Abstract
Growth hormone (GH) function is mediated through multiple endocrine pathways. In the liver, GH also transcriptionally activates hepatocyte nuclear factor-6 (HNF-6; OC-1), a liver-enriched transcription factor that regulates the expression of genes essential to hepatic function. We hypothesize that GH modulates hepatic function in the normal and injured liver through HNF-6 and HNF-6 target genes. CD1 mice received PBS or GH for the 1-, 7-, and 28-day course of Sham operation or bile duct ligation (BDL). Proliferation-, metabolic-, and profibrotic-specific hepatic functions were assessed with a focus on candidate HNF-6 transcriptional target genes. Confirmation of HNF-6 regulation was done by analysis of target gene expression in liver infected with recombinant adenovirus AdHNF-6 expression vectors. GH administration upregulated HNF-6 expression throughout the course of liver injury. This was associated with increased expression of HNF-6 proliferative target genes cyclin D1 and metabolic gene Cyp7A1 and downregulation of profibrogenic TGFb2R. Hepatic function improved such as enhanced hepatocyte proliferation, higher cholesterol clearance throughout the course of injury, and attenuated fibrogenic response at day 28 of BDL. GH treatment also transcriptionally increased albumin expression in an HNF-6-independent manner. This was associated with enhanced serum albumin levels. In conclusion, the GH/HNF-6 axis is a potential in vivo mechanism underlying GH diverse function in the liver to modulate the liver repair response to BDL.
Keywords: bile duct ligation, liver regeneration, liver fibrosis, cholestasis, cholesterol metabolism, cyclin D1, Cyp7A1, TGFb2R
the liver performs the essential functions of detoxification, bile transport, and metabolic homeostasis. Functional analyses of promoter regions of many hepatocyte-specific genes reveal the presence of multiple cis-DNA sequences with binding sites for different families of trans-activating hepatocyte nuclear factors (HNF) that act cooperatively to modulate hepatic gene function (7). Among these regulatory proteins, HNF-6 (also known as OC-1) transcriptionally activates target genes by using the ONECUT-homeodomain sequence to localize HNF-6 protein to the nuclear compartment and to bind to specific DNA sequences of hepatic target gene promoters. Many of HNF-6 target genes serve important function in hepatic glucose, protein, and lipid metabolism, as well as hepatocyte DNA synthesis (37).
Hepatic HNF-6 is in turn regulated by growth hormone (GH) through GH-mediated STAT-5 transcriptional activation of HNF-6 promoter (21). Since the liver is a primary target organ for GH effect in basic hepatic function (5, 41) as well as in potentiating liver regeneration following partial hepatectomy (PH) (4, 27), we hypothesize that GH regulation of in vivo hepatic function in normal liver physiology as well as during liver injury can be mediated in part by HNF-6 and HNF-6 target genes. This would be a potential additional mechanism to the previously demonstrated hepatic GH actions mediated through IGF-I (11, 17).
Using biliary obstruction as the model of liver injury, we sought to define the in vivo biological role of HNF-6 in the liver repair response to biliary injury. We targeted candidate HNF-6 genes with proliferation-, metabolic-, and profibrotic-specific functions that are likely to be involved in the acute phase of injury (first week) and the chronic phase (4 wk) of the liver repair response to bile duct ligation (BDL) injury.
METHODS
Animal procedures.
Male CD1 mice 6–8 wk old were kept in a 12-h light-dark cycle with free access to standard chow and water. All animals received humane care according to the criteria outlined in the Guide for the Care and Use of Laboratory Animals by the National Academy of Sciences and the National Institutes of Health. Experiments were conducted under IRB approved.
GH administration and BDL.
All procedures were performed under ketamine (100 mg/kg body wt ip) and xylazine (5 mg/kg body wt ip) anesthesia. For short-term 24-h treatment, mice received an initial intraperitoneal injection of human recombinant GH (obtained from the National Institute of Diabetes and Digestive and Kidney Diseases National Hormone and Peptide Program) at 4 μg/g body wt followed by a 3 μg/g body wt injection every 6 h. For long-term administration (1 and 4 wk), the Alzet miniosmotic pumps (Duret, Cupertino, CA) were preloaded with PBS or GH (200 μl volume for a continuous rate of 5 μg/h) and implanted in a subcutaneous pocket in the back.
To assess the effect of GH on liver injury, the common bile duct was ligated and divided between ligatures. In Sham mice (n = 4), the duct was exposed without scission. Mice from the day 1 GHBDL group (n = 8–10) received the initial GH intraperitoneal injection at the conclusion of the surgery and every 6 h after. Mice from the days 7 (n = 8–10) and 28 (n = 10–12) GHBDL groups underwent minipump implantation at the time of BDL.
Two hours before euthanasia, mice were weighed and 50 μg/g body wt of 5-bromo-2-deoxyuridine (BrdU) was injected intraperitoneally. Serum was collected by cardiac puncture. The liver was dissected out, weighed, and processed for total RNA and protein extraction or for paraffin fixation and embedding.
Liver weight assessment.
The liver was dissected intact and all visibly dilated bile ducts from BDL liver were punctured to drain all residual bile. A wet liver weight (wt liver) was obtained and liver mass was reported as a percent of body weight at the time of euthanasia (wt liver/body wt × 100).
Adenovirus infection.
Mice underwent tail vein injection of 200 μl of mock virus or 2 × 1011 particles of recombinant adenovirus vectors expressing bacterial LacZ (AdLacZ) (n = 3) or mouse HNF-6 (AdHNF-6) cDNA (n = 4) and euthanized after 24 h. The preparation of the replication defective AdHNF-6 or AdLacZ have been described (36).
Immunostaining.
Assessments for BrdU incorporation and HNF-6 expression were described previously (18). Staining for HNF-6 was detected by using the diaminobenzidine substrate (Vector Laboratories). The percent of proliferating hepatocyte was calculated as percent of BrdU-positive hepatocytes over total hepatocyte count of 1,000–2,000 hepatocytes/mouse.
Western blot analyses.
TGFb2R, cyclin D1, Cyp7A1 (Santa Cruz Biotechnologies), α-smooth muscle actin (α-SMA), and β-actin (Sigma-Aldrich) antibodies were used. The immune complexes were detected with horseradish-conjugated secondary antibody (Fisher) followed by chemiluminescence (ECL + plus, Amersham Biosciences)
Real-time RT-PCR to determine mRNA expression levels.
Total liver RNA was extracted by using RNA-STAT-60 (Tel-Test B, Friendswood, TX). Following DNaseI (Ambion, Austin, Texas) digestion, cDNA was synthesized by use of the cDNA Synthesis Kit (Bio-Rad, Hercules, CA) and purified through a Qiagen column. Reactions were amplified with the appropriate primer sets and analyzed in triplicate by use of a MyiQ Single Color Real-Time PCR Detection System (Bio-Rad). The relative expression of the genes of interest was calculated by a mathematical delta-delta method developed by PE Applied Biosystems. Levels were normalized to housekeeping gene cyclophilin for each gene and reported relative to Sham-operated PBS control liver (set at 1).
