Abstract
The length at which a muscle operates in vivo (operational length) and the length at which it generates maximal force (optimal length) may be quite different. We studied active and passive length-tension characteristics of external anal sphincter (EAS) in vivo and in vitro to determine the optimal and operational length of rabbit EAS. For the in vitro studies, rings of EAS (n = 4) were prepared and studied in a muscle bath under isometric conditions. For in vivo studies, female rabbits (n = 19) were anesthetized and anal canal pressure was recorded by use of a sleeve sensor placed in the custom-designed catheter holders of 4.5-, 6-, and 9-mm diameters. Measurements were obtained at rest and during EAS electrical stimulation. Sarcomere length of EAS muscle was measured by laser diffraction technique with no probe and three probes in the anal canal. In vitro studies revealed 2,054 mN/cm2 active tension at optimal length. In vivo studies revealed a probe size-dependent increase in anal canal pressure and tension. Maximal increase in anal canal tension with stimulation was recorded with the 9-mm probe. Increases in anal canal tension with increase in probe size were completely abolished by pancuronium bromide. EAS muscle sarcomere length without and with 9-mm probe in the anal canal were 2.11 ± 0.08 and 2.99 ± 0.07 μm, respectively. Optimal sarcomere length, based on the thin filament length measured by thin filament analysis, is 2.44 ± 0.10 μm. These data show that the operational length of EAS is significantly shorter than its optimal length. Our findings provide insight into EAS function and we propose the possibility of increasing anal canal pressure by surgical manipulation of the EAS sarcomere length.
Keywords: anal continence, fecal incontinence, sarcomere length
smooth muscle of the internal anal sphincter (IAS) and striated muscle of the external anal sphincter (EAS) and puborectalis muscle play essential roles in the fecal continence mechanism. Current understanding is that the resting anal canal pressure is mostly related to the IAS and the increase in anal canal pressure with voluntary squeeze is related to contraction of striated EAS muscle (5, 8, 25). Pressure recording of the anal canal using side-hole manometry and station pull-through technique show that, both at rest and during voluntary squeeze, the highest pressure is located in the part of the anal canal that is surrounded by the EAS, suggesting that EAS is most likely the strongest component of the anal continence mechanism (22). The EAS is also the most commonly affected muscle in women with fecal incontinence because it frequently gets damaged during childbirth-related trauma and episiotomy (29).
The EAS muscle in humans shows both resting or sustained slow (tonic) and rapid (phasic) contractions. The rapid contractions occur during voluntary squeeze and reflex responses to visceral (rectal distension) and cutaneous (perianal) stimuli. Both slow and fast contractions of the EAS are the result of activity in the cortex, brain stem, and spinal motor neurons. These neurons innervate the EAS muscle through pudendal nerves. The neural input is only one of the determinants of force of contraction generated by a skeletal muscle; the other is the sarcomere length. The maximal force by a muscle is generated at its optimal sarcomere length. The length-tension relationship of a muscle determines the optimal length (Lo), defined as the sarcomere length at which the thick and thin filament overlap is complete (11, 12).
Krier et al. (17) studied the length-tension relationship of the cat EAS muscle in vitro. However, in vivo length at which a muscle operates (operational length) can be quite different from its Lo, and it appears that various muscles in the body operate at different sarcomere lengths to suit their functional needs (4). The goal of our study was to determine the length-tension properties of the IAS and EAS in vivo. Furthermore, we sought to determine both operational and optimal sarcomere lengths of the EAS muscle in vivo. Our studies indicate that the EAS muscle operates at a low sarcomere length, which is suited to perform function unique to the sphincter muscle.
METHODS
The institutional animal care and use committee at the Veterans Affairs San Diego Healthcare Systems approved the study protocol, and all experiments were conducted in accordance with the Guide and Use of Laboratory Animals (National Institutes of Health, Bethesda, MD). Adult New Zealand white female rabbits (n = 19; 4–5 kg) were anesthetized with an intramuscular injection of ketamine (35 mg/kg) and xylazine (5 mg/kg) and an intravenous (IV) catheter was placed (ear vein) for maintenance of anesthesia and administration of drugs. We used separate group of animals for the in vivo and in vitro studies.
In vitro studies.
Female New Zealand White rabbits were euthanized and the anal canal was harvested. It was immediately placed in the modified Krebs solution at 37°C. The Krebs solution was bubbled with 95% O2-5% CO2 and contained (in mM) 137.4 Na+, 5.9 K+, 2.5 Ca2+, 1.2 Mg+, 134 Cl−, 5.5 HCO−, 1.2 H2PO4, and 11.5 glucose. The pH of the Krebs solution ranged from 7.35 to 7.40. The EAS muscle was identified as a striated muscle band around the circumference of the anal canal. After the skin, mucosa, and connective tissue were dissected, four rings of the EAS muscle, each ∼2.5 mm in width, were mounted under isometric conditions in a 50-ml water-jacketed organ bath containing modified Krebs solution. One end of the ring was secured to an electrode holder, and the other end was fastened to an isometric force transducer (model 60-2995, Harvard Apparatus, Holliston, MA). A baseline tension of 0.5 g was applied and tension was allowed to equilibrate for 45–60 min. Each ring was stretched in 10% increments of its initial length, Li. The EAS muscle rings were maintained at the stretched length for a period of 15–20 min to allow for passive forces to decay to a new steady-state level (stress-relaxation). Electrical field stimulation (EFS) of increasing intensity (20–150 mA in steps of 5 mA, train length 2 s, pulse duration 1.5 ms) was applied to the muscle rings at each length until the maximal contraction force was elicited during stimulation (80–90 mA). The EFS was delivered by a Grass stimulator (Grass Technologies, West Warwick, RI; model S-48) interfaced to a Hewlett Packard bipolar amplifier (model 6826A, Waltham, MA) through platinum wire electrodes placed on either side of each muscle ring. Output data from the force transducer were acquired by using BioPac MP100A-CE (BioPac Systems, Goleta, CA). The stretched length that produced maximum contraction was considered as the Lo. The effect of neuromuscular blocking agent pancuronium bromide (PB; Hospira, Lake Forest, IL; 2 μM) on the force of contraction was also studied (17). The cross-sectional area of the muscle rings was determined by estimating the thickness (using high-frequency intravascular ultrasound) and the width of the muscle rings. The EAS muscle tension was then normalized by cross-sectional area and denoted in millinewtons per centimeter squared (mN/cm2).
