Abstract
Tumor necrosis factor (TNF) and epidermal growth factor (EGF) are key regulators in the intricate balance maintaining intestinal homeostasis. Previous work from our laboratory shows that TNF attenuates ligand-driven EGF receptor (EGFR) phosphorylation in intestinal epithelial cells. To identify the mechanisms underlying this effect, we examined EGFR phosphorylation in cells lacking individual TNF receptors. TNF attenuated EGF-stimulated EGFR phosphorylation in wild-type and TNFR2−/−, but not TNFR1−/−, mouse colon epithelial (MCE) cells. Reexpression of wild-type TNFR1 in TNFR1−/− MCE cells rescued TNF-induced EGFR inhibition, but expression of TNFR1 deletion mutant constructs lacking the death domain (DD) of TNFR1 did not, implicating this domain in EGFR downregulation. Blockade of p38 MAPK, but not MEK, activation of ERK rescued EGF-stimulated phosphorylation in the presence of TNF, consistent with the ability of TNFR1 to stimulate p38 phosphorylation. TNF promoted p38-dependent EGFR internalization in MCE cells, suggesting that desensitization is achieved by reducing receptor accessible to ligand. Taken together, these data indicate that TNF activates TNFR1 by DD- and p38-dependent mechanisms to promote EGFR internalization, with potential impact on EGF-induced proliferation and migration key processes that promote healing in inflammatory intestinal diseases.
Keywords: intestinal inflammation, epithelial cells, growth factors, cytokines, p38
the single layer of epithelial cells lining the gut forms an essential barrier to a wide range of noxious substances found in the intestinal lumen. Integrity of this barrier is regulated by a number of soluble growth factors and cytokines (35, 47). Imbalances in the expression of molecules such as tumor necrosis factor (TNF) or epidermal growth factor (EGF) have been observed both clinically and in experimental models of inflammatory bowel disease (IBD) and necrotizing enterocolitis (NEC) (11, 19, 39, 42); such dysregulated signal transduction may contribute to defects in mucosal repair and potentiate disease states.
TNF, a potent proinflammatory cytokine, is produced as a 26-kDa homotrimeric transmembrane precursor that is cleaved by metalloproteinase (MMP) activity into a soluble 17-kDa polypeptide (6, 7, 33) to bind two distinct transmembrane receptors, the 55-kDa low-affinity TNF receptor (TNFR) 1, and the 75-kDa high-affinity TNFR2 (26, 28, 31). These two receptors mediate distinct effects in intestinal epithelial cells, with TNFR1 promoting growth arrest and cytokine-mediated inflammation and TNFR2 promoting cellular proliferation and migration (14, 28, 29). These diverging affinities and responses suggest preferential binding to TNFR2 at physiological concentrations and increased relative TNFR1 activation during states of heightened TNF levels such as inflammation (44, 49).
TNFR1 has two well-characterized cytoplasmic domains, the Death Domain (DD) and the neutral sphingomyelinase domain (NSD) (1). The DD is an 80-amino acid span localized to the COOH-terminal portion of the receptor. It is thought to be critical for generation of cytotoxic death signals, anti-viral responses, and acid sphingomyelinase activation, which promotes inflammation and apoptosis through induction of NF-κB and other pathways (5, 43, 45). The NSD is a nine-amino acid motif immediately adjacent to the DD (1). Activation of the NSD causes activation of inflammatory signals including MAPK pathways (1, 2, 46).
To maintain both absorptive and barrier functions in the face of inflammation or other damage, intestinal epithelial tissues express a number of molecules that drive restitution and repair. EGF receptor (EGFR, aka ErbB-1), a well-characterized promoter of intestinal cell growth and response to damage, is a 170-kDa transmembrane protein containing an intrinsic cytoplasmic tyrosine kinase domain and docking sites for various signaling effector molecules (4, 27). Following binding to EGF or other ligands, the tyrosine phosphorylation on the cytoplasmic tail increases and stimulates a variety of downstream cascades. Signal termination is accomplished through receptor internalization, ubiquitinylation and proteolytic degradation, and/or inactivation by tyrosine phosphatases. EGFR is widely expressed in mammalian epithelial tissues and initiates signals for cellular growth and survival, cellular migration, and wound healing (8, 20, 24, 41, 60). During intestinal inflammation, EGF has been used effectively in the treatment of rodent models of NEC, as well as in clinical trials for ulcerative colitis (19, 42), suggesting that this signaling axis is an important target for IBD and NEC therapy.
In addition to their individual effects on cell growth, proliferation, and apoptosis, TNF and EGF regulate intestinal homeostasis via complex signaling cross-talk mechanisms. TNF, for example, promotes MMP activity, leading to release of EGF family members and promotion of EGFR activation (32, 37, 53). In contrast, previous findings in our laboratory have shown that TNF decreases EGF-stimulated EGFR phosphorylation (28). Similarly, a recent report by Yarden and colleagues (59) demonstrated p38-dependent TNF-induced internalization of EGFR in two cancer cell lines. Here we define TNF inhibition of EGF-stimulated EGFR phosphorylation as a TNFR1 DD-dependent process. Furthermore, both TNF-induced EGFR inhibition and internalization require p38 MAPK activity. These findings have important implications for understanding injury repair mechanisms in a high TNF environment, such as the gastrointestinal mucosa in a number of inflammatory disorders.
MATERIALS AND METHODS
Cells.
Conditionally immortalized mouse colon epithelial (MCE) cells were established from the colonic epithelium of H-2Kb-tsA58 Immortomice by Robert Whitehead at the Vanderbilt University Digestive Disease Research Center novel cell line core (54). These cells express a heat-labile SV40 large T antigen under the control of an interferon-γ-inducible promoter. Young adult mouse colon (YAMC), TNFR1−/− MCE, TNFR2−/− MCE, and EGFR−/− MCE knockout cells were generated from TNFR1, TNFR2, or EGFR-null mice crossed with Immortomice as previously described (14). Receptor deletion was confirmed by PCR and immunofluorescence analysis. Intestinal epithelial cell (IEC)-6 cells are a well-known epithelial cell line derived from rat small intestine (American Type Culture Collection, Manassas, VA).
Cell culture.
