Abstract
There is increased prevalence of abdominal pain and diarrhea and decreased gastric sensation with increased body mass index (BMI). Our hypothesis is that increased BMI is associated with increased colonic motility and sensation. The study aim was to assess effect of BMI on colonic sensory and motor functions and transit. We used a database of colonic tone, compliance, and perception of distensions measured by intracolonic, barostat-controlled balloon, and gastrointestinal transit was measured by validated scintigraphy in healthy obese and nonobese subjects. Regression analysis was applied to assess the association of BMI with colonic sensory and motor functions. We included adjustments for sex differences, age, height, balloon volumes during distension, and psychological stress. Among 165 participants (87 women, 78 men), increased BMI was associated with decreased colonic compliance (P < 0.006, adjusted), decreased pain rating during distensions (P = 0.02, adjusted), and a higher threshold for pain (P = 0.042, adjusted). Sensation for gas, colonic tone, and contraction after meal ingestion were not significantly associated with BMI. Transit was assessed in 72 participants (41 women, 31 men); colonic transit was faster with BMI >30 kg/m2 (P = 0.003 unadjusted, P = 0.08 adjusted for gender). In conclusion, BMI >25 kg/m2 is associated with decreased colonic compliance and pain sensation; colonic transit is accelerated particularly with BMI >30 kg/m2 in women. These data suggest that colonic dysfunction may contribute to diarrhea, but the cause of increased abdominal pain in obesity is not explained by the studies of colonic sensation and requires further study of afferent, spinal, and central mechanisms.
Keywords: colon, sensation, motility
increased body mass is associated with decreased sensation in the upper gastrointestinal tract. This decreased sensation has been documented in response to different stimuli: increased tolerance to gastric distention with water-filled balloons (21, 22), decreased satiation, and lower fullness scores 30 min after a challenge with a liquid nutrient ingested to the point of full satiation (3). The mechanisms underlying these observations have not yet been elucidated, but several lines of evidence suggest that there is alteration in central control of brain nuclei and neurotransmitters that alter satiation responses in obesity and may also alter gastrointestinal functions. For example, neuroimaging studies show a “weaker” signal after meal ingestion in the brain centers that control satiety in overweight or obese subjects compared with lean controls (19, 20, 32), and the responses of satiation and orexigenic signals such as ghrelin, glucagon-like peptide-1, peptide YY, or CCK to meal intake (14, 46, 47) are lower in obese subjects than in controls.
It is unknown whether, in addition to decreased sensation in the upper gastrointestinal tract, obesity is associated with altered motor function or sensation in the lower gut. Population-based studies from the USA and Australia have evaluated symptoms suggestive of gastrointestinal dysfunction and have shown increased frequency of abdominal pain and diarrhea in overweight and obese subjects (12, 42, 43). However, it is difficult to elucidate whether this increased frequency of symptoms observed among subjects with increased body mass is related to 1) obesity per se, such as alteration in visceral sensation or motor function, 2) environmental factors, such as the intake of an excessive amount of food with high carbohydrate and fat content that may induce osmotically driven diarrhea (29), 3) effects of medications, such as lipase inhibitors or metformin, or 4) comorbidities associated with obesity, such as cholelithiasis or even intestinal angina secondary to the atherosclerosis associated with obesity.
The aim of this study was to assess the effect of body mass on colonic motor and sensory functions and on gastrointestinal and colonic transit times, measured in controlled conditions with validated methods in overweight and obese subjects who were otherwise healthy and free of medications that are known to alter motor or sensory function in the gastrointestinal tract.
MATERIALS AND METHODS
Data Source
Data used in this study are derived from a database that includes underweight, normal weight, overweight, and obese, but otherwise healthy, subjects who participated in several clinical trials that involved assessment of colonic tone and perception of colonic distensions and/or assessment of gastrointestinal transit with the use of standardized protocols (5, 6, 10, 15, 16, 23). Only data obtained before drug treatment or from participants receiving a nonactive placebo in a parallel group design study (never receiving potentially active medications in the study) were used for the present analyses. These studies were performed and analyzed by the same investigators (I. Busciglio and D. D. Burton for barostat and transit studies, respectively) at the General Clinical Research Center at Mayo Clinic, Rochester, Minnesota from September 2001 to May 2006.
Assessment of Colonic Motor and Sensory Function
Colonic motor and sensory function was assessed using the barostat technique as described previously (6, 10). All participants received a standard oral colonic lavage solution on the basis of polyethylene glycol and electrolytes over the prior 24 h. They attended the research center at around 7 AM after having fasted for 8 h. The combined barostat balloon and multilumen manometric assembly was placed by one investigator (M. Camilleri) in the left colon via unsedated flexible sigmoidoscopy and with the aid of a Teflon-coated guide wire and fluoroscopy. The position of the barostat tube was in the descending (>75%) or the junction of the sigmoid and descending colon. There were no differences in mean differences for all parameters analyzed depending on location of the balloon, and, hence, all the data are presented as one set.
Colonic wall compliance and resting tone, thresholds for first perception of gas and pain, and the intensity of perception during phasic distensions were measured using the same protocol before receiving any drug in all included studies and were used for the present analysis. We have also analyzed the postprandial colonic contractile response assessed in participants who were randomized to receive nonactive placebo in clinical trials.
