Abstract
Adrenergic and serotonergic (ADR-SER) mechanisms alter gut (GI) function; these effects are mediated through G protein transduction. Candidate genetic variations in ADR-SER were significantly associated with somatic scores in irritable bowel syndrome (IBS) and gastric emptying but not small bowel or colonic transit. Our aim was to assess whether candidate ADR-SER genes are associated with motor and sensory GI functions in IBS and subgroups on the basis of bowel dysfunction. In 122 patients with IBS and 39 healthy controls, we assessed gastrointestinal somatic symptoms and affect by validated questionnaires. We measured: gastric volume (GV), maximum tolerated volume, rectal compliance, sensation thresholds and ratings, and genetic variations including α2A (C-1291G), α2C (Del 332–325), GNβ3 (C825T), and 5-HTTLPR. Demographics and genotype distributions were similar in the patients with IBS subgrouped on bowel function. There were significant associations between 5-HTTLPR SS genotype and absence of IBS symptoms and between 5-HTTLPR LS/SS genotype and increased rectal compliance and increased pain ratings, particularly at 12 and 24 mmHg distensions. GNβ3 was associated only with fasting GV; we did not detect associations between α2A genotype and the gastrointestinal sensory or motor functions tested. We concluded that 5-HTTLPR LS/SS genotype is associated with both increased pain sensation and increased rectal compliance though the latter effect is unlikely to contribute to increased pain sensation ratings with LS/SS genotype. The data suggest the hypotheses that the endophenotype of visceral hypersensitivity in IBS may be partly related to genetic factors, and the association of GNβ3 with fasting GV may explain, in part, the reported association of GNβ3 with dyspepsia.
Keywords: adrenergic, serotonergic, GNβ3, SLC6A4, G protein, receptor
adrenergic mechanisms alter gastrointestinal reflexes involved in intestinal propulsion, coordinated motor activity in the small bowel and colon, and gastrointestinal tone and fluid secretion (12, 46). Norepinephrine is also involved in control of pain including supraspinal inhibition of pain, e.g., due to a change in the behavioral state (37) including stress, which contributes to gut motility disorders (10).
Among α2 receptors, the α2A subtype appears to be critically important for control of motor functions, such as gastrointestinal transit in mice (40) and colonic circular smooth muscle in canine colon (47). The α2 receptor agonist, clonidine, inhibits gastric and colonic tone, phasic contractility, and sensation in the rectum and colon in response to balloon distension in healthy humans (4, 43).
Serotonergic (5-HT) mechanisms also alter gut absorptive, motor, and sensory functions via actions in the gut, spinal cord, and brain stem (27). Ligand-receptor interaction results in cellular functions with an estimated 80% of these interactions mediated by G proteins. Thus it is conceivable that genetic variation in G proteins may influence the effects of neurotransmitters including norepinephrine and serotonin. Holtmann et al. (22) observed an association between GNβ3 genotype and dyspepsia though the association with a variety of functional gastrointestinal disorders was not confirmed (1).
It has been demonstrated that α2 adrenoceptors modulate neuronal serotonin release in mouse brain, suggesting that the two control mechanisms interact in the nervous system (39). Serotonergic and noradrenergic systems interact to modulate pain perception in the spinal cord and brain peripherally and to activate the hypothalamo-pituitary-adrenal axis (32). In humans, a combination of serotonin and norepinephrine reuptake inhibition has a more profound effect on human gastric and colonic sensory and motor functions relative to selective serotonin reuptake (14, 15). These and other data suggest that serotonergic and adrenergic mechanisms may interact to alter gastrointestinal motor and sensory functions, and these may involve G protein transduction.
Genetic variations in the mechanisms that control adrenergic receptors, G proteins, and the reuptake of serotonin are reported to be associated with different irritable bowel syndrome (IBS) or dyspepsia phenotypes (24, 28), associated somatic scores (28), and depressive episodes (25) although results are inconsistent (1, 44). In a previous study of symptom phenotype, we observed that single nucleotide polymorphisms (SNPs) in genes for α2C receptor and 5-HTTLPR (5-hydroxytryptamine transporter long polymorphic region in the promoter for serotonin transporter protein, SERT) either alone or in combination are associated with constipation-predominant IBS [IBS-C (28)] and with high somatic symptom scores in these patients (28). SERT is also termed SLC6A4. There is also evidence that the adrenergic and serotonergic systems interact in mediating functional dyspepsia (34).
In a recently published study of 251 patients with dyspepsia or IBS or in healthy states, associations were demonstrated between candidate genes affecting adrenergic and serotonergic functions and gastric emptying or accommodation but not with small bowel or colonic transit (22).
Our hypothesis is that sensory functions in health and IBS are modulated by genetic variations in α2AR, 5-HTTLPR, and GNβ3. Our aim was to assess whether genetic variations in adrenergic and serotonergic control and G protein are associated with sensory functions in health and IBS.
MATERIALS AND METHODS
Study Design
All participants underwent studies of satiation and rectal sensation, and, to facilitate interpretation of these sensory responses, we also measured gastric volumes and rectal compliance.
Participants and Questionnaires
This study recruited 163 participants, of which 161 provided DNA for studies. Thus a total of 122 patients with IBS (Rome II positive, 3 male) and 39 healthy controls were assessed. The study was approved by Mayo Clinic Institutional Review Board. All participants signed informed consent. To characterize the subtype of IBS on the basis of predominant bowel function, we used validated bowel symptom questionnaires (41), reviewed the electronic medical record (by S. McKinzie), or conducted direct physician (M. Camilleri) interview and examination. Participants were allowed to be on stable doses of the following medications: thyroid replacement, estrogen replacement, low-dose aspirin (81 mg/day), and birth control pills or depot estrogen injections. Exclusion criteria included use of medication for IBS or constipation within 7 days before measurements, any structural or metabolic diseases/conditions that affect the gastrointestinal system, or participation in another clinical study within the prior 30 days.
