Abstract
Capillary electrophoretic separation of samples of physiological origin typically have both poor resolution and efficiency due to destacking. We have previously reported a stacking method for concentration of catecholamines in artifical dialysate, or Ringer’s solution. However, pH-mediated sample stacking of other cations has not been investigated. In this report, pH-mediated stacking has been extended to eletripan, dofetilide, doxazosin, sildenafil, UK-103,320, UK-202,581, and CP-122,288. These compounds were chosen without prior structural screening except that they were cationic at the pH of our background electrolyte (BGE). Capillary electrophoretic behavior of samples in BGE is compared with those of samples in Ringer’s solution with and without pH-mediated acid stacking. Results indicate that the peak heights and efficiencies for acid-stacked samples are increased compared to the unstacked samples in Ringer’s solution or BGE. For example, the peak efficiencies for 5 s injections of eletriptan in BGE and Ringer’s solution are 138 000 and 72000 plates, respectively. In contrast, a 10 s injection of eletriptan followed by acid injection for 16 s produces a peak with 246 000 plates. Evaluation of the stacking effect was performed by comparison of the peak height at similar peak efficiencies for samples in Ringer’s solution with and without stacking. Using this method, pH-mediated acid stacking provides a 10- to 27-fold sensitivity enhancement for the seven cations.
Keywords: pH-mediated on-column concentration, Sample stacking
1 Introduction
The detection limits of capillary electrophoresis (CE) for absorbance-based measurements are often limited by the short optical path length of the capillary. In addition, the small volume of the capillary (low microliters) necessitates the injection of very low sample volumes (low nanoliters) in order to avoid overloading. Sample concentration can be used to improve the detection limits of CE. The goal is to concentrate the sample before separation so that the maximum concentration effect is obtained with mimimum loss in efficiency. One approach is to concentrate the sample on an LC column before separation by CE [1–6]. Isotachophoresis has also been used with some success [7].
The most common approach to on-column concentration is by field-amplified sample stacking. In this technique, the sample is diluted in a low-conductivity sample matrix such as water before analysis. The electric field strength is greater in the low conductivity region of the sample plug than the BGE. Therefore, the sample ions will move faster in the sample zone than in the BGE. When they reach the interface of the sample buffer and BGE, they slow down and stack into a narrower zone resulting in enhanced peak efficiency with increased peak height. Large volumes of sample can be injected producing up to a hundredfold concentration of cations oranions [8–12].
Sweeping is another approach to sample concentration. The concentration effect here is based upon analyte partitioning into a pseudostationary phase for electrokinetic chromatography [13–16]. Using this technique, a 5000-fold sensitivity enhancement has been reported for neutrals [13]. Recently, sweeping of cations and anions has been reported [15]. In addition, a new variation of this method, termed cation-selective exhaustive injection and sweeping (CSEI-sweep-MEKC), has been reported to produce up to a millionfold sensitivity enhancement [16]. However, sweeping techniques are only applicable to hydrophobic analytes that partition strongly into the micelle pseudophase. While sweeping and CSEI-sweep-MEKC produce dramatic improvements in detection limits for CE, the sample matrices used for these technique are limited. For example, samples must either match the ionic strength of the BGE (sweeping) or be comprised of low-ionic strength sample matrix such as water (CSEI-sweep-MEKC). Field-amplified sample stacking also requires samples to be in a low-conductivity sample matrix such as water. Therefore, these concentration methods are most useful under very controlled sample buffer conditions.