Ribonuclease protection assay.
Total RNA were hybridized with [32P] UTP-labeled antisense RNA probes and digested with RNase ONE (Promega). Syntheses for antisense mouse HNF-6 have been described (31).
Statistics.
Data are shown as means ± SD. Intergroup differences were evaluated by analysis of variance. A P value ≤0.05 is considered to be significant.
RESULTS
HNF-6 expression is upregulated in the liver following GH treatment.
To examine the downstream hepatic effects of GH, we performed RNAse protection assay in PBS-or GH-treated Sham mice, which showed that, in addition to IGF-I induction (data not shown), GH administration (n = 4) led to consistent upregulation of HNF-6 liver expression (Fig. 1A).
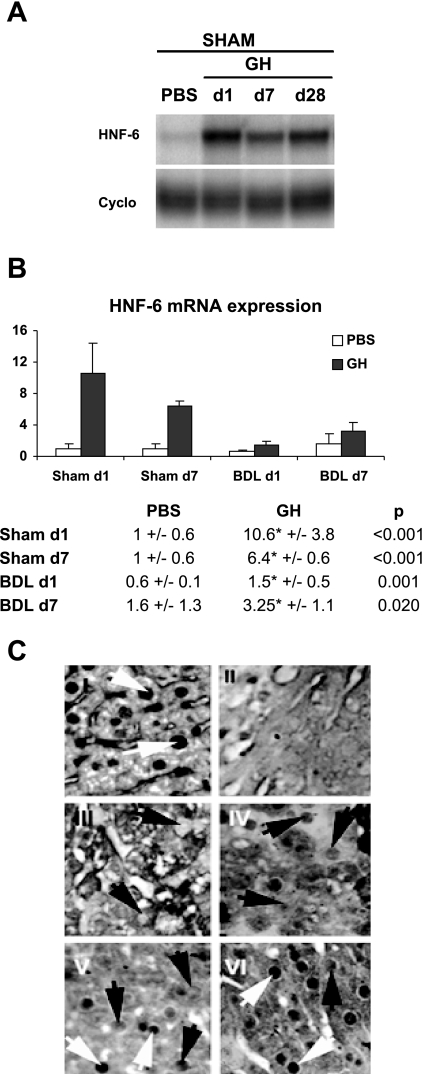
Fig. 1.
Growth hormone (GH) treatment enhances hepatic hepatic nuclear factor (HNF)-6 expression. A: sham-operated mice were treated with PBS or GH for 1, 7, or 28 days (n = 4). Representative RNAse protection assay showed that GH upregulates HNF-6 gene expression. B: mice were treated with PBS or GH for 1 or 7 days following Sham operation (n = 4) or bile duct ligation (BDL; n = 8–10). Real-time PCR analyses showed that HNF-6 was downregulated following BDL whereas GH treatment led to sustained increases in HNF-6 mRNA expression relative to PBS-treated groups. *Significant differences between PBS/BDL and GH/BDL groups with P values ≤0.05. C: representative HNF-6 immunostaining of liver micrographs showed normal level of HNF-6 protein staining, which is localized to hepatocyte nuclei of unoperated liver (I, white arrow showing normal hepatocyte nuclei staining). In contrast, HNF-6 expression was extinguished in hepatocyte nuclei from BDL day 1 (d1) liver as previously reported ( 18) (III, black arrows showing hepatocytes lacking of nuclear staining). Consistent with the recovery in HNF-6 gene expression after 7 days of BDL, faint HNF-6 staining was detected in nuclei of hepatocytes from day 7 (d7) BDL liver (IV, black arrow showing weak staining in hepatocyte nuclei). HNF-6 nuclear staining in BDL days 1 and 7 liver (III and IV) were diminished relative to GH-treated BDL day 1 (V) and day 7 liver; respectively (VI; black and white arrows). C, II: micrographs of Sham-operated liver treated with irrelevant antibody showed no HNF-6 signals.
Mice next underwent Sham surgery (n = 4) or BDL (n = 8–10) with PBS or GH treatment for the 1- and 7-day time course. As previously reported, real-time PCR analysis of day 1 PBS-treated BDL liver showed downregulation of HNF-6 transcripts (Fig. 1B) (relative expression from baseline 1 ± 0.6 to 0.6 ± 0.1 with BDL; P = 0.05) (18). By day 7 of BDL, HNF-6 gene expression was recovered in the liver whereas sustained increases in hepatic HNF-6 gene expression were seen in GH-treated Sham and BDL groups relative to PBS-treated liver. Immunostaining of liver microsections (Fig. 1C) showed that HNF-6 nuclear expression was extinguished at day 1 of BDL (Fig. 1C, III) with some recovery of its nuclear protein expression in day 7 BDL liver (Fig. 1C, IV) but at levels that were lower than GH-treated BDL day 1 and 7 liver (Fig. 1C, V and VI, respectively), thus demonstrating that, in addition to its effects on IGF-I transcription, GH treatment can also partially maintain HNF-6 nuclear protein expression in hepatocytes.
GH administration attenuates the course of acute liver injury.
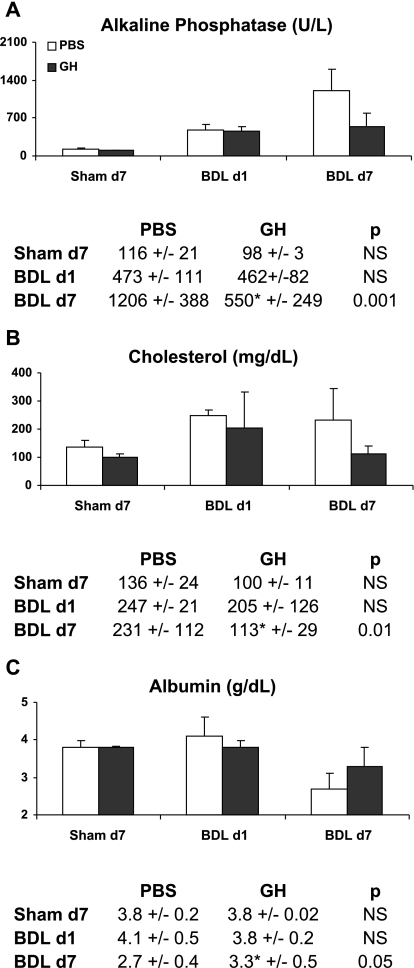
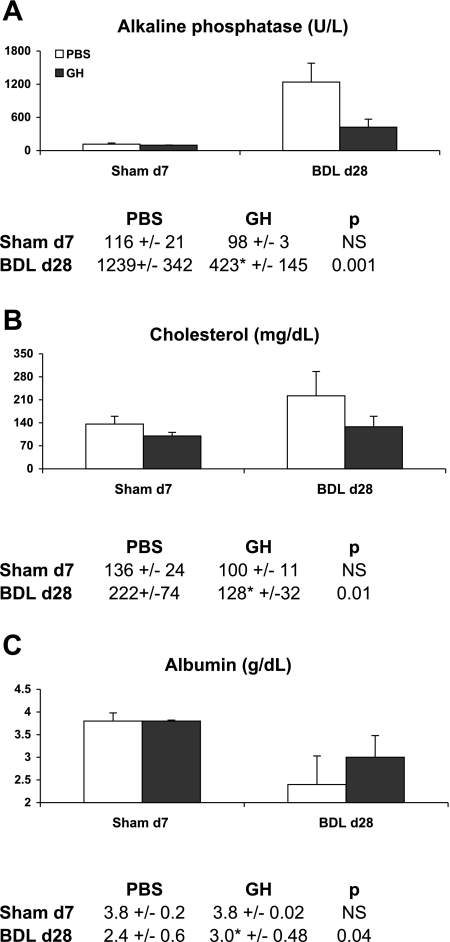
Serum alkaline phosphatase levels (a measure of canalicular injury) increased with the progression of injury and peaked at day 7 of BDL, but compared with PBS/BDL mice the levels in GH/BDL mice were significantly reduced (Fig. 2A), consistent with diminished cholestatic injury. In contrast, serum transaminase or lactate dehydrogenase levels did not differ between GH- and PBS-treated BDL groups (data not shown), suggesting that the extent of parenchymal necrosis/damage are similar between the groups. Reflecting liver function impairment following BDL liver injury, serum cholesterol levels (Fig. 2B) were abnormally increased by day 1 of BDL and serum albumin levels (Fig. 2C) deteriorated by day 7 of BDL whereas GH-treated BDL mice had significantly lower cholesterol and higher albumin levels at day 7 compared with PBS/BDL mice (Fig. 2, B and C).
