Abstract
Previously, we have reported that endothelial nitric oxide synthase (eNOS) promoter activity is decreased in pulmonary arterial endothelial cells (PAECs) in response to hydrogen peroxide (H2O2). Thus the objective of this study was to identify the cis-element(s) and transcription factor(s) responsible for oxidant-mediated downregulation of the eNOS gene. Initial promoter experiments in PAECs treated with H2O2 revealed a significant decrease in activity of a promoter fragment containing 840 bp of upstream sequence of the human eNOS gene fused to a luciferase reporter. However, a promoter construct containing only 640 bp of upstream sequence had a significantly attenuated response to H2O2 challenge. As the 840-bp promoter construct had a putative binding site for the transcription factor activator protein-1 (AP-1) that was lacking in the 640-bp construct, we evaluated the effect of H2O2 on promoter activity after mutation of the AP-1 binding sequence (TGAGTCA at −661 to TGAGTtg in the 840-bp construct). Similar to the results seen with the 640 bp, the AP-1 mutant promoter had a significantly attenuated response to H2O2. EMSA revealed decreased binding of AP-1 during H2O2 treatment. Supershift analysis indicated that the AP-1 complex consisted of a c-Jun and FosB heterodimer. Furthermore, in vitro EMSA analysis indicated the c-Jun binding was significantly decreased after H2O2 exposure. Using chromatin immunoprecipitation analysis, we demonstrated decreased binding of AP-1 to the eNOS promoter in vivo in response to H2O2. These data suggest a role of decreased AP-1 binding likely through c-Jun in the H2O2-mediated decrease in eNOS promoter activity.
Keywords: oxidative stress, gene regulation, pulmonary hypertension
normal pulmonary vascular tone is regulated by a complex interaction of vasoactive substances produced by the vascular endothelium (12, 16, 20). These include nitric oxide (NO) and endothelin-1 (ET-1). NO is an endothelium-derived relaxing factor synthesized by the oxidation of l-arginine after activation of endothelial nitric oxide synthase (eNOS) (35). Studies using null mice have confirmed the importance of eNOS in the control of blood vessel tone (23), remodeling (37), hemostasis (14), angiogenesis (27), and the mobilization of endothelial progenitor cells (3). NO activates soluble guanylate cyclase, leading to elevation of cGMP and vasorelaxation (15). This is the primary basis for blood-flow and -pressure regulation. Loss of NO bioavailability is a key feature of endothelial dysfunction. Multiple pathways can reduce NO bioavailability by altering its synthesis or biodegradation. Excessive production of reactive oxygen species (ROS) and hydrogen peroxide (H2O2) seems to be a major mechanism of reduced vascular NO levels (19). Increased production of ROS has been implicated in the pathogenesis of cardiovascular diseases such as atherosclerosis, restenosis, hypertension, diabetic vascular complication, and heart failure (6, 9, 28, 46). Abnormal regulation of endothelial function has been implicated in the pathophysiology of a number of pulmonary hypertensive disorders. For example, in persistent pulmonary hypertension of the newborn (PPHN), pulmonary vascular resistance does not decrease normally at birth, resulting in pulmonary hypertension, right-to-left shunting, and hypoxemia (43). Newborns who die of PPHN exhibit an increase in the thickness of the smooth muscle layer within small pulmonary arteries and extension of this muscle to nonmuscular arteries (21), and there is also a decrease in eNOS expression (17). Surgical ligation of the ductus arterious in the fetal lamb generates a model of PPHN that resembles the human condition (2, 4, 50). Previously, our laboratory has reported an increase in both superoxide (8) and H2O2 (49) in this ovine model of PPHN. We also demonstrated that ET-1-mediated increases in H2O2 within fetal pulmonary arterial smooth muscle cells (FPASMC) produced decreases in eNOS promoter activity and eNOS activity in endothelial cells (FPAEC) cocultured with FPASMC (47). A similar effect was observed when FPAEC were challenged directly with H2O2 (47).
The eNOS promoter lacks a typical TATA box, but it has potential binding sites for a number of transcription factors including activator protein-1 (AP-1), Sp1, GATA, NF-κB, AP-2, and also shear stress-response elements and sterol-regulatory elements (29, 48). The Sp1 binding site at −104 is involved in the basal expression of eNOS (25, 45, 56), and we (47) have previously reported a decrease in eNOS promoter activity and eNOS expression in response to H2O2 treatment. Thus the purpose of this study was to identify the cis-element(s) and transcription factor(s) responsible for H2O2-mediated downregulation of the eNOS gene. Here, we demonstrate the involvement of AP-1 in H2O2-mediated downregulation of eNOS promoter in pulmonary arterial endothelial cells (PAECs).
MATERIAL AND METHODS
Cell culture.
Primary cultures of PAECs from late-gestation fetal lambs were isolated by the explant technique as we (48) have described previously. Briefly, the heart and lungs were obtained from fetal (138–140 days gestation) lambs after death. These fetal lambs had not undergone previous surgery or study. The main and branching pulmonary arteries were removed, and the exterior of the vessels were rinsed with 70% ethanol prepared using sterile distilled water water. The vessel was opened longitudinally, and the interior was rinsed with PBS to remove blood. The endothelium was lightly scraped away, placed in Dulbecco's modified Eagle's-H16 media (DME-H16) containing 10% FBS and antibiotics (penicillin and streptomycin), and incubated at 37°C in 21% O2-5% CO2-balance N2. After 5 days, islands of endothelial cells (ECs) were cloned to ensure purity. Basic fibroblast growth factor (10 ng/ml) was added to the medium every other day. When confluent, the cells were passaged to maintain them in culture or frozen in liquid nitrogen. EC identity was confirmed by the typical cobblestone appearance, contact inhibition, specific uptake of acetylated low-density lipoprotein labeled with 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine, and positive staining for von Willebrand factor (DAKO, Carpinteria, CA). Ovine FPAECs were studied between passages 3 and 10.
Cell transfection and promoter assays.
