Abstract
During acetaminophen (APAP) hepatotoxicity, increased expression of multidrug resistance-associated proteins 2, 3, and 4 (Mrp2-4) occurs. Mrp4 is the most significantly upregulated transporter in mouse liver following APAP treatment. Although the expression profiles of liver transporters following APAP hepatotoxicity are well characterized, the regulatory mechanisms contributing to these changes remain unknown. We hypothesized that Kupffer cell-derived mediators participate in the regulation of hepatic transporters during APAP toxicity. To investigate this, C57BL/6J mice were pretreated with clodronate liposomes (0.1 ml iv) to deplete Kupffer cells and then challenged with APAP (500 mg/kg ip). Liver injury was assessed by plasma alanine aminotransferase and hepatic transporter protein expression was determined by Western blot and immunohistochemistry. Depletion of Kupffer cells by liposomal clodronate increased susceptibility to APAP hepatotoxicity. Although increased expression of several efflux transporters was observed after APAP exposure, only Mrp4 was found to be differentially regulated following Kupffer cell depletion. At 48 and 72 h after APAP dosing, Mrp4 levels were increased by 10- and 33-fold, respectively, in mice receiving empty liposomes. Immunohistochemistry revealed Mrp4 staining confined to centrilobular hepatocytes. Remarkably, Kupffer cell depletion completely prevented Mrp4 induction by APAP. Elevated plasma levels of TNF-α and IL-1β were also prevented by Kupffer cell depletion. These findings show that Kupffer cells protect the liver from APAP toxicity and that Kupffer cell mediators released in response to APAP are likely responsible for the induction of Mrp4.
Keywords: multidrug resistance protein, hepatotoxicity, macrophage, transporter, cytokine, Abcc4
acetaminophen (APAP) ingestion accounts for 40% of all cases of acute liver failure in the United States and the incidence of both intentional and unintentional APAP overdoses has been increasing in recent years (28, 30). When taken at supratherapeutic doses, more APAP is bioactivated by cytochrome P-450s (Cyp450s) to the reactive metabolite N-acetyl-p-benzoquinone imine (NAPQI) than under therapeutic doses, resulting in centrilobular hepatic damage. This hepatocellular injury is multifactorial, resulting from oxidative stress and formation of protein covalent adducts, ultimately leading to cell death. In addition to these factors, activation of resident macrophages in the liver, known as Kupffer cells, has been implicated in APAP hepatotoxicity (29, 35).
Until recently, the general consensus was that following their activation, Kupffer cells release inflammatory mediators, including cytokines, chemokines, growth factors, and reactive oxygen species, which promote the progression of liver injury (29, 35). Recently, liposome-encapsulated clodronate (clodronate liposomes) have been used to investigate Kupffer cell functions. Clodronate liposomes are phagocytosed by Kupffer cells, subsequently leading to their apoptosis (45). Depletion of Kupffer cells using clodronate liposomes sensitizes mice to APAP hepatotoxicity (25). The hepatoprotective role of Kupffer cells in APAP toxicity has been attributed to the production and release of several protective mediators, primarily the acute phase cytokines tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6), in addition to IL-10, IL-1β, and cyclooxygenase-2 (COX-2)-derived mediators (9–11, 33, 37, 42, 49).
Previous work in our laboratory has documented the simultaneous increase in expression of efflux transporters and decrease in expression of uptake transporters in mice during liver injury induced by APAP and carbon tetrachloride (CCl4). More specifically, mRNA and protein expression of multidrug resistance-associated proteins 2-4 (Mrp2-4) are increased following APAP. Sodium taurocholate cotransporting polypeptide (Ntcp) expression is reduced following exposure to a hepatotoxic dose of CCl4 (1, 2). Decreased expression of organic anion-transporting polypeptides (Oatps) was observed at the mRNA level following exposure to either toxicant (2). Similar changes in efflux transporter expression have been observed in the livers of humans exposed to hepatotoxic doses of APAP (5).
Altered transporter expression is hypothesized to be a compensatory response to liver injury and may contribute to the acquired resistance exhibited by mice that are reexposed to even higher doses of APAP. This phenomenon is known as autoprotection. We have hypothesized that transport protein induction contributes to this acquired resistance by enhancing the export of toxic substrates or signaling molecules necessary for communication between adjacent hepatocytes and other cell types in the liver during critical periods of cell replication and tissue repair. Although the expression profile of hepatic transport proteins following drug-induced liver injury has been well characterized, the signaling pathways mediating these changes remain to be determined.
A substantial amount of evidence supports a role for cytokines in the regulation of hepatic transporter expression in acute liver injury. The expression of several cytokines including TNF-α, IL-1β, IL-6, IL-10, and IL-13 increases in the liver following APAP-induced hepatotoxicity (9, 16, 33, 48). Cytokines have been implicated in the regulation of transporter expression in mice with cholestatic liver disease (6, 17, 19). Additionally, depletion of Kupffer cells prevents the downregulation of Ntcp mRNA and protein following endotoxin exposure in rats and also blocks decreases in Oatp1a1, Oatp1a4, Oatp1b2, and bile salt export pump mRNA that occur during hepatic ischemia-reperfusion in mice (41, 43). Together, these data suggest a role for inflammatory cytokines in the regulation of transporter expression following chemical liver injury.
Since Kupffer cells are the main source of cytokines and other inflammatory mediators in the liver, the aim of this study was to determine the collective involvement of Kupffer-cell-derived mediators in the regulation of hepatic transporter expression during APAP-induced liver injury. To study the contribution of signaling molecules originating from Kupffer cells in the regulation of hepatocellular transporters, mice were treated with clodronate liposomes or empty liposomes prior to administration of a hepatotoxic dose of APAP. Protein expression levels of several uptake (Oatp1a1, Oatp1a4, Oatp1b2, Ntcp) and efflux (Mrp1-4, P-glycoprotein) transport proteins were determined by Western blot analysis at different time points after APAP. Zonal localization of Mrp4 protein, which was differentially regulated in Kupffer cell-depleted and nondepleted mouse livers, was determined immunohistochemically. Our findings support a role for Kupffer cell-derived mediators in the regulation of hepatic transport protein expression during drug-induced liver injury and warrant further studies to identify the specific mediators involved in this response.
MATERIALS AND METHODS
Animal care and treatment.
Male 10- to 12-wk-old C57BL/6J mice were purchased from Jackson Laboratories (Bar Harbor, ME). Upon arrival, mice were acclimated for 1 wk and maintained in a 12-h dark-light cycle, temperature- and humidity-controlled environment. Clodronate was a gift of Roche Diagnostics (Mannheim, Germany). It was encapsulated in liposomes as previously described (44). Mice were dosed intravenously with 100 μl of clodronate liposomes or empty liposomes 48 h prior to APAP treatment. Animals were fasted for 16–18 h prior to challenge with APAP. APAP was dissolved in 50% propylene glycol-water. Groups of mice (n = 3–6) were administered APAP (500 mg/kg, 5 ml/kg ip) or vehicle control. Livers were collected 48 h after liposome treatment (at time zero of APAP administration) and also 6, 24, 48, or 72 h after APAP. A portion of the liver was fixed in 10% zinc formalin and the remaining liver tissue was snap frozen in liquid nitrogen. Frozen tissues were stored at −80°C until assayed. All animal studies were conducted in accordance with National Institutes of Health standards and the Guide for the Care and Use of Laboratory Animals. Studies were approved by the University of Connecticut Institutional Animal Care and Use Committee (IACUC Protocol no. A06-028).
