Introduction
ADME (adsorption, distribution, metabolism, elimination) studies on new drug entities, toxicological studies on environmental contaminants, and evaluation of chemicals in the workplace all involve gathering similar types of information. Such studies rely on a combination of in vitro, ex vivo, and in vivo techniques. Among the in vitro and ex vivo systems are isolated enzymes, microsomes, cell cultures, tissue homogenates, tissue slices, and isolated organs. Information about the role of specific enzymes and tissues, types of intermediates formed, and pathways for xenobiotic biotransformation are gained through these types of studies. However, because they represent parts rather than the whole physiological system, the types of information gained do not reflect the entire scheme of biotransformation within the intact organism.
Traditional in vivo techniques include sampling blood and other biological fluids, analysis of elimination products to determine final disposition of the parent compound and metabolites, and tissue sampling requiring one or more animals per time point. These studies provide general information about the metabolism and final disposition of a compound, but do not offer details about specific enzymes, intermediates, or in vivo metabolic pathways. An overall limitation to traditional approaches is that results obtained in vitro sometimes do not correlate with those found in vivo.
Microdialysis sampling has several characteristics that complement traditional in vitro and in vivo pharmacokinetic and metabolism techniques. This approach is accomplished by implanting a probe consisting of a hollow fiber dialysis membrane into the organ or biological matrix of interest. The short length of hollow fiber is affixed to pieces of narrow bore tubing that serve as inlet and oulet conduits for the probe. A solution, termed the perfusate, is pumped slowly through the probe. The perfusate is an aqueous solution that closely matches the ionic composition of the extracellular fluid (ECF). When the perfusate is correctly matched to the ECF, there should be no net exchange of water or ions across the membrane. Low molecular weight compounds can diffuse into or out of the probe lumen in response to concentration gradients and are pumped to the analysis system.
A diagram of the process is shown in Figure 1. For clarity, the diagram shows only one analyte (A), although several compounds are typically sampled at the same time in a microdialysis experiment. Large molecules such as proteins (P) and small molecules bound to proteins (P-A) are excluded by the membrane. Those molecules entering the lumen of the membrane are swept along by the perfusate and exit the probe. The solution leaving the probe, termed the dialysate, is collected for analysis. The probe can be thought of as an artificial blood vessel in that it can both deliver and remove compounds from the local area. Delivery of the parent compound via the probe permits study of local metabolism without systemic involvement. Figure 2 shows a basic diagram of a microdialysis system for sampling from an awake, freely moving animal.
Figure 1.
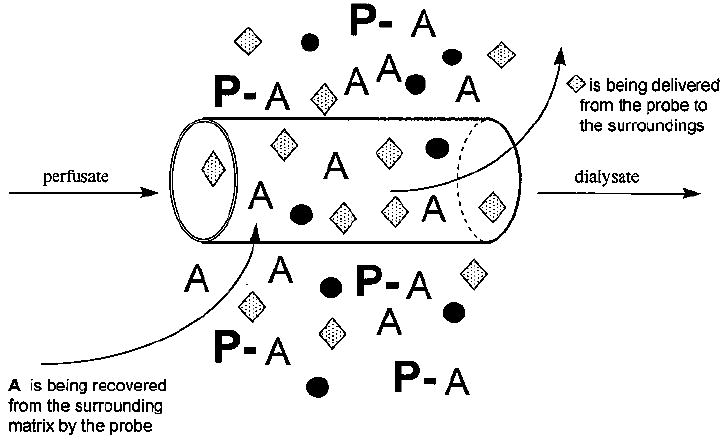
The microdialysis process.37
Figure 2.
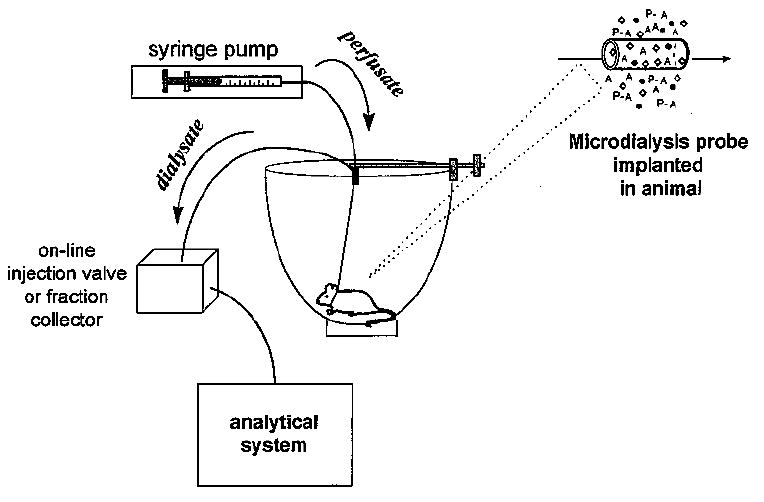
General diagram of microdialysis system for sampling from an awake, freely moving animal. The analysis may be performed on a variety of analytical systems, and coupling between the analytical and microdialysis systems may be on- or off-line.
Microdialysis is an established in vivo tool in the neurosciences and has been used in vitro and in peripheral tissues for a variety of applications of pharmacological and metabolic interest. Samples obtained by microdialysis represent a local profile of low molecular mass hydrophilic substances in the area surrounding the probe. For compounds of pharmaceutical interest, the dialysate reflects the free fraction of the compound of interest, which is the therapeutically active portion of the dose. Microdialysis sampling does not change the net fluid balance in the surrounding matrix or tissue, so higher temporal resolution can be achieved than with traditional techniques. Also, because there is no net fluid loss, samples can be collected continuously for hours or days from a single animal. Most importantly, each animal can serve as its own control, reducing the number of experimental animals needed.
Typically, sampling in vitro from various incubation mixtures or in vivo from blood or other fluids involves manipulation of small volume samples and multiple step cleanup procedures, which produces poor temporal resolution relative to physiological or pharmacological events. Elimination products and tissue samples also require extensive sample cleanup prior to analysis. Because microdialysis sampling provides protein-free samples, loss of analyte during protein precipitation is avoided. Enzymes are also excluded from the dialysate sample; thus, there is no further enzymatic degradation of the sample.
Optimization of Microdialysis Sampling
Probe Design, Surgery, Tissue Response
The small size of the dialysis probe, nominally 300 μm o.d. with an active window in the range 4–10 mm in length, causes minimal perturbation to the tissue. Therefore, the technique can be used in awake, freely moving animals, and the integrity of tissues, organs, and systems is maintained. Several different probe geometries have been developed to facilitate in vivo sampling from various sites. Those commonly used are shown in Figure 3. The designs are of two general types: parallel and serial. Parallel perfusion probes are constructed of rigid or flexible cannula material and may have either a side-by-side or concentric arrangement. Probes with rigid cannulae are especially suited for intracerebral sampling, whereas those with flexible cannulae work well for venous sampling. Serial perfusion probes include linear and loop styles. They are typically used for sampling soft peripheral tissue such as skin, muscle, tumor, and liver. The shunt probe suspends a linear probe within a larger tube, allowing sampling from flowing fluids (such as bile or blood) without permanent diversion of the fluid.1,2
Figure 3.
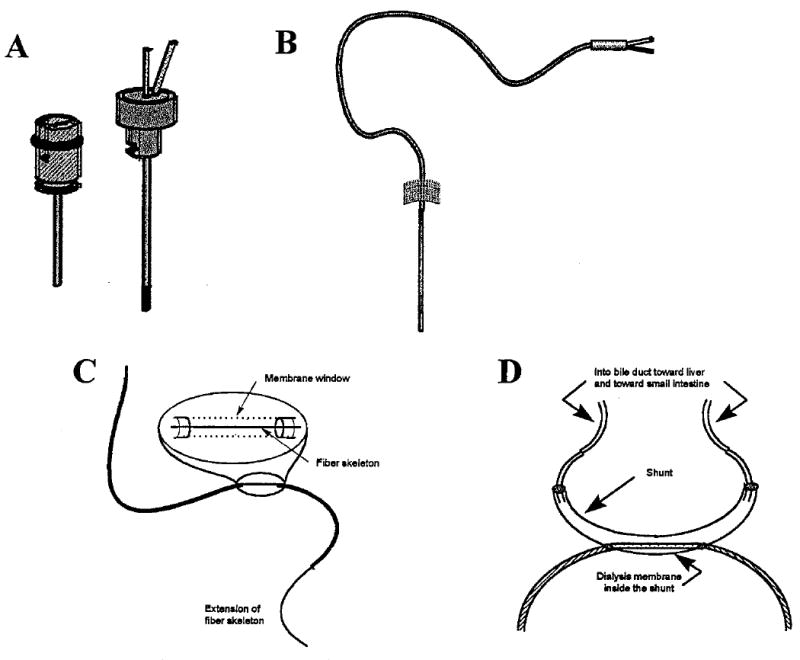
Microdialysis probe geometries (probes not drawn to scale). A. Intracerebral guide cannula and pin-style brain probe. B. Flexible probe for intravenous use. C. Linear probe for peripheral tissues. D. Shunt probe designed for sampling from bile duct of rat. (Used with permission of Bioanalytical Systems, Inc., West Lafayette, IN.)
Several modified probe designs have also been reported, including a spinal loop dialysis catheter for use in sampling the spinal intrathecal space of unanesthetized rats3,4 and a flexible intravenous probe for use with unanesthetized small animals.5,6 In 1995, Kanthan et al.7 described a method for microdialysis sampling in the human brain. This technique utilizes a Codman bolt kit, typically used to monitor intracranial pressures, and a custom-designed microdialysis probe with a probe sheath to accommodate variations in skull thickness. Using this system, five monoaminergic neurotransmitters7 and glucose-induced decreases in glutamate levels8 were monitored during acute focal ischemia.
Although the small size of the microdialysis probe causes minimal perturbation to the tissue, the surgery to implant the probe is invasive. Experimental animals are anesthetized during probe implantation, and the anatomical location of the target tissue dictates the duration of anesthesia and the severity of the surgical invasion. For example, probe implantation into the dermis or muscle is much less invasive to the animal than implantation in the liver or bile duct.
A few groups have examined tissue response to probe implantation and to indwelling probes. For intracerebral microdialysis, compromise of the blood–brain barrier (BBB) upon implantation of the guide cannula and/or probe must be considered. Benveniste and Hüttemeier9 and deLange et al.10 have reviewed the literature concerning BBB compromise and brain tissue response to probe implantation. One approach to evaluating the integrity of the BBB is to employ markers that do not normally cross the barrier. Several of these types of studies, which suggested that the BBB was generally intact within a short time after the surgery, are discussed in the reviews already mentioned. Other studies, however, have concluded that the BBB remains compromised for 24 h or longer after implantation takes place.11-13 A second approach used to assess changes in the BBB is to monitor alterations in the transport of a compound across the BBB after injection of compounds known to open the barrier. Again, conflicting results have been reported.12,14 A study by Allen et al.14 suggested that the rate of insertion of the probe into the tissue affects the amount of BBB damage and could account, to some extent, for the differing results.
Several publications from the laboratory of C. Lunte15-17 have reported the responses of different tissues to the presence of a microdialysis probe over time. The tissues examined include dermis,15 muscle and tumor,16 and liver.17 Dermis, muscle, and liver showed the expected acute inflammatory response, with the initial invasion of neutrophiles followed by macrophages. Tumor tissue showed little or no inflammatory response up to 72 h. Davies and Lunte17 tested probes of various designs in liver tissue and found that using a ‘needle’ with a diameter smaller than that of the membrane minimized the extent of cell damage.
Quantitation Issues and Extraction Efficiency
Microdialysis sampling is not typically performed under equilibrium conditions, so the concentration of the analyte determined in the dialysate is some fraction of its actual concentration in the surrounding sample matrix. The relationship between the concentration of analyte in the dialysate and that of the sample matrix may be thought of as the extraction efficiency (EE) of the probe. Among the parameters that influence EE are temperature, perfusate flow rate, chemical and physical properties of the dialysis membrane, probe geometry, membrane surface area, and properties of the analyte. The diffusion rate of the analyte within the matrix also affects EE. In vivo uptake into cells, metabolic rate, extent of tissue vascularization, and blood flow will influence diffusion through the tissue.18-20 Fortunately, under normal conditions of microdialysis sampling, these parameters remain constant; thus, a steady state is rapidly achieved although equilibrium is not established. Therefore, the EE of the probe for a given set of parameters is constant and the direction of net flux of the analyte across the membrane is determined by the concentration gradient of the analyte. In practice, the EE of the probe may be determined by recovery or delivery experiments.
