Abstract
Background
Cecal or distal colonic concentration of butyrate has been used as an index of butyrate production from various fermentable carbohydrates. However, we previously found that cecal concentration of butyrate does not correlate with the rate of synthesis of butyrate in the cecal lumen. As part of a larger study of the cellular effects of cecal infusions of butyrate, we sought to rule out the null hypothesis that cecal infusion of butyrate also would not alter butyrate concentration in the cecum.
Methods
Piglets (n = 10) were fed sow milk replacement formula plus inulin (3 g × L-1). After 6 days of oral feeding, the piglets were randomly assigned into 2 equal groups: (I) Cecal infusion of phosphate-buffered NaCl and (II) cecal infusion of butyrate (2.13 μmol × kg-1 × min-1). The concentration of butyrate was measured by gas chromatography in the cecum and distal colon.
Results
There was no effect of cecal butyrate infusion on butyrate concentration (mM; I vs II) in the cecum (5.7 ± 0.4 vs 5.3 ± 1.1) or distal colon (3.3 ± 0.6 vs 4.1 ± 0.8) or on the ratio of cecal butyrate concentration to the sum of the concentrations of butyrate, acetate, propionate, and valerate (0.101 ± 0.004 vs 0.083 ± 0.011). There was no effect of cecal butyrate infusion on the concentration of any of these short chain fatty acids.
Conclusions
At an entry rate into the cecum within the physiological range, butyrate had no effect on cecal or distal colonic luminal concentration of butyrate.
Keywords: butyrate, cecum, short chain fatty acids, colon, luminal concentration
Sugars, starches, or fibers, not digested in the small intestine, are subjected to bacterial fermentation in the colon, which produces butyrate and other short chain fatty acids; these fatty acids are normally efficiently absorbed in the colon and have several potential biological effects.1 In cultured neoplastic cells of colonic origin, butyrate generally causes a suppression of the cell cycle, although the effect may be concentration dependent.2,3 In these studies, the medium concentration of butyrate is in the range found in the colon lumen (e.g., 0.5-2 mM),2 presumably because of the assumption that colonic mucosal uptake of butyrate will be proportional to luminal concentration. Indeed, cecal, colonic, or even fecal concentrations of butyrate are generally used as indices of butyrate production from various fermentable starches or fibers (eg, Lupton and Kurtz PP and Cummings).4,5 In our stable isotope tracer studies of the rate of appearance (synthesis) of butyrate into the colonic lumen,6 we found that the fractional uptake of cecum-derived butyrate was virtually complete in all piglets. We also found that cecal butyrate concentration was not different among different dietary groups, which varied in butyrate synthesis rate.6 Unpublished data analysis based on that study6 showed no linear correlation of cecal butyrate concentration with either luminal production rate or estimated net cecal mucosal uptake. However, there certainly is experimental error inherent in any technique for measuring colonic luminal synthesis of butyrate. Therefore, we believed it would be useful to examine the relationship between the rate of infusion of exogenous butyrate into the cecum and cecal concentration. We nominally sought to rule out the null hypothesis that cecal butyrate infusion, at a physiological rate, would not alter cecal butyrate concentration.
Materials and Methods
Animals, Feedings, and Design
Piglets were studied, which also were the subjects of a previously published study of the effect of butyrate and/or inulin on intestinal cell proliferation7; therefore, some experimental details will not be given here.
Fifteen standard Yorkshire/Hampshire piglets were studied at The University of Vermont, where the Institutional Animal Care and Use Committee approved the research protocol. On approximately day 12 of life, the piglets were transported from the pig farm to the laboratory. For 6 days, the piglets then were fed orally a sow milk replacer (SMR) formula (Control formula, C; SPF Lac, Sterile milk replacer, PetAG Inc., Hampshire, IL).7
This study is based on piglets in 3 study groups: (1) The control group (C) received only the SMR formula for both the preoperative period of 6 days and also postoperatively after the insertion of a cecal catheter on day 7 (n = 5). For 4 days postoperatively, this group received a cecal infusion (1 mL × h-1) of phosphate-buffered saline (PBS; pH 7.4; phosphate, 10 mmol × L-1; NaCl, 138 mmol × L-1 ; KCl, 2.7 mmol × L-1; P-3813, SIGMA, St. Louis, MO). (2) The experimental group, I, was fed SMR and inulin (3 g × L-1) for the entire study and a cecal infusion of PBS postoperatively at an identical rate to group C (n = 5). (3) The experimental group (B + I), was fed SMR + inulin for the entire study period and received a cecal butyrate infusion postoperatively (2.13 mol kg-1 min-1) (n = 5). This report will not consider the data on piglets in a fourth study group, B, which was fed SMR for the entire experiment but, for 4 days postoperatively, received a cecal infusion of butyrate. We were only able to obtain cecal fluid in 2 piglets in this group.