All participants underwent bowel disease questionnaires [including somatic symptom questionnaires (41)], hospital anxiety and depression inventory (HAD) (48), and a general quality of life instrument [SCL-90 (19)]. Participants also filled in questionnaires to assess their state of anxiety, relaxation, and fear of pain with the use of the 30-item Fear of Pain Questionnaire [FPQ-III (35)] and a revised version of the 36-item Anxiety Sensitivity Index [ASI-R (42)] on the days of rectal sensation tests. The observations using all of the same measurements, as well as full gastrointestinal transit, have been reported elsewhere (9). The results are only used here to assess association with candidate genes.
Satiation by the Nutrient Drink Test
A standardized nutrient drink test to measure satiation and postprandial symptoms was used (13). The method has been used extensively in previous studies, and a liquid nutrient drink, Ensure (1 Kcal/ml, 11% fat, 73% carbohydrate, and 16% protein), was used to identify maximum tolerated volume at full satiation, as well as postprandial fullness, nausea, bloating, and pain 30 min after the meal.
Gastric Volume by 99mTc-SPECT
We used a single photon emission computed tomography (SPECT) method (5) developed, validated, and extensively used in our laboratory to measure the gastric volume during fasting and after 300-ml liquid nutrients (300 kcal). The primary endpoint was postprandial change in gastric volume, and secondary endpoints were fasting and postprandial gastric volumes.
Rectal Compliance and Sensation by Barostat
Rectal barostat equipment and procedure.
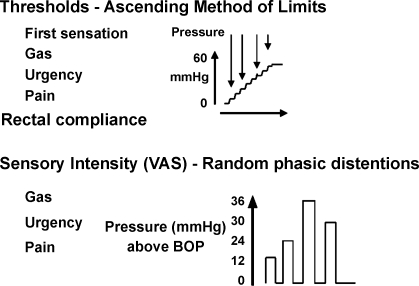
The method and performance characteristics have been extensively described elsewhere (17, 40). Ascending method of limits was used to measure rectal compliance and sensory thresholds. Random-order phasic distensions were used to assess sensory ratings, as in prior studies (4, 45). The methods are detailed in Fig. 1.
Fig. 1.
Experimental design for measuring rectal compliance and sensation using ascending method of limits and random-order phasic distensions, respectively. Anxiety/depression sensory ratings were recorded on each day of testing. VAS, visual analog scales; BOP, baseline operating pressure.
Methods for measuring rectal compliance and sensation.
All subjects presented to the research center after bowel preparation (Fleet phosphate enema, self-administered at least 1 h before reporting to the center) and an overnight fast. A catheter, which was attached a polyethylene bag, was inserted into the rectum so that the middle of the balloon was located ∼10 cm from the anal verge. To decrease the effects of abdominal viscera on the balloon volume, the subjects were placed in a semiprone position and the foot end of the bed elevated 15 degrees. The bag was unfolded by transient inflation with 75 ml of air, followed by complete deflation. After a 20–30-min recovery period, the catheter was connected to a barostat (G&J Electronics, Toronto, Ontario, Canada), and the pressure in the bag increased from 4 mmHg in steps of 1 mmHg for 1 min per step until respiratory excursions were observed. The baseline operating pressure (BOP) was set 2 mmHg above the minimal distension pressure at which respiratory excursions were clearly recorded from the barostat tracing. If respiratory variations were not seen by 18 mmHg, BOP was set at 12 mmHg. An initial “conditioning” distension of the rectum was then performed in which the pressure was increased from 0 mmHg in steps of 4 mmHg for 15 s per step until 20 mmHg was reached. Previous studies have shown that an initial conditioning distension to 20 mmHg renders subsequent assessments of compliance and perception more reproducible (23). The bag was then deflated to 0 mmHg, and the subjects were allowed to rest for 10 min before proceeding to the ascending method of limits. The experimental design is shown in Fig. 1.
Ascending method of limits: compliance and sensory thresholds.
Rectal compliance and sensory thresholds were measured by ramp inflation, starting at 0 mmHg and increasing the pressure each minute in steps of 4 mmHg to a maximum of 60 mmHg. Thresholds for first sensation, gas, urgency, and pain (Fig. 1) were indicated by the subjects by pressing a button at the distension pressure at which sensations were perceived. Ramp inflation was terminated as soon as the subjects reported the first sensation of pain. Following this procedure, the bag was deflated to BOP and participants rested for 10 min.
Random-order phasic distensions: sensory ratings.
After the protocol of ascending method of limits, phasic distensions of 12, 24, 30, and 36 mmHg above BOP were each applied once in random order. Each distension was maintained for 1 min with an interstimulus interval of 2 min during which the balloon was deflated to BOP. This approach has been shown to be reliable in multiple previous studies (4, 14, 15, 45) because the intensity ratings are generally proportional to the magnitude of the distension pressures. Study participants were blinded to the distension order, which was provided by the study statistician (A. R. Zinsmeister). Participants were asked to mark separate 100-mm visual analog scales (VAS) 30 s after the onset of the distension for the sensations of gas, urgency, and pain. These scales were anchored at each end by the descriptions “unnoticeable” and “unbearable.” Pressure was immediately released if the subject reported greater than 80 mm of pain on the VAS scale, and higher distension pressures were not subsequently administered. During the assessment of sensation, the interaction between the subject and the study investigator (I. Busciglio) was kept to a minimum.
Data analysis.
The following measurements were obtained: 1) the sensory thresholds for first sensation, gas, urgency, and pain during ascending method of limits, 2) the three individual sensation scores (gas, urgency, and pain) in response to the three random phasic distensions (13, 25, 32, and 36 mmHg above BOP), and 3) rectal compliance.
Rectal pressure-volume relationships were analyzed with the use of a linear interpolation method that was recently described and validated (21). A summary value for each compliance curve was thus calculated for each subject, specifically the pressure observed at one-half of the maximum observed volume (Pr1/2), where a smaller Pr1/2 corresponds to higher compliance.