Assessment of Kupffer cell depletion.
To determine the Kupffer cell status in the liver of clodronate and empty liposome-treated mice, livers were assayed for phagocytic activity and for expression of the macrophage cell surface marker, F4/80 (3). At 48 h after liposome treatment only, India ink was administered to mice (100 μl iv) to determine the phagocytic activity in the liver. Carbon particles (India ink) are internalized and accumulate within macrophages following intravenous administration (46). At 20 min after ink injection the livers were removed, fixed in 10% zinc formalin, embedded in paraffin, and stained with hematoxylin and eosin. F4/80 staining was performed on liver sections collected 48 h after liposome-only treatment and at all time points (6, 24, 48, 72 h) after APAP treatment as described previously (24). Briefly, sections were incubated with rat anti-mouse F4/80 antibody (ab6640, Abcam, Cambridge, MA) diluted 1:50 for 30 min. Protein antibody complexes were visualized by use of the rat Vectastain Elite ABC kit and developed with 3,3′-diaminobenzidine (DAB) according to the manufacturer's instructions (Vector Laboratories, Burlingame, CA). Tissues were counterstained with hematoxylin. Negative control staining was performed by incubating sections with rat IgG control antibody (Vector Laboratories). The uptake of carbon particles and F4/80-positive cells in the livers were detected by light microscopy. Liver sections were imaged with a Nikon Optiphot microscope (Donsanto, Natick, MA) equipped with a Spot Insight 2MP Firewire Color Mosaic Camera and Spot Software v4.5 (Diagnostic Instruments, Sterling Heights, MI).
ALT activity assay.
Infinity ALT Liquid Stable Reagent (Thermotrace, Melbourne, Australia) was used to determine plasma alanine aminotransferase (ALT) activity as a biochemical indicator of hepatocellular necrosis according to the manufacturer's protocol.
Histopathology.
Liver samples were fixed in 10% zinc formalin followed by paraffin embedding. Liver sections (5 μm) were stained with hematoxylin and eosin. Sections were examined by light microscopy for the presence and severity of hepatocellular degeneration and necrosis. Centrilobular liver injury was scored by a grading system described previously (32).
Preparation of microsomal and crude membrane fractions.
Liver plasma membrane and microsomal fractions were prepared as previously described (1). The resulting pellets of the crude membrane or microsomal fraction were resuspended in sucrose-Tris buffer. Protein concentration was determined by use of Bio-Rad protein assay reagents (Bio-Rad Laboratories, Hercules, CA).
APAP in vitro activation and glucuronidation.
The capacity of liver microsomes to metabolize APAP into NAPQI or APAP-glucuronide (GLUC) was measured as described by Manautou et al. (32) and Court and Greenblatt (12) respectively, with modifications. Briefly, at 48 h after treatment with empty or clodronate liposomes, mouse liver microsomes were prepared and incubated for 30 min with 0.83 mM NADP, 15 mM MgCl2, 20 mM glucose-6-phosphate, 4 IU glucose-6-phosphate dehydrogenase, and 20 mM APAP. To measure the in vitro activation of APAP to its reactive metabolite NAPQI, microsomal incubations were supplemented with N-acetyl cysteine (NAC) and the resulting APAP-NAC conjugate was extracted and analyzed by HPLC as described previously (32). To assess APAP glucuronidation, microsomal incubations were supplemented with uridine diphosphoglucuronic acid and the resulting APAP-GLUC conjugate was also analyzed by HPLC as described previously (32). The concentrations of APAP-NAC and APAP-GLUC in these incubations were determined by comparison to a standard curve of known APAP concentrations.
Hepatic NPSH assay.
Hepatic nonprotein sulfhydryl (NPSH) content in whole liver homogenates was measured as an indicator of reduced glutathione (GSH) content. The colorimetric procedure of Ellman was used for NPSH quantification (15, 32). Hepatic NPSH content was determined by comparison to a GSH standard curve. GSH comprises ∼95% of all the nonprotein sulfhydryls in liver, and correspondence between HPLC analysis for GSH and the Ellman assay is 96.8% (20).
Western blot analysis.
Membrane proteins were electrophoretically resolved by using polyacrylamide gels and transblotted overnight at 4°C onto PVDF-Plus membrane (Micron Separations). Immunochemical detection of Cyp2e1, Cyp3a11, Cyp1a2, UDP-glucuronosyltransferase (Ugt) 1a6, glutamate-cysteine ligase catalytic subunit (Gclc), glutamate-cysteine ligase modifier subunit (Gclm), Oatp1a1, Oatp1a4, Oatp1b2, Ntcp, Mrp1-4, P-glycoprotein (P-gp), and β-actin proteins was carried out using conditions described in Table 1 and as described by Aleksunes et al. (1). Protein bands were detected and quantified using Quantity One 1-D Analysis Software (Bio-Rad Laboratories).
Table 1.
Antibody sources and dilutions used for Western blot analysis
| Antigen | MW, kDa | Antibody | Source | Primary Ab Concentration |
|---|---|---|---|---|
| Cyp1a2 | ∼60 | sc-30085 | Santa Cruza | 1:1,000 |
| Cyp2e1 | ∼60 | ab28146 | Abcamb | 1:5,000 |
| Cyp3a11 | ∼45 | PA1-343 | Affinity Bio.c | 1:5,000 |
| Ugt1a6 | ∼60 | sc-27434 | Santa Cruza | 1:1,000 |
| Gcc | ∼75 | — | Washingtond | 1:20,000 |
| Gclm | ∼28 | — | Washingtond | 1:20,000 |
| Oatp1a1 | ∼80 | KS 219 | KUMCe | 1:1,000 |
| Oatp1a4 | ∼80 | KS 221 | KUMCe | 1:1,000 |
| Oatp1b2 | ∼85 | KS 223 | KUMCe | 1:2,000 |
| Ntcp | ∼51 | K4 | Univ. Hospitalf | 1:5,000 |
| Mrp1 | ∼190 | MRPr1 | VUMCg | 1:2,000 |
| Mrp2 | ∼190 | M2III-5 | VUMCg | 1:600 |
| Mrp3 | ∼180 | M3II-2 | VUMCg | 1:2,000 |
| Mrp4 | ∼160 | M4I-10 | VUMCg | 1:2,000 |
| P-gp | ∼170 | C219 | Abcamb | 1:2,000 |
| b-actin | ∼45 | ab8227 | Abcamb | 1:2,000 |
Santa Cruz Biotechnologies, Santa Cruz, CA;
Abcam, Cambridge, MA;
Affinity Bioreagents, Golden, CO;
University of Washington, Seattle, WA, Terry Kavanagh;
University of Kansas Medical Center, Kansas City, KS, Curtis Klaassen;
University Hospital, Zurich, Switzerland, Bruno Stieger;
Vrije Universiteit Medical Center, Amsterdam, The Netherlands, George Scheffer.
Mrp4 immunohistochemistry.
Immunohistochemical detection of Mrp4 protein was performed in sections of formalin-fixed, paraffin-embedded livers as described above for the detection of F4/80 protein with the following modifications. Antigen retrieval was performed by incubating slides in citrate buffer (10 mM) at 95°C for 10 min. Sections were incubated with rat anti-mouse Mrp4 antibody (M4I-10) diluted 1:100 overnight at 4°C. Protein antibody complexes were visualized by use of the rat Vectastain Elite ABC kit and developed with DAB according to the manufacturer's instructions (Vector Laboratories). Tissues were counterstained with hematoxylin. Negative control staining was performed by incubating sections with rat IgG control antibody (Vector Laboratories). Images were captured as described for F4/80 immunohistochemistry.