We generated promoter constructs by PCR using human genomic DNA as a template. The reverse primer common to all constructs binds immediately upstream of the ATG initiation codon of eNOS (5′-GTTACTGTG CGT CCA CTCTGCTGCC-3′). Forward primers were 5′-TGTAGTTTCCCTAGTCCCCC-3′ (840-bp fragment) and 5′-GGTGTGGGGGTTCCAGGAAA-3′ (650-bp fragment). A 2-bp mutation was introduced into the AP-1 consensus sequence within the 840-bp construct. The sequence TGAGTCA at −661 in the human eNOS promoter (29) was changed to TGAGTtg by site-directed mutagenesis as we (48) described previously. FPAECs were cotransfected with 1.6 μg of test plasmid and 0.4 μg of β-galactosidase plasmid (as an internal control to normalize for transfection efficiency) on a 10-cm2 tissue culture plate at 90% confluency with Effectene Transfection Reagent (Qiagen, Valencia, CA) according to the manufacturer's instructions. On the next day, the cells were split onto six-well plates and allowed to adhere for 36 h. The culture medium was then replaced with fresh DMEM containing 0 to 200 μM H2O2 for 16–18 h. The luciferase activity of 20 μl of protein extract was determined using Luciferase Assay System (Promega, Madison, WI), and β-galactosidase of 10 μl of protein extract was determined using β-galactosidase enzyme assay system (Promega) as transfection efficiency control.
Nuclear extract preparation and EMSA.
Nuclear extracts were prepared using the NE-PER nuclear extraction kit (Pierce Biotechnology, Rockford, IL). EMSA were performed using biotinylated double-stranded oligonucleotides corresponding to the AP-1 site at −661 of the eNOS promoter (29). We also used an AP-1 mutant oligonucleotide (AP-1mut) where a 2-bp mutation was introduced into the AP-1 consensus sequence at −661 of the human eNOS promoter. The single-stranded oligonucleotides were biotinylated using Biotin 3′ End DNA Labeling Kit (Pierce Biotechnology) to incorporate 1–3 biotinylated ribonucleotides (Biotin-11-UTP) onto the 3′ end of DNA strands using terminal deoxynucleotidyl transferase (TdT) and then annealed to make it double stranded. The sequence of the different oligonucleotides are AP-1 −677 5′-TTT TGT GTC CCC CAC TTG AGT CAT GGG GGT-3′ −648 and AP-1mut −677 5′-TTT TGT GTC CCC CAC TTG AGT TGT GGG GGT-3′ −648 [Integrated DNA Technologies (IDT), Coralville, IA]. Binding reactions involved incubating 10 μg of nuclear extract with biotinylated oligonucleotide and 1 μg poly(dI-dC) for 20 min at room temperature. In addition, the binding of recombinant purified c-Jun was examined in presence of H2O2. Purified c-Jun (1.2 μg, Promega) was treated with 10 μM H2O2 for 20 min. Binding reaction was then set up by adding biotinylated AP-1 oligonucleotide and 1 μg poly(dI-dC) for 20 min at room temperature. The DNA-protein complexes were resolved on a 5% nondenaturing polyacrylamide gel in 1× Tris-borate-EDTA (TBE) buffer and then transferred to nylon membrane, and biotinylated oligonucleotide was detected using a LightShift Chemiluminescent EMSA Kit (Pierce Biotechnology). Competition reactions were done with 10-, 50-, and 100-fold excess of unlabeled oligonucleotides. Supershift experiments were conducted by incubating nuclear extracts overnight at 4°C with 4 μg of polyclonal antibodies specific to c-Jun (cat. no. sc-1694X), FosB (cat. no. sc-48X), and Fra-1 (cat. no. sc-605X) all from Santa Cruz Biotechnology (Santa Cruz, CA) followed by the addition of biotinylated oligonucleotide. The complexes were visualized using an Image Station 440 CF (Eastman Kodak).
Chromatin immunoprecipitation.
Chromatin immunoprecipitation (ChIP) assays were performed essentially following the method of Braunstein et al. (7). FPAEC cells (∼1.0 × 106) cultured in 10-cm plates were treated with H2O2 (100 μM) for 2 h and then cross-linked with 1% formaldehyde for 10 min, harvested, and sonicated to 200∼1,000-bp fragments. Supernatants were incubated overnight at 4°C with an anti-c-Jun antibody. After ethanol precipitation, DNA was resuspended in 20 μl for each 106 cells, and 1 μl was used as PCR template. The primer pairs used to amplify the regions containing the AP-1-binding site in the −700/−1 promoter region, respectively, were 5′-TGCCTCAGCCCTAGTCTCTC-3′/5′-GATAGAGGCCCAGCAAGGAT-3′ (for the AP-1-binding site at −661 resulting in a fragment of 225 bp). A DNA sample from sonicated lysates that underwent reverse cross-link and phenol/chloroform extraction was used as a positive control (input).
Analysis of cell death.
FPAECs were treated with 0 to 200 μM H2O2 overnight. After incubation, the medium was collected and centrifuged for 5 min at 500 g, and the supernatant was stored at 4°C until assay. Relative cytotoxicity was quantified by measurement of release of the soluble cytoplasmic enzyme lactate dehydrogenase (LDH). LDH activity in cell-free supernatant was measured using a commercial kit (Roche Applied Science, Indianapolis, IN). After a given time course, 50-μl aliquots of cell-free medium were transferred from all wells to a fresh 96-well plate, and 50 μl of reconstituted substrate mix were added to each well. The plate was incubated for 30 min at room temperature in the dark, and the absorbance was recorded at 490 nm. Each culture sample was measured in quadruplicate, with a minimum of three samples per experimental group. Relative cytotoxicity was determined by comparison of absorbance of the experimental group with absorbance of a control cell group treated with 2% Triton X-100 cell lysis buffer according to the manufacturer's protocol.
At high concentration, H2O2 may be a toxic chemical. Thus we initially performed LDH-release assays to examine potential cytotoxic effects of H2O2. However, at all doses examined, H2O2 did not induce significant cytotoxicity (untreated = 0.0%; 100 μM for 2 h = 0.86% ± 0.06%; 200 μM overnight = 2.7% ± 0.2%; 2% Triton X-100 = 100% cell death).
Statistical analyses.
The means ± SE were calculated for all samples, and significance was determined either by the unpaired t-test (for 2 groups) or ANOVA with Newman-Keuls post hoc testing (for ≥3 groups). The statistical significance of differences was set at P < 0.05. Statistical analysis was performed using GraphPad Prism version 4.01 for Windows (GraphPad Software, San Diego, CA).
RESULTS
Effect of H2O2 on eNOS promoter activity.