In vivo studies.
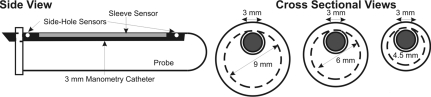
For the in vivo studies, an incision was made on the anal skin to expose the EAS muscle. Two custom-designed copper wire hook electrodes were placed in the EAS muscle for electrical stimulation. The anal canal pressure was measured by manometric methods; a 3-mm diameter sleeve sensor was placed in the custom-designed probe holders of 4.5-, 6-, and 9-mm diameter, as shown in Fig. 1. The probe holder size selection was based on the rabbit fecal pellet size, which typically ranges from 10–11 mm. The anal canal pressures were recorded with the pressure-sensing surface of the sleeve sensor facing in the posterior midline direction. The sleeve sensor measures the highest pressure along its length. Therefore our probe system measures the highest pressure along the length of anal canal, irrespective of the muscle contributing to that pressure (6). A pulse generator (model S48, Grass Technologies) connected to a constant current unit (model CCU1A, Grass Technologies), with currents ranging from 1 to 5 mA, in steps of 1 mA, at a frequency 50 Hz with pulse duration of 0.2 ms, was used for electrical stimulation. Sodium nitroprusside (SNP; Sigma Chemical, St. Louis, MO) (1.5 μg/kg) and PB (0.4 mg/kg) were used during in vivo studies to evaluate the contribution of IAS and EAS to the anal canal pressure (5, 27). Animals were maintained at 24 breaths/min on an artificial dual phase control respiratory pump (model 613; Harvard Apparatus, Holliston, MA) via an endotracheal tube. Heart rate was monitored continuously during the entire experiment.
Fig. 1.
Schematics of manometry catheter and anal probe holder. Anal canal pressure is recorded via a 3-mm manometry catheter. The manometry catheter is placed inside the probe holders of diameters ranging from 4.5 to 9 mm. The sleeve sensor records the maximum pressure along the length of the anal canal.
The effect of caffeine on the EAS muscle contraction during maximal stimulation was determined in four animals. For these experiments animals received IV injection of 10 mM/kg of caffeine (Sigma Chemical) in 5 ml of saline (2) and the EAS muscle was stimulated at the maximal current intensity used in our experiment.
Ultrasound images of anal canal were obtained to determine the anal canal radius and muscle thickness. Ultrasound imaging was performed via a 6-Fr, 20-MHz transducer (EU-M30S; Olympus Optical; Tokyo, Japan). The probe was placed inside the lumen and advanced into the rectum and then gradually pulled back into that part of the anal canal where both internal and EASs were clearly visualized. The images were recorded on videotape and analysis was performed at a later time to calculate the radius of the anal canal and muscle thickness of the EAS (24).
Measurements of sarcomere length.
At the end of the recording period, the anal canal with intact sphincters and with either no probe or one of the anal probes placed inside the lumen was removed. The anal canal with the probe in place was fixed in 10% phosphate-buffered formalin solution for 24 −48 h to fix the muscle and other tissues around the probe. At the end of fixation period, specimens were rinsed in phosphate-buffered saline and placed in 15% sulfuric acid (8–12 h) to partially digest the connective tissues surrounding the muscle. After acid digestion, the EAS muscle fibers were isolated by microdissection, mounted on microscopic glass slides, and subjected to laser diffraction for determination of the sarcomere lengths (20). The muscle fiber bundle was transilluminated by a He-Ne laser (model 05-LHR-171, Melles-Griot, Irvine, CA), and sarcomere length was calculated via the grating equation: nλ = d·sinθ, where n is the diffraction order (±1, ±2, ±3, etc.), λ is the laser wave length (0.632 μm), d is the grating spacing (which equals sarcomere length), and θ is the diffraction angle measured by use of a photodiode array.
EAS thin filament length measurements.
These measurements were performed in the rabbit EAS muscle cryosections. The specimens were fixed and stained for actin (phalloidin) and α-actinin by use of appropriate antibodies and immunofluorescence technique (1). Images were acquired by confocal microscopy and thin filament lengths were determined from the width of the phalloidin bands, by distributed deconvolution analysis, via a custom plug-in written for the program Image (NIH) (21). This method determines the best fit of a probe-specific intensity (model) distribution to 1D myofibril fluorescence intensity profiles (line scans). It estimates the spread of light along the line scan, and the positions and intensities of each probe with adjustable intensity distribution models for the entire thin filament array. Image regions containing at least three stretched sarcomeres were identified on the basis of the appearance of phalloidin gaps (H zones). Thin filament length was defined as half the width of the phalloidin bands.