Candidate Genes Tested via SNPs
SNPs tested were: α2A (C-1291G), α2C (Del 322–325), 5-HTTLPR, and GNβ3 (C825T). The methods have been published in prior reports from this laboratory (1, 22, 28).
Data and Statistical Analysis
The initial analysis examined the overall association of symptom phenotype (health vs. overall IBS), and separately the four phenotypes [health, IBS-C, diarrhea-predominant IBS (IBS-D), and mixed bowel dysfunction IBS], with each genotype using contingency table analyses (chi-square test or Fishers exact test as warranted). The primary endpoints for evaluating the association of genetic variations with sensory function in IBS and health were maximum tolerated nutrient drink volume (MTV) and symptoms, sensory thresholds, and ratings in response to distensions. The secondary endpoints were fasting and postprandial gastric volumes [as surrogates for the tone of the stomach, which are factors that influence satiation in dyspepsia (18)] and rectal compliance.
Analyses were done separately for each candidate gene. In the analyses of the individual physiologic response endpoints, the genotypes were categorized as wild-type vs. nonwild-type and were included as predictor variables of these sensory functions in separate analyses of covariance (ANCOVA) or proportional hazards regression models (for the sensory thresholds to account for the small number of “censored” values, i.e., the maximum distension did not evoke the particular sensory type). In addition, a genotype by disease group (IBS vs. health) interaction term was included in each of the models to examine whether the association of genotype with sensory function was similar in patients with IBS vs. healthy subjects. A repeated measures ANCOVA (for the repeated distension levels) was used to assess the association of genotype with sensory VAS ratings separately for sensations of gas, urgency, and pain. The following covariates were included in the repeated measures models: age, body mass index (BMI), HAD scale scores, SCL-90-R somatization scale score, the somatic symptom checklist (SSC) score, and, for the sensory threshold models, the ratings for “tired,” “worried,” “peace,” and “active” attributes.
The aim in these hypothesis-generating analyses was to explore potential associations that would warrant further study, and thus no adjustment in the alpha level for multiple tests was made. In particular, P values between 0.05 and 0.1 were considered suggestive of potential associations that might deserve further study with larger numbers of subjects and were specifically reported.
Some genotypes such as 5-HTTLPR LS and SS were grouped because of the low prevalence (<20%) of SS genotype in people in the Midwest of the United States who are predominantly of Northern European extraction (28). With the sample size available, the study was not adequately powered to detect potential associations with SS genotype alone. Moreover, the prior observation by Lesch et al. (29) suggested that the S allele is associated with decreased production of the serotonin transporter protein, justifying the combination LS/SS. Similarly, the prevalence of GG α2A is 7% and TT GNβ3 is 3% in controls studied from the same geographical region and similar ethnicity in the Midwest USA around southeastern Minnesota (1, 28).
RESULTS
Participants.
Table 1 shows key demographics and psychological data in 161 participants (39 controls and 122 patients with IBS, the latter categorized by IBS subgroup on the basis of bowel function). Data on gastrointestinal transit, rectal sensation, and compliance for each of these groups have been published elsewhere (9).
Table 1.
Key demographics, psychological and genotype data in 161 participants
| Healthy Controls | C-IBS | D-IBS | M-IBS | |
|---|---|---|---|---|
| Number (no. male) | 39 (0) | 49 (0) | 44 (3) | 29 (0) |
| Age, yr | 33.5±1.6 | 38.4±1.5 | 35.4±1.6 | 37.2±2.2 |
| BMI, kg/m2 | 25.1±0.7 | 25.1±0.4 | 28.9±1.0 | 27.9±0.8 |
| Anxiety score, HADS | 3.2±0.4 | 4.7±0.5 | 5.0±0.5 | 5.0±0.4 |
| Depression score, HADS | 0.6±0.2 | 1.6±0.3 | 1.4±0.2 | 1.9±0.4 |
| Somatization T score | 41.3±1.0 | 47.6±0.9 | 49.3±1.2 | 48.8±1.5 |
| SCL-90 general severity index T score | 37.6±1.3 | 44.0±1.3 | 44.6±1.5 | 44.6±1.7 |
| Somatic symptom score | 0.3±0.05 | 0.4±0.04 | 0.5±0.05 | 0.4±0.05 |
| Number taking SSRI, stable dose | 5 | 9 | 11 | 6 |
| Genotype | ||||
| α2A CC, n (%) | 25 (64.1) | 21(42.9) | 24 (54.6) | 12 (41.4) |
| α2A GC/GG, n (%) | 14 (35.9) | 28 (57.1) | 20 (45.4) | 17 (58.6) |
| α2C* wild-type, n (%) | 38 (97.4) | 45 (91.8) | 42 (95.4) | 25 (86.2) |
| α2C* (Del 322–325), n (%) | 1 (2.6) | 4 (8.2) | 2 (4.6) | 4 (13.8) |
| GNβ3 CC, n (%) | 19 (48.7) | 27(55.1) | 19 (43.2) | 11 (37.9) |
| GNβ3 TC/TT, n (%) | 20 (51.3) | 22 (44.9) | 25 (56.8) | 18 (62.1) |
| SLC6A4 LL, n (%) | 16 (41.0) | 21(42.9) | 16 (36.4) | 7 (24.1) |
| SLC6A4 LS/SS, n (%) | 23 (59.0) | 28 (57.1) | 28 (63.4) | 22 (75.9) |
Applicable values are means ± SE.
Not considered in further analyses due to small numbers of α2C (Del 322–325). IBS, irritable bowel syndrome; C-IBS, constipation-predominant IBS; D-IBS, diarrhea-predominant IBS; M-IBS, mixed bowel dysfunction IBS; BMI, body mass index; HADS, hospital anxiety and depression scale; SCL-90, syptom check list 90 revised; SSRI, selective serotonin reuptake inhibitor.
Association between candidate genes and symptom phenotype.