Calibration of Microdialysis Probes
A major concern in the wider adoption of microdialysis sampling for pharmaceutical studies is the calibration of the microdialysis probes. Microdialysis is generally performed under nonequilibrium conditions where the recovery of a compound depends on many parameters. Recoveries ranging from <1% to >99% can be achieved. It is not always clear whether a high or low recovery is preferable. High recoveries are favored from the analytical perspective because higher concentrations are obtained. A high recovery also results in less depletion of the concentration around the probe because the situation is closer to equilibrium (i.e., the concentrations inside and outside the probe are the same). However, high recovery is typically achieved either using very slow perfusion rates or long dialysis membranes. Using a very slow perfusion rate leads to a loss of temporal resolution because of the relatively large sample volume requirement of most analytical methods. Higher recoveries can be obtained with large sampling windows; however, a loss of spatial resolution is the drawback. Low recoveries, on the other hand, result in less concentrated samples for analysis and a greater decrease in the analyte concentration around the microdialysis probe. However, these limitations may be offset by the fact that higher perfusion rates and shorter dialysis membranes can be used.
Although an in vitro determination of recovery provides a crude estimate of the behavior of a microdialysis probe with a given compound, extrapolating such a value to an in vivo experiment is impossible21-24 because the nature of the tissue to be sampled and its interactions with the analyte affect the recovery. For many experiments, accurate calibration of the microdialysis probe is not necessary. For example, if the desired information is the relative change in concentration brought about by some experimental manipulation, only the concentration independence and stability of the recovery need be known. On the other hand, if a relative distribution of a compound to various sites is the information desired, then the behavior of each probe used must be normalized versus the other probes. Only when an absolute concentration is needed must each probe be calibrated for an accurate in vivo recovery. As this is often the most difficult step in a microdialysis experiment, careful experimental design should be practiced. If the desired information can be obtained from relative rather than absolute concentrations, the calibration issue is greatly simplified.
Calibration for Hydrodynamic Systems
Systems in which the sample is a flowing fluid, for example the blood and bile, present a special case for microdialysis sampling. Mass transfer in the sample is dominated by diffusion through the probe membrane rather than by diffusion through the surrounding tissue for such systems.25 Diffusion must occur through a thin layer of stagnant fluid around the membrane. The thickness of this diffusion layer is a function of the velocity of the sample fluid. At low sample flow rates, the diffusion layer is thicker and diffusion through it is the rate-limiting step for microdialysis sampling. As the sample flow rate increases, the diffusion layer collapses and diffusion through the dialysis membrane begins to dominate. Eventually, diffusion through the dialysis membrane becomes the rate-limiting step, after which further increases in the sample flow rate do not affect the microdialysis process.
For hydrodynamic systems, an in vitro calibration can provide accurate recovery values if the perfusion flow rate and sample temperature are well controlled and the characteristics of the membrane do not change during the experiment. Because the membranes are hydrophilic and used in an aqueous environment, physical changes in the membrane are not likely. However, one must always be concerned that physiological responses to probe implantation may result in changes to the membrane environment. For example, there have been reports of the formation of clots around microdialysis probes implanted intravenously. Careful experimental design will usually prevent such problems, but one must always keep this possibility in mind.
Calibration in Tissues
A more difficult case is the use of microdialysis sampling in tissues, particularly those that are poorly perfused. It has been demonstrated that the recovery of a microdialysis probe depends on mass transport in three regions: the probe lumen, the dialysis membrane, and the sample medium.18,23,24 The first two regions can be characterized in vitro and controlled. Mass transport in the probe lumen is limiting only when using very low perfusion rates, whereas transport through the dialysis fiber is limiting only when transport through the sample is rapid. Rapid transport through the sample occurs in most hydrodynamic systems as well as in highly perfused tissues. However, in most tissues, transport through the tissue determines the recovery of the microdialysis probes.18,24 Under these conditions, calibration performed in vitro may not be valid in vivo. A number of approaches to the calibration of microdialysis probes in vivo have been described. The two most common methods involve (1) adding an internal standard to the perfusate, which is commonly known as “retrodialysis,” or (2) estimating the equilibrium condition by adding varying concentrations of analyte to the perfusate, which is known as the method of zero net flux. Alternatively, very slow perfusion rates can be employed, often circumventing the need for calibration.
Slow Perfusion
Justice et al.26 have shown that at perfusion rates of <50 nL/min, recovery is >95% for compounds with molecular weights <500 Da. At such a slow perfusion rate, the error introduced by assuming 100% recovery is insignificant. One drawback to this approach is the difficulty in the collection and analysis of the dialysate sample. Off-line analysis generally requires at least 2 μL of sample. The use of on-line systems, such as microdialysis coupled to capillary electrophoresis or capillary liquid chromatography, makes it possible to analyze nanoliter volumes of sample.
Retrodialysis
In the retrodialysis method, an internal standard is added to the perfusate and the rate of delivery of this compound to the tissue is measured during microdialysis sampling.27-30 The assumption is made that the recovery of the analyte is equal to the delivery of the internal standard. This method requires that the internal standard and the analyte behave in exactly the same manner. This requirement is usually met with regard to the diffusion coefficient of the two compounds, but is generally overlooked in terms of metabolism. Essentially, the internal standard and the analyte must be metabolized in an identical manner to provide a proper calibration of the probe. Of course, if this requirement were met, the internal standard could disrupt the experiment because it is being added to the system in relatively highly concentrations exactly at the site of sampling.
Method of Zero Net Flux
In the method of zero net flux, various concentrations of analyte are added successively to the perfusate.31,32 The amount of analyte either lost or gained during dialysis is then determined. If the perfusion concentration is greater than the in vivo concentration, a loss has occurred; if the perfusate concentration is less than the in vivo concentration, there has been a gain. Only when the perfusate concentration and the in vivo concentration match has there been no net change in concentration because the system is at equilibrium. A plot of the change in concentration of dialysate versus the initial concentration of perfusate is made; this should result in a straight line. The slope of this line is the inverse of the concentration recovery and the y-intercept is the actual sample concentration. At this concentration, there is no net diffusion of the analyte into or out of the microdialysis probe, because the concentration in the sample and in the probe lumen is the same.
The method of zero net flux is more accurate than retrodialysis in that the actual compound of interest is used for calibration; however, it is a very time-consuming process. The precision of this method is highly dependent on the precision of the individual concentrations determined and the number of perfusion concentrations used. Several hours–up to 12 in some cases–are required to collect sufficient data at the various concentrations of standard added to the perfusate to provide an accurate estimate of the equilibrium state. During this time, the concentration of the analyte in the sample must remain constant. This requirement limits the use of the method to the determination of basal levels of endogenous compounds and steady-state concentrations of drugs during constant infusion experiments.
Delivery
Determining the delivery of the actual compound of interest is a variation of retrodialysis that overcomes the uncertainty introduced by using a standard to mimic the analyte.15,33 First, the fact that the delivery is equal to the recovery is verified in vitro. This step serves to confirm the viability of the microdialysis probe. If the delivery and recovery are not equal for a given microdialysis probe, that probe is discarded. After the probe is implanted and the animal has recovered from surgery, the deliveries of the test compound and any other analytes of interest are determined prior to the start of the experiment. Finally, if possible, the deliveries are determined again after completion of the experiment. This procedure will verify any changes in the behavior of the probe during the course of the experiment. If the viability of the probe is tested prior to implantation, its behavior will not change during the course of the experiment within the constraints of tissue response, as was discussed previously. The shortcoming of this approach is that recovery changes resulting from the experiment are not detected. For example, administration of a vasodilator or constrictor can change recovery in a poorly perfused tissue by changing the blood flow. In general, changes in recovery as a result of experimental manipulation are more common when studying endogenous than exogenous compounds.
The various calibration methods are summarized in Table 1. Before undertaking in vivo calibration, the experimentalist should consider what information is desired from the microdialysis experiment. The need for in vivo calibration will generally fall into one of three categories. In the first, two states (such as basal versus excited or formulation A versus formulation B) are compared. In these cases, changes in analyte concentration usually provide sufficient information. Here, one does not need an exact EE value but, rather, a constant EE over the time course of the experiment. In this case, the behavior of the probe can be tracked using retrodialysis. In the second category, one needs to know only the order of magnitude of the analyte concentration in ECF. In other words, is the analyte present at 10 ng/mL or 100 ng/mL? In vitro calibration may be sufficient because it usually differs by not more than two or three times the in vivo value. If the EE is 60% in vitro and 30% in vivo, the concentration of analyte in the in vivo dialysate corrected for the in vitro EE will be within the order of magnitude of the analyte concentration in ECF. In the third category, where an accurate in vivo concentration is required, the in vivo EE must be determined for the analyte in the target tissue. The approaches that can be used to obtain this information include very low flow rates, zero net flux, and in vivo delivery of the analyte.
Table 1.
Approaches to In Vivo Calibration of Microdialysis Probes
| method | description | considerations | reference |
|---|---|---|---|
| extrapolation to zero flow rate | An estimate of analyte concentration surrounding the probe is obtained by extrapolating to zero flow rate, where analyte concentration in the tissue and in the probe would presumably be in equilibrium. | Requires determining the recovery of the probe at several flow rates with the analyte at a steady-state concentration in the matrix throughout the determinations. | 35 |
| use of very low flow rate | This extension of the aforementioned method carries out microdialysis sampling at a flow rate (ca. 50 nL/min) where the probe recovery is >95% so that assuming 100% extraction efficiency introduces negligible error. | Practical considerations include long sampling intervals to collect sufficient volume for analysis and problems manipulating small-volume samples such as evaporation. | 26 |
| zero net flux | With the analyte at a steady-state concentration in the tissue, the probe is perfused with a succession of solutions containing known concentrations of the analyte. A plot of the change in perfusate concentration versus the initial perfusate concentration should be linear with the slope being the inverse of the probe recovery and the y-intercept being the point of zero net flux; that is, the concentration of the analyte in the tissue. | Tissue concentration of the analyte must remain at steady state, whereas the EE of the probe is determined for several different initial analyte concentrations in the perfusate. | 31, 32 |
| retrodialysis | Uses the in vivo delivery of an internal standard (usually an analog or mimic of the analyte) in the perfusate as the basis for probe calibration. The method assumes that the in vivo recovery of the analyte has a constant known relationship to the in vivo delivery of internal standard. In some cases that relationship is determined in vitro and assumed to hold in vivo. | The advantage of this method is convenience, especially in terms of time. The experimentalist should consider that, in a biologically active matrix, delivery of an internal standard that binds to receptors or is metabolized in the same way as the analyte will introduce unwanted perturbations in the tissue area being sampled. | 27-30, 36 |
| in vivo delivery of the analyte | A variation of retrodialysis that overcomes the uncertainty introduced when an analog is used as the internal standard. Essentially, the in vivo delivery of the analyte to the target tissue is determined before the actual experiment. If possible, the delivery is determined again after completion of the experiment to confirm that the probe behavior has not changed. | The method is simple, convenient, and has a minimal time requirement. This method assumes that the extraction efficiency of the probe in the tissue of interest is constant regardless of the direction of the concentration gradient of the analyte. The assumption should be validated for analytes and tissues of interest. | 15, 37 |
Analytical Considerations
Several features of microdialysis sampling are of considerable importance in the development of the analytical method. Because of the low flow rates employed, microdialysis typically results in small sample volumes of 1–10 μL. The process also inherently dilutes the samples as they are collected. Therefore, small volume samples, often with low analyte concentration (1 pM-1 μM), are typical and present a considerable challenge to the analyst.