Samples of physiological origin are typically high in salt content. Therefore, field amplification and sweeping techniques are not suitable approaches for their concentration. Moreover, the high ionic strength of physiological samples generally produces destacking of the sample zone. The analytes migrate slower in the highly conductive sample zone than in the BGE. When the analytes reach the interface of the sample zone and the BGE, they speed up resulting in band broadening. This typically results in poor separation of complex physiological samples since the resolution is compromised by decreased efficiency. In addition, the peak height decreases thereby adversely affecting the detection limits of the method. Perhaps the most effective concentration of sample in a high-ionic strength matrix has been performed using a nonsweeping variation of micellar CE [17]. In this method, the high ionic strength of the sample is used to stack the anionic micellar phase and neutral analytes. Although a several hundredfold concentration of corticosteroids has been reported, this technique is only suitable for hydrophobic analytes that strongly partition into the micelles. In performing analysis of serum, Shihabi [18] has illustrated the advantages of addition of acetonitrile to the samples producing a 10- to 20-fold concentration effect. Another approach to sample analysis of physiological origin has been to use a combination of LC and CE for on-line chromatographic sample pretreatment [19]. While these techniques are feasible with urine or serum, small-volume samples, where extraction or dilution are unsuitable, necessitate the need for other sample stacking methods.
Microdialysis samples are an example where the small volumes (typically a few μL) of low-concentration, high-ionic strength samples demand a new stacking method. Our approach to overcome the problems of analysis of high-ionic strength samples is to use on-column sample concentration with pH-mediated stacking [20–23]. For acid stacking of cations, a weak acid buffer (acetate) is used as the BGE with electrokinetic injection of sample followed by electrokinetic injection of a strong acid (HCl). Ringer’s solution (a mixture of NaCl, CaCl2 and KCl) matches the components and ionic strength of dialysate and is used as artificial dialysate. The high-ionic strength of the sample zone comprised of Ringer’s solution makes the conductivity of the sample zone 1.8-fold higher than the acetate BGE zone. Upon injection of sample, the chloride in the Ringer’s solution is displaced by the acetate in the BGE. When the acid is injected, the high mobility of the protons allows them to overtake the sample zone and titrate the acetate to acetic acid. When the acid injection is stopped, titration of the sample zone is terminated and separation of cations can begin. A sevenfold increase in detection limits has been reported using this technique [21].
Analysis of anions can be performed using an ammonium salt buffer with reversed electroosmotic flow and separation polarity. Sample injection is followed immediately by injection of a base thereby titrating the ammonium in the sample zone to ammonia. A 66-fold sensitivity enhancement has been reported using this method for the analysis of the anions p-hydroxybenzoic acid, vanillic acid, p-coumaric acid, and syringic acid [22]. Schwer and Lottspeich [24] used a similar stacking mechanism for stacking peptides at extreme pH values. In this method, the sample zone as sandwiched between zones of OH− and H+. These zones migrated towards each other forming a low-conductivity zone over which the sample was stacked by a field amplification effect. The authors suggested that the OH− and H+ may also act as a terminator producing an isotachophoretic effect in addition to the field amplification.
While there may be an ITP effect at the beginning of the acid injection during pH-mediated acid stacking, this is not the main stacking phenomena. First, the electrophoretic current decreases dramatically during the acid injection and only returns to the level of BGE after the neutralized zone exits the capillary [21, 22]. Second, electro-kinetic injection is needed for pH-mediated acid stacking to occur [21], whereas ITP stacking occurs using hydrodynamic injection to set up the discontinuous electrolyte zones. The main driving force for pH-mediated acid stacking is field amplification resulting from titration of the buffer in the sample zone. In this report, the general utility of acid stacking for cationic analytes is demonstrated. Previous work has focused on the stacking of catecholamines. Here, seven noncatecholamine cations, provided without structural constraint other than being cations below pH 8, are shown to be amenable to acid stacking. A standard method is proposed for evaluation of the pH-mediated stacking effect based upon comparison with results in Ringer’s solution.
2 Materials and methods
2.1 Chemicals
Eletriptan, dofetilide, doxazosin, sildenafil, UK-103,320, UK-202,581, and CP-122,288 were donated by Pfizer UK (Sandwich, Kent, UK) and used as received. Stock solutions were made by dissolving sample standards in 100% methanol. The BGE was 100 mM sodium acetate (Fisher Scientific, Fair Lawn, NJ, USA) titrated to pH 4.75 with concentrated acetic acid. Ringer’s solution was comprised of 155 mM NaCl, 2.3 mM CaCl2, and 5.5 mM KCl. BGE and Ringer’s solutions were prepared in deionized water from a LABCONCO Water Pro Plus water system (Fisher Scientific). All solutions were stored at 4°C when not in use.
