Abstract
Despite memory failures being a central feature of Amnestic Mild Cognitive Impairment (a-MCI), there is limited research into the nature of the memory impairment associated with this condition. A further understanding could lead to refinement of criteria needed to qualify for this designation and aid in prediction of who will progress to development of clinical Alzheimer’s disease. Dual process models posit that recognition memory is supported by the dissociable processes of recollection and familiarity. The present study sought to evaluate recognition memory in a-MCI in the framework of the dual process model. Patients with a-MCI and age- and education-matched controls were tested on three memory paradigms. Two paradigms were modifications of the process-dissociation procedure in which recollection required either memory of word-pair associations (associative) or the font color of words at study (featural). A final paradigm utilized the task-dissociation methodology comparing performance for item and visual spatial source memory. All three tasks revealed that familiarity was impaired to at least the same extent as recollection. As familiarity is thought to be spared in normal aging, its measurement may provide a relatively specific marker for the early pathological changes of Alzheimer’s disease.
Keywords: Alzheimer’s disease, memory, dual process, process-dissociation procedure, task-dissociation
Introduction
Mild Cognitive Impairment (MCI) has been conceptualized as a transitional state from normal aging to clinical Alzheimer’s disease (AD) or other forms of dementia. Consistent with this notion a number of studies have found that people with MCI develop AD at a rate much higher than age-matched controls without cognitive impairment [10–15%/year versus 1–2%/year, respectively; (Gauthier et al., 2006; Petersen, 2004)]. Further, pathological studies support that a significant percentage of patients classified with MCI have AD pathology (Guillozet, Weintraub, Mash, & Mesulam, 2003; Kordower et al., 2001; Mitchell et al., 2002; Petersen et al., 2006). However, notwithstanding the controversy as to whether MCI should be classified as ‘early AD’ (Morris, 2006; Morris et al., 2001), not all cases using current criteria appear to develop clinical AD. This is most evident in community-based studies in which there is a much lower rate of conversion to dementia and even a proportion of patients reverting back to a non-impaired designation over time (Ganguli, Dodge, Shen, & DeKosky, 2004; Larrieu et al., 2002). Additionally, there is not complete consensus on what specific criteria to use with regard to psychometrics and other factors. Thus, MCI represents a heterogeneous population which is, in part, driven by the manner in which the criteria are applied and the population studied.
In the last several years, MCI has been divided into subgroups; amnestic and non-amnestic types. These groups have been further divided based on whether there is the presence of a single or multiple domains of cognitive impairment (Petersen, 2004). Although recent work has suggested that a proportion of all classes of MCI will develop AD (Busse, Hensel, Guhne, Angermeyer, & Riedel-Heller, 2006), the majority of work on this diagnostic construct has focused on amnestic-MCI (a-MCI) presuming that this entity represents the transitional state to clinical AD. Despite this large body of work and the preeminent position of memory impairment in its classification, there remains somewhat limited investigation into the nature of the memory impairment beyond standard clinical psychometrics. A more detailed understanding of the phenotype of early AD memory impairment may be useful in further refining the criteria for MCI to reduce heterogeneity and aid in distinguishing those with true AD pathology. An approach informed by novel memory measures developed in the cognitive neuroscience literature may be of particular utility for this endeavor.
One major theory in the memory literature is that of the dual process model of recognition memory. While accounts vary in detail, the general notion is that recognition judgments are subserved by the functionally distinct processes of recollection and familiarity (Curran, Tepe, & Piatt, 2006; Eichenbaum, Yonelinas, & Ranganath, 2007; Jacoby, 1991; Mandler, 1980; Yonelinas, 2002). Recollection is defined as a conscious retrieval of associations and context while familiarity is an acontextual sense of prior exposure. Familiarity is usually conceived of as varying in strength and may be best described by signal detection or global matching models while recollection involves a qualitative, high threshold recovery of associates. While this model is not without disagreement (Donaldson, 1996; Slotnick & Dodson, 2005; Squire, Wixted, & Clark, 2007; Wixted, 2007), converging data from a variety of different modalities of enquiry are supportive, including studies of rodents and primates (Brown & Aggleton, 2001; Eichenbaum, 2004), healthy young and elderly subjects (Davidson & Glisky, 2002; Howard, Bessette-Symons, Zhang, & Hoyer, 2006; Yonelinas, 2002), patients with amnesia (Holdstock et al., 2002; Yonelinas et al., 2002b), functional MRI [fMRI; see (Eichenbaum, Yonelinas, & Ranganath, 2007), for review], and event-related potentials [ERPs; see (Rugg & Curran, 2007), for review]. Much of this work has supported an anatomic dissociation within the medial temporal lobes in support of these processes. Recollection has been linked to hippocampal function while familiarity appears related to perirhinal activity, as well as possibly that of the lateral entorhinal cortex (Brown & Aggleton, 2001; Eichenbaum, Yonelinas, & Ranganath, 2007). However, not all work has supported this anatomic dissociation with some suggesting that these regions differentially support weak and strong memories, orthogonal to recollection and familiarity [(Wais, Wixted, Hopkins, & Squire, 2006; Wixted & Squire, 2004); see (Squire, Wixted, & Clark, 2007) for review].
In addition to the hippocampus, recollection, considered a more controlled process, appears dependent on neocortical structures, including prefrontal cortex. For example, patients with frontal lobe injury appear to have a relatively selective impairment of associative or source-based memories (thought to be dependent on recollection) (Johnson, O'Connor, & Cantor, 1997; Wheeler, Stuss, & Tulving, 1995; Yonelinas, 2002). Relative sparing of item memory (thought to be dependent on familiarity) in these patients suggests that the prefrontal cortex plays a less critical role for familiarity and that it is a more automatic memory process than recollection (Jacoby, 1991).
A number of studies have examined the impact of aging on recollection and familiarity. Using a variety of different methodologies, the majority of these studies support a general decline in recollection with little or no impairment in familiarity relative to younger subjects (Davidson & Glisky, 2002; Howard, Bessette-Symons, Zhang, & Hoyer, 2006; Jacoby, 1999; Jennings & Jacoby, 1997; Light, Patterson, Chung, & Healy, 2004; Parkin & Walter, 1992; Yonelinas, 2002). Recent work has suggested that the sparing of familiarity is associated with intact and, perhaps, compensatory increases in perirhinal activity (Cabeza et al., 2004; Daselaar, Fleck, Dobbins, Madden, & Cabeza, 2006). On the other hand, recollection decline appears related to measures of frontal lobe function (Davidson & Glisky, 2002; Parkin & Walter, 1992). The relationship of prefrontal dysfunction with age-associated memory impairment is consistent with fMRI studies demonstrating aberrant recruitment during the encoding and retrieval of older participants relative to their younger counterparts (Cabeza, Anderson, Locantore, & McIntosh, 2002; Grady et al., 1995; Logan, Sanders, Snyder, Morris, & Buckner, 2002; Rosen et al., 2002). It is also consistent with imaging findings that the largest age-related changes involve prefrontal-striatal structure (for review, see (Buckner, 2004; Raz & Rodrigue, 2006)). The direct role of the hippocampus in decline of recollection with healthy aging is less clear (Van Petten, 2004), but likely is, in part, related to disruption of prefrontal-hippocampal communication, if not intrinsic hippocampal dysfunction (Albert, 2002; Buckner, 2004).
Studies of recollection and familiarity in patients with AD have been limited; however, most work suggests an impairment of memories supported by both recollection and familiarity (Budson, Desikan, Daffner, & Schacter, 2000; Gallo, Sullivan, Daffner, Schacter, & Budson, 2004; Smith & Knight, 2002). This finding is consistent with the significant neurofibrillary tangle (NFT) pathology in the medial temporal lobes, involving both hippocampal and extra-hippocampal structures, by the time of clinical AD diagnosis (Braak & Braak, 1991; Delacourte et al., 1999).
