Abstract
To test the hypothesis that colonic Na+ transport is altered in the 5/6 nephrectomized rat model of chronic renal failure (CRF), we measured Na+ fluxes across distal colon from control (CON), CRF, and CRF rats treated with the angiotensin II (ANG II) receptor antagonist losartan (+LOS). We also evaluated overall fluid and Na+ balance and compared colonic protein and mRNA expression profiles for electroneutral [sodium-hydrogen exchanger (NHE)] and electrogenic Na+ transport [epithelial sodium channel (ENaC)] in these groups. Consistent with a 60% enhancement in colonic Na+ absorption in CRF, urinary Na+ excretion increased by about 50% while serum Na+ homeostasis was maintained. These CRF-induced changes in Na+ handling were normalized by treatment with LOS. Net Na+ absorption was also stimulated in in vitro tissues from CON rats following acute serosal addition of ANG II (10−7 M), and this increase was blocked by AT1 antagonism but not by an AT2 antagonist. In CRF, colonic protein and mRNA expression variably increased for apical NHE2, NHE3, and ENaC α-, β-, γ-subunits, whereas expression of basolateral NHE1 and Na+-K+-ATPase (α-isoform) remained unaltered. Upregulation of the ENaC subunit mRNA was attenuated somewhat by LOS treatment. Previously, we showed that colonic AT1 receptor protein is upregulated twofold in CRF, and here we find that AT1 and AT2 mRNA and AT2 protein abundance is unchanged in CRF. We conclude that Na+ absorption in CRF rat distal colon is increased due to elevated expression of proteins mediating electroneutral and electrogenic uptake and that it is partially mediated by AT1 receptors.
Keywords: nephrectomy, angiotensin II, losartan, AT2 receptor, EIPA
the distal colon has a significant role in electrolyte balance, often in ways that complement those provided by renal pathways or in a manner that compensates for renal dysfunction. For example, in the rat model of chronic renal failure (CRF) induced by surgically removing 5/6 of renal mass, distal colonic K+ (12, 14, 15) and anion (11, 13) secretory pathways are stimulated, which partially offsets compromised renal excretion of these solutes. These CRF-induced reversals of net transport, from absorption to secretion, are sensitive to AT1 receptor antagonism in vivo and in vitro (11, 14–16), suggesting a role for the renin-angiotensin system in colonic adaptation to renal failure. Remarkably, these changes in epithelial transport appear to be mediated by local upregulation of colonic AT1 receptors rather than changes in systemic aldosterone or angiotensin II (ANG II) titers (12).
Whether distal colonic sodium transport is also affected in the 5/6 nephrectomy model of CRF has not been established, yet there are several compelling reasons to expect that this may be the case. First, exogenous ANG II alters sodium absorption in other intestinal segments (5, 25, 26) and in renal epithelia (29, 34, 35, 37) in a bimodal manner, with low concentrations of the octapeptide stimulating and higher doses inhibiting net Na+ absorption. Second, renal Na+ excretion is increased in CRF (23), and AT1 blockade alters Na+ transporter gene expression in CRF (20). Third, critical elements mediating Na+ transport in the distal colon, such as the epithelial sodium channel (ENaC) (2, 7) and sodium-hydrogen exchangers (NHEs) (7), are regulated by ANG II in renal epithelia. Finally, distal colonic Na+ transport is regulated by aldosterone, which alters the relative abundance of sodium exchanger/sodium channel protein and mRNA (10, 18).
On the basis of these observations, we speculated that distal colon Na+ transport in the CRF rat model is also regulated by ANG II, like K+ and anion transport. To test this hypothesis, we compared Na+ transport and mRNA expression profiles of AT1 and AT2 receptors and of some critical genes encoding transporters that mediate Na+ absorption in normal and CRF rat distal colon. We report here that Na+ absorption by the rat distal colon is increased in this model of CRF and that these changes correlate with elevated expression of mRNA transcripts encoding proteins mediating electroneutral (NHE2, NHE3) and electrogenic (ENaC) Na+ uptake across the apical membrane. Additionally, AT1 and AT2 receptor mRNA expression were not altered in CRF, and AT2 receptor protein was not changed in CRF colon [unlike AT1 receptor protein which increases twofold (12)]. The increases in ENaC mRNA expression, but not NHE message, are partly mitigated by AT1 receptor antagonism, further suggesting a role for ANG II mediating a stimulation of colonic Na+ absorption in CRF.
MATERIALS AND METHODS
Animals.
All animal experimentation was conducted in accordance with protocols approved by the University of Florida and in accordance with the NIH Guide for the Care and Use of Laboratory Animals. Male Sprague-Dawley rats (275–300 g; Harlan, Indianapolis, IN) were used in the following studies and had free access to water and Purina rat chow 5001 at all times. A 5/6 nephrectomy was performed to produce a surgical resection CRF model as described previously but in one surgical step rather than two (14), and flux studies were performed 6 wk after this procedure. In one experimental series, a group of CRF rats was treated with losartan (10 mg/kg), an ANG II receptor antagonist, added to the drinking water for 7 days from the end of week 5 to week 6 (+LOS). Control series of experiments were conducted using intestinal tissue from normal control rats (CON) that did not undergo any treatment. All rats were euthanatized by an intraperitoneal injection of pentobarbital (150 mg/kg body wt), and blood was collected by cardiac puncture for serum Na+ (Roche Hitachi Modular P800 chemistry analyzer; Roche Diagnostics, Indianapolis, IN) and creatinine determination (kit 555A; Sigma Chemical, St. Louis, MO). Sodium and creatinine were also determined in a urine specimen collected in 3 ml of 3.5 N HCl over 24 h immediately before euthanasia. Creatinine clearance, which was used as an indicator of renal function, was determined for each rat with the serum and urine creatinine measurements. Systolic blood pressure was monitored in all animals before and after losartan administration with the use of a tail sphygmomanometer (Harvard Apparatus, South Natick, MA).
Transport studies.
Immediately following euthanasia and exsanguination of the rats, the entire distal colon was removed, cleansed in ice-cold isotonic saline, and partially stripped of the serosal muscularis using blunt dissection. Flat sheets of tissue were mounted in modified Ussing chambers with an exposed tissue area of 0.64 cm2. Transepithelial fluxes of 22Na+ (Perkin Elmer Life Sciences, Boston, MA) were measured across colonic tissues bathed on both sides by 10 ml of buffered saline (pH 7.4) at 37°C and circulated by bubbling with 95% O2-5% CO2. The standard saline contained the following solutes (in mmol/l): 139.4 Na+, 5.4 K+, 1.2 Mg2+, 123.2 Cl−, 21.0 HCO3−, 1.2 Ca2+, 0.6 H2PO4−, 2.4 HPO2−, and 10 glucose. The magnitude and direction of the net flux (J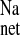 ) was calculated as the difference between the two unidirectional fluxes (mucosal to serosal, J
) was calculated as the difference between the two unidirectional fluxes (mucosal to serosal, J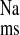 and serosal to mucosal, J
and serosal to mucosal, J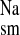 ) measured at 15-min intervals for a control period of 45 min (Per I), under short-circuit conditions. In some series, Per I was followed by a second 45-min flux period (Per II) to determine the acute effects of Na+ transport inhibitors and losartan or ANG II added in vitro. Untreated tissue preparations showed that there were no significant time-dependent alterations in Na+ transport or in the electrical parameters over the duration of the two-flux-period design (not shown). The concentration of losartan (10−4 M) used here is known to have a maximum inhibitory effect on both K+ and Cl− secretion across this tissue preparation in vitro (15). Similarly, we used relatively high concentrations of exogenous ANG II due to rapid tissue hydrolysis of the octapeptide, which results in an effective tissue concentration that is two orders of magnitude less that that of the bathing solution (14, 15). The unidirectional tracer fluxes were determined on conductance-matched tissues (GT ≤ 20%). The electrical parameters of the tissue were also recorded at 15-min intervals throughout the entire experiment. Tissue conductance (GT, mS/cm2) was calculated as the ratio of the open-circuit potential (mV) to the short-circuit current (Isc, μA/cm2).