Measurement of plasma cytokines and chemokines.
Plasma levels of IL-1β, IL-4, IL-6, IL-10, IL-13, granulocyte colony-stimulating factor (GM-CSF), IFN-γ, keratinocyte-derived chemokine (KC), MCP-1, and TNF-α were simultaneously measured by a multiplex antibody assay (Bio-Plex Mouse Cytokine Assay, Bio-Rad Laboratories) according to the manufacturer's protocol. The fluorescence of each reaction mixture was detected via a Luminex 200 instrument with Luminex IS 100 software (Luminex, Austin, TX). Cytokine concentrations were determined by comparison to standard curves derived from recombinant cytokine standards.
Statistical analysis.
For protein data, semiquantitative results are expressed as mean protein expression ± standard error, and the data are normalized to pooled control values (0 h). Data were analyzed by either the Student's t-test or a one-way analysis of variance followed by Newman-Keuls multiple-range test where appropriate. P values <0.05 were considered significant.
RESULTS
Clodronate liposome-induced Kupffer cell depletion.
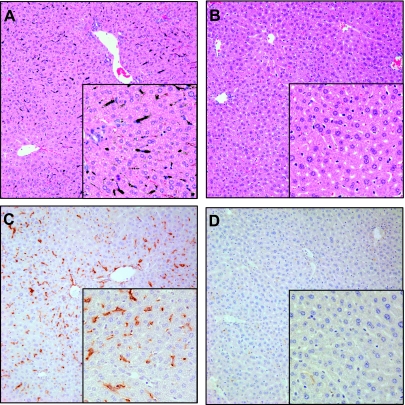
Clodronate liposomes depleted Kupffer cells throughout the liver lobule by 48 h as measured by uptake of carbon particles. Substantial uptake of carbon particles was observed in empty liposome-treated mice (Fig. 1A). This was inhibited by treatment with clodronate liposomes (Fig. 1B). The effect of liposomal clodronate on Kupffer cells was further confirmed by staining for F4/80, a cell surface marker for murine macrophages. Although numerous F4/80-positive macrophages are seen in the livers of empty liposome-treated mice (Fig. 1C), staining appears to be completely absent after clodronate liposome treatment (Fig. 1D). Immunostaining of livers collected 24–72 h after APAP exposure demonstrated that F4/80-positive Kupffer cells were depleted in clodronate liposome-treated mice throughout the study (data not shown). Similar to a prior report (47), a small number of F4/80-positive macrophages began to reappear at 72 h after APAP treatment or 5 days following clodronate treatment (data not shown). No reactivity was detected with isotope control antibody (data not shown).
Fig. 1.
Depletion of Kupffer cells by clodronate liposome treatment. Mice were injected with empty liposomes (A and C) or clodronate liposomes (B and D). After 48 h some mice were euthanized 20 min after injection of India ink for the determination of carbon particle uptake in hematoxylin and eosin-stained liver sections (A and B). Other mice were euthanized at 48 h after liposome treatment and liver sections were stained with F4/80 antibody (C and D). Images were taken at ×20 and ×40 (insets) magnification.
Effect of Kupffer cell depletion on APAP-induced hepatotoxicity.
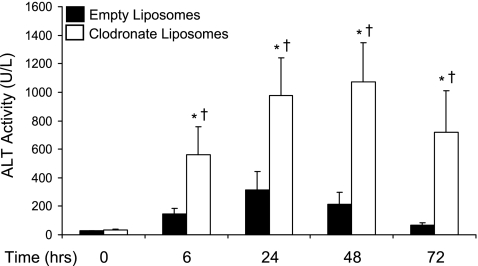
Liver injury was significantly higher in clodronate liposome-pretreated mice compared with empty liposome controls at all time points after APAP treatment (Fig. 2). In mice with normal Kupffer cell function, ALT values peaked at 24 h after APAP treatment (mean ALT 315 U/l) and began to return to normal thereafter. Kupffer cell depleted mice exhibited peak ALT activity at 48 h following APAP exposure (mean ALT 1071 U/l), which remained elevated through the 72-h time point. Similarly, elimination of Kupffer cells with clodronate liposomes resulted in higher severity of hepatocellular necrosis, as determined by histopathological analysis (Table 2). Livers of empty liposome-pretreated mice exhibited milder damage compared with clodronate liposome-pretreated mice. In nearly all time points, a higher percentage of clodronate liposome-pretreated mice had a more severe histopathology grade of 2.
Fig. 2.
Plasma alanine aminotransferase (ALT) activity after acetaminophen (APAP) treatment. Plasma was isolated from empty or clodronate liposome-pretreated mice 6, 24, 48, and 72 h following dosing with APAP (500 mg/kg) or vehicle. The data are presented as mean plasma ALT (U/l) ± SE (n = 3–12 animals). *Statistical difference (P < 0.05) from pooled control mice of the same liposome treatment (0 h); †statistical difference (P < 0.05) from empty liposome APAP-treated mice.
Table 2.
Histopathological analysis of liver injury after APAP treatment
| Treatment |
Histopathology Grade |
% ≥2 | |||||
|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | ||
| 6 h | |||||||
| Empty liposome control | 3 | 0% (0/3) | |||||
| Empty liposome APAP* | 1 | 4 | 0% (0/5) | ||||
| Clodronate liposome control | 3 | 0% (0/3) | |||||
| Clodronate liposome APAP* | 4 | 0% (0/4) | |||||
| 24 h | |||||||
| Empty liposome control | 3 | 0% (0/3) | |||||
| Empty liposome APAP* | 4 | 2 | 33% (2/6) | ||||
| Clodronate liposome control | 3 | 0% (0/3) | |||||
| Clodronate liposome APAP*† | 1 | 5 | 83% (5/6) | ||||
| 48 h | |||||||
| Empty liposome control | 3 | 0% (0/3) | |||||
| Empty liposome APAP* | 2 | 4 | 66% (4/6) | ||||
| Clodronate liposome control | 3 | 0% (0/3) | |||||
| Clodronate liposome APAP* | 1 | 4 | 80% (4/5) | ||||
| 72 h | |||||||
| Empty liposome control | 3 | 0% (0/3) | |||||
| Empty liposome APAP* | 3 | 3 | 50% (3/6) | ||||
| Clodronate liposome control | 3 | 0% (0/3) | |||||
| Clodronate liposome APAP* | 2 | 3 | 60% (3/5) | ||||
The table shows the number of mice receiving the indicated histological grade per treatment group. Mice pretreated with empty or clodronate liposomes received 500 mg/kg acetaminophen (APAP) or vehicle. After 6, 24, 48, and 72 h mice were euthanized and a portion of the liver was fixed in 10% zinc formalin, paraffin embedded, and stained with hematoxylin and eosin. Liver sections were analyzed by light microscopy and scored (0–5) according to the severity of degenerative and necrotic damage as previously described (32).
Statistical difference (P < 0.05) from pooled control mice of the same liposome treatment (0 h);
statistical difference (P < 0.05) from empty liposome APAP-treated mice.
Effect of Kupffer cell depletion on APAP bioactivation.