2.2 CE
Experiments were performed using an ISCO 3850 capillary electrophoresis unit (ISCO, Lincoln, NE, USA). Detection was set at 220 nm. For separation we used a fused-silica capillary with 75 μm ID and 70 cm length (Polymicro Technologies, Phoenix, AZ). The distance from the injection end to the detection window was 50 cm. Samples were introduced by electrokinetic injection at 5 kV. Eletriptan, dofetilide, and doxazosin were injected as a mixture, sildenafil and UK-103,320 were injected as a mixture, and UK-202,581 and CP-122,288 were injected separately. This was to assure resolution of all compounds. Separations were performed at 20 kV. The capillary was manually flushed using a 100 μL syringe and a flushing port on the CE system. At the beginning of the day the capillary was flushed with 100 μL of 0.1 N NaOH followed sequentially by the same volumes of deionized water and BGE. High voltage was applied for 10 min to equilibrate the capillary before experiments were performed. Data were collected with a SP4400 Datajet integrator interfaced with a Labnet connection to a computer with the program Chromnet (Spectra-Physics, San Jose, CA, USA).
3 Results and discussion
3.1 Comparison of the behavior in BGE and Ringer’s solution with and without acid stacking
The cationic compounds discussed here were obtained from Pfizer UK without prior structural screening. Compounds were selected based only on the fact that they were cations below pH 8. Samples were made with analytes dissolved in BGE or Ringer’s solution. Experiments were performed with analytes in BGE and the results compared to experiments performed with the samples in Ringer’s solution with and without acid stacking. Peak efficiency was calculated using the equation N = 5.54 (mt/W1/2)2. Figure 1 presents electropherograms for eletriptan, dofetilide, and doxazosin in BGE, Ringer’s solution and with acid stacking. Electropherograms of analyte in BGE had sharp, narrow peaks while those of the analytes in Ringer’s solution were skewed with lower efficiency and peak height. The differences in migration times between samples in BGE, and Ringer’s solution with and without acid