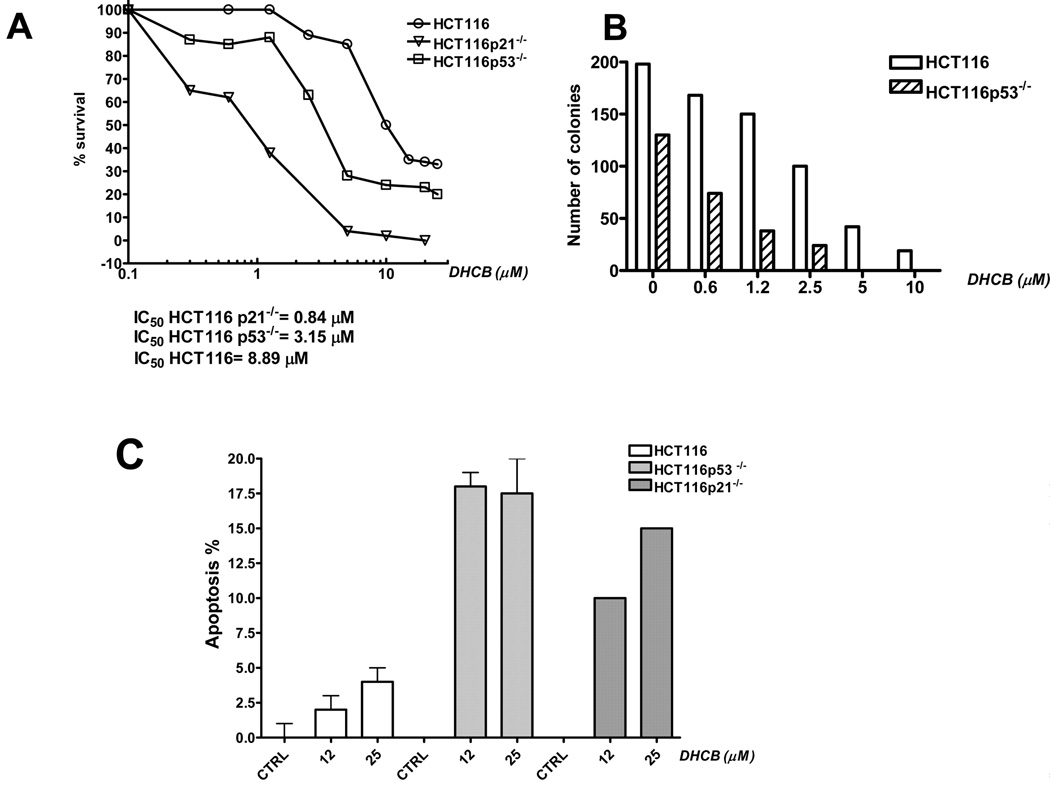
Finally, to confirm the essential role of p53 and p21 in the biological activity of DHCB, we compared its activity in the parental HCT116 cells, in p53 deficient HCT116 cells (HCT116 p53−/−) and in p21 deficient HCT116 cells (HCT116 p21−/−). As shown in Fig. 6A, both p53 and p21 deficiency significantly increased sensitivity of HCT116 cells to DHCB. Consistently, HCT116 p53−/− cells formed fewer colonies than the parental HCT116 cells in the presence of DHCB (Fig. 6B). (HCT116 p21−/− cells failed to form any colonies, therefore we could not assessed the effect of DHCB on the clonogenic potential in these cells). Finally, the extent of apoptosis induced by DHCB was higher in both p53 and p21 deficient HCT116 cells (Fig. 6C), demonstrating that p53 and p21 protect cells from apoptosis induced by DHCB. Similar data were obtained with CCI (Supplemental Fig. 5).
Fig. 6.
p53 and p21 protect cells from DHCB-induced apoptosis. A: Survival of HCT116, HCT116p53−/− and HCT116p21−/− cells treated with cucurbitacins weas calculated form the MTT data. B) Clonogenic assay was performed with HCT116 and HCT116 p53 knockout cells that were left untreated or were treated with increasing concentrations of DHCB as indicated. C: Apoptosis was determined by PI staining in HCT116 cells, HCT116p53−/− and HCT116p21−/− cells 72 hours after treatment with DHCB.