There have been no studies which directly estimate recollection and familiarity in a-MCI. Some have reported failures of item recognition in addition to associative memory which would be suggestive of an impairment in both memory processes (Bennett, Golob, Parker, & Starr, 2006; Dudas, Clague, Thompson, Graham, & Hodges, 2005; Hudon et al., 2006). Alternatively, one study suggested a sparing of familiarity based on control-level performance on a forced-choice memory task using highly similar foils (Westerberg et al., 2006).
The present study sought to determine the degree to which recollection and familiarity are impaired in a-MCI relative to healthy controls. Although a heterogeneous population, it is expected that a significant percentage of our a-MCI patients have early AD pathology and will eventually develop clinical AD (as in other memory disorders clinics, our conversion rate is approximately 15%/year). In this context, we predicted that both recollection and familiarity would be lower than that of our control group. The rationale for this hypothesis comes from the fact that, at least for those destined to develop AD, there is expected to be early pathological changes of the disease. Interestingly, the earliest areas of NFT involvement in AD are the transentorhinal and entorhinal cortices (Braak & Braak, 1991; Delacourte et al., 1999). The transentorhinal cortex may be considered part of the medial aspect of the perirhinal cortex although exact boundaries remain controversial (Suzuki & Amaral, 2003). Regardless, perirhinal pathology is likely an early feature of the disease (Mitchell et al., 2002; Van Hoesen, Augustinack, Dierking, Redman, & Thangavel, 2000) and appears highly correlated with entorhinal pathology, including in patients with MCI (Mitchell et al., 2002). Given the putative role of perirhinal processing in familiarity-based memory, we expect this memory process to be impaired. In addition, pathology to the entorhinal cortex, which provides the main input to the hippocampus, as well as the hippocampus proper, should contribute to impaired recollection.
To measure these memory processes participants performed three separate recognition memory paradigms. Two tests followed the logic of the ‘process-dissociation procedure’ developed by Jacoby (Jacoby, 1991). These tasks create test stimuli in which recollection opposes familiarity, allowing for determination of the relative strength of these processes. The third paradigm involves the ‘task dissociation’ methodology [for review, see (Yonelinas, 2002)] in which we compared visual-spatial source memory (dependent on recollection) with item recognition memory (dependent on a mixture of recollection and familiarity, but may be performed accurately in the absence of recollection). Relative sparing of performance on the latter task would suggest a more selective impairment of recollection. All of the process measurement techniques are associated with assumptions and potential confounds despite general agreement of these methods across studies (Yonelinas et al., 2002b). We used more than one paradigm in the present study to provide converging data across different stimuli and estimation techniques.
Methods
Participants
Sixteen patients with a-MCI and 21 healthy controls participated in the study. Patients were recruited from the Alzheimer’s Disease Research Center (ADRC) of the University of Pittsburgh. As part of their enrollment in the ADRC, each patient underwent an extensive evaluation, including medical history and physical examination, neurological history and examination, semi-structured psychiatric evaluation, and neuropsychological assessment. Additionally, relevant blood work and brain imaging studies were evaluated. Clinical diagnosis was determined by review of the above data at a consensus conference attended by neurologists, neuropsychologists, and psychiatrists [see (Lopez et al., 2000) for more detailed description of the ADRC evaluation process]. This evaluation and consensus discussion is performed annually.
Diagnosis of MCI was made essentially following the criteria outlined by Petersen and others (Petersen, 2004; Petersen et al., 2001; Winblad et al., 2004). Patients had to have a memory problem, generally intact cognitive functioning and activities of daily living, objective evidence of memory impairment on cognitive testing, and not qualify for a diagnosis of dementia. There was no strict cut-off for the degree of memory impairment, but generally these patients performed greater than 1.5 SD’s below our age-adjusted means on verbal and/or non-verbal memory tests. Rather than a strict cut-off, clinical judgment accounting for the premorbid status of the patient and performance on other cognitive tests weighs into decisions of objective impairment (Petersen, 2004). Patients with both single-domain and multiple-domain a-MCI were included in the present study (10 single-domain, 6 multiple-domain). The University of Pittsburgh ADRC since 2000 has classified patients as MCI-amnestic type and MCI-multiple cognitive domain type. The former corresponds to the designation of single domain a-MCI. The latter includes patients who have deterioration in at least one cognitive domain (not including memory), or evidence of impairment in at least two domains, without sufficiently severe cognitive function impairment or compromised instrumental activities of daily living (IADL’s) to constitute dementia. Patients with the MCI-multiple cognitive domain type who had memory impairment were classified as multiple-domain a-MCI. Controls were age- and education-matched to the patients with a-MCI. Controls were recruited from the community and the majority of participants had been evaluated in the University of Pittsburgh ADRC by similar procedures as the patients, including consensus conference designation of ‘healthy control.’ Inclusion criteria included age between 55–85, > 7 years education, and English speaking at an early age. Participants were excluded if they had a history of clinical stroke, traumatic brain injury, alcohol or drug abuse/dependence, prior electroconvulsive therapy, and any significant disease or medical/psychiatric condition that was felt to impact neuropsychological performance. The study was approved by the Institutional Review Board of the University of Pittsburgh.
For the purposes of the present study all patients underwent at least the following psychometric testing: (1) Mini-Mental Status Exam (Folstein, Folstein, & McHugh, 1975); (2) Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) Word List Memory test (Morris et al., 1989); (3) category fluency (animals) (Spreen & Strauss, 1998) (4) Controlled Oral Word Association Test [COWAT; (Ivnik, Malec, Smith, Tangalos, & Petersen, 1996)]; (5) Trail Making Test A and B (Reitan, 1958); (6) a 15- or 30-item version of the Boston Naming Test [BNT; (Kaplan, Goodglass, & Weintraub, 1983)]; and (7) Digit Span subtest of the Wechsler Adult Intelligence Scale III (Wechsler, 1997). Four control participants completed only a portion of the above tests. However, all four had been adjudicated by the ADRC as a healthy control at their annual follow-up visit, did not have a drop in the MMSE from prior years, had normal medical and neurological examinations, had normal memory testing, and did not voice complaints with regard to their cognition. Demographic and psychometric data are presented in Table 1. As can be seen, patients with a-MCI had significant memory impairment. Tests for non-memory domains were either above the mean of our normative sample or within 1 SD below the mean. Nonetheless, patients with a-MCI performed statistically worse than our control group on several non-memory tests.
Table 1.
Demographic and Neuropsychological Data
| Controls | a-MCI | Norms | |
|---|---|---|---|
| Age | 71.2 (8.9) | 72.2 (6.6) | |
| Education | 16.1 (3.2) | 16.9 (2.6) | |
| Female:Male | 13:8 | 6:10 | |
| MMSE | 29.6 (.9) | 28.1* (1.8) | 28.9 (1.3) |
| Trails A | 28.4 (9.1) | 34.8 (11.2) | 40.6 (13.1) |
| Trails B | 60.6 (15.7) | 77.1 (32.9) | 87.2 (31.6) |
| Digits Forwards Max. | 6.9 (1.1) | 7.0 (1.0) | 6.7 (1.4) |
| Digits Backwards Max. | 5.7 (1.1) | 4.6** (1.0) | 5.2 (1.4) |
| CERAD Encoding | 22.7 (3.4) | 16.6** (3.3) | 22.3 (3.8) |
| CERAD Recall | 7.2 (1.8) | 2.4** (2.0) | 7.4 (2.1) |
| Category Fluency (animals) | 21.9 (4.9) | 16.3** (3.6) | 19.0 (5.1) |
| COWAT (FAS) | 47.9 (15.8) | 38.3* (11.8) | 43.5 (13.1) |
Note: Standard deviations are in parentheses.
p < 0.05
p < 0.01, compared to the control group.