From the analytical perspective, there are two primary considerations with respect to extraction efficiency. First, except in cases of local delivery via the probe, the dialysate concentration will be less than the actual tissue concentration of the analyte. Thus, the limit of detection must be less than the lowest in vivo concentration expected. Second, extraction efficiency increases as perfusion rate decreases. The slower the perfusion rate, the closer the dialysate concentration of the analyte will be to that in the sample matrix surrounding the probe.34
Increased temporal resolution is one of the advantages of microdialysis over blood sampling and traditional tissue sampling. For traditional blood sampling, the total fluid that can be withdrawn is limited, so the number of points that can be obtained is small. In traditional tissue sampling, several animals must be sacrificed at each time point, and, thus, the frequency of time points is usually small to minimize the total number of animals used in each study. With microdialysis sampling, the same type of study can be conducted using only a few animals and, because there is no fluid loss or need to remove tissue, data can be collected continuously. The animal can also serve as its own control.
The temporal resolution of a microdialysis study is defined by the time interval at which the microdialysis samples are collected; it is dependent on the sensitivity and sample volume requirements of the analytical method as well as the recovery of the probe. Microdialysis provides a continuous flow of sample, whereas most analytical systems require discrete samples. Individual samples can be collected off-line with a fraction collector, but the sample volumes are very small due to the low perfusate flow rates used (typically 1 μL/min or less). If the analytical system requires a larger sample for injection or the method is not sensitive and requires a preconcentration step, larger volumes may be collected, albeit at the expense of temporal resolution. Overall, the analytical method with the lowest detection limit and smallest sample volume requirement affords the best temporal resolution for microdialysis experiments.
Another approach to overcoming problems associated with handling small volume samples is on-line coupling of the microdialysis system with the analytical system. This method minimizes the delay between sample collection and analysis and eliminates the problems of evaporation that can occur during any physical manipulation of small volume samples. Near real-time data on physiological events can be obtained, but the temporal resolution achieved by the coupled system will depend on the time needed for analysis (i.e., the analysis time must be less than the duration of the physiological event). Reviews of on-line coupling of microdialysis with LC and microseparation techniques are available.38,39
Three-Way Trade-Offs
When microdialysis sampling is applied in vivo, three previously independent systems become interlinked: the animal, the microdialysis sampling system, and the analytical system. The experimentalist must be aware that once these systems are linked, the conditions that were optimal for each element independently must now be considered in relation to the others. Frequently, the sample volume requirement of the analytical method necessitates increasing the microdialysis perfusion rate, which in turn lowers the extraction efficiency of the probe, thus providing samples with lower concentrations of analyte. Using a lower perfusion rate to increase probe efficiency and analyte concentration results in longer sampling times. Some degree of temporal resolution is lost through this compromise. In addition, the increased recovery may deplete compounds of low molecular mass in the tissue adjacent to the probe, in turn perturbing the biological system. The anatomical location and spatial resolution needed for obtaining the desired information influence the probe design and active window length. Although implantation of a microdialysis probe may cause little disruption of the target organ, the necessary anesthesia and extent of the surgical procedure also impact the biological system. The successful use of microdialysis sampling in vivo will depend on achieving a suitable balance among these systems.
The trade-offs among perfusate flow rate, concentration detection limit, and sample volume requirement will decide the temporal resolution that can be achieved for the experiment. A clear statement of the experimental question should dictate the balancing of the microdialysis sampling and analytical method parameters.
Analytical Methodologies
One of the main advantages of microdialysis sampling is that it can be used in conjunction with a wide range of analytical techniques. Non-separation-based methods can be used to monitor one analyte at a time, and separation-based methods allow the detection of multiple analytes in each sample. This section will provide a brief overview of the considerations that must be heeded when coupling microdialysis sampling to various analytical techniques. Specific references will also be given in cases where the use of the analytical technique or the method of coupling to microdialysis sampling is novel.
Non-Separation-Based Sampling Approaches
In an attempt to further understand the complex nature of biological events, the idea of making real-time analytical measurements has attracted much attention. These types of measurements are achievable when using a continuous detection device, such as a biosensor. Microdialysis provides a possible alternative to implantable sensors. When online biosensors are used with microdialysis sampling, the apparatus typically consists of a syringe pump, a microdialysis probe, and a sensor or an enzyme reactor, followed by an electrochemical detector. The enzyme is present to provide specificity and to generate a redox-active species that can be detected electrochemically.
Although the response time of an on-line biosensor system coupled to microdialysis sampling is slower than that of an implantable modified microelectrode, the former is more quantitative and provides a higher conversion of analytes, a longer lifetime, and easier calibration. One disadvantage of biosensors is fouling of the electrode, usually by proteins. Coupling microdialysis sampling with a biosensor overcomes this problem because of the size-exclusion nature of the membrane. In vivo implantation of biosensors places size restraints on the dimensions of the electrode. Using a microdialysis probe in vivo reduces these restrictions on the biosensor.
One example of this methodology is the monitoring of extracellular l-glutamate with a microdialysis-based online biosensor subsequent to stimulation of cultured nerve cells.40 A sensitivity of 24.3 nA/μM and a limit of detection of 7.2 nM for l-glutamate was reported with this system. In vivo levels of l-glutamate were also continuously monitored using microdialysis coupled to an enzyme-amperometric biosensor following cardiac arrest and K+-induced local depolarization.41 A limit of detection of 0.5 μmol/L was reported. In both of these examples, glutamate oxidase was employed to oxidize l-glutamate in the presence of oxygen, generating hydrogen peroxide, which was detected electrochemically. Similar sensors were applied to the real-time monitoring of lactate in the subcutaneous tissue of rabbits and humans through the use of microdialysis sampling and a lactate amperometric sensor;42 glucose was monitored continuously with a glucose-oxidase-immobilized enzyme reactor.43
However, biosensors are not always used for continuous monitoring. Using injection valves, discrete microdialysis samples were injected into flow injection systems and analyzed with amperometric-based biosensors for l-lactate44 and glucose.45 A miniaturized flow injection thermal biosensor coupled with microdialysis sampling has been reported for the determination of glucose in subcutaneous fluid with a sampling rate of 42 samples/h.46 Advantages of a flow injection analysis system are the ability to provide for on-line sample dilution, required when analyzing concentrated samples, and the capacity to periodically recalibrate the biosensor with standard solutions. Other non-separation-based methods without continuous monitoring have been reported, including immunoassays47-49 and mass spectrometric analyses.50,51 On-line interfacing of microdialysis and mass spectrometry (MS) has been reported for the analysis of tris(2-chloroethyl) phosphate in rat plasma,52 along with the utilization of microdialysis for on-line sample cleanup of nucleic acid53 and protein and peptide samples.54
Separation-Based Approaches
Microdialysates consist primarily of relatively small hydrophilic analytes in highly ionic aqueous samples. In general, these characteristics have made liquid chromatography (LC) the analytical method of choice to couple to microdialysis sampling. Reversed-phase or ion-exchange are the modes of liquid chromatography that are most compatible with direct injection of aqueous microdialysis samples. The mode of chromatography chosen for analysis is dependent on the physiochemical properties of the analyte. The type of column used (length, particle size, and internal diameter) is determined by the sampling interval desired and the required sensitivity. In a typical LC assay, 5–10 μL of sample are needed, which means that the temporal resolution is 5–10 min if a perfusion flow rate of 1 μL/min is employed. If lower microdialysate flow rates are used to increase recovery, temporal resolution is further decreased. Applications of on-line coupling of microdialysis with LC have been reviewed elsewhere.38,39
Capillary electrophoresis (CE) is another separation method that can be used for the analysis of microdialysis samples. The low sample volume requirement of CE is compatible with the small volumes generated by microdialysis sampling. Typical injection volumes in CE are 1–10 nL. However, 1–5-μL samples are generally required because of difficulties associated with the physical manipulation of submicroliter volumes, in particular, problems with surface tension and evaporation.
One disadvantage of CE is its incompatibility with high ionic strength samples. In CE the optimal procedure to achieve stacking, or compression of the injection zone, is to prepare the sample in an injection buffer that is 10-fold more dilute than the background electrolyte. The high ionic strength of the microdialysis sample causes anti-stacking and, therefore, lower detection sensitivity. In contrast, LC is amenable to high ionic strength samples.
There have been several reports of the on-line coupling of microdialysis and capillary electrophoresis with UV55 and laser-induced fluorescence (LIF) detection.56-58 The first reported coupling of microdialysis with CE and LIF detection involved the separation of an investigational antineoplastic, SR 4233, from its main metabolite, SR 4317.55 Figure 4 shows a schematic of the on-line microdialysis/capillary electrophoresis system employed in this study. Resolution of the two compounds was achieved in <60 s, with an overall temporal resolution of 90 s. During the on-line study, a 4-mg/kg intravenous injection of SR 4233 was administered to the rat. A pharmacokinetic curve (Figure 5) was then constructed, and a 15.3 ± 1.0 min half-life of elimination and 1.1 ± 0.2 min half-life of distribution were determined for SR 4233. Another method of detection for use with the on-line coupling of microdialysis and CE that has been explored in our laboratory is electrochemical detection.59 This detection system provides higher sensitivity than does ultraviolet (UV) detection and is comparable to LIF. Furthermore, the number of potential analytes that can be studied at high sensitivity is greatly increased because more compounds are electroactive than are naturally fluorescent.
Figure 4.
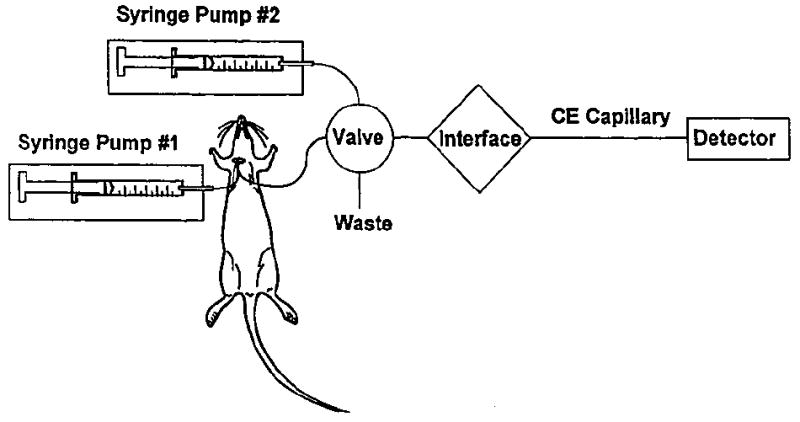
Schematic of the on-line microdialysis/capillary electrophoresis system used for the analysis of SR4233.56
Figure 5.
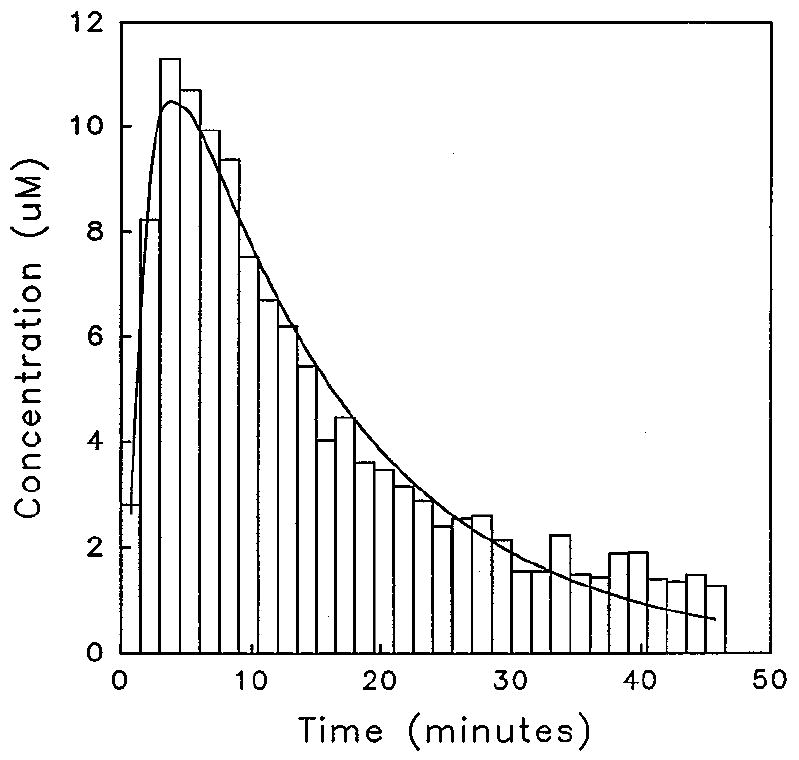
Pharmacokinetic curve following a 4-mg/kg ip dose of SR4233 obtained using an on-line microdialysis/capillary electrophoresis system.56
Scope of Applications
As the number of research studies incorporating microdialysis sampling has continued to increase, secondary literature has appeared in the form of books60 and review articles.38,39,61-65 An exhaustive examination of the microdialysis literature is beyond the scope of this review. Many of these studies involve monitoring levels of endogenous compounds in response to the administration of drugs or other stimuli. Others report monitoring of the parent compound and/or metabolites in the dialysate. The small size and minimal invasiveness of microdialysis probes make the technique ideal for simultaneous monitoring in more than one organ or tissue. In the following overview of applications, we have focused on presenting a few examples to illustrate the breadth of possibilities for using microdialysis sampling in pharmacokinetic and metabolism studies.