) measured at 15-min intervals for a control period of 45 min (Per I), under short-circuit conditions. In some series, Per I was followed by a second 45-min flux period (Per II) to determine the acute effects of Na+ transport inhibitors and losartan or ANG II added in vitro. Untreated tissue preparations showed that there were no significant time-dependent alterations in Na+ transport or in the electrical parameters over the duration of the two-flux-period design (not shown). The concentration of losartan (10−4 M) used here is known to have a maximum inhibitory effect on both K+ and Cl− secretion across this tissue preparation in vitro (15). Similarly, we used relatively high concentrations of exogenous ANG II due to rapid tissue hydrolysis of the octapeptide, which results in an effective tissue concentration that is two orders of magnitude less that that of the bathing solution (14, 15). The unidirectional tracer fluxes were determined on conductance-matched tissues (GT ≤ 20%). The electrical parameters of the tissue were also recorded at 15-min intervals throughout the entire experiment. Tissue conductance (GT, mS/cm2) was calculated as the ratio of the open-circuit potential (mV) to the short-circuit current (Isc, μA/cm2).
RNA isolation and real-time PCR.
Immediately following euthanasia, a 50–100-mg segment of distal colon was preserved from each rat in RNAlater (Ambion, Austin, TX), and total RNA was isolated using TRIzol (Invitrogen, Carlsbad, CA) according to the recommendations of the manufacturer. The TURBO DNA-free kit (Ambion) was used to remove residual genomic DNA contamination from the total RNA samples. Gene-specific oligonucleotide primers were designed from rat reference sequences available in GenBank using Primer 3 (31) and are shown in Table 1. Real-time PCR was performed using the QuantiTect SYBR Green RT-PCR kit (Qiagen, Valencia, CA) with the DNA Engine Opticon continuous fluorescence detection system (MJ Research, San Francisco, CA). Briefly, 100 ng of total RNA was added to 25 μl of 2× QuantiTect SYBR Green RT-PCR Master mix, 250 ng of each gene-specific primer and 0.5 μl of QuantiTect RT mix. The reactions were adjusted to 50 μl total volume with RNase-free water and incubated at 50°C for 30 min. Reverse transcriptase was deactivated, and the HotStarTaq DNA polymerase was activated by incubation at 95°C for 15 min. Reactions were then subjected to 45 cycles of denaturation at 94°C for 15 s, annealing at 55°C for 30 s, and extension at 72°C for 30 s. Fluorescence data were collected after each extension step. Following each PCR run, a melting curve analysis was performed with built-in software of the DNA Engine Opticon System to verify the specificity of the RT-PCR product. GAPDH was used as an internal reference to normalize for the amount of RNA added to the reverse transcription reaction. Standard curves for GAPDH and each target gene shown in Table 1 were generated from serial dilutions of a distal colon total RNA pool. Regression analysis was performed on each data set, and the resultant slopes were compared between each target gene and GAPDH to ensure amplification efficiencies were similar. Average threshold cycle (Ct) number for GAPDH was also compared among all experimental groups to demonstrate stability of expression and consequently validate its use (4, 6, 36) as a reference gene (16.0 ± 0.15, 16.2 ± 0.14, and 16.1 ± 0.02 for the CON, CRF, and CRF + LOS groups, respectively; P = 0.41). Gene expression data are expressed as fold changes relative to controls using the 2−ΔΔCt as previously described (27).
Table 1.
Oligonucleotides designed from genes of interest and utilized for real-time PCR
| Protein | Gene ID | Reference Sequence | mRNA Coordinates | Primer | 5′-oligonucleotide sequence-3′ | Amplicon Size, bp |
|---|---|---|---|---|---|---|
| NHE1 | Slc9a1 | NM_012652 | 1992–2011 | Forward | CACCAGTGGAACTGGACCTT | 211 |
| 2202–2183 | Reverse | ACATGGGGAAGTGCTTCTTG | ||||
| NHE2 | Slc9a2 | NM_012653 | 2173–2192 | Forward | CGCAGGGCTTCTACTTCAAC | 235 |
| 2407–2388 | Reverse | CGTCCACCTCATTCTTCCAT | ||||
| NHE3 | Slc9a3 | NM_012654 | 1885–1905 | Forward | GGAACAGAGGCGGAGGAGCAT | 322 |
| 2206–2187 | Reverse | GAAGTTGTGTGCCAGATTCT | ||||
| ENaC-α | Scnn1a | NM_031548 | 1443–1462 | Forward | TACCCTAAGCCCAAGGGAGT | 226 |
| 1668–1649 | Reverse | TGTTCTGCAAGGACAGCATC | ||||
| ENaC-β | Scnn1b | NM_012648 | 1505–1524 | Forward | ACCCTGAGCAGGAAGGGTAT | 222 |
| 1726–1707 | Reverse | ACAGGAGGCCACTAGCTTGA | ||||
| ENaC-γ | Scnn1 g | NM_017046 | 680–699 | Forward | GCACGTTCATGAGTCGAAGA | 206 |
| 885–866 | Reverse | AGAAGCAGGTCACCAGCAGT | ||||
| Na+/K+-ATPase | Atp1a1 | NM_012504 | 3207–3231 | Forward | TGCCTTCCCCTACTCCCTTCTCATC | 323 |
| (α 1 subunit) | 3529–3505 | Reverse | CTTCCCCGCTGTCGTCCCCGTCCAC | |||
| AT1 | Agtr1a | NM_030985 | 392–411 | Forward | GGAAACAGCTTGGTGGTGAT | 241 |
| 632–614 | Reverse | CGATGCTGAGACACGTGAG | ||||
| AT2 | Agtr2 | NM_012494 | 252–272 | Forward | CAATCTGGCTGTGGCTGACTT | 101 |
| 352–331 | Reverse | TGCACATCACAGGTCCAAAGAG | ||||
| GAPDH | Gapd | NM_017008 | 1270–1289 | Forward | TCCCTCAAGATTGTCAGCAA | 308 |
| 1577–1558 | Reverse | AGATCCACAACGGATACATT |
NHE, sodium-hydrogen exchanger; ENaC, epithelial sodium channel.
Immunoblotting.