The prevalence of the α2C (Del 322–325) was <6%, providing insufficient power to explore associations of interest in this study.
There was a significant association between 5-HTTLPR genotype and subject phenotype (P = 0.034); specifically, higher proportions of subjects with the LL or the LS genotype were in the constipation phenotype; in contrast, a higher proportion of subjects with the 5-HTTLPR SS genotype were in the healthy group. A significant association between phenotype and the other two genotypes of interest, GNβ3 and α2A, was not observed (for either overall IBS vs. health or the four phenotype subgroups). Although IBS status was moderately associated with SCL-90-R scores (partial r2 = 12%, P < 0.001) and modestly associated with somatic symptom, anxiety, and depression scores (partial r2 values about 3–4%, P < 0.05), no association of genotypes with these scores was observed.
Fasting gastric volumes.
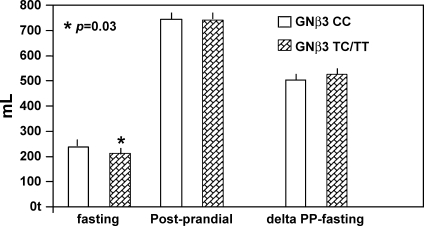
GNβ3 was associated with fasting gastric volume, with the TC/TT genotype being associated with lower fasting gastric volume (Fig. 2, P = 0.03); the association with postprandial change in gastric volume was not significant (P = 0.095).
Fig. 2.
Association of GNβ3 genotype with gastric volumes. After adjusting for age, body mass index (BMI), hospital anxiety and depression scale score, somatization, and group [irritable bowel syndrome (IBS) vs. health], fasting gastric volume is significantly associated (P = 0.03), and delta gastric volume borderline is significantly associated (P = 0.095). PP, postprandial.
Satiation and postprandial gastric volumes.
There was no significant association between satiation volume, aggregate symptom scores, or postprandial changes in gastric volume and either of the three candidate genes of interest in participants with IBS and healthy participants.
Rectal compliance, thresholds, and sensation ratings in different IBS groups and health.
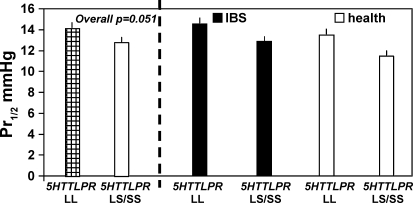
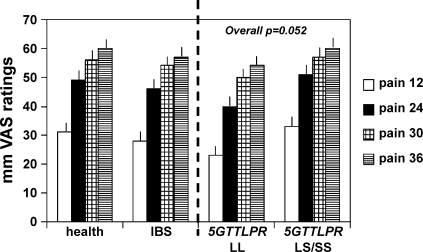
In Table 2, rectal compliance, thresholds, and sensation ratings are summarized for healthy participants and IBS patients; these data are further tabulated for the combined group on the basis of specific genotype of interest (Table 3). Note that the only significant associations are for 5-HTTLPR genotype with rectal compliance (Fig. 3) and sensation ratings for pain (Table 3, Fig. 4), with LS/SS genotype being associated with lower Pr1/2 (signifying higher compliance) and higher pain ratings. Figure 4 shows that the association of pain ratings and 5-HTTLPR genotype is predominantly noted at 12 and 24 mmHg distensions.
Table 2.
Rectal functions: compliance, sensation ratings and thresholds
| Endpoint† | Health, n = 39 | IBS, n = 122 |
|---|---|---|
| Compliance | ||
| Pr1/2, mmHg | (37) 12.3±0.8 | (118) 13.5±0.4 |
| Maximum balloon volume, ml | (37) 234±16 | (118) 238±10 |
| Sensation ratings | ||
| Gas at 30 mmHg | (36) 63±4 | (111) 59±2 |
| Gas at 36 mmHg | (33) 66±5 | (106) 62±3 |
| Pain at 30 mmHg | (36) 56±5 | (111) 54±3 |
| Pain at 36 mmHg | (33) 60±4 | (106) 57±3 |
| Urgency at 30 mmHg | (36) 74±3 | (110) 73±2 |
| Urgency at 36 mmHg | (33) 81±2 | (105) 76±2 |
| Thresholds* | ||
| 1st Sensation | (37) 7.8±0.6 | (118) 8.1±0.4 |
| Gas | (37) 13.6±1.5 | (118) 13.9±0.8 |
| Urgency | (37) 17.2±1.1 | (117) 19.1±0.9 |
| Pain | (37) 28.8±2.1 | (118) 30.2±1.1 |
n Values are means ± SE;
means ± SE ignoring censored status. Pr1/2, pressure observed at one-half of the maximum observed volume. Number in parentheses refers to number of participants undergoing the study.
Table 3.