Briefly, the operating pressure (OP) was set at 2 mmHg above the minimum distending pressure (the pressure at which respiratory excursions during deep inspiration are accompanied by a noticeable deflection in the balloon volume). After a conditioning distention (25) and an equilibration period at the OP, we measured colonic compliance. Compliance was assessed as the volume response to 4-mmHg increments in intraballoon pressures from 0 to 44 mmHg. During assessment of compliance, participants were asked to report first perception of gas and pain. After compliance measurement, resting colonic tone was assessed at the OP, followed by phasic balloon distensions of 8, 16, 24, and 32 mmHg above the OP, performed in random order. During phasic distensions, participants were asked to rate the intensity of gas and pain perception using 100-mm visual analog scales (VASs) (6, 10).
Postprandial colonic contraction was assessed by measuring colonic volume 60 min after ingestion of a 750-ml milkshake containing 1,000 Kcal (53% fat, 35% carbohydrate, and 12% protein).
Levels of arousal and stress were assessed before assessment of colonic sensation in all studies. These assessments were included since levels of arousal and stress just before the conduct of the sensation measurements have been shown to influence perception of colonic distention (17). Thus levels of arousal and stress were assessed using four 100-mm horizontal VASs with the words 1) tired-energetic, 2) active-drowsy, 3) peaceful-tense, and 4) worried-relaxed anchored the left and right ends of each line, respectively (17).
Assessment of Gastrointestinal Transit by Scintigraphy
Gastric emptying and small bowel and colonic transit of solids were assessed using scintigraphy and the same protocol (6, 10). Only data from participants receiving a nonactive placebo were used for the present analysis.
Briefly, after an overnight fast, participants ingested an 111In-charcoal capsule coated with a pH-sensitive methacrylate that dissolves when it reaches the colon (4), allowing colonic transit measurement with a gamma camera. After the capsule emptied from the stomach, participants ingested two scrambled eggs labeled with 99mTc-sulfur colloid with one slice of whole wheat bread and one glass of whole milk (total 300 Kcal) to measure gastric emptying and the colonic filling at 6 h postmeal as a measurement of small bowel transit. Subjects ingested standardized meals for lunch and dinner at 4 and 8 h after the radiolabeled meal. Anterior and posterior abdominal images of 2-min duration were obtained every hour for the first 8 h and at 24, 32, and 48 h.
Data Analysis
BMI.
The data on weight and height obtained at the study site were used to calculate BMI, weight in kilograms divided by the square of height in meters (kg/m2). BMI was entered as a continuous variable in all statistical analysis. However, for the purpose of data display, participants were categorized on the basis of BMI as underweight (<18.5 kg/m2), normal (≥18.5 and <25 kg/m2), overweight (≥25 and <30 kg/m2), and obese (≥30 kg/m2), as defined by the National Heart, Lung, and Blood Institute and the World Health Organization (34a, 38).
Colonic tone and compliance.
Colonic tone, intraballoon volume at the OP, was calculated by averaging the colonic volume throughout the periods of tone assessment, as in previous studies (2, 3, 48). Postprandial change in tone was calculated as the difference between postprandial colonic volume and colonic volume before meal (48). Compliance was summarized by the pressure corresponding to 50% of maximum volume [Phalf, (3)].
Gastrointestinal transit.
Gastric emptying was summarized by proportion of 99mTc emptied from the stomach at 4 h and orocecal transit as the colonic filling with 99mTc at 6 h. Colonic transit was summarized by the geometric center (GC) at 24 h, which is calculated by multiplying the proportion of 111In 24 h after administration in each colonic region, ascending (AC), transverse (TC), descending (DC), rectosigmoid (RS), and stool, by its weighting factor, as indicated in the formula: GC = %AC × 1 + %TC × 2 + %DC × 3 + %RS × 4 + %stool × 5/100.
A low GC value, therefore, indicates slow transit, and a high GC, a rapid colonic transit. These have been shown in previous studies to be the most robust endpoints among scintigraphic transit measurements (8).
Statistical Analysis
The effects of BMI on the different parameters assessed, except for sensation thresholds, were explored using multiple (linear) regression with age, sex differences, and height as covariates to adjust for potential confounding. The effect of BMI on pressure thresholds for first perception of gas and pain was assessed using proportional hazard regression to account for censored values (i.e., maximum pressure level reached without the sensation of interest reported). Arousal and stress scores as well as colonic volumes during distensions were included also as covariates in analyses of perception to colonic distensions. The arousal and stress scores were not included as covariates in appraisal of tone or compliance since they were not significant covariates in the assessment of compliance or tone in these studies or in multiple previous studies already published. All numerical variables including BMI were included as continuous in all the analysis. All tests were two-sided, and the significance level was set at 5%. Since there were 165 participants, the logistic regression and covariate analyses included >10 participants for each factor considered in the analysis of colonic sensation and tone. Similarly, there were >10 participants for each of the covariates (sex, height, age, and BMI) appraised among the 72 participants who underwent colonic transit.
Descriptive statistics are presented as the regression means ± SE, unless otherwise stated.
RESULTS
Study Population
Colonic sensory-motor analyses.
165 subjects (87 women and 78 men) underwent assessment of colonic motor and sensory function. Median age was 28 years [interquartile range (IQR): 23–37, range: 18–63], and median BMI was 25 kg/m2 (IQR: 22–28.5, range: 17–44). The number of subjects in each BMI category is shown in Table 1.
Table 1.