During the study, body weight, formula intake, and stool characteristics were monitored. Diarrhea was quantified as previously described.8,9
Details of the surgical procedures have been described previously.6,7,10-13 Anesthesia was induced (intramuscular telazol, 6 mg × kg-1, and xylazine, 4 mg × kg-1), and general anesthesia was maintained with isoflurane by means of an endotracheal tube. A cecal cannula was inserted very close to the ileocecal valve.10 This acetyl copolymer cannula was modified from one originally designed for sampling excreta from the ileum.14 The modifications included a lengthening of the portion of the cannula exiting the abdominal wall as previously described,10 but also, for the experiments described in this and our most previous study,7 we added an additional removable extension that was fitted to the cannula after it exited the abdominal wall, so that the fitting connecting it to infusion tubing was completely enclosed by flexible metal tubing. After 4 days of cecal infusion (day 5), the piglets were anesthetized with the same telazol/xylazine combination followed by maintenance of general anesthesia with isoflurane. A laparotomy was performed, and incisions were made in the cecum (8-10 cm from the ileocecal valve) and distal colon (10-15 cm from the rectum), and the respective luminal contents were removed and frozen at -80°C. for subsequent analysis.
Measurement of Cecal and Distal Colonic Concentration of Short Chain Fatty Acids
We followed closely our previously published technique but used a new gas chromatography system (6890N Network GC System, Agilent Technologies, Wilmington, DE). With each run of cecal or distal colon samples, we ran a set of external standards in the concentration range of our samples. We defined percent “recovery” as the following: estimated concentration/known concentration × 100. Percent recovery averaged (coefficient of variation) 52.2% (10.8%), 82% (8.5%), 97.1% (8.2%), and 118.6% (7.8%) for acetate, propionate, butyrate, and valerate. For acetate, we are reporting values corrected for recovery.
Data Analysis and Statistics
Because we could not study the piglets in the piglets receiving control formula and butyrate infusion, our main statistical analysis was a 2-sample t test between the 2 inulin groups. However, for reference to a study in humans examining the effect of fructooligosaccharides on fecal short chain fatty acid concentrations,15 we compared the 2 inulin-fed groups with the control group using 1-way ANOVA, and we compared Group I with Group C using the 2-sample t test. All statistical analyses were completed using SPSS (SPSS Base 10.0, SPSS Inc., Chicago, IL). All results are expressed as mean ± SEM. A P value of .05 was accepted as statistically significant.
Results
Luminal Concentrations of Butyrate and Other Short Chain Fatty Acids in the Cecum and Distal Colon
In the inulin-fed groups, there was no effect of cecal butyrate infusion on butyrate concentration (mM; I vs I + B) in the cecum (5.7 ± 0.4 vs 5.3 ± 1.1; Figure 1) and distal colon (3.3 ± 0.6 vs 4.1 ± 0.8). The fractional concentration of butyrate was defined as the ratio of butyrate concentration to the sum of the concentrations of butyrate, acetate, propionate, and valerate. Butyrate infusion did not affect this ratio either in the cecum (0.101 ± 0.004 vs 0.083 ± 0.011; Figure 2) or colon (0.115 ± 0.013 vs 0.107 ± 0.016). There also was no effect of cecal butyrate infusion on the concentration of acetate, propionate, and valerate.
Figure 1.
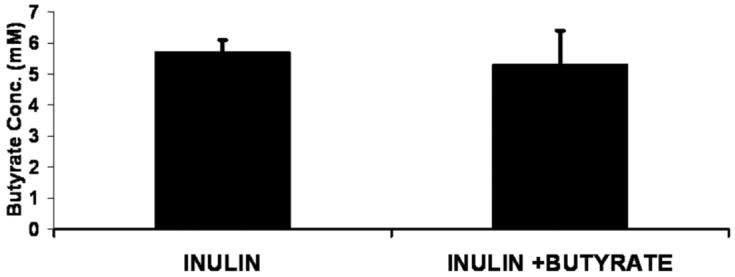
Cecal butyrate concentration in the cecum in piglets fed inulin or fed inulin plus cecal butyrate infusion (I + B; I vs I + B, NS).
Figure 2.
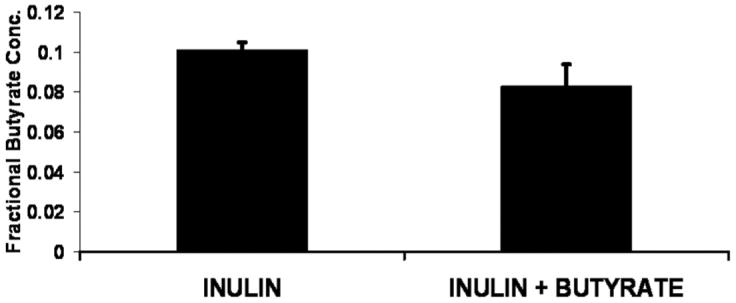
Fractional cecal concentration of butyrate (the ratio of butyrate concentration to the sum of the concentrations of butyrate, acetate, propionate, and valerate). I, fed inulin, no butyrate infusion; I + B, fed inulin plus cecal butyrate infusion (I vs I + B, NS).