Participants completed the three experimental paradigms below in the same order. Short breaks were taken between tests. All completed Experiment 1. Three controls and two patients with a-MCI did not complete Experiment 2 and 3, as these tests were added to the experimental protocol after their participation. An additional control participant did not complete Experiment 2 due to computer malfunction, and one patient with a-MCI was not included in analysis of Experiment 3 due to performance (all items were endorsed as “new”).
Experiment 1: Associative Process-Dissociation Procedure
Materials and Design
The design was adapted from Gallo and colleagues (Gallo, Sullivan, Daffner, Schacter, & Budson, 2004). We selected 240 words from the MRC Psycholinguistic database (http://www.psy.uwa.edu.au/mrcdatabase/uwa_mrc.htm) that had a high familiarity (M = 551.7) and concreteness (M = 595.0). Words were 4 to 8 letters (M = 5.2) and were of a modest frequency (M = 52.2 occurrences per million by Francis and Kucera norms). These words were randomly divided into 120 word-pairs. These pairs were then grouped into 6 lists of 20 word-pairs. An ‘intact’ and ‘re-arranged’ version of each pair was created; the latter was made by switching the second word of two pairs. All pairs were reviewed to assure that they were not obviously related to each other.
Participants studied 80 word pairs; half one-time and half repeated three-times (1x; 3x). Two repetition conditions allowed for assessment of the differential effect of study on measures of recollection and familiarity in the patients with a-MCI relative to the control group (Experiment 3 also utilized this strategy). An additional 2 word-pairs were added to the beginning and end of each list to serve as buffers. There were 120 test pairs. Forty pairs were presented exactly as studied (intact); half studied one-time and half studied three-times. Another 40 pairs were re-arranged pairs; half studied one-time and half studied three-times (at study they were in their original pairings so that each word of the re-arranged pair was studied 1x or 3x). Finally, 40 pairs were ‘novel’, in which neither word had appeared in the studied list. Test items were presented in a pseudorandom order such that a particular test type was not presented more than three times in succession. There were six counterbalanced lists such that each pair served as an intact, re-arranged, or novel pair in either the 1x or 3x conditions.
Procedure
Study pairs were presented in capital letters and black font on a white background using Psyscope software. The study task was self-paced and for each pair the participant was told that they should form a mental image of the referents of the two words and decide which is larger in size. Depending on their answer, they were told to push a button marked “1” or “2”. This keypress would trigger the presentation of the next pair. The incidental encoding task was chosen to assure semantic encoding of the items. Participants were also informed that they would be tested on these items at a later time.
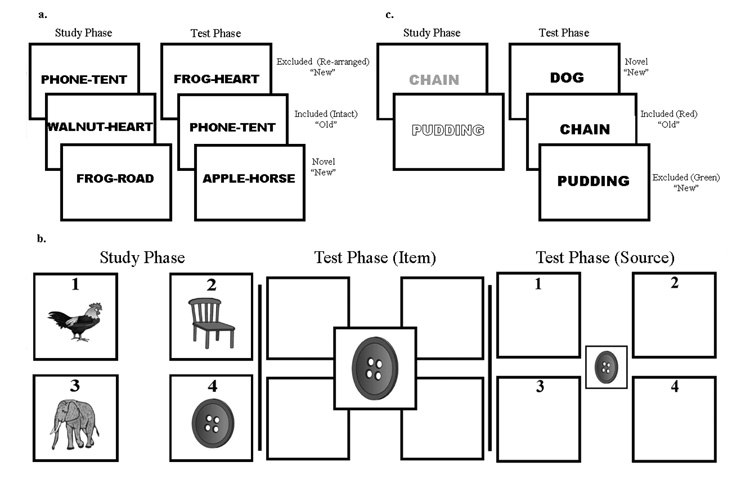
After a three minute delay, the test phase began. Test pairs were presented in the same manner as at study. Participants were given instructions to decide whether words were “old” or “new” based on the study list. They were told to only call pairs “old” if presented exactly as was seen at study. Subjects were explicitly told to call pairs “new” even if the words making up the pair were studied as part of a different pair. Participants had to demonstrate understanding of these instructions before the test trial began. A keypress of a button marked “OLD” or “NEW” triggered the next test item (see Figure 1a).
Figure 1. Experimental paradigms.
Schemas for the (a) associative process-dissociation procedure task, (b) the item versus visual spatial source task, and (c) the featural process-dissociation task are displayed. For the two process-dissociation tasks, only included items are to be given an old response. In the study phase of the featural task, the outlined and grey fonts represent green and red words on the actual task, respectively. For the displayed version, subjects are asked to only call words studied in red “old.”
Analysis
The current paradigm allows for estimation of recollection and familiarity based on the logic of the process-dissociation procedure. The essence of this method is the creation of a class of items in which recollection, as defined by associative or source memory, opposes familiarity (in this case, the re-arranged test items). In the original formulation, subjects study two lists which are followed by either of two test conditions, an ‘inclusion’ or ‘exclusion’ test. In the inclusion test, subjects are instructed to endorse studied items from either list as “old” and non-studied items as “new”. In the exclusion test, only items from one of the two study lists were to be endorsed as “old”. Thus, items from the other list (the ‘excluded’ items) would only be incorrectly endorsed as “old” if they were familiar, but the subject was unable to recollect which study list it was from (i.e. source memory). In other words, the probability that an excluded item would be endorsed as “Old” is equal to the probability that it was familiar (F) in the absence of recollection [R; p(excluded) = F − FR = (1 − R)F]. Alternatively, in the inclusion test the probability that items from the same list (the ‘included’ items) would be correctly endorsed as “old” is equal to the probability that the item was either recollected and/or simply familiar [p(included) = R + F − RF = R + (1 − R)F]. These equations can then be solved for both R and F [R = p(included) − p(excluded); F = p(excluded)/(1−R)].
In the current paradigm, intact and re-arranged pairs represent the ‘included’ and ‘excluded’ items, respectively. Since words from both item groups are intermixed in the study phase, recollection and familiarity can be calculated from a single test phase (using exclusion test conditions), as has been done by others (Smith & Knight, 2002; Wilding & Rugg, 1997). This variation avoids some of the methodological concerns with testing the same subjects under two different test conditions (with potentially different levels of bias) or deriving estimates from a population of interest by having half of the participants perform under one of the two test conditions. Measures of R and F were calculated for the overall performance, as well as 1x and 3x conditions. To account for differences in base rates of false alarms (“old” responses to novel pairs) between the two groups, F was calculated using a measure of discrimination (d’) derived from signal detection theory (Davidson, Anaki, Saint-Cyr, Chow, & Moscovitch, 2006; Yonelinas, Regehr, & Jacoby, 1995). Additionally, an overall bias (C) was measured to assess for potential differences in the response tendencies of the two groups. In this paradigm incorrect “old” endorsements can occur for both the excluded and novel pairs; thus, there are twice as many ‘nonstudied’ pairs as studied ones. To account for this difference and provide an appropriate measure of C, false alarms to both classes of nonstudied pairs were weighted so that their impact on bias was equivalent to the studied pairs (Budson, Wolk, Chong, & Waring, 2006; MacMillan & Creelman, 2005).