In Vitro Studies
Microdialysis sampling does not change the net fluid balance of the surrounding sample matrix and provides clean samples in which analytes are separated from further enzymatic action. Thus, microdialysis is useful for in vitro drug development and evaluation applications. A series of reports on in vitro applications of microdialysis sampling has been published. These applications include tablet dissolution testing with both single and multiple vessel configurations,66,67 erythrocyte membrane partitioning studies,68 and enzyme kinetic studies.69 Gunaratna and Kissinger70 used microdialysis to follow metabolism kinetics in liver microsome incubations. In this application, an in vitro microdialysis system, shown in Figure 6, was used to monitor the metabolic profiles of three metabolites of diazepam following its incubation in liver microsomes (Figure 7). Several studies have been published in which microdialysis sampling was compared to traditional methods for determining drug binding to plasma proteins.71-73 More recently, Yang and Elmquist74 demonstrated the feasibility of determining the plasma binding of a highly lipophilic drug, cyclosporin A, using microdialysis sampling.
Figure 6.
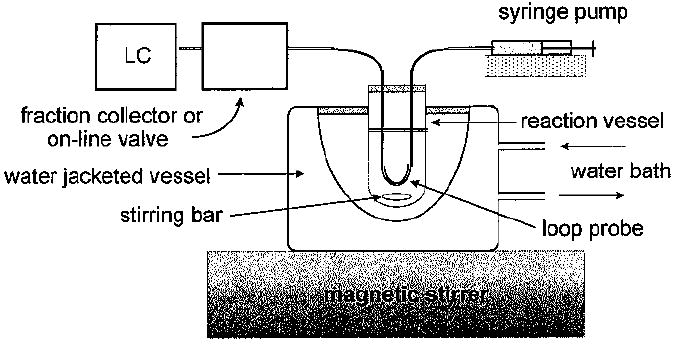
In vitro microdialysis sampling system for microsome or similar incubation process.70
Figure 7.
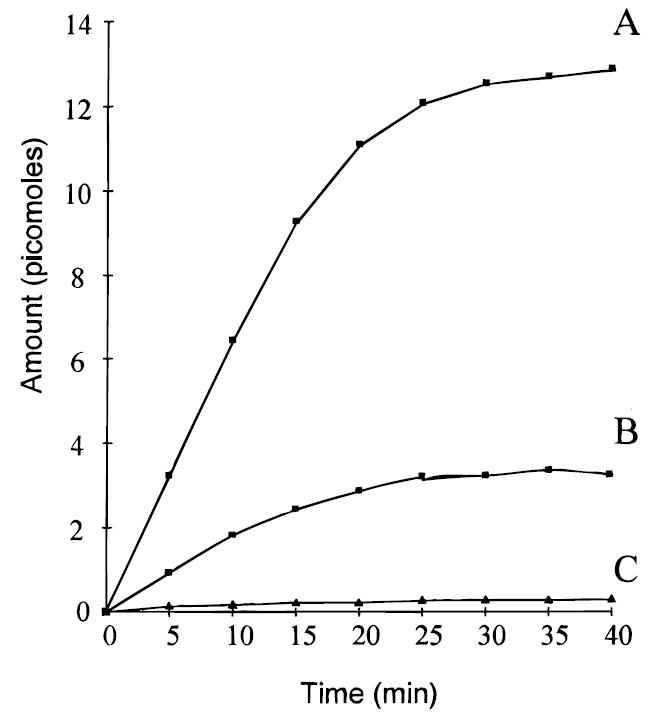
Metabolic profiles of diazapam metabolites sampled by microdialysis from liver microsome incubation mixture. Diazepam concentration was 145 M: (A) temazepam; (B) N-desmethyldiazepam; (C) oxazepam.70
Several researchers have employed in vitro microdialysis sampling to minimize sample preparation when monitoring fermentation and related bioprocesses. Mannino et al.75 used an on-line microdialysis system to provide clean samples from milk products. They detected sugars amperometrically at a chemically modified electrode. Torto and co-workers76 have demonstrated the utility of in situ microdialysis sampling for monitoring bioprocesses without perturbation of the process. Microdialysis sampling coupled on-line to LC with pulsed electrochemical detection was used to monitor an enzymatic hydrolysis that produced a mixture of oligosaccharides. Using the same system, Torto et al.77 evaluated three microdialysis sampling modes with respect to their suitability for quantitative on-line monitoring of bioprocesses in small-volume (5 mL) bioreactors. They concluded that stopped-flow microdialysis modes were advantageous for small-volume reactors to avoid significant depletion of the analyte.
In Vivo Studies
Brain
Microdialysis sampling has been used to study a wide variety of endogenous and exogenous analytes in brain. The large number of papers published on this topic is beyond the scope of this review, so a brief overview of recent work from the laboratory of S. Lunte will be presented.
Tryptophan metabolism along the kynurenine pathway has been one area of interest in our research group. This pathway is responsible for approximately 90% of tryptophan metabolism in mammals and yields the formation of kynurenic acid, an endogenous glycine site NMDA receptor antagonist. Malone et al.78 utilized CE with electrochemical detection for the determination of tryptophan and kynurenine in brain microdialysates. In that study, an intraperitoneal injection of tryptophan was administered to an anaesthetized rat, and the levels of tryptophan and kynurenine in rat hippocampus were monitored for 6 h by microdialysis sampling. A three- to fourfold increase in dialysate tryptophan levels was observed, resulting in a maximum concentration of 2–3 μM tryptophan at approximately 90 min after ip administration. Sixty minutes after dosing, kynurenine was found to reach a maximum concentration of 1.3 μM. It was concluded that the high sensitivity and low volume requirements of CE with electrochemical detection allowed good temporal resolution and high recoveries to be realized. Kynurenic acid, however, cannot be effectively detected electrochemically; therefore, alternate methods for its determination were investigated. Capillary electrophoresis with LIF detection following zinc complexation was evaluated for monitoring the levels of kynurenic acid in brain microdialysis samples.79 Kynurenic acid is essentially unable to penetrate the BBB; therefore, its presence in brain is dependent on its bioprecursor kynurenine, a major tryptophan metabolite. In this study, the increase in hippocampal kynurenic acid was monitored following a systemic injection of one of its bioprecursors, tryptophan or kynurenine. One of the greatest potential advantages of this methodology compared with those currently in use is its increased temporal resolution. Microdialysate samples could be analyzed every 10 min rather than the 30–60-min sampling intervals used with LC.
Another project involved the investigation of substance P metabolism in brain using microdialysis sampling followed by CE with postcolumn derivatization and laser-induced fluorescence detection.80 A microdialysis probe implanted in the striatum of an anesthetized rat was perfused with substance P, and dialysate samples were analyzed for substance P and its metabolites. By introducing substance P into the brain via the microdialysis probe, metabolite formation in the immediate vicinity of the probe could be monitored.
Ocular
One of the major constraints in developing improved intraocular drug delivery systems has been the lack of information concerning the uptake, disposition, and elimination of drugs from the eye.81 Ocular microdialysis is one technique that could provide such information; however, few studies using this method have been reported.
Continuous sampling of drugs in the vitreous humor of anesthetized rabbits has been reported.81 This study involved the pharmacokinetic analysis of ganciclovir and acyclovir using a concentric microdialysis probe implanted in the vitreous humor. Ganciclovir is a purine nucleoside analog approved for the treatment of cytomegalovirus retinitis.
Ganciclovir or acyclovir was given as an intravitreal injection (100 mL) following implantation of the guide cannula. The microdialysis probe was then inserted into the guide cannula, and dialysates were collected every 20 min. It was found that the initial equilibration phase was over within the first 4 h, and that the microdialysis technique did not result in a breakdown of the blood–retinal barrier. The short vitreous half-lives of ganciclovir (2.62 ± 0.44 h) and acyclovir (2.98 ± 0.24 h) in albino rabbits suggest a transretinal mechanism of vitreous clearance for these compounds. In pigmented rabbits, however, the rate of elimination increased to 8.63 and 5.59 h for ganciclovir and acyclovir, respectively, as a result of binding to melanin. This research indicates that ocular microdialysis can be useful in assessing the vitreous concentrations of drugs and providing information on the uptake, disposition, and elimination of drugs from the eye.
Transdermal
Traditionally, transdermal drug delivery has been studied in vitro using isolated skin preparations; however, with the use of microdialysis sampling, in vivo studies can be performed.15 In one such study, intradermal microdialysis was used to investigate the transdermal absorption of cyclosporin in rats.82 Cyclosporin is an immunosuppressive agent that has been shown to be beneficial in treating several dermatological diseases. Due to the toxic effects found with systemic administration, topical application has been explored, but it is limited because of the poor ability of cyclosporin to penetrate the dermis. In this study, the use of an absorption enhancer, 1-[2-(decylthio)ethyl]azacyclopentan-2-one (HPE-101), and glycerin to increase the transdermal absorption of cyclosporin was evaluated. HPE-101 and glycerin were both found to significantly enhance the absorption of topically applied cyclosporin in rat skin. The authors concluded that utilizing an in vivo microdialysis method obviates the need for concern about hydration and microbial growth as with in vitro methods using excised skin.
Muscle
Two recent publications have focused on the use of microdialysis sampling correlated with traditional blood sampling to devise models for predicting the free concentration of drugs in muscle tissue from the plasma levels. Nolting and others83 monitored piperacillin, a β-lactam antibiotic, in muscle tissue by microdialysis while collecting serial blood samples. They concluded that microdialysis sampling was suitable for determining concentrations of unbound drug in tissue. Kovar et al.84 also used microdialysis sampling in muscle tissue to compare free tissue levels of ceftriazone, a highly bound drug, with free plasma levels determined from blood samples. Free levels were much higher in plasma than in muscle. To accurately predict free tissue levels of ceftriazone from plasma levels, the model required factors to account for nonlinear protein binding.
Adipose
Felländer and associates85 examined the effect of α- and β-adrenoceptor blocking agents on lipolysis in human adipose tissue using microdialysis sampling during surgery. They found that in humans both α- and β-adrenogenic mechanisms modulated the response of adipose tissue to surgical trauma. In another study of lipolytic activity, Darimont et al.86 used in vivo microdialysis sampling in rat adipose tissue. The effect of moxestrol, a potent estrogenic analog, on lipolysis was monitored by measuring extracellular glycerol in parametrial fat pads of estrus, diestrus, and ovariectomized animals.
Blood
The possibility of producing protein-free samples and conducting long-term continuous sampling without fluid loss is especially attractive for microdialysis sampling from blood vessels. In addition to commercially available venous probes, flexible probes for sampling from blood have been constructed by Telting-Diaz et al.87 and Evrard et al.5
Nakashima and colleagues88 applied microdialysis sampling to the study of in vivo plasma protein binding of valproate. Microdialysis probes were implanted in the femoral veins of rabbits prior to dosing the animals with valproate. Whole blood samples were collected simultaneously and prepared for analysis of the free fraction using ultrafiltration. No difference was found in the elimination half-lives determined from microdialysis and from blood sampling. Further, they found that in vivo dialysate concentrations could be corrected for probe efficiency using recovery values obtained from in vitro experiments performed in stirred rabbit plasma at 37 °C.