Relationship of genotype to satiation volume, gastric volumes, rectal compliance, sensation ratings, and thresholds
| Endpoint | α2A CC | α2A GC/GG | GNβ3 CC | GNβ3 TC/TT | SLC6A4 LL | SLC6A4 LS/SS |
|---|---|---|---|---|---|---|
| Number | 82 | 79 | 76 | 85 | 60 | 101 |
| Satiation (MTV) volume, l | 1.03±0.05 | 1.06±0.03 | 1.02±0.03 | 1.08±0.04 | 1.07±0.04 | 1.04±0.03 |
| Fasting gastric volume, ml | 224±7 | 240±10 | 244±10 | 221±6 | 232±11 | 232±7 |
| Δ Postmeal gastric vol, ml | 521±9 | 516±10 | 507±10 | 528±9 | 513±10 | 521±9 |
| Compliance, Pr1/2, mmHg | 13.0±0.5 | 13.4±0.6 | 13.0±0.6 | 13.3±0.5 | 14.3±0.6* | 12.6±0.5 |
| Sensation rating gas at 30 mmHg | 61±3 | 59±3 | 63±3 | 58±3 | 56±3 | 63±3 |
| Rating gas at 36 mmHg | 65±4 | 61±3 | 65±4 | 61±3 | 59±4 | 65±3 |
| Rating pain at 30 mmHg | 56±3 | 53±3 | 56±4 | 53±3 | 50±4 | 57±3 |
| Rating pain at 36 mmHg | 59±4 | 56±3 | 58±4 | 58±3 | 54±4 | 60±3 |
| Rating urgency at 30 mmHg | 76±2 | 71±2 | 75±2 | 76±2 | 72±2 | 76±2 |
| Rating urgency at 36 mmHg | 80±2 | 74±2 | 78±2 | 74±2 | 77±2 | 74±2 |
| Threshold† 1st sensation, mmHg | 8.1±0.5 | 8.0±0.5 | 7.3±0.4 | 8.7±0.5 | 8.3±0.6 | 7.8±0.4 |
| Threshold gas, mmHg | 13.5±0.8 | 14.1±1.1 | 12.5±0.8 | 14.9±1.0 | 14.0±1.1 | 13.7±0.9 |
| Threshold urgency, mmHg | 18.0±1.0 | 19.3±1.0 | 17.6±0.9 | 19.6±1.0 | 19.2±1.1 | 18.3±0.9 |
| Threshold pain, mmHg | 29.6±1.5 | 30.1±1.4 | 29.1±1.5 | 30.5±1.4 | 32.0±1.6 | 28.6±1.3 |
Applicable values are means ± SE.
P < 0.05 vs. SLC6A4 LS/SS;
mean ± SE (includes censored values). MTV, maximum tolerated volume.
Fig. 3.
Rectal compliance in health and IBS on the basis of SLC6A4 gene. The LS/SS genotype is associated with higher compliance (lower Pr1/2, P = 0.051). Data show least square means adjusted for age, BMI, somatization, and group (health vs. IBS).
Fig. 4.
Association of SLC6A4 genotype with pain sensation ratings at the different levels of distension (P = 0.052 for overall test across all 4 distension levels); note that the LL genotype is associated with lower pain sensation ratings especially with 12 and 24 mmHg (above BOP) distensions.
DISCUSSION
This study has provided novel observations on the association of sensory functions in IBS and candidate genes of interest. Our study also explored the potential association of these candidate genes and symptom phenotype. We generally confirmed the prior studies from our laboratory (1, 28) regarding the lack of association of the three genotypes individually and IBS symptom phenotype. Thus a significant association between phenotype and the other two genotypes of interest, GNβ3 and α2A, was not observed. However, there was a higher proportion of subjects with the 5-HTTLPR LL and LS genotype with the constipation phenotype, whereas a higher proportion of subjects with the SS genotype was in the healthy group. In general, the long allele was associated with more effective 5-HT reuptake (29), and it is conceivable that this reduces effects of endogenous 5-HT on motor and secretory functions that may ultimately lead to constipation.
The overall conclusions reached in the prior study from our laboratory (28) of the association of SERT genotype and IBS were confirmed by a recent systematic review and meta-analysis by van Kerkhoven et al. (44) that included patients of European or Asian extraction. Moreover, our present data showed that 5-HTTLPR SS genotype relative to combined LL/LS genotype was protective from association with IBS. This is also consistent with the findings in the studies by Pata et al. (36) and Niesler et al. (33), although the Peto plot in the paper by van Kerkhoven et al. (44) showed that an overall odds ratio for the association of SS genotype with IBS in the 8 studies in the literature was very close to 1.
Although IBS status was moderately associated with somatization scores (by SCL-90-R) and modestly associated with somatic symptoms and anxiety and depression scores (9), no association of genotypes with these scores was observed in the present study. Thus our present data do not appear to confirm that SLC6A4 SS genotype is more likely to be associated with depression among patients with IBS, as was previously reported by another group (25). However, it is important to note that the proportion of patients on antidepressants in our patient cohort was low, and we have previously demonstrated by a detailed analysis (9) that the patient cohort included in this study was less psychologically compromised than a series of patients with IBS studied at another referral center (20).
The association of GNβ3 genotype and fasting gastric volume is consistent with epidemiological association of GNβ3 genotype with dyspepsia in two reports in the literature (24, 28). In a previous study (22), we also observed significant association between GNβ3 genotype and gastric emptying of solids at 4 h. The physiological factors that are significantly associated with the development of postprandial symptoms in dyspepsia are fasting gastric volume and accelerated gastric emptying at 1 h and delayed gastric emptying at 4 h (18). Together with the previous study (22), our present observations on the association of GNβ3 genotype and fasting gastric volume are consistent with the hypothesis that the epidemiological association of GNβ3 genotype with dyspepsia may be related to an association with abnormal gastric functions.
We have previously observed associations of the same genetic variations with gastric emptying in 251 participants (60 male and 191 female): 82 healthy, 20 patients with IBS with mixed bowel habit, 49 IBS-C, 67 IBS-D, and 33 functional dyspepsia (22). Those participants were selected from a database of people who had undergone gastrointestinal motility tests. On the other hand, the present prospective study focused primarily on the associations of these candidate genes with sensory functions in health and in patients with IBS.
In general, the satiation volume and postprandial gastric volumes were not significantly different in health and this group of patients with IBS (whole group and subtypes). As described and discussed extensively elsewhere (9), we found that ∼16% demonstrated rectal hyposensitivity, ∼21% demonstrated hypersensitivity, and the remainder demonstrated normosensitivity in this cohort of IBS patients relative to the concurrently recruited controls, all of whom were female. This study focused on relationships between the candidate genes and sensation in IBS, specifically satiation and rectal sensation. Associations with gastric volume and rectal compliance were of secondary interest and were included to facilitate interpretation of any associations identified between genotype and the sensory endpoints.