Fig. 2.
GH treatment improves liver function at day 7 BDL. The graph and accompanying table showed mean ± SD of days 1 and 7 serum levels of alkaline phosphatase (A), cholesterol (B), and albumin (C) in PBS- or GH-treated Sham and BDL mice.
Consistent with GH somatotrophic effects, PBS/BDL mice lost weight and remained in negative weight balance through the first week of injury whereas GH-treated BDL mice exhibited less drastic weight loss and earlier weight recovery from the acute injury (data not shown).
Overall, these data showed that GH treatment attenuated acute liver injury, improved the deterioration in liver function, and enhanced recovery from the postsurgical catabolic state.
GH mediates HNF-6 regulation of hepatocyte proliferation.
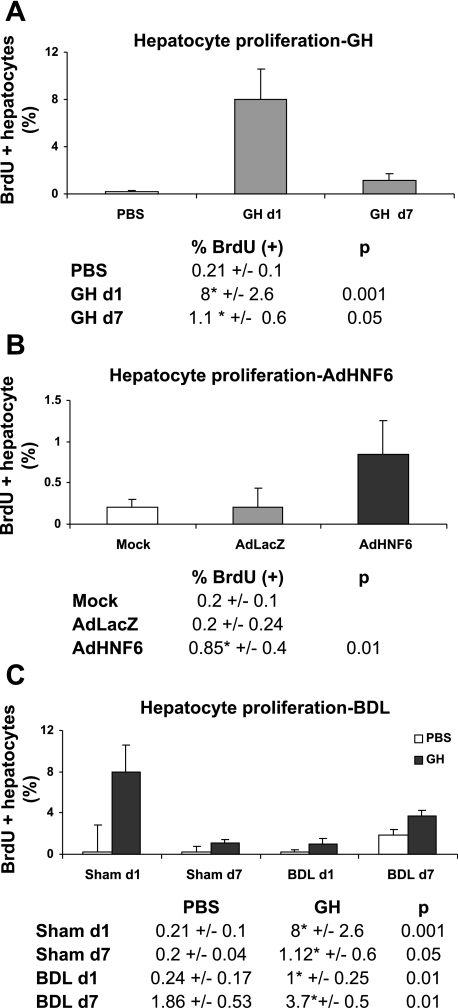
In GH-treated Sham-operated liver, spontaneous hepatocyte proliferation at both days 1 and 7 was observed (Fig. 3A). Similar to the hepatic growth response to GH, adenoviral-mediated overexpression of HNF-6 (39) also induced spontaneous hepatocyte DNA synthesis (Fig. 3B), suggesting that GH-mediated hepatocyte proliferation is in part secondary to HNF-6 direct effects on cell proliferation. Of note, hepatocyte nuclear factors can participate in a cooperative regulatory network and GH has been implicated in the modulation of this network to govern hepatic gene expression (20, 30, 41). When HNF-6 in vivo expression was increased by using AdHNF-6 (36) or GH expression system (39) in the normal mouse liver, no significant changes in the expression profile of HNF-4a, Foxa2, C/EBPa, C/EBPb, or HNF-1a was observed, demonstrating that, among the potential hepatocyte nuclear factor-dependent transcriptional pathways mediated by GH in vivo, HNF-6 is the dominant in vivo transcriptional effector for GH.
Fig. 3.
Increasing hepatic HNF-6 expression enhances in vivo hepatocyte proliferation. Livers were immunostained for 5-bromo-2-deoxyuridine (BrdU) incorporation. Hepatocyte proliferation were graphed and reported as means ± SD percent of BrdU-positive hepatocytes in 1) Sham/PBS or Sham/GH for 1 or 7 days (n = 4; A) showing that GH enhanced spontaneous hepatocyte proliferation; 2) mock-, AdLacZ-, or AdHNF-6-treated liver (n = 4; B), showing that HNF-6 overexpression enhanced hepatic regeneration; and 3) PBS- or GH- treated Sham or BDL day 1 or 7 liver (C), showing increases in hepatocyte proliferation in GH-treated liver. Sham data (A) are replotted for comparison.
We next assessed whether GH effect on liver growth could be sustained during bile duct injury in the first week of BDL (Fig. 3C). Consistent with the course of BDL injury characterized by progressive increases in hepatocyte proliferative response by the end of the first week of BDL (23), increases in hepatocyte proliferation were detected at day 7 over Sham liver whereas GH treatment of BDL liver was associated with even higher hepatocyte proliferative activities. Consistent with previous data showing that maintaining hepatic HNF-6 expression using expression vector AdHNF-6 inhibited biliary cell proliferation during the first 24 h of BDL (18), GH-treated BDL day 1 liver also exhibited similar inhibition of the biliary cell proliferative response although this effect was lost by day 7 of injury (data not shown). With the above data demonstrating that GH treatment increased HNF-6 expression in BDL liver and that AdHNF-6 can induce hepatocyte proliferation, these findings suggest that the growth-enhancing effects of GH in liver regeneration to BDL injury can be in part linked to HNF-6-mediated pathways.