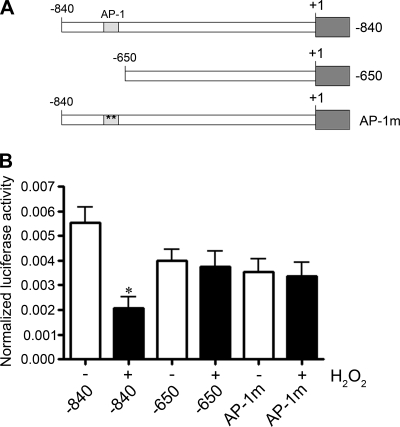
We initially examined the effect of H2O2 on eNOS transcription using several promoter constructs from the human eNOS gene fused upstream of a luciferase reporter (Fig. 1A). When FPAECs were transfected with constructs containing 840 bp of upstream promoter sequence (−840 eNOS promoter construct) and then treated with 200 μM H2O2 for 16–18 h, there was a 65% decrease (P < 0.05) in eNOS promoter activity. However, when FPAECs were transfected with a promoter construct containing only 640 bp of upstream sequence (lacking an AP-1 binding site at −661), the H2O2-mediated decrease in eNOS promoter activity was attenuated. To determine the effect of AP-1 on the regulation of eNOS promoter activity, we transfected FPAEC with a promoter construct identical to the −840 eNOS promoter construct except for a 2-bp mutation in the wild-type AP-1 sequence at −661 (from TGAGTCA to TGAGTtg). We were unable to detect any decrease in promoter activity with H2O2 (Fig. 1B). The 650-bp construct and AP-1mut construct both lack a functional AP-1 (−661) sequence, suggesting that AP-1 is regulating eNOS promoter activity in response to H2O2. In addition, in the absence of H2O2 treatment, there was a 30–35% decrease (although these decreases did not reach statistical significance) in promoter activity of the −650 and AP-1mut constructs compared with the −840 construct, suggesting AP-1 may be also be involved in basal regulation of the eNOS promoter.
Fig. 1.
Transcriptional activity of the human endothelial nitric oxide synthase (eNOS) promoter in response to H2O2 in fetal pulmonary arterial endothelial cells (FPAEC). A: the constructs used contain −840 or −650 [without the activator protein-1 (AP-1) binding site] of eNOS promoter DNA upstream of the transcription start site. AP-1 mutant oligonucleotide (AP-1mut) is identical to the −840-bp construct except for a 2-bp mutation in the AP-1 consensus sequence at −661. B: FPAECs were transfected either with the −840, the −650, or the AP-1mut eNOS promoter construct fused to a luciferase reporter gene. Cells were then treated with 200 μM H2O2 for 16 h. The luciferase activity and β-galactosidase activity were measured in the cell lysate, and the ratio of luciferase activity to β-galactosidase activity was used to determine the normalized eNOS promoter activity. H2O2 significantly decreased the activity of the −840 human eNOS promoter fragment but did not affect the AP-1mut and −650 constructs. Values expressed are means ± SE, n = 6. *P < 0.05 vs. untreated.
Effect of H2O2 on AP-1 binding.
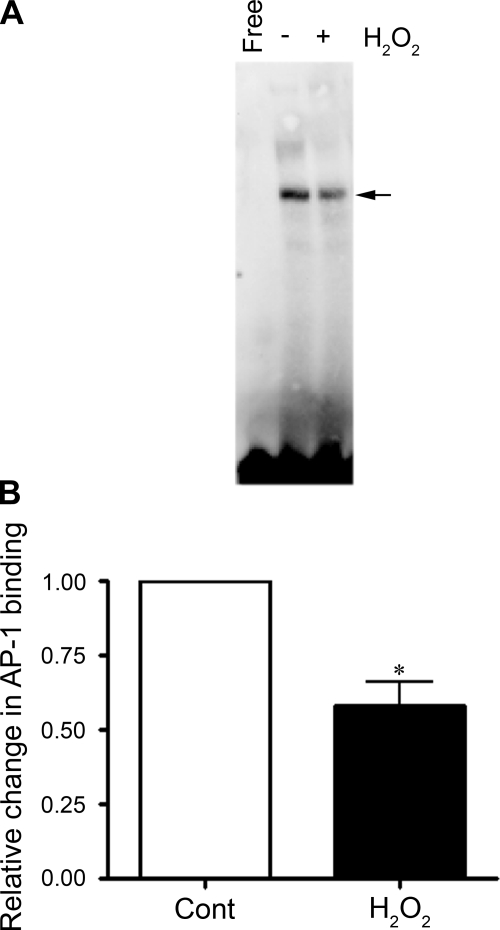
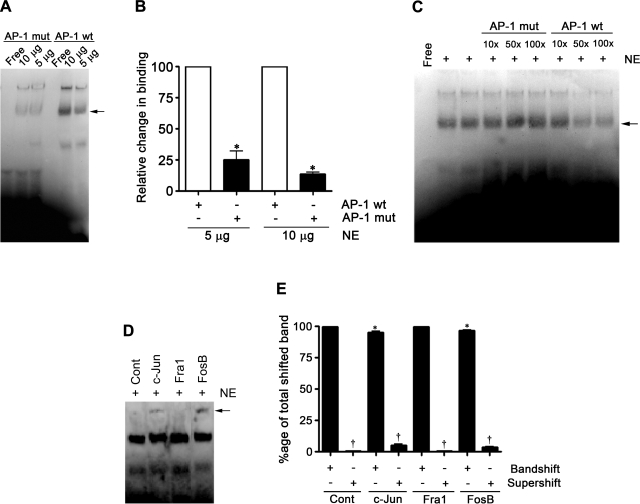
We next examined the effect of H2O2 on binding of AP-1 at −661 of the eNOS promoter. We performed EMSA using nuclear extracts prepared from FPAECs treated with 100 μM H2O2 for 2 h. Compared with control extracts, we observed a significant (42%) decrease in the binding of AP-1 (Fig. 2, A and B). We then carried out a study to confirm the specificity of AP-1 binding to an AP-1 oligonucleotide prepared from the eNOS promoter sequence. We mutated two nucleotides from the AP-1 consensus sequence, specifically CA to TG. The binding of the AP-1 complex was significantly decreased with this mutation (Fig. 3, A and B), and the specific AP-1 complex was competed out with 50× and 100× excesses of unlabeled wild-type AP-1 oligonucleotide but not with 10×, 50×, and 100× excesses of AP-1mut (Fig. 3C). To determine the composition of the AP-1 complex, we used anti-c-Jun, -FosB, and -Fra-1 antibodies in supershift analyses. The AP-1 complex was found to supershift with anti-c-Jun and anti-FosB antibodies but not with anti-Fra-1 (Fig. 3, D and E), suggesting that the AP-1 complex is a dimer of c-Jun and FosB. In addition, we found that the intensity of the band shift in c-Jun and FosB lanes was significantly decreased in the presence of the antibodies to c-Jun and FosB but not Fra-1 (Fig. 3, D and E).