Experiment 2: Item versus Visual Spatial Source Memory
Materials and Design
This paradigm was loosely adapted from Dudas and colleagues (Dudas, Clague, Thompson, Graham, & Hodges, 2005). One-hundred and sixty color pictures were selected from the corpus of Rossion and Pourtois such that half were of living and half of non-living items (Rossion & Pourtois, 2004). These were divided into two lists of 80 items (half living; half non-living). In each list, items were grouped into fours (two living; two non-living).
Participants studied 20 groups of four items in which the items were randomly assigned to each of four quadrants. The assignment of quadrant and study order were randomized. There were 80 test items, all non-living (40 studied; 40 novel). Test items were pseudo-randomized, such that not more than three studied or novel items were presented in a row. Eight counterbalanced lists were used such that each study item appeared in all four quadrants and served as both a studied and novel item.
Procedure
Using Psyscope software, study items were presented in one of 4 quadrants labeled 1 through 4 (see Figure 1b). Each screen presented two non-living and two living items. Participants were asked to verbally name the two non-living items and then say and press a button corresponding to the quadrant of both of the non-living items. This encoding task was used to assure semantic processing of the items. In addition, they were informed that they would be tested on the item and location later. After the two keypresses, the next group of items would appear.
The test phase began after a three minute delay. Test items were presented in the center of the screen and participants were told to identify the item as “old” or “new” with reference to the prior study list and press the corresponding key (“OLD”, “NEW”). If “NEW” was pressed, the next test item would appear. If “OLD” was pressed, the test item would shrink and numbers would appear in each of the quadrants. The participant was to designate by keypress which of the four quadrants the item was previously studied in. Once they responded, the next test item appeared.
Analysis
Item memory was measured by d’ to account for differences in the base false alarm rate of the two groups. Visual spatial source memory was calculated both as a ratio of correct quadrant judgments over the number of correct item memory judgments and over the total number of items. As in Experiment 1, C was also calculated.
Experiment 3: Featural Process-Dissociation Procedure
Materials and Design
We selected 90 additional words (none from Experiment 1) that had a high familiarity (M = 599.5) and concreteness (M = 560.5). Words were 4 to 8 letters (M = 5.2) and were of a modest frequency (M = 46.9 occurrences per million by Francis and Kucera norms). These words were randomly divided into 6 lists of 15 words. Four additional words with similar characteristics served as buffers at the beginning and end of study lists.
Participants studied 60 words (not including buffers); half presented in green font and half in red font. For each font color half were studied once (1x) and the other half four times (4x). There were 90 test words (60 studied and 30 novel). Order at study and test was pseudorandomized, such that no item type repeated more than three times in a row. Six counter-balanced lists were used; each word served as a red, green, or novel word for both the 1x and 4x repetitions.
Procedure
Study words were presented in capital letters and in either red or green font on a white background using Psyscope software (see Figure 1c). The study task was self-paced and for each word the participant had to read the word out loud, state the font color, and decide whether the referent of the item was pleasant or unpleasant to them. Depending on their answer, they were told to push a button marked “Pleasant” or “Unpleasant”. This keypress would trigger the presentation of the next word. The incidental encoding task was used to ensure semantic elaboration of items and attention to word color. Participants were informed that they would later be tested on the words and their font color.
After a three minute delay, the test phase began. Test words were all presented in black font on a white background. Participants were given instructions to decide if the word was previously studied. They were told to only call items “old” if they were presented in one of the two font colors (e.g. only call items “old” if they were previously presented in the green font). Studied words in the other font and all novel words were to be called “new”. Test instructions alternated between red and green for each counterbalanced list and participants had to demonstrate understanding of this instruction before the test trial began. A keypress of a button marked “OLD” or “NEW” triggered the next test item.
Analysis
Similar to experiment 1, R and F were calculated using the process-dissociation procedure equations. Study words of the color font that participants were instructed to endorse as “old” at test represent the included items. Words of the other font are the ‘excluded’ items. A total measure of C was calculated using the same procedure as Experiment 1.
Results
Experiment 1
The proportion of “old” judgments to intact, re-arranged, and novel pairs are displayed in Table 2. As can be seen, controls tended to respond “old” to a higher proportion of intact items [total: t(35) = 2.47, p < 0.05] while patients with a-MCI had a higher number of false alarm (“old” responses to novel pairs; t(35) = 3.29, p < 0.01]. Patients with a-MCI also had a higher exclusion error rate (“old” responses to ‘excluded’ items), but this did not reach significance [total: t(35) < 1, p > 0.3]. Based on these responses, measures of R and F were calculated for each condition (see Table 2 and Figure 2).
Table 2.
Proportion “old” responses, bias (C), recollection, and familiarity on associative process-dissociation procedure task
| Controls (n=21) | a-MCI (n=16) | |||||
|---|---|---|---|---|---|---|
| Condition | Total | 1x | 3x | Total | 1x | 3x |
| Intact | .75 (.03) | .62 (.04) | .88 (.02) | .62 (.04) | .49 (.04) | .75 (.05) |
| Re-arranged | .40 (.03) | .36 (.03) | .44 (.05) | .45 (.04) | .37 (.05) | .53 (.05) |
| Novel | .02 (.01) | .13 (.04) | ||||
| C | .21(.06) | .19 (.10) | ||||
| Recollection | .35 (.04) | .27 (.04) | .44 (.06) | .18 (.04) | .14 (.03) | .23 (.05) |
| Familiarity (d’) | 2.31 (.13) | 1.98 (.15) | 2.96 (.20) | 1.38 (.14) | 1.05 (.13) | 1.88 (.20) |
Note: Standard errors of each mean are in parentheses.
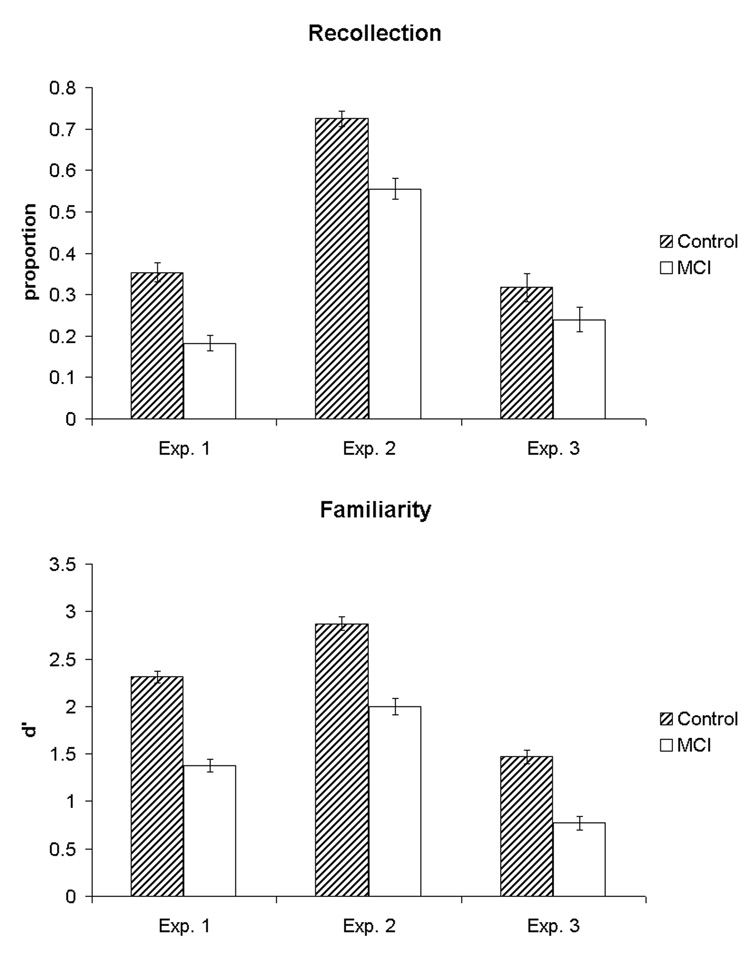
Figure 2. Measures of recollection and familiarity for the three experimental paradigms.