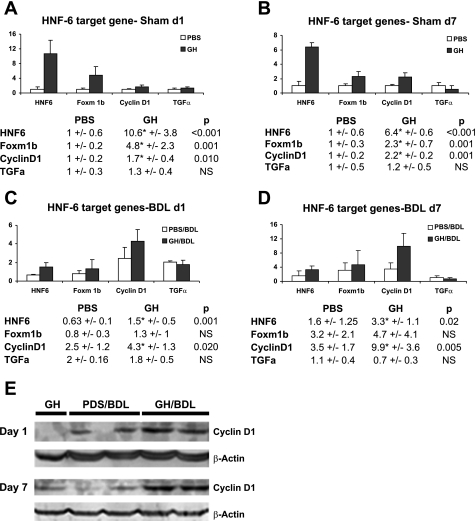
GH enhances HNF-6 expression to induce cyclin D1 during the acute phase of BDL.
TGF-α and cyclin D1 have been shown to be direct in vitro transcriptional targets for HNF-6 (37). In Hepa-6 cells treated with HNF-6 small interfering RNA, DNA replication was impaired (37) whereas AdHNF-6 liver transduction (n = 4) accelerated hepatocyte proliferation in normal liver (Fig. 3B) and PH liver (37), suggesting that GH may likely mediate HNF-6 proliferative effects through these genes. The Forkhead Box proliferation-specific transcription factor Foxm1b controls cell cycle entry to induce hepatocyte proliferation by direct regulation of cyclin D1 promoter function (40) as well as by cooperation with HNF-6 to stimulate cyclin D1 transcription (37). GH also enhanced Foxm1b expression, which stimulated hepatocyte proliferation in regenerating PH liver (20), suggesting that Foxm1b may also mediate GH-induction of hepatocyte proliferation following BDL.
We therefore examined whether these genes can participate in GH-dependent liver regeneration during BDL. Figure 4 showed that, in Sham liver, HNF-6 upregulation at 24 h (Fig. 4A) or 7 days (Fig. 4B) of GH treatment was associated with similar upregulation of Foxm1b and cyclin D1 but not of TGF-α. Following BDL (Fig. 4, C and D), although persistent upregulation for cyclin D1 was clearly observed at days 1 (Fig. 4C) and 7 (Fig. 4D), this response was mixed for Foxm1b and absent for TGF-α. Enhanced cyclin D1 gene expression in day 1 and 7 GH-treated BDL liver were associated with similar increases in cyclin D1 protein expression (Fig. 4E), suggesting that, beyond IGF-I, GH growth-promoting function on liver regeneration during BDL can be in part mediated by its effect on HNF-6 transcriptional activation of cyclin D1.
Fig. 4.
GH induces cyclin D1 expression in uninjured and BDL liver. A and B: real-time PCR analyses of PBS- or GH- treated day 1 (A) or 7 (B) Sham liver showed that increased HNF-6 expression in GH-treated Sham liver was associated with upregulation of Foxm1b and cyclin D1 but not TGF-α gene expression. C and D: PBS- or GH- treated day 1 (C) or 7 (D) liver gene expression showed that increased HNF-6 expression in GH/BDL liver was associated with upregulation of cyclin D1 but not Foxm1b or TGF-α expression. E: representative Western blot micrographs of day 1 and 7 Sham/GH, PBS/BDL, and GH/BDL liver showed enhanced cyclin D1 protein expression in GH/BDL liver relative to PBS/BDL liver at both time points.
GH enhances HNF-6 dependent and HNF-6 independent metabolic and repair gene transcription during acute BDL liver injury.
Since GH treatment improved cholesterol metabolism, albumin synthesis, and cholestasis by day 7 of BDL (Fig. 2), we next examined whether GH can activate HNF-6 potential target genes with related function at the day 7 time point.
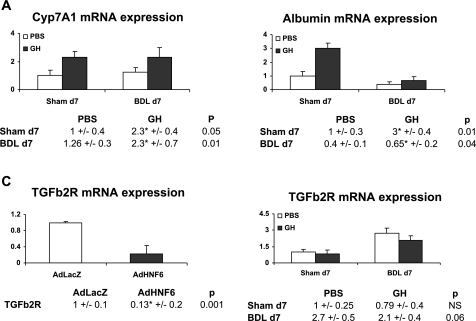
These genes include Cyp7A1 encoding the cholesterol-7a hydroxylase enzyme, which catalyzes cholesterol conversion to bile acid and which is an authentic HNF-6-transcriptional target (39). Biliary injury was associated with acute downregulation of HNF-6 and Cyp7A1 expression within the first day of biliary injury (data not shown) as previously reported (39). By day 7 of BDL, adaptive restoration of HNF-6 expression was accompanied by normalization of Cyp7A1 gene expression whereas GH treatment further increased Cyp7A1 expression in Sham and BDL mice (Fig. 5A).
Fig. 5.
Transcriptional induction of hepatic genes. A and B: PBS- or GH-treated Sham and BDL day 7 liver analyses for Cyp7A1 (A) and albumin (B) gene expression showed upregulation of both genes in Sham and in BDL liver. C: compared with AdLacZ, AdHNF-6-infected liver (n = 4) showed downregulation of TGFb2R gene, suggesting that HNF-6 negatively regulated TGFb2R transcription. D: PBS- or GH-treated Sham and BDL day 7 liver showed subtle downregulation in TGFb2R gene expression in day 7 GH/BDL liver (P = 0.06).
We also analyzed the promoter regions of genes regulating bile flow and protein synthesis for the presence of HNF-6 binding sites and found that the genes encoding NTCP, ATP binding cassette transporter proteins ABCB11 (or BSEP) and ABCC1 (MRP2 or cMOAT), albumin, and TGFb2R are potential HNF-6-regulated genes. We next evaluated their expression in the previously AdHNF-6-infected liver. In contrast to a 26-fold induction in Cyp7A1, the expression of NTCP, BSEP, albumin, or MRP2 did not change substantially (data not shown), suggesting that these genes are unlikely to be direct in vivo target genes for HNF-6. Consistent with these results, GH-treated Sham and BDL liver did not show increases in NTCP, BSEP, and MRP2 transcripts (data not shown). Interestingly, however, GH treatment led to significant upregulation in albumin (Fig. 5B) gene expression in Sham and day 7 BDL liver, suggesting that GH attenuation of liver damage can be linked to non HNF-6-mediated upregulation of albumin gene in hepatocytes to enhance albumin synthesis.
TGFb2R was also a candidate target gene for HNF-6. Consistent with this possibility, TGFb2R transcripts were significantly downregulated in AdHNF-6 infected liver (Fig. 5C). Although TGFb2R expression was not diminished in GH-treated Sham liver (Fig. 5D), GH-treated BDL liver showed subtle downregulation of TGFb2R expression with differences nearing statistically significance (P = 0.06).
GH effects on hepatic genes in the chronic phase of BDL.
We have thus far demonstrated that GH treatment improved liver metabolic function and enhanced hepatocyte proliferation by the first week of BDL. This was associated with upregulation of HNF-6 target genes cyclin D1 and Cyp7A1. Furthermore, improved albumin biochemical profile was associated with parallel increases in albumin gene expression.