Fig. 2.
Effect of H2O2 on transcription factor binding to the AP-1 cis-element in the human eNOS promoter. EMSA analyses were performed using a biotinylated AP-1 oligonucleotide corresponding to −661 of the human eNOS promoter. The biotinylated oligonucleotide was incubated for 20 min with nuclear extracts from FPAECs treated with 100 μM H2O2 for 2 h. The reaction was run on a 5% native polyacrylamide gel and transferred to a nylon membrane, and the biotinylated oligonucleotide was detected by using a streptavidin-horseradish peroxidase antibody and chemiluminescent substrate. Arrow indicates the position of the AP-1 complex. Lane marked Free is biotinylated oligonucleotide without any extract. The bar graph shows relative changes in band intensity for FPAECs exposed to H2O2 for 2 h. Band intensities were calculated by densitometric analysis. Binding is expressed as fold changes relative to untreated control (Cont). The values expressed are means ± SE, n = 6. *P < 0.05 vs. untreated.
Fig. 3.
Determination of the transcription factors constituting the AP-1 complex in FPAEC. A: EMSA using biotinylated probes containing sequences for AP-1 and AP-1mut, where 2 bp of the AP-1 consensus sequence TGAGTCA were changed to TGAGTtg and incubated with 5 or 10 μg of nuclear extracts (NE) from PAECs. Panel is representative of 3 independent experiments. B: densitometric analysis used to quantify the band intensity of the band shift of the AP-1 and AP-1mut oligonucleotide sequences in A identifies a significantly decreased binding to the AP-1mut compared with the wild-type (wt) sequence. *P < 0.05 vs. wild-type AP-1 sequence. C: to check the specificity of AP-1, binding competition was performed with 10×, 50×, and 100× excess of cold AP-1 wild-type or AP-1mut oligonucleotide. There was a decrease in the binding of AP-1 with 50× and 100× excess of wild-type but not with mutant. D: supershift assays were conducted by incubating NE with 4 μg of polyclonal antibodies specific to c-Jun, FosB, and Fra-1 overnight at 4°C. The supershifted complex is shown with an arrow. The complex is found to be composed of c-Jun and FosB but not Fra-1. Panel is representative of 3 independent supershift experiments. E: densitometric analysis was used to quantify the band intensities of the band shift and supershift and determine relative changes in band intensity. Binding is expressed as percentage of total shifted band. The values are expressed as means ± SE, n = 4. *P < 0.05 vs. band shift no antibody, †P < 0.05 vs. supershift no antibody.
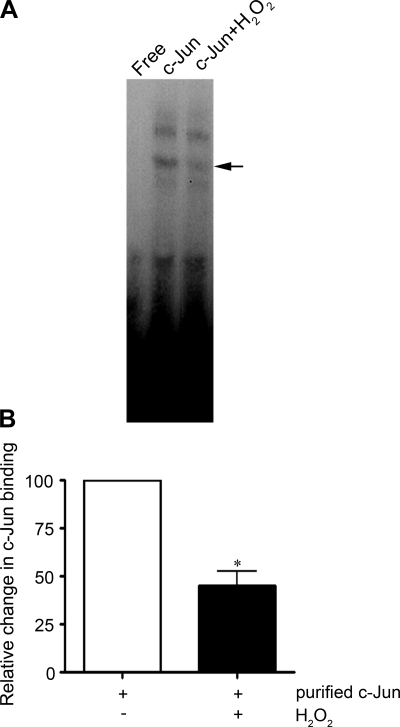
It has been established that c-Jun contains conserved redox-sensitive cysteine residues in the DNA-binding domain (1). Previously, it has been reported that c-Jun and AP-1 activity can be altered by S-nitrosylation of cysteine (30) or by chemical oxidation of cysteine residues (52). Thus we analyzed the effect of H2O2 on the ability of purified recombinant c-Jun to bind a consensus AP-1 sequence in vitro after challenge with H2O2. Our data indicate that H2O2 (10 μM for 20 min) induced a significant (50%; P < 0.05 vs. untreated) decrease in c-Jun binding during H2O2 treatment (Fig. 4, A and B), suggesting that the oxidation of c-Jun may be responsible for the loss of AP-1 transcription activity after H2O2 challenge.
Fig. 4.
Effect of H2O2 on the binding activity of recombinant c-Jun. A: recombinant c-Jun (1.2 μg) was treated with 10 μM H2O2 (for 20 min) and then used in the binding reaction. There is a decrease in the c-Jun-to-the-AP-1 consensus sequence after H2O2 exposure. A representative image from 3 separate experiments is shown. B: the bar graph shows relative changes in c-Jun binding with and without H2O2 treatment. Band intensities were calculated by densitometric analysis. Binding is expressed as fold changes relative to untreated control. The values expressed are means ± SE, n = 4. *P < 0.05 vs. untreated.
Effect of H2O2 on the binding of AP-1 to the endogenous eNOS promoter in vivo.
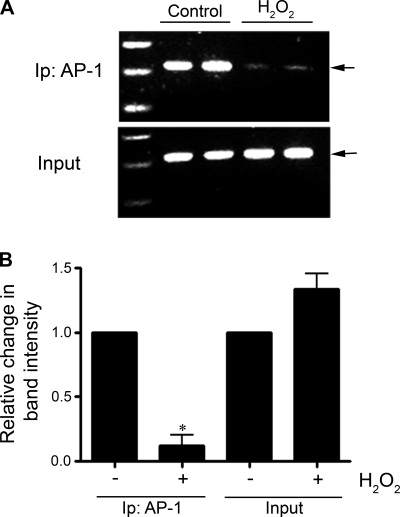
To examine the effect of H2O2 on the binding of AP-1 to the endogenous promoter in vivo, ChIP analysis was used. FPAECs were treated with H2O2 (100 μM, 2 h), and the cells were processed. Similar to the luciferase and EMSA data, we found that H2O2 induced a significant decrease in the binding of AP-1 to the eNOS promoter in vivo (Fig. 5).