YAMC and EGFR−/− MCE cells were maintained on rat tail collagen-coated plates (Mediatech, Herndon, VA). All cells were maintained as a monolayer in RPMI 1640 medium with 5% fetal bovine serum, 5 U/ml mouse interferon-γ (Integren, Norcross, GA), 100 U/ml penicillin and streptomycin, 5 μg/ml insulin, 5 μg/ml transferrin, and 5 ng/ml selenous acid (BD Biosciences, San Jose, CA) at 33°C (permissive conditions) under 5% CO2. Before experiments, cells were transferred to RPMI 1640 medium with 0.5% fetal bovine serum and 100 U/ml penicillin and streptomycin at 37°C (nonpermissive conditions) for 16–18 h.
Treatment protocol.
After incubation in nonpermissive conditions, cells were pretreated with TNF (100 ng/ml) for 45 min and then treated with EGF (10 ng/ml) for 1 min, unless otherwise noted. Inhibitors (SB220025 or U0126, 10 μM each) were added to the media 30 min before TNF treatment. TNFR1 agonist antibody was used at 2 μg/ml and was added to media 45 min before treatment with EGF. In all experiments, cells remained in nonpermissive conditions during treatments.
Antibodies, inhibitors, cytokines, and growth hormones.
Murine TNF was purchased from PeproTech (Rocky Hill, NJ). Mouse EGF was a gift from Stanley Cohen (Vanderbilt University, Nashville, TN). EGFR phophospecific antibodies P-Y845, P-Y1045, P-Y1068, and P-Y1173, anti-rabbit and anti-mouse horseradish peroxidase-conjugated secondary antibodies, phospho-Akt, and phospho-focal adhesion kinase (FAK) antibodies were purchased from Cell Signaling Technology (Beverly, MA). We have previously characterized the EGFR phosphospecific antibodies for specificity (23). Anti-EGFR antibody was purchased from Upstate Biotechnology (Charlottesville, VA). Anti-active ERK polyclonal antibody and the MEK inhibitor U0126 were purchased from Promega (Madison, WI). Horseradish peroxidase-conjugated mouse anti-phosphotyrosine (PY20) was purchased from BD Transduction Laboratories (Franklin Lakes, NJ). Anti-FAK, anti-TNFR1, and anti-TNFR2 antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-human TNFR1 agonist antibody was purchased from R&D Systems (Minneapolis, MN). The p38 inhibitor SB220025 was purchased from Calbiochem (La Jolla, CA). The proteasome inhibitor MG132 was purchased from Sigma Aldrich (St. Louis, MO).
Cell lysates and Western blotting.
Cell monolayers were washed twice with ice cold PBS and scraped on ice into cold lysis buffer (1% Triton, 10% 200 μM HEPES, pH 7.4). Cellular lysates were cleared and boiled in Laemmli sample buffer (30). Proteins were separated by SDS-PAGE and transferred to nitrocellulose membranes. Membranes were blocked for 1 h in Tris-buffered saline (50 mM Tris, 150 mM NaCl, and 25 mM KCl, pH 8.0) with 0.05% Tween 20 (TBST) and 5% nonfat dry milk, incubated with primary antibody overnight at 4°C, and incubated with secondary antibody (except for PY20) for 45 min. Horseradish peroxidase was detected with the Western Lightning enhanced chemiluminescence kit (PerkinElmer Life Sciences, Wellesley, MA).
Immunoprecipitation.
Cellular lysates were precleared by incubating with protein AG agarose beads (Santa Cruz Biotechnology) for 30 min followed by centrifugation. Supernatants were incubated with 2 μg anti-EGFR antibody for 1 h at 4°C, and then beads were added for 1 h at 4°C. Immunocomplexes were collected by centrifugation, washed three times in lysis buffer, and boiled in Laemmli sample buffer (3, 30) for SDS-PAGE Western blot analysis.
Constructs.
TNFR1 was cloned by RT-PCR with mRNA isolated from YAMC cells to generate full-length wildtype (WT) mouse TNFR1. TNFR1 mutant constructs were generated using the site directed mutagenesis kit TaqMan (Applied Biosystems, Branchburg, NJ). The ΔDD construct was terminated at aa-348, and the ΔCT construct was terminated at aa-228, where CT is cytoplasmic tail. Constructs were cloned into the LZRS retroviral vector and amplified with the use of Phoenix ecotropic packaging cells before infection of TNFR1−/− MCE cells. Infected cells were sorted on the basis of green fluorescent protein expression.
EGFR internalization and fluorescence microscopy.
YAMC cells were plated on four-well chamber slides (Nalge Nunc International, Rochester, NY) and treated with SB220025 (10 μM), TNF (100 ng/ml), or SB220025 and TNF together for 45 min. EGF (10 ng/ml, 30 min) served as the positive control for EGFR internalization. Cells were fixed with methanol at −20°C for 5 min, solubilized with PBS + 0.2% Triton X-100, and blocked with 10% goat serum. Slides were stained with anti-EGFR and Cy3-labeled rabbit secondary antibody (Jackson Laboratories, Bar Harbor, ME). EGFR localization was detected by Apoptome optical sectioning with the use of an Axiovert 200 microscope (Zeiss, Thornwood, NY).
TNFR1 and TNFR2 identification by fluorescence microscopy.
YAMC, TNFR1−/− MCE, and TNFR2−/− MCE cells were cultured on chamber slides. Cells were washed with PBS and incubated in 10% normal donkey serum (Zymed Laboratories, San Francisco, CA) for 1 h to decrease nonspecific antibody binding. Cells were stained with anti-TNFR1 or TNFR2 (1:1,000) in PBS with 5% donkey serum overnight at 4°C, then with donkey anti-rabbit IgG-FITC (1:2,000, Zymed Laboratories).
Replicates and statistical analysis.
All data are representative of at least three independent experiments. Statistical significance of differences between mean values was assessed with a Student's t-test analysis. Minimum level of significance was set at 0.05.
RESULTS
TNF inhibits EGF-stimulated EGFR phosphorylation.