In the control group, the concentration of butyrate in the cecal and distal colonic fluid was, respectively, 5.4 ± 1.3 mM and 3.2 ± 0.8 mM and did not differ statistically either compared with Group I or both Groups I and I + B. Similarly, the fractional concentration of butyrate in the cecal fluid (0.103 ± 0.015) and distal colonic fluid (0.099 ± 0.021) was not different in the control group compared with both of the other 2 groups or to Group I. Finally, the concentrations of acetate, propionate, valerate, and the fractional concentration of each of these short chain fatty acids were not significantly different among the 3 groups or between Groups C and I.
Discussion
This study, along with our previous study, suggests that cecal luminal concentration of butyrate does not change in response to the rate of entry of butyrate into the cecum of piglets, whether by means of bacterial production of butyric acid6 or exogenous infusion of butyrate. However, our conclusions are limited to the particular rate of infusion of butyrate we used. Because concentration is a “state” measurement and isotopically measured synthetic rates6 or infusion rates are “rate” measurements, it may seem obvious that concentration would not necessarily reflect rate of entry of butyrate into the cecum from endogenous (bacteria) or exogenous sources. Nevertheless, many investigators use measurements of cecal, colonic, or fecal butyrate concentration as an index of production. Our previous study6 compared the effects of 4 feeding regimens on both luminal synthesis of butyrate measured with a stable isotope technique and cecal butyrate concentration. Feeding a high dose of lactulose seemed to intensify fermentation as indicated by an approximate doubling of the rate of butyrate synthesis, on average, compared with controls, without raising the cecal butyrate concentration, which actually appeared to be lower in the lactulose-feeding group.6 Inulin feeding did not significantly change the rate of synthesis of butyrate, the mean in the group being 27% to 38% higher than the control mean, depending on whether the data were normalized for body weight or not. In the present study, we infused butyrate at a rate designed to approximately double the normal rate of butyrate synthesis. In inulin-fed piglets, we observed no effect of the rate of butyrate entering the cecum (from an exogenous source) on cecal or colon concentrations of butyrate or other short chain fatty acids. Thus, taken together, these two studies suggest that it might not be accurate to judge the propensity of different fermentable starches or fiber to produce butyrate, based on the static measurement of butyrate concentration in the colon lumen. In this regard, as an ancillary goal, we also examined the effect of inulin feeding on cecal and distal colonic short chain fatty acid concentrations; we observed no effect in contrast to reported effects, in humans, of a larger intake of fructooligosaccharide on fecal concentrations.15
Data derived from our studies could affect how one carries out future studies of butyrate’s effects on the colonocyte, when the goal is to use butyrate, not as a reagent to induce differentiation and inhibit histone deacetylase, but to explore its potential in vivo effects. If luminal concentration has no particular bearing on the rate of uptake of butyrate by colonic mucosa, perhaps consideration needs to be given to exploring ranges of concentration in cell culture2 rather than trying to mimic the luminal concentration. However, one can envision that luminal concentration of butyrate also may have special importance. There could be inflammatory signaling pathways elicited by the concentration of butyrate or other organic acids in the lumen. For example, either lactate or acetate infused at high concentrations can cause reversible injury to the colonic mucosa of piglets,16 and a large, single, intraluminal dose of butyrate (600 or 3000 μcmol/kg) into the proximal colon of the newborn rat caused severe colonic necrosis.17
In conclusion, we showed that cecal butyrate infusion at a rate equal to the normal production rate does not change butyrate concentration in the cecum or distal colon. Thus, colonic concentration of butyrate does not appear to be a reliable index of the rate of entry of exogenous or endogenous (bacteria-derived) butyrate6 into the lumen.
Acknowledgments
We appreciate the technical assistance of Rhonda Maple and Karen Everingham, and Morton Alling IV and Heather Joyce of the Animal Resources Center, University of Vermont.
Financial disclosure: This study was supported by NIH Grant DK61775.