Fig. 5.
The effect of H2O2 on AP-1 binding to the endogenous ovine eNOS promoter in FPAEC. Chromatin immunoprecipitation (ChIP) assays were conducted with FPAECs treated with H2O2 (100 μM, 2 h) followed by PCR using specific primers for the eNOS promoter. A: representative gel showing a decrease in PCR product with H2O2 treatment. There is a decrease in the binding of AP-1 to the eNOS promoter with H2O2 treatment. B: bar graph indicating that there is a significant decrease in the band intensity after H2O2 treatment, although there is no change in the levels of input DNA. Data are means ± SE, n = 6. *P < 0.05 vs. untreated. Ip, immunoprecipitation analysis.
DISCUSSION
NO is one of the primary components for blood-flow and -pressure regulation, and loss of NO bioavailability is a key feature of endothelial dysfunction. Multiple pathways can reduce NO bioavailability by altering its synthesis or biodegradation, but excessive production of ROS including H2O2 seems to be a major mechanism of reduced vascular NO levels (19). Increased production of ROS has been implicated in the pathogenesis of cardiovascular diseases such as atherosclerosis, restenosis, hypertension, diabetic vascular complication, and heart failure (6, 9, 28, 46). In PPHN, there is a decrease in eNOS expression (17). Surgical ligation of the ductus arterious in the fetal lamb generates a model of PPHN that resembles this condition in humans (2, 4, 50). Previously, our laboratory has reported a decrease in eNOS mRNA and protein and an increase in ET-1 expression in this ovine model of PPHN (4). These changes in gene expression are associated with an increase in both superoxide (8) and H2O2 (49), which are associated with increased activity of the NADPH oxidase complex. Our (47) previous in vitro studies demonstrated that exogenous ET-1 stimulated an increase in H2O2 within FPASMC, which led to decrease in eNOS promoter activity and eNOS activity in FPAEC cocultured with FPASMC. In addition, eNOS expression decreased when FPAECs were treated with H2O2 (47). Together, these data suggest a link between increased ROS production and decreased eNOS expression in PPHN lambs. However, the mechanisms involved are incompletely understood.
The eNOS promoter has binding sites for a variety of transcription factors in the setting of a TATA-less promoter, including Sp1, Ets, GATA, NF-1, AP-1, and shear stress-response element (29). A number of transcription factors including NF-κB, AP-1, Sp1, and GATA are involved in the regulation of eNOS expression basally or in response to exogenous stimuli (18, 48, 56). Of the above-mentioned factors, AP-1 and Sp1 are redox-sensitive transcription factors (31, 32, 40). AP-1 is known to be involved in the upregulation of eNOS transcription by cyclosporine A (33), hypoxia (22), and shear stress (48). Sp1 is known to be involved in the upregulation of eNOS expression by insulin (13), lysophosphatidylcholine (10, 53), and cyanidin-3-glucoside (54), and Sp1 is also involved in the basal transcription of the eNOS gene (45, 56). Recently, we (26) have reported that AP-1 upregulates eNOS expression when ECs were treated with antioxidant nordihydroguaiaretic acid (NDGA). Thus we were interested to study the potential roles of AP-1 in modulating eNOS promoter activity in response to H2O2 treatment. We found a decrease in promoter activity when FPAECs, transfected with our −840 promoter construct, were treated with H2O2. The −840 construct contains an AP-1 binding site at −661, and mutation of 2 bp within the consensus AP-1 binding sequence specifically disrupts DNA binding (5, 24). Previously, we (48) have shown that introduction of the same mutations within the AP-1 sequence (AP-1mut) prevented shear-induced increase in promoter activity in FPAEC. In this study, the AP-1mut construct did not exhibit H2O2-mediated decreases in eNOS promoter activity (Fig. 1B), suggesting the involvement of AP-1 in the redox regulation of eNOS transcription. Similarly the −650 construct, which is truncated just before the AP-1 site at −661, did not appear to respond significantly to H2O2 (Fig. 1B), suggesting AP-1 is the major player responsible for the H2O2-mediated decreases in eNOS expression.
For EMSA, we used the sequence from −677 to −648 of the human eNOS promoter as a probe. This region includes the AP-1 binding site. AP-1 binding decreased in response to 100 μM H2O2 treatment (Fig. 2, A and B). Again, we found that the binding of AP-1 decreased significantly when we mutated the AP-1 consensus sequence (Fig. 3, A and B), suggesting the complex we observed in EMSA was formed at the AP-1 element at −661 of the eNOS promoter. The AP-1 transcription factors consist of Jun (c-Jun, JunB, JunD, and v-Jun) homodimers or heterodimers of Jun with Fos (c-Fos, FosB, Fra-1, and Fra-2), and in previous studies it has been shown that regulation of c-Jun expression in ECs regulates eNOS expression. For example, in cyclosporine A-treated human umbilical vein endothelial cells (33) and shear stress-treated PAECs (48), increased c-Jun/AP-1 binding activity at the eNOS promoter AP-1 site are associated with the upregulation of eNOS mRNA and protein. By contrast, in high glucose-treated human aortic ECs, c-Jun/AP-1 binding to eNOS promoter is accompanied by decreased eNOS mRNA (42). In addition, it has been shown in the human hepatoma cell line HuH-7 there is an increase in c-Jun expression and AP-1 DNA binding with 0.5 to 5 mM H2O2 (55). This treatment was also found to be associated with a dose-dependent increase in necrosis (55). However, although our PAECs were treated with 100 to 200 μM H2O2, we were unable to detect any increase in necrosis as measured by LDH release. Indeed, we (51) have previously found that doses of H2O2 of ≥250 μM are necessary to cause increases in PAEC cell death both by necrosis and apoptosis. Furthermore, we found that the cell death was also associated with increased mitochondrial-derived superoxide (51). However, at the doses we have used in this study, no effects on mitochondrial activity were observed (51).