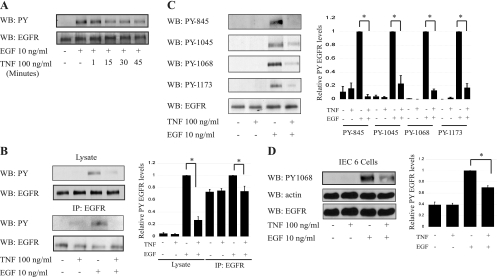
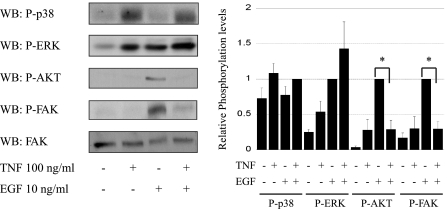
Our laboratory (28) and others (59) have reported that TNF decreases EGF-stimulated EGFR activation. To determine the optimal conditions for TNF inhibition of EGFR phosphorylation, YAMC cells were incubated with TNF for various times (Fig. 1A) and then exposed to EGF for 1 min in this and subsequent experiments, except where otherwise indicated. Whole cell lysates and EGFR immunoprecipitates were prepared and subjected to Western blot analysis for tyrosine-phosphorylated EGFR. TNF substantially attenuated EGFR phosphorylation in response to EGF, with maximal effect seen at 45 min (Fig. 1A). Decreased EGF-induced phosphorylation was observed both in whole cell lysate and immunoprecipitated EGFR (Fig. 1B). To determine whether this response is phosphosite specific, we performed Western blot analysis using phosphospecific EGFR antibodies that we have previously characterized (Y845, Y1045, Y1068, and Y1173) (23). A marked decrease in phosphorylation was detected when cells were pretreated with TNF before EGF exposure (Fig. 1C). Similar downregulation of EGF-dependent EGFR activation was seen in IEC-6 cells pretreated with TNF (Fig. 1D). Thus, as EGFR tyrosine phosphorylation was nonspecifically inhibited, we asked whether downstream target signaling was similarly affected. EGF-stimulated Akt and FAK phosphorylation were both blocked by TNF pretreatment (Fig. 2). TNF exposure for shorter times causes activation of Akt as we previously reported [(58), (data not shown)], but by 45 min cellular signals return to basal levels, allowing us to isolate effects on EGF-stimulated signaling. TNF induced ERK and p38 MAPK activation (detected by phosphorylation) as strongly as EGF, suggesting that blockade of EGFR signals through these pathways is not a target of inhibition.
Fig. 1.
Tumor necrosis factor (TNF) inhibits epidermal growth factor (EGF)-stimulated EGF receptor (EGFR) phosphorylation. A: young adult mouse colon (YAMC) cells were exposed to 100 ng/ml TNF for the indicated times before 1-min EGF stimulation, and lysates were subjected to Western blot analysis for phosphotyrosine (PY). B and C: lysates or EGFR immunoprecipitates (as indicated) from YAMC cells pretreated with 100 ng/ml TNF for 45 min before EGF exposure were subjected to Western blot analysis with antibodies specific for PY (B), EGFR, or indicated EGFR phosphorylation sites (C). D: intestinal epithelial cell (IEC)-6 cells were treated with TNF and EGF as above and subjected to Western blot analysis for PY-1068. Densitometry shows averaged results from 3 or more experiments. WB, Western blot; IP, immunoprecipitation. *P < 0.04 vs. EGF treatment. EGFR and actin blots shown as loading controls.
Fig. 2.
TNF inhibits a subset of EGFR-stimulated signaling. YAMC cells were pretreated with TNF for 45 min and then exposed to EGF as in Fig. 1. Whole cell lysates were analyzed by Western blot analysis using the indicated phosphospecific antibodies. Focal adhesion kinase (FAK) is shown as loading control. Densitometry shows averaged results from 3 or more experiments. *P < 0.006 vs. EGF treatment.
Blockade of EGFR phosphorylation by TNF requires TNFR1.
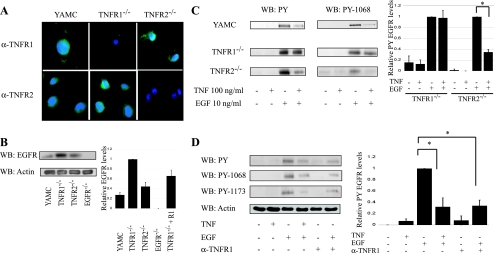
Most studies suggest that distinct cellular responses are regulated by TNFR1 and TNFR2. Thus we tested the roles of these receptors in attenuation of EGF-stimulated EGFR activation, using TNFR1−/− MCE or TNFR2−/− MCE, which lack their respective TNFRs but express EGFR at levels equal to or higher than in YAMC cells (Fig. 3, A and B). As in YAMC cells, which express both TNFR1 and TNFR2, TNF blocked ligand-stimulated EGFR phosphorylation in TNFR2−/− MCE cells. In contrast, TNF had no effect on EGFR phosphorylation in cells lacking TNFR1 (Fig. 3C). Furthermore, a TNFR1-specific agonist antibody inhibited EGFR activation as efficiently as TNF in YAMC cells (Fig. 3D).
Fig. 3.
Tumor necrosis factor receptor 1 (TNFR1) is required for TNF-induced EGFR inhibition. YAMC cells, TNFR1−/− mouse colon epithelial (MCE), or TNFR2−/− MCE cells were subjected to immunofluorescence analysis for TNF receptor expression (A) and Western blot analysis for EGFR expression (B). C: cells were treated as above, and EGFR phosphorylation was determined. D: YAMC cells were incubated with a TNFR1 agonist antibody for 45 min before EGF stimulation. Densitometry shows averaged results from 3 or more experiments. *P < 0.02 vs. EGF treatment. Actin blot is shown as a loading control.
TNFR1 mediates EGFR blockade through the DD.
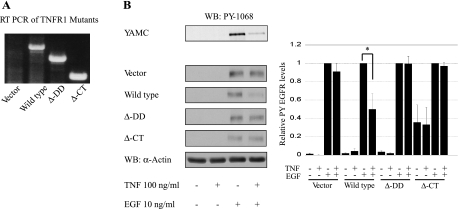
TNFR1 contains two well-characterized signal transduction regulatory domains, the DD and the NSD. We examined their role in TNF-induced EGFR inhibition by infecting mutant human TNFR1 viral constructs lacking the DD (ΔDD) or both the DD and NSD (ΔCt) into TNFR1−/− MCE cells. Cells were exposed to EGF and TNF as above, and lysates were subjected to Western blot analysis for EGFR phosphorylation. As shown in Fig. 4, TNF attenuated EGFR activation only in cells expressing TNFR1 containing the DD.