References
- 1.Roy CC, Kien CL, Bouthillier L, Levy E. Short-chain fatty acids: ready for prime time? Nutr Clin Pract. 2006;21:351–366. doi: 10.1177/0115426506021004351. [DOI] [PubMed] [Google Scholar]
- 2.Singh B, Halestrap A, Paraskeva C. Butyrate can act as a stimulator of growth or inducer of apoptosis in human colonic epithelial cell lines depending on the presence of alternative energy sources. Carcinogenesis. 1997;18:1265–1270. doi: 10.1093/carcin/18.6.1265. [DOI] [PubMed] [Google Scholar]
- 3.Davie JR. Inhibition of histone deacetylase activity by butyrate. J Nutr. 2003;133(7 Suppl):2485S–2493S. doi: 10.1093/jn/133.7.2485S. [DOI] [PubMed] [Google Scholar]
- 4.Lupton JR, Kurtz PP. Relationship of colonic luminal short-chain fatty acids and pH to in vivo cell proliferation in rats. J Nutr. 1993;123:1522–1530. doi: 10.1093/jn/123.9.1522. [DOI] [PubMed] [Google Scholar]
- 5.Cummings JH. Fermentation in the human large intestine: evidence and implications for health. Lancet. 1983;11:1206–1208. doi: 10.1016/s0140-6736(83)92478-9. [DOI] [PubMed] [Google Scholar]
- 6.Kien CL, Schmitz-Brown M, Solley T, Sun D, Frankel WL. Increased colonic luminal synthesis of butyric acid is associated with lowered colonic cell proliferation in piglets. J Nutr. 2006;136:64–69. doi: 10.1093/jn/136.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kien CL, Blauwiekel R, Bunn JY, Jetton TL, Frankel WL, Holst JJ. Cecal infusion of butyrate increases intestinal cell proliferation in piglets. J Nutr. 2007;137:916–922. doi: 10.1093/jn/137.4.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kien CL, Murray RD, Qualman SJ, Marcon M. Lactulose feeding in piglets: a model for persistent diarrhea and colitis induced by severe sugar malabsorption. Dig Dis Sci. 1999;44:1476–1484. doi: 10.1023/a:1026672306929. [DOI] [PubMed] [Google Scholar]
- 9.Kien CL, Chang JC, Cooper JR, Frankel WL. Effects of prefeeding a prebiotic on diarrhea and colonic cell proliferation in piglets fed lactulose. JPEN J Parenter Enteral Nutr. 2004;28:22–26. doi: 10.1177/014860710402800122. [DOI] [PubMed] [Google Scholar]
- 10.Kien CL, Ailabouni AH, Murray RD, Powers PA, McClead RE, Kepner J. Technical note: pig model for studying nutrient assimilation by the intestine and colon. J Anim Sci. 1997;75:2161–2164. doi: 10.2527/1997.7582161x. [DOI] [PubMed] [Google Scholar]
- 11.Kien CL, Chang JC, Cooper JR. Quantitation of colonic luminal synthesis of butyric acid in piglets. J Pediatr Gastroenterol Nutr. 2002;35:324–328. doi: 10.1097/00005176-200209000-00016. [DOI] [PubMed] [Google Scholar]
- 12.Ebner S, Schoknecht P, Reeds P, Burrin D. Growth and metabolism of gastrointestinal and skeletal muscle tissues in protein-malnourished neonatal pigs. Am J Physiol. 1994;266:R1736–R1743. doi: 10.1152/ajpregu.1994.266.6.R1736. [DOI] [PubMed] [Google Scholar]
- 13.Reeds PJ, Burrin DG, Jahoor F, Wykes L, Henry J, Frazer EM. Enteral glutamate is almost completely metabolized in first pass by the gastrointestinal tract of infant pigs. Am J Physiol. 1996;270:E413–E418. doi: 10.1152/ajpendo.1996.270.3.E413. [DOI] [PubMed] [Google Scholar]
- 14.Walker WR, Morgan GL, Maxwell CV. Ileal cannulation in baby pigs with a simple t-cannula. J Anim Sci. 1986;62:407–411. [Google Scholar]
- 15.Whelan K, Judd PA, Preedy VR, Simmering R, Jann A, Taylor MA. Fructooligosaccharides and fiber partially prevent the alterations in fecal microbiota and short-chain fatty acid concentrations caused by standard enteral formula in healthy humans. J Nutr. 2005;135:1896–1902. doi: 10.1093/jn/135.8.1896. [DOI] [PubMed] [Google Scholar]
- 16.Argenzio RA, Meuten DJ. Short-chain fatty acids induce reversible injury of porcine colon. Dig Dis Sci. 1991;36:1459–1468. doi: 10.1007/BF01296816. [DOI] [PubMed] [Google Scholar]
- 17.Nafday SM, Chen W, Peng L, Babyatsky MW, Holzman IR, Lin J. Short-chain fatty acids induce colonic mucosal injury in rats with various postnatal ages. Pediatr Res. 2005;57:201–204. doi: 10.1203/01.PDR.0000150721.83224.89. [DOI] [PubMed] [Google Scholar]