Distribution of BMI overall and separated by sexes
| BMI, kg/m2 | 18.5 < 25 N(%) | 25–30 N(%) | >30 N(%) |
|---|---|---|---|
| Participants in colonic barostat studies | |||
| Females (N = 87) | 56 (64%) | 23 (26%) | 8 (9%) |
| Males (N = 78) | 29 (37%) | 32 (41%) | 17 (22%) |
| Total N = 165 | 85 (52%) | 55 (33%) | 25 (15%) |
| Participants in gut transit studies | |||
| Females (N = 41) | 24 (59%) | 13 (32%) | 4 (10%) |
| Males (N = 31) | 10 (32%) | 14 (45%) | 7 (23%) |
| Total N = 72 | 34 (47%) | 27 (38%) | 11 (15%) |
BMI, body mass index.
Transit analyses.
72 subjects (41 women and 31 men) completed gastrointestinal transit measurements and received placebo treatment. Median age was 29.5 yr (IQR: 24–36, range: 18–62), and median BMI was 25.5 kg/m2 (IQR: 23–28, range: 17–40).
The number of subjects in each BMI category is shown in Table 1.
Colonic Sensory-Motor Function
Compliance.
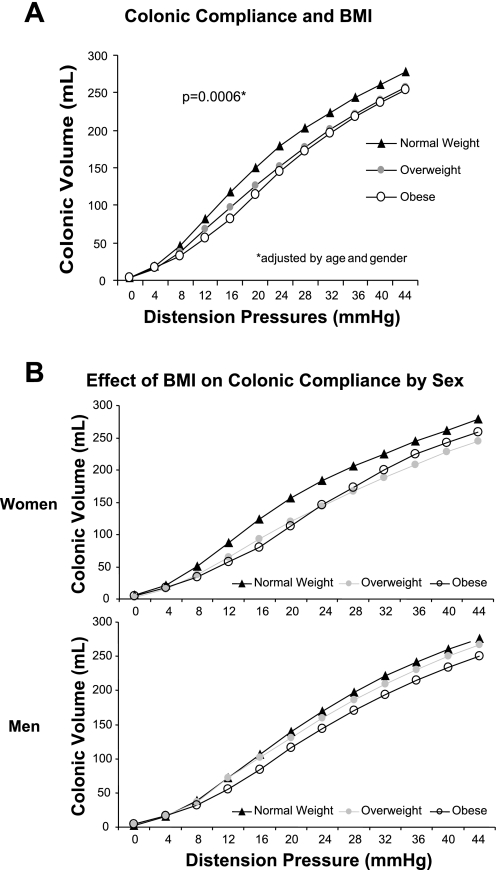
Colonic compliance was significantly associated by BMI [P = 0.0006, adjusted by age (P = 0.053) and gender (P = 0.0253)]. Hence, in heavier subjects, the P1/2 (the pressure at half the maximal volume) was higher and, thus the pressure/volume curve was shifted to the right (decreased colonic compliance) compared with lower weight subjects (Fig. 1A). Height (P = 0.74) did not have any effect on compliance curves. We also explored whether the observed effect of BMI on compliance (P1/2) was similar for women and men by adding an interaction term (BMI × sex) in the regression model. There was no significant interaction (P = 0.54) between BMI and sex. Thus there were no significant differences in the observed influence of BMI on colonic compliance in men and women although this influence appears to be stronger in women (Fig. 1B).
Fig. 1.
A: colonic compliance curves for normal, overweight, and obese subjects. The P50, the pressure at which half of the maximum volume is reached, was significantly higher in overweight or obese participants, indicating decreased compliance or higher stiffness of the colonic wall (P = 0.0006, adjusted by age and sex). B: colonic compliance data plotted for women and men showing similar patterns with higher P1/2 in obesity and no significant differences between women and men. BMI, body mass index.
Colonic resting tone.
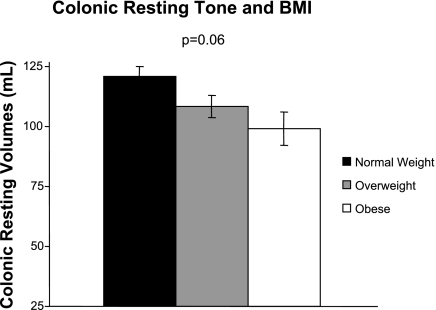
Colonic resting tone (colonic volume at the OP) was not significantly influenced by BMI (P = 0.06). Heavier participants tended to have lower colonic resting volumes (increased tone) although this did not reach statistical significance after adjusting for OP, which was higher with increased BMI (P < 0.0001), age (P = 0.018), and sex (P = 0.020), three variables that affected colonic resting tone (Fig. 2).
Fig. 2.
Mean colonic volumes at the operating pressure in fasting conditions (colonic resting tone) in normal, overweight, and obese subjects. Colonic volumes decreased with increased BMI, suggesting increased colonic tone, although this did not reach statistical significance (P = 0.06).
Colonic contractile response to feeding.
Colonic contractile response was not associated with BMI (P = 0.72) even after adjusting for preprandial colonic tone (P = 0.0007), age (P = 0.98), or sex (P = 0.79).
Sensory ratings in response to random order colonic distensions and sensory thresholds.
As anticipated from prior work, both pain and gas VAS scores were influenced, besides BMI, by baseline arousal-stress levels (P = 0.0007 for pain and P = 0.004 for gas) and age (P = 0.02 for pain and P = 0.037 for gas). Therefore, these were included as covariates in the analysis of the effect of BMI on colonic sensation.