Fig. 4.
TNF-induced EGFR inhibition requires the TNFR1 death domain (DD). A: TNFR1−/− cells were stably infected with pRK7 vector, wild-type TNFR1, ΔDD TNFR1, or ΔCt TNFR1 constructs, where Ct is the cycle threshold, as described in materials and methods, and then expression was validated by RT-PCR. B: mutant TNFR1 cell lines were exposed to TNF and EGF as above. Lysates were subjected to Western blot analysis for EGFR phosphorylation. Densitometry shows averaged results from 3 or more experiments. *P < 0.05 vs. EGF treatment. Actin is shown as a loading control.
TNF inhibition of EGFR activation requires p38 MAPK activity.
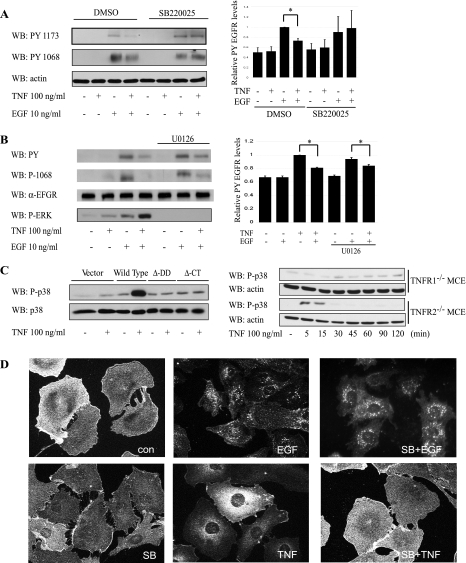
A recent report by Zwang and colleagues (59) describes p38-dependent transient EGFR internalization in response to UV irradiation or TNF in HeLa and SW480 cells. To test whether this mechanism explains our findings, we pretreated YAMC cells with a pharmacological p38 inhibitor (SB220025) 30 min before treatment with TNF and EGF as above. TNF exposure attenuated ligand-stimulated EGFR phosphorylation in vehicle-treated but not SB220025-treated cells, implicating a p38 MAPK-dependent process (Fig. 5A). In contrast, blockade of MEK-ERK1/2 signaling with the MEK inhibitor U0126 had no effect on TNF-induced EGFR inhibition (Fig. 5B), suggesting that p38 is acting independently of other MAPKs. Similar results were obtained with another MEK inhibitor (PD98059, data not shown).
Fig. 5.
TNFR1-mediated inhibition of EGFR phosphorylation requires p38 MAPK activity. A: YAMC cells were pretreated with p38 inhibitor (SB220025) (SB) 30 min before TNF and EGF as above. Cells were lysed and probed by Western blot analysis for EGFR phosphorylation. B: YAMC cells were pretreated with MEK inhibitor (U0126) and exposed to TNF before EGF. Cells were lysed, and Western blot analysis for EGFR phosphorylation was performed. C: TNFR1−/− cells were transiently transfected with vector, wild-type TNFR1, ΔDD TNFR1, or ΔCt TNFR1. Cells were treated with TNF, lysed, and subjected to Western Blot analysis using antibody specific for phospho (active)-p38. Right: TNFR1−/− MCE and TNFR2−/− MCE cells were treated with TNF for the times indicated, and cellular lysates were analyzed by Western blot analysis for P-p38 and total actin. D: EGFR internalization in YAMC cells was assessed by immunofluorescence localization analysis following 45-min TNF or EGF treatment, with or without SB220025. Densitometry shows averaged results from 3 or more experiments. *P < 0.03 vs. EGF treatment. Actin, total EGFR, and total p38 are included as loading controls. Con, control.
As both TNFR1 DD signaling and p38 are required for TNF-induced EGFR desensitization, we tested the requirement for TNFR1 and its DD in p38 activation in colon epithelial cells. TNFR1−/− and TNFR2−/− MCE cells were treated with TNF for 0–120 min, and p38 phosphorylation in whole cell lysates was assessed by Western blot analysis. Only cells expressing TNFR1 displayed increased p38 phosphorylation above baseline in response to TNF treatment (Fig. 5C). Furthermore, TNF promoted p38 activation in TNFR1−/− MCE cells reconstituted with WT, but not ΔDD or ΔCt, TNFR1 (Fig. 5C).
To determine whether TNF-induced p38 activation causes EGFR internalization as previously described (59), YAMC cells were treated for 45 min with TNF, SB220025, or both. Cells were fixed and subjected to immunofluorescence analysis for EGFR subcellular localization. Thirty-minute EGF exposure served as a positive control for internalization. Both EGF and TNF treatment promoted EGFR internalization. However, p38 blockade with SB220025 prevented TNF-induced EGFR internalization (Fig. 5D), suggesting that TNFR1-induced EGFR inhibition may be via this mechanism. In contrast, EGF-driven receptor internalization was not blocked by p38 inhibitor.
TNF-stimulated EGFR inhibition occurs independent of proteasome inhibition.
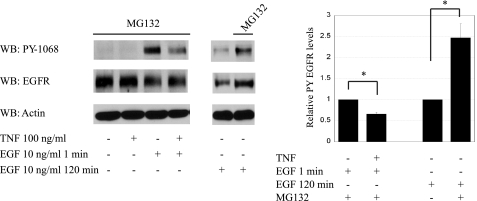
EGFR can be internalized either constitutively or by a ligand-binding-induced mechanism (55). Once internalized, the receptor is sorted by incompletely understood mechanisms through multivesicular endosomes (12, 18, 25) and designated for either destruction or recycling to the plasma membrane (10, 16). Our data show that TNF inhibits EGFR via p38-dependent EGFR internalization. To test whether this mechanism impacts receptor degradation as well as internalization, we pretreated YAMC cells with a proteasome inhibitor (MG132, 10 μM) 30 min before treatment with TNF and EGF as above. MG132 attenuated EGF-induced EGFR degradation but did not affect the ability of TNF to inhibit EGFR phosphorylation (Fig. 6). Thus altered receptor degradation is not the primary mechanism for TNF-induced EGFR desensitization. This result is consistent with the observation that TNF does not alter total EGFR levels in YAMC cells (Figs. 1, A–D, and 5C).