stacking were the result of running the experiments on different days. Experiments run on the same day resulted in 2.4% coefficient of variation in migration times. Acid stacking (Fig. 1C) resulted in peaks with significantly greater peak height and efficiency due to the on-column concentration produced with this technique. The large broad response at 8.0 min was produced by the acid which elutes with the EOF. This response at the neutral marker increased with increasing acid injection. Eletriptan standards underwent degradation after preparation and the additional peaks at 6.2 min are breakdown products of this drug. Note that these degradation products were at low concentration in the sample and were not observed without the aid of acid stacking. In addition, the breakdown products were not observed with injection of only Ringer’s solution or other analytes.
Figure 1.
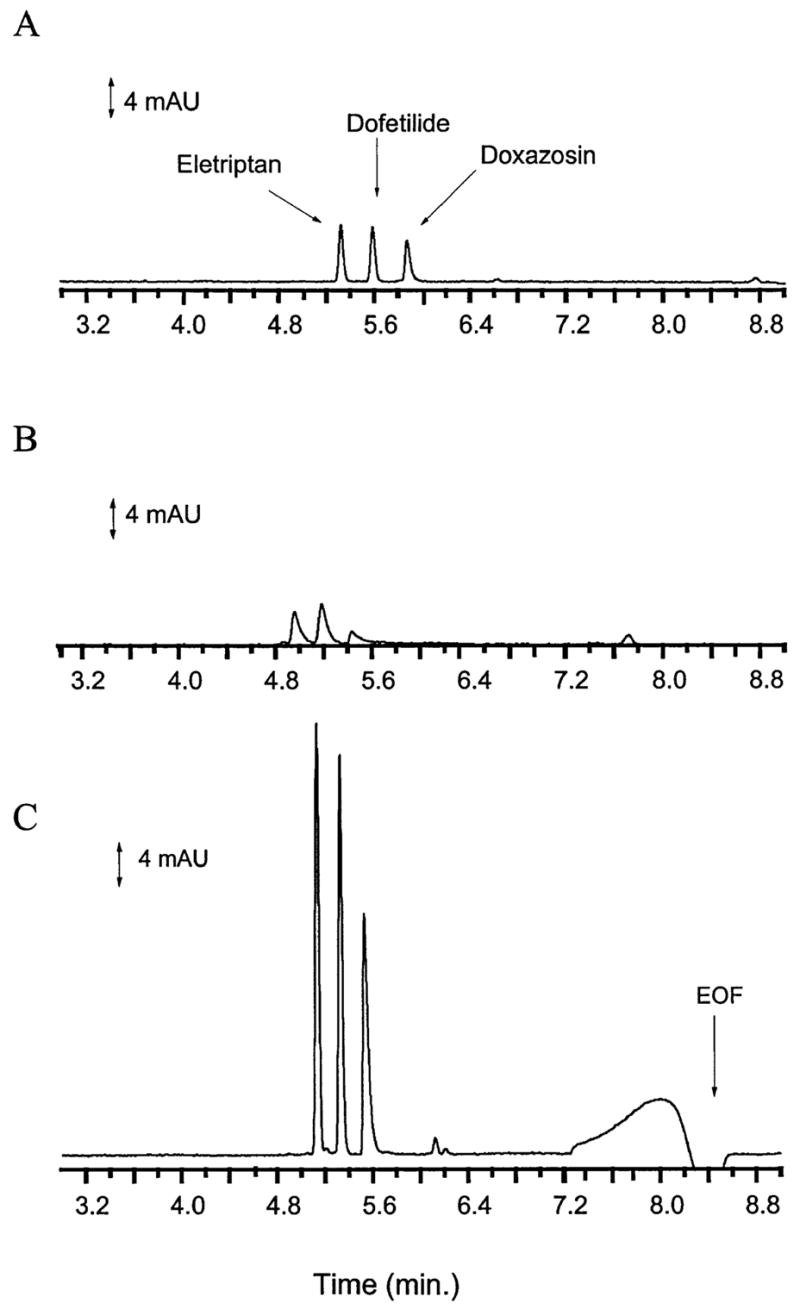
30 μg/mL eletriptan, dofetilide and doxazosin in (A) BGE, (B) Ringer’s solution, and (C) Ringer’s solution with acid stacking. BGE and Ringer’s solution were injected for 12 and 10 s, respectively. Acid stacking was performed with injection of sample for 50 s followed by injection of acid for 80 s. Injection was performed electrokinetically at 5 kV. The BGE was 100 mM sodium acetate buffer, pH 4.75, with a separation performed at 20 kV.
Table 1 presents the peak efficiency and peak height (P.M.) of eletriptan at various injection times with samples in BGE, Ringer’s solution and with acid stacking. Because all seven compounds studied produced the same trend, results for only one compound are presented. As expected, the efficiency was relatively constant until zone broadening due to sample injection exceeds the separation efficiency. The effects of Ringer’s solution on sample destacking can be seen by comparing the efficiencies in Table 1 for eletriptan in BGE with the compound in Ringer’s solution. For example, with a 1 s injection no broadening due to sample injection was expected. However, the peak efficiency was lower at 1 s for eletriptan in Ringer’s solution than for a 15 s injection of the sample in BGE.
Table 1.