Immunodetection of the NHEs, the ENaC subunits, Na+-K+-ATPase, and the AT2 receptor protein on Western blots was performed using commercial primary and secondary antibodies [NHE1, NHE3, AT2 from Santa Cruz Biotechnology (Santa Cruz, CA), ENaC-γ from Alpha-Diagnostics (San Antonio, TX), and NHE2, ENaC-α, ENaC-β, and Na+-K+-ATPase-α isoform from Millipore (Billerica, MA)]. Briefly, protein samples (100 or 250 μg) were loaded onto a 5% stacking/10% SDS polyacrylamide gel and separated by electrophoresis at 200 V. The proteins were electrophoretically transferred to a nitrocellulose membrane at 60 V for 3 h at 4°C. After a 45-min blocking incubation, the blots were incubated overnight in the same blocking solution containing the primary antibody followed by a 30-min incubation with the secondary antibody. After each incubation, the blots were extensively washed with Tris buffer containing 0.1% Tween-20. For detection, the membranes were reacted for 1 min with chemiluminescence reagent (Amersham, Piscataway, NJ) and exposed to autoradiographic hyperfilm-ECL (Amersham). The intensity of the resulting band in each case was quantified using Image J (NIH). Membranes were stripped by incubating in Restore Western Stripping Buffer (Pierce, Rockford, IL) for 20 min at 37°C and reprobed as described above with a monoclonal antibody to GAPDH (Ambion) for 1 h.
Reagents.
Losartan was a generous gift from Merck (Rahway, NJ), and PD-123319 was a product of Research Biochemicals International (Natick, MA). [Asn1, Val5]-ANG II was purchased from Peninsula Laboratories (Belmont, CA), and all other reagents, including EIPA and (N-Amidino-3, 5-diamino-6-chloropyrazinecarboxyamide) amiloride were purchased from Sigma.
Statistical analyses.
For the flux studies, a paired or unpaired t-test was used for the comparison of two means, whereas multiple means were compared by a one-way ANOVA followed by Bonferroni's t-test. Results are presented as the mean ± 1 SE for the number of matched tissue pairs employed. For all analyses, differences were considered significant if P ≤ 0.05. For real-time PCR data, one-way ANOVA was performed on the rank-transformed ΔCt values with the General Linear Models procedures of SAS. Significant treatment effects were separated by Student-Newman-Keuls sequential range test and orthogonal contrasts (32).
RESULTS
Sodium and water balance in the CRF rat.
A comparison of sodium and water balance in CRF and in + LOS rats with CON revealed that, whereas serum Na+ homeostasis was maintained in both CRF and + LOS groups, urinary Na+ excretion was significantly higher only in CRF rats, despite comparable dietary Na+ intake among the three groups (Table 2). Both fluid intake and urinary output were significantly elevated in all CRF rats compared with CON and were unaffected by losartan administration. Although losartan treatment ameliorated the increases in serum creatinine by ∼30%, renal function (as judged by creatinine clearance) was significantly reduced in both losartan-treated and untreated CRF rats as shown previously (11, 14). Blood pressure, which was also increased in CRF, was significantly reduced by losartan treatment, again as expected (11).
Table 2.
Comparison of overall fluid and sodium balance, blood pressure, and renal function in CON, CRF, and + LOS groups
| Group | CON | CRF | + LOS |
|---|---|---|---|
| SNa+, mmol/l | 146.5±1.8 | 147.9±1.7 | 145.2±1.7 |
| UNa+, μmol/24 h | 1237±109 | 1929±285* | 1289±98 |
| SCr, μmol/l | 43.3±4.3 | 125.0±7.3* | 84.2±6.1*† |
| Cr Clear, ml/min | 2.50±0.28 | 0.94±0.08* | 1.38±0.18* |
| UV, ml/24 h | 13.5±1.1 | 50.2±3.8* | 44.8±4.2* |
| H2O intake, ml/24 h | 36.7±1.8 | 79.5±9.2* | 71.5±6.9* |
| Food intake, g/24 h | 21.3±0.5 | 22.9±0.9 | 21.4±1.0 |
| Δ Body wt, g | 75.7±10.3 | 50.2±8.0 | 52.7±11.9 |
| BP, mmHg | 121.7±9.5 | 195.0±10.5* | 150.0±3.4† |
Values are means ± SE. CON, control; CRF, chronic renal failure; +LOS, losartan; SNa+, serum sodium; UNa+, urinary sodium excretion; SCr, serum creatinine; Cr Clear, creatinine clearance; UV, urinary volume excretion; Δ Body Wt, the gain in body weight over the 6-week period following 5/6 nephrectomy; BP, mean arterial systolic blood pressure.
Significant difference from control, P ≤ 0.05;
significant difference from CRF, P ≤ 0.05.
Enhanced sodium absorption in CRF rats.
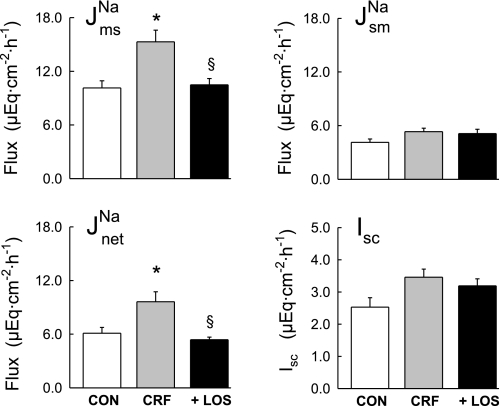
Fluxes of 22Na+ were measured across short-circuited colonic segments removed from CON, CRF and + LOS. As shown in Fig. 1, there was a significant increase in net Na+ absorption (J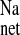 ) in the CRF rats (gray bars in Fig. 1) compared with controls, which occurred by virtue of a significant increase in J
) in the CRF rats (gray bars in Fig. 1) compared with controls, which occurred by virtue of a significant increase in J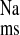 . In the CRF colon there was also an increase in Isc (Fig. 1), but this did not reach statistical significance (P = 0.064).
. In the CRF colon there was also an increase in Isc (Fig. 1), but this did not reach statistical significance (P = 0.064).
Fig. 1.
Comparison of Na+ transport and short-circuit current (Isc) across isolated, short-circuited distal colon from control rats with 2 intact kidneys (CON, n = 7), 5/6 nephrectomized rats with chronic renal failure (CRF, n = 11), and rats with renal failure that were treated with losartan (10 mg/kg) for 7 days before euthanasia (+LOS, n = 10). Error bars represent ±1 SE of the mean of data in each group. An asterisk indicates a significant difference compared with CON, and § indicates a significant difference from CRF in a one-way ANOVA (P ≤ 0.05). Transepithelial conductance (GT) was not significantly affected in CRF (9.00 ± 0.62 mS/cm2) or + LOS (8.23 ± 0.66 mS/cm2) compared with CON (8.79 ± 0.83 mS/cm2). The fluxes and electrical parameters measured in + LOS are not significantly different compared with CON. J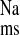 , mucosal to serosal flux; J
, mucosal to serosal flux; J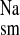 , serosal to mucosal flux; J
, serosal to mucosal flux; J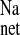 , net flux.
, net flux.