Significant genotype-function associations were noted for fasting gastric volume (with GNβ3), rectal compliance, and pain sensation (with 5-HTTLPR). When the association with a specific candidate gene is demonstrated for a motor function, it is important to interpret with caution the association of that gene with a sensory function. In this study, we observed associations of increased rectal compliance (lower Pr1/2) and increased pain sensation ratings during rectal distension in those with 5-HTTLPR LS/SS genotype relative to LL genotype. A decreased compliance may contribute to higher pain sensation ratings (6). However, since we observed increased compliance and higher pain sensation ratings, we conclude that the influence of 5-HTTLPR LS/SS genotype on rectal compliance does not explain the increase in pain sensation observed with this genotype.
Therefore, this supports the hypothesis that 5-HTTLPR LS/SS genotype increases pain sensation. The association of the genotype with increased pain ratings is not observed with the higher distension pressures, probably reflecting a “ceiling” effect, that is, that 30 and 36 mmHg above BOP (see Fig. 4 and data in Table 3) constitutes a significant stimulus that overwhelms the hypothetical effect of the 5-HTTLPR genotype proposed here. The LS/SS genetic variation would be expected to reduce reuptake of serotonin, and, if the latter is mediating pain sensation, it would result in higher pain sensation ratings.
It is unclear whether the lack of a significant association of 5-HTTLPR LS/SS genotype with sensations of gas and urgency reflects a true biological difference in the influence of SLC6A4 on sensation. It is conceivable that these sensations are mediated by different neural pathways, but this requires further study to explore whether, apart from the genotype, the tissue expression of these mechanisms is also associated with motor and sensory functions in IBS. This concept has been explored in experimental animal studies.
In studies conducted in guinea pigs with 2, 4, 6-trinitrobenzene sulfonic acid-induced colitis, mucosal SERT (SLC6A4) expression was reduced, and this led to increased 5-HT availability and motor functions (30). The role of SERT expression in tissues from patients with IBS is still unclear. Although Bellini et al. (3) suggested that platelet SERT should reflect colonic SERT in IBS and observed a significant relationship with IBS-D, the assumption has not been validated or rigorously tested. Coates et al. (16) suggested that there was an association between IBS-C or IBS-D and reduced SERT mRNA mucosal expression; however, we were unable to replicate the association between IBS symptom phenotype and SERT mRNA in sigmoid colon mucosa (7).
The lack of association of α2A genotype on sensation of gas, urgency, or pain is not altogether surprising since upregulation of α2A receptors and norepinephrine appears to be mostly associated with injury (37), which may be less relevant in IBS. Injury induces expression of novel noradrenergic receptors, sprouting of sympathetic nerve fibers, and pronociceptive changes in the ionic channel properties of primary afferent nociceptors. α2C adrenoceptors on axon terminals of excitatory interneurons of the spinal dorsal horn possibly contribute to spinal control of pain (38); however, the genetic variation in the α2C receptor is too infrequent to allow us to explore its contribution to the endophenotype. On the other hand, α2A receptors appear to be more important in gastrointestinal motor functions (40, 47).
We perceive that studies of physiological genetics or “endophenotype” may help identify susceptibility genes for complexly inherited traits like IBS, as has been reported in common mental disorders such as schizophrenia, bipolar disorder, and severe major depression. In all of these complex, multi-factorial conditions, identification of disease-promoting genes may be facilitated by studies of disturbed functions rather than symptom phenotype. The schizophrenia literature illustrates the usefulness of endophenotypes in genetic analyses of mental disorders (11) and in understanding these disorders at the cellular and molecular levels (2). As with those mental disorders, there is evidence from familial clustering and twin studies to support a heritable component in IBS (26), although this is somewhat controversial (31). Although our laboratory focuses predominantly on peripheral organ functions, investigation of genetic influences on brain structure or functions in the development of IBS using neuroimaging may provide further advances in understanding, as in schizophrenia (2).
In conclusion, we propose that such studies, as well as a recent report on the effect of genetic control of cannabinoid metabolism on gut functions (8), may usher in studies of endophenotype in IBS and help decipher the role of genetics and candidate mechanisms in the etiology, pathophysiology, or manifestations of IBS.
GRANTS
Dr. Camilleri is funded in part by grants RO1 DK-54681 and K24 DK-02638 from National Institutes of Health.
Acknowledgments
We thank Adil E. Bharucha, for helpful discussions and Cindy Stanislav for secretarial assistance.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Andresen V, Camilleri M, Kim HJ, Stephens DA, Carlson PJ, Talley NJ, Saito YA, Urrutia R, Zinsmeister AR. Is there an association between GNbeta3—C825T genotype and lower functional gastrointestinal disorders? Gastroenterology 130: 1985–1994, 2006. [DOI] [PubMed] [Google Scholar]