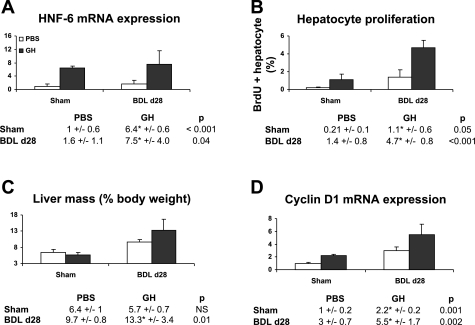
We next evaluated whether GH continues to mediate these effects into the chronic injury phase. Mice underwent PBS or GH treatment during a 28-day course of BDL (n = 10–12). GH-treated day 28 BDL liver showed increases in HNF-6 gene expression relative to PBS/BDL liver (Fig. 6A). Stabilization of compensatory liver growth beyond the first week of BDL as previously shown (13) is again demonstrated by comparable hepatocyte proliferative activities between days 7 (Fig. 3C) and 28 BDL (Fig. 6B) liver. In these animals, GH effects in enhancing hepatocyte proliferation are maintained (Fig. 6B). Consistent with GH effects on hepatocyte replication, higher compensatory increases in the wet liver mass were seen in GH-treated BDL liver (Fig. 6C). This was in turn associated with significant upregulation of cyclin D1 gene expression (Fig. 6D).
Fig. 6.
GH-mediated HNF-6 proliferative effects are maintained into the chronic phase of liver injury. A and D: mice underwent PBS and GH treatment for day 28 of BDL (n = 10–12). Gene analyses showed that GH-treated liver maintained higher expression levels of HNF-6 (A) and cyclin D1 (D) relative to PBS liver (□). B: BrdU labeling experiments of liver from PBS/BDL and GH/BDL day 28 mice showed that GH treatment enhanced hepatocyte DNA synthesis. Previous Sham day 7 data are replotted in A, B, and D for comparison. C: PBS- or GH-Sham operation and PBS- or GH-BDL day 28 liver mass (as percent of body weight at the time of euthanasia) showed higher liver mass in GH/BDL mice.
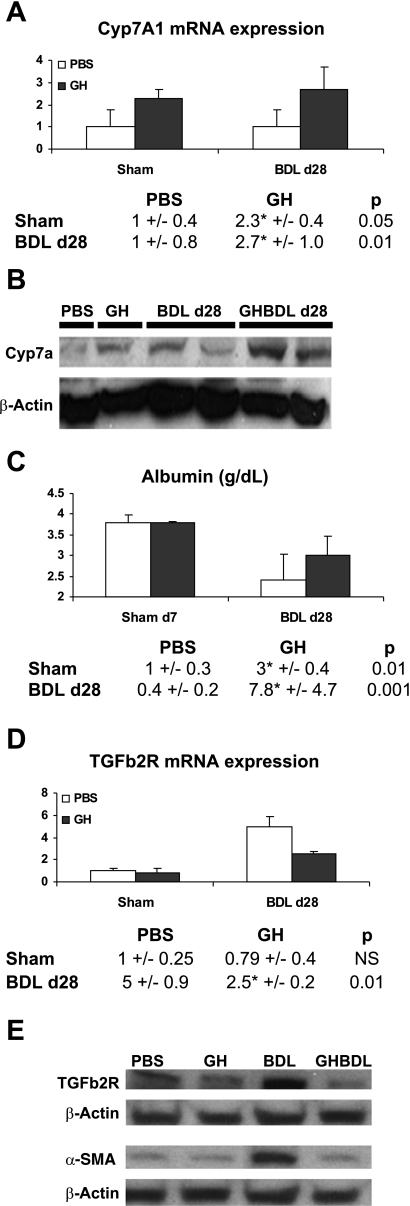
GH effects were also sustained with increases in Cyp7A1 transcripts (Fig. 7A), the corresponding Cyp7A1 protein (Fig. 7B) as well as reciprocal changes in serum cholesterol levels at day 28 of BDL (Fig. 8B). Similarly, albumin transcripts remained elevated in GH-treated BDL liver (Fig. 7C), paralleling the increase in serum albumin levels (Fig. 8C).
Fig. 7.
GH effects on Cyp7A1, albumin, TGFb2R transcription, and fibrogenesis during chronic liver injury. A and C: PBS- or GH-treated day 28 BDL liver analyses for Cyp7A1 (A) and albumin (C) gene expression showed upregulation of both genes with GH treatment. Sham day 7 data are replotted for comparison. B: Western blot analyses of PBS- and GH-treated Sham and BDL liver showed that GH increased Cyp7A1 protein expression in GH/Sham and GH/BDL day 28 liver relative to PBS liver. D: TGFb2R gene expression analyses showed increased TGFb2R expression with progression of injury after 28 days of BDL and that GH treatment led to downregulation of TGFb2R transcripts. E: representative Western blot micrographs for TGFb2R and α-smooth muscle actin showed baseline expression in PBS- and GH-treated Sham liver, upregulation of these markers with advanced liver injury in BDL liver and suppression of their expression in GH/BDL mice at day 28.
Fig. 8.
GH attenuates liver injury in chronic BDL injury. Serum alkaline phosphatase (A), cholesterol (B), and albumin (C) analyses from day 28 PBS- or GH-treated BDL mice showed that GH-treated liver had diminished cholestasis, normal cholesterol, and enhanced albumin levels compared with PBS/BDL liver.
GH also led to sustained improvement in cholestasis as reflected in lower serum alkaline phosphatase levels (Fig. 8A) without significant differences in transaminase levels (data not shown) and significantly higher overall body weight in GH-treated BDL mice (data not shown).
By 4 wk of BDL, although TGFb2R transcripts were increased in PBS-treated BDL liver, significant suppression of TGFb2R transcripts in GH-treated liver (Fig. 7D) was seen. Together with previous data showing negative regulation of TGFb2R expression in AdHNF-6 liver (Fig. 5C), these results suggest that TGFb2R is transcriptionally regulated by HNF-6.
The downregulation in TGFb2R gene expression also led to parallel inhibition of TGFb2R protein expression (Fig. 7E) which was in turn associated with similar downregulation of α-SMA protein expression (Fig. 7E), showing that TGFb2R signaling pathways potentially mediated by hepatocytes and biliary cells in inducing myofibroblastic transdifferentiation of hepatic stellate cells and portal fibroblasts (10, 29, 43) during fibrogenesis can be suppressed in GH-treated BDL mice, thus providing a mechanism for previously published data showing that GH attenuated fibrosis in cholestatic rats (33).
DISCUSSION
In this manuscript, we analyzed the spectrum of HNF-6-regulated responses in the liver following biliary injury. We found that HNF-6 controls hepatic target genes such as cyclin D1, Cyp7A1, and TGFb2R and that enhancing HNF-6 expression by GH during BDL was associated with upregulation of hepatic cyclin D1 and Cyp7A1 and in turn with increased hepatocyte proliferation and improved cholesterol clearance and with downregulation of TGFb2R to suppress the fibrogenic response. GH also mediated the transcriptional activation of albumin through HNF-6 independent manner to improve hepatic synthetic function. These correlative data thus suggest that GH/HNF-6 axis is a potential relevant in vivo pathway underlying the complexity of GH diverse function in target organs (22) and provides an additional molecular underpinning for the protective effects of GH during liver injury that extend beyond the traditional GH/STAT-5/IGF-I axis (11, 17).