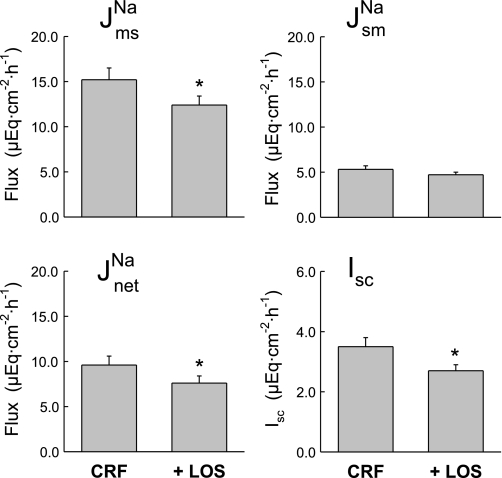
The CRF-induced increase in distal colon sodium transport was significantly reduced by treating the animals with the AT1 receptor antagonist, losartan. As shown by the solid bars in Fig. 1, J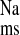 and net sodium fluxes in the + LOS rats were significantly smaller than those of the CRF rats, but not significantly different from the CON rats. These effects of chronic AT1 antagonism on sodium transport in vivo were similar to the acute effects of adding losartan to the distal colon in vitro. Thus when 10−4 M losartan (serosal) was added to tissues from CRF rats during Per II of the flux measurements, J
and net sodium fluxes in the + LOS rats were significantly smaller than those of the CRF rats, but not significantly different from the CON rats. These effects of chronic AT1 antagonism on sodium transport in vivo were similar to the acute effects of adding losartan to the distal colon in vitro. Thus when 10−4 M losartan (serosal) was added to tissues from CRF rats during Per II of the flux measurements, J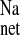 was significantly reduced by a reduction in J
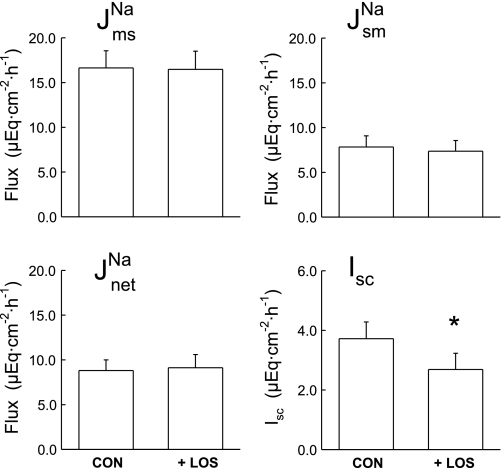
was significantly reduced by a reduction in J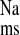 as shown in Fig. 2. Acute losartan addition to these CRF tissues also significantly reduced Isc, and GT was unaffected. In contrast, serosal addition of the AT2 receptor antagonist, PD123319 (10−4 M), had no effect on the unidirectional or net fluxes of Na+ across CRF distal colon (data not shown). It is also notable that acute losartan addition to tissues removed from control rats had no effects on Na+ fluxes, but a losartan-induced decrease in Isc was evident as shown in Fig. 3.
as shown in Fig. 2. Acute losartan addition to these CRF tissues also significantly reduced Isc, and GT was unaffected. In contrast, serosal addition of the AT2 receptor antagonist, PD123319 (10−4 M), had no effect on the unidirectional or net fluxes of Na+ across CRF distal colon (data not shown). It is also notable that acute losartan addition to tissues removed from control rats had no effects on Na+ fluxes, but a losartan-induced decrease in Isc was evident as shown in Fig. 3.
Fig. 2.
Acute serosal losartan (10−4 M) reduces net sodium absorption and Isc across distal colonic tissues removed from CRF rats (n = 11). Error bars represent ±1 SE above or below the mean of data in each group. An asterisk indicates a significant difference between the control period of 45 min (Per I) (CRF) and the second 45-min flux period (Per II) (+LOS), using a paired t-test, P ≤ 0.05. Transepithelial conductance (GT) was not significantly changed in Per II (8.23 ± 0.66 mS/cm2) compared with Per I (9.00 ± 0.62 mS/cm2).
Fig. 3.
Acute serosal losartan (10−4 M) does not alter sodium fluxes across distal colonic tissues removed from CON rats (n = 9), but Isc is significantly decreased. Error bars represent ±1 SE above or below the mean of data in each group. An asterisk indicates a significant difference between Per I (CON) and Per II (+LOS), using a paired t-test, P ≤ 0.05. Transepithelial conductance (GT) was not significantly changed in Per II (9.94 ± 1.05 mS/cm2) compared with Per I (10.37 ± 1.08 mS/cm2).
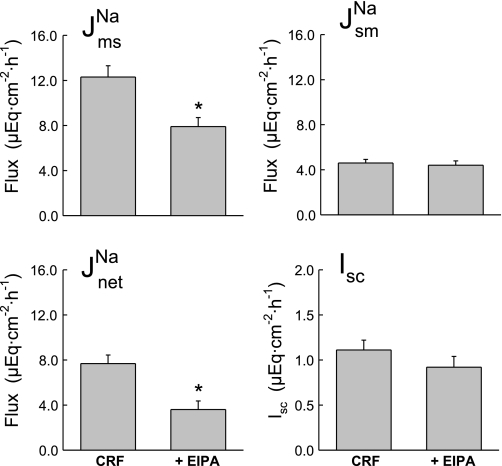
To generally assess the relative contribution of electroneutral NHE mediated by apical NHE isoforms NHE2 and NHE3 to J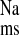 , we also examined the effect of mucosal EIPA in the CRF distal colon. As shown in Fig. 4, addition of 50 μM EIPA to the mucosal side of CRF distal colon produced significant decreases in J
, we also examined the effect of mucosal EIPA in the CRF distal colon. As shown in Fig. 4, addition of 50 μM EIPA to the mucosal side of CRF distal colon produced significant decreases in J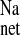 and J
and J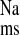 without significantly affecting J
without significantly affecting J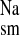 or Isc. The decrease in J
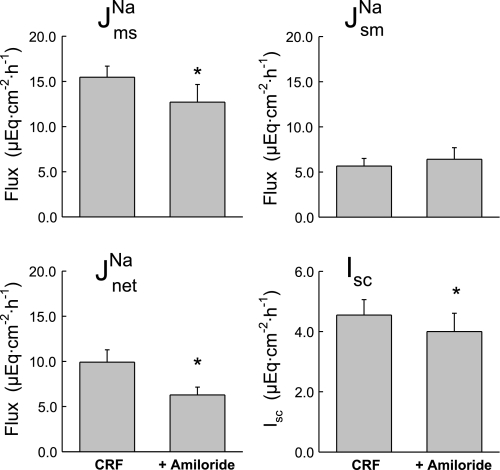
or Isc. The decrease in J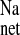 of more than 1 μEq·cm−2·h−1 in the absence of a similar reduction in Isc suggests that sodium absorption in the CRF rat distal colon is mediated by both electroneutral EIPA-sensitive (NHE) and EIPA-insensitive (ENaC) components because 50 μM of this amiloride analog should effectively inhibit most apical NHE (21, 30). When the ENaC blocker amiloride was added to these CRF tissues in a similar series of experiments shown in Fig. 5, we also observed a reduction in J
of more than 1 μEq·cm−2·h−1 in the absence of a similar reduction in Isc suggests that sodium absorption in the CRF rat distal colon is mediated by both electroneutral EIPA-sensitive (NHE) and EIPA-insensitive (ENaC) components because 50 μM of this amiloride analog should effectively inhibit most apical NHE (21, 30). When the ENaC blocker amiloride was added to these CRF tissues in a similar series of experiments shown in Fig. 5, we also observed a reduction in J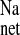 and J
and J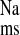 in addition to a significant reduction in Isc. We conclude from the transport and inhibition studies that net sodium absorption is enhanced in the distal colon of CRF rats by increases in J
in addition to a significant reduction in Isc. We conclude from the transport and inhibition studies that net sodium absorption is enhanced in the distal colon of CRF rats by increases in J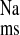 (both EIPA-sensitive and -insensitive) that are, in part, dependent on AT1 mediated pathways.
