Abstract
Current emphasis on translational application of genetic models of lung disease has renewed interest in the measurement of the gravimetric filtration coefficient (Kf) as a means to assess vascular permeability changes in isolated perfused lungs. The Kf is the product of the hydraulic conductivity and the filtration surface area, and is a sensitive measure of vascular fluid permeability when the pulmonary vessels are fully recruited and perfused. We have observed a remarkable consistency of the normalized baseline Kf values between species with widely varying body weights from mice to sheep. Uniformity of Kf values can be attributed to the thin alveolar capillary barrier required for gas exchange and the conserved matching of lung vascular surface area to the oxygen requirements of the body mass. An allometric correlation between the total lung filtration coefficient (Kf,t) and body weight in several species (r2 = 1.00) had a slope that was similar to those reported for alveolar and pulmonary capillary surface areas and pulmonary diffusion coefficients determined by morphometric methods in these species. A consistent Kf is dependent on accurately separating the filtration and vascular volume components of lung weight gain, then Kf is a consistent and repeatable index of lung vascular permeability.
Keywords: capillary permeability, vascular surface area, hydraulic conductivity
the current emphasis in translational application of genetic modifications and signaling pathways in the study of lung diseases has renewed interest in the measurement of the gravimetric filtration coefficient (Kf) in isolated lungs to evaluate changes in vascular permeability. In a recent editorial focus, Dr. Bhattacharya (5) elegantly summarized four basic assumptions for using Kf to assess lung microvascular injury, as well as conditions that could alter these assumptions and lead to errors in interpretation. In this perspective, we focus on the basis of the extremely consistent values obtained for Kf between species when normalized to lung or body weight.
The Kf is defined as the product of the hydraulic conductivity (Lp) and filtration surface area (SA), and essentially measures the endothelial barrier permeability to convective water transport (7). As a measure of vascular permeability, Kf is inherently more sensitive than protein flux because convective transport through paracellular pathways is a function of the fourth power of the pathway radius (r4), compared with r2 for protein flux (20, 26, 27). We have previously reviewed the merits of Kf for measuring lung microvascular permeability and the most reliable method for extracting the filtration component from the rate of lung weight gain (20).
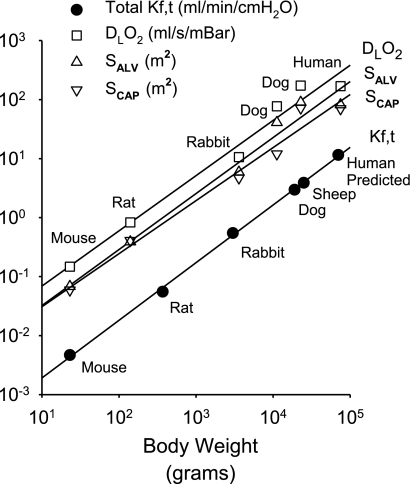
Kf normalized to lung or body mass is remarkably consistent across species. This consistency can largely be attributed to the matching of the microvascular surface area for filtration to the gas exchange surface area, the latter designed to achieve a gas exchange that will support oxidative metabolism of a given body mass (31). Kf is normalized to 100 g of lung weight for comparison of values between experiments, but a total lung filtration coefficient (Kf,t) can be calculated for the total lung weight and filtration surface area. Kf,t then represents a hydraulic conductance equivalent of the capillary oxygen conductance embodied in the pulmonary diffusion capacity for oxygen for the whole animal. As shown in Fig. 1, K f,t varies as a function of body weight (BW) for mouse (11, 16, 32), rat (2, 3, 18), rabbit (1, 4), dog (21, 29), and sheep (8, 22) as well as a predicted value for a 70-kg human. The relationship of Kf,t to body mass is similar to that for alveolar (SALV) or pulmonary capillary (SCAP) surface areas and pulmonary diffusion coefficient for oxygen (DLO2) determined by Weibel and associates using morphometric methods (31). Weibel has previously shown that these variables have a relationship of the form Y = A·BWB, where A is the intercept and B is the slope of the logarithmic plot. Relative to BW, the total lung Kf,t had a slope of 0.98 ml·min−1·cmH2O−1·g−1 (r2 = 1.00), which was comparable to slopes of 0.95 m2/g (r2 = 0.98), 0.90 m2/g (r2 = 0.98), and 0.94 ml·s−1·mBar−1·g−1 (r2 = 0.98) for SALV, SCAP, and DLO2, respectively. The average SCAP/SALV ratio determined by Weibel for the species shown was 0.87 ± 0.04. However, small extra-alveolar arterioles and venules (20- to 50-μm diameter) likely filter fluid and contribute an additional surface area of ∼13% (25). This additional surface area for filtration and the 20-fold greater hydraulic conductance of these conduit vessels compared with alveolar capillary endothelium undoubtedly account for the greater slope of Kf,t compared with SCAP normalized to BW (17).