For experiment 2, the recollection measure is derived from the proportion of correct visual spatial source judgments over the number of correctly recognized items (this calculation is perhaps closest to the independence assumption used for calculation of recollection with the other two experimental paradigms). The familiarity measure for experiment 2 is based on item memory performance, which is not process pure, but presented here given its greater dependence on familiarity relative to the source memory task.
To explore group differences for each memory process and the effect of repetition, 2 × 2 ANOVA’s were calculated with factors of group (control, a-MCI) and repetition (1x, 3x) for both recollection and familiarity. A main effect of group [F(1,35) = 7.9, p < 0.01] reflects greater recollection in the control than a-MCI group. Consistent with increased recollection in the 3x condition, a main effect of repetition was also found [F(1,35) = 21.1, p < 0.001]. An interaction between group and repetition did not reach significance [F(1,35) = 2.2, p > 0.1] although there was a trend for the control group to benefit more from repetition. The analogous ANOVA for familiarity yielded main effects of group [F(1,35) = 19.8, p < 0.001] and repetition [F(1,35) = 80.9, p < 0.001], but no interaction [F(1,35) < 1.0, p > 0.4]. The group effect was due to poorer familiarity in the patients with a-MCI than controls.
Consistent with the ANOVA, independent t-tests demonstrated significantly greater recollection for the control participants in the overall, 1x, and 3x comparisons [total: t(35) = 2.84, p < 0.01; 1x: t(35) = 2.28; p < 0.05; 3x: t(35) = 2.76, p < 0.01]. In addition, familiarity was also greater in the control groups regardless of repetition [total: t(35) = 4.98, p < 0.001; 1x: t(35) = 4.62, p < 0.001; 3x: t(35) = 3.78, p < 0.01]. There was no difference in response bias between the two groups [t(35) < 1.0, p > 0.9].
Thus, consistent with our hypothesis, both recollection and familiarity were impaired in a-MCI. To more directly compare the degree of impairment and account for level of difficulty in making judgments based on these processes (e.g. familiarity judgments may be easier), z-scores were calculated for the a-MCI group referenced to the control mean and standard deviation (Nunally & Bernstein, 1994; Yonelinas, 2002). Control-referenced z-scores for recollection and familiarity were −0.84 and −1.61, respectively. A paired-samples t-test revealed a significantly lower z-score for the familiarity measure than recollection [t(15) = 3.86, p < 0.01]. Overall, familiarity appears to be, if anything, more impaired than recollection.
Experiment 2
Item memory performance and measures of visual spatial source memory are in Table 3 and Figure 2. Studied and novel items are presented as the proportion endorsed “old.” Performance on the quadrant judgment was measured with the denominator either as the total number of trials or the number of trials in which the participant made a correct recognition judgment (hits). Controls performed significantly better than patients with a-MCI on the item memory aspect of the task as measured by discrimination [t(29) = 3.95, p < 0.001]. Although both groups performed clearly better than chance (.25), controls demonstrated better visual spatial source memory than patients with a-MCI using hits as the denominator [t(29) = 2.79, p < 0.01]. When calculated over the total number of test items, a similar advantage was seen for the controls [t(29) = 3.26, p < 0.01]. There was no statistical difference in bias between the two groups [t(29) < 1.0, p > 0.5].
Table 3.
Proportion “old” responses, discrimination, and bias (C) on item versus visual spatial source task
| Condition | Controls (n=17) | a-MCI (n=14) |
|---|---|---|
| Studied | .78 (.03) | .68 (.04) |
| Novel | .02 (.01) | .08 (.02) |
| d’ | 2.87 (.15) | 2.00 (.16) |
| Source/Hits | .72 (.04) | .56 (.05) |
| Source/Total | .57 (.04) | .38 (.04) |
| C | .59 (.07) | .51 (.10) |
Note: Standard errors of each mean are in parentheses.
As in experiment 1, control-referenced z-scores were calculated for the patients with a-MCI to control for task difficulty and assess whether there was a difference in performance on a task dependent on recollection (source memory) versus one relatively dependent on familiarity (item memory). The control-referenced z-score for the item memory task was −1.43. Z-scores for the source/hits and source/total were −1.03 and −1.07, respectively. Comparison of the item memory z-score to either of the visual spatial source measures did not reveal statistically significant differences [item vs. source/hits: t(13) = 1.36, p > 0.1; item vs. source/total, t(13) = 1.86, > 0.08]. Nonetheless, there was no evidence for sparing of item memory relative to the visual spatial source task, suggesting that there is not selective sparing of familiarity in a-MCI.
Experiment 3
The proportion of ‘old’ responses to included, excluded, and novel words are displayed in Table 4. In general, correct endorsements of the included items were higher in the controls, but were not statistically different [total included: t(29) < 1.0, p > .3]. False alarms to the novel items were, again, higher in the patients with a-MCI [t(29) = 3.64, p < 0.01]. Errors to the excluded items were similar in the two groups [total excluded: t(29) < 1.0, p > 0.6] although the controls demonstrated some degree of suppression of these errors in the 4x versus 1x condition.
Table 4.
Proportion “old” responses, bias (C), recollection, and familiarity on featural process-dissociation procedure task
| Controls (n=18) | a-MCI (n=13) | |||||
|---|---|---|---|---|---|---|
| Condition | Total | 1x | 4x | Total | 1x | 4x |
| Included | .59 (.05) | .44 (.06) | .74 (.05) | .52 (.05) | .40 (.06) | .65 (.06) |
| Excluded | .27 (.03) | .31 (.04) | .24 (.04) | .30 (.04) | .30 (.05) | .31 (.07) |
| Novel | .05 (.01) | .17 (.04) | ||||
| C | .44 (.07) | .37 (.10) | ||||
| Recollection | .32 (.07) | .19 (.06) | .50 (.07) | .24 (.06) | .14 (.04) | .38 (.08) |
| Familiarity (d’) | 1.47 (.14) | 1.38 (.15) | 1.84 (.28) | .77 (.15) | .59 (.15) | .90 (.22) |
Note: Standard errors of each mean are in parentheses.
As with experiment 1, 2 × 2 ANOVA’s were calculated with factors of group (control, a-MCI) and repetition (1x, 4x) for both memory processes. There was a significant main effect of repetition [F(1,29) = 49.03, p < 0.001] for recollection, but there was not a group effect [F(1,29) < 1.0, p > 0.3]. Although the level of recollection of the control group appeared to benefit more from repetition than the patients with a-MCI, as with experiment 1, the interaction of group with repetition did not reach significance [F(1,29) < 1.0, p > 0.3]. The analogous ANOVA for familiarity revealed main effects of group [F(1,29) = 11.74, p < 0.01] and repetition [F(1,29) = 5.07, p < 0.05], consistent with a familiarity advantage in the control group relative to the patients with a-MCI and enhanced familiarity with repetition. There was no interaction between repetition and group [F(1,29) < 1, p > 0.6].
Recollection and familiarity measures were also submitted to independent t-tests (see Figure 2). Despite the advantage of recollection for the controls in all conditions, none of these comparisons reached statistical significance [total: t(29) < 1.0, p > 0.4; 1x: t(29) < 1.0, p > 0.5; 4x: t(29) = 1.11, p > 0.2], consistent with the above ANOVA. The lack of significance is likely due to relatively high variance in recollection performance in both groups, particularly the controls. In distinction, familiarity was significantly greater for the controls than patients with a-MCI in each repetition condition [total: t(29) = 3.32, p < 0.01; 1x: t(29) = 3.71, p < 0.01; 4x: t(29) = 2.50, p < 0.05]. Finally, there was no difference in C between the two groups [t(29) < 1.0, p > 0.5].
