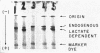Abstract
The effect of growth conditions on the specific activity of nicotinamide adenine dinucleotide (NAD)-linked lactate dehydrogenase (LDH) in extracts of Staphylococcus aureus strain SG 511A was examined. Kinetic and electrophoretic experiments, with extracts prepared from aerobically and anaerobically grown cells, provided evidence for only one physiologically significant enzyme. The aerobic level of NAD-linked LDH of S. aureus remained constant and was independent of the carbon source. In contrast, the level of LDH produced in an anaerobic environment was variable and was dependent on the carbon source. Growth anaerobically on pyruvate, as the sole fermentable carbon source, resulted in a maximal level of LDH activity, a value about eightfold greater than the aerobic level. Anaerobic growth either on pyruvate plus glucose or on glucose alone, however, resulted in approximately a threefold decrease in this maximum. Experiments with a heme-requiring auxotroph derived from S. aureus demonstrated that the aerobic level of LDH activity was dependent on a functional respiratory chain.
Full text
PDF
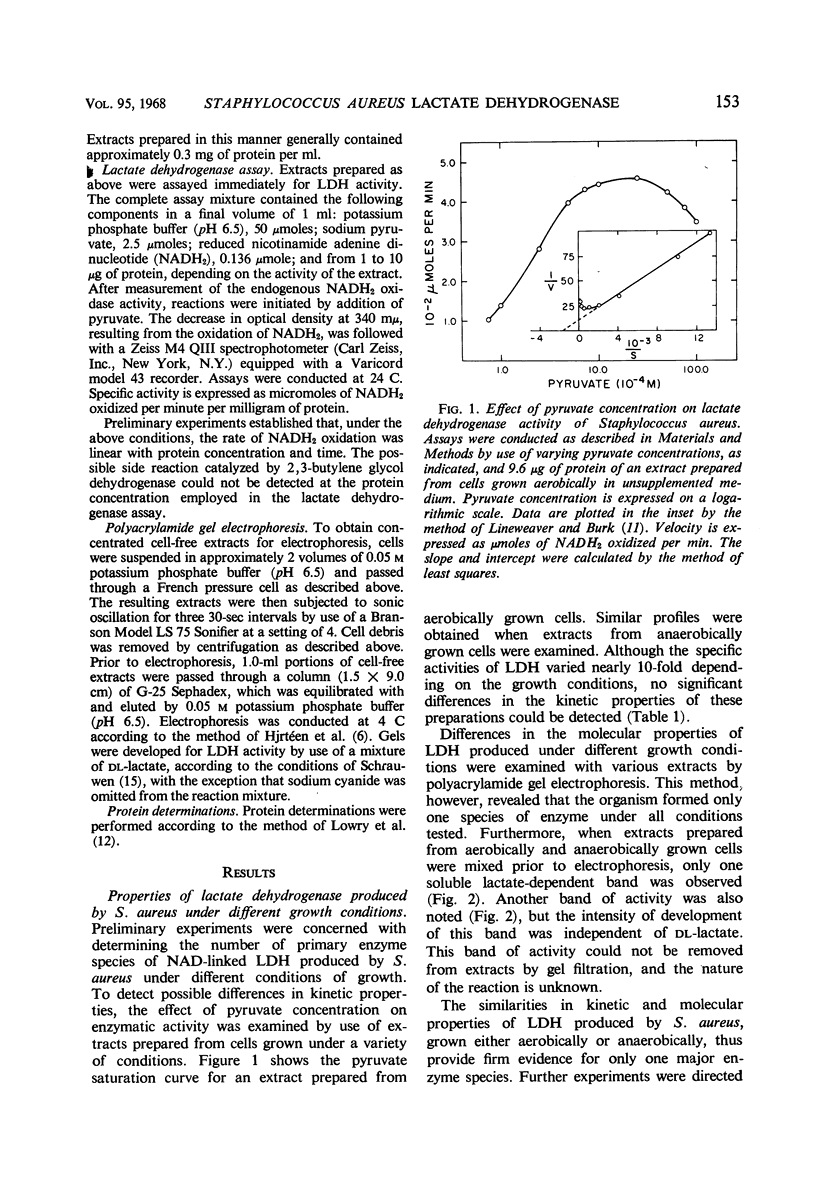
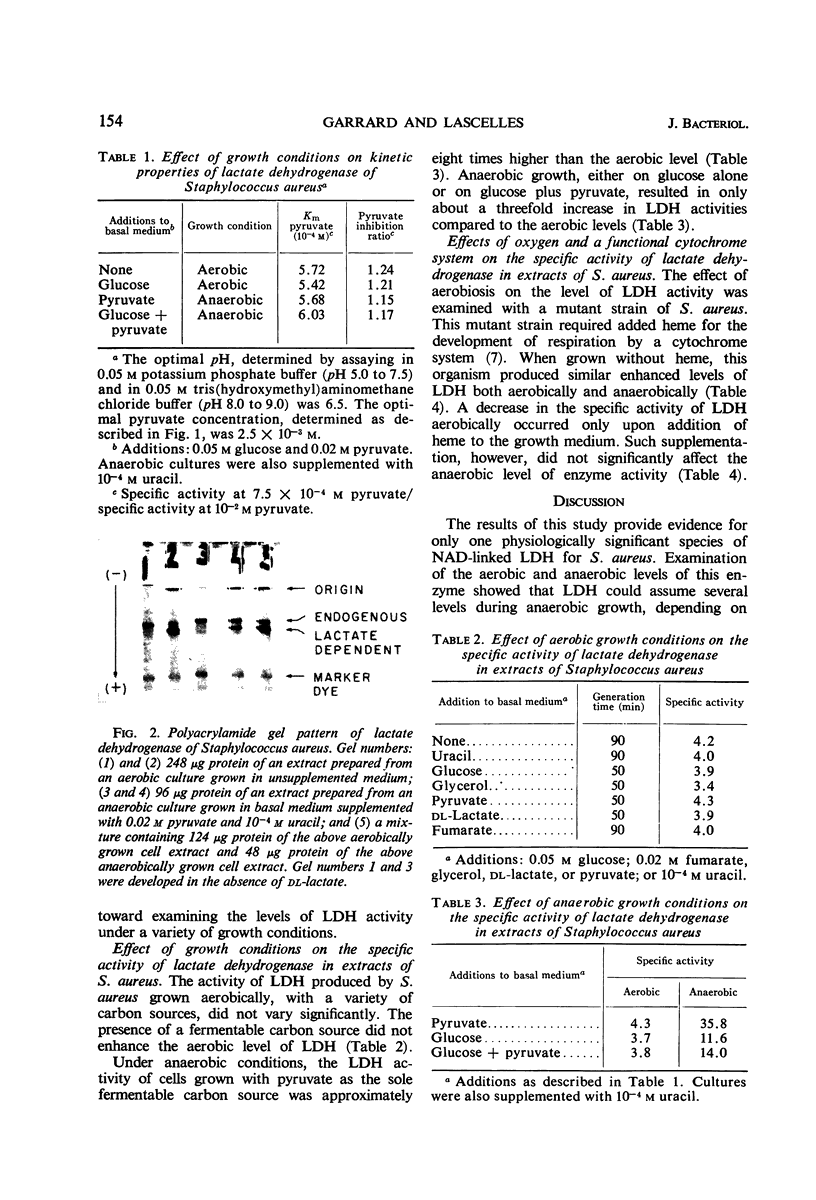


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CHANG J. P., LASCELLES J. NITRATE REDUCTASE IN CELL-FREE EXTRACTS OF A HAEMIN-REQUIRING STRAIN OF STAPHYLOCOCCUS AUREUS. Biochem J. 1963 Dec;89:503–510. doi: 10.1042/bj0890503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COLLINS F. M., LASCELLES J. The effect of growth conditions on oxidative and dehydrogenase activity in Staphylococcus aureus. J Gen Microbiol. 1962 Nov;29:531–535. doi: 10.1099/00221287-29-3-531. [DOI] [PubMed] [Google Scholar]
- Cahn R. D., Zwilling E., Kaplan N. O., Levine L. Nature and Development of Lactic Dehydrogenases: The two major types of this enzyme form molecular hybrids which change in makeup during development. Science. 1962 Jun 15;136(3520):962–969. doi: 10.1126/science.136.3520.962. [DOI] [PubMed] [Google Scholar]