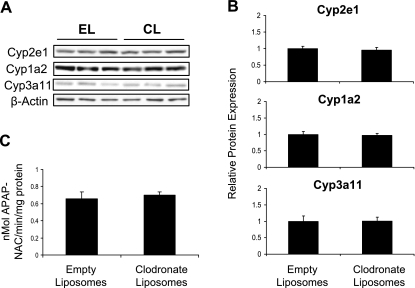
To determine whether the increased susceptibility of Kupffer cell-depleted mice to APAP toxicity might be attributed to heightened activity of APAP bioactivation pathways, expression and function of the major Cyp450 enzymes responsible for the metabolism of APAP to the reactive intermediate NAPQI in the liver were investigated at 48 h following liposome dosing. Mice used for these analyses did not receive APAP. Expression of Cyp2e1, Cyp1a2, and Cyp3a11 proteins was not altered by clodronate liposome treatment (Fig. 3, A and B). Similarly, liver microsomes from empty and clodronate liposome-treated mice had the same capacity to bioactivate APAP to NAPQI (Fig. 3C). In support of these observations, a previous study demonstrated similar APAP-protein adducts formed in livers from empty and clodronate liposome-pretreated mice (25). Therefore, the increased susceptibility of Kupffer cell-depleted mice to APAP hepatotoxicity does not appear to involve increases in metabolic activation of APAP to its reactive metabolite.
Fig. 3.
Analysis of APAP bioactivation pathways in Kupffer cell-depleted and nondepleted mice. Mice were injected with empty liposomes (EL) or clodronate liposomes (CL) and euthanized after 48 h. Western blots were performed by using liver membrane fractions. The data are presented as representative blots with each lane representing an individual mouse (A) and as mean relative Cyp2e1, Cyp1a2, or Cyp3a11 protein expression ± SE (n = 3) as quantified from the blots in A (B). Microsomes were prepared and assayed for APAP bioactivation (C), with data presented as means ± SE (n = 3).
Effect of Kupffer cell depletion on APAP detoxification pathways.
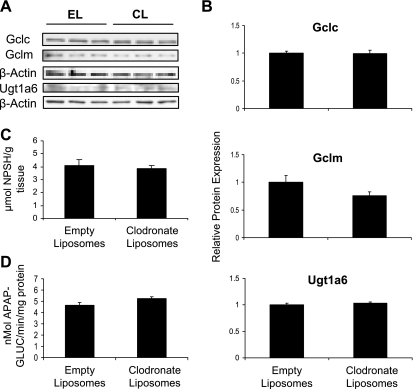
Alternatively, the enhanced susceptibility of Kupffer cell-depleted mice to APAP hepatotoxicity could be attributed to reduced APAP detoxification. Therefore, we investigated the capacity of the livers from these mice to detoxify APAP by conjugation with GSH and by glucuronidation. Glutamate cysteine ligase is the rate-limiting enzyme in GSH synthesis. Protein expression of the two subunits of glutamate cysteine ligase, Gclc and Gclm, was not altered by clodronate liposome treatment (Fig. 4, A and B). In agreement with this, there is no difference in hepatic nonprotein sulfhydryl content between Kupffer cell-depleted and nondepleted mice (Fig. 4C). Protein expression of Ugt1a6, a major Ugt involved in APAP glucuronidation in mouse liver, was similar between empty and clodronate liposome-treated mice (Fig. 4, A and B). Similarly, in vitro APAP glucuronidation was the same in both groups of mice (Fig. 4D).
Fig. 4.
Analysis of APAP detoxification pathways in Kupffer-cell depleted and nondepleted mice. Mice were injected with empty liposomes or clodronate liposomes and euthanized after 48 h. Western blots were performed by using cytosolic fractions (for Gclc and Gclm) or liver membrane fractions (for Ugt1a6). The data are presented as representative blots with each lane representing an individual mouse (A) and as mean relative Gclc, Gclm, or Ugt1a6 protein expression ± SE (n = 3) as quantified from the blots in A (B). Hepatic nonprotein sulfhydryl (NPSH) content was determined (C), and microsomes were prepared and assayed for APAP glucuronidation (D). The NPSH and glucuronidation data are presented as means ± SE (n = 3).
Western blot analysis of uptake transport proteins.
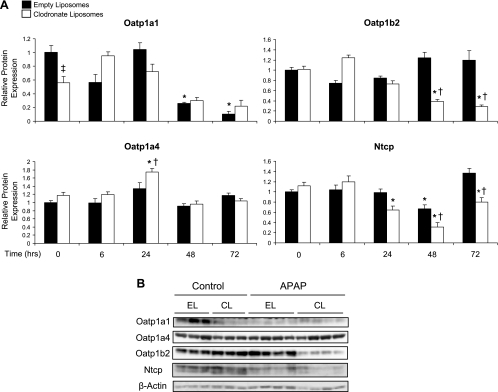
To evaluate the effect of Kupffer cell depletion on APAP-induced changes in uptake transporter expression, Western blot analysis was performed. Basal expression of Oatp1a1 was lower in Kupffer cell-depleted mice (56% of nondepleted controls). Oatp1a1 protein expression was reduced at 48 and 72 h following APAP treatment (26 and 10% of control, respectively) in empty liposome-pretreated mice (Fig. 5A). Lower Oatp1a1 protein levels were also seen in clodronate liposome-pretreated mice. However, these changes were not statistically significant. Oatp1a4 protein levels were slightly increased (1.75-fold) at 24 h in clodronate liposome-pretreated mice, whereas Oatp1b2 protein expression was reduced to 39 and 28% of control at 48 and 72 h, respectively. Ntcp protein expression was reduced in both Kupffer cell-depleted and nondepleted mouse livers at 48 h after APAP treatment (66 and 65% of control, respectively) (Fig. 5A). Ntcp protein levels remained reduced in clodronate liposome-pretreated mice at 72 h (80% of control). Representative 48-h Western blots are shown in Fig. 5B.
Fig. 5.
Western blot analysis of hepatic uptake transport proteins. Western blots were performed by using liver membrane fractions from liposome-pretreated mice at 6, 24, 48, and 72 h after APAP (500 mg/kg) or vehicle treatment (control). The data are presented as mean relative Oatp1a1, Oatp1a4, Oatp1b2, or Ntcp protein expression ± SE (n = 4–9) (A) and as representative blots at 48 h, with each lane representing an individual mouse (B). *Statistical difference (P < 0.05) from pooled control (0 h) mice of the same liposome treatment; †statistical difference (P < 0.05) from empty liposome APAP-treated mice; ‡statistical difference (P < 0.05) from empty liposome control mice.
Western blot analysis of efflux transport proteins.
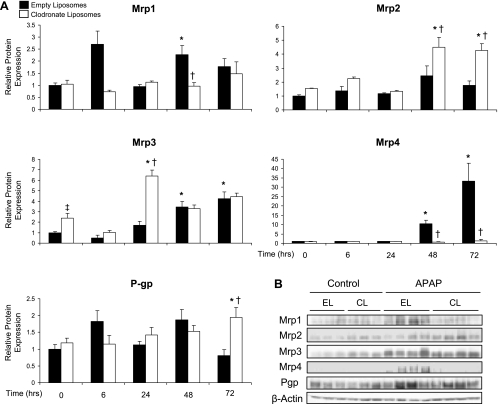
A similar pattern of increased expression of efflux transporters to that previously reported by our laboratory was observed here following Western blot analysis of crude liver membrane preparations (1). Mrp1 protein expression was significantly increased in empty liposome-pretreated mice (2.3-fold) 48 h after APAP, but not in clodronate liposome-pretreated mice (Fig. 6A). Mrp2 protein was elevated by ∼4.5-fold at 48 and 72 h in Kupffer cell-depleted mice. Elevated Mrp3 levels were observed after APAP in both groups of pretreated mice. Also, Mrp3 expression was 2.4-fold higher in clodronate liposome-treated mice than in empty liposome controls that did not receive APAP. Interestingly, Mrp4 was the most highly upregulated efflux transporter following APAP exposure in empty liposome-pretreated mice (10.4- and 33.3-fold at 48 and 72 h, respectively), and this upregulation was completely prevented by elimination of Kupffer cells (Fig. 6A). In addition to these changes, P-gp protein expression was elevated twofold at 72 h in clodronate liposome-pretreated mice. Representative 48-h Western blots are shown in Fig. 6B.