Data analysis.
All pressures were measured in reference to atmospheric pressure. Rest pressure was determined as the average pressure recorded 30 s before the EAS muscle stimulation. The maximum pressure during EAS stimulation was the peak pressure recorded during the 10-s stimulation period. Delta pressure was defined as the difference between maximum and rest pressure.
The force of contraction of the EAS muscle was determined by calculating muscle tension (Tm) by using the following equation: Tm = P × rm/tm, where P is the intraluminal pressure, rm is the inner radius of the EAS muscle, and tm is the EAS muscle thickness. The rm and tm were measured from the ultrasound images of the rabbit anal canal and further confirmed by measuring these parameters in a freshly harvested specimen. The EAS muscle tension represents the average circumferential force per unit area of the circular muscle and is denoted in millinewtons per centimeter squared (3). The EAS muscle active tension at rest was calculated by subtracting anal canal tension after PB from the total tension. The IAS active tension at rest was obtained by subtracting anal canal tension in the presence of PB and SNP from the one after the PB.
Statistical analysis.
Data are shown as means ± SE. For length-tension studies, a two-way repeated-measures ANOVA with post hoc Tukey's test (SPSS) was performed.
RESULTS
Length tension of the EAS: in vitro studies.
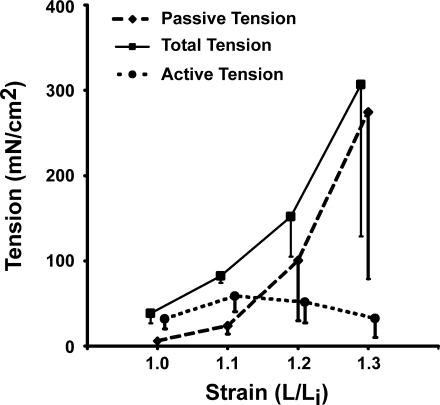
EFS of EAS muscle rings at the indicated stimulation parameters induced a tetanic contraction that lasted for the duration of stimulus. As expected, the amplitude of contraction increased with the increase in the stimulation current. With the increase in the length of EAS muscle rings, there was a linear increase in the force of contraction at the maximal electrical stimulus. The maximum force during EFS was reproducible and so was the passive length-tension curve. Peak force and active force generated at Lo were 2,895 and 2,054 mN/cm2, respectively. The latter suggests that 71% of the total muscle force at Lo was due to active muscle contraction and the remainder due to passive properties of the EAS muscle and connective tissue (Fig. 2). PB did not affect the EFS-induced contraction of EAS muscle (peak force before and after PB were 2,114 ± 123 and 2,090 ± 204 mN/cm2, respectively). The latter suggests that the EFS induced contraction was mediated by direct muscle fiber depolarization rather than through the neuromuscular junction activation.
Fig. 2.
Effect of increasing length (L) and electrical field stimulation (EFS) on rabbit external anal sphincter (EAS) in vitro contractions (n = 4). x-axis: Normalized change in length (strain; Li is the initial length). y-axis: Tension in mN/cm2. EFS parameters: current 80–90 mA, train length 2 s, pulse duration 1.5 ms, pulse frequency 0.3 Hz.
Length tension of the EAS: in vivo studies.
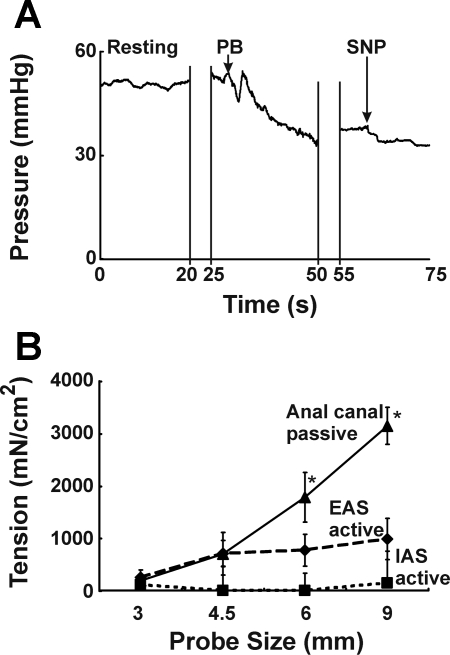
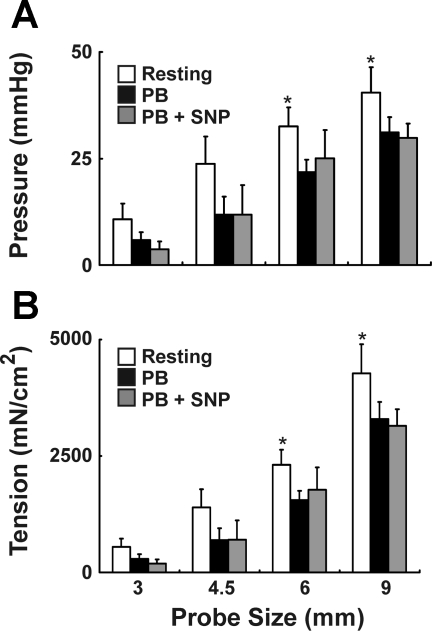
Anal canal pressures were measured, both at rest and during electrical stimulation of the EAS. The representative tracings are shown in Figs. 3A and 4A. The anal canal pressure and tension at rest increased with increase in the probe size (Fig. 5). The maximal tension was observed with the 9-mm probe (4,277 ± 627 mN/cm2). Insertion of the 12-mm probe in the anal canal was met with significant resistance in our preliminary experiments and therefore the largest probe tested was 9 mm. We tried 12-mm probes in a few rabbits only and did not test the smaller probes after it. For the other animals we tried both sequences (small to large as well as large to small) and found no difference in our pressure responses. In the absence of electrical stimulation of the EAS muscle, PB administration caused a small decrease in the anal canal pressure at each probe size, which was attributed to the active EAS muscle contraction. In the presence of PB, administration of SNP resulted in either a small or no decrease in the anal canal pressure at each probe size. The latter was attributed to the contribution of smooth muscle sphincter, i.e., IAS to total anal pressure (Figs. 3A and 5A). The active EAS muscle tension increased with the increase in probe size. On the other hand, active IAS tension did not increase with the increase in the probe size (Fig. 3B). The anal canal pressure in the presence of combined PB and SNP was attributed to the passive tension of anal canal tissues, including that of IAS and EAS muscles, connective tissue, and perianal skin. With increasing probe size there was significant increase in the passive anal canal tension, and differences were significant with probe sizes of 6 and 9 mm (Fig. 3B).