To compare the relative decline in recollection and familiarity, control-referenced z-scores were calculated for these estimates. Total recollection and familiarity z-scores were −0.28 and −1.15, respectively. A paired-sample t-test revealed that the familiarity z-score was significantly lower than that of recollection [t(12) = 3.13, p < 0.01]. Once again, the estimate of familiarity was just as, if not more, impaired than recollection.
Discussion
To our knowledge, this is the first study to measure recollection and familiarity using process estimation methods in a-MCI. Consistent with our prediction, the present data support the notion that a-MCI is associated with impairment in both of these memory processes. The three experimental memory paradigms were associated with remarkably similar results despite differences in the materials studied and the nature of the estimates. Two out of the three tests showed clear reduction in responses thought to reflect recollection in the a-MCI group. The one non-significant result (experiment 3) demonstrated the same trend with recollection higher in the control participants. Additionally, patients with a-MCI consistently demonstrated significant reduction of familiarity relative to control participants. Indeed, levels of familiarity impairment tended to be to a greater extent than recollection when controlling for task difficulty.
Our prediction that both recollection and familiarity would be impaired was based on the assumption that a significant percentage of our a-MCI patients are destined to develop clinical AD and likely already have AD pathology. Consonant with this notion, several autopsy studies have identified varying stages of AD pathology in patients with a-MCI (Guillozet, Weintraub, Mash, & Mesulam, 2003; Morris et al., 2001; Petersen et al., 2006). Critically for the present study, pathology at these earlier stages of disease involves medial temporal structures which have been linked to both recollection and familiarity.
Our expectation of failures of recollection follows from the common finding of NFT involvement of the entorhinal cortex and hippocampus in patients with a-MCI (Guillozet, Weintraub, Mash, & Mesulam, 2003; Morris et al., 2001). Early involvement of these regions is virtually an invariable finding in the progression of AD (Braak & Braak, 1991; Delacourte et al., 1999). Given the central role attributed to the hippocampus in recollection-based memory, it follows that this memory process should be affected. Further, the area of most intense and earliest pathology in the entorhinal cortex is to layer II neurons which form the perforant pathway, the main input into the hippocampus. Such pathology could result in a functional decoupling of the hippocampus, contributing to failures of recollection-based memory.
The finding of reduced recollection is coherent with a number of studies which have suggested impairment in associative memory and recollection in patients with a-MCI or very mild AD (Budson, Desikan, Daffner, & Schacter, 2000; Dudas, Clague, Thompson, Graham, & Hodges, 2005; Gallo, Sullivan, Daffner, Schacter, & Budson, 2004; Rauchs et al., 2007; Westerberg et al., 2006). It should, however, be kept in mind that the designation of a-MCI is generally made with evidence of free recall impairment on standard psychometrics. It is likely that delayed recall is dependent on recollection and not supported to a great extent by familiarity-based processes. Thus, to some degree the way this group is defined selects for patients with impaired recollection.
Of most interest is our finding of reduced familiarity in the patients with a-MCI relative to the controls. Again, consideration of the early pathology of AD predicts this finding. Indeed, the earliest area of NFT involvement is the transentorhinal region which arguably includes the perirhinal cortex (Braak & Braak, 1991; Delacourte et al., 1999; Van Hoesen, Augustinack, Dierking, Redman, & Thangavel, 2000). Further, perirhinal NFT involvement appears to highly correlate with entorhinal pathology and the degree of pathology in these regions also correlates with memory performance in MCI and early AD (Mitchell et al., 2002). Given the putative role of perirhinal cortex in familiarity-based memory, it is, thus, not surprising that impairment of this form of memory would be an early finding in the disease course.
Nonetheless, this result stands in apparent distinction from a few reports which have suggested a relative sparing of this memory process in early AD (Bartock et al., 1997; Dalla Barba, 1997; Lekeu et al., 2003; Rauchs et al., 2007; Tendolkar et al., 1999) or a-MCI (Westerberg et al., 2006). While it is not entirely clear why these results differ, methodological issues are likely important. For example, two of the studies used another process estimation technique in which participants are asked to determine introspectively whether items they think are ‘old’ are associated with recollection or just familiarity by giving a ‘remember’ or ‘know’ response, respectively (Gardiner, 1988). For example, Dalla Barba et al. (1997) found that patients with mild AD had a reduced proportion of remember responses, but a largely equivalent proportion of know responses relative to controls. They argued that this result was consistent with a sparing of familiarity in the patients with AD. However, because the nature of the task only allows for a know response in the absence of recollection, it is likely that simply examining the absolute level of know responses underestimates familiarity. Following the assumption that these processes are independent, many advocate calculating familiarity as a proportion of items given a know response over the number of items in which such a response can be given [1 minus proportion remember; (Yonelinas, 2002)]. Given the much higher remember rate of the controls, equivalent know responses suggests a significantly higher estimate of familiarity in the controls. Additionally, patients with AD in this study had a shorter study-test retention interval than controls further confounding comparisons.
Several other studies have examined gist memory, which is thought dependent on familiarity, in patients with mild AD (Budson, Desikan, Daffner, & Schacter, 2000; Budson, Desikan, Daffner, & Schacter, 2001; Pierce, Sullivan, Schacter, & Budson, 2005). Consistent with our finding of familiarity impairment, these reports have generally demonstrated a significant decrement in gist memory. However, data from this work has also been felt supportive of the notion that familiarity is affected to a lesser degree than recollection. This follows from findings that conditions which increase recollection in controls and oppose gist-based memory errors, paradoxically increase these errors in patients (Budson, Desikan, Daffner, & Schacter, 2000; Pierce, Sullivan, Schacter, & Budson, 2005). It is argued that in these situations, familiarity drives memory responses in the patients with AD to a greater extent than the controls. Unfortunately, these studies do not provide a clear way to quantitate familiarity and a greater reliance on this memory process does not unambiguously determine the degree of impairment relative to recollection. It is worth noting that in the present work, study repetition, in absolute terms, resulted in a larger increase in exclusion errors (or lack of decrease in experiment 3) for the patients with a-MCI relative to controls, analogous to the results of these gist memory studies.
Of most relevance to the current study population, another report suggested sparing of familiarity in a-MCI based on control level performance on a difficult forced-choice recognition task (Westerberg et al., 2006). While this study did not directly estimate familiarity, these investigators compared performance on a forced-choice format to a “yes-no” item recognition test. By using foils that were highly perceptually similar to that of the studied items, the relative dependence on familiarity and recollection varied across the two tasks. Namely, “yes-no” performance is essentially completely supported by recollection while forced-choice discrimination is weighted towards familiarity, but also potentially with a contribution of recollection. Essentially, this experimental model is a type of task dissociation paradigm. Consistent with intact familiarity, but impaired recollection, these authors reported that patients with a-MCI had control level performance on the forced-choice task, but were impaired on the “yes-no” recognition task.