Altogether these data demonstrated that enhanced induction of p53 and p21 in cells that harbor kRasV12 contributes to the resistance of these cells to cucurbitacins.
4 Discussion
In this study we tested the antitumorigenic activities of two cucurbitacins, CCR and DHCB, that we recently isolated from the roots of Cayaponia tayuya [3]. We have shown before that both cucurbitacins exert potent anti-inflammatory activity both in vitro and in vivo [2, 3, 5]. Here we examined their anti-tumorigenic activity in colon cancer cell lines, and compared their potency with CCI, a cucurbitacin with established anti-tumorigenic activity [11, 30]. All three cucurbitacins showed overlapping biological activity in colon cancer cells. Among the cucurbitacins tested, CCI was effective at the lowest concentrations, followed by DHCB and CCR. It therefore appears that the acetyl group in the side chain that structurally differentiates CCR and DHCB (Supplemental Fig.1) is important for the anti-tumorigenic activity of cucurbitacins and that the presence of double bonds in the side chain and in the first ring of CCI increases the antitumorigenic activity of cucurbitacins.
We showed that all three cucurbitacins tested (CCR, DHCB, and CCI) induced dramatic changes in cytoskeleton. The actin filament organization was rapidly disrupted upon treatment of cells with cucurbitacins. In addition, we showed that cucurbitacins induced the expression of actin (data not shown), and gelsolin, an actin severing protein, confirming extensive cytoskeletal reorganization in treated cells.
Cucurbitacins have been shown to disrupt the actin cytoskeleton before [33, 34], however we also observed extensive bundling of tubulin filaments in cucurbitacin treated cells, pointing to a perturbed dynamics of microtubule stabilization in treated cells. Microtubules play a crucial role in biological processes such as motility, cell division and mitotic spindle formation, and are therefore an important target for anticancer drugs. Treatment of cells with microtubule targeting agents has been shown to result in cell cycle arrest, and induction of apoptosis, which accounts for the extensive use of microtubule-interfering agents in tumor chemotherapy [35–37]. Indeed, collapsed cytoskeleton was accompanied with strong induction of G2/M or G0/G1 arrest in cucurbitacin treated cells.
Of interest, microtubules appear to be a preferred target of naturally occurring cytotoxic substances produced by plants, including paclitaxel and vinca alkaloids [38]. Drugs that target microtubule often bind directly to tubulin; whether this is the case for cucurbitacins remains to be determined. In this regard, morphological changes in cells treated with taxol, a prototypical microtubule binding drug, and cucurbitacins were distinct (data not shown), suggesting a different mechanism of action of these drugs.
An important and a novel finding presented in this paper is that the presence of activated kRas modulates the sensitivity of colon cancer cells to cucurbitacins. We used the HCT116 cells and their isogenic clone with deleted mutant Ras allele, HKe-3 cells [25], and IECiKRas cells, nontransformed intestinal cells with IPTG-inducible expression of kRasV12 [28], to demonstrate that the presence of oncogenic kRas offer strong protection from cucuribtacin-induced apoptosis. Consistent with our data, CCI has been shown to efficiently inhibit the growth of Src-NIH3T3 tumors in vivo, but had no effect on Ras-NIH3T3 tumors [30].
We have previously shown that oncogenic kRas modulates apoptosis in response to a variety of chemopreventive and chemotherapeutic drugs. In contrast to cucurbitacins, the presence of oncogenic kRas sensitized cells to agents such as butyrate [20], 5FU [22] and HDAC inhibitors [21]. We showed that inhibition of gelsolin and STAT1 in cells with oncogenic Ras is at least in part responsible for enhanced apoptosis of k-ras transformed cells. Signaling pathways whereby oncogenic Ras protects from cucurbitacin-induced apoptosis remain to be determined. We showed that cucurbitacins induced the expression of p53 and p21 primarily in cells that harbor mutant Ras, which were resistant to cucuributacins, and confirmed that p53 and p21 deficient cells undergo accelerated apoptosis in response to cucurbitacins. These data therefore demonstrated that enhanced induction of p53/p21 in cells that harbor mutant kRas contributes to the resistance of these cells to cucurbitacins.