Fig. 6.
Western blot analysis of hepatic efflux transport proteins. Western blots were performed by using liver membrane fractions from liposome-pretreated mice at 6, 24, 48, and 72 h after APAP (500 mg/kg) or vehicle treatment (control). The data are presented as mean relative Mrp1, Mrp2, Mrp3, Mrp4, or P-gp protein expression ± SE (n = 4–9) (A) and as representative blots at 48 h with each lane representing an individual mouse (B). *Statistical difference (P < 0.05) from pooled control (0 h) mice of the same liposome treatment; †statistical difference (P < 0.05) from empty liposome APAP-treated mice; ‡statistical difference (P < 0.05) from empty liposome control mice.
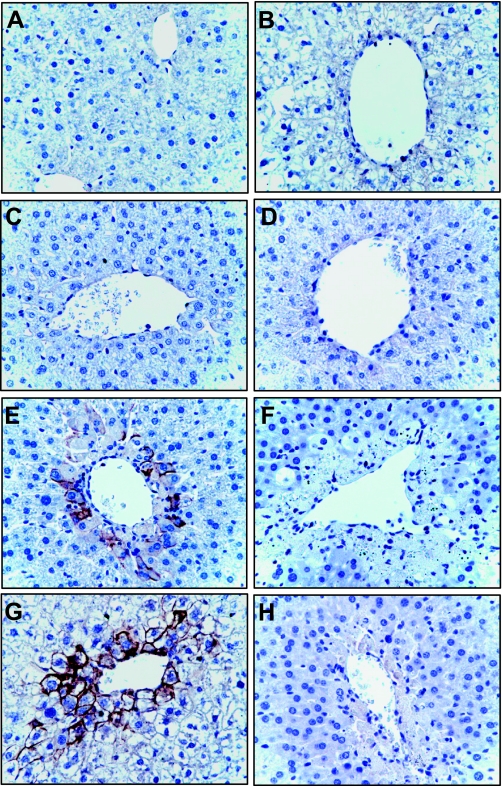
Immunohistochemical localization of Mrp4.
Mrp4 protein expression was further investigated immunochemically due to its differential regulation in the livers of Kupffer cell-depleted and nondepleted mice. Mrp4 protein expression is extremely low in normal mouse liver, as is seen by the minimal staining in control livers in Fig. 7, A and B. No change in Mrp4 staining was seen at 24 h after APAP in either treatment group (Fig. 7, C and D). By contrast, strong Mrp4 staining can be observed in centrilobular hepatocytes colocalized with damage 48 h after APAP treatment in livers with normal Kupffer cell function (Fig. 7E). Mrp4 staining intensity is even greater at 72 h in the same treatment group (Fig. 7G). By contrast, Kupffer cell-depleted livers exhibited minimal Mrp4 staining at all time points after APAP treatment (Fig. 7, D, F, and H). No reactivity was detected with isotope control antibody (data not shown).
Fig. 7.
Immunohistochemical analysis of Mrp4. Mice pretreated with empty liposomes (A, C, E, and G) and clodronates liposome (B, D, F, and H) were challenged with APAP (500 mg/kg) or vehicle (A and B) and euthanized at 24 (C and D), 48 (E and F), and 72 (G and H) h. Mrp4 staining was performed on formalin fixed paraffin-embedded liver sections. Tissues were counterstained with hematoxylin and eosin. Images were taken at ×40 magnification.
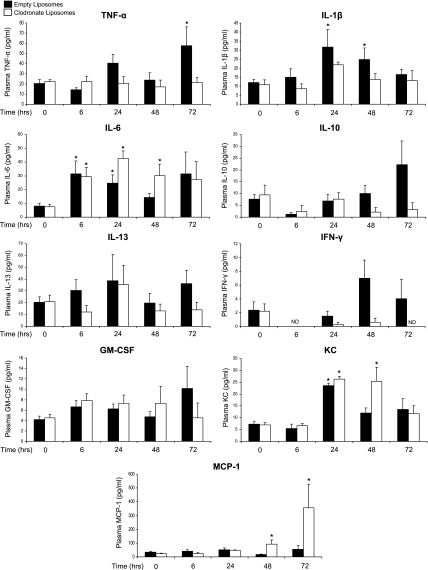
Plasma cytokine and chemokine levels after APAP treatment in Kupffer-cell depleted mice.
Alterations in liver cytokine and chemokine production with Kupffer cell depletion may contribute to the differential regulation of Mrp4 and susceptibility to APAP toxicity. Therefore, plasma levels of several cytokines and chemokines were measured via multiplex cytokine assays. APAP treatment increased plasma levels of TNF-α, IL-1β, IL-6, and KC (Fig. 8). Trends toward elevated IL-10 and IFN-γ were also observed. Elimination of Kupffer cells prevented the increase in plasma TNF-α and IL-1β, whereas IL-6 levels were similarly elevated in both groups of APAP-treated mice at 6 and 24 h. MCP-1 plasma levels were highly increased in Kupffer cell-depleted mice at 48 and 72 h after APAP. No change in IL-13 or GM-CSF was detected, and IL-4 was not detected in any of the treatment groups.
Fig. 8.
Effect of Kupffer cell depletion on APAP-induced cytokine and chemokine plasma levels. Mice were pretreated with either empty or clodronate liposomes and challenged with APAP (500 mg/kg) or vehicle. Plasma was collected at all time points after APAP administration (0–72 h). Plasma levels of IL-1β, IL-4, IL-6, IL-10, IL-13, GM-CSF, IFN-γ, KC, MCP-1, and TNF-α were measured by Bio-plex Cytokine Assays. The data are presented as means ± SE (n = 3–6 animals). ND, not detectable. *Statistical difference (P < 0.05) from pooled control mice of the same liposome treatment (0 h).
DISCUSSION
In the present study, the role of Kupffer cell function in altering expression of hepatic transport proteins during drug-induced liver injury was examined. We have previously documented the induction of Mrp2, 3, and 4 efflux transporters in mouse liver after toxic APAP treatment. These transporters are responsible for the export of a wide variety of endo- and xenobiotics across the canalicular (Mrp2) and basolateral (Mrp3, 4) membranes of hepatocytes (1, 2). The heightened susceptibility of Kupffer cell-depleted mice to APAP hepatotoxicity confirms previous findings by Ju et al. (25). Administration of clodronate liposomes to achieve selective in vivo depletion of Kupffer cells was similarly used here to investigate the impact of Kupffer cells on the expression of hepatic transporters during APAP-induced liver injury. As demonstrated before, treatment of mice with clodronate liposomes was effective in eliminating F4/80-positive cells from all regions of the liver lobule (25, 45).