It is not certain why this result deviates from the current findings, but could, again, be due to the nature of the task or differences in the population of MCI patients. The latter is an important issue in all studies of MCI given the heterogeneity of these patients. Differences in the nature of the population from which patients are recruited and how criteria are applied are amongst factors that can impact the proportion of patients who truly have early AD pathology (Petersen, 2004). Further, the degree of impairment within the MCI range can vary somewhat across studies. It is possible that our patients, despite having a similar MMSE, may have been further along in the AD pathologic process than the population reported by Westerberg et al., resulting in greater perirhinal NFT involvement and, consequently, greater impairment of familiarity. Alternatively, our population may have had a different proportion of patients who will eventually convert to AD, which would have obvious implications for their memory performance. Nonetheless, they also reported less impairment on the forced-choice than yes-no recognition task for a group of patients with mild AD, suggesting that although familiarity was impaired, it was to a lesser extent than recollection. Thus, additional factors outside of participant heterogeneity are needed to explain the divergence of our results. Important differences in the nature of the paradigms are also likely to be critical. For example, the Westerberg et al. study used a very short study-test interval relative to the current one. Perhaps, familiarity is more vulnerable to delay in a-MCI patients than in controls.
Another important consideration in evaluation of memory performance in patients with a-MCI, as well as AD, is the degree to which other cognitive domains are involved. As in the Westerberg et al. study, our participants included both single and multiple domain a-MCI patients. Several non-memory tests were below that of our control group despite being well within 1.0 SD’s of the normative population. Mild involvement of these other domains, such as executive functioning or language, could influence memory encoding and/or retrieval, reducing measures of recollection and familiarity. Such factors may be more likely to reduce recollection given that this memory process is generally found to be more affected by encoding manipulations than familiarity, which is often conceptualized as a more automatic process (Yonelinas, 2002). It is worth noting that our signal domain a-MCI patients produced essentially the same pattern of memory performance as the overall analysis.
Other work has been more consistent with our finding of reduced familiarity in a-MCI. For example, item recognition memory has been found to be impaired in several studies of this population (Barbeau et al., 2004; Bennett, Golob, Parker, & Starr, 2006; Dudas, Clague, Thompson, Graham, & Hodges, 2005). Similar to findings in mild AD, gist-based familiarity has also been reported to be decreased in a-MCI (Chapman et al., 2002; Hudon et al., 2006). Interestingly, a recent study segregated patients with a-MCI into two groups based on their performance on an item picture recognition task (the groups had similar recall performance). They found that those with impairment on the recognition task had a resting SPECT scan with a pattern of hypo-perfusion consistent with AD while those who performed well on the task did not (Guedj et al., 2006).
The latter study underscores the potential utility of directly measuring familiarity in a-MCI. This form of memory may be more specific to the pathology of AD relative to tasks which tax recollection, such as free recall. For example, patients with vascular dementia may have impaired recall, but intact item recognition suggesting a sparing of familiarity (Tierney et al., 2001). Non-AD causes of a-MCI may affect recollection without familiarity due to direct hippocampal pathology (e.g. hippocampal sclerosis) or dysfunction of prefrontal-subcortical networks (e.g. cerebrovascular disease, depression). These non-AD causes of memory impairment may be less likely to affect familiarity due to their lack of involvement of the perirhinal cortex and the fact that familiarity appears less dependent on the pre-frontally mediated control processes necessary for accurate recollection.
Importantly, familiarity impairment also may differentiate the memory loss of healthy aging from that due to the early pathology of AD. As described above, familiarity-based memory, as opposed to recollection, appears largely intact in healthy aging (Davidson & Glisky, 2002; Yonelinas, 2002). This may be reflected by normal or increased perirhinal activity reported in healthy elderly subjects in fMRI memory studies (Cabeza et al., 2004; Daselaar, Fleck, Dobbins, Madden, & Cabeza, 2006). Thus, familiarity, perhaps as a marker of perirhinal integrity, may be a specific measure for differentiating those with very early AD pathology from those with memory decline due to ‘normal’ aging; the latter often attributed to dysfunction of frontal-subcortical networks and/or the hippocampus.
As early diagnosis of AD has become an important goal in the field, differentiating normal from pathological cognitive aging is critical. One might expect this to be most challenging for people at the border of the diagnostic criteria for a-MCI. Operationalizations which require 1.0 or 1.5 SD’s below the mean on memory performance to meet diagnostic criteria will obviously capture some people who are not experiencing pathological aging and are just part of the normal distribution. This issue is perhaps reflected in the phenomenon of reversion to normal in population studies in which a significant proportion of these patients likely do not have AD pathology.
Consistent with the aim of finding memory measures that are more specific for early AD, some have proposed that the nature of the memory impairment be better defined in criteria for a-MCI or AD (Dubois & Albert, 2004; Dubois et al., 2007). One proposal is that a-MCI be qualified by the type of amnesia. It would be expected that those with a ‘medial temporal’ form of amnesia would be more likely to develop AD than those with a ‘frontal form.’ Familiarity may be a specific marker for medial temporal pathology (perirhinal) while impairment on tests that are more dependent on recollection, such as free recall, may not be; impairment could result from either medial temporal (hippocampal) or frontal-subcortical dysfunction. It is worth pointing out that many of the standard recognition memory tests used in clinical practice, which may better tap familiarity function than recall, are easier than those administered here and may suffer from ceiling effects in differentiating groups, especially when diagnosis is made early in the disease course.
Another finding from the present data is that repetition does benefit performance for both recollection and familiarity in patients with a-MCI. While in absolute terms it appeared this effect was less robust for recollection compared to controls, there was still a substantial improvement. The benefit of repetition on familiarity was similar between the groups. Whether further repetition would lead to more of a dissociation between these memory processes could be explored in the future. Regardless, these data suggest that rehearsal of information can enhance episodic memory subserved by both processes in patients with a-MCI, which could direct adaptive strategies.
The present results need to be discussed in the context of the measurement tools utilized for this study. As is the case for all of the estimation techniques, the process-dissociation procedure operates under a number of assumptions, which if incorrect, could impact the validity of the current findings. Perhaps the most critical assumption is that recollection and familiarity operate independently. While other frequently used estimation methods, such as the receiver operator characteristic procedure [ROC; (Yonelinas, 1994)] and the independence remember/know method (Yonelinas & Jacoby, 1995), also make this assumption, recollection and familiarity may relate in other ways [e.g. redundancy or exclusivity; see (Mayes, Montaldi, & Migo, 2007)]. These other relationships can affect estimation, particularly for the familiarity measure. If all recollected items are familiar (redundancy), we would expect to see an even greater divergence of familiarity between the two groups here. The opposite would be the case if items are either recollected or familiar, but never both (exclusivity). While the veracity of the independence assumption remains in doubt, findings of convergence across estimation methods that do not require this assumption (e.g. task dissociation methods) provide support for the claim (Yonelinas, 2002). Further, a recent study reported impaired familiarity with intact recollection after an anterior temporal lobe lesion, including the perirhinal cortex (Bowles et al., 2007). These data, which will need to be corroborated, complete a double dissociation, as pure hippocampal lesions have been reported to be associated with an isolated recollection deficit [(Holdstock et al., 2002; Mayes et al., 2004; Yonelinas et al., 2002a); although see (Squire, Wixted, & Clark, 2007)].
Another important limitation of the process-dissociation method and the task dissociation paradigm utilized here is that the measure of recollection is dependent on the requirement of the task. In other words, one could still recollect some aspect of the prior study episode, but not the particular feature that is necessary for accurate performance of the task (e.g. that the word was presented in green). Thus, non-criterial recollection could result in underestimation of this memory process. While this may have occurred in the present study, there is no principled reason to believe that the relationship of non-criterial to criterial recollection was different in the two groups and, thus, the difference in recollection memory would not be expected to be distorted.