Fig. 6.
TNF-stimulated EGFR inhibition occurs independent of proteasome-mediated receptor degradation. YAMC cells were pretreated with proteasome inhibitor (MG132) 30 min before TNF and/or EGF as indicated. Cells were lysed and probed by Western blot analysis for EGFR phosphorylation. Actin is included as a loading control. *P < 0.01 vs. EGF treatment and no MG132, respectively.
DISCUSSION
In this study we provide evidence that signaling through TNFR1, but not TNFR2, inhibits EGF-stimulated EGFR phosphorylation in murine colon epithelial cells by a mechanism requiring the TNFR1 DD and p38 MAPK. We show that TNF activation of p38 through TNFR1 promotes internalization of EGFR in murine colon epithelial cells. Internalization under these conditions was concomitant with EGFR inhibition but independent of proteasome activity.
TNF-induced EGFR blockade may be an important signaling mechanism in the acute injury initiated by the innate immune response with implications for both acute and chronic IBDs. TNF is a key proinflammatory cytokine expressed at high levels in IBD and NEC (11, 39); conversely, EGF has been shown effective in treatment of both experimental NEC and clinical ulcerative colitis (19, 42). The data presented here indicate that activation of TNFR1, the receptor likely to be preferentially activated in the presence of high TNF levels during active disease, induces EGFR desensitization via p38-driven internalization. Taken in the context of our recent work showing that p38, which is elevated in several IBD models (51), is also required for ligand-stimulated ubiquitinylation/degradation of EGFR (14), these results suggest that signaling through this MAPK regulates EGFR at multiple levels.
P38 is involved in cellular stress responses and apoptosis (15, 57), as well as wound healing in epithelial cells with impact on migration and proliferation (17, 23, 40). Our data showing that TNFR1-stimulated EGFR internalization in colon epithelial cells requires p38 are in agreement with a recent study(59) describing EGFR internalization following cell stressors such as UV irradiation and TNF exposure. In that paper, p38 phosphorylation of the cytoplasmic tail of the receptor was linked to transient receptor endocytosis (59). Similarly, Vergarajauregui and colleagues (50) have demonstrated EGFR internalization as a result of forced p38 activation with anisomycin. Thus transient receptor endocytosis may be an important mechanism by which TNFR1 attenuates responses to EGF in IECs.
TNFR1 has two well-characterized cytoplasmic domains, the NSD and the DD (1). Our data show that the DD is necessary for both p38 activation and EGFR inhibition by pathological TNF concentrations (Figs. 4 and 5). The 80-amino acid cytoplasmic DD region of TNFR1 is thought to be critical for generation of cytotoxic death signals, anti-viral responses, and activation of acid sphingomyelinase, which promotes inflammation and apoptosis through induction of NF-κB and other pathways (5, 43, 45). Previous studies (9, 13) in mouse fibrosarcoma cells have shown that the DD was competent to promote p38 signaling; our data extend this by showing conclusively that, in the absence of the TNFR1 DD, p38 phosphorylation does not occur, despite pathological TNF concentrations. Because p38 is a key regulator of EGFR signaling through both TNF-induced internalization [Fig. 5 and (59)] and EGFR processing (23), our data further define the important role of the TNFR1 DD in colon epithelial cell physiology.
EGFR is found predominantly on the cell surface but constantly undergoes shuttling and recycling between the plasma membrane and the endosomal compartment. Following ligand binding, EGFR is quickly internalized through a coated pit pathway to the endosomal compartment of the cell and then sorted through incompletely understood mechanisms through multivesicular endosomes (12, 18, 25). Once internalized, the receptor is sorted for either destruction or recycling to the plasma membrane (10, 16). Because EGFR is internalized shortly after ligand binding, it is thought that significant EGFR signaling can occur within the endosomal compartment (55). Although active EGFR is able to signal when internalized, EGFR internalized while still inactive is presumably unable to bind ligands present at the cell surface. In addition, receptor trafficking is slower for nonligand bound EGFR by 5–10-fold (52, 56), maintaining the receptor in a ligand inaccessible compartment for longer periods of time. Our laboratory and others (50, 59) have seen that p38 activation can induce EGFR internalization (Fig. 6). Additionally, a recent report from our laboratory showed that EGFR-stimulated p38 activation is required for EGF-induced cobalamin (Cbl) activation and subsequent receptor ubiquitinylation and degradation (23). However, TNF-induced p38 activation in the present study was not associated with changes in EGFR levels (Fig. 1) or Cbl phosphorylation (data not shown). Thus this cytokine-inducible pathway appears to be independent of lysosomal EGFR degradation and may involve p38 activation with different kinetics or in a different subcellular compartment than that required for EGF-driven receptor downregulation.
EGFR signaling is integral to the IEC response to damage and inflammation. In vitro, EGF induces epithelial migration (24) and intestinal epithelial proliferation (23). In animal models of IBD and NEC, EGFR-dependent responses reduce disease severity and promote recovery. Mice lacking TGF-α show a significantly increased susceptibility to dextran sodium sulphate (DSS)-induced colitis (22), whereas, in contrast, mice treated with exogenous EGF or overexpressing TGF-α are protected from DSS-induced colitis (21, 38). Neonatal rat pups pretreated with EGF show a reduced incidence of experimental NEC compared with controls (19), and 8-wk-old rabbits with jejunal resections treated with EGF exhibit an increase in glucose absorption (34). Thus there is a key role for EGFR in both the healing process from and prevention of intestinal epithelial injury.