Effect of injection time on efficiency and peak height for eletriptan in BGE, Ringer's solution and with acid stacking
| Injection time (s) (mAU) sample/acid | Migration time (s) | W1/2(s) | N/1000 | Peak height (mAU) |
|---|---|---|---|---|
| BGE | ||||
|
| ||||
| 1 | 306 ±17 | 2.0 ± 0.15 | 125 ± 5.5 | 0.36 ± 0.62 |
| 5 | 304 ± 20 | 1.9± 0.15 | 138 ± 66 | 1.5 ± 0.23 |
| 10 | 303 ±19 | 2.4 ± 0.5 | 94 ± 23 | 5.3 ± 1.1 |
| 12 | 309 ± 22 | 2.1 ± 0.06 | 125 ± 22 | 5.8 ± 0.92 |
| 15 | 287 ± 9.3 | 3.2 ± 1.2 | 59 ± 33 | 7.0 ± 0.85 |
| 20 | 302 ± 27 | 5.7 ± 1.3 | 17 ± 9.2 | 6.6 ± 1.1 |
|
| ||||
| Ringer’s solution | ||||
|
| ||||
| 1 | 281 ± 4.5 | 3.2 ± 0.26 | 46 ± 0.26 | 0.57 ± 0.81 |
| 5 | 281 ± 1.5 | 2.5 ± 0.11 | 72 ± 5.9 | 2.4 ± 0.58 |
| 10 | 280 ±1.7 | 2.7 ± 0.10 | 60 ± 4.5 | 3.8 ± 0.35 |
| 15 | 273 ±1.5 | 3.1 ± 0.23 | 43 ± 5.9 | 4.8 ± 0.36 |
| 20 | 273 ± 2.0 | 3.5 ± 0.50 | 35 ± 11 | 5.0 ± 0.46 |
|
| ||||
| Acid stacking | ||||
|
| ||||
| 5/8 | 288 ± 11 | 1.6± 0.12 | 184 ± 35 | 3.3 ± 1.1 |
| 10/16 | 288 ±12 | 1.4± 0.12 | 246 ± 34 | 5.0 ± 0.89 |
| 20/32 | 323 ± 2.6 | 1.9 ± 2.5 | 160 ± 8.6 | 14 ± 3.3 |
| 30/48 | 295 ±2.1 | 1.5 ± 0.10 | 217 ± 23 | 20 ± 1.0 |
| 40/64 | 300 ± 2.0 | 1.6 ± 0.03 | 191 ± 4.6 | 26 ± 1.1 |
| 50/80 | 306 ± 2.1 | 2.1 ± 0.03 | 148 ± 5.0 | 37 ± 2.0 |
| 70/112 | 321 ± 4.3 | 2.4 ± 0.27 | 102 ± 20 | 38 ± 6.8 |
| 90/144 | 328 ± 2.5 | 2.6 ± 0.07 | 90 ± 6.3 | 35 ± 5.7 |
| 120/192 | 338 ± 6.1 | 3.1 ± 0.06 | 66 ± 2.1 | 46 ± 3.7 |
Values are presented as mean ± standard deviation from experiments run in triplicate
Sample stacking was performed at the previously optimized ratio of acid to sample injection times of 1.6 [21]. The sample injection times were increased proportionally from 1 to 120 s with the results presented in Table 1. With sample stacking the efficiencies were higher not only than those when the samples were in Ringer’s solution, but also when compared to samples in BGE. For example, a 10 s sample injection followed by a 16 s acid injection results in a peak efficiency of 246 000 plates for eletriptan. For the same injection time (10 s) the efficiencies were 60 000 plates in Ringer’s solution without sample stacking, and 94000 plates in BGE. Therefore, even when compared to samples in BGE, the efficiency was increased when a Ringer’s sample was acid-stacked.
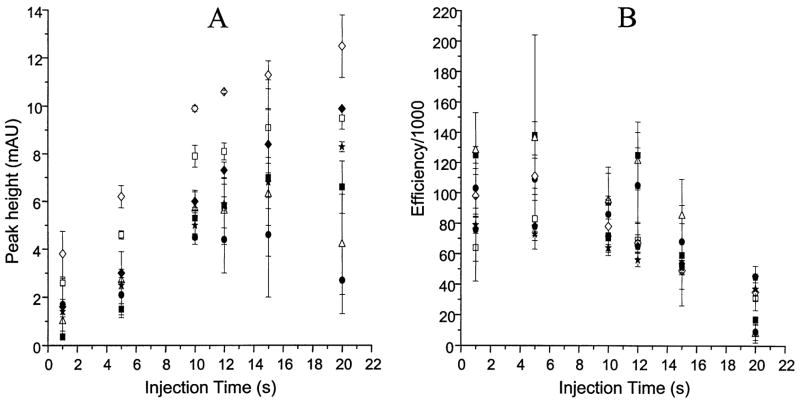
Figure 2 presents plots of the peak height and efficiency versus the sample injection time for the seven compounds in BGE. The efficiency was constant until the point at which too much sample was injected resulting in a loss of efficiency. Compounds in Ringer’s solution exhibit a similar behavior (Fig. 3). However, due to destacking the peak heights and efficiencies were decreased compared to results with BGE. In addition, because the destacking effect was so prominent, the decrease in efficiency was observed at shorter injection times. Therefore, the efficiencies and peak heights in Ringer’s solution also dropped off at shorter injection times than those in BGE. The result was a decrease in slopes of the peak height plots with injection times as low as 5–10 s, with peak efficiency following the same trend.