Fig. 3.
A: effect of pancuronium bromide (PB) alone and in combination with sodium nitroprusside (SNP) on the anal canal resting pressure is shown in the tracings with a 9-mm anal probe. B: effect of probe size on EAS active, internal anal sphincter (IAS) active, and anal canal passive tensions. *Statistically significant increase (P < 0.05) in passive tension with increase in probe size (6 and 9 mm compared with 3 mm, n = 4).
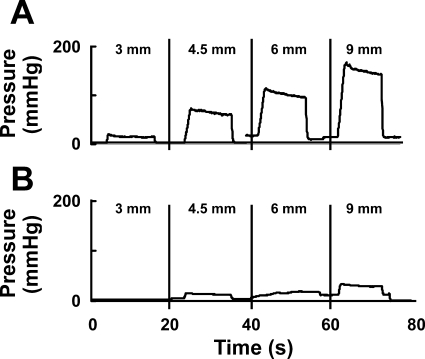
Fig. 4.
Representative tracings with 4 different anal probe sizes. Tracings show anal canal pressure with different probe sizes during electrical stimulation with 5 mA before (A) and after (B) neuromuscular blockade with PB. Note that, for a given electrical stimulus, increase in probe size resulted in an increase in the anal canal pressure.
Fig. 5.
Effect of probe size, PB, and PB + SNP on anal canal resting pressure (A) and tension (B). *Significant increase (P < 0.05) in resting pressure and tension with increase in probe size (6 and 9 mm compared with 3 mm, n = 4).
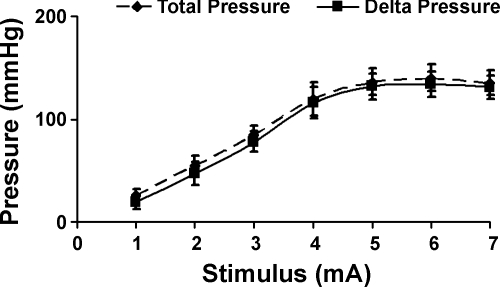
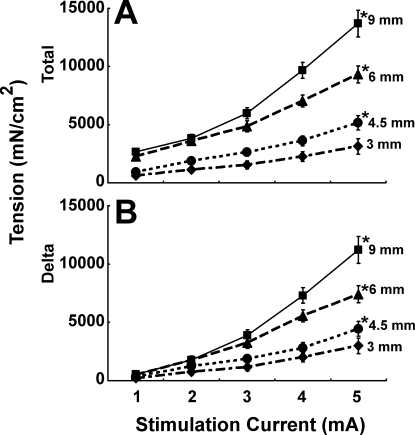
Electrical stimulation of the EAS muscle resulted in the intensity dependent increase in the anal canal pressure and tension (Figs. 6 and 7). We tested 1- to 7-mA stimuli in our pilot experiments and found that the maximum pressure was achieved at the 5-mA current intensity (Fig. 6). For all electrical stimulus intensities, anal canal pressure and tension increased with the increase in the probe size (Fig. 7). Maximal anal canal pressure-tension was observed with the 9-mm probe. The difference between the anal canal pressure prior to and during the EAS stimulation was attributed to active contraction of EAS muscle and it was almost completely blocked by PB (Fig. 4B). The latter suggests that the majority of EAS contraction was related to the activation of muscle through the neuromuscular junction.
Fig. 6.
EAS electrical stimulus-response curve. EAS was subjected to electrical stimulation (1–7 mA) and anal canal pressure was recorded with a 3-mm probe as described in methods (n = 6). Note maximal response at 5 mA stimulus.
Fig. 7.
Anal canal total (A) and delta (B) tension recorded after stretching EAS with probes of increasing diameter and increasing electrical current. At maximal stimulus, tensions for 4.5-, 6-, and 9-mm probes were significantly (P < 0.05) greater than with the 3-mm probe (n = 15).
Effect of caffeine on anal canal pressure during stimulation of EAS.