The cascade of events that occurs during APAP hepatotoxicity involves interaction and communication among the different cell types within the liver. These interactions may influence the balance between hepatocyte death or recovery. As seen in this study and in previous work, the presence of Kupffer cells is critical for tipping this balance in the direction of recovery and repair (25). In the past, Kupffer cell function and activation has been reported to exacerbate APAP-induced liver injury. This comes from studies in rodents receiving gadolinium chloride prior to APAP. Although gadolinium chloride also depletes Kupffer cells, it only targets a subpopulation of cells and its mechanism of inactivation is different from that of clodronate liposomes (18, 25, 27, 29, 35). In this study, the altered susceptibility of Kupffer cell-depleted mice to APAP toxicity was not due to compensatory changes in metabolic pathways involved in APAP bioactivation or detoxification. The higher toxicity in Kupffer cell-depleted mice is not due to altered transport of APAP or its conjugates either, since the majority of the administered dose of APAP is eliminated within the first 24 h. This is well before any noticeable changes in transport protein expression occur. The protective role of Kupffer cells may be attributed to mediators released by these resident liver macrophages. As discussed earlier, TNF-α, IL-1β, IL-6, IL-10, IL-13, and COX-2-derived mediators have been implicated as hepatoprotective mediators during liver injury. These mediators exert their protective effects in part through participating in the compensatory hepatocellular proliferation that occurs following liver injury, induction of cytoprotective heat shock proteins, and induction of antioxidant defenses and through downregulation of excessive proinflammatory mediator production (9–11, 23, 33).
In addition to playing a prominent role in influencing the susceptibility of the liver to toxicant-induced injury, Kupffer cell products have also been implicated in the regulation of hepatic transporter expression in other forms of liver disease. Most previous work investigating the role of cytokines in regulating transporter expression was focused on liver conditions such as cholestasis and ischemia-reperfusion, with minimal emphasis on chemical-induced liver injury (6, 19, 43). In this study, Kupffer cell depletion prevented the induction of Mrp4 protein expression in mice following toxic APAP exposure. Mrp4 is the most significantly upregulated efflux transporter in mouse liver after APAP exposure and is also increased in human livers following APAP overdose (1, 5). Mrp4 is responsible for the basolateral export of bile acids and their conjugates, cyclic nucleotides (cAMP and cGMP), prostaglandins (PGE1 and PGE2), and glutathione (4, 34, 36, 38, 39, 50). The altered expression of Oatp1a1 and Mrp3 by Kupffer cell depletion in the absence of APAP treatment suggests that these macrophages also play a role in the basal regulation of these transporters. Endogenous substrates of Oatp1a1 include bile acid and steroid conjugates, and Mrp3 transports bile acids and glucuronide conjugates, among other substrates (7, 8, 14, 51). For several of the uptake and efflux transporters, the changes in protein expression by APAP treatment were more pronounced in Kupffer cell-depleted mice compared with mice with normal Kupffer cell function (e.g., Oatp1b2, Ntcp, Mrp2). This could be attributed to greater liver injury in these mice. Our previous studies have revealed a direct relationship between the extent of hepatic damage and the degree of change in transporter expression (2).
The concurrent upregulation of efflux transporters and decrease in uptake transporter expression may aid in reducing the accumulation of toxic chemicals and by-products of cellular injury within hepatocytes. Alternatively, these changes may accelerate the export of substrates that function as signaling molecules, aiding in the recovery process by promoting tissue repair and regeneration. Because of these potential implications, the lack of Mrp4 induction in Kupffer cell-depleted mice may be a contributing factor to their delayed recovery from liver injury. The regulation of transporters, particularly Mrp4, may represent a novel mechanism by which inflammatory mediators exert their protective effects. We hypothesize that following APAP-induced hepatocellular damage the ensuing release of mediators from Kupffer cells activates signaling pathways to increase Mrp4 expression, resulting in enhanced export of substrates through Mrp4 that may play key roles in the recovery process.
The use of clodronate encapsulated in liposomes for in vivo depletion of Kupffer cells allowed us to examine the global role of these macrophages in transporter protein regulation during drug-induced liver injury. To gain a better understanding of which inflammatory mediators may be participating in Kupffer cell-dependent regulation of transporters following APAP treatment, we measured plasma levels of IL-1β, IL-4, IL-6, IL-10, IL-13, GM-CSF, IFN-γ, KC, MCP-1, and TNF-α. These particular cytokines and chemokines were examined because of their well-documented role in regulating expression of transporters in other disease states and/or their role in APAP hepatotoxicity (6, 9, 19, 40, 48). Elevated plasma levels of TNF-α and IL-1β in the empty liposome-pretreated mice, but not the clodronate liposome mice, suggest that these two cytokines may contribute not only to protection from APAP toxicity but also to Mrp4 induction. Additional studies are warranted to further evaluate the involvement of TNF-α and/or IL-1β in regulating Mrp4 expression during APAP-induced liver injury. IL-6, a cytokine that also been shown to regulate hepatic transporters (40), was elevated in the plasma of both empty and clodronate liposome-pretreated mice. This observation suggests that IL-6 may play a role regulating transporters that were either decreased (Ntcp) or increased (Mrp3) in both groups of liposome-pretreated mice. Analysis of the neutrophil chemoattractant KC revealed increased levels in both empty and clodronate liposome-pretreated mice at 24 h, which remained elevated in the Kupffer cell-depleted mice at 48 h after APAP treatment. This prolonged elevation of plasma KC levels in these mice may be a compensatory response to recruit more leukocytes into the liver for tissue repair. Alternatively, increased recruitment of neutrophils may lead to enhanced liver injury and delayed recovery, since in vivo neutrophil depletion with monoclonal antibodies has been shown to protect from APAP-induced liver injury (22, 31). The levels of MCP-1, a potent macrophage chemoattractant, were highly elevated only in plasma of Kupffer cell-depleted mice. The C-C chemokine receptor CCR2 is the primary receptor for MCP-1. It has been previously shown that both CCR2 and MCP-1 play a hepatoprotective role during APAP toxicity (13, 21). Elevated levels of MCP-1 in Kupffer cell-depleted mice may lead to increased recruitment of monocytes to repopulate the liver and to aid in the resolution of tissue injury in these mice.
Kupffer cells provide important regulatory functions within the liver, including development of tolerance to orally ingested antigens and containment of systemic immune responses (26). The data presented here provide strong evidence for an additional regulatory role of Kupffer cells in the altered expression of the basolateral efflux transporter Mrp4 during drug-induced liver injury. Alterations in Mrp4 may promote paracrine signaling to adjacent hepatocytes and other nonparenchymal cells to facilitate the coordinated response of several cell types that is critical during the recovery process from drug-induced hepatotoxicity. Additional studies focused specifically on TNF-α and IL-1β through the use of knockout mice or pharmacological blockade are needed to aid in determining the exact role of these Kupffer cell products in regulating Mrp4 expression during APAP-induced liver injury. Equally important will be to study the susceptibility of Mrp4 knockout mice to APAP hepatotoxicity.
GRANTS
S. N. Campion is a PhRMA Foundation Predoctoral Fellow. This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-069557 (J. E. Manautou) and DK-068039 (N. J. Cherrington).