One of the main target of cucurbitacins is activated STAT3, and it has been suggested that their biological activity is negligible in the absence of constitutive STAT3 signaling [30]. As the aberrant activation of STAT3 is required for growth and survival of many tumors [39, 40], cucurbitacins were considered promising agents for the treatment of tumors whose survival and growth depend on STAT3. However, in this study we demonstrated that cucurbitacins could also exert biological activity in a p-STAT3-independent manner. We showed that cucurbitacins exert strong anti-proliferative and pro-apoptotic activity in colon cancer cell lines that do not have STAT3 phoshorylated on tyrosine. In fact, the IC50 for CCI in colon cancer cell lines that we examined was lower than concentrations of CCI that were effective in inhibiting phosphorylation of STAT3 and JAK2 in lung cancer cell lines with constitutive STAT3 activation [30]. It has been suggested that the ability of CCI to inhibit proliferation is independent of STAT3 activation state, while the ability of cucurbitacins to induce apoptosis depends on tyrosine phosphorylated STAT3 [11]. In contrast, our results demonstrated that activated STAT3 is not required for cucurbitacins to induce apoptosis. Consistent with our data, cucurbitacin I has been shown to disassemble the actin cytoskeleton and to inhibit lysophosphatidic acid-mediated upregulation of CTGF in STAT3 knock out fibroblasts [33]. In addition, cucurbitacin glucosides (B and E) have been shown to inhibit proliferation in two breast cancer cell lines that lack activated STAT3 and have been actually shown to induce a rapid and sustain tyrosine phosphorylation of STAT3 in these two cell lines [34]. Thus, the precise role of activated STAT3 in the responsiveness of cells to cucurbitacins remains to be determined.
Supplementary Material
Supplemental Fig. 1. Dihydrocucurbitacin B (DHCB), curcurbitacin R (CCR), and cucurbitacin I (CCI) with unique structural features indicated in red.
Supplemental Fig. 2: The expression of p-STAT3 and total STAT3 in HCT116 cells that were left untreated (0) or were treated with IL6 as indicated. THP1 cells treated with IL6 for 15 minutes were used as a positive control for p-STAT3.
Supplemental Fig. 3: Activation of caspase-3 in HCT116 and Hke-3 cells upon treatment with DHCB.
Supplemental Fig. 4: Induction of p53, gelsolin and p21 by cucurbitacins. Cells were treated as indicated and the levels of p53, gelsolin, p21 and tubulin, were determined by immunoblotting.
Supplemental Fig. 5: The effect of p53 and p21 deficiency on sensitivity of cells to CCI. Cells were treated as indicated; the survival was calculated from the MTT data (A), p21 deficiency demonstrated in HCT116p21−/− cells (B), and apoptosis was determined by PI staining (C).