For these experiments, the effect of IV caffeine on anal canal tension during maximal EAS stimulation was determined. At each stimulus, there was small increase in the anal canal pressure following caffeine, but this increase was not statistically significant (anal canal tensions recorded for 5-mA stimulus before and after caffeine infusion were 671 ± 47 and 871 ± 140 mN/cm2, respectively). The latter suggests that our electrical stimulation technique resulted in near maximal stimulation of the EAS muscle.
Sarcomere length measurement.
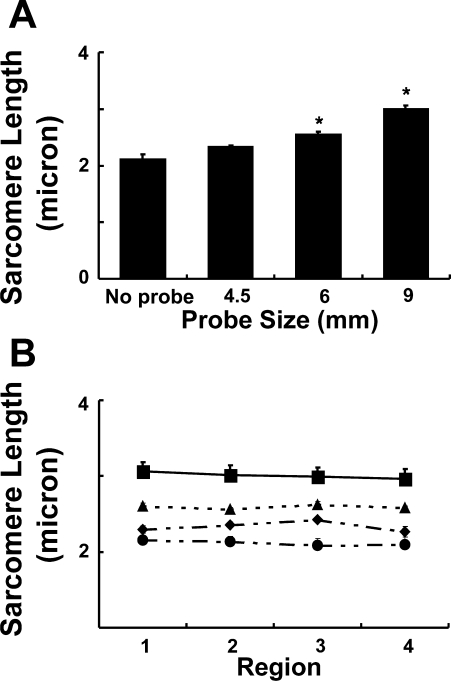
In the absence of any probe in the anal canal, mean sarcomere length of the EAS muscle was 2.11 ± 0.08 μm. The addition of probe in the anal canal resulted in the probe size-dependent increase in the sarcomere length (Fig. 8A). The mean sarcomere length with the 9-mm probe was 2.99 ± 0.07 μm. To determine whether there was nonuniformity of sarcomere length around the EAS circumference, measurements were made at the 3, 6, 9, and 12 o'clock positions around the anal canal. There was no significant difference in these measurements for any of the probe sizes (Fig. 8B). To define the heterogeneity in sarcomere length from the superficial to deep muscle layers, we also measured the sarcomere length at various depths of the EAS muscle that was stretched with a 9-mm probe and found no significant difference in the sarcomere length of these muscle fibers (sarcomere lengths were 2.97 ± 0.04 and 2.94 ± 0.05 μm for fibers obtained from the deep and the superficial muscle layers, respectively).
Fig. 8.
The anal canal was harvested without and with probes of increasing diameter (4.5, 6, 9 mm) and immersion fixed in phosphate-buffered formalin. EAS muscle fibers were dissected, fixed on microscopic slides, and subjected to laser diffraction. A: stretch-dependent increase in EAS sarcomere length (n = 4–6 for each probe size). B: regional evaluation of sarcomere length at 12, 3, 6, and 9 o'clock positions around the circumference of the anal canal (n = 4–6 for each probe size). *Statistically significant increase (P < 0.05).
Thin filament length analysis.
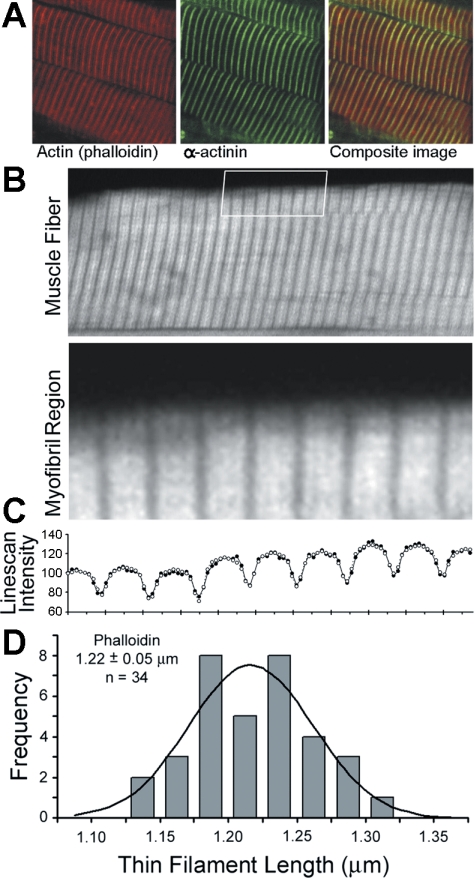
Confocal images of EAS frozen sections stained for actin (phalloidin) and α-actinin show the characteristic regular striations of cross-striated skeletal muscle (Fig. 9A). Phalloidin bands were uniform in width and bisected by α-actinin at the Z line. Clear gaps in the phalloidin staining (H zones) were visible in sufficiently stretched myofibrils indicating that thin filaments were precisely specified in length. The uniform and distinct appearance of the phalloidin bands allowed us to quantify thin filament lengths using distributed deconvolution analysis, a nonlinear curve-fitting method that determines thin filament lengths with better than 50 nm accuracy (see methods). For each myofibril region (Fig. 9B), the phalloidin intensities along the myofibril (line scan) were measured (Fig. 9C, •) and observed to consist of a single intensity profile that is blurred by the microscope and repeated for each Z line. Three functions describing the distribution of phalloidin binding within a single profile, the spread of light in the microscope, and the distribution of Z lines were combined to generate a “myofibril intensity profile” and best fit to the myofibril line scan. The myofibril intensity profiles (Fig. 9C, ○) accurately reproduce the myofibril line scans, resulting in residual values of ∼5%. The function used to quantify phalloidin staining assumes that phalloidin bound uniformly along the thin filament and includes a parameter corresponding to the thin filament length (21). This analysis (Fig. 9, B–D) confirmed the appearance of regular-width phalloidin bands and determined that the thin filament length is 1.22 ± 0.05 μm (n = 34). This length indicates that the rabbit EAS muscle should have an optimal sarcomere length of 2.44 ± 0.10 μm (2× thin filament length) and should have a plateau region on its force-length curve at sarcomere lengths of 2.29–2.59 μm (optimal sarcomere length ± a bare zone of 150 nm). The thin filament lengths predict that any sarcomere length greater than 2.59 μm should be on the descending limb of the length-tension curve.