(both EIPA-sensitive and -insensitive) that are, in part, dependent on AT1 mediated pathways.
Fig. 4.
Mucosal EIPA (50 μM) partially reduces net sodium absorption in the distal colon of CRF rats (n = 7). Indomethacin was present in this series (5 μM, serosal) to better resolve any changes in Isc that might result from EIPA inhibition of electrogenic Na+ absorption. Error bars represent ±1 SE above or below the mean of data in each group. An asterisk indicates a significant difference between Per I (CRF) and Per II (+EIPA), using a paired t-test, P ≤ 0.05. Transepithelial conductance (GT) was not significantly changed in Per II (7.82 ± 0.90 mS/cm2) compared with Per I (8.62 ± 0.77 mS/cm2).
Fig. 5.
Mucosal amiloride (10−4 M) partially reduces net sodium absorption and Isc in the distal colon of CRF rats (n = 7). Error bars represent ±1 SE above or below the mean of data in each group. An asterisk indicates a significant difference between Per I (CRF) and Per II (+Amiloride), using a paired t-test, P ≤ 0.05. Transepithelial conductance (GT) was not significantly changed in Per II (9.58 ± 1.09 mS/cm2) compared with Per I (10.13 ± 1.13 mS/cm2).
Effects of ANG II on colonic sodium absorption in vitro.
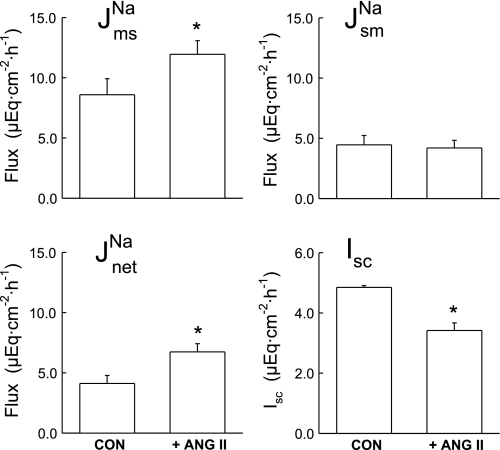
Since the foregoing results suggested that ANG II may be involved in mediating the CRF-enhanced Na+ absorption, we next examined the effects of acute ANG II addition to colonic tissues removed from control rats. It is notable here that we added ANG II at 10−7 M to the in vitro tissue preparation because significant hydrolysis of ANG II occurs at 37°C in colonic tissues (12, 14). We previously demonstrated that the difference in concentration of ANG II between the bathing solution and the extracellular tissue space is approximately two orders of magnitude; thus we reasoned that the effective concentration of ANG II at the site of the receptor is likely to be in the order of 10−9 M. As shown in Fig. 6, ANG II (10−7 M, serosal) stimulated net Na+ absorption ∼60% via an increase in J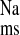 , and Isc was significantly reduced by about 40% (from 5.48 ± 0.49 to 3.42 ± 0.25 μEq·cm−2·h−1), whereas GT remained unchanged. The ANG II-induced reduction in Isc is likely due to an increase in electrogenic K+ secretion or a reduction in electrogenic Cl− secretion as we have observed in other studies (14, 15).
, and Isc was significantly reduced by about 40% (from 5.48 ± 0.49 to 3.42 ± 0.25 μEq·cm−2·h−1), whereas GT remained unchanged. The ANG II-induced reduction in Isc is likely due to an increase in electrogenic K+ secretion or a reduction in electrogenic Cl− secretion as we have observed in other studies (14, 15).
Fig. 6.
Angiotensin II (ANG II) stimulation of sodium transport by rat distal colon in vitro. ANG II (10−7 M) was added at the end of Per I (CON) to the serosal side of control rat colonic tissue (n = 7). Error bars represent ±1 SE above or below the mean of data in each group. An asterisk indicates a significant difference between Per I and II using a paired t-test, P ≤ 0.05. Transepithelial conductance (GT) was not significantly affected in Per II (8.00 ± 0.57 mS/cm2) compared with Per I (8.57 ± 0.48 mS/cm2).
ANG II receptor and sodium transporter expression in CRF.
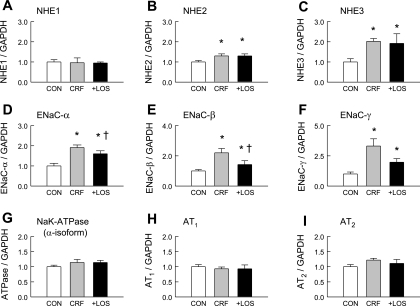
Previously, we detected no differences in plasma ANG II, aldosterone, or K+ concentrations, or in tissue content of ANG II between normal and CRF rats (14). However, we did find a twofold increase in the number of specific ANG II binding sites in CRF colonic mucosa (14) as well as a twofold increase in AT1 receptor protein expression compared with normal controls (12). As shown in Fig. 7, however, CRF rats and CRF + LOS have comparable levels of AT1 mRNA expression. It is also notable that the expression of AT2 mRNA (Fig. 7) and protein (see Fig. 8) was the same in CRF and CRF + LOS rats treated with losartan.
Fig. 7.
mRNA expression of AT1, AT2, and selected sodium transporters in the distal colon of control rats (CON), rats in CRF, and CRF rats treated with losartan (10 mg/kg) for 1 wk before study (+LOS). *Significant difference compared with control, P ≤ 0.05. †Significant difference compared with CRF, P ≤ 0.05. NHE, sodium-hydrogen exchanger; ENaC, epithelial sodium channel.
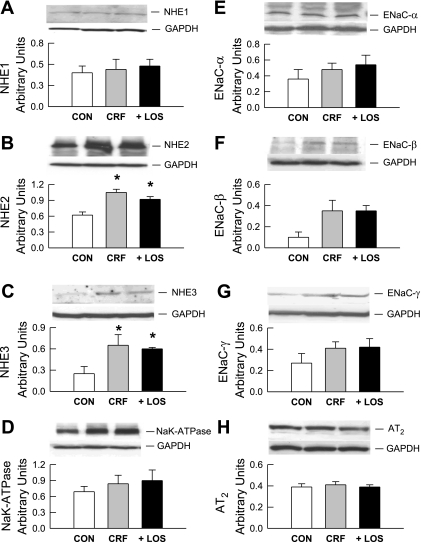
Fig. 8.
Immunoblot analyses of Na+/H+ exchangers (NHE1, NHE2, NHE3), Na+ channel subunits (ENaC-α, ENaC-β, ENaC-γ), ATPase, and AT2 receptor in protein extracts of distal colon from CON, CRF, and + LOS rats. Membranes were reprobed for GAPDH as an internal loading control. Results were quantitated by densitometry from n = 4 to 6 tissues in each group and given in arbitrary units. Significant differences in CRF and + LOS from CON rats were established by a one-way ANOVA followed by Bonferroni's t-test and are indicated by an asterisk.
We next asked whether the CRF-induced increases in sodium absorption are correlated with altered expression of genes encoding proteins mediating transepithelial Na+ transport in the rat distal colon. Accordingly, we measured the relative mRNA abundance of NHE1, NHE2, NHE3, the three subunits of ENaC (α, β, γ), and the α-isoform of Na+-K+ ATPase (Atp1a1) in CRF, + LOS, and normal control distal colon. These results, also presented in Fig. 7, show that there are significant increases in the mRNA expression of NHE2 and NHE3 and in all of the ENaC subunits. There were no alterations in NHE1 or in Na+-K+-ATPase mRNA associated with CRF. Interestingly, in CRF + LOS, mRNA expression of the three ENaC subunits was attenuated although this did not reach significance for the γ-subunit (P = 0.08). In contrast, the CRF-induced increases in NHE2, and NHE3 mRNA expression persisted during losartan treatment of CRF rats, whereas NHE1 remained unchanged.