Acknowledgments
We thank Bert Vogelstein for the gift of HCT116 p21−/− and HCT116 p53−/− cells, Raymond DuBois for the IEC-ikRas cells, and Georg Wisniewski for reading the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Chen JC, Chiu MH, Nie RL, Cordell GA, Qiu SX. Cucurbitacins and cucurbitane glycosides: structures and biological activities. Nat Prod Rep. 2005;22:386–399. doi: 10.1039/b418841c. [DOI] [PubMed] [Google Scholar]
- 2.Escandell JM, Recio MC, Manez S, Giner RM, Cerda-Nicolas M, Rios JL. Cucurbitacin R reduces the inflammation and bone damage associated with adjuvant arthritis in lewis rats by suppression of tumor necrosis factor-alpha in T lymphocytes and macrophages. J Pharmacol Exp Ther. 2007;320:581–590. doi: 10.1124/jpet.106.107003. [DOI] [PubMed] [Google Scholar]
- 3.Recio MC, Prieto M, Bonucelli M, Orsi C, Manez S, Giner RM, et al. Anti-inflammatory activity of two cucurbitacins isolated from Cayaponia tayuya roots. Planta Med. 2004;70:414–420. doi: 10.1055/s-2004-818968. [DOI] [PubMed] [Google Scholar]
- 4.Prieto JM, Giner RM, Recio MC, Manez S, Rios JL. Dual inhibition of cyclooxygenase-1 and 5-lipoxygenase by aerial part of Bupleurum fruticescens methanol extract. Fitoterapia. 2004;75:179–186. doi: 10.1016/j.fitote.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 5.Escandell JM, Recio MC, Manez S, Giner RM, Cerda-Nicolas JM, Gil-Benso R, et al. Dihydrocucurbitacin B inhibits delayed-type hypersensitivity reactions by suppressing lymphocyte proliferation. J Pharmacol Exp Ther. 2007 doi: 10.1124/jpet.107.122671. [DOI] [PubMed] [Google Scholar]
- 6.Escandell JM, Recio MC, Manez S, Giner RM, Cerda-Nicolas M, Rios JL. Dihydrocucurbitacin B, isolated from Cayaponia tayuya, reduces damage in adjuvant-induced arthritis. Eur J Pharmacol. 2006;532:145–154. doi: 10.1016/j.ejphar.2005.12.028. [DOI] [PubMed] [Google Scholar]
- 7.Siqueira JM, Jr, Peters RR, Gazola AC, Krepsky PB, Farias MR, Rae GA, et al. Anti-inflammatory effects of a triterpenoid isolated from Wilbrandia ebracteata Cogn. Life Sci. 2007;80:1382–1387. doi: 10.1016/j.lfs.2006.12.021. [DOI] [PubMed] [Google Scholar]
- 8.Bowman T, Garcia R, Turkson J, Jove R. STATs in oncogenesis. Oncogene. 2000;19:2474–2488. doi: 10.1038/sj.onc.1203527. [DOI] [PubMed] [Google Scholar]
- 9.Bromberg J. Stat proteins and oncogenesis. J Clin Invest. 2002;109:1139–1142. doi: 10.1172/JCI15617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klampfer L. Signal transducers and activators of transcription (STATs): Novel targets of chemopreventive and chemotherapeutic drugs. Curr Cancer Drug Targets. 2006;6:107–121. doi: 10.2174/156800906776056491. [DOI] [PubMed] [Google Scholar]
- 11.Sun J, Blaskovich MA, Jove R, Livingston SK, Coppola D, Sebti SM. Cucurbitacin Q: a selective STAT3 activation inhibitor with potent antitumor activity. Oncogene. 2005;24:3236–3245. doi: 10.1038/sj.onc.1208470. [DOI] [PubMed] [Google Scholar]
- 12.Klampfer L. The role of signal transducers and activators of transcription in colon cancer. Front Biosci. 2008;13:2888–2899. doi: 10.2741/2893. [DOI] [PubMed] [Google Scholar]
- 13.Krasilnikov M, Ivanov VN, Dong J, Ronai Z. ERK and PI3K negatively regulate STAT-transcriptional activities in human melanoma cells: implications towards sensitization to apoptosis. Oncogene. 2003;22:4092–4101. doi: 10.1038/sj.onc.1206598. [DOI] [PubMed] [Google Scholar]