Acknowledgments
The authors thank Bruno Stieger (University Hospital, Zurich, Switzerland) for providing the K4 polyclonal Ntcp antibody, Terrance Kavanagh (University of Washington) for providing the Gclc and Gclm antibodies, and Curtis Klaassen for providing the Oatp antibodies. The authors also thank Jeff Volek and Michael Puglisi (University of Connecticut) for assistance with the Bio-Plex assay.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Aleksunes LM, Scheffer GL, Jakowski AB, Pruimboom-Brees IM, Manautou JE. Coordinated expression of multidrug resistance-associated proteins (Mrps) in mouse liver during toxicant-induced injury. Toxicol Sci 89: 370–379, 2006. [DOI] [PubMed] [Google Scholar]
- 2.Aleksunes LM, Slitt AM, Cherrington NJ, Thibodeau MS, Klaassen CD, Manautou JE. Differential expression of mouse hepatic transporter genes in response to acetaminophen and carbon tetrachloride. Toxicol Sci 83: 44–52, 2005. [DOI] [PubMed] [Google Scholar]
- 3.Austyn JM, Gordon S. F4/80, a monoclonal antibody directed specifically against the mouse macrophage. Eur J Immunol 11: 805–815, 1981. [DOI] [PubMed] [Google Scholar]
- 4.Bai J, Lai L, Yeo HC, Goh BC, Tan TM. Multidrug resistance protein 4 (MRP4/ABCC4) mediates efflux of bimane-glutathione. Int J Biochem Cell Biol 36: 247–257, 2004. [DOI] [PubMed] [Google Scholar]
- 5.Barnes SN, Aleksunes LM, Augustine L, Scheffer GL, Goedken M, Jakowski AB, Pruimboom-Brees IM, Cherrington NJ, Manautou JE. Induction of hepatobiliary efflux transporters in acetaminophen-induced acute liver failure cases. Drug Metab Dispos 35: 1963–1969, 2007. [DOI] [PubMed] [Google Scholar]
- 6.Bohan A, Chen WS, Denson LA, Held MA, Boyer JL. Tumor necrosis factor alpha-dependent up-regulation of Lrh-1 and Mrp3(Abcc3) reduces liver injury in obstructive cholestasis. J Biol Chem 278: 36688–36698, 2003. [DOI] [PubMed] [Google Scholar]
- 7.Borst P, Zelcer N, van de Wetering K. MRP2 and 3 in health and disease. Cancer Lett 234: 51–61, 2006. [DOI] [PubMed] [Google Scholar]
- 8.Bossuyt X, Muller M, Meier PJ. Multispecific amphipathic substrate transport by an organic anion transporter of human liver. J Hepatol 25: 733–738, 1996. [DOI] [PubMed] [Google Scholar]
- 9.Bourdi M, Masubuchi Y, Reilly TP, Amouzadeh HR, Martin JL, George JW, Shah AG, Pohl LR. Protection against acetaminophen-induced liver injury and lethality by interleukin 10: role of inducible nitric oxide synthase. Hepatology 35: 289–298, 2002. [DOI] [PubMed] [Google Scholar]
- 10.Chiu H, Gardner CR, Dambach DM, Brittingham JA, Durham SK, Laskin JD, Laskin DL. Role of p55 tumor necrosis factor receptor 1 in acetaminophen-induced antioxidant defense. Am J Physiol Gastrointest Liver Physiol 285: G959–G966, 2003. [DOI] [PubMed] [Google Scholar]
- 11.Chiu H, Gardner CR, Dambach DM, Durham SK, Brittingham JA, Laskin JD, Laskin DL. Role of tumor necrosis factor receptor 1 (p55) in hepatocyte proliferation during acetaminophen-induced toxicity in mice. Toxicol Appl Pharmacol 193: 218–227, 2003. [DOI] [PubMed] [Google Scholar]
- 12.Court MH, Greenblatt DJ. Molecular basis for deficient acetaminophen glucuronidation in cats. An interspecies comparison of enzyme kinetics in liver microsomes. Biochem Pharmacol 53: 1041–1047, 1997. [DOI] [PubMed] [Google Scholar]
- 13.Dambach DM, Watson LM, Gray KR, Durham SK, Laskin DL. Role of CCR2 in macrophage migration into the liver during acetaminophen-induced hepatotoxicity in the mouse. Hepatology 35: 1093–1103, 2002. [DOI] [PubMed] [Google Scholar]
- 14.Eckhardt U, Schroeder A, Stieger B, Hochli M, Landmann L, Tynes R, Meier PJ, Hagenbuch B. Polyspecific substrate uptake by the hepatic organic anion transporter Oatp1 in stably transfected CHO cells. Am J Physiol Gastrointest Liver Physiol 276: G1037–G1042, 1999. [DOI] [PubMed] [Google Scholar]
- 15.Ellman GL Tissue sulfhydryl groups. Arch Biochem Biophys 82: 70–77, 1959. [DOI] [PubMed] [Google Scholar]
- 16.Gardner CR, Laskin JD, Dambach DM, Chiu H, Durham SK, Zhou P, Bruno M, Gerecke DR, Gordon MK, Laskin DL. Exaggerated hepatotoxicity of acetaminophen in mice lacking tumor necrosis factor receptor-1. Potential role of inflammatory mediators. Toxicol Appl Pharmacol 192: 119–130, 2003. [DOI] [PubMed] [Google Scholar]
- 17.Geier A, Dietrich CG, Voigt S, Kim SK, Gerloff T, Kullak-Ublick GA, Lorenzen J, Matern S, Gartung C. Effects of proinflammatory cytokines on rat organic anion transporters during toxic liver injury and cholestasis. Hepatology 38: 345–354, 2003. [DOI] [PubMed] [Google Scholar]
- 18.Hardonk MJ, Dijkhuis FW, Hulstaert CE, Koudstaal J. Heterogeneity of rat liver and spleen macrophages in gadolinium chloride-induced elimination and repopulation. J Leukoc Biol 52: 296–302, 1992. [DOI] [PubMed] [Google Scholar]
- 19.Hartmann G, Cheung AK, Piquette-Miller M. Inflammatory cytokines, but not bile acids, regulate expression of murine hepatic anion transporters in endotoxemia. J Pharmacol Exp Ther 303: 273–281, 2002. [DOI] [PubMed] [Google Scholar]
- 20.Hissin PJ, Hilf R. A fluorometric method for determination of oxidized and reduced glutathione in tissues. Anal Biochem 74: 214–226, 1976. [DOI] [PubMed] [Google Scholar]
- 21.Hogaboam CM, Bone-Larson CL, Steinhauser ML, Matsukawa A, Gosling J, Boring L, Charo IF, Simpson KJ, Lukacs NW, Kunkel SL. Exaggerated hepatic injury due to acetaminophen challenge in mice lacking C-C chemokine receptor 2. Am J Pathol 156: 1245–1252, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ishida Y, Kondo T, Kimura A, Tsuneyama K, Takayasu T, Mukaida N. Opposite roles of neutrophils and macrophages in the pathogenesis of acetaminophen-induced acute liver injury. Eur J Immunol 36: 1028–1038, 2006. [DOI] [PubMed] [Google Scholar]
- 23.James LP, Lamps LW, McCullough S, Hinson JA. Interleukin 6 and hepatocyte regeneration in acetaminophen toxicity in the mouse. Biochem Biophys Res Commun 309: 857–863, 2003. [DOI] [PubMed] [Google Scholar]
- 24.Ju C, Pohl LR. Immunohistochemical detection of protein adducts of 2,4-dinitrochlorobenzene in antigen presenting cells and lymphocytes after oral administration to mice: lack of a role of Kupffer cells in oral tolerance. Chem Res Toxicol 14: 1209–1217, 2001. [DOI] [PubMed] [Google Scholar]