Fig. 9.
A: confocal images of immunostained EAS frozen sections for actin, α-actinin, and the 2 superimposed, i.e., composite image. B: rabbit EAS muscle fiber stained with phalloidin reveals uniform thin filament lengths. The boxed region (shown below at ×4 magnification) indicates the myofibril region analyzed by distributed deconvolution. C: myofibril line scan shown with observed intensity (•) and expected intensity (○) as explained in the text. D: histogram of phalloidin length parameters determined by distributed deconvolution analysis (gray bars). Line, expected number of observations based on a normal distribution.
DISCUSSION
Our data show the following: 1) In vitro study shows an increase in the EFS-mediated EAS muscle contractions with an increase in muscle length, which was not blocked by PB (Fig. 2). 2) In vivo study shows a) a probe size-dependent increase in resting anal canal pressure that is related to the contribution from the EAS muscle and passive anal canal resistance (Fig. 5) and b) a probe size- and an electrical stimulus intensity-dependent increase in the anal canal pressure that was abolished by neuromuscular blockade, i.e., PB. 3) An increase in the active muscle contraction of the EAS with an increase in the probe size during electrical stimulation of EAS is severalfold higher compared with the resting anal canal pressure (Fig. 7). 4) There is a probe-dependent increase in the EAS sarcomere length (Fig. 8). Our data suggest that the operational or the in vivo sarcomere length of the EAS muscle is significantly shorter than its Lo.
Krier et al. (17) studied the length-tension characteristics of the cat EAS muscle strips in vitro, and their study revealed that the EAS muscle is similar to other skeletal muscles in that it shows a bell-shaped curve with ascending and descending limbs. The major finding of their study was that at Lo the passive tension generated by the EAS muscle accounted for 40% of the total tension. In our experiments, at Lo, passive tension accounted for 21% of the total tension. The difference between our study and Krier's, who studied cats, could be related to the species difference. The high passive tension of the muscle is thought to be due to the excessive amount of collagen in the EAS muscle. Krier et al. came to the conclusion that the passive properties of the EAS muscle must play a significant role in the anal closure mechanism. However, their conclusion was based on the assumption that in vivo, and under physiological conditions, the EAS muscle operates at Lo. However, under normal physiological conditions different skeletal muscles in the body may operate at optimal, suboptimal, or supraoptimal lengths (4). When operating at suboptimal length (i.e., on the ascending limb of the force length curve), an increase in the muscle length increases the force of contraction; the myocardium is an example of such a muscle (15). Respiratory diaphragm, on the other hand, operates at the Lo (9), whereas the flexors and extensors of the arm operate at suboptimal and supraoptimal portions of the length-tension curve, respectively (18, 19).
Our novel probe system allowed us to measure, for the first time, in vivo length-tension properties of the IAS and the EAS at rest, and of the EAS muscle during stimulation. Technique somewhat similar to ours was used by Biancani et al. (3) to study the length-tension characteristics of the lower esophageal sphincter muscle in vivo. Increase in the sarcomere length with the increase in the probe size suggests that the technique we used was effective in increasing the in vivo muscle sarcomere length. We employed PB and SNP to delineate the roles of skeletal and smooth muscles, respectively, in the genesis of anal canal pressure at rest. PB is a neuromuscular blocking agent and previous studies in rabbits have demonstrated complete skeletal muscle paralysis at the employed doses (10, 28). Nitric oxide donors such as SNP have been shown to relax IAS smooth muscle in the animal models (16). SNP can affect both the smooth and skeletal muscle contraction; therefore we always used PB first (to eliminate the skeletal muscle contribution) and then used SNP to determine whether there was any leftover smooth muscle contribution to the anal canal pressure. Our data show that the anal canal pressure at rest is relatively small in the rabbit, compared with the opossum (5) and humans (7). With the increase in anal canal probe size there is an increase in the anal canal pressure in the rabbit but majority of this increase is related to the passive properties of the anal canal tissue because PB and SNP do not inhibit it. At rest, with the increase in the probe size there is small increase in the anal canal tension generated by the EAS muscle. The EMG activity of the EAS at rest shows small tonic activity (data not shown), indicating a small neural input under basal conditions. In our experiments, large increases in the anal canal pressure were observed during electrical stimulation of the EAS, and with the increase in the probe size the increase in anal canal pressure and EAS muscle tension became larger. It is well known that the tension generated by a muscle is proportional to the degree of thin-thick filament overlap and the sarcomere length (12, 26). Sarcomere length measurements of the EAS muscle in our experiment provide evidence of this phenomenon. The biggest probe size we could