A developing literature suggests that there is complex and tightly controlled cross-talk between TNFRs and EGF. One example of this interaction is through TNF-α-converting enzyme (TACE). TACE is a membrane-bound protein containing MMP and disintegrin domains involved in TNF-induced cleavage of TGF-α (37). TNF can transactivate EGFR by promoting MMP-dependent cleavage of TGF-α and other EGFR ligands into their active forms (32, 37, 53). Mice that are deficient in active TACE have epithelial defects, including in the intestinal tract, which are similar to those reported in mice lacking EGFR (37). A second avenue for TNF-induced regulation of EGFR is the stimulation of protein tyrosine phosphatase activity. ME-180 cells treated with TNF showed an increase in protein tyrosine phosphatase (PTP)1B, a 37-kDa protein with tyrosine-specific protein phosphatase activity in cervical cancer cells (36, 48). Because of this association, we examined two common nonreceptor PTPs, Src-homology 2 domain containing phosphatase (SHP)-1 and SHP-2, but found no increase in either PTP with TNF treatment (data not shown). Our findings expand the understanding of the relationship between TNF and EGFR by identifying a third mechanism by which TNF signals through the TNFR1 DD to activate p38 and stimulate internalization of EGFR in colon epithelial cells.
In summary, we have shown that TNF negatively regulates EGF-dependent EGFR tyrosine phosphorylation and activity in a p38-dependent manner. This effect is a specific response to stimulation of TNFR1 and requires the presence of the DD-containing cytoplasmic tail region of the receptor. These data provide evidence of negative regulation of EGFR signal transduction by the proinflammatory cytokine TNF in murine IEC, with implications for impaired injury response mechanisms in a number of gastrointestinal disorders such as IBD and NEC. Understanding these interactions may lead to important new approaches to pharmacological prevention and/or treatment of IBDs.
GRANTS
This work was supported by National Institutes of Health Grant 5R01DK056008-09 to D. B. Polk (PI), NIH Grant 2T32HL07256-26 to Pediatrics Training Grant, Arnold Strauss (PI) and D. B. Polk (Mentor), Vanderbilt University Hazinski/Turner Award to S. McElroy (PI), and 2P30 DK058404-06 (Vanderbilt Digestive Disease Research Center) to D. B. Polk (PI).
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Adam-Klages S, Schwandner R, Adam D, Kreder D, Bernardo K, Kronke M. Distinct adapter proteins mediate acid versus neutral sphingomyelinase activation through the p55 receptor for tumor necrosis factor. J Leukoc Biol 63: 678–682, 1998. [DOI] [PubMed] [Google Scholar]
- 2.Adam D, Wiegmann K, Adam-Klages S, Ruff A, Kronke M. A novel cytoplasmic domain of the p55 tumor necrosis factor receptor initiates the neutral sphingomyelinase pathway. J Biol Chem 271: 14617–14622, 1996. [DOI] [PubMed] [Google Scholar]
- 3.Allen A Physiology of the Gastrointestinal Tract, edited by Johnson LR and Christensen J. New York: Raven, 1981, chapt. 21, p. 617–639.
- 4.Arteaga CL Overview of epidermal growth factor receptor biology and its role as a therapeutic target in human neoplasia. Semin Oncol 29: 3–9, 2002. [DOI] [PubMed] [Google Scholar]
- 5.Baker SJ, Reddy EP. Modulation of life and death by the TNF receptor superfamily. Oncogene 17: 3261–3270, 1998. [DOI] [PubMed] [Google Scholar]
- 6.Beutler B, Cerami A. Cachectin and tumour necrosis factor as two sides of the same biological coin. Nature 320: 584–588, 1986. [DOI] [PubMed] [Google Scholar]
- 7.Black RA, Rauch CT, Kozlosky CJ, Peschon JJ, Slack JL, Wolfson MF, Castner BJ, Stocking KL, Reddy P, Srinivasan S, Nelson N, Boiani N, Schooley KA, Gerhart M, Davis R, Fitzner JN, Johnson RS, Paxton RJ, March CJ, Cerretti DP. A metalloproteinase disintegrin that releases tumour-necrosis factor-alpha from cells. Nature 385: 729–733, 1997. [DOI] [PubMed] [Google Scholar]
- 8.Blay J, Brown KD. Epidermal growth factor promotes the chemotactic migration of cultured rat intestinal epithelial cells. J Cell Physiol 124: 107–112, 1985. [DOI] [PubMed] [Google Scholar]
- 9.Boone E, Vandevoorde V, De Wilde G, Haegeman G. Activation of p42/p44 mitogen-activated protein kinases (MAPK) and p38 MAPK by tumor necrosis factor (TNF) is mediated through the death domain of the 55-kDa TNF receptor. FEBS Lett 441: 275–280, 1998. [DOI] [PubMed] [Google Scholar]
- 10.Bucci C, Thomsen P, Nicoziani P, McCarthy J, van Deurs B. Rab7: a key to lysosome biogenesis. Mol Biol Cell 11: 467–480, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caplan MS, Hsueh W. Necrotizing enterocolitis: role of platelet activating factor, endotoxin, and tumor necrosis factor. J Pediatr 117: S47–S51, 1990. [DOI] [PubMed] [Google Scholar]
- 12.Chang CP, Lazar CS, Walsh BJ, Komuro M, Collawn JF, Kuhn LA, Tainer JA, Trowbridge IS, Farquhar MG, Rosenfeld MG, Wiley HS, Gill GN. Ligand-induced internalization of the epidermal growth factor receptor is mediated by multiple endocytic codes analogous to the tyrosine motif found in constitutively internalized receptors. J Biol Chem 268: 19312–19320, 1993. [PubMed] [Google Scholar]
- 13.Chang L, Karin M. Mammalian MAP kinase signalling cascades. Nature 410: 37–40, 2001. [DOI] [PubMed] [Google Scholar]
- 14.Corredor J, Yan F, Shen CC, Tong W, John SK, Wilson G, Whitehead R, Polk DB. Tumor necrosis factor regulates intestinal epithelial cell migration by receptor-dependent mechanisms. Am J Physiol Cell Physiol 284: C953–C961, 2003. [DOI] [PubMed] [Google Scholar]