The monitoring of clinically relevant analytes in the blood of critically ill patients provides information vital to management and therapeutic decisions in their care. Rabenstein et al.89 adapted the bile shunt probe introduced by Scott and Lunte1 to obtain microdialysis samples from central vein catheters in greyhounds. The microdialysis samples and blood samples were analyzed for glucose and lactate. In a related study, the same group used their probe system with a biosensor array to determine glucose and lactate.2
Tumor
The systemic concentration-time profile of a drug does not necessarily reflect its profile in the extracellular space of tissues. Antineoplastic drugs, because of their especially high cytotoxicity, and tumor tissue, being poorly perfused, are a case in point. A microdialysis probe implanted directly in tumor mass can monitor actual tissue levels of the drug and provide information concerning in-tumor metabolism, if any, of the drug. For example, Palsmeier and Lunte16,33 applied microdialysis sampling to the investigation of the disposition and metabolism of SR-4233, a benzotriazine compound with preferential cytotoxicity for hypoxic tissue. They found that, using a systemic dose, much less drug reached the tumor than reached healthy muscle tissue. However, when the same amount of drug reached both tumor and muscle, which was accomplished by delivering the drug via a probe, the levels of reductive metabolites formed were significantly higher in tumor than in muscle.
Other oncological applications of microdialysis sampling include monitoring of carboplatin in cutaneous melanoma and in subcutaneous space to evaluate the portion of the dose reaching the cancerous tissue,90 methotrexate transport in brain tumors,91 and the influence of an angiogenesis inhibitor on the uptake of temozolomide.92
Transplacental
Ward and Pollack93 used microdialysis sampling to monitor the effect of methanol on uteroplacental blood flow. The microdialysis probe was implanted through the uterine wall into the amniotic fluid of near-term pregnant rats. Methanol was administered as an iv bolus dose or an iv infusion over several hours. In addition to producing data suggesting that maternal exposure to methanol may produce hypoxia in fetuses, their work demonstrated the value of microdialysis sampling as a tool for investigating the flux of compounds across placenta.
Bile
A shunt probe design introduced by Scott and Lunte1 provides microdialysis samples from the bile duct of rats. Briefly, the microdialysis probe is suspended inside a tube that is implanted as a bile duct shunt. The shunt directs bile flow past the microdialysis membrane, then returns the bile to the duct prior to its entry into the small intestine. Because bile flow is maintained, continuous monitoring of compounds excreted into the bile is possible while maintaining enterohepatic circulation.
Scott and Lunte1 monitored phenol and its metabolites in bile using the shunt probe. Hadwiger et al.94 obtained pharmacokinetic profiles and parameters for tacrine and its hydroxylated metabolite in bile using the shunt probe design. Gunaratna and colleagues95 demonstrated the use of this probe design in awake animals, choosing acetaminophen and its metabolites as the analytes.
Liver
Ischemia/reperfusion injury involves reactive oxidant species that are difficult to measure directly. Layton and colleagues96 monitored two antioxidant species, uric acid and ascorbic acid, by microdialysis sampling in the liver of anesthetized rats during ischemia and reperfusion. As expected, the extracellular levels of both anti-oxidants decreased during and immediately following ischemia. High ascorbic acid levels were noted immediately after implantation of the microdialysis probe; however, this initially elevated level decreased to a stable basal level after 90 min.
Various drug delivery approaches are being evaluated for their ability to increase therapeutic effectiveness while reducing side effects associated with systemic doses. Sato et al.97 found microdialysis sampling useful in evaluating microfibrous collagen sheets for local drug delivery. They studied the delivery of etoposide to liver tissues in rats in the presence and absence of cyclosporin A. Etoposide with or without cyclosporin A, a P-glycoprotein-mediated transport inhibitor, was incorporated in a microfibrous collagen sheet applied to the surface of the liver lobe over the site of the microdialysis probe. The microfibrous collagen sheet maintained drug concentrations in the liver with only low concentrations observed in plasma. Inclusion of cyclosporin A further prolonged the drug concentration in liver tissue.
Simultaneous Multiple-Probe Studies in the Same Organ
Malagié et al.98 compared the effect of a combination treatment of WAY 100635 (a 5-HT1A receptor antagonist) and fluoxetine (a 5-HT reuptake inhibitor) on two brain regions simultaneously. Microdialysis probes were implanted in the frontal cortex and ventral hippocampus prior to iv administration of either saline or WAY 100635, followed by ip administration of fluoxetine or saline. The 5-HT levels were monitored simultaneously in the cortex and hippocampus. Fluoxetine alone did not change the extracellular 5-HT levels in either region studied. When fluoxetine and WAY-100635 were coadministered, 5-HT levels in the frontal cortex but not the hippocampus increased by >200% compared with pre-dosing levels.
The spatial resolution of microdialysis sampling can provide information not readily accessible by other methods. Davies and Lunte99 demonstrated this in a study of phenol metabolism in liver tissue. Three linear microdialysis probes placed in different regions (anterior, middle, and posterior) of the median lobe of rat livers revealed differences in metabolic profiles for the regions following iv administration of phenol (Figure 8). The levels of the conjugated metabolites phenol-glucuronide and glutathionyl-hydroquinone were significantly lower at the anterior position than at other positions, even though the levels of phenol reaching each region were the same (Figures 9 and 10).
Figure 8.
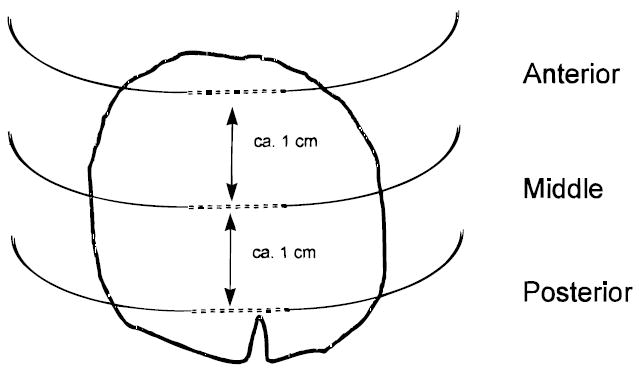
Placement of microdialysis probes implanted into the median lobe of the liver (viewed from the ventral surface) used to monitor phenol metabolism.37
Figure 9.
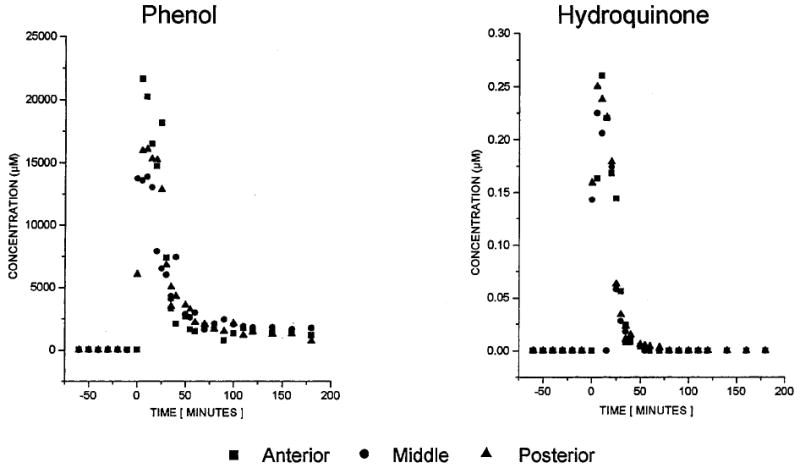
Typical concentration–time course for phenol (left) and hydroquinone (right) during and after a 20-min iv infusion of phenol (administration corresponding to 0–20 min).37
Figure 10.
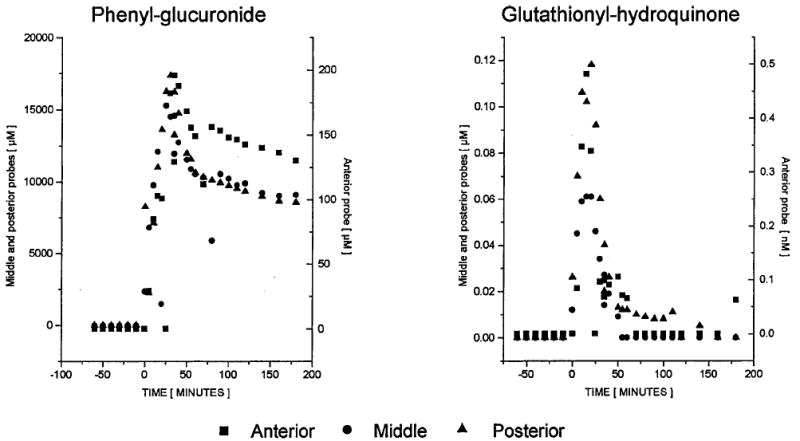
Typical concentration–time course for phenyl-glucuronide (left) and glutathionyl-hydroquinone (right) during and after a 20-min iv infusion of phenol (administration corresponding to 0–20 min). Note: scale for the anterior probe is the right-hand axis.37
Simultaneous Multiple-Probe Studies in Different Organs
Several studies have used simultaneous microdialysis sampling to study drug penetration of the BBB. Simultaneous microdialysis sampling from brain and blood was used by Malhortra et al.100 to determine the penetration of an experimental NMDA antagonist into brain tissue. They found good agreement between the microdialysis-based pharmacokinetic parameters and those determined using conventional blood sampling. Nakashima et al.101 evaluated simultaneous microdialysis sampling from blood and brain as a method for determining relative pharmacokinetics and metabolism of l-DOPA in the presence and absence of aromatic amino acid decarboxylase inhibitors. They found in vivo microdialysis to be a simple, reliable technique for the simultaneous determination of pharmacokinetics and metabolism in a target tissue and systemic circulation.
Other dual-site microdialysis sampling experiments have simultaneously examined l-DOPA in blood and muscle,102,103 SDZ ICM 567 (a 5-HT3 receptor antagonist) in blood and muscle,104 lactate in muscle and adipose tissue in relation to insulin action,105 and antibiotics in human muscle and subcutaneous space.106 Reports of simultaneous microdialysis sampling from three sites have also been published. These include disposition of phenol in bile, blood, and liver;1 distribution kinetics of carbamazepine in brain, blood, and liver;107 aluminum chelation by hydroxypyridinones in blood, liver, and brain;108 methotrexate levels in kidney, liver, and muscle;109,110 and lactate and xanthines in blood, liver, and small intestine during endotoxic shock.111
Multiple-site microdialysis sampling has proven useful in the investigation of pharmacokinetic drug–drug interactions.112 Ekstrøm et al.112 placed microdialysis probes in the blood, liver, and kidney of anesthetized rats to examine the interactions of methotrexate and the non-steroidal antiinflammatory drug (NSAID) naproxen. When animals were pretreated with naproxen, approximately a twofold increase in the areas under the curve (AUCs) of time versus concentration for methotrexate and 7-hydroxymethotrexate, the major metabolite, was found in all three tissues.
Conclusions
Quantitation or calibration of microdialysis probes and the challenge of analyzing the small volumes typical of microdialysis sampling are issues that must continue to be investigated. For microdialysis data to be quantitative, calibration of the probe is necessary. Approaches such as extrapolation to zero flow rates, the use of very low perfusion flow rates, and zero net flux tend to be time consuming. Retrodialysis and in vivo delivery are less time consuming; however, validation of these methods prior to initiation of the microdialysis study is necessary. In general, for applications where absolute tissue concentrations are required, the time involved in calibrating the probe can overshadow many of the other advantages of microdialysis sampling.