Figure 2.
Effect of injection time on peak height and efficiency for compounds in BGE. Legend: (■) eletriptan; (△) dofetilide; (●) doxazosin; (*) sildenafil; (◆) UK-103,320, (◇) CP-122,288, and (□) UK-202,581.
Figure 3.
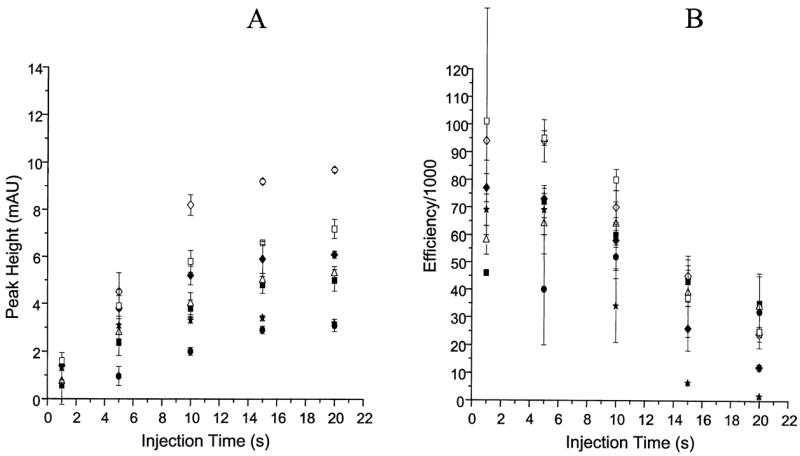
Effect of injection time on peak height and efficiency for compounds in Ringer’s solution. Legend as in Fig. 2.
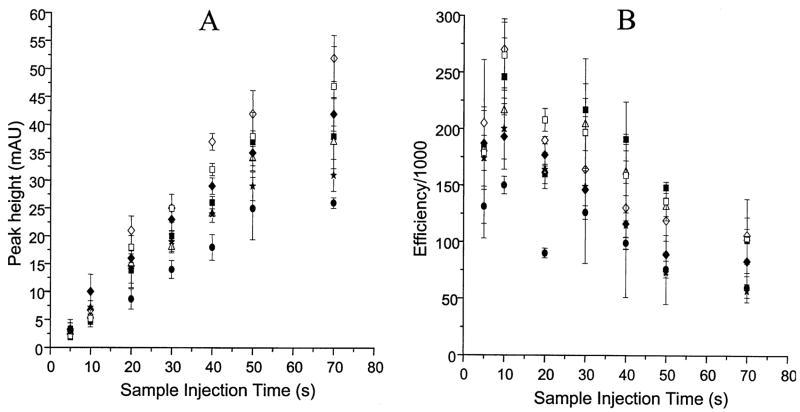
Acid stacking allowed longer sample injection times to be used with higher efficiency than samples in BGE or Ringer’s solution (Fig. 4). For example, while the slope of the graph for the sample in Ringer’s solution showed a rapid loss of efficiency, the plot was linear up to 50 s with acid stacking. Therefore, acid stacking allows a much greater amount of sample to be injected without significant loss of peak efficiency.
Figure 4.
Effect of sample injection time on peak hight and efficiency for compounds in Ringer’s solution with acid stacking. Acid injection times are as in Table 1. Legend as in Fig. 2.