- 15.Daly JM, Olayioye MA, Wong AM, Neve R, Lane HA, Maurer FG, Hynes NE. NDF/heregulin-induced cell cycle changes and apoptosis in breast tumour cells: role of PI3 kinase and p38 MAP kinase pathways. Oncogene 18: 3440–3451, 1999. [DOI] [PubMed] [Google Scholar]
- 16.de Renzis S, Sonnichsen B, Zerial M. Divalent Rab effectors regulate the sub-compartmental organization and sorting of early endosomes. Nat Cell Biol 4: 124–133, 2002. [DOI] [PubMed] [Google Scholar]
- 17.Dieckgraefe BK, Weems DM, Santoro SA, Alpers DH. ERK and p38 MAP kinase pathways are mediators of intestinal epithelial wound-induced signal transduction. Biochem Biophys Res Commun 233: 389–394, 1997. [DOI] [PubMed] [Google Scholar]
- 18.Dikic I, Giordano S. Negative receptor signalling. Curr Opin Cell Biol 15: 128–135, 2003. [DOI] [PubMed] [Google Scholar]
- 19.Dvorak B, Halpern MD, Holubec H, Williams CS, McWilliam DL, Dominguez JA, Stepankova R, Payne CM, McCuskey RS. Epidermal growth factor reduces the development of necrotizing enterocolitis in a neonatal rat model. Am J Physiol Gastrointest Liver Physiol 282: G156–G164, 2002. [DOI] [PubMed] [Google Scholar]
- 20.Egan LJ, de Lecea A, Lehrman ED, Myhre GM, Eckmann L, Kagnoff MF. Nuclear factor-kappa B activation promotes restitution of wounded intestinal epithelial monolayers. Am J Physiol Cell Physiol 285: C1028–C1035, 2003. [DOI] [PubMed] [Google Scholar]
- 21.Egger B, Carey HV, Procaccino F, Chai NN, Sandgren EP, Lakshmanan J, Buslon VS, French SW, Buchler MW, Eysselein VE. Reduced susceptibility of mice overexpressing transforming growth factor alpha to dextran sodium sulphate induced colitis. Gut 43: 64–70, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Egger B, Procaccino F, Lakshmanan J, Reinshagen M, Hoffmann P, Patel A, Reuben W, Gnanakkan S, Liu L, Barajas L, Eysselein VE. Mice lacking transforming growth factor alpha have an increased susceptibility to dextran sulfate-induced colitis. Gastroenterology 113: 825–832, 1997. [DOI] [PubMed] [Google Scholar]
- 23.Frey MR, Dise RS, Edelblum KL, Polk DB. p38 kinase regulates epidermal growth factor receptor downregulation and cellular migration. EMBO J 25: 5683–5692, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frey MR, Golovin A, Polk DB. Epidermal growth factor-stimulated intestinal epithelial cell migration requires Src family kinase-dependent p38 MAPK signaling. J Biol Chem 279: 44513–44521, 2004. [DOI] [PubMed] [Google Scholar]
- 25.Futter CE, Pearse A, Hewlett LJ, Hopkins CR. Multivesicular endosomes containing internalized EGF-EGF receptor complexes mature and then fuse directly with lysosomes. J Cell Biol 132: 1011–1023, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heller RA, Kronke M. Tumor necrosis factor receptor-mediated signaling pathways. J Cell Biol 126: 5–9, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jorissen RN, Walker F, Pouliot N, Garrett TP, Ward CW, Burgess AW. Epidermal growth factor receptor: mechanisms of activation and signalling. Exp Cell Res 284: 31–53, 2003. [DOI] [PubMed] [Google Scholar]
- 28.Kaiser GC, Polk DB. Tumor necrosis factor alpha regulates proliferation in a mouse intestinal cell line. Gastroenterology 112: 1231–1240, 1997. [DOI] [PubMed] [Google Scholar]
- 29.Kaiser GC, Yan F, Polk DB. Conversion of TNF alpha from antiproliferative to proliferative ligand in mouse intestinal epithelial cells by regulating mitogen-activated protein kinase. Exp Cell Res 249: 349–358, 1999. [DOI] [PubMed] [Google Scholar]
- 30.Laemmli UK Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685, 1970. [DOI] [PubMed] [Google Scholar]
- 31.Loetscher H, Schlaeger EJ, Lahm HW, Pan YC, Lesslauer W, Brockhaus M. Purification and partial amino acid sequence analysis of two distinct tumor necrosis factor receptors from HL60 cells. J Biol Chem 265: 20131–20138, 1990. [PubMed] [Google Scholar]
- 32.Mauviel A Cytokine regulation of metalloproteinase gene expression. J Cell Biochem 53: 288–295, 1993. [DOI] [PubMed] [Google Scholar]
- 33.Moss ML, Jin SL, Milla ME, Bickett DM, Burkhart W, Carter HL, Chen WJ, Clay WC, Didsbury JR, Hassler D, Hoffman CR, Kost TA, Lambert MH, Leesnitzer MA, McCauley P, McGeehan G, Mitchell J, Moyer M, Pahel G, Rocque W, Overton LK, Schoenen F, Seaton T, Su JL, Warner J, Williard D, Becherer JD. Cloning of a disintegrin metalloproteinase that processes precursor tumour-necrosis factor-alpha. Nature 385: 733–736, 1997. [DOI] [PubMed] [Google Scholar]
- 34.O'Loughlin E, Winter M, Shun A, Hardin JA, Gall DG. Structural and functional adaptation following jejunal resection in rabbits: effect of epidermal growth factor. Gastroenterology 107: 87–93, 1994. [DOI] [PubMed] [Google Scholar]
- 35.Okamoto T, Akaike T, Sawa T, Miyamoto Y, van der Vliet A, Maeda H. Activation of matrix metalloproteinases by peroxynitrite-induced protein S-glutathiolation via disulfide S-oxide formation. J Biol Chem 276: 29596–29602, 2001. [DOI] [PubMed] [Google Scholar]
- 36.Perez M, Haschke B, Donato NJ. Differential expression and translocation of protein tyrosine phosphatase 1B-related proteins in ME-180 tumor cells expressing apoptotic sensitivity and resistance to tumor necrosis factor: potential interaction with epidermal growth factor receptor. Oncogene 18: 967–978, 1999. [DOI] [PubMed] [Google Scholar]
- 37.Peschon JJ, Slack JL, Reddy P, Stocking KL, Sunnarborg SW, Lee DC, Russell WE, Castner BJ, Johnson RS, Fitzner JN, Boyce RW, Nelson N, Kozlosky CJ, Wolfson MF, Rauch CT, Cerretti DP, Paxton RJ, March CJ, Black RA. An essential role for ectodomain shedding in mammalian development. Science 282: 1281–1284, 1998. [DOI] [PubMed] [Google Scholar]