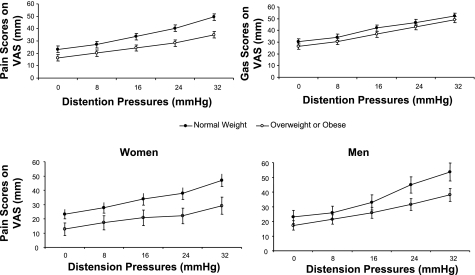
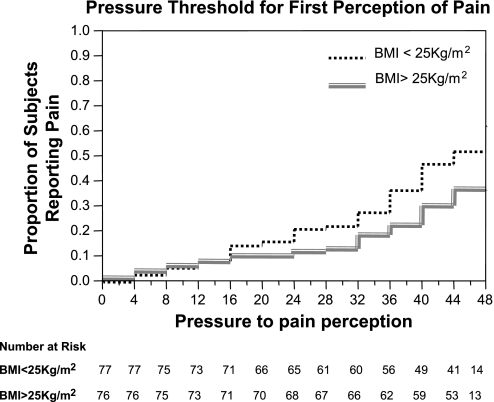
BMI was significantly associated with pain VAS scores during colonic distensions (P = 0.0166) as well as with the pressure threshold for first perception of pain (P = 0.042), adjusting for age, sex, colonic volume during distensions, and arousal-stress levels at the time of distensions. Thus subjects with higher BMI reported lower pain ratings during colonic distensions (Fig. 3, top) and had higher pressure thresholds for reporting first perception of pain (Fig. 4).
Fig. 3.
Pain and gas scores as measured by visual analog scales (VAS) in response to pressure-based colonic distentions. Reported pain intensity was significantly reduced in overweight or obese subjects compared with lean controls at each pressure level [P = 0.02, adjusted by age, sex, intracolonic volume, and arousal-stress levels (top)], and the effect was not significantly different in women and men (bottom).
Fig. 4.
Kaplan-Meier curves for reporting first perception of pain grouped by BMI (below and above sample median, 25 kg/m2). Subjects with higher BMI had a decreased risk of reporting pain during colonic distentions.
We did not observe a significant association of BMI and gas sensation VAS scores (P = 0.07, Fig. 3, top) or pressure thresholds for first perception of gas (P = 0.64).
The effect of BMI on pain did not significantly differ between sexes (P = 0.5 for the interaction term BMI*gender included in the regression model). Thus pain scores were lower with higher BMI, and the effect of higher BMI was not significantly different in men and women (Fig. 3, bottom).
Gastrointestinal and Colonic Transit
There was no significant association of BMI with gastric emptying and small bowel transit (P = 0.53 and P = 0.19, respectively). However, colonic transit as measured by GC at 24 h tended to be faster in participants who weighed >30 kg/m2 (P = 0.003 unadjusted, P = 0.08 adjusted by sex, Table 2).
Table 2.
Transit parameters by BMI
| BMI, kg/m2 | 18.5 < 25 (%) | 25–30 (%) | >30(%) | P |
|---|---|---|---|---|
| GC 4 h (% emptied) | 96±1 | 95±1.5 | 95±2.3 | NS |
| CF 6 h (% in colon) | 47±5 | 41±6 | 58±9 | NS |
| Colonic GC 24 h | 2.4±0.2 | 2.5±0.2 | 2.9±0.3 | NS |
Values are means ± SE. CF, colonic filling; GC, geometric center; NS, not significant.
DISCUSSION
In this study we have shown that perception of pain in response to experimental colonic pressure-based distensions decreases as BMI increases and that colonic volumes in response to pressure distensions were lower (suggesting decreased compliance) with higher BMI. We also observed a trend to faster colonic transit with increased BMI. These data provide some insights relative to the observed increase in the prevalence of diarrhea in association with obesity in epidemiological studies. However, the present studies do not provide a clear explanation for the reports of abdominal pain in obesity in epidemiological studies.
The lower gut sensation of pain during distension (not gas) is consistent with the reduced upper gastrointestinal tract sensation in obesity (see Introduction for details). The present data suggest that, after controlling for other factors such as age, sex, intracolonic balloon volume, or arousal-stress levels, the experimental pressure-based distensions elicited lower pain intensity in people with increased body mass. The reduced sensation ratings for pain were confirmed by the observation that they also had a higher pressure threshold to report pain. These differences in pain perception seem unrelated to differences in colonic compliance since compliance was reduced in subjects with increased body mass. Typically, one would expect that reduced compliance would result in increased perception, conceivably because of activation at lower pressures of the tension receptors that lead to heightened visceral perception (13). An important precaution in our studies appraising colonic thresholds and sensory ratings was the inclusion of volume of the balloon at the specified pressure distensions as a covariate in the analyses. Hence, we avoided a potential bias in the assessment of sensation by starting the assessment of thresholds from 0 mmHg, and we “corrected” for the observed differences in compliance across groups by including the volume of the balloon at the standardized distension pressures above baseline OP.
The mechanism for increased body mass to be associated with decreased perception of pressure-based distensions in the face of decreased colon compliance is unclear. One hypothesis is that there was decreased “distention” of the colonic wall in response to a standard pressure because of pericolonic or abdominal wall fat. It is conceivable that this might actually lead to reduced activation of stretch receptors in the colonic wall. However, this hypothesis is not consistent with our data since, even with controlling for intracolonic volumes as covariates (which may have been influenced by visceral or abdominal wall fat), increased body mass was still independently associated with decreased pain ratings in response to each pressure distention and with higher pressure thresholds for perception of pain. Other factors, including those that might independently affect stretch or tension receptors, need to be further investigated to understand the mechanisms for decreased perception observed in subjects with increased body mass.