use for our studies was 9 mm because insertion of 12-mm probes was met with significant resistance in our pilot experiments. With the 9-mm probe the active tension generated by EAS muscle was higher than by the 6-mm probe, suggesting that the EAS muscle length we achieved with the maximal size probe was still on the ascending limb of the length-tension curve. Therefore we did not achieve Lo of the EAS muscle in vivo using our technique. On the other hand, the EAS sarcomere length of 2.99 μm with the 9-mm probe is greater than the optimal sarcomere length for EAS muscle as measured by the thin filament analysis. The optimal sarcomere length for most of the other rabbit skeletal muscle is also ∼2.40 μm (4, 14). There are several possibilities for this discrepancy; the first possibility is that our EAS muscle stimulation technique did not induce maximal muscle contraction. Maximal muscle activation is usually induced by nerve stimulation. The reason to stimulate muscle rather than nerve was related to the fact that we could not clearly identify the nerves supplying to the EAS muscle in the rabbit. However, others have successfully used direct muscle stimulation technique to study the length-tension characteristics (13). Furthermore, administration of caffeine did not increase the force of contraction of EAS muscle, which should have been the case if our technique did not induce maximal muscle activation. Therefore, we believe that our muscle activation technique was adequate. The second possibility is that the sarcomere length of rabbit EAS muscle, which has never been studied before, is different from the other skeletal muscles. However, it is unlikely because according to the thin filament length analysis the optimal sarcomere length ranges from 2.44 to 2.54 μm. The third possibility, not explored in our studies, is that some of the tension generated at higher muscle lengths is related to the stretch-sensitive release of intracellular calcium (30). Finally, it is possible that the EAS muscle fibers in the nondistended state are not oriented in the precisely circumferential direction and with anal canal distension they reorient themselves in the circumferential direction, thereby producing a relatively more efficient circumferential squeeze.
Irrespective of the precise Lo for the EAS muscle; our data are clear in that the operational length of the EAS muscle is significantly shorter than its Lo. Furthermore, at the in vivo operational length the passive force generated by the EAS muscle is relatively small (as shown by our in vitro study). In general, the reason for different muscles in the body to work at different operational lengths is related to the functional needs of the organ system. What is the advantage for the EAS muscle to work at low operational length? The sphincters in general have two functions: 1) they provide barriers for the movements of contents from one cavity to the other by remaining tonically contracted and 2) they open to allow movements of contents from one cavity to another during relaxation. Therefore, by operating at the low muscle lengths the sphincter muscle offers minimal passive resistance to the anal canal opening. Our length-tension curve shows that the passive tension in the EAS muscle increases rapidly after Lo, which would result in a significant resistance to the anal canal opening if the EAS muscle were to operate at Lo. It is not known whether all the sphincters in the body follow above principles. However, our own observation with the puborectalis muscle, which also provides a “sphincterlike” function to the anal canal, show that indeed it also operates at a low sarcomere length (unpublished data).
In conclusion, our novel probe system allowed us to determine the length-tension properties of the EAS in rabbit, and we propose that a similar system may be used to study the length-tension relation of the EAS muscle in humans. Our finding that the in vivo operational length of EAS muscle is significantly smaller than its Lo has considerable clinical relevance. Overlapping sphincteroplasty of the EAS muscle is a commonly performed surgical procedure to treat fecal incontinence (23). There is significant debate with regard to the degree of muscle overlap required for an effective EAS repair. The degree of muscle overlap is likely to be the major determinant of in vivo operational sarcomere length. Future studies should explore the ideal sarcomere length required to achieve maximum gain in the EAS muscle function. Furthermore, it may be possible to gain anal canal closure function by surgical adjustments of the EAS sarcomere length in the fecal incontinent patients with an anatomically intact but poorly contracting EAS muscle due to partial pudendal nerve injury.
GRANTS
This research was supported by a Public Health System Grant NIH 5R24 HD-050837-03.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Bang ML, Li X, Littlefield R, Bremner S, Thor A, Knowlton KU, Lieber RL, Chen J. Nebulin-deficient mice exhibit shorter thin filament lengths and reduced contractile function in skeletal muscle. J Cell Biol 173: 905–916, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Batkai S, Racz IB, Ivanics T, Toth A, Hamar J, Slaaf DW, Reneman RS, Ligeti L. An in vivo model for studying the dynamics of intracellular free calcium changes in slow- and fast-twitch muscle fibres. Pflügers Arch 438: 665–670, 1999. [DOI] [PubMed] [Google Scholar]