Hepatocyte replication during liver regeneration is dependent on cytokine- and cytokine-mediated pathways (38), with HGF and TGF-α growth factors participating in the later pathways to stimulate cell cycle progression. The in vivo relevance of TGF-α is mixed considering that TGF-α had minimal effect on inducing spontaneous hepatocyte proliferation in quiescent liver (15) and that TGF-α-null mice exhibited no obvious defect in liver regeneration to PH (32). Consistent with these data, GH did not enhance hepatic TGF-α in the resting and BDL-injured liver. With respect to HGF, although the hepatocyte is the major cellular target for GH action and IGF-I production, it poorly expresses IGF-I receptor (22), suggesting that IGF-I has no direct proliferation effect on hepatocytes. It has been proposed that GH/IGF-I's growth-promoting effect on hepatocytes is instead mediated through hepatocytic IGF-I induction of HGF from hepatic stellate cells (34). Although liver regeneration following PH was severely impaired in GH-deficient mice (27), normal liver mass restitution was not affected in IGF-I- and IGF-I receptor-deficient mice, although transient delays in hepatocyte proliferation were seen (12, 27), suggesting that IGF-I is not essential to hepatocyte regeneration and that GH-mediated liver growth can occurs through IGF-I independent pathways. Consistent with this, GH did not enhance HGF expression in either Sham or BDL liver (data not shown), suggesting that the GH/IGF-I/HGF-mediated pathway is not a major mechanism for GH proliferative expansion of hepatocytes in BDL liver. Instead, GH upregulated HNF-6 transcriptional activity to increase cyclin D1 expression through the GH/HNF-6/cyclin D1 pathway, to induce spontaneous hepatocyte proliferation in quiescent liver and to enhance hepatocyte replication in injured BDL liver. It is interesting that hepatocytes proliferated to AdHNF-6 or GH expression vectors in the Sham liver, bypassing the necessity of priming signals from the injured liver (42). Cyclin D1 is important in cell cycle S phase entry and is a critical mediator of many of the factors implicated in regulating liver regeneration following PH (1, 35, 37, 40). Direct activation of cyclin D1 and coordinated induction of cyclin D1 expression and hepatocyte proliferation by HNF-6 suggests that cyclin D1 is also important in HNF-6- and GH-directed mediation of hepatocyte replication in normal liver and BDL liver.
Foxm1b is a proliferation-specific transcription factor that stimulates S- and M-phase cell cycle genes (40, 44) and expression of which is enhanced by GH to accelerate hepatocyte proliferation during PH (20). To our surprise, we were, however, unable to demonstrate a clear effect of GH on Foxm1b gene expression in the BDL liver, suggesting that Foxm1b does not play a prominent role in potentiating liver regeneration in this model of injury. A potential explanation is that the underlying mechanisms for liver regeneration to PH are distinct from other forms of liver damage (14).
We also identified GH transcriptional regulation of Cyp7A1 gene through the GH/HNF-6/Cyp7A1 axis as a potential in vivo mechanism for cholesterol clearance. Consistent with the importance of Cyp7A1 in cholesterol catabolism, abnormal Cyp7A1 expression in rats was associated with age-related defect of cholesterol disposal (16), whereas CYP7A1 polymorphism is a risk factor for hyperlipidemia (19) and gallstone disease (8). We demonstrated that both AdHNF-6- and GH-mediated increases in HNF-6 expression induced Cyp7A1 mRNA expression in the quiescent liver. Following BDL, downregulation in the expression of HNF-6 and its corresponding Cyp7A1 target gene (39) were inversely related to an acute rise in serum cholesterol. With further liver injury, progressive recovery in HNF-6 mRNA expression along with similar adaptive recovery of Cyp7A1 expression was associated with stabilization in the abnormal cholesterol profile in BDL mice at days 7 and 28 of injury. Remarkably, in association with HNF-6 and Cyp7A1 upregulation, GH-treated BDL mice maintained normal serum cholesterol levels, suggesting that the improvement in the metabolic profile of GH-treated BDL liver is likely mediated by GH transcriptional activation of Cyp7A1 through HNF-6.
The GH/HNF-6 pathway may also negatively regulate TGFb2R gene expression. Increasing HNF-6 expression in normal liver by AdHNF-6 vectors or in BDL liver by GH administration was associated with downregulation of TGFb2R transcripts, suggesting that HNF-6 is involved in the transcriptional inhibition of TGFb2R. In support of the possibility of TGFb2R-negative transcriptional regulation by HNF-6, TGFb2R expression and TGF-β signaling pathways were upregulated in embryonic liver of HNF-6-null mice (6, 28). Our results demonstrating parallel suppression of α-SMA expression, a marker for hepatic stellate cells and portal fibroblast-dependent fibrogenesis (10, 29, 43), thus provide a mechanistic basis for previously seen antifibrotic effects of GH administration in BDL rats (33). It is possible that diminished HNF-6 function in GH-treated liver can alter cross talk between hepatocytes or biliary cells and mesenchymal cells in the form of downregulating the TGFb2R-mediated recruitment pathways for the fibroblastic response by the liver epithelial cells during biliary fibrogenesis.
GH can also target hepatic genes such as albumin. Through albumin transcriptional stimulation, GH treatment may improve protein synthesis as seen by higher albumin serum levels. Although GH did not noticeably improve liver parenchymal injury as reflected by liver lactate dehydrogenase or transaminase levels, it reduced the extent of cholestatic injury during BDL as shown by lower serum alkaline phosphatase levels. The molecular mechanism for this finding remains to be defined.
The importance of hepatocyte replication during hepatic parenchymal injury is illustrated for example in IL-6-deficient mice where the hepatocyte proliferation defect can be linked to accelerated liver failure following PH or BDL (9, 13) or clinically in cirrhotic patients by the association of increased proliferating cell nuclear antigen expression with compensated chronic active hepatitis but decreased expression with deterioriated liver failure (26). Our results showed a similar link between higher hepatocyte proliferative indexes, relative preservation of hepatic function, and attenuation of liver injury. Although GH and/or the GH/HNF-6 axis may mediate hepatocyte proliferation independently of its effects on the metabolic and repair genes, it is possible that the observed improvement in metabolic and cholestatic response is simply a manifestation of better preservation of functional liver mass in GH-treated injured liver as a consequence of GH promoting effect on hepatocyte growth or, as yet unexplored, of GH or HNF-6 potential protective effect in diminishing apoptotic injury from Fas-mediated toxic bile acid injury during BDL (25), although the lack in difference of transaminase levels suggest otherwise. Of note, although GH/IGF-I have been reported to modulate in vitro biliary epithelial cells proliferation and protective response to bile salt-associated apoptotic injury (2, 3), a sustained inducing effect of GH administration on biliary cell proliferation could not be demonstrated in our experimental model.