There is evidence that impaired sensory nerve function occurs among subjects with increased body mass although most studies deal with somatic rather than visceral sensation (9, 55). Insulin resistance may play a role in the decreased sensation observed in obese subjects. Thus, in one study of somatic sensation, warm and cold perception thresholds, reflecting C-fibers and Aδ-fibers, respectively, were increased in normoglycemic obese subjects with reduced insulin sensitivity compared with controls with normal insulin sensitivity (9). Our study included healthy subjects with normal basic laboratory tests including normal fasting blood glucose. However, we cannot exclude subclinical insulin resistance, and, hence, it is conceivable that insulin resistance associated with increased body mass may partly explain the decreased colonic sensation observed in our subjects.
It has also been suggested that an increased activity of the endogenous opiate system in obese subjects might play a role in the lowered sensation observed in response to electrical cutaneous stimulation (54). Future studies may explore the role of endogenous opiate mechanisms in subjects with increased body mass. Although there is no evidence of increased endogenous opiate levels in obesity, it is possible that, as with upregulation of central opiate receptors in the control of appetite in obesity (34), an upregulation of peripheral or central opiate receptors involved in sensation may change visceral sensation in obesity.
Autonomic factors may conceivably influence sensation, particularly in view of differing levels of arousal and stress in the subjects. Even if BMI is associated with differences in autonomic function, the analysis of covariance accounted for differences in the potential effects of arousal and stress. Overall, the present studies suggest that the increased prevalence of abdominal pain in obesity cannot be explained by the decreased perception of pain to colonic distension, and, therefore, other mechanisms have to be considered, particularly central mechanisms. Given the substantial literature on differences in the central regulation of eating behavior in obese subjects, including recent neuroimaging studies showing altered activation of cortical brain areas involved in satiety control (19, 20, 28, 32), it is conceivable that there may be central inhibitory modulation of afferent signals from the gastrointestinal tract. Clearly, further studies should address the role of central mechanisms in the development of abdominal pain in obesity.
There is, however, a potential pitfall in the assessment of sensation in this study that evaluated a convenience sample of participants participating in clinical trials, even though they were randomized to placebo. Thus patient perceptions could have been altered by placebo and influenced sensory ratings and conceivably even motor physiology though one would not expect those changes to vary by BMI. Therefore, it may be necessary to evaluate the same functions in those not receiving placebo to ensure that a placebo effect is excluded. However, this large database in people without gastrointestinal symptoms represents a unique opportunity to commence to explore the study hypothesis, which we perceive to be relevant, given the epidemiological evidence of abdominal pain and diarrhea in obesity and the complexity of the validated studies conducted.
It is unclear whether the observed change in colonic compliance represents a true or apparent increased stiffness of the colonic wall. As stated above, the presence of intraabdominal fat could limit colon expansion in response to standard application of an intraluminal distension pressure. Increased stiffness has also been described in the vascular system and heart of subjects with increased body mass (30, 41, 49). However, it seems that factors other than the mechanical effect of the presence of fat may contribute to the increased stiffness observed in blood vessels and cardiac walls. For instance, it has been suggested that hyperinsulinemia, which promotes sodium reabsorption (40, 44), stimulates the sympathetic nervous system (36, 52), and induces smooth muscle cell growth (1), might contribute to increased vascular stiffness. On the other hand, insulin bound to its receptor induces nitric oxide release from endothelium, facilitating vascular wall relaxation and decreasing blood pressure (33). In obesity, a state of insulin resistance, the effects of insulin bound to its receptor may be reduced, decreasing vascular wall relaxation (33). Finally, leptin, which is increased with increased fat mass, might mediate increased vascular wall stiffness associated with obesity since it promotes smooth muscle cell proliferation (35), even independently of body mass (39).
In summary, one could pose the hypothesis that, as in the vascular system, biological factors operating in obesity may lead to a state of smooth cell proliferation and/or lack of smooth muscle relaxation, and that result could increase stiffness of the colonic wall. Future studies will be needed to clarify the underlying mechanisms of the decreased colonic wall compliance observed in our study.
A third observation of the present study pertains to gastrointestinal and colonic transit. There was no significant association of BMI with gastric emptying and small bowel transit relative to healthy adults, after controlling for age, height, and sex. Effects of body mass on gastric emptying have already been evaluated previously, with inconclusive results. Some studies have shown a more rapid gastric emptying in obese subjects compared with lean controls (24, 45, 50, 51, 53), whereas others have reported normal (18) or even slow (26, 27, 31, 37) gastric emptying in obese subjects.
On the other hand, we did observe an overall acceleration of colonic transit with increasing BMI (P = 0.003), which was significantly influenced by sex, such that, after adjustment, the observation was only borderline significant (P = 0.08). This clearly requires further study, given the sample size (n = 72) of the present study. However, this accelerated colonic transit may contribute to the increased frequency of diarrhea reported in obese participants in population-based studies.
In conclusion, BMI over 25 kg/m2 is associated with decreased colonic compliance, decreased colonic sensation, and possibly accelerated colonic transit in those over BMI 30 kg/m2. These data suggest that factors other than colonic function such as afferent, spinal, or central mechanisms modulating pain perception might contribute to the observed increased prevalence of abdominal pain in obesity. This will require further study.