Typically, AP-1 forms complexes with Jun (c-Jun, JunB, JunD, and v-Jun) and Fos (c-Fos, FosB, Fra-1, and Fra-2). In this study, we found that the AP-1 complex is composed of c-Jun and FosB, since it was supershifted with c-Jun- and FosB-specific antibodies (Fig. 3, D and E). Recently, it has been reported that overexpression of c-Jun increased eNOS expression in uterine artery ECs (36). Both c-Jun and c-Fos contain conserved redox-sensitive cysteine residues in the DNA-binding domains (1), and changes in redox potential can regulate AP-1 activity via these residues. Previously, cellular and in vitro studies using NO donor compounds have demonstrated that NO can inhibit the activity of c-Jun and bind to AP-1 sequence (34, 44). Furthermore, the activity of the AP-1 complex can be inhibited through S-nitrosylation of cysteine residues of c-Jun by NO (30) or by chemical oxidation of c-Jun cysteine residues (52). In confirmation of previous studies, we also observed decreased binding of purified recombinant c-Jun following H2O2 treatment (Fig. 4, A and B). For our promoter analysis study, we used a human eNOS promoter transfected into ovine FPAECs. Sequence analysis of the recently cloned ovine eNOS promoter identified a consensus AP-1 binding at −682 to −676 (36). To confirm the effect of H2O2 on AP-1 binding to the ovine eNOS promoter in vivo, we performed ChIP assays with FPAECs treated with 100 μM H2O2 for 2 h. We demonstrated a decrease in AP-1 binding, indicating that H2O2 also regulates AP-1 binding to the eNOS promoter in vivo.
It has been reported that H2O2 can increase eNOS promoter activity, eNOS mRNA stability, as well as eNOS activity as measured by the conversion of l-arginine to l-citrulline in bovine aortic endothelial cells (BAECs) (11, 41). Previously, our group (47) has shown that eNOS expression increased at lower H2O2 concentration and that BAECs have twofold higher catalase activity than FPAECs. In the same report, it has been shown that when BAECs and FPAECs were transfected with an eNOS promoter construct and treated with the same amount of H2O2, BAECs had increased eNOS promoter activity, whereas FPAECs had decreased eNOS promoter activity. Thus the effective concentration of H2O2 inside ovine FPAECs may be higher than BAECs as they have lower catalase activity. This suggests that the antioxidant capacity of the cells may also affect the eNOS expression. Recently, we have reported that, in the lamb, lung antioxidant enzymes catalase, SOD1, and SOD2 are regulated both by development and by increased pulmonary blood flow (39). Additional research is therefore warranted to characterize further the complex redox regulation of eNOS expression in ECs.
Overall, our data suggest a major role for AP-1 in the downregulation of eNOS expression in FPAEC treated with H2O2. We conclude that H2O2 decreases AP-1 binding at the eNOS promoter, resulting in decreased promoter activity. It has been reported previously that AP-1 is involved in modulating eNOS expression in response to anoxia (38) and hypoxia (22). However, we believe this to be the first report suggesting that AP-1 plays a major role in regulating eNOS expression in response to H2O2. By characterizing the molecular mechanisms of redox regulation of eNOS expression in vivo, we hope to identify potential therapies to reduce or prevent endothelial dysfunction in diseases such as PPHN where decreased eNOS expression is associated with increased ROS production.
GRANTS
This research was supported, in part, by National Heart, Lung, and Blood Institute Grants HL-60190, HL-67841, HL-72123, and HL-70061 and a Fondation Leducq Grant, all to S. M. Black.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Abate C, Patel L, Rauscher FJ 3rd, Curran T. Redox regulation of fos and jun DNA-binding activity in vitro. Science 249: 1157–1161, 1990. [DOI] [PubMed] [Google Scholar]
- 2.Abman SH, Shanley PF, Accurso FJ. Failure of postnatal adaptation of the pulmonary circulation after chronic intrauterine pulmonary hypertension in fetal lambs. J Clin Invest 83: 1849–1858, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aicher A, Heeschen C, Mildner-Rihm C, Urbich C, Ihling C, Technau-Ihling K, Zeiher AM, Dimmeler S. Essential role of endothelial nitric oxide synthase for mobilization of stem and progenitor cells. Nat Med 9: 1370–1376, 2003. [DOI] [PubMed] [Google Scholar]
- 4.Black SM, Johengen MJ, Soifer SJ. Coordinated regulation of genes of the nitric oxide and endothelin pathways during the development of pulmonary hypertension in fetal lambs. Pediatr Res 44: 821–830, 1998. [DOI] [PubMed] [Google Scholar]
- 5.Bohmann D, Bos TJ, Admon A, Nishimura T, Vogt PK, Tjian R. Human proto-oncogene c-jun encodes a DNA binding protein with structural and functional properties of transcription factor AP-1. Science 238: 1386–1392, 1987. [DOI] [PubMed] [Google Scholar]
- 6.Brandes RP, Kreuzer J. Vascular NADPH oxidases: molecular mechanisms of activation. Cardiovasc Res 65: 16–27, 2005. [DOI] [PubMed] [Google Scholar]
- 7.Braunstein M, Rose AB, Holmes SG, Allis CD, Broach JR. Transcriptional silencing in yeast is associated with reduced nucleosome acetylation. Genes Dev 7: 592–604, 1993. [DOI] [PubMed] [Google Scholar]
- 8.Brennan LA, Steinhorn RH, Wedgwood S, Mata-Greenwood E, Roark EA, Russell JA, Black SM. Increased superoxide generation is associated with pulmonary hypertension in fetal lambs: a role for NADPH oxidase. Circ Res 92: 683–691, 2003. [DOI] [PubMed] [Google Scholar]