- 3.Biancani P, Zabinski MP, Behar J. Pressure tension, and force of closure of the human lower esophageal sphincter and esophagus. J Clin Invest 56: 476–483, 1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burkholder TJ, Lieber RL. Sarcomere length operating range of vertebrate muscles during movement. J Exp Biol 204: 1529–1536, 2001. [DOI] [PubMed] [Google Scholar]
- 5.Culver PJ, Rattan S. Genesis of anal canal pressures in the opossum. Am J Physiol Gastrointest Liver Physiol 251: G765–G771, 1986. [DOI] [PubMed] [Google Scholar]
- 6.Dent J A new technique for continuous sphincter pressure measurement. Gastroenterology 71: 263–267, 1976. [PubMed] [Google Scholar]
- 7.Diamant NE, Kamm MA, Wald A, Whitehead WE. AGA technical review on anorectal testing techniques. Gastroenterology 116: 735–760, 1999. [DOI] [PubMed] [Google Scholar]
- 8.Duthie HL, Watts JM. Contribution of the external anal sphincter to the pressure zone in the anal canal. Gut 6: 64–68, 1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farkas GA, Cerny FJ, Rochester DF. Contractility of the ventilatory pump muscles. Med Sci Sports Exerc 28: 1106–1114, 1996. [DOI] [PubMed] [Google Scholar]
- 10.Fok TF, al-Essa M, Monkman S, Dolovich M, Girard L, Coates G, Kirpalani H. Delivery of metered dose inhaler aerosols to paralyzed and nonparalyzed rabbits. Crit Care Med 25: 140–144, 1997. [DOI] [PubMed] [Google Scholar]
- 11.Gordon AM, Huxley AF, Julian FJ. Tension development in highly stretched vertebrate muscle fibres. J Physiol 184: 143–169, 1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gordon AM, Huxley AF, Julian FJ. The variation in isometric tension with sarcomere length in vertebrate muscle fibres. J Physiol 184: 170–192, 1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Granzier HL, Akster HA, Ter Keurs HE. Effect of thin filament length on the force-sarcomere length relation of skeletal muscle. Am J Physiol Cell Physiol 260: C1060–C1070, 1991. [DOI] [PubMed] [Google Scholar]
- 14.Higuchi H, Yanagida T, Goldman YE. Compliance of thin filaments in skinned fibers of rabbit skeletal muscle. Biophys J 69: 1000–1010, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holmes JW, Hunlich M, Hasenfuss G. Energetics of the Frank-Starling effect in rabbit myocardium: economy and efficiency depend on muscle length. Am J Physiol Heart Circ Physiol 283: H324–H330, 2002. [DOI] [PubMed] [Google Scholar]
- 16.Jonas-Obichere M, Scholefield JH, Acheson A, Mundey M, Tyler H, Wilson VG. Comparison of the effects of nitric oxide donors and calcium channel blockers on the intrinsic myogenic tone of sheep isolated internal anal sphincter. Br J Surg 92: 1263–1269, 2005. [DOI] [PubMed] [Google Scholar]
- 17.Krier J, Meyer RA, Percy WH. Length-tension relationship of striated muscle of cat external anal sphincter. Am J Physiol Gastrointest Liver Physiol 256: G773–G778, 1989. [DOI] [PubMed] [Google Scholar]
- 18.Lieber RL, Loren GJ, Friden J. In vivo measurement of human wrist extensor muscle sarcomere length changes. J Neurophysiol 71: 874–881, 1994. [DOI] [PubMed] [Google Scholar]
- 19.Lieber RL, Ponten E, Burkholder TJ, Friden J. Sarcomere length changes after flexor carpi ulnaris to extensor digitorum communis tendon transfer. J Hand Surg [Am] 21: 612–618, 1996. [DOI] [PubMed] [Google Scholar]
- 20.Lieber RL, Yeh Y, Baskin RJ. Sarcomere length determination using laser diffraction. Effect of beam and fiber diameter. Biophys J 45: 1007–1016, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Littlefield R, Fowler VM. Measurement of thin filament lengths by distributed deconvolution analysis of fluorescence images. Biophys J 82: 2548–2564, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu J, Guaderrama N, Nager CW, Pretorius DH, Master S, Mittal RK. Functional correlates of anal canal anatomy: puborectalis muscle and anal canal pressure. Am J Gastroenterol 101: 1092–1097, 2006. [DOI] [PubMed] [Google Scholar]
- 23.Malouf AJ, Norton CS, Engel AF, Nicholls RJ, Kamm MA. Long-term results of overlapping anterior anal-sphincter repair for obstetric trauma. Lancet 355: 260–265, 2000. [DOI] [PubMed] [Google Scholar]
- 24.Mittal RK, Liu J, Puckett JL, Bhalla V, Bhargava V, Tipnis N, Kassab G. Sensory and motor function of the esophagus: lessons from ultrasound imaging. Gastroenterology 128: 487–497, 2005. [DOI] [PubMed] [Google Scholar]
- 25.Rao SS Pathophysiology of adult fecal incontinence. Gastroenterology 126: S14–S22, 2004. [DOI] [PubMed] [Google Scholar]
- 26.Rassier DE, MacIntosh BR, Herzog W. Length dependence of active force production in skeletal muscle. J Appl Physiol 86: 1445–1457, 1999. [DOI] [PubMed] [Google Scholar]
- 27.Rattan S, Sarkar A, Chakder S. Nitric oxide pathway in rectoanal inhibitory reflex of opossum internal anal sphincter. Gastroenterology 103: 43–50, 1992. [DOI] [PubMed] [Google Scholar]
- 28.Saito T, Yamamoto I, Huang XL, Yukawa N, Osawa M, Takeichi S. Effects of muscle relaxants on EEG, ABR and EMG in rabbits. Hum Exp Toxicol 18: 718–723, 1999. [DOI] [PubMed] [Google Scholar]
- 29.Sultan AH, Kamm MA, Hudson CN, Thomas JM, Bartram CI. Anal-sphincter disruption during vaginal delivery. N Engl J Med 329: 1905–1911, 1993. [DOI] [PubMed] [Google Scholar]
- 30.Yeung EW, Allen DG. Stretch-activated channels in stretch-induced muscle damage: role in muscular dystrophy. Clin Exp Pharmacol Physiol 31: 551–556, 2004. [DOI] [PubMed] [Google Scholar]