In summary, these data suggested that, beyond hepatoprotective mechanisms that have been linked to IGF-I, GH may mediate HNF-6-regulated target gene expression to attenuate acute and chronic liver injury. This could be linked to its effect on enhancing hepatocyte proliferation through cyclin D1, in improving cholesterol metabolism through Cyp7A1, and in downregulating the hepatic fibrogenic response through TGFb2R. Other protective effects of GH were to diminish cholestasis and enhance protein synthesis through its transcriptional effects on albumin. Although the confirmation of the importance of these HNF-6-mediated effects awaits further analyses of liver-specific HNF-6 conditional null mice, these results helped to further our understanding of the diverse molecular mechanisms for GH by invoking the potential of GH/HNF-6 in vivo participation during biliary injury with the implication that GH or alternative HNF-6-enhancing modalities may be of therapeutic use in delaying cholestatic and fibrotic liver injury.
GRANTS
This research was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants K08 DK-02964-04 and R21 DK-070784-02 to A. Holterman.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Allan AL, Albanese C, Pestell RG, LaMarre J. Activating transcription factor 3 induces DNA synthesis and expression of cyclin D1 in hepatocytes. J Biol Chem 276: 27272–27280, 2001. [DOI] [PubMed] [Google Scholar]
- 2.Alvaro D, Macarri G, Mancino MG, Marzioni M, Bragazzi M, Onori P, Corradini SG, Invernizzi P, Franchitto A, Attili AF, Gaudio E, Benedetti A. Serum and biliary insulin-like growth factor I and vascular endothelial growth factor in determining the cause of obstructive cholestasis. Ann Intern Med 147: 451–459, 2007. [DOI] [PubMed] [Google Scholar]
- 3.Alvaro D, Metalli VD, Alpini G, Onori P, Franchitto A, Barbaro B, Glaser SS, Francis H, Cantafora A, Blotta I, Attili AF, Gaudio E. The intrahepatic biliary epithelium is a target of the growth hormone/insulin-like growth factor 1 axis. J Hepatol 43: 875–883, 2005. [DOI] [PubMed] [Google Scholar]
- 4.Asakawa K, Hizuka N, Takano K, Horikawa R, Sukegawa I, Toyoda C, Shizume K. Human growth hormone stimulates liver regeneration in rats. J Endocrinol Invest 12: 343–347, 1989. [DOI] [PubMed] [Google Scholar]
- 5.Cheung L, Andersen M, Gustavsson C, Odeberg J, Fernandez-Perez L, Norstedt G, Tollet-Egnell P. Hormonal and nutritional regulation of alternative CD36 transcripts in rat liver—a role for growth hormone in alternative exon usage (Abstract). BMC Mol Biol 8: 60, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clotman F, Jacquemin P, Plumb-Rudewiez N, Pierreux CE, Van der Smissen P, Dietz HC, Courtoy PJ, Rousseau GG, Lemaigre FP. Control of liver cell fate decision by a gradient of TGF beta signaling modulated by Onecut transcription factors. Genes Dev 19: 1849–1854, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Costa RH, Kalinichenko VV, Holterman AX, Wang X. Transcription factors in liver development, differentiation, and regeneration. Hepatology 38: 1331–1347, 2003. [DOI] [PubMed] [Google Scholar]
- 8.Couture P, Otvos JD, Cupples LA, Wilson PW, Schaefer EJ, Ordovas JM. Association of the A-204C polymorphism in the cholesterol 7alpha-hydroxylase gene with variations in plasma low density lipoprotein cholesterol levels in the Framingham Offspring Study. J Lipid Res 40: 1883–1889, 1999. [PubMed] [Google Scholar]
- 9.Cressman DE, Greenbaum LE, DeAngelis RA, Ciliberto G, Furth EE, Poli V, Taub R. Liver failure and defective hepatocyte regeneration in interleukin-6-deficient mice. Science 274: 1379–1383, 1996. [DOI] [PubMed] [Google Scholar]
- 10.Cui X, Shimizu I, Lu G, Itonaga M, Inoue H, Shono M, Tamaki K, Fukuno H, Ueno H, Ito S. Inhibitory effect of a soluble transforming growth factor beta type II receptor on the activation of rat hepatic stellate cells in primary culture. J Hepatol 39: 731–737, 2003. [DOI] [PubMed] [Google Scholar]
- 11.Davey HW, Xie T, McLachlan MJ, Wilkins RJ, Waxman DJ, Grattan DR. STAT5b is required for GH-induced liver IGF-I gene expression. Endocrinology 142: 3836–3841, 2001. [DOI] [PubMed] [Google Scholar]
- 12.Desbois-Mouthon C, Wendum D, Cadoret A, Rey C, Leneuve P, Blaise A, Housset C, Tronche F, Le Bouc Y, Holzenberger M. Hepatocyte proliferation during liver regeneration is impaired in mice with liver-specific IGF-IR knockout. FASEB J 20: 773–775, 2006. [DOI] [PubMed] [Google Scholar]
- 13.Ezure T, Sakamoto T, Tsuji H, Lunz JG 3rd, Murase N, Fung JJ, Demetris AJ. The development and compensation of biliary cirrhosis in interleukin-6-deficient mice. Am J Pathol 156: 1627–1639, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fausto N Lessons from genetically engineered animal models. V. Knocking out genes to study liver regeneration: present and future. Am J Physiol Gastrointest Liver Physiol 277: G917–G921, 1999. [DOI] [PubMed] [Google Scholar]
- 15.Fausto N, Campbell JS, Riehle KJ. Liver regeneration. Hepatology 43: S45–S53, 2006. [DOI] [PubMed] [Google Scholar]
- 16.Galman C, Matasconi M, Persson L, Parini P, Angelin B, Rudling M. Age-induced hypercholesterolemia in the rat relates to reduced elimination but not increased intestinal absorption of cholesterol. Am J Physiol Endocrinol Metab 293: E737–E742, 2007. [DOI] [PubMed] [Google Scholar]
- 17.Held MA, Cosme-Blanco W, Difedele LM, Bonkowski EL, Menon RK, Denson LA. Alterations in growth hormone receptor abundance regulate growth hormone signaling in murine obstructive cholestasis. Am J Physiol Gastrointest Liver Physiol 288: G986–G993, 2005. [DOI] [PubMed] [Google Scholar]
- 18.Holterman AX, Tan Y, Kim W, Yoo KW, Costa RH. Diminished hepatic expression of the HNF-6 transcription factor during bile duct obstruction. Hepatology 35: 1392–1399, 2002. [DOI] [PubMed] [Google Scholar]
- 19.Hubacek JA, Bobkova D. Role of cholesterol 7alpha-hydroxylase (CYP7A1) in nutrigenetics and pharmacogenetics of cholesterol lowering. Mol Diagn Ther 10: 93–100, 2006. [DOI] [PubMed] [Google Scholar]