Western blot analyses of the NHEs, the ENaC subunits, and Na+-K+-ATPase show that protein expression patterns largely tracked mRNA expression patterns in each case (Fig. 8). Thus although we found no changes in NHE1 or Na+-K+-ATPase protein expression among the three rat groups, protein expression of both NHE2 and NHE3, as well as the three ENaC subunits, were increased in CRF, albeit to varying degrees (Fig. 8). However, in CRF, differences in ENaC protein expression due to losartan treatment were more difficult to resolve by the qualitative nature of immunoblot analyses compared with mRNA expression of these subunits. We conclude from these findings that the increased sodium absorption in the distal colon of CRF rats is likely due to increases in both electroneutral (NHE2 and NHE3) and electrogenic (ENaC) sodium transport across the apical membrane, with the latter avenues being largely sensitive to AT1 antagonism. In addition, the increase in urinary Na+ excretion in CRF is likely a consequence of this enhanced colonic absorption.
DISCUSSION
The 5/6 nephrectomy model of CRF in rats results in numerous changes in the morphology and function of the remnant kidney including hypertrophy, decreased creatinine clearance, increased urine output subsequent to dysfunction of the urinary concentrating mechanism, proteinuria, and altered electrolyte handling (11, 23). Regarding the latter, urinary Na+ excretion is increased in CRF rats, and this has been proposed to result from impaired proximal tubular Na+ handling following decreased expression of Na+ transporters in this nephron segment (23). It is also notable that several of the transport alterations concomitant with CRF in the nephron exhibit a dependence on ANG II type 1 receptors (23).
Renal failure in the CRF rat model also leads to changes in intestinal electrolyte transport that may mitigate impaired renal function. For example, we have previously shown that K+ and a number of anions, including Cl−, which are normally absorbed by the rat distal colon, are secreted by colonic epithelia of CRF rats, and these reversals in the direction of net transport are mediated by an upregulation of AT1 receptor protein (11, 13, 14, 16, 17). In the present study we show that Na+ absorption is enhanced in the distal colon of CRF rats resulting in a significant increase in urinary Na+ excretion, and these changes correlate with increases in the expression of apical membrane sodium uptake pathways (NHE2, NHE3, and ENaC subunits) in distal colonic tissues. We further observed that the CRF-induced increases in colonic Na+ absorption and urinary Na+ excretion were blocked by losartan (a specific AT1 receptor antagonist), suggesting that the AT1 receptor plays a regulatory role in this response. Losartan treatment was also associated with a small improvement in renal function and a significant lowering of blood pressure in CRF as we have previously reported (11). In contrast, the twofold increase in fluid intake and the more than threefold increase in urinary volume excretion in CRF were unaltered by treatment with the AT1 receptor antagonist.
Enhanced colonic Na+ absorption in CRF.
Under normal conditions, sodium absorption by rat distal colon occurs by uptake across the apical membrane via electroneutral Na+-H+ exchange (18, 22, 38) followed by extrusion across the basolateral membrane mediated by Na+-K+-activated ATPase (22, 38). However, in certain situations, such as hyperaldosteronism induced by dietary Na+ depletion, electrogenic Na+ absorption is induced, which is mediated by the apical epithelial Na+ channel (ENaC), whereas the electroneutral absorption mechanisms appear to be suppressed (10, 19, 22). These changes in transport have been shown to be mediated (in part) by changes in transporter mRNA and protein abundance; apical NHEs are downregulated and apical ENaC is elevated in dietary sodium restriction (18).
In the CRF rat model induced by 5/6 nephrectomy, the increase in net sodium absorption across the distal colon was chiefly due to an increase in the mucosal to serosal unidirectional flux of Na+. This increase in J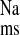 could result from increases in the influx of Na+ across the apical membrane, enhanced extrusion across the basolateral membrane, or some combination of thereof. Given that mRNA encoding basolateral Na+-K+ ATPase and NHE1 and expression of these transport proteins did not appear to be altered in CRF, whereas mRNA transcripts and protein expression of apically situated NHE2, NHE3, and all ENaC subunits were upregulated, we suggest that changes in apical Na+ uptake mediated by electroneutral, Na+-H+ exchange (NHE3 and NHE2) and electrogenic (ENaC) avenues may be largely responsible for the increase in colonic Na+ absorption in CRF. In accordance with this interpretation, we observed that 50 μM mucosal EIPA produced a significant reduction in J
could result from increases in the influx of Na+ across the apical membrane, enhanced extrusion across the basolateral membrane, or some combination of thereof. Given that mRNA encoding basolateral Na+-K+ ATPase and NHE1 and expression of these transport proteins did not appear to be altered in CRF, whereas mRNA transcripts and protein expression of apically situated NHE2, NHE3, and all ENaC subunits were upregulated, we suggest that changes in apical Na+ uptake mediated by electroneutral, Na+-H+ exchange (NHE3 and NHE2) and electrogenic (ENaC) avenues may be largely responsible for the increase in colonic Na+ absorption in CRF. In accordance with this interpretation, we observed that 50 μM mucosal EIPA produced a significant reduction in J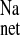 and J
and J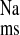 without significantly reducing Isc. Since this amiloride analog is reported to inhibit all isoforms of NHE-mediated Na+ uptake in normal rat distal colon with a Ki (inhibition kinetic constant) of less than 1 μM (21, 30) we suggest that the remaining (EIPA-insensitive) component of J
without significantly reducing Isc. Since this amiloride analog is reported to inhibit all isoforms of NHE-mediated Na+ uptake in normal rat distal colon with a Ki (inhibition kinetic constant) of less than 1 μM (21, 30) we suggest that the remaining (EIPA-insensitive) component of J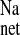 and J
and J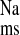 in the CRF distal colon is mediated by ENaC, and, consistent with this, we also observed reductions in J
in the CRF distal colon is mediated by ENaC, and, consistent with this, we also observed reductions in J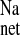 , J
, J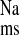 , and in Isc with mucosal amiloride addition. Although it has been clearly shown that all three ENaC subunits are required for full channel activity (1), and, interestingly, in rat distal colon aldosterone stimulates electrogenic Na+ transport by increased expression of the β- and γ-subunits but not the α-subunit (10), our data suggest that enhanced expression of all three subunits produces increased ENaC activity in CRF distal colon.
, and in Isc with mucosal amiloride addition. Although it has been clearly shown that all three ENaC subunits are required for full channel activity (1), and, interestingly, in rat distal colon aldosterone stimulates electrogenic Na+ transport by increased expression of the β- and γ-subunits but not the α-subunit (10), our data suggest that enhanced expression of all three subunits produces increased ENaC activity in CRF distal colon.