GRANTS
This study was supported in part by nursing support available through the Clinical Research Unit, Mayo Clinic CTSA grant RR024150 (Physiology Core) from National Institutes of Health (NIH). M. Camilleri is supported by grants R01-DK54681, RO1-DK-67071, and K24-DK02638 from NIH.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Begum N, Song Y, Rienzie J, Ragolia L. Vascular smooth muscle cell growth and insulin regulation of mitogen-activated protein kinase in hypertension. Am J Physiol Cell Physiol 275: C42–C49, 1998. [DOI] [PubMed] [Google Scholar]
- 2.Bharucha AE, Camilleri M, Haydock S, Ferber I, Burton D, Cooper S, Tompson D, Fitzpatrick K, Higgins R, Zinsmeister AR. Effects of a serotonin 5-HT(4) receptor antagonist SB-207266 on gastrointestinal motor and sensory function in humans. Gut 47: 667–674, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bharucha AE, Camilleri M, Zinsmeister AR, Hanson RB. Adrenergic modulation of human colonic motor and sensory function. Am J Physiol Gastrointest Liver Physiol 273: G997–G1006, 1997. [DOI] [PubMed] [Google Scholar]
- 4.Camilleri M, Colemont LJ, Phillips SF, Brown ML, Thomforde GM, Chapman N, Zinsmeister AR. Human gastric emptying and colonic filling of solids characterized by a new method. Am J Physiol Gastrointest Liver Physiol 257: G284–G290, 1989. [DOI] [PubMed] [Google Scholar]
- 5.Chial HJ, Camilleri M, Burton D, Thomforde G, Olden KW, Stephens D. Selective effects of serotonergic psychoactive agents on gastrointestinal functions in health. Am J Physiol Gastrointest Liver Physiol 284: G130–G137, 2003. [DOI] [PubMed] [Google Scholar]
- 6.Chial HJ, Camilleri M, Ferber I, Delgado-Aros S, Burton D, McKinzie S, Zinsmeister AR. Effects of venlafaxine, buspirone, and placebo on colonic sensorimotor functions in healthy humans. Clin Gastroenterol Hepatol 1: 211–218, 2003. [DOI] [PubMed] [Google Scholar]
- 8.Cremonini F, Mullan BP, Camilleri M, Burton DD, Rank MR. Performance characteristics of scintigraphic transit measurements for studies of experimental therapies. Aliment Pharmacol Ther 16: 1781–1790, 2002. [DOI] [PubMed] [Google Scholar]
- 9.Delaney CA, Mouser JV, Westerman RA. Insulin sensitivity and sensory nerve function. Clin Exp Neurol 31: 19–37, 1994. [PubMed] [Google Scholar]
- 10.Delgado-Aros S, Chial HJ, Camilleri M, Szarka LA, Weber FT, Jacob J, Ferber I, McKinzie S, Burton DD, Zinsmeister AR. Effects of a κ-opioid agonist, asimadoline, on satiation and GI motor and sensory functions in humans. Am J Physiol Gastrointest Liver Physiol 284: G558–G566, 2003. [DOI] [PubMed] [Google Scholar]
- 11.Delgado-Aros S, Cremonini F, Castillo JE, Chial HJ, Burton DD, Ferber I, Camilleri M. Independent influences of body mass and gastric volumes on satiation in humans. Gastroenterology 126: 432–440, 2004. [DOI] [PubMed] [Google Scholar]
- 12.Delgado-Aros S, Locke GR, Camilleri M, Talley NJ, Fett SL, Zinsmeister AR, Melton LJ 3rd. Obesity is associated with increased risk of gastrointestinal symptoms: a population-based study. Am J Gastroenterol 99: 1801–1806, 2004. [DOI] [PubMed] [Google Scholar]
- 13.Distrutti E, Azpiroz F, Soldevilla A, Malagelada JR. Gastric wall tension determines perception of gastric distention. Gastroenterology 116: 1035–1042, 1999. [DOI] [PubMed] [Google Scholar]
- 14.Erdmann J, Leibl M, Wagenpfeil S, Lippl F, Schusdziarra V. Ghrelin response to protein and carbohydrate meals in relation to food intake and glycerol levels in obese subjects. Regul Pept 135: 23–29, 2006. [DOI] [PubMed] [Google Scholar]
- 15.Esfandyari T, Camilleri M, Busciglio I, Burton D, Baxter K, Zinsmeister AR. Effects of a cannabinoid receptor agonist on colonic motor and sensory functions in humans: a randomized, placebo-controlled study. Am J Physiol Gastrointest Liver Physiol 293: G137–G145, 2007. [DOI] [PubMed] [Google Scholar]
- 16.Esfandyari T, Camilleri M, Ferber I, Burton D, Baxter K, Zinsmeister AR. Effect of a cannabinoid agonist on gastrointestinal transit and postprandial satiation in healthy human subjects: a randomized, placebo-controlled study. Neurogastroenterol Motil 18: 831–838 2006. [DOI] [PubMed] [Google Scholar]
- 17.Ford MJ, Camilleri M, Zinsmeister AR, Hanson RB. Psychosensory modulation of colonic sensation in the human transverse and sigmoid colon. Gastroenterology 109: 1772–1780, 1995. [DOI] [PubMed] [Google Scholar]