- 14.Klampfer L, Huang J, Corner G, Mariadason J, Arango D, Sasazuki T, et al. Oncogenic Ki-ras inhibits the expression of interferon-responsive genes through inhibition of STAT1 and STAT2 expression. J Biol Chem. 2003;278:46278–46287. doi: 10.1074/jbc.M304721200. [DOI] [PubMed] [Google Scholar]
- 15.Andreyev HJ, Norman AR, Oates J, Cunningham D. Why do patients with weight loss have a worse outcome when undergoing chemotherapy for gastrointestinal malignancies? Eur J Cancer. 1998;34:503–509. doi: 10.1016/s0959-8049(97)10090-9. [DOI] [PubMed] [Google Scholar]
- 16.Abdellatif M, MacLellan WR, Schneider MD. p21 Ras as a governor of global gene expression. J Biol Chem. 1994;269:15423–15426. [PubMed] [Google Scholar]
- 17.Barbacid M. ras genes. Annu Rev Biochem. 1987;56:779–827. doi: 10.1146/annurev.bi.56.070187.004023. [DOI] [PubMed] [Google Scholar]
- 18.Malumbres M, Pellicer A. RAS pathways to cell cycle control and cell transformation. Front Biosci. 1998;3:d887–d912. doi: 10.2741/a331. [DOI] [PubMed] [Google Scholar]
- 19.Khosravi M, Weaver DD, Bull MJ, Lachman R, Rimoin DL. Lethal syndrome of skeletal dysplasia and progressive central nervous system degeneration. Am J Med Genet. 1998;77:63–71. [PubMed] [Google Scholar]
- 20.Klampfer L, Huang J, Sasazuki T, Shirasawa S, Augenlicht L. Oncogenic Ras promotes butyrate-induced apoptosis through inhibition of gelsolin expression. J Biol Chem. 2004;279:36680–36688. doi: 10.1074/jbc.M405197200. [DOI] [PubMed] [Google Scholar]
- 21.Klampfer L, Huang J, Shirasawa S, Sasazuki T, Augenlicht L. Histone Deacetylase Inhibitors Induce Cell Death Selectively in Cells That Harbor Activated kRasV12: The Role of Signal Transducers and Activators of Transcription 1 and p21. Cancer Res. 2007;67:8477–8485. doi: 10.1158/0008-5472.CAN-07-0210. [DOI] [PubMed] [Google Scholar]
- 22.Klampfer L, Swaby LA, Huang J, Sasazuki T, Shirasawa S, Augenlicht L. Oncogenic Ras increases sensitivity of colon cancer cells to 5-FU-induced apoptosis. Oncogene. 2005;24:3932–3941. doi: 10.1038/sj.onc.1208552. [DOI] [PubMed] [Google Scholar]
- 23.Ahnen DJ. Colon cancer prevention by NSAIDs: what is the mechanism of action? Eur J Surg Suppl. 1998:111–114. doi: 10.1080/11024159850191544. [DOI] [PubMed] [Google Scholar]
- 24.Markowitz S, Hines JD, Lutterbaugh J, Myeroff L, Mackay W, Gordon N, et al. Mutant K-ras oncogenes in colon cancers Do not predict Patient's chemotherapy response or survival. Clin Cancer Res. 1995;1:441–445. [PubMed] [Google Scholar]
- 25.Shirasawa S, Furuse M, Yokoyama N, Sasazuki T. Altered growth of human colon cancer cell lines disrupted at activated Ki-ras. Science. 1993;260:85–88. doi: 10.1126/science.8465203. [DOI] [PubMed] [Google Scholar]
- 26.Waldman T, Kinzler KW, Vogelstein B. p21 is necessary for the p53- mediated G1 arrest in human cancer cells. Cancer Res. 1995;55:5187–5190. [PubMed] [Google Scholar]
- 27.Bunz F, Dutriaux A, Lengauer C, Waldman T, Zhou S, Brown JP, et al. Requirement for p53 and p21 to sustain G2 arrest after DNA damage. Science. 1998;282:1497–1501. doi: 10.1126/science.282.5393.1497. [DOI] [PubMed] [Google Scholar]
- 28.Sheng H, Shao J, Dubois RN. K-Ras-mediated increase in cyclooxygenase 2 mRNA stability involves activation of the protein kinase B1. Cancer Res. 2001;61:2670–2675. [PubMed] [Google Scholar]
- 29.Sowa Y, Orita T, Minamikawa S, Nakano K, Mizuno T, Nomura H, et al. Histone deacetylase inhibitor activates the WAF1/Cip1 gene promoter through the Sp1 sites. Biochem Biophys Res Commun. 1997;241:142–150. doi: 10.1006/bbrc.1997.7786. [DOI] [PubMed] [Google Scholar]