Fig. 1.
Allometric relationship of total lung filtration coefficient (Kf,t), alveolar surface area (SALV), pulmonary capillary surface area (SCAP), and pulmonary diffusion coefficients (DLO2) for oxygen to body mass of several species. SALV, SCAP, and DLO2 data are from Weibel (31).
The excellent correlation of Kf,t with body weight suggests that the baseline hydraulic conductivity is approximately the same in mouse and dog lungs. In contrast to the large proportional increase in pulmonary surface area with body mass, Weibel observed that the mean harmonic thickness of the alveolar capillary barrier only increased from 0.32 μm in mouse to 0.5 μm in human, with a slope of only 0.05 μm/g relative to body weight (10). Thus, the thinnest possible septal barrier is conserved between species to minimize diffusion distances and results in consistency of fluid exchange. Although normalized baseline Kf values are similar in adult lungs of various species, a thinner alveolar capillary barrier could account for the slightly higher baseline Kf and lower threshold for vascular pressure injury in mice compared with dogs and for the lungs of younger compared with older animals (1, 9, 13, 28).
We can conclude that Kf,t measures the total transvascular fluid exchange across a perfused surface area that is proportional to that available for gas exchange, as also suggested by lung mass resection studies (30). A further conclusion is that an increase in Kf from a baseline value can be attributed to an increase in endothelial hydraulic conductance rather than recruitment of additional surface area, provided that the pulmonary vascular surface area is fully recruited at baseline. In previous studies in isolated dog lungs, changes in blood flow from 0.03 to 2.5 l·min−1·100 g−1 lung weight had no significant effect on baseline Kf in uninjured lungs (24). Increasing the perfusion flow in these same lungs after injury caused significant increases in Kf due to recruitment of injured and vasoconstricted lung segments. In this situation, the magnitude of the increase in hydraulic conductance is partially offset by a decrease in surface area, unless equivalent perfusion rates are maintained between Kf measurements in the baseline and experimental injury states (24). In smaller species such as mouse and rat, some decrease in baseline Kf was observed at very low perfusion rates, presumably due to derecruitment, so a baseline perfusate flow of at least 300 ml·min−1·100 g−1 lung weight in rats was recommended (20). Conceivably, very high perfusion and shear rates could activate endothelial cells and increase Kf, but previous isolated lung baseline Kf measurements over a wide range of flows do not support a shear-induced permeability response in uninjured lungs (24, 30).
We have previously emphasized the necessity of maintaining the increased weight transient for 18–20 min to minimize errors due to vascular stress relaxation during baseline Kf measurements and completely separate the vascular volume and filtration components of lung weight gain (19). A wide range of baseline Kf values has been published for lungs of the same species using methods that include a variable amount of the vascular stress relaxation component of lung weight gain to the filtration measurement. These other methods produce Kf values that do not correlate as well with body weight as the Kf,t data we show here. Only during severe lung vascular injury when the filtration component is large compared with the vascular volume component of lung weight gain can valid filtration rates be measured using much shorter periods of weight gain. Since alveolar surface tension forces and interstitial fluid pressures increase as edema accumulates, a modest increase in Kf occurs after a lung weight gain of ∼40–50% due to initiation of alveolar flooding (6). Thus, only the Kf measurements in uninjured, fully recruited lungs without edema can be used as a reliable indicator of filtration surface area.
The possibility of endothelial signaling induced by the increased vascular pressure during a Kf measurement was recently emphasized by Bhattacharya (5). Endothelial responses to pressure were not considered during original applications of Kf because the endothelium was considered to be an inert physical barrier. However, seminal intravital microscopy studies by Bhattacharya, Kuebler, and associates (12, 14, 15) demonstrated increases in both endothelial calcium and endothelial P-selectin expression after a microvascular pressure increase of only 10 cmH2O. Although a 10 cmH2O pressure elevation is commonly used for Kf measurement, a smaller vascular pressure increase would minimize potential endothelial activation. However, previous studies indicated no acute increase in Kf at microvascular filtration pressures lower than 42 cmH2O in dog lungs or 30 cmH2O in mouse lungs (13, 23, 28). Baseline Kf measured using a 10 cmH2O increase in microvascular pressure were not significantly different between transient receptor potential-4 (TRPV4) channel knockout and wild-type mice even though these mechanogated channels were essential for high vascular or airway pressure injury to the lung (11, 13). These results suggest that modest transient microvascular pressure increases do not cause endothelial activation sufficient to induce an acute vascular permeability increase, but when more subtle proinflammatory endothelial signaling and longer term inflammatory cell effects must be considered, then the increases in vascular pressure should be kept at a minimum.