This pattern of simultaneous upregulation of ENaC and NHE in CRF is notably different from that produced by Na+ depletion (aldosterone mediated) in the rat distal colon, which, as noted above, results in reduced apical NHE expression and enhanced apical ENaC expression (18). Although we have interpreted the upregulation of NHE and ENaC expression in CRF distal colon in terms of ANG II type 1 receptor mechanisms as observed in renal epithelia (7, 24), it is also possible that other regulatory factors/pathways also contribute to enhanced colonic Na+ absorption in CRF. One strong possibility is that the expression of apical NHEs is modulated by a metabolic acidosis in the CRF rats that is a common complication of progressive renal failure (30). In the distal colon of rats with metabolic acidosis induced by NH4Cl ingestion, both NHE2 and NHE3 mRNA expression and function were found to be increased in the absence of changes in basolateral NHE1 expression (28). We observed similar changes in CRF rat distal colon in the present study, and it is certainly possible that a concomitant metabolic acidosis in CRF initiated the increases in NHE2 and NHE3 gene and protein expression. In this regard, we have also noted that this CRF model exhibits some signs of a nascent acidosis including an increase in anion gap, titratable acid, and phosphate excretion, with a lower urinary pH (9). Perhaps, the absence of losartan attenuation of the NHEs is a further indicator that a metabolic acidosis promotes these changes in NHE expression in the CRF distal colon, but further studies are required to make these distinctions.
Role of ANG II and ANG II receptors in CRF-enhanced Na+ absorption.
On the basis of the present findings, we suggest that ANG II and its receptors also play a significant role in the CRF-induced stimulation of net Na+ absorption across the rat distal colon. Functionally, chronic AT1 receptor antagonism with losartan blocked the increase in J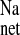 in CRF rat colon solely by a reduction in J
in CRF rat colon solely by a reduction in J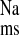 , clearly implicating ANG II involvement in this response. Acute AT1 antagonism in vitro (10−4 M losartan, serosal) also reduced net sodium absorption in CRF tissues via a reduction in J
, clearly implicating ANG II involvement in this response. Acute AT1 antagonism in vitro (10−4 M losartan, serosal) also reduced net sodium absorption in CRF tissues via a reduction in J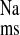 , whereas AT2 receptor antagonism (10−4 M PD123319, serosal) had no effect on sodium fluxes. Finally, the significance of AT1 receptors to CRF-stimulated Na+ absorption in the rat distal colon is further suggested by the effects of chronic AT1 antagonism on the mRNA expression profiles of genes encoding selected sodium transporters. Losartan treatment of CRF rats was associated with reductions in α-, β-, and γ- ENaC subunits compared with CRF alone without changing NHE1 or Na+-K+-ATPase mRNA or protein levels.
, whereas AT2 receptor antagonism (10−4 M PD123319, serosal) had no effect on sodium fluxes. Finally, the significance of AT1 receptors to CRF-stimulated Na+ absorption in the rat distal colon is further suggested by the effects of chronic AT1 antagonism on the mRNA expression profiles of genes encoding selected sodium transporters. Losartan treatment of CRF rats was associated with reductions in α-, β-, and γ- ENaC subunits compared with CRF alone without changing NHE1 or Na+-K+-ATPase mRNA or protein levels.
Additional evidence for the involvement of ANG II and specifically AT1 receptor-mediation of CRF-induced changes in colonic electrolyte transport is provided by previous studies from our laboratory using this CRF rat model (11, 12, 14–16). In summary, the CRF-induced changes in ion transport can be correlated with a twofold increase in specific 125I-ANG II binding (14) and a twofold upregulation in AT1 receptor protein in CRF colonic tissue compared with normal tissue (12). In the case of K+ transport, the changes in K+ fluxes were shown to be temporally correlated with the increase in AT1 receptor protein both of which occurred within 48 h after the surgical procedure and were sustained for at least a six-week period (14). No changes in plasma concentrations of ANG II or aldosterone were observed in CRF rats, and neither could we detect any changes in tissue ANG II content when CRF and control distal colon were compared (14) at 6 wk. We have argued that this ANG II modulation of CRF colonic transport is independent of aldosterone action for several additional reasons. Although aldosterone stimulates K+ secretion across the distal colon (3), it does not stimulate Cl− secretion across this segment (33), and in CRF we consistently observe a net secretory flux of Cl− (11, 17). In the present study, we found no increase in Na+-K+-ATPase (α-subunit) protein or mRNA expression in CRF, which is a typical effect of chronic aldosterone stimulation of transepithelial Na+ transport. Finally, acute addition of ANG II to short-circuited tissues from both CRF and control rats results in a rapid change in Isc, reflecting immediate alterations in electrolyte transport (8, 14, 15), which is inconsistent with an aldosterone-mediated effect on colonic transport, at least at the genomic level.
It is clear from the present study that, despite the fact AT1 receptor protein mass is increased twofold in CRF colon compared with control (12), there is no change in AT1 mRNA and neither is there a change in AT2 protein or AT2 mRNA expression. Consequently, we hypothesize that there may be a decreased degradation rate of AT1 receptor protein in CRF colonic tissue in light of no change in AT1 mRNA. Taken together, however, the results of the present study indicate that AT1 receptor agonism is chiefly responsible for ANG II-associated alterations in electrolyte transport in CRF distal colon.
In conclusion, ANG II has a variety of agonistic activities in a number of tissues giving rise to a broad spectrum of physiological responses. Although we cannot exclude other neuro/immune/paracrine components or in vivo hemodynamic influences, the results presented in this report are the first to demonstrate that CRF-induced stimulation of colonic Na+ absorption is due to AT1 modulation of Na+ transporter and channel proteins at the message level.
GRANTS
This work was supported by NIH-NIDDK (DK56245 to M. Hatch).
Acknowledgments
Candi Morris and Bonnie Murphey provided expert technical assistance. We also thank Dr. M. L. Green for assistance with the real-time PCR methods.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Anantharam A, Palmer LG. Determination of epithelial Na+ channel subunit stoichiometry from single-channel conductances. J Gen Physiol 130: 55–70, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beutler KT, Masilamani S, Turban S, Nielsen J, Brooks HL, Ageloff S, Fenton RA, Packer RK, Knepper MA. Long-term regulation of ENaC expression in kidney by angiotensin II. Hypertension 41: 1143–1150, 2003. [DOI] [PubMed] [Google Scholar]
- 3.Binder HJ, Sandle GI. Electrolyte absorption and secretion in the mammalian colon. In: Physiology of the Gastrointestinal Tract, 2nd ed., edited by Johnson LR. New York: Raven, 1987, p. 1389–1418.