Another problem commonly encountered arises from the fact that the small sample volumes obtained from microdialysis sampling are usually less than that required for traditional analytical methodologies such as liquid chromatography. To obtain identical temporal resolution using standard separation methods, it is possible to increase the sample volume by increasing the microdialysis perfusion rate. However, this method decreases the concentration extraction efficiency of the probe. One solution to this problem has been the use of analytical systems with lower sample volume requirements, including microbore LC and capillary electrophoresis. Other approaches have employed preconcentration and stacking methods for LC and CE to overcome the problems of low concentration samples associated with using higher microdialysis perfusion flow rates.
Researchers have shown the feasibility of implanting microdialysis probes in a number of tissues; however, little has been published concerning the physiological response of the tissue to this probe implantation or the way in which tissue response may ultimately affect the pharmacokinetics or metabolic activity. Advances in probe design and dialysis membrane materials that result in less tissue damage as well as biocompatible materials for the construction of microdialysis probes continue to be of interest.
The simultaneous use of multiple probes is another area that has not yet been fully explored. This approach would permit investigations of tissue distribution in near real time while reducing the total number of animals necessary for a study in comparison with traditional methods. The challenge in multiple-probe experiments is preventing the tangling of numerous fluid lines. Multi-channel swivel systems exist for this purpose, but the channel volumes and resistance to turning increase with each additional channel. An alternative to multi-channel swivel systems is the use of a turntable to counter the animal’s rotations.113 These types of studies have become more practical with the commercial availability of swivel-free animal containment systems, such as the Raturn system from Bioanalytical Systems, Inc.
The capacity of microdialysis, performed in vivo, to provide continuous sampling over several days without detriment to the fluid volume of the animal, and the possibility of obtaining such samples simultaneously from multiple sites have the potential for significant reductions in the number of animals necessary for pharmacokinetic and distribution studies. When this technique is coupled on-line to a rapid LC or CE separation method, near real-time data can be obtained. Overall, the increasing application of microdialysis sampling to tissues other than brain, which has been demonstrated in the last decade, is likely to continue, especially as advances in analytical methods for small volume samples and suitable techniques for probe calibration are developed and refined.
Acknowledgments
Financial support from the National Institutes of Health (SBIR Grant R43-GM52272 with Bioanalytical Systems; RO1) is gratefully acknowledged. The authors thank Bioanalytical Systems, the Kansas Technology Enterprise Corporation, and the Center for Bioanalytical Research at the University of Kansas for their support, and also acknowledge the assistance of N. Harmony in editing and preparing the manuscript.
References and Notes
- 1.Scott DO, Lunte CE. In vivo microdialysis sampling in the bile, blood, and liver of rats to study the disposition of phenol. Pharm Res. 1993;10:335–342. doi: 10.1023/a:1018971818689. [DOI] [PubMed] [Google Scholar]
- 2.Dempsey E, Diamond D, Smyth MR, Malone MA, Rabenstein K, McShane A, McKenna M, Keaveny TV, Freaney R. In vitro optimisation of a microdialysis system with potential for on-line monitoring of lactate and glucose in biological samples. Analyst. 1997;122:185–189. doi: 10.1039/a606029c. [DOI] [PubMed] [Google Scholar]
- 3.Marsala M, Malmberg A, Yaksh T. The spinal loop dialysis catheter: characterization of use in the unanesthetized rat. J Neurosci. 1995;62:43–53. doi: 10.1016/0165-0270(95)00053-4. [DOI] [PubMed] [Google Scholar]
- 4.Khan I, Marsala M, Printz M, Taylor P, Yaksh T. Intrathecal nicotinic agonist-elicited release of excitatory amino acids as measured by in vivo spinal microdialysis in rats. J Pharm Exp Ther. 1996;278:97–106. [PubMed] [Google Scholar]
- 5.Evrard PA, Deridder G, Verbeeck RK. Intravenous microdialysis in the mouse and the rat: development and pharmacokinetic application of a new probe. Pharm Res. 1996;13:12–17. doi: 10.1023/a:1016056628685. [DOI] [PubMed] [Google Scholar]
- 6.Evrard PA, Deridder G, Verbeeck RK. Concentration-dependent plasma protein binding of flurbiprofen in the rat: an in vivo microdialysis study. Pharm Res. 1996;13:18–22. doi: 10.1023/a:1016008712756. [DOI] [PubMed] [Google Scholar]
- 7.Kanthan R, Shuaib A, Goplen G, Miyashita H. A new method of in vivo microdialysis of the human brain. J Neurosci Meth. 1995;60:151–155. doi: 10.1016/0165-0270(95)00006-g. [DOI] [PubMed] [Google Scholar]
- 8.Kanthan R, Shuaib A, Greibel R, El-Alazounni H, Miyashita H, Kalra J. Glucose-induced decrease in glutamate levels in ischemic human brain by in vivo microdialysis. Neurosci Lett. 1996;209:207–209. doi: 10.1016/0304-3940(96)12642-2. [DOI] [PubMed] [Google Scholar]
- 9.Benveniste H, Hüttemeier PC. Microdialysis-Theory and application. Progr Neurobiol. 1990;35:195–215. doi: 10.1016/0301-0082(90)90027-e. [DOI] [PubMed] [Google Scholar]
- 10.de Lange EC, Danhof M, de Boer AG, Breimer DD. Methodological considerations of intracerebral microdialysis in pharmacokinetic studies on drug transport across the blood-brain barrier. Brain Res Rev. 1997;25:27–49. doi: 10.1016/s0165-0173(97)00014-3. [DOI] [PubMed] [Google Scholar]
- 11.Major O, Shdanova T, Duffek L, Nagy Z. Continuous monitoring of blood-brain barrier opening to Cr51-EDTA by microdialysis following probe injury. Acta Neurochir Suppl (Wien) 1990;51:46–48. doi: 10.1007/978-3-7091-9115-6_16. [DOI] [PubMed] [Google Scholar]
- 12.Westergren I, Mystrom B, Hamberger A, Johansson BB. Intracerebral microdialysis and the blood-brain barrier. J Neurochem. 1995;64:229–234. doi: 10.1046/j.1471-4159.1995.64010229.x. [DOI] [PubMed] [Google Scholar]
- 13.Morgan ME, Singhal D, Anderson BD. Quantitative assessment of blood-brain barrier damage during microdialysis. J Pharm Exp Ther. 1996;277:1167–1176. [PubMed] [Google Scholar]
- 14.Allen DD, Crooks PA, Yokel RA. 4-Trimethylammonium antipyrine: a quaternary ammonium nonradionuclide marker for blood-brain barrier integrity during in vivo microdialysis. J Pharmacol Toxicol Meth. 1992;28:129–135. doi: 10.1016/1056-8719(92)90074-b. [DOI] [PubMed] [Google Scholar]
- 15.Ault JM, Riley CM, Meltzer NM, Lunte CE. Dermal microdialysis sampling in vivo. Pharm Res. 1994;11:1631–1639. doi: 10.1023/a:1018922123774. [DOI] [PubMed] [Google Scholar]
- 16.Palsmeier RK, Lunte CE. Microdialysis sampling of tumors for study of the metabolism of antineoplastic agents. Cancer Bull. 1994;46:58–66. [Google Scholar]
- 17.Davies MI, Lunte CE. Microdialysis sampling for hepatic metabolism studies: Impact of microdialysis probe design and implantation technique on liver tissue. Drug Metab Dispos. 1995;23:1072–1079. [PubMed] [Google Scholar]
- 18.Morrison PF, Bungay PM, Hsiao JK, Ball BA, Mefford IN, Bedrick RL. Quantitative microdialysis: analysis of transients and application to pharmacokinetics in brain. J Neurochem. 1991;57:103–119. doi: 10.1111/j.1471-4159.1991.tb02105.x. [DOI] [PubMed] [Google Scholar]
- 19.Smith AD, Justice JB. The effect of inhibition of synthesis, release, metabolism and uptake on the microdialysis extraction fraction of dopamine. J Neurosci Meth. 1994;54:75–82. doi: 10.1016/0165-0270(94)90161-9. [DOI] [PubMed] [Google Scholar]
- 20.Stenken JA, Lunte CE, Southard MZ, Ståhle L. Factors that influence microdialysis recovery. Comparison of experimental and theoretical microdialysis recoveries in rat liver. J Pharm Sci. 1997;86:958–966. doi: 10.1021/js960269+. [DOI] [PubMed] [Google Scholar]
- 21.Ståhle L, Segersvärd S, Ungerstedt U. A comparison between three methods for estimation of extracellular concentrations of exogenous and endogenous compounds by microdialysis. J Pharmacol Meth. 1990;25:41–52. doi: 10.1016/0160-5402(91)90021-v. [DOI] [PubMed] [Google Scholar]
- 22.Amberg G, Lindefors N. Intracerebral microdialysis: II. Mathematical studies of diffusion kinetics. J Pharmacol Meth. 1989;22:157–183. doi: 10.1016/0160-5402(89)90012-0. [DOI] [PubMed] [Google Scholar]
- 23.Benveniste H, Hansen AJ, Ottosen NS. Determination of brain interstitial concentrations by microdialysis. J Neurochem. 1989;52:1741–1750. doi: 10.1111/j.1471-4159.1989.tb07252.x. [DOI] [PubMed] [Google Scholar]
- 24.Bungay PM, Morrison PF, Dedrick RL. Steady-state theory for quantitative microdialysis of solutes and water in vivo and in vitro. Life Sci. 1990;46:105–119. doi: 10.1016/0024-3205(90)90043-q. [DOI] [PubMed] [Google Scholar]
- 25.Stenken JA, Topp EM, Southard MZ, Lunte CE. Examination of microdialysis sampling in a well-characterized hydrodynamic system. Anal Chem. 1993;65:2324–2328. doi: 10.1021/ac00065a026. [DOI] [PubMed] [Google Scholar]
- 26.Menacherry S, Hubert W, Justice JB., Jr In vivo calibration of microdialysis probes for exogenous compounds. Anal Chem. 1992;64:577–583. doi: 10.1021/ac00030a003. [DOI] [PubMed] [Google Scholar]
- 27.Larsson CI. The use of an “internal standard” for control of the recovery in microdialysis. Life Sci. 1991;49:PL73–PL78. doi: 10.1016/0024-3205(91)90082-m. [DOI] [PubMed] [Google Scholar]
- 28.Wang Y, Wong SL, Sawchuk RJ. Microdialysis calibration using retrodialysis and zero-net flux: application to a study of the distribution of zidovudine to rabbit cerebrospinal fluid and thalamus. Pharm Res. 1993;10:1411–1419. doi: 10.1023/a:1018906821725. [DOI] [PubMed] [Google Scholar]
- 29.Wong SL, Wang Y, Sawchuck RL. Analysis of zidovudine distribution to specific regions in rabbit brain using microdialysis. Pharm Res. 1992;9:332–338. doi: 10.1023/a:1015834701136. [DOI] [PubMed] [Google Scholar]
- 30.Yokel RA, Allen DD, Burgio DE, McNamara PJ. Antipyrine as a dialyzable reference to correct differences in efficiency among and within sampling devices during in vivo microdialysis. J Pharmacol Toxicol Meth. 1992;27:135–142. doi: 10.1016/1056-8719(92)90034-x. [DOI] [PubMed] [Google Scholar]