3.2 Quantitation of the stacking effect
There are several methods that can be used to quantify the concentration effect produced by pH-mediated stacking. One method is to choose the same injection time to compare the concentration effect. The peak height in Ringer’s solution with 50 s injection was 5.5 mAU. Injections of 50 s sample/80 s acid resulted in a peak height of 37 mAU. This comparison yields a 7-fold concentration enhancement. However, the peak without stacking suffers from very poor efficiency (7800 plates) compared to the peaks obtained with acid stacking (131 000). Another limitation of the comparison is that the injection time chosen can be somewhat arbitrary.
Another approach to calculate the stacking effect is to compare the peak height at the same efficiency for the mixture in Ringer’s solution with the acid-stacked result. The efficiency in Ringer’s solution was chosen at the time that corresponded to the highest efficiency. For example, the efficiency for a 5 s injection of eletriptan in Ringer’s solution was 72 000 plates with a peak height of 2.4 mAU.
With increased injection time the peak efficiency dropped rapidly. A similar efficiency using acid stacking (102 000 plates) was found with injection of 70 s sample/112s acid resulting in a peak height of 38 mAU. Therefore, a 16-fold on-column concentration increase was produced with acid stacking of eletriptan.
Table 2 lists the efficiencies in Ringer’s solution with and without acid stacking for the seven compounds studied. The concentration effect is shown as the ratio of the peak height for acid stacking with respect to the compound in Ringer’s solution. The injection times corresponding to the efficiencies in the table are also presented. The same injection times were chosen since all seven compounds exhibited the same trend as eletriptan. Note that the injection times for acid-stacked samples were dramatically longer than the maximum injection times before efficiency deteriorated for unstacked samples. The greater amount of Ringer’s solution that can be injected onto the capillary with acid stacking allows a 10-to 27-fold sensitivity enhancement compared to samples in Ringer’s solution without acid stacking. In addition, for all cases the efficiency was higher for acid stacking relative to normal electrokinetic injection in Ringer’s solution.
Table 2.
Concentration effect for acid stacking
| Compound | ITRinger’s Sol. (s) | NRinger’s Sol. (Efficiency/1000) | ITstacking (Sample/acid) | Nstacking (Efficiency/1000) | PHstacking |
|---|---|---|---|---|---|
| PHRinger’s Sol. | |||||
| Eletriptan | 5 | 72 | 70/112 | 102 | 16 |
| Dofetilide | 5 | 64 | 70/112 | 104 | 13 |
| Doxazosin | 5 | 40 | 70/112 | 60 | 27 |
| UK-103,320 | 5 | 73 | 70/112 | 83 | 11 |
| Sildenafil | 5 | 69 | 70/112 | 53 | 10 |
| CP-122,288 | 5 | 94 | 70/112 | 106 | 12 |
| UK-202,581 | 5 | 95 | 70/112 | 103 | 12 |
IT, injection time
PH, peak height
4 Concluding remarks
Acid stacking is not compound-specific and is applicable to cations other than catecholamines. The peak efficiencies of acid-stacked samples are increased compared to those in either Ringer’s solution or BGE. Moreover, since the resolution of the separation is a function of the peak efficiency, separations of physiological solutions should show enhanced resolution when acid stacking is used. While the stacking effect here is close to an order to magnitude, other methods such as field-amplified sample stacking using low-conductivity sample buffer and sweeping provide a greater stacking effect. However, because it is not limited to samples of similar or lower conductivity than the BGE, pH-mediated stacking can be used for on-column concentration of physiological solutions. In addition, acid stacking is applicable to more hydrophilic analytes than sweeping.
Acknowledgments
This work was supported in part by NIH grant R01GM44900. DJW acknowledges the support from the National Cancer Institute Training Grant T32CA09424.