- 38.Procaccino F, Reinshagen M, Hoffmann P, Zeeh JM, Lakshmanan J, McRoberts JA, Patel A, French S, Eysselein VE. Protective effect of epidermal growth factor in an experimental model of colitis in rats. Gastroenterology 107: 12–17, 1994. [DOI] [PubMed] [Google Scholar]
- 39.Reinecker HC, Steffen M, Witthoeft T, Pflueger I, Schreiber S, MacDermott RP, Raedler A. Enhanced secretion of tumour necrosis factor-alpha, IL-6, and IL-1 beta by isolated lamina propria mononuclear cells from patients with ulcerative colitis and Crohn's disease. Clin Exp Immunol 94: 174–181, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sharma GD, He J, Bazan HE. p38 and ERK1/2 coordinate cellular migration and proliferation in epithelial wound healing: evidence of cross-talk activation between MAP kinase cascades. J Biol Chem 278: 21989–21997, 2003. [DOI] [PubMed] [Google Scholar]
- 41.Sibilia M, Fleischmann A, Behrens A, Stingl L, Carroll J, Watt FM, Schlessinger J, Wagner EF. The EGF receptor provides an essential survival signal for SOS-dependent skin tumor development. Cell 102: 211–220, 2000. [DOI] [PubMed] [Google Scholar]
- 42.Sinha A, Nightingale J, West KP, Berlanga-Acosta J, Playford RJ. Epidermal growth factor enemas with oral mesalamine for mild-to-moderate left-sided ulcerative colitis or proctitis. N Engl J Med 349: 350–357, 2003. [DOI] [PubMed] [Google Scholar]
- 43.Tartaglia LA, Ayres TM, Wong GH, Goeddel DV. A novel domain within the 55 kd TNF receptor signals cell death. Cell 74: 845–853, 1993. [DOI] [PubMed] [Google Scholar]
- 44.Tartaglia LA, Pennica D, Goeddel DV. Ligand passing: the 75-kDa tumor necrosis factor (TNF) receptor recruits TNF for signaling by the 55-kDa TNF receptor. J Biol Chem 268: 18542–18548, 1993. [PubMed] [Google Scholar]
- 45.Tartaglia LA, Rothe M, Hu YF, Goeddel DV. Tumor necrosis factor's cytotoxic activity is signaled by the p55 TNF receptor. Cell 73: 213–216, 1993. [DOI] [PubMed] [Google Scholar]
- 46.Tcherkasowa AE, Adam-Klages S, Kruse ML, Wiegmann K, Mathieu S, Kolanus W, Kronke M, Adam D. Interaction with factor associated with neutral sphingomyelinase activation, a WD motif-containing protein, identifies receptor for activated C-kinase 1 as a novel component of the signaling pathways of the p55 TNF receptor. J Immunol 169: 5161–5170, 2002. [DOI] [PubMed] [Google Scholar]
- 47.Tokumaru S, Higashiyama S, Endo T, Nakagawa T, Miyagawa JI, Yamamori K, Hanakawa Y, Ohmoto H, Yoshino K, Shirakata Y, Matsuzawa Y, Hashimoto K, Taniguchi N. Ectodomain shedding of epidermal growth factor receptor ligands is required for keratinocyte migration in cutaneous wound healing. J Cell Biol 151: 209–220, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tonks NK, Diltz CD, Fischer EH. Purification of the major protein-tyrosine-phosphatases of human placenta. J Biol Chem 263: 6722–6730, 1988. [PubMed] [Google Scholar]
- 49.Vandenabeele P, Declercq W, Beyaert R, Fiers W. Two tumour necrosis factor receptors: structure and function. Trends Cell Biol 5: 392–399, 1995. [DOI] [PubMed] [Google Scholar]
- 50.Vergarajauregui S, San Miguel A, Puertollano R. Activation of p38 mitogen-activated protein kinase promotes epidermal growth factor receptor internalization. Traffic 7: 686–698, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Waetzig GH, Seegert D, Rosenstiel P, Nikolaus S, Schreiber S. p38 mitogen-activated protein kinase is activated and linked to TNF-alpha signaling in inflammatory bowel disease. J Immunol 168: 5342–5351, 2002. [DOI] [PubMed] [Google Scholar]
- 52.Waterman H, Sabanai I, Geiger B, Yarden Y. Alternative intracellular routing of ErbB receptors may determine signaling potency. J Biol Chem 273: 13819–13827, 1998. [DOI] [PubMed] [Google Scholar]
- 53.Werb Z, Yan Y. A cellular striptease act. Science 282: 1279–1280, 1998. [DOI] [PubMed] [Google Scholar]
- 54.Whitehead RH, VanEeden PE, Noble MD, Ataliotis P, Jat PS. Establishment of conditionally immortalized epithelial cell lines from both colon and small intestine of adult H-2Kb-tsA58 transgenic mice. Proc Natl Acad Sci USA 90: 587–591, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wiley HS Trafficking of the ErbB receptors and its influence on signaling. Exp Cell Res 284: 78–88, 2003. [DOI] [PubMed] [Google Scholar]
- 56.Worthylake R, Opresko LK, Wiley HS. ErbB-2 amplification inhibits down-regulation and induces constitutive activation of both ErbB-2 and epidermal growth factor receptors. J Biol Chem 274: 8865–8874, 1999. [DOI] [PubMed] [Google Scholar]
- 57.Xia Z, Dickens M, Raingeaud J, Davis RJ, Greenberg ME. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science 270: 1326–1331, 1995. [DOI] [PubMed] [Google Scholar]
- 58.Yan F, Polk DB. Probiotic bacterium prevents cytokine-induced apoptosis in intestinal epithelial cells. J Biol Chem 277: 50959–50965, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zwang Y, Yarden Y. p38 MAP kinase mediates stress-induced internalization of EGFR: implications for cancer chemotherapy. EMBO J 25: 4195–4206, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zyzak LL, MacDonald LM, Batova A, Forand R, Creek KE, Pirisi L. Increased levels and constitutive tyrosine phosphorylation of the epidermal growth factor receptor contribute to autonomous growth of human papillomavirus type 16 immortalized human keratinocytes. Cell Growth Differ 5: 537–547, 1994. [PubMed] [Google Scholar]