- 30.Blaskovich MA, Sun J, Cantor A, Turkson J, Jove R, Sebti SM. Discovery of JSI-124 (cucurbitacin I), a selective Janus kinase/signal transducer and activator of transcription 3 signaling pathway inhibitor with potent antitumor activity against human and murine cancer cells in mice. Cancer Res. 2003;63:1270–1279. [PubMed] [Google Scholar]
- 31.Brown JM, Attardi LD. The role of apoptosis in cancer development and treatment response. Nat Rev Cancer. 2005;5:231–237. doi: 10.1038/nrc1560. [DOI] [PubMed] [Google Scholar]
- 32.Weiss RH. p21Waf1/Cip1 as a therapeutic target in breast and other cancers. Cancer Cell. 2003;4:425–429. doi: 10.1016/s1535-6108(03)00308-8. [DOI] [PubMed] [Google Scholar]
- 33.Graness A, Poli V, Goppelt-Struebe M. STAT3-independent inhibition of lysophosphatidic acid-mediated upregulation of connective tissue growth factor (CTGF) by cucurbitacin I. Biochem Pharmacol. 2006;72:32–41. doi: 10.1016/j.bcp.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 34.Tannin-Spitz T, Grossman S, Dovrat S, Gottlieb HE, Bergman M. Growth inhibitory activity of cucurbitacin glucosides isolated from Citrullus colocynthis on human breast cancer cells. Biochem Pharmacol. 2007;73:56–67. doi: 10.1016/j.bcp.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 35.Joshi HC. Microtubule dynamics in living cells. Curr Opin Cell Biol. 1998;10:35–44. doi: 10.1016/s0955-0674(98)80084-7. [DOI] [PubMed] [Google Scholar]
- 36.Bhat KM, Setaluri V. Microtubule-associated proteins as targets in cancer chemotherapy. Clin Cancer Res. 2007;13:2849–2854. doi: 10.1158/1078-0432.CCR-06-3040. [DOI] [PubMed] [Google Scholar]
- 37.Pasquier E, Andre N, Braguer D. Targeting microtubules to inhibit angiogenesis and disrupt tumour vasculature: implications for cancer treatment. Curr Cancer Drug Targets. 2007;7:566–581. doi: 10.2174/156800907781662266. [DOI] [PubMed] [Google Scholar]
- 38.Jordan MA, Wilson L. Microtubules as a target for anticancer drugs. Nat Rev Cancer. 2004;4:253–265. doi: 10.1038/nrc1317. [DOI] [PubMed] [Google Scholar]
- 39.Jing N, Tweardy DJ. Targeting Stat3 in cancer therapy. Anticancer Drugs. 2005;16:601–607. doi: 10.1097/00001813-200507000-00002. [DOI] [PubMed] [Google Scholar]
- 40.Turkson J, Bowman T, Garcia R, Caldenhoven E, De Groot RP, Jove R. Stat3 activation by Src induces specific gene regulation and is required for cell transformation. Mol Cell Biol. 1998;18:2545–2552. doi: 10.1128/mcb.18.5.2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Fig. 1. Dihydrocucurbitacin B (DHCB), curcurbitacin R (CCR), and cucurbitacin I (CCI) with unique structural features indicated in red.
Supplemental Fig. 2: The expression of p-STAT3 and total STAT3 in HCT116 cells that were left untreated (0) or were treated with IL6 as indicated. THP1 cells treated with IL6 for 15 minutes were used as a positive control for p-STAT3.
Supplemental Fig. 3: Activation of caspase-3 in HCT116 and Hke-3 cells upon treatment with DHCB.
Supplemental Fig. 4: Induction of p53, gelsolin and p21 by cucurbitacins. Cells were treated as indicated and the levels of p53, gelsolin, p21 and tubulin, were determined by immunoblotting.
Supplemental Fig. 5: The effect of p53 and p21 deficiency on sensitivity of cells to CCI. Cells were treated as indicated; the survival was calculated from the MTT data (A), p21 deficiency demonstrated in HCT116p21−/− cells (B), and apoptosis was determined by PI staining (C).