- 4.Bustin SA Quantification of mRNA using real-time reverse transcription PCR (RT-PCR): trends and problems. J Mol Endocrinol 29: 23–39, 2002. [DOI] [PubMed] [Google Scholar]
- 5.Davies NT, Munday KA, Parsons BJ. The effect of angiotensin on rat intestinal fluid transfer. J Endocrinol 48: 39–46, 1970. [DOI] [PubMed] [Google Scholar]
- 6.Dheda K, Huggett JF, Bustin SA, Johnson MA, Rook G, Zumla A. Validation of housekeeping genes for normalizing RNA expression in real-time PCR1. Biotechniques 37: 12–114, 116, 118–119, 2004. [DOI] [PubMed] [Google Scholar]
- 7.Dixit MP, Xu L, Xu H, Bai L, Collins JF, Ghishan FK. Effect of angiotensin-II on renal Na+/H+ exchanger-NHE3 and NHE2. Biochim Biophys Acta 1664: 38–44, 2004. [DOI] [PubMed] [Google Scholar]
- 8.Grahnquist L, Chen M, Gerasev A, Aizman R, Celsi G. Regulation of K+ transport in the rat distal colon via angiotensin II subtype receptors and K+ -pathways. Acta Physiol Scand 171: 145–151, 2001. [DOI] [PubMed] [Google Scholar]
- 9.Green ML, Hatch M, Freel RW. Ethylene glycol induces hyperoxaluria without metabolic acidosis in rats. Am J Physiol Renal Physiol 289: F536–F543, 2005. [DOI] [PubMed] [Google Scholar]
- 10.Greig ER, Baker EH, Mathialahan T, Boot-Handford RP, Sandle GI. Segmental variability of ENaC subunit expression in rat colon during dietary sodium depletion. Pflügers Arch 444: 476–483, 2002. [DOI] [PubMed] [Google Scholar]
- 11.Hatch M, Freel RW, Shahinfar S, Vaziri ND. Effects of the specific angiotensin II receptor antagonist losartan on urate homeostasis and intestinal urate transport. J Pharmacol Exp Ther 276: 187–193, 1996. [PubMed] [Google Scholar]
- 12.Hatch M, Freel RW, Vaziri ND. AT1 receptor up-regulation in intestine in chronic renal failure is segment specific. Pflügers Arch 437: 881–887, 1999. [DOI] [PubMed] [Google Scholar]
- 13.Hatch M, Freel RW, Vaziri ND. Intestinal excretion of oxalate in chronic renal failure. J Am Soc Nephrol 5: 1339–1343, 1994. [DOI] [PubMed] [Google Scholar]
- 14.Hatch M, Freel RW, Vaziri ND. Local upregulation of colonic angiotensin II receptors enhances potassium excretion in chronic renal failure. Am J Physiol Renal Physiol 274: F275–F282, 1998. [DOI] [PubMed] [Google Scholar]
- 15.Hatch M, Freel RW, Vaziri ND. Losartan antagonism of angiotensin-II-induced potassium secretion across rat colon. Pflügers Arch 436: 717–724, 1998. [DOI] [PubMed] [Google Scholar]
- 16.Hatch M, Freel RW, Vaziri ND. Regulatory aspects of oxalate secretion in enteric oxalate elimination. J Am Soc Nephrol 10, Suppl 14: S324–S328, 1999. [PubMed] [Google Scholar]
- 17.Hatch M, Vaziri ND. Enhanced enteric excretion of urate in rats with chronic renal failure. Clin Sci (Lond) 86: 511–516, 1994. [DOI] [PubMed] [Google Scholar]
- 18.Ikuma M, Kashgarian M, Binder HJ, Rajendran VM. Differential regulation of NHE isoforms by sodium depletion in proximal and distal segments of rat colon. Am J Physiol Gastrointest Liver Physiol 276: G539–G549, 1999. [DOI] [PubMed] [Google Scholar]
- 19.Inagaki A, Yamaguchi S, Ishikawa T. Amiloride-sensitive epithelial Na+ channel currents in surface cells of rat rectal colon. Am J Physiol Cell Physiol 286: C380–C390, 2004. [DOI] [PubMed] [Google Scholar]
- 20.Kim EJ, Jung YW, Kwon TH. Angiotensin II AT1 receptor blockade changes expression of renal sodium transporters in rats with chronic renal failure. J Korean Med Sci 20: 248–255, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krishnan S, Rajendran VM, Binder HJ. Apical NHE isoforms differentially regulate butyrate-stimulated Na absorption in rat distal colon. Am J Physiol Cell Physiol 285: C1246–C1254, 2003. [DOI] [PubMed] [Google Scholar]
- 22.Kunzelmann K, Mall M. Electrolyte transport in the mammalian colon: mechanisms and implications for disease. Physiol Rev 82: 245–289, 2002. [DOI] [PubMed] [Google Scholar]
- 23.Kwon TH, Frokiaer J, Fernandez-Llama P, Maunsbach AB, Knepper MA, Nielsen S. Altered expression of Na transporters NHE-3, NaPi-II, Na-K-ATPase, BSC-1, and TSC in CRF rat kidneys. Am J Physiol Renal Physiol 277: F257–F270, 1999. [DOI] [PubMed] [Google Scholar]
- 24.Kwon TH, Nielsen J, Kim YH, Knepper MA, Frokiaer J, Nielsen S. Regulation of sodium transporters in the thick ascending limb of rat kidney: response to angiotensin II. Am J Physiol Renal Physiol 285: F152–F165, 2003. [DOI] [PubMed] [Google Scholar]
- 25.Levens NR Control of intestinal absorption by the renin-angiotensin system. Am J Physiol Gastrointest Liver Physiol 249: G3–G15, 1985. [DOI] [PubMed] [Google Scholar]
- 26.Levens NR, Peach MJ, Carey RM, Poat JA, Munday KA. Response of rat jejunum to angiotensin II: role of norepinephrine and prostaglandins. Am J Physiol Gastrointest Liver Physiol 240: G17–G24, 1981. [DOI] [PubMed] [Google Scholar]
- 27.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25: 402–408, 2001. [DOI] [PubMed] [Google Scholar]
- 28.Lucioni A, Womack C, Musch MW, Rocha FL, Bookstein C, Chang EB. Metabolic acidosis in rats increases intestinal NHE2 and NHE3 expression and function. Am J Physiol Gastrointest Liver Physiol 283: G51–G56, 2002. [DOI] [PubMed] [Google Scholar]
- 29.Morduchowicz GA, Sheikh-Hamad D, Dwyer BE, Stern N, Jo OD, Yanagawa N. Angiotensin II directly increases rabbit renal brush-border membrane sodium transport: presence of local signal transduction system. J Membr Biol 122: 43–53, 1991. [DOI] [PubMed] [Google Scholar]
- 30.Rajendran VM, Binder HJ. Characterization of Na-H exchange in apical membrane vesicles of rat colon. J Biol Chem 265: 8408–8414, 1990. [PubMed] [Google Scholar]
- 31.Rozen S, Skaletsky H. Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol 132: 365–386, 2000. [DOI] [PubMed] [Google Scholar]
- 32.Steel RG, Torrie JH. Principles and Procedures of Statistics. New York: McGraw-Hill, 1980.
- 33.Turnamian SG, Binder HJ. Regulation of active sodium and potassium transport in the distal colon of the rat. Role of the aldosterone and glucocorticoid receptors. J Clin Invest 84: 1924–1929, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang T, Chan YL. Mechanism of angiotensin II action on proximal tubular transport. J Pharmacol Exp Ther 252: 689–695, 1990. [PubMed] [Google Scholar]
- 35.Wang T, Giebisch G. Effects of angiotensin II on electrolyte transport in the early and late distal tubule in rat kidney. Am J Physiol Renal Fluid Electrolyte Physiol 271: F143–F149, 1996. [DOI] [PubMed] [Google Scholar]
- 36.Wong ML, Medrano JF. Real-time PCR for mRNA quantitation. Biotechniques 39: 75–85, 2005. [DOI] [PubMed] [Google Scholar]
- 37.Yanagawa N Angiotensin II and proximal tubule sodium transport. Renal Physiol Biochem 14: 208–215, 1991. [DOI] [PubMed] [Google Scholar]
- 38.Zachos NC, Tse M, Donowitz M. Molecular physiology of intestinal Na+/H+ exchange. Annu Rev Physiol 67: 411–443, 2005. [DOI] [PubMed] [Google Scholar]