References
- 1.Novic M, Gucek M. J Chromatogr A. 2000;868:135–139. doi: 10.1016/s0021-9673(99)01217-0. [DOI] [PubMed] [Google Scholar]
- 2.Debets AJJ, Mazereeuw M, Voogt WH, van Iperen DJ, Lingeman H, Hupe KP, Brinkman UAT. J Chromatogr A. 1992;608:151–158. [Google Scholar]
- 3.Figeys D, Ducret A, Aebersold R. J Chromatogr A. 1997;763:295–306. doi: 10.1016/s0021-9673(96)00847-3. [DOI] [PubMed] [Google Scholar]
- 4.Veraart JR, Gooijer C, Lingeman H, Velthorst NH, Brinkman UA. J Chromatogr A. 1998;719:199–208. doi: 10.1016/s0378-4347(98)00410-1. [DOI] [PubMed] [Google Scholar]
- 5.Arce L, Kuban P, Rios A, Valcarcel M, Karlberg B. Anal Chim Acta. 1999;390:39–44. [Google Scholar]
- 6.Thomlinson AJ, Benson LM, Guzman NA, Naylor S. J Chromatogr A. 1996;744:3–15. [Google Scholar]
- 7.Dankova M, Kaniansky D, Fanali S, Ivanyi F. J Chromatogr A. 1999;838:31–43. doi: 10.1016/s0021-9673(98)00974-1. [DOI] [PubMed] [Google Scholar]
- 8.Albin M, Grossman PD, Moring SE. Anal Chem. 1993;65:489A–497A. [Google Scholar]
- 9.Chien R, Burgi DS. Anal Chem. 1992;64:489A–496A. [Google Scholar]
- 10.Quirino JP, Terabe S. Electrophoresis. 2000;21:355–359. doi: 10.1002/(SICI)1522-2683(20000101)21:2<355::AID-ELPS355>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 11.McGrath G, Smyth WF. J Chromatogr B. 1996;681:125–131. doi: 10.1016/0378-4347(95)00486-6. [DOI] [PubMed] [Google Scholar]
- 12.Albert M, Debusschere L, Demesmay C, Rocca JL. J Chromatogr A. 1997;757:291–296. doi: 10.1016/s0021-9673(97)00413-5. [DOI] [PubMed] [Google Scholar]
- 13.Quirino JP, Terabe S. Science. 1998;16:465–468. doi: 10.1126/science.282.5388.465. [DOI] [PubMed] [Google Scholar]
- 14.Quirino JP, Terabe S, Otsuka K, Vincent JB, Vigh G. J Chromatogr A. 1999;838:3–10. [Google Scholar]
- 15.Quirino JP, Terabe S. Anal Chem. 1999;71:1638–1644. [Google Scholar]
- 16.Quirino JP, Terabe S. Anal Chem. 2000;72:1023–1030. doi: 10.1021/ac990344b. [DOI] [PubMed] [Google Scholar]
- 17.Palmer J, Munro NJ, Landers JP. Anal Chem. 1999;71:1679–1687. doi: 10.1021/ac981302a. [DOI] [PubMed] [Google Scholar]
- 18.Shihabi ZK. J Chromatogr A. 1999;853:3–9. doi: 10.1016/s0021-9673(99)00316-7. [DOI] [PubMed] [Google Scholar]
- 19.Veraart JR, Gooijer C, Lingeman H, Velthorst NH, Brinkman UAT. Chromatographia. 1997;44:581–588. [Google Scholar]
- 20.Hadwiger ME, Torchia SR, Park S, Biggin ME, Lunte CE. J Chromatogr B. 1996;681:241–249. doi: 10.1016/0378-4347(95)00549-8. [DOI] [PubMed] [Google Scholar]
- 21.Park S, Lunte CE. J Microcol Sep. 1998;10:511–517. [Google Scholar]
- 22.Zhao Y, Lunte CE. Anal Chem. 1999;71:3985–3991. doi: 10.1021/ac990242l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xiong Y, Park S, Swerdlow H. Anal Chem. 1998;70:3605–3611. doi: 10.1021/ac980376j. [DOI] [PubMed] [Google Scholar]
- 24.Schwer C, Lottspeich F. J Chromatogr. 1992;623:345–355. doi: 10.1016/0021-9673(92)80375-5. [DOI] [PubMed] [Google Scholar]