- GARDNER J. F., LASCELLES J. The requirement for acetate of a streptomycin-resistant strain of Staphylococcus aureus. J Gen Microbiol. 1962 Sep;29:157–164. doi: 10.1099/00221287-29-1-157. [DOI] [PubMed] [Google Scholar]
- Goodfriend T. L., Sokol D. M., Kaplan N. O. Control of synthesis of lactic acid dehydrogenases. J Mol Biol. 1966 Jan;15(1):18–31. doi: 10.1016/s0022-2836(66)80206-1. [DOI] [PubMed] [Google Scholar]
- Hjertén S., Jerstedt S., Tiselius A. Some aspects of the use of "continuous" and "discontinuous" buffer systems in polyacrylamide gel electrophoresis. Anal Biochem. 1965 May;11(2):219–223. doi: 10.1016/0003-2697(65)90008-4. [DOI] [PubMed] [Google Scholar]
- Jones R. G., Lascelles J. The relationship of 4-hydroxybenzoic acid to lysine and methionine formation in Escherichia coli. Biochem J. 1967 Jun;103(3):709–713. doi: 10.1042/bj1030709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAPLAN N. O. LACTATE DEHYDROGENASE--STRUCTURE AND FUNCTION. Brookhaven Symp Biol. 1964 Dec;17:131–153. [PubMed] [Google Scholar]
- KAPLAN N. O. Symposium on multiple forms of enzymes and control mechanisms. I. Multiple forms of enzymes. Bacteriol Rev. 1963 Jun;27:155–169. doi: 10.1128/br.27.2.155-169.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- PESCE A., MCKAY R. H., STOLZENBACH F., CAHN R. D., KAPLAN N. O. THE COMPARATIVE ENZYMOLOGY OF LACTIC DEHYDROGENASES. I. PROPERTIES OF THE CRYSTALLINE BEEF AND CHICKEN ENZYMES. J Biol Chem. 1964 Jun;239:1753–1761. [PubMed] [Google Scholar]
- Richardson G. M. The nutrition of Staphylococcus aureus. Necessity for uracil in anaerobic growth. Biochem J. 1936 Dec;30(12):2184–2190. doi: 10.1042/bj0302184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILSON A. C., CAHN R. D., KAPLAN N. O. Functions of the two forms of lactic dehydrogenase in the breast muscle of birds. Nature. 1963 Jan 26;197:331–334. doi: 10.1038/197331a0. [DOI] [PubMed] [Google Scholar]
- WOLIN M. J. FRUCTOSE-1,6-DIPHOSPHATE REQUIREMENT OF STREPTOCOCCAL LACTIC DEHYDROGENASES. Science. 1964 Nov 6;146(3645):775–777. doi: 10.1126/science.146.3645.775. [DOI] [PubMed] [Google Scholar]