- 31.Lönnroth P, Jansson P, Smith U. A microdialysis method allowing characterization of intercellular water space in humans. Am J Physiol. 1987;253:E228–E231. doi: 10.1152/ajpendo.1987.253.2.E228. [DOI] [PubMed] [Google Scholar]
- 32.Ståhle L. Drug distribution studies with microdialysis: I. Tissue dependent difference in recovery between caffeine and theophylline. Life Sci. 1991;49:1835–1842. doi: 10.1016/0024-3205(91)90486-u. [DOI] [PubMed] [Google Scholar]
- 33.Palsmeier RK, Lunte CE. Microdialysis sampling in tumor and muscle: Study of the disposition of 3-amino-1,2,4-benzotriazine-1,4-di-N-oxide (SR 4233) Life Sci. 1994;55:815–825. doi: 10.1016/0024-3205(94)00565-6. [DOI] [PubMed] [Google Scholar]
- 34.Lindefors N, Amberg G, Ungerstedt U. Intracerebral microdialysis. I. Experimental studies of diffusion kinetics. J Pharmacol Meth. 1989;22:141–156. doi: 10.1016/0160-5402(89)90011-9. [DOI] [PubMed] [Google Scholar]
- 35.Jacobson I, Sandberg M, Hamberger A. Mass transfer in brain dialysis devices-a new method for the estimation of extracellular amino acid concentration. J Neurosci Meth. 1985;15:263–268. doi: 10.1016/0165-0270(85)90107-4. [DOI] [PubMed] [Google Scholar]
- 36.Van Belle K, Dzeka T, Sarre S, Ebinger G, Michotte Y. In vitro and in vivo microdialysis calibration for the measurement of carbamazepine and its metabolites in rat brain tissue using the internal reference technique. J Neurosci Meth. 1993;49:167–173. doi: 10.1016/0165-0270(93)90120-g. [DOI] [PubMed] [Google Scholar]
- 37.Davies MI. Ph. D. Dissertation. The University of Kansas; Lawrence, Kansas: 1995. Microdialysis Sampling for In Vivo Hepatic Metabolism Studies. [Google Scholar]
- 38.Riley CM, Ault JM, Lunte CE. On-line microdialysis sampling. In: Riley CM, Lough WJ, Wainer IW, editors. Pharmaceutical and Biomedical Applications of Liquid Chromatography. Elsevier; Oxford, UK: 1994. pp. 193–239. [Google Scholar]
- 39.Davies MI, Lunte CE. Microdialysis sampling coupled on-line to microseparation techniques. Chem Soc Rev. 1997;26:215–222. [Google Scholar]
- 40.Niwa O, Torimitsu K, Morita M, Osbourne P, Yamamoto K. Concentration of extracellular l-glutamate released from cultured nerve cells measured with a small-volume on-line sensor. Anal Chem. 1996;68:1865–1870. doi: 10.1021/ac951154d. [DOI] [PubMed] [Google Scholar]
- 41.Zilkha E, Obrenovitch T, Koshy A, Kusakabe H, Bennetto H. Extracellular glutamate: on-line monitoring using microdialysis coupled to enzyme-amperometric analysis. J Neurosci Meth. 1995;60:1–9. doi: 10.1016/0165-0270(94)00214-2. [DOI] [PubMed] [Google Scholar]
- 42.Volpe G, Moscone D, Compagnone D, Palleschi G. In vivo continuous monitoring of l-lactate coupling subcutaneous microdialysis and an electrochemical biocell. Sensors Actuators B. 1995;24–25:138–141. [Google Scholar]
- 43.Yao T, Suzuki S, Nishino H, Nakahara T. On-line ampoerometric assay of glucose, l-glutamate, and acetylcholine using microdialysis probes and immobilized enzyme reactors. Electroanalysis. 1995;7:1114–1117. [Google Scholar]
- 44.Palmisano F, Centonze D, Quinto M, Zambonin P. A microdialysis fiber based sampler for flow injection analysis: determination of l-lactate in biofluids by an electrochemically synthesized bilayer membrane based biosensor. Biosens Bioelectron. 1996;11:419–425. doi: 10.1016/0956-5663(96)82737-0. [DOI] [PubMed] [Google Scholar]
- 45.Csoregi E, Laurell T, Katakis I, Heller A, Gorton L. Online glucose monitoring by using microdialysis sampling and amperometric detection based on “wired” glucose oxidate in carbon paste. Mikrochim Acta. 1995;121:31–40. [Google Scholar]
- 46.Amine A, Digua K, Xie B, Danielsson B. A microdialysis probe coupled with a miniaturized thermal glucose sensor for in vivo monitoring. Anal Lett. 1995;28:2275–2286. [Google Scholar]
- 47.Muller M, v Osten B, Schmid R, Piegler E, Gerngross I, Buchegger H, Eichler H. Theophylline kinetics in peripheral tissues in vivo in humans. Naunyn-Schmeidebergs Arch Pharmacol. 1995;352:438–441. doi: 10.1007/BF00172782. [DOI] [PubMed] [Google Scholar]
- 48.Desrayaud S, Boschi G, Rips R, Schermann J. Dose-dependent delivery of colchicine to the rat hippocampus by microdialysis. Neurosci Lett. 1996;205:9–12. doi: 10.1016/0304-3940(96)12374-0. [DOI] [PubMed] [Google Scholar]
- 49.Wagstaff J, Gibb J, Hanson G. Dopamine D2-receptors regulate neurotensin release from nucleus accumbens and striatum as measured by in vivo microdialysis. Brain Res. 1996;721:196–203. doi: 10.1016/0006-8993(96)00132-1. [DOI] [PubMed] [Google Scholar]
- 50.Emmett M, Andren P, Caprioli R. Specific molecular mass detection of endogenously released neuropeptides using in vivo microdialysis/mass spectrometry. J Neurosci Meth. 1995;62:141–147. doi: 10.1016/0165-0270(95)00070-4. [DOI] [PubMed] [Google Scholar]
- 51.Boismenu D, Mamer O, Stemarie L, Vachon L, Montgomery J. In vivo hydroxylation of the neurotoxin, 1-methyl-4-phenylpyridinium, and the effect of monoamine oxidase inhibitors: electrospray-MS analysis of intrastriatal mi-crodialysates. J Mass Spectrom. 1996;31:1101–1008. doi: 10.1002/(SICI)1096-9888(199610)31:10<1101::AID-JMS397>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 52.Deterding LJ, Dix K, Burka LT, Tomer KB. On-line coupling of in-vivo microdialysis with tandem mass spectrometry. Anal Chem. 1992;64:2636–2641. doi: 10.1021/ac00045a029. [DOI] [PubMed] [Google Scholar]
- 53.Liu C, Wu Q, Harms A, Smith R. On-line microdialysis sample cleanup for electrospray ionization mass spectrometry of nucleic acid samples. Anal Chem. 1996;68:3295–3299. doi: 10.1021/ac960286j. [DOI] [PubMed] [Google Scholar]
- 54.Wu Q, Liu C, Smith R. On-line microdialysis desalting for electrospray ionization mass spectrometry of proteins and peptides. Rapid Commun Mass Spectrom. 1996;10:835–840. [Google Scholar]
- 55.Lada MW, Shaller G, Carriger MH, Vickroy TW, Kennedy RT. On-line interface between microdialysis and capillary zone electrophoresis. Anal Chim Acta. 1995;307:217–225. [Google Scholar]
- 56.Hogan BL, Lunte SM, Stobaugh JF, Lunte CE. Online coupling of in vivo microdialysis sampling with capillary electrophoresis. Anal Chem. 1994;66:596–602. doi: 10.1021/ac00077a004. [DOI] [PubMed] [Google Scholar]
- 57.Zhou SY, Zuo H, Stobaugh JF, Lunte CE, Lunte SM. Continuous in vivo monitoring of amino acid neurotransmitters by microdialysis sampling with on-line derivatization and capillary electrophoresis separation. Anal Chem. 1995;67:594–599. doi: 10.1021/ac00099a017. [DOI] [PubMed] [Google Scholar]
- 58.Lada MW, Kennedy RT. Quantitative in vivo monitoring of primary amines in rat caudate nucleus using microdialysis and capillary electrophoresis with laser-induced fluorescence detection. Anal Chem. 1996;68:2790–2797. doi: 10.1021/ac960178x. [DOI] [PubMed] [Google Scholar]
- 59.Zhou J, Zuo H, Heckert DM, Lunte CE, Lunte SM. On-line coupling of in vivo microdialysis sampling with capillary electrophoresis/electrochemistry. Anal Chim Acta. doi: 10.1021/ac00077a004. in press. [DOI] [PubMed] [Google Scholar]
- 60.Robinson TE, Justice JB, editors. Microdialysis in the Neurosciences, Vol. 7, Techniques in the Behavioral and Neural Sciences. Elsevier; Amsterdam: 1991. [Google Scholar]
- 61.Westerink BH. Brain microdialysis and its application for the study of animal behaviour (review) Behav Brain Res. 1995;70:103–124. doi: 10.1016/0166-4328(95)80001-8. [DOI] [PubMed] [Google Scholar]
- 62.Lunte CE. Microdialysis and target organ exposure. In: Johnson DE, editor. Drug Toxicodynamics. Marcel Dekker; New York: in press. [Google Scholar]
- 63.Fettweis G, Borlak J. Topics in xenobiochemistry–application of microdialysis techniques in pharmacokinetic studies. Xenobiotica. 1996;26:473–485. doi: 10.3109/00498259609046725. [DOI] [PubMed] [Google Scholar]
- 64.Lunte SM, Lunte CE. Microdialysis sampling for pharmacological studies: HPLC and CE analysis. In: Grushka E, Brown P, editors. Advances in Chromatography. Marcel Dekker; New York: 1996. pp. 383–432. [PubMed] [Google Scholar]
- 65.Elmquist WF, Sawchuk RJ. Application of microdialysis in pharmacokinetic studies. Pharm Res. 1997;14:267–288. doi: 10.1023/a:1012081501464. [DOI] [PubMed] [Google Scholar]
- 66.Shah KP, Chang M, Riley CM. Automated analytical systems for drug development studies. IIsA system for dissolution testing. J Pharm Biomed Anal. 1994;12:1519–1527. doi: 10.1016/0731-7085(94)00103-0. [DOI] [PubMed] [Google Scholar]
- 67.Shah KP, Chang M, Riley CM. Automated analytical systems for drug development studies. 3. Multivessel dissolution testing system based on microdialysis sampling. J Pharm Biomed Anal. 1995;13:1235–1241. doi: 10.1016/0731-7085(95)01529-t. [DOI] [PubMed] [Google Scholar]
- 68.Knaub SR, Chang MF, Lunte CE, Topp EM, Riley CM. Automated analytical systems for drug development studies. Part IV. A microdialysis system to study the partitioning of lomefloxacin across an erythrocyte membrane in vitro. J Pharm Biomed Anal. 1995;14:121–129. doi: 10.1016/0731-7085(95)01597-3. [DOI] [PubMed] [Google Scholar]
- 69.Zhou J, Shearer EC, Hong J, Riley CM, Schowen R. Automated analytical systems for drug development studies. V. A system for enzyme kinetic studies. J Pharm Biomed Anal. 1996;14:1691–1698. doi: 10.1016/0731-7085(96)01792-x. [DOI] [PubMed] [Google Scholar]
- 70.Gunaratna C, Kissinger PT. Application of microdialysis to study the in vitro metabolism of drugs in liver microsomes. J Pharm Biomed Anal. 1997;16:239–248. doi: 10.1016/s0731-7085(97)00042-3. [DOI] [PubMed] [Google Scholar]
- 71.Herrara AM, Scott DO, Lunte CE. Microdialysis sampling for determination of plasma protein binding of drugs. Pharm Res. 1990;7:1077–1076. doi: 10.1023/a:1015955503708. [DOI] [PubMed] [Google Scholar]
- 72.Ekblom M, Hammarlund-Udenaes M, Lundqvist T, Sjöberg P. Potential use of microdialysis sampling in pharmacokinetics: a protein binding study. Pharm Res. 1992;9:155–158. doi: 10.1023/a:1018960617549. [DOI] [PubMed] [Google Scholar]
- 73.Sarre S, Van Belle K, Smolders I, Krieken G, Michotte Y. The use of microdialysis for the determination of plasma protein binding of drugs. J Pharm Biomed Anal. 1992;10:735–739. doi: 10.1016/0731-7085(91)80073-i. [DOI] [PubMed] [Google Scholar]