In summary, Kf is determined by the hydraulic conductivity of the thin endothelial layer and the large pulmonary vascular surface area of the blood gas barrier. These anatomic features are optimized for efficient gas exchange to meet the oxygen requirements of the animals' body mass. The relationship of alveolar capillary surface area for gas exchange to body mass leads to the remarkable consistency of Kf across species when normalized to lung or body weight. When the pulmonary vascular surface area is fully recruited, the Kf measurement is then a reliable and repeatable method used to track vascular permeability changes in isolated lungs.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant P01-HL-66299.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Adkins WK, Hernandez LA, Coker PJ, Buchanan B, Parker JC. Age affects susceptibility to pulmonary barotrauma in rabbits. Crit Care Med 19: 390–393, 1991. [DOI] [PubMed] [Google Scholar]
- 2.Alvarez DF, Gjerde EA, Townsley MI. Role of EETs in regulation of endothelial permeability in rat lung. Am J Physiol Lung Cell Mol Physiol 286: L445–L451, 2004. [DOI] [PubMed] [Google Scholar]
- 3.Alvarez DF, King JA, Townsley MI. Resistance to store depletion-induced endothelial injury in rat lung after chronic heart failure. Am J Respir Crit Care Med 172: 1153–1160, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anglade D, Corboz M, Menaouar A, Parker JC, Sanou S, Bayat S, Benchetrit G, Grimbert FA. Blood flow vs. venous pressure effects on filtration coefficient in oleic acid-injured lung. J Appl Physiol 84: 1011–1023, 1998. [DOI] [PubMed] [Google Scholar]
- 5.Bhattacharya J Interpreting the lung microvascular filtration coefficient. Am J Physiol Lung Cell Mol Physiol 293: L9–L10, 2007. [DOI] [PubMed] [Google Scholar]
- 6.Bhattacharya J, Nakahara K, Staub NC. Effect of edema on pulmonary blood flow in the isolated perfused dog lung lobe. J Appl Physiol 48: 444–449, 1980. [DOI] [PubMed] [Google Scholar]
- 7.Curry FE Mechanics and thermodynamics of transcapillary exchange. In: The Cardiovascular System, edited by Renkin EM and Michel CC. Bethesda, MD: American Physiological Society, 1984, p. 309–374.
- 8.Dodd-o JM, Pearse DB. Effect of the NADPH oxidase inhibitor apocynin on ischemia-reperfusion lung injury. Am J Physiol Heart Circ Physiol 279: H303–H312, 2000. [DOI] [PubMed] [Google Scholar]
- 9.Fu Z, Heldt GP, West JB. Increased fragility of pulmonary capillaries in newborn rabbit. Am J Physiol Lung Cell Mol Physiol 284: L703–L709, 2003. [DOI] [PubMed] [Google Scholar]
- 10.Gehr P, Mwangi DK, Ammann A, Maloiy GM, Taylor CR, Weibel ER. Design of the mammalian respiratory system. V. Scaling morphometric pulmonary diffusing capacity to body mass: wild and domestic mammals. Respir Physiol 44: 61–86, 1981. [DOI] [PubMed] [Google Scholar]
- 11.Hamanaka K, Jian MY, Weber DS, Alvarez DF, Townsley MI, Al Mehdi AB, King JA, Liedtke W, Parker JC. TRPV4 initiates the acute calcium-dependent permeability increase during ventilator-induced lung injury in isolated mouse lungs. Am J Physiol Lung Cell Mol Physiol 293: L923–L932, 2007. [DOI] [PubMed] [Google Scholar]
- 12.Ichimura H, Parthasarathi K, Issekutz AC, Bhattacharya J. Pressure-induced leukocyte margination in lung postcapillary venules. Am J Physiol Lung Cell Mol Physiol 289: L407–L412, 2005. [DOI] [PubMed] [Google Scholar]
- 13.Jian MY, King JA, Al Mehdi AB, Liedtke W, Townsley MI. High vascular pressure-induced lung injury requires P450 epoxygenase-dependent activation of TRPV4. Am J Respir Cell Mol Biol 38: 386–392, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuebler WM, Ying X, Bhattacharya J. Pressure-induced endothelial Ca2+ oscillations in lung capillaries. Am J Physiol Lung Cell Mol Physiol 282: L917–L923, 2002. [DOI] [PubMed] [Google Scholar]