- 25.Ju C, Reilly TP, Bourdi M, Radonovich MF, Brady JN, George JW, Pohl LR. Protective role of Kupffer cells in acetaminophen-induced hepatic injury in mice. Chem Res Toxicol 15: 1504–1513, 2002. [DOI] [PubMed] [Google Scholar]
- 26.Knolle PA, Gerken G. Local control of the immune response in the liver. Immunol Rev 174: 21–34, 2000. [DOI] [PubMed] [Google Scholar]
- 27.Kono H, Fujii H, Asakawa M, Yamamoto M, Maki A, Matsuda M, Rusyn I, Matsumoto Y. Functional heterogeneity of the Kupffer cell population is involved in the mechanism of gadolinium chloride in rats administered endotoxin. J Surg Res 106: 179–187, 2002. [DOI] [PubMed] [Google Scholar]
- 28.Larson AM, Polson J, Fontana RJ, Davern TJ, Lalani E, Hynan LS, Reisch JS, Schiodt FV, Ostapowicz G, Shakil AO, Lee WM. Acetaminophen-induced acute liver failure: results of a United States multicenter, prospective study. Hepatology 42: 1364–1372, 2005. [DOI] [PubMed] [Google Scholar]
- 29.Laskin DL, Gardner CR, Price VF, Jollow DJ. Modulation of macrophage functioning abrogates the acute hepatotoxicity of acetaminophen. Hepatology 21: 1045–1050, 1995. [PubMed] [Google Scholar]
- 30.Lee WM Acetaminophen and the U. S. Acute Liver Failure Study Group: lowering the risks of hepatic failure. Hepatology 40: 6–9, 2004. [DOI] [PubMed] [Google Scholar]
- 31.Liu ZX, Han D, Gunawan B, Kaplowitz N. Neutrophil depletion protects against murine acetaminophen hepatotoxicity. Hepatology 43: 1220–1230, 2006. [DOI] [PubMed] [Google Scholar]
- 32.Manautou JE, Hoivik DJ, Tveit A, Hart SG, Khairallah EA, Cohen SD. Clofibrate pretreatment diminishes acetaminophen's selective covalent binding and hepatotoxicity. Toxicol Appl Pharmacol 129: 252–263, 1994. [DOI] [PubMed] [Google Scholar]
- 33.Masubuchi Y, Bourdi M, Reilly TP, Graf ML, George JW, Pohl LR. Role of interleukin-6 in hepatic heat shock protein expression and protection against acetaminophen-induced liver disease. Biochem Biophys Res Commun 304: 207–212, 2003. [DOI] [PubMed] [Google Scholar]
- 34.Mennone A, Soroka CJ, Cai SY, Harry K, Adachi M, Hagey L, Schuetz JD, Boyer JL. Mrp4-/- mice have an impaired cytoprotective response in obstructive cholestasis. Hepatology 43: 1013–1021, 2006. [DOI] [PubMed] [Google Scholar]
- 35.Michael SL, Pumford NR, Mayeux PR, Niesman MR, Hinson JA. Pretreatment of mice with macrophage inactivators decreases acetaminophen hepatotoxicity and the formation of reactive oxygen and nitrogen species. Hepatology 30: 186–195, 1999. [DOI] [PubMed] [Google Scholar]
- 36.Reid G, Wielinga P, Zelcer N, van der Heijden I, Kuil A, de Haas M, Wijnholds J, Borst P. The human multidrug resistance protein MRP4 functions as a prostaglandin efflux transporter and is inhibited by nonsteroidal antiinflammatory drugs. Proc Natl Acad Sci USA 100: 9244–9249, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reilly TP, Brady JN, Marchick MR, Bourdi M, George JW, Radonovich MF, Pise-Masison CA, Pohl LR. A protective role for cyclooxygenase-2 in drug-induced liver injury in mice. Chem Res Toxicol 14: 1620–1628, 2001. [DOI] [PubMed] [Google Scholar]
- 38.Rius M, Hummel-Eisenbeiss J, Hofmann AF, Keppler D. Substrate specificity of human ABCC4 (MRP4)-mediated cotransport of bile acids and reduced glutathione. Am J Physiol Gastrointest Liver Physiol 290: G640–G649, 2006. [DOI] [PubMed] [Google Scholar]
- 39.Rius M, Thon WF, Keppler D, Nies AT. Prostanoid transport by multidrug resistance protein 4 (MRP4/ABCC4) localized in tissues of the human urogenital tract. J Urol 174: 2409–2414, 2005. [DOI] [PubMed] [Google Scholar]
- 40.Siewert E, Dietrich CG, Lammert F, Heinrich PC, Matern S, Gartung C, Geier A. Interleukin-6 regulates hepatic transporters during acute-phase response. Biochem Biophys Res Commun 322: 232–238, 2004. [DOI] [PubMed] [Google Scholar]
- 41.Sturm E, Havinga R, Baller JF, Wolters H, van Rooijen N, Kamps JA, Verkade HJ, Karpen SJ, Kuipers F. Kupffer cell depletion with liposomal clodronate prevents suppression of Ntcp expression in endotoxin-treated rats. J Hepatol 42: 102–109, 2005. [DOI] [PubMed] [Google Scholar]
- 42.Sun H, Li JY, Yu JR. Protective effect of interleukin-1 beta on acetaminophen induced liver damage in mice. Sheng Li Xue Bao 49: 153–159, 1997. [PubMed] [Google Scholar]
- 43.Tanaka Y, Chen C, Maher JM, Klaassen CD. Kupffer cell-mediated downregulation of hepatic transporter expression in rat hepatic ischemia-reperfusion. Transplantation 82: 258–266, 2006. [DOI] [PubMed] [Google Scholar]
- 44.Van Rooijen N, Sanders A. Liposome mediated depletion of macrophages: mechanism of action, preparation of liposomes and applications. J Immunol Methods 174: 83–93, 1994. [DOI] [PubMed] [Google Scholar]
- 45.Van Rooijen N, van Kesteren-Hendrikx E. “In vivo” depletion of macrophages by liposome-mediated “suicide.” Methods Enzymol 373: 3–16, 2003. [DOI] [PubMed] [Google Scholar]
- 46.Witmer-Pack MD, Crowley MT, Inaba K, Steinman RM. Macrophages, but not dendritic cells, accumulate colloidal carbon following administration in situ. J Cell Sci 105: 965–973, 1993. [DOI] [PubMed] [Google Scholar]
- 47.Yamamoto T, Naito M, Moriyama H, Umezu H, Matsuo H, Kiwada H, Arakawa M. Repopulation of murine Kupffer cells after intravenous administration of liposome-encapsulated dichloromethylene diphosphonate. Am J Pathol 149: 1271–1286, 1996. [PMC free article] [PubMed] [Google Scholar]
- 48.Yee SB, Bourdi M, Masson MJ, Pohl LR. Hepatoprotective role of endogenous interleukin-13 in a murine model of acetaminophen-induced liver disease. Chem Res Toxicol 20: 734–744, 2007. [DOI] [PubMed] [Google Scholar]
- 49.Yee SB, Bourdi M, Masson MJ, Pohl LR. Hepatoprotective role of endogenous interleukin-13 in a murine model of acetaminophen-induced liver disease. Chem Res Toxicol 20: 734–744, 2007. [DOI] [PubMed] [Google Scholar]
- 50.Zelcer N, Reid G, Wielinga P, Kuil A, van der Heijden I, Schuetz JD, Borst P. Steroid and bile acid conjugates are substrates of human multidrug-resistance protein (MRP) 4 (ATP-binding cassette C4). Biochem J 371: 361–367, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zelcer N, Saeki T, Bot I, Kuil A, Borst P. Transport of bile acids in multidrug-resistance-protein 3-overexpressing cells cotransfected with the ileal Na+-dependent bile-acid transporter. Biochem J 369: 23–30, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]