- 9.Cai H NAD(P)H oxidase-dependent self-propagation of hydrogen peroxide and vascular disease. Circ Res 96: 818–822, 2005. [DOI] [PubMed] [Google Scholar]
- 10.Cieslik K, Zembowicz A, Tang JL, Wu KK. Transcriptional regulation of endothelial nitric-oxide synthase by lysophosphatidylcholine. J Biol Chem 273: 14885–14890, 1998. [DOI] [PubMed] [Google Scholar]
- 11.Drummond GR, Cai H, Davis ME, Ramasamy S, Harrison DG. Transcriptional and posttranscriptional regulation of endothelial nitric oxide synthase expression by hydrogen peroxide. Circ Res 86: 347–354, 2000. [DOI] [PubMed] [Google Scholar]
- 12.Fineman JR, Soifer SJ, Heymann MA. Regulation of pulmonary vascular tone in the perinatal period. Annu Rev Physiol 57: 115–134, 1995. [DOI] [PubMed] [Google Scholar]
- 13.Fisslthaler B, Benzing T, Busse R, Fleming I. Insulin enhances the expression of the endothelial nitric oxide synthase in native endothelial cells: a dual role for Akt and AP-1. Nitric Oxide 8: 253–261, 2003. [DOI] [PubMed] [Google Scholar]
- 14.Freedman JE, Sauter R, Battinelli EM, Ault K, Knowles C, Huang PL, Loscalzo J. Deficient platelet-derived nitric oxide and enhanced hemostasis in mice lacking the NOSIII gene. Circ Res 84: 1416–1421, 1999. [DOI] [PubMed] [Google Scholar]
- 15.Fridovich I Superoxide radical and superoxide dismutases. Annu Rev Biochem 64: 97–112, 1995. [DOI] [PubMed] [Google Scholar]
- 16.Furchgott RF, Vanhoutte PM. Endothelium-derived relaxing and contracting factors. FASEB J 3: 2007–2018, 1989. [PubMed] [Google Scholar]
- 17.Giaid A, Saleh D. Reduced expression of endothelial nitric oxide synthase in the lungs of patients with pulmonary hypertension. N Engl J Med 333: 214–221, 1995. [DOI] [PubMed] [Google Scholar]
- 18.Grumbach IM, Chen W, Mertens SA, Harrison DG. A negative feedback mechanism involving nitric oxide and nuclear factor kappa-B modulates endothelial nitric oxide synthase transcription. J Mol Cell Cardiol 39: 595–603, 2005. [DOI] [PubMed] [Google Scholar]
- 19.Guzik TJ, Harrison DG. Vascular NADPH oxidases as drug targets for novel antioxidant strategies. Drug Discov Today 11: 524–533, 2006. [DOI] [PubMed] [Google Scholar]
- 20.Hassoun PM, Thappa V, Landman MJ, Fanburg BL. Endothelin 1: mitogenic activity on pulmonary artery smooth muscle cells and release from hypoxic endothelial cells. Proc Soc Exp Biol Med 199: 165–170, 1992. [DOI] [PubMed] [Google Scholar]
- 21.Haworth SG, Reid L. Persistent fetal circulation: newly recognized structural features. J Pediatr 88: 614–620, 1976. [DOI] [PubMed] [Google Scholar]
- 22.Hoffmann A, Gloe T, Pohl U. Hypoxia-induced upregulation of eNOS gene expression is redox-sensitive: a comparison between hypoxia and inhibitors of cell metabolism. J Cell Physiol 188: 33–44, 2001. [DOI] [PubMed] [Google Scholar]
- 23.Huang PL, Huang Z, Mashimo H, Bloch KD, Moskowitz MA, Bevan JA, Fishman MC. Hypertension in mice lacking the gene for endothelial nitric oxide synthase. Nature 377: 239–242, 1995. [DOI] [PubMed] [Google Scholar]
- 24.Kaluzova M, Pastorekova S, Svastova E, Pastorek J, Stanbridge EJ, Kaluz S. Characterization of the MN/CA 9 promoter proximal region: a role for specificity protein (SP) and activator protein 1 (AP1) factors. Biochem J 359: 669–677, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karantzoulis-Fegaras F, Antoniou H, Lai SL, Kulkarni G, D'Abreo C, Wong GK, Miller TL, Chan Y, Atkins J, Wang Y, Marsden PA. Characterization of the human endothelial nitric-oxide synthase promoter. J Biol Chem 274: 3076–3093, 1999. [DOI] [PubMed] [Google Scholar]
- 26.Kumar S, Wedgwood S, Black SM. Nordihydroguaiaretic acid increases endothelial nitric oxide synthase expression via the transcription factor AP-1. DNA Cell Biol 26: 853–862, 2007. [DOI] [PubMed] [Google Scholar]
- 27.Lee PC, Salyapongse AN, Bragdon GA, Shears LL 2nd, Watkins SC, Edington HD, Billiar TR. Impaired wound healing and angiogenesis in eNOS-deficient mice. Am J Physiol Heart Circ Physiol 277: H1600–H1608, 1999. [DOI] [PubMed] [Google Scholar]
- 28.Li JM, Shah AM. Endothelial cell superoxide generation: regulation and relevance for cardiovascular pathophysiology. Am J Physiol Regul Integr Comp Physiol 287: R1014–R1030, 2004. [DOI] [PubMed] [Google Scholar]
- 29.Marsden PA, Heng HH, Scherer SW, Stewart RJ, Hall AV, Shi XM, Tsui LC, Schappert KT. Structure and chromosomal localization of the human constitutive endothelial nitric oxide synthase gene. J Biol Chem 268: 17478–17488, 1993. [PubMed] [Google Scholar]
- 30.Marshall HE, Merchant K, Stamler JS. Nitrosation and oxidation in the regulation of gene expression. FASEB J 14: 1889–1900, 2000. [DOI] [PubMed] [Google Scholar]
- 31.Meyer M, Pahl HL, Baeuerle PA. Regulation of the transcription factors NF-kappa B and AP-1 by redox changes. Chem Biol Interact 91: 91–100, 1994. [DOI] [PubMed] [Google Scholar]
- 32.Meyer M, Schreck R, Baeuerle PA. H2O2 and antioxidants have opposite effects on activation of NF-kappa B and AP-1 in intact cells: AP-1 as secondary antioxidant-responsive factor. EMBO J 12: 2005–2015, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Navarro-Antolin J, Rey-Campos J, Lamas S. Transcriptional induction of endothelial nitric oxide gene by cyclosporine A. A role for activator protein-1. J Biol Chem 275: 3075–3080, 2000. [DOI] [PubMed] [Google Scholar]