- 15.Kuebler WM, Ying X, Singh B, Issekutz AC, Bhattacharya J. Pressure is proinflammatory in lung venular capillaries. J Clin Invest 104: 495–502, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miyahara T, Hamanaka K, Weber DS, Drake DA, Anghelescu M, Parker JC. Phosphoinositide 3-kinase, Src, and Akt modulate acute ventilation-induced vascular permeability increases in mouse lungs. Am J Physiol Lung Cell Mol Physiol 293: L11–L21, 2007. [DOI] [PubMed] [Google Scholar]
- 17.Parker JC Hydraulic conductance of lung endothelial phenotypes and Starling safety factors against edema. Am J Physiol Lung Cell Mol Physiol 292: L378–L380, 2007. [DOI] [PubMed] [Google Scholar]
- 18.Parker JC, Ivey CL. Isoproterenol attenuates high vascular pressure induced permeability increases in isolated rat lungs. J Appl Physiol 83: 1962–1967, 1998. [DOI] [PubMed] [Google Scholar]
- 19.Parker JC, Prasad R, Allison RA, Wojchiechowski WV, Martin SL. Capillary filtration coefficients using laser densitometry and gravimetry in isolated dog lungs. J Appl Physiol 74: 1981–1987, 1993. [DOI] [PubMed] [Google Scholar]
- 20.Parker JC, Townsley MI. Evaluation of lung injury in rats and mice. Am J Physiol Lung Cell Mol Physiol 286: L231–L246, 2004. [DOI] [PubMed] [Google Scholar]
- 21.Parker JC, Townsley MI, Rippe B, Taylor AE, Thigpen J. Increased microvascular permeability in dog lungs due to high peak airway pressures. J Appl Physiol 57: 1809–1816, 1984. [DOI] [PubMed] [Google Scholar]
- 22.Pearse DB, Wagner EM, Permutt S. Effect of ventilation on vascular permeability and cyclic nucleotide concentrations in ischemic sheep lungs. J Appl Physiol 86: 123–132, 1999. [DOI] [PubMed] [Google Scholar]
- 23.Rippe B, Townsley M, Thigpen J, Parker JC, Korthuis RJ, Taylor AE. Effects of vascular pressure on the pulmonary microvasculature in isolated dog lungs. J Appl Physiol 57: 233–239, 1984. [DOI] [PubMed] [Google Scholar]
- 24.Shibamoto T, Parker JC, Taylor AE, Townsley MI. Derecruitment of filtration surface area in paraquat-injured isolated dog lungs. J Appl Physiol 68: 1581–1589, 1990. [DOI] [PubMed] [Google Scholar]
- 25.Staub NC Pulmonary edema. Physiol Rev 54: 678–811, 1974. [DOI] [PubMed] [Google Scholar]
- 26.Taylor AE, Granger DN. Exchange of macromolecules across the microcirculation. In: Handbook of Physiology, edited by Renkin EM and Michel CC. Bethesda, MD: Am. Physiol. Soc., 1984, p. 467–520.
- 27.Taylor AE, Parker JC. The interstitial spaces and lymph flow. In: Handbook of Physiology: The Respiratory System. Circulation and Nonrespiratory Function, edited by Fishman AP and Fisher AB. Bethesda, MD: Am. Physiol. Soc., 1985, p. 167–320.
- 28.Townsley MI, Fu Z, Mathieu-Costello O, West JB. Pulmonary microvascular permeability: responses to high vascular pressure after induction of pacing induced heart failure in dogs. Circ Res 77: 317–325, 1995. [DOI] [PubMed] [Google Scholar]
- 29.Townsley MI, Lin CF, Sahawneh TM, Song W. Interaction of chemical and high pressure injury in isolated canine lung. J Appl Physiol 69: 1657–1664, 1990. [DOI] [PubMed] [Google Scholar]
- 30.Townsley MI, Parker JC, Korthuis RJ, Taylor AE. Alterations in hemodynamics and Kf,c during lung mass resection. J Appl Physiol 63: 2460–2466, 1987. [DOI] [PubMed] [Google Scholar]
- 31.Weibel ER Morphologic basis of alveolar-capillary gas exchange. Physiol Rev 53: 419–495, 1973. [DOI] [PubMed] [Google Scholar]
- 32.Yoshikawa S, King JA, Lausch RN, Penton AM, Eyal FG, Parker JC. Acute ventilator-induced vascular permeability and cytokine responses in isolated and in situ mouse lungs. J Appl Physiol 97: 2190–2199, 2004. [DOI] [PubMed] [Google Scholar]