- 74.Yang H, Elmquist WF. The binding of cyclosporin A to human plasma: An in vitro microdialysis study. Pharm Res. 1996;13:622–627. doi: 10.1023/a:1016066609489. [DOI] [PubMed] [Google Scholar]
- 75.Mannino S, Cosio MS, Zimei P. Microdialysis sampling and high performance liquid chromatography with amperometric detection for sugar analysis in milk products. Electroanalysis. 1996;8:353–355. [Google Scholar]
- 76.Torto N, Buttler T, Gorton L, Marko-Varga G, Stålbrand H, Tjerneld F. Monitoring enzymatic hydrolysis of ivory nut mannan using on-line microdialysis sampling and anion-exchange chromatography with integrated pulsed electrochemical detection. Anal Chim Acta. 1995;313:15–24. [Google Scholar]
- 77.Torto N, Marko-Varga G, Gorton L, Stålbrand H, Tjerneld F. On-line quantitation of enzymatic mannan hydrolysates in small-volume bioreactors by microdialysis sampling and column liquid chromatography-integrated puled electrochemical detection. J Chromatogr A. 1996;725:165–175. [Google Scholar]
- 78.Malone M, Zuo H, Lunte SM, Smyth M. Determination of tryptophan and kynurenine in brain microdialysis samples by capillary electrophoresis with electrochemical detection. J Chromatogr A. 1995;700:73–80. [Google Scholar]
- 79.Hansen DK, Lunte SM. Determination of kynurenic acid by capillary electrophoresis with laser-induced fluorescence detection. J Chromatogr A. 1997;781:81–89. doi: 10.1016/s0021-9673(97)00534-7. [DOI] [PubMed] [Google Scholar]
- 80.Kostel KL, Lunte SM. Evaluation of capillary electrophoresis with postcolumn derivatization and laser-induced fluorescence detection for the determination of substance P and its metabolites. J Chromatogr B. 1997;695:27–38. doi: 10.1016/s0378-4347(97)00173-4. [DOI] [PubMed] [Google Scholar]
- 81.Hughes P, Krishnamoorthy R, Mitra A. Vitreous disposition of two acycloguanosine antivirals in the albino and pigmented rabbit models: a novel ocular microdialysis technique. J Ocul Pharmacol Ther. 1996;12:209–224. doi: 10.1089/jop.1996.12.209. [DOI] [PubMed] [Google Scholar]
- 82.Nakashima M, Zhao MF, Ohya H, Sakuva M, Sasaki H, Matsuyama K, Ichikawa M. Evaluation of in-vivo transdermal absorption of cyclosporin with absorption ehancer using intradermal microdialysis in rats. J Pharm Pharmacol. 1996;48:1143–1146. doi: 10.1111/j.2042-7158.1996.tb03910.x. [DOI] [PubMed] [Google Scholar]
- 83.Nolting A, Costa TD, Vistelle R, Rand K, Derendorf H. Determination of free extracellular concentrations of piperacillin by microdialysis. J Pharm Sci. 1996;85:369–372. doi: 10.1021/js950304x. [DOI] [PubMed] [Google Scholar]
- 84.Kovar A, Costa TD, Derendorf H. Comparison of plasma and free tissue levels of ceftriazone in rats by microdialysis. J Pharm Sci. 1997;86:52–56. doi: 10.1021/js960244a. [DOI] [PubMed] [Google Scholar]
- 85.Felländer G, Eleborg L, Bolinder J, Nordenström J. Microdialysis of adipose tissue during surgery: effect of local alpha and beta-adrenoceptor blockade on blood flow and lipolysis. J Clin Endocrinol Metab. 1996;81:2919–2924. doi: 10.1210/jcem.81.8.8768852. [DOI] [PubMed] [Google Scholar]
- 86.Darimont C, Delansorne R, Paris J, Ailhaud G, Negrel R. Influences of estrogenic status on the lipolytic activity of parametrial adipose tissue in vivo: an in situ microdialysis study. Endocrinology. 1997;138:1092–1096. doi: 10.1210/endo.138.3.4984. [DOI] [PubMed] [Google Scholar]
- 87.Telting-Diaz M, Scott DO, Lunte CE. Intravenous microdialysis sampling in awake, freely moving rats. Anal Chem. 1992;64:806–810. doi: 10.1021/ac00031a019. [DOI] [PubMed] [Google Scholar]
- 88.Nakashima M, Takeuchi N, Hamada M, Matsuyama K, Ichikawa M, Goto S. In vivo microdialysis for pharmacokinetics investigations: a plasma protein binding study of valproate in rabbits. Biochem Pharmacol Bull. 1994;17:1630–1634. doi: 10.1248/bpb.17.1630. [DOI] [PubMed] [Google Scholar]
- 89.Rabenstein K, McShane A, McKenna M, Dempsey E, Keaveny TV, Freaney R. An intravascular microdialysis sampling system suitable for application in continuous biochemical monitoring of glucose and lactate. Technol Health Care. 1996;4:67–76. [PubMed] [Google Scholar]
- 90.Blöchl-Daum B, Müller M, Meisinger V, Eichler H, Fassolt A, Pehamberger H. Measurement of extracellular fluid carboplatin kinetics in melanoma metastases with microdialysis. Br J Cancer. 1996;73:920–924. doi: 10.1038/bjc.1996.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Devineni D, Klein-Szanto A, Gallo JM. In vivo microdialysis to characterize drug transport in brain tumors: analysis of methotrexate uptake in rat glioma-2 (RG-2)-bearing rats. Cancer Chemother Pharmacol. 1996;38:499–507. doi: 10.1007/s002800050518. [DOI] [PubMed] [Google Scholar]
- 92.Devineni D, Klein-Szanto A, Gallo JM. Uptake of temozolomide in a rat glioma model in the presence and absence of the angiogenesis inhibitor TNP-470. Cancer Res. 1996;56:1983–1987. [PubMed] [Google Scholar]
- 93.Ward KW, Pollack GM. Use of intrauterine microdialysis to investigate methanol-induced alteration in uteroplacental blood flow. Toxicol Appl Pharmacol. 1996;140:203–210. doi: 10.1006/taap.1996.0214. [DOI] [PubMed] [Google Scholar]
- 94.Hadwiger ME, Telting-Diaz M, Lunte CE. Liquid chromatographic determination of tacrine and its metabolites in rat bile microdialysates. J Chromatogr B. 1994;655:235–241. doi: 10.1016/0378-4347(94)00099-9. [DOI] [PubMed] [Google Scholar]
- 95.Gunaratna C, Lunte SM, Zuo H. Shunt probe: a new microdialysis probe design for in vivo drug metabolism studies. Curr Separations. 1994;13:80–83. [Google Scholar]
- 96.Layton ME, Wood JG, Yan ZY, Forster J. Ischemia/reperfusion alters uric acid and ascorbic acid levels in liver. J Surg Res. 1996;64:1–5. doi: 10.1006/jsre.1996.0297. [DOI] [PubMed] [Google Scholar]
- 97.Sato H, Kitazawa H, Adachi I, Horikoshi I. Microdialysis assessment of microfibrous collagen containing a P-glycoprotein-mediated transport inhibitor, cyclosporine A, for local delivery of the etoposide. Pharm Res. 1996;13:1565–1569. doi: 10.1023/a:1016096016642. [DOI] [PubMed] [Google Scholar]
- 98.Malagié I, Trillant A, Douvier E, Anmella M, Dessalles M, Jancquot C, Gardier AM. Regional differences in the effect of the combined treatment of WAY 100635 and fluoxetine: An in vivo microdialysis study. Naunyn-Schmeidebergs Arch Pharmacol. 1996;354:785–790. doi: 10.1007/BF00166906. [DOI] [PubMed] [Google Scholar]
- 99.Davies MI, Lunte CE. Simultaneous microdialysis sampling from multiple sites in the liver for the study of phenol metabolism. Life Sci. 1996;59:1001–1013. doi: 10.1016/0024-3205(96)00407-9. [DOI] [PubMed] [Google Scholar]
- 100.Malhotra B, Lemaire M, Brouillard J, Sawchuk R. High-performance liquid chromatographic analysis of (S)-α-amino-5-phosphonomethyl[1,1′-biphenyl]-3-propanoic acid (EAB 5151) in brain and blood microdialysate (on-line) and in plasma ultrafiltrate of freely moving rats. J Chromatogr B. 1996;679:167–176. doi: 10.1016/0378-4347(96)00020-5. [DOI] [PubMed] [Google Scholar]
- 101.Nakashima M, Zhao MF, Nakashima MN, Sakurai M, Sasaki H, Matsuyama K, Ichikawa M. In vivo microdialysis to determine the relative pharmacokinetics of drugs. Biochem Pharmacol Bull. 1996;19:988–994. doi: 10.1248/bpb.19.988. [DOI] [PubMed] [Google Scholar]
- 102.Deleu D, Sarre S, Michotte Y, Ebinger G. Simultaneous in vivo microdialysis in plasma and skeletal muscle: a study of the pharmacokinetic properties of levodopa by noncompartmental analysis. J Pharm Sci. 1994;83:25–28. doi: 10.1002/jps.2600830107. [DOI] [PubMed] [Google Scholar]
- 103.Sarre S, Deleu D, Van Belle K, Ebinger G, Michotte Y. Quantitative microdialysis for studying the in vivo L-DOPA kinetics in blood and skeletal muscle of the dog. Pharm Res. 1995;12:746–750. doi: 10.1023/a:1016271911983. [DOI] [PubMed] [Google Scholar]
- 104.Van Amsterdam C, Boukhabza A, Ofner B, Pacha W, Lemaire M. Measurement of free concentration of SDZ ICM 567 in blood and muscle using microdialysis sampling. Biopharm Drug Dispos. 1995;16:521–527. doi: 10.1002/bdd.2510160609. [DOI] [PubMed] [Google Scholar]
- 105.Yang WP, Oshida Y, Wu W, Sato J, Ohsawa I, Sato Y. Effect of daily voluntary running on in vivo insulin action in rat skeletal muscle and adipose tissue as determined by the microdialysis technique. Int J Sports Med. 1995;16:99–104. doi: 10.1055/s-2007-972973. [DOI] [PubMed] [Google Scholar]
- 106.Müller M, Haag O, Burgdorff T, Georgopoulos A, Weninger W, Jansen B, Stanek G, Pehamberger H, Agneter E, Eichler HG. Characterization of peripheral-compartment kinetics of antibiotics by in vivo microdialysis in humans. Antimicrob Agents Chemother. 1996;40:2703–2709. doi: 10.1128/aac.40.12.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Van Belle K, Sarre S, Ebinger G, Michotte Y. Brain, liver and blood distribution kinetics of carbamazepine and its metabolic interaction with clomipramine in rats: A quantitative microdialysis study. J Pharmacol Exp Ther. 1995;272:1217–1222. [PubMed] [Google Scholar]
- 108.Yokel RA. Aluminum chelation by 3-hydrozypyridin-4-ones in the rat demonstrated by microdialysis. Biol Trace Element Res. 1996;53:193–203. doi: 10.1007/BF02784555. [DOI] [PubMed] [Google Scholar]
- 109.Ekstrøm PO, Andersen A, Warren DJ, Giercksky KE, Slørdal L. Pharmacokinetics of different doses of methotrexate at steady state by in situ microdialysis in a rat model. Cancer Chemother Pharmacol. 1995;36:283–289. doi: 10.1007/BF00689044. [DOI] [PubMed] [Google Scholar]
- 110.Ekstrøm PO, Andersen A, Warren DJ, Giercksky KE, Slørdal L. Determination of extracellular methotrexate tissue levels by microdialysis in a rat model. Cancer Chemother Pharmacol. 1996;37:394–400. doi: 10.1007/s002800050403. [DOI] [PubMed] [Google Scholar]
- 111.Oldner A, Goiny M, Ungerstedt U, Sollevi A. Splanchnic homeostasis during endotoxin challenge in the pig as assessed by microdialysis and tonometry. Shock. 1996;6:188–193. [PubMed] [Google Scholar]
- 112.Ekstrøm PO, Giercksky KE, Andersen A, Slørdal L. Alterations in methotrexate pharmacokinetics by naproxen in the rat as measured by microdialysis. Life Sci. 1997;60:PL359–364. doi: 10.1016/s0024-3205(97)00241-5. [DOI] [PubMed] [Google Scholar]
- 113.Molhotra BK, Lemaire M, Sawchuk RJ. Investigation of the distribution of EAB 515 to cortical ECF and CSF in freely moving rats utilizing microdialysis. Pharm Res. 1997;11:1223–1232. doi: 10.1023/a:1018921906993. [DOI] [PubMed] [Google Scholar]