- 18.French SJ, Murray B, Rumsey RD, Sepple CP, Read NW. Preliminary studies on the gastrointestinal responses to fatty meals in obese people. Int J Obes Relat Metab Disord 17: 295–300, 1993. [PubMed] [Google Scholar]
- 19.Gautier JF, Chen K, Salbe AD, Bandy D, Pratley RE, Heiman M, Ravussin E, Reiman EM, Tataranni PA. Differential brain responses to satiation in obese and lean men. Diabetes 49: 838–846, 2000. [DOI] [PubMed] [Google Scholar]
- 20.Gautier JF, Del Parigi A, Chen K, Salbe AD, Bandy D, Pratley RE, Ravussin E, Reiman EM, Tataranni PA. Effect of satiation on brain activity in obese and lean women. Obes Res 9: 676–684, 2001. [DOI] [PubMed] [Google Scholar]
- 21.Geliebter A, Hashim SA. Gastric capacity in normal, obese, and bulimic women. Physiol Behav 74: 743–746, 2001. [DOI] [PubMed] [Google Scholar]
- 22.Geliebter A, Westreich S, Gage D. Gastric distention by balloon and test-meal intake in obese and lean subjects. Am J Clin Nutr 48: 592–594, 1988. [DOI] [PubMed] [Google Scholar]
- 23.Gonenne J, Camilleri M, Ferber I, Burton D, Baxter K, Keyashian K, Foss J, Wallin B, Du W, Zinsmeister AR. Effect of alvimopan and codeine on gastrointestinal transit: a randomized controlled study. Clin Gastroenterol Hepatol 3: 784–791, 2005. [DOI] [PubMed] [Google Scholar]
- 24.Gryback P, Naslund E, Hellstrom PM, Jacobsson H, Backman L. Gastric emptying of solids in humans: improved evaluation by Kaplan-Meier plots, with special reference to obesity and gender. Eur J Nucl Med 23: 1562–1567, 1996. [DOI] [PubMed] [Google Scholar]
- 25.Hammer HF, Phillips SF, Camilleri M, Hanson RB. Rectal tone, distensibility, and perception: reproducibility and response to different distensions. Am J Physiol Gastrointest Liver Physiol 274: G584–G590, 1998. [DOI] [PubMed] [Google Scholar]
- 26.Horowitz M, Collins PJ, Cook DJ, Harding PE, Shearman DJ. Abnormalities of gastric emptying in obese patients. Int J Obes 7: 415–421, 1983. [PubMed] [Google Scholar]
- 27.Horowitz M, Collins PJ, Shearman DJ. Effect of increasing the caloric/osmotic content of the liquid component of a mixed solid and liquid meal on gastric emptying in obese subjects. Hum Nutr Clin Nutr 40: 51–56, 1986. [PubMed] [Google Scholar]
- 28.Le DS, Pannacciulli N, Chen K, Del Parigi A, Salbe AD, Reiman EM, Krakoff J. Less activation of the left dorsolateral prefrontal cortex in response to a meal: a feature of obesity. Am J Clin Nutr 84: 725–731, 2006. [DOI] [PubMed] [Google Scholar]
- 29.Levy RL, Linde JA, Feld KA, Crowell MD, Jeffery RW. The association of gastrointestinal symptoms with weight, diet, and exercise in weight-loss program participants. Clin Gastroenterol Hepatol 3: 992–996, 2005. [DOI] [PubMed] [Google Scholar]
- 30.Mackey RH, Sutton-Tyrrell K, Vaitkevicius PV, Sakkinen PA, Lyles MF, Spurgeon HA, Lakatta EG, Kuller LH. Correlates of aortic stiffness in elderly individuals: a subgroup of the Cardiovascular Health Study. Am J Hypertens 15: 16–23, 2002. [DOI] [PubMed] [Google Scholar]
- 31.Maddox A, Horowitz M, Wishart J, Collins P. Gastric and oesophageal emptying in obesity. Scand J Gastroenterol 24: 593–598, 1989. [DOI] [PubMed] [Google Scholar]
- 32.Matsuda M, Liu Y, Mahankali S, Pu Y, Mahankali A, Wang J, DeFronzo RA, Fox PT, Gao JH. Altered hypothalamic function in response to glucose ingestion in obese humans. Diabetes 48: 1801–1806, 1999. [DOI] [PubMed] [Google Scholar]
- 33.Montagnani M, Quon MJ. Insulin action in vascular endothelium: potential mechanisms linking insulin resistance with hypertension. Diabetes Obes Metab 2: 285–292, 2000. [DOI] [PubMed] [Google Scholar]
- 34.Morley JE, Levine AS. The role of the endogenous opiates as regulators of appetite. Am J Clin Nutr 35: 757–761, 1982. [DOI] [PubMed] [Google Scholar]
- 34a.National Institutes of Health. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults—the evidence report. Obes Res 6, Suppl 2: 51S–209S, 1998. [PubMed] [Google Scholar]
- 35.Oda A, Taniguchi T, Yokoyama M. Leptin stimulates rat aortic smooth muscle cell proliferation and migration. Kobe J Med Sci 47: 141–150, 2001. [PubMed] [Google Scholar]
- 36.O'Hare JA, Minaker KL, Meneilly GS, Rowe JW, Pallotta JA, Young JB. Effect of insulin on plasma norepinephrine and 3,4-dihydroxyphenylalanine in obese men. Metabolism 38: 322–329, 1989. [DOI] [PubMed] [Google Scholar]