- 20.Krupczak-Hollis K, Wang X, Dennewitz MB, Costa RH. Growth hormone stimulates proliferation of old-aged regenerating liver through forkhead box m1b. Hepatology 38: 1552–1562, 2003. [DOI] [PubMed] [Google Scholar]
- 21.Lahuna O, Rastegar M, Maiter D, Thissen JP, Lemaigre FP, Rousseau GG. Involvement of STAT5 (signal transducer and activator of transcription 5) and HNF-4 (hepatocyte nuclear factor 4) in the transcriptional control of the hnf6 gene by growth hormone. Mol Endocrinol 14: 285–294, 2000. [DOI] [PubMed] [Google Scholar]
- 22.Lanning NJ, Carter-Su C. Recent advances in growth hormone signaling. Rev Endocr Metab Disord 7: 225–235, 2006. [DOI] [PubMed] [Google Scholar]
- 23.Liu Z, Sakamoto T, Ezure T, Yokomuro S, Murase N, Michalopoulos G, Demetris AJ. Interleukin-6, hepatocyte growth factor, and their receptors in biliary epithelial cells during a type I ductular reaction in mice: interactions between the periductal inflammatory and stromal cells and the biliary epithelium. Hepatology 28: 1260–1268, 1998. [DOI] [PubMed] [Google Scholar]
- 24.McElduff A, Poronnik P, Baxter RC, Williams P. A comparison of the insulin and insulin-like growth factor I receptors from rat brain and liver. Endocrinology 122: 1933–1939, 1988. [DOI] [PubMed] [Google Scholar]
- 25.Miyoshi H, Rust C, Roberts PJ, Burgart LJ, Gores GJ. Hepatocyte apoptosis after bile duct ligation in the mouse involves Fas. Gastroenterology 117: 669–677, 1999. [DOI] [PubMed] [Google Scholar]
- 26.Nakajima T, Kagawa K, Ueda K, Ohkawara T, Kimura H, Kakusui M, Deguchi T, Okanoue T, Kashima K, Ashihara T. Evaluation of hepatic proliferative activity in chronic liver diseases and hepatocellular carcinomas by proliferating cell nuclear antigen (PCNA) immunohistochemical staining of methanol-fixed tissues. J Gastroenterol 29: 450–454, 1994. [DOI] [PubMed] [Google Scholar]
- 27.Pennisi PA, Kopchick JJ, Thorgeirsson S, LeRoith D, Yakar S. Role of growth hormone (GH) in liver regeneration. Endocrinology 145: 4748–4755, 2004. [DOI] [PubMed] [Google Scholar]
- 28.Plumb-Rudewiez N, Clotman F, Strick-Marchand H, Pierreux CE, Weiss MC, Rousseau GG, Lemaigre FP. Transcription factor HNF-6/OC-1 inhibits the stimulation of the HNF-3alpha/Foxa1 gene by TGF-beta in mouse liver. Hepatology 40: 1266–1274, 2004. [DOI] [PubMed] [Google Scholar]
- 29.Qi Z, Atsuchi N, Ooshima A, Takeshita A, Ueno H. Blockade of type beta transforming growth factor signaling prevents liver fibrosis and dysfunction in the rat. Proc Natl Acad Sci USA 96: 2345–2349, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rastegar M, Lemaigre FP, Rousseau GG. Control of gene expression by growth hormone in liver: key role of a network of transcription factors. Mol Cell Endocrinol 164: 1–4, 2000. [DOI] [PubMed] [Google Scholar]
- 31.Rausa FM, Tan Y, Zhou H, Yoo KW, Stolz DB, Watkins SC, Franks RR, Unterman TG, Costa RH. Elevated levels of hepatocyte nuclear factor 3beta in mouse hepatocytes influence expression of genes involved in bile acid and glucose homeostasis. Mol Cell Biol 20: 8264–8282, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Russell WE, Kaufmann WK, Sitaric S, Luetteke NC, Lee DC. Liver regeneration and hepatocarcinogenesis in transforming growth factor-alpha-targeted mice. Mol Carcinog 15: 183–189, 1996. [DOI] [PubMed] [Google Scholar]
- 33.Scopa CD, Koureleas S, Tsamandas AC, Spiliopoulou I, Alexandrides T, Filos KS, Vagianos CE. Beneficial effects of growth hormone and insulin-like growth factor I on intestinal bacterial translocation, endotoxemia, and apoptosis in experimentally jaundiced rats. J Am Coll Surg 190: 423–431, 2000. [DOI] [PubMed] [Google Scholar]
- 34.Skrtic S, Wallenius K, Gressner AM, Jansson JO. Insulin-like growth factor signaling pathways in rat hepatic stellate cells: importance for deoxyribonucleic acid synthesis and hepatocyte growth factor production. Endocrinology 140: 5729–5735, 1999. [DOI] [PubMed] [Google Scholar]
- 35.Streetz KL, Luedde T, Manns MP, Trautwein C. Interleukin 6 and liver regeneration. Gut 47: 309–312, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tan Y, Adami G, Costa RH. Maintaining HNF6 expression prevents AdHNF3beta-mediated decrease in hepatic levels of Glut-2 and glycogen. Hepatology 35: 790–798, 2002. [DOI] [PubMed] [Google Scholar]
- 37.Tan Y, Yoshida Y, Hughes DE, Costa RH. Increased expression of hepatocyte nuclear factor 6 stimulates hepatocyte proliferation during mouse liver regeneration. Gastroenterology 130: 1283–1300, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Taub R Liver regeneration: from myth to mechanism. Nat Rev Mol Cell Biol 5: 836–847, 2004. [DOI] [PubMed] [Google Scholar]
- 39.Wang M, Tan Y, Costa RH, Holterman AX. In vivo regulation of murine CYP7A1 by HNF-6: a novel mechanism for diminished CYP7A1 expression in biliary obstruction. Hepatology 40: 600–608, 2004. [DOI] [PubMed] [Google Scholar]
- 40.Wang X, Quail E, Hung NJ, Tan Y, Ye H, Costa RH. Increased levels of forkhead box M1B transcription factor in transgenic mouse hepatocytes prevent age-related proliferation defects in regenerating liver. Proc Natl Acad Sci USA 98: 11468–11473, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Waxman DJ, O'Connor C. Growth hormone regulation of sex-dependent liver gene expression. Mol Endocrinol 20: 2613–2629, 2006. [DOI] [PubMed] [Google Scholar]
- 42.Webber EM, Bruix J, Pierce RH, Fausto N. Tumor necrosis factor primes hepatocytes for DNA replication in the rat. Hepatology 28: 1226–1234, 1998. [DOI] [PubMed] [Google Scholar]
- 43.Wells RG, Kruglov E, Dranoff JA. Autocrine release of TGF-beta by portal fibroblasts regulates cell growth. FEBS Lett 559: 107–110, 2004. [DOI] [PubMed] [Google Scholar]
- 44.Ye H, Holterman AX, Yoo KW, Franks RR, Costa RH. Premature expression of the winged helix transcription factor HFH-11B in regenerating mouse liver accelerates hepatocyte entry into S phase. Mol Cell Biol 19: 8570–8580, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]