- 34.Nikitovic D, Holmgren A, Spyrou G. Inhibition of AP-1 DNA binding by nitric oxide involving conserved cysteine residues in Jun and Fos. Biochem Biophys Res Commun 242: 109–112, 1998. [DOI] [PubMed] [Google Scholar]
- 35.Palmer RM, Ashton DS, Moncada S. Vascular endothelial cells synthesize nitric oxide from l-arginine. Nature 333: 664–666, 1988. [DOI] [PubMed] [Google Scholar]
- 36.Qian XX, Mata-Greenwood E, Liao WX, Zhang H, Zheng J, Chen DB. Transcriptional regulation of endothelial nitric oxide synthase expression in uterine artery endothelial cells by c-Jun/AP-1. Mol Cell Endocrinol 279: 39–51, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rudic RD, Shesely EG, Maeda N, Smithies O, Segal SS, Sessa WC. Direct evidence for the importance of endothelium-derived nitric oxide in vascular remodeling. J Clin Invest 101: 731–736, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rui T, Kvietys PR. NFkappaB and AP-1 differentially contribute to the induction of Mn-SOD and eNOS during the development of oxidant tolerance. FASEB J 19: 1908–1910, 2005. [DOI] [PubMed] [Google Scholar]
- 39.Sharma S, Grobe AC, Wiseman DA, Kumar S, Englaish M, Najwer I, Benavidez E, Oishi P, Azakie A, Fineman JR, Black SM. Lung antioxidant enzymes are regulated by development and increased pulmonary blood flow. Am J Physiol Lung Cell Mol Physiol 293: L960–L971, 2007. [DOI] [PubMed] [Google Scholar]
- 40.Shi J, Wang X, Qiu J, Si Q, Sun R, Guo H, Wu Q. Roles of NF-kappaB and SP-1 in oxidative stress-mediated induction of platelet-derived growth factor-B by TNFalpha in human endothelial cells. J Cardiovasc Pharmacol 44: 26–34, 2004. [DOI] [PubMed] [Google Scholar]
- 41.Shimizu S, Nomoto M, Naito S, Yamamoto T, Momose K. Stimulation of nitric oxide synthase during oxidative endothelial cell injury. Biochem Pharmacol 55: 77–83, 1998. [DOI] [PubMed] [Google Scholar]
- 42.Srinivasan S, Hatley ME, Bolick DT, Palmer LA, Edelstein D, Brownlee M, Hedrick CC. Hyperglycaemia-induced superoxide production decreases eNOS expression via AP-1 activation in aortic endothelial cells. Diabetologia 47: 1727–1734, 2004. [DOI] [PubMed] [Google Scholar]
- 43.Steinhorn RH, Millard SL, Morin FC 3rd. Persistent pulmonary hypertension of the newborn. Role of nitric oxide and endothelin in pathophysiology and treatment. Clin Perinatol 22: 405–428, 1995. [PubMed] [Google Scholar]
- 44.Tabuchi A, Sano K, Oh E, Tsuchiya T, Tsuda M. Modulation of AP-1 activity by nitric oxide (NO) in vitro: NO-mediated modulation of AP-1. FEBS Lett 351: 123–127, 1994. [DOI] [PubMed] [Google Scholar]
- 45.Tang JL, Zembowicz A, Xu XM, Wu KK. Role of Sp1 in transcriptional activation of human nitric oxide synthase type III gene. Biochem Biophys Res Commun 213: 673–680, 1995. [DOI] [PubMed] [Google Scholar]
- 46.Touyz RM, Schiffrin EL. Reactive oxygen species in vascular biology: implications in hypertension. Histochem Cell Biol 122: 339–352, 2004. [DOI] [PubMed] [Google Scholar]
- 47.Wedgwood S, Black SM. Endothelin-1 decreases endothelial NOS expression and activity through ETA receptor-mediated generation of hydrogen peroxide. Am J Physiol Lung Cell Mol Physiol 288: L480–L487, 2005. [DOI] [PubMed] [Google Scholar]
- 48.Wedgwood S, Mitchell CJ, Fineman JR, Black SM. Developmental differences in the shear stress-induced expression of endothelial NO synthase: changing role of AP-1. Am J Physiol Lung Cell Mol Physiol 284: L650–L662, 2003. [DOI] [PubMed] [Google Scholar]
- 49.Wedgwood S, Steinhorn RH, Bunderson M, Wilham J, Lakshminrusimha S, Brennan LA, Black SM. Increased hydrogen peroxide downregulates soluble guanylate cyclase in the lungs of lambs with persistent pulmonary hypertension of the newborn. Am J Physiol Lung Cell Mol Physiol 289: L660–L666, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wild LM, Nickerson PA, Morin FC 3rd. Ligating the ductus arteriosus before birth remodels the pulmonary vasculature of the lamb. Pediatr Res 25: 251–257, 1989. [DOI] [PubMed] [Google Scholar]
- 51.Wiseman DA, Wells SM, Hubbard M, Welker JE, Black SM. Alterations in zinc homeostasis underlie endothelial cell death induced by oxidative stress from acute exposure to hydrogen peroxide. Am J Physiol Lung Cell Mol Physiol 292: L165–L177, 2007. [DOI] [PubMed] [Google Scholar]
- 52.Xanthoudakis S, Miao G, Wang F, Pan YC, Curran T. Redox activation of Fos-Jun DNA binding activity is mediated by a DNA repair enzyme. EMBO J 11: 3323–3335, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xing F, Jiang Y, Liu J, Zhao K, Mo Y, Qin Q, Wang J, Ouyang J, Zeng Y. Role of AP1 element in the activation of human eNOS promoter by lysophosphatidylcholine. J Cell Biochem 98: 872–884, 2006. [DOI] [PubMed] [Google Scholar]
- 54.Xu JW, Ikeda K, Yamori Y. Upregulation of endothelial nitric oxide synthase by cyanidin-3-glucoside, a typical anthocyanin pigment. Hypertension 44: 217–222, 2004. [DOI] [PubMed] [Google Scholar]
- 55.Xu Y, Bradham C, Brenner DA, Czaja MJ. Hydrogen peroxide-induced liver cell necrosis is dependent on AP-1 activation. Am J Physiol Gastrointest Liver Physiol 273: G795–G803, 1997. [DOI] [PubMed] [Google Scholar]
- 56.Zhang R, Min W, Sessa WC. Functional analysis of the human endothelial nitric oxide synthase promoter. Sp1 and GATA factors are necessary for basal transcription in endothelial cells. J Biol Chem 270: 15320–15326, 1995. [DOI] [PubMed] [Google Scholar]