- 2.Bearden CE, van Erp TG, Thompson PM, Toga AW, Cannon TD. Cortical mapping of genotype-phenotype relationships in schizophrenia. Hum Brain Mapp 28: 519–532, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bellini M, Rappelli L, Blandizzi C, Costa F, Stasi C, Colucci R, Giannaccini G, Marazziti D, Betti L, Baroni S, Mumolo MG, Marchi S, Del Tacca M. Platelet serotonin transporter in patients with diarrhea-predominant irritable bowel syndrome both before and after treatment with alosetron. Am J Gastroenterol 98: 2705–2711, 2003. [DOI] [PubMed] [Google Scholar]
- 4.Bharucha AE, Camilleri M, Zinsmeister AR, Hanson RB. Adrenergic modulation of human colonic motor and sensory function. Am J Physiol Gastrointest Liver Physiol 273: G997–G1006, 1997. [DOI] [PubMed] [Google Scholar]
- 5.Bouras EP, Delgado-Aros S, Camilleri M, Castillo EJ, Burton DD, Thomforde GM, Chial HJ. SPECT imaging of the stomach: comparison with barostat, and effects of sex, age, body mass index, and fundoplication. Single photon emission computed tomography. Gut 51: 781–786, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Camilleri M, Coulie B, Tack JF. Visceral hypersensitivity: facts, speculations, and challenges. Gut 48: 125–131, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Camilleri M, Andrews CN, Bharucha AE, Carlson PJ, Ferber I, Stephens D, Smyrk TC, Urrutia R, Aerssens J, Thielemans L, Goehlmann H, Van Den Wyngaert I, Coulie B. Alterations in expression of p11 and SERT in mucosal biopsy specimens of patients with irritable bowel syndrome. Gastroenterology 132: 17–25, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Camilleri M, Carlson P, McKinzie S, Grudell A, Busciglio I, Burton D, Baxter K, Ryks M, Zinsmeister AR. Genetic variation in endocannabinoid metabolism, gastrointestinal motility and sensation. Am J Physiol Gastrointest Liver Physiol 294: G13–G19, 2008. [DOI] [PubMed] [Google Scholar]
- 9.Camilleri M, McKinzie S, Busciglio I, Low PA, Sweetser S, Burton D, Baxter K, Ryks M, Zinsmeister AR. Prospective study of motor, sensory, psychological and autonomic functions in patients with irritable bowel syndrome. Clin Gastroenterol Hepatol. In press. [DOI] [PMC free article] [PubMed]
- 10.Camilleri M, Neri M. Motility disorders and stress. Dig Dis Sci 34: 1777–1786, 1989. [DOI] [PubMed] [Google Scholar]
- 11.Cannon TD, Keller MC. Endophenotypes in the genetic analyses of mental disorders. Annu Rev Clin Psychol 2: 267–290, 2006. [DOI] [PubMed] [Google Scholar]
- 12.Chang EB, Bergenstal RM, Field M. Diarrhea in streptozocin-treated rats. Loss of adrenergic regulation of intestinal fluid and electrolyte transport. J Clin Invest 75: 1666–1670, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chial HJ, Camilleri C, Delgado-Aros S, Burton D, Thomforde G, Ferber I, Camilleri M. A nutrient drink test to assess maximum tolerated volume and postprandial symptoms: effects of gender, body mass index and age in health. Neurogastroenterol Motil 14: 249–253, 2002. [DOI] [PubMed] [Google Scholar]
- 14.Chial HJ, Camilleri M, Burton D, Thomforde G, Olden KW, Stephens D. Selective effects of serotonergic psychoactive agents on gastrointestinal functions in health. Am J Physiol Gastrointest Liver Physiol 284: G130–G137, 2003. [DOI] [PubMed] [Google Scholar]
- 15.Chial HJ, Camilleri M, Ferber I, Delgado-Aros S, Burton D, McKinzie S, Zinsmeister AR. Effects of venlafaxine, buspirone and placebo on colonic sensorimotor functions in healthy humans. Clin Gastroenterol Hepatol 1: 211–218, 2003. [DOI] [PubMed] [Google Scholar]
- 16.Coates MD, Mahoney CR, Linden DR, Sampson JE, Chen J, Blaszyk H, Crowell MD, Sharkey KA, Gershon MD, Mawe GM, Moses PL. Molecular defects in mucosal serotonin content and decreased serotonin reuptake transporter in ulcerative colitis and irritable bowel syndrome. Gastroenterology 126: 1657–1664, 2004. [DOI] [PubMed] [Google Scholar]
- 17.Cremonini F, Houghton LA, Camilleri M, Ferber I, Fell C, Cox V, Castillo EJ, Alpers DH, Dewit OE, Gray E, Lea R, Zinsmeister AR, Whorwell PJ. Barostat testing of rectal sensation and compliance in humans: comparison of results across two centres and overall reproducibility. Neurogastroenterol Motil 17: 810–820, 2005. [DOI] [PubMed] [Google Scholar]
- 18.Delgado-Aros S, Camilleri M, Cremonini F, Ferber I, Stephens D, Burton DD. Contributions of gastric volumes and gastric emptying to meal size and post-meal symptoms in functional dyspepsia. Gastroenterology 127: 1685–1694, 2004. [DOI] [PubMed] [Google Scholar]
- 19.Derogatis LR, Lipman RS, Covi L. SCL-90: an outpatient psychiatric rating scale—preliminary report. Psychopharmacol Bull 9: 13–28, 1973. [PubMed] [Google Scholar]
- 20.Dorn SD, Palsson OS, Thiwan SI, Clark WC, Kanazawa M, van Tilburg Drossman DA, Scarlett Y, Levy RL, Ringel Y, Crowell MD, Olden KW, Whitehead WE. Increased colonic pain sensitivity in irritable bowel syndrome is the result of an increased tendency to report pain rather than increased neurosensory sensitivity. Gut 56: 1202–1209, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Floyd BNI, Camilleri M, Andresen V, Esfandyari T, Busciglio I, Zinsmeister AR. Comparison of mathematical methods for calculating colonic compliance in humans: power exponential, computer-based, and manual linear interpolation models. Neurogastroenterol Motil 20: 330–335, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grudell AB, Camilleri M, Carlson P, Gorman H, Ryks M, Baxter K, Zinsmeister AR. An exploratory study of the association of adrenergic and serotonergic genotype and gastrointestinal motor functions. Neurogastroenterol Motil 20: 213–219, 2008. [DOI] [PubMed] [Google Scholar]