- 37.Portincasa P, Di Ciaula A, Palmieri V, Van Berge-Henegouwen GP, Palasciano G. Effects of cholestyramine on gallbladder and gastric emptying in obese and lean subjects. Eur J Clin Invest 25: 746–753, 1995. [DOI] [PubMed] [Google Scholar]
- 38.Report of a WHO Expert Committee. Physical status: the use, and interpretation of anthropometry. World Health Organ Tech Rep Ser 854: 1–452, 1995. [PubMed] [Google Scholar]
- 39.Singhal A, Farooqi IS, Cole TJ, O'Rahilly S, Fewtrell M, Kattenhorn M, Lucas A, Deanfield J. Influence of leptin on arterial distensibility: a novel link between obesity and cardiovascular disease? Circulation 106: 1919–1924, 2002. [DOI] [PubMed] [Google Scholar]
- 40.Stenvinkel P, Bolinder J, Alvestrand A. Effects of insulin on renal haemodynamics and the proximal and distal tubular sodium handling in healthy subjects. Diabetologia 35: 1042–1048, 1992. [DOI] [PubMed] [Google Scholar]
- 41.Sutton-Tyrrell K, Newman A, Simonsick EM, Havlik R, Pahor M, Lakatta E, Spurgeon H, Vaitkevicius P. Aortic stiffness is associated with visceral adiposity in older adults enrolled in the study of health, aging, and body composition. Hypertension 38: 429–433, 2001. [DOI] [PubMed] [Google Scholar]
- 42.Talley NJ, Howell S, Poulton R. Obesity and chronic gastrointestinal tract symptoms in young adults: a birth cohort study. Am J Gastroenterol 99: 1807–1814, 2004. [DOI] [PubMed] [Google Scholar]
- 43.Talley NJ, Quan C, Jones MP, Horowitz M. Association of upper and lower gastrointestinal tract symptoms with body mass index in an Australian cohort. Neurogastroenterol Motil 16: 413–419, 2004. [DOI] [PubMed] [Google Scholar]
- 44.ter Maaten JC, Bakker SJ, Serne EH, ter Wee PM, Donker AJ, Gans RO. Insulin's acute effects on glomerular filtration rate correlate with insulin sensitivity whereas insulin's acute effects on proximal tubular sodium reabsorption correlation with salt sensitivity in normal subjects. Nephrol Dial Transplant 14: 2357–2363, 1999. [DOI] [PubMed] [Google Scholar]
- 45.Tosetti C, Corinaldesi R, Stanghellini V, Pasquali R, Corbelli C, Zoccoli G, Di Febo G, Monetti N, Barbara L. Gastric emptying of solids in morbid obesity. Int J Obes Relat Metab Disord 20: 200–205, 1996. [PubMed] [Google Scholar]
- 46.Vazquez Roque MI, Camilleri M, Stephens DA, Jensen MD, Burton DD, Baxter KL, Zinsmeister AR. Gastric sensorimotor functions and hormone profile in normal weight, overweight, and obese people. Gastroenterology 131: 1717–1724, 2006. [DOI] [PubMed] [Google Scholar]
- 47.Verdich C, Toubro S, Buemann B, Lysgard MJ, Juul HJ, Astrup A. The role of postprandial releases of insulin and incretin hormones in meal-induced satiety—effect of obesity and weight reduction 1. Int J Obes Relat Metab Disord 25: 1206–1214, 2001. [DOI] [PubMed] [Google Scholar]
- 48.Viramontes BE, Camilleri M, McKinzie S, Pardi DS, Burton D, Thomforde GM. Gender-related differences in slowing colonic transit by a 5-HT3 antagonist in subjects with diarrhea-predominant irritable bowel syndrome. Am J Gastroenterol 96: 2671–2676, 2001, [DOI] [PubMed] [Google Scholar]
- 49.Wildman RP, Mackey RH, Bostom A, Thompson T, Sutton-Tyrrell K. Measures of obesity are associated with vascular stiffness in young and older adults. Hypertension 42: 468–473, 2003. [DOI] [PubMed] [Google Scholar]
- 50.Wisen O, Johansson C. Gastrointestinal function in obesity: motility, secretion, and absorption following a liquid test meal. Metabolism 41: 390–395, 1992. [DOI] [PubMed] [Google Scholar]
- 51.Wright RA, Krinsky S, Fleeman C, Trujillo J, Teague E. Gastric emptying and obesity. Gastroenterology 84: 747–751, 1983. [PubMed] [Google Scholar]
- 52.Young JB Effect of experimental hyperinsulinemia on sympathetic nervous system activity in the rat. Life Sci 43: 193–200, 1988. [DOI] [PubMed] [Google Scholar]
- 53.Zahorska-Markiewicz B, Jonderko K, Lelek A, Skrzypek D. Gastric emptying in obesity. Hum Nutr Clin Nutr 40: 309–313, 1986. [PubMed] [Google Scholar]
- 54.Zahorska-Markiewicz B, Kucio C, Pyszkowska J. Obesity and pain. Hum Nutr Clin Nutr 37: 307–310, 1983. [PubMed] [Google Scholar]
- 55.Zahorska-Markiewicz B, Zych P, Kucio C. Pain sensitivity in obesity. Acta Physiol Pol 39: 183–187 1988. [PubMed] [Google Scholar]