- 23.Hammer HF, Phillips SF, Camilleri M, Hanson RB. Rectal tone, distensibility, and perception: reproducibility and response to different distensions. Am J Physiol Gastrointest Liver Physiol 274: G584–G590, 1998. [DOI] [PubMed] [Google Scholar]
- 24.Holtmann G, Siffert W, Haag S, Mueller N, Langkafel M, Senf W, Zotz R, Talley NJ. G protein beta 3 subunit 825 CC genotype is associated with unexplained (functional) dyspepsia. Gastroenterology 126: 971–979, 2004. [DOI] [PubMed] [Google Scholar]
- 25.Jarrett ME, Kohen R, Cain KC, Burr RL, Poppe A, Navaja GP, Heitkemper MM. Relationship of SERT polymorphisms to depressive and anxiety symptoms in irritable bowel syndrome. Biol Res Nurs 9: 161–169, 2007. [DOI] [PubMed] [Google Scholar]
- 26.Kalantar JS, Locke 3rd GR, Zinsmeister AR, Beighley CM, Talley NJ. Familial aggregation of irritable bowel syndrome: a prospective study. Gut 52: 1703–1707, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim DY, Camilleri M. Serotonin: a mediator of the brain-gut connection. Am J Gastroenterol 95: 2698–2709, 2000. [DOI] [PubMed] [Google Scholar]
- 28.Kim HJ, Camilleri M, Carlson PJ, Cremonini F, Ferber I, Stephens D, McKinzie S, Zinsmeister AR, Urrutia R. Association of distinct alpha(2) adrenoceptor and serotonin transporter polymorphisms with constipation and somatic symptoms in functional gastrointestinal disorders. Gut 53: 829–837, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, Benjamin J, Müller CR, Hamer DH, Murphy DL. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science 274: 1527–31, 1996. [DOI] [PubMed] [Google Scholar]
- 30.Linden DR, Chen JX, Gershon MD, Sharkey KA, Mawe GM. Serotonin availability is increased in mucosa of guinea pigs with TNBS-induced colitis. Am J Physiol Gastrointest Liver Physiol 285: G207–G216, 2003. [DOI] [PubMed] [Google Scholar]
- 31.Mohammed I, Cherkas LF, Riley SA, Spector TD, Trudgill NJ. Genetic influences in irritable bowel syndrome: a twin study. Am J Gastroenterol 100: 1340–1344, 2005. [DOI] [PubMed] [Google Scholar]
- 32.Nakagawa I, Omote K, Kitahata LM, Collins JG, Murata K. Serotonergic mediation of spinal analgesia and its interaction with noradrenergic systems. Anesthesiology 73: 474–478, 1990. [DOI] [PubMed] [Google Scholar]
- 33.Niesler B, Fell C, Kapeller J, et al. SERT-P polymorphism in the serotonin transporter gene and irritable bowel syndrome: what's going on? Gastroenterology 132: A675, 2007. [Google Scholar]
- 34.O'Mahony S, Dinan TG, Keeling PW, Chua AS. Central serotonergic and noradrenergic receptors in functional dyspepsia. World J Gastroenterol 12: 2681–2687, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Osman A, Breitenstein JL, Barrios FX, Gutierrez PM, Kopper BA. The fear of pain questionnaire III: further reliability and validity with nonclinical samples. J Behav Med 25: 155–173, 2002. [DOI] [PubMed] [Google Scholar]
- 36.Pata C, Erdal ME, Derici E, Yazar A, Kanik A, Ulu O. Serotonin transporter gene polymorphism in irritable bowel syndrome. Am J Gastroenterol 97: 1780–1784, 2002. [DOI] [PubMed] [Google Scholar]
- 37.Pertovaara A Noradrenergic pain modulation. Prog Neurobiol 80: 53–83, 2006. [DOI] [PubMed] [Google Scholar]
- 38.Philipp M, Brede M, Hein L. Physiological significance of α-2-adrenergic receptor subtype diversity: one receptor is not enough. Am J Physiol Regul Integr Comp Physiol 283: R287–R295, 2002. [DOI] [PubMed] [Google Scholar]
- 39.Scheibner J, Trendelenburg AU, Hein L, Starke K. Alpha2-adrenoceptors modulating neuronal serotonin release: a study in alpha2-adrenoceptor subtype-deficient mice. Br J Pharmacol 132: 925–933, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Scheibner J, Trendelenburg AU, Hein L, Starke K, Blandizzi C. Alpha 2-adrenoceptors in the enteric nervous system: a study in alpha 2A-adrenoceptor-deficient mice. Br J Pharmacol 135: 697–704, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Talley NJ, Phillips SF, Wiltgen CM, Zinsmeister AR, Melton 3rd LJ. Assessment of functional gastrointestinal disease: the bowel disease questionnaire. Mayo Clin Proc 65: 1456–1479, 1990. [DOI] [PubMed] [Google Scholar]
- 42.Taylor S, Cox BJ. An expanded anxiety sensitivity index: evidence for a hierarchic structure in a clinical sample. J Anxiety Disord 12: 463–483, 1998. [DOI] [PubMed] [Google Scholar]
- 43.Thumshirn M, Camilleri M, Choi MG, Zinsmeister AR. Modulation of gastric sensory and motor functions by nitrergic and alpha2-adrenergic agents in humans. Gastroenterology 116: 573–585, 1999. [DOI] [PubMed] [Google Scholar]
- 44.Van Kerkhoven LA, Laheij RJ, Jansen JB. Meta-analysis: a functional polymorphism in the gene encoding for activity of the serotonin transporter protein is not associated with the irritable bowel syndrome. Aliment Pharmacol Ther 26: 979–986, 2007. [DOI] [PubMed] [Google Scholar]
- 45.Viramontes BE, Malcolm A, Camilleri M, Szarka LA, McKinzie S, Burton DD, Zinsmeister AR. Effects of an alpha(2)-adrenergic agonist on gastrointestinal transit, colonic motility, and sensation in humans. Am J Physiol Gastrointest Liver Physiol 281: G1468–G1476, 2001. [DOI] [PubMed] [Google Scholar]
- 46.Weems WA, Szurszewski JH. Modulation of colonic motility by peripheral neural inputs to neurons of the inferior mesenteric ganglion. Gastroenterology 73: 273–278, 1977. [PubMed] [Google Scholar]
- 47.Zhang L, Keef KD, Bradley ME, Buxton IL. Action of alpha 2A-adrenergic receptors in circular smooth muscle of canine proximal colon. Am J Physiol Gastrointest Liver Physiol 262: G517–G524, 1992. [DOI] [PubMed] [Google Scholar]
- 48.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 67: 361–370, 1983. [DOI] [PubMed] [Google Scholar]