A major strength of the current study is the use of three different paradigms, each producing very similar results despite the use of different stimuli and forms of associative memory to measure recollection. Most critically, we found consistent results across two different types of measurement methods (process-dissociation procedure and task dissociation) that differ in underlying assumptions. Task dissociation does not make any specific claims about the independence of recollection and familiarity, but still produced a qualitatively similar outcome to the process-dissociation method (regardless of whether hits or total items were used in the denominator to calculate source performance). This convergence across methods echoes the large literature on these different measurement techniques (Yonelinas, 2002). Despite the strong internal consistency, further work using other methodologies, such as ROC, remember/know, and response deadlines, should be pursued to confirm the present findings.
Such convergence is particularly important given the variability of results and interpretations noted above in the limited literature studying recollection and familiarity in mild AD and a-MCI. Indeed, several strategies could be pursued in the future to limit such inconsistencies across studies. First, as already mentioned, the use of multiple estimation techniques should be pursued in each study population. These paradigms should ideally vary in their basic assumptions. Disagreements in measurement may provide insight into the validity of these assumptions, as well as deepening our understanding of these memory processes. Second, most of the work in the literature involves relatively small numbers of participants and given the heterogeneity in these patient groups, as well as in healthy aging populations, attempts at larger samples should be pursued. Ideally, this would involve multiple sites to further reduce bias in selection process. Third, structural imaging in patient and control groups may further explain variability. For example, the degree of cerebrovascular disease in patient and control groups could have an important modulating effect on performance. Fourth, longitudinal study would impart additional valuable information. In a-MCI patients, and even ‘healthy’ controls, such assessment would allow for determination of whether or not these participants truly are at the beginnings of AD, or have memory impairment on another basis. Longitudinal testing would also allow for determination of whether recollection and familiarity decline in a linear fashion or whether their relationship varies at different stages of disease, which might be expected given the temporal topography of AD pathology. Finally, future studies should control for the relative difficulty of making responses based on familiarity versus recollection. Familiarity judgments are often felt to be ‘easier’ than ones requiring recollection. Control-referenced z-scores, as used here, is one approach. Most prior work has simply compared the magnitude of impairment for these memory processes. Relatedly, a broad range of task difficulty should be pursued as the relative balance of recollection and familiarity likely varies with the overall difficulty of the task. These strategies are just a partial list of potential ways to try to more definitively adjudicate between some of the diverging results in the literature. Obviously, further knowledge of these memory processes and refinement of our operationalizations will ultimately lead to more precise measurement.
A few additional points which could impact the present data bear mentioning. First, the present experiments involved self-paced semantic, encoding tasks in an attempt to ensure that participants encoded the material in a similar manner. While this methodology has the advantage of controlling encoding conditions to some extent, it can also introduce differences in the study test interval based on the speed with which it is performed. We did not find a statistical difference in the encoding time per study item for each paradigm [exp. 1: 3036.9 ms vs. 3403.8 ms, controls vs a-MCI; exp. 2: 3953.8 ms vs 4654.5 ms 1; exp. 3: 3011.6 ms vs. 3266.4 ms; all p’s > .1], but the magnitude of the study time was generally longer for the MCI patients. This potentially increased time may have had a differential effect on recollection and familiarity. Future work could use a set encoding time although this too creates potential issues with the degree to which both groups elaborate within the presentation time. The three minute delay between study and test also could have introduced additional differences in rehearsal; participants were generally engaged in conversation with the experimenter during this time, but this could have differed between the two groups.
Finally, although we hypothesized that familiarity would be significantly impaired in a-MCI due to the location of early AD pathology, we did not have a specific prediction regarding the degree of this impairment relative to recollection. Our finding of greater impairment of familiarity than recollection may be considered a somewhat unexpected outcome, as medial temporal regions thought to be involved in both processes are associated with NFT pathology early in AD. However, this result should be viewed with caution, as we did not find statistical support for it in the task dissociation paradigm despite the data being in the same direction as that with the process-dissociation procedure tasks. Thus, one potential explanation for this finding is related to the process dissociation assumptions and methodology. Another possibility is that the recollection impairment associated with aging reduces the baseline of performance for this memory process to which the patients with a-MCI are compared, allowing for a greater drop in familiarity performance in the patients. The most interesting speculation is that given the intense involvement of extra-hippocampal medial temporal structures early in the disease process, familiarity is more involved than recollection. Volumetric study with structural MRI may add further insight to this issue.
In conclusion, using process estimation and task dissociation techniques, we found that both recollection and familiarity are reduced in a-MCI. This result suggests that familiarity impairment is an early cognitive manifestation of AD pathology. Given the assumptions inherent in all estimation techniques, further confirmation with other methodologies would provide additional support for this result. Nonetheless, the apparent sparing of familiarity in aging and possibly other conditions, such as cerebrovascular disease, suggest that measurement of this memory process may offer a relatively specific marker for early AD. The predictive value of familiarity for the presence of early AD pathology will require longitudinal studies of patients with a-MCI (or even a healthy elderly cohort) to measure conversion to clinical AD or the use of imaging techniques providing in vivo visualization of AD pathology [e.g. Pittsburgh compound-B (Klunk et al., 2004)].
Acknowledgements
We thank Daniel L. Schacter, David A. Gallo, and Andrew E. Budson for suggestions at various stages of this work. We also thank Kathryn L Dunfee for assistance in manuscript preperation. The research was supported by the National Institute of Aging K23 AG028018 and P50 AG05133.
Footnotes
Note that rt’s were not recorded for one control and one patient with a-MCI in experiment 2 due to computer failure.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albert MS. Memory decline: The boundary between aging and age-related disease. Annals of Neurology. 2002;51(3):282–284. [PubMed] [Google Scholar]
- Barbeau E, Didic M, Tramoni E, Felician O, Joubert S, Sontheimer A, et al. Evaluation of visual recognition memory in MCI patients. Neurology. 2004;62(8):1317–1322. doi: 10.1212/01.wnl.0000120548.24298.db. [DOI] [PubMed] [Google Scholar]
- Bartock J, Wilson C, Giordani B, Keys B, Persad C, Foster N, et al. Varying patterns of verbal recall, recognition, and response bias with progression of Alzheimer's disease. Aging, Neuropsychology, and Cognition. 1997;4(4):266–272. doi: 10.1080/13825589708256651. [DOI] [PubMed] [Google Scholar]
- Bennett IJ, Golob EJ, Parker ES, Starr A. Memory evaluation in mild cognitive impairment using recall and recognition tests. J Clin Exp Neuropsychol. 2006;28(8):1408–1422. doi: 10.1080/13803390500409583. [DOI] [PubMed] [Google Scholar]
- Bowles B, Crupi C, Mirsattari SM, Pigott SE, Parrent AG, Pruessner JC, et al. Impaired familiarity with preserved recollection after anterior temporal-lobe resection that spares the hippocampus. Proc Natl Acad Sci U S A. 2007;104(41):16382–16387. doi: 10.1073/pnas.0705273104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological staging of Alzheimer-related changes. Acta Neuropathologica. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Brown CM, Aggleton JP. Recognition memory: what are the roles of the perirhinal cortex and hippocampus? Nature Reviews Neuroscience. 2001;2:51–61. doi: 10.1038/35049064. [DOI] [PubMed] [Google Scholar]
- Buckner RL. Memory and executive function in aging and AD: multiple factors that cause decline and reserve factors that compensate. Neuron. 2004;44(1):195–208. doi: 10.1016/j.neuron.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Budson AE, Desikan R, Daffner KR, Schacter DL. When false recognition is unopposed by true recognition: Gist-based memory distortion in Alzheimer's disease. Neuropsychology. 2000;14(2):277–287. doi: 10.1037//0894-4105.14.2.277. [DOI] [PubMed] [Google Scholar]
- Budson AE, Desikan R, Daffner KR, Schacter DL. Perceptual false recognition in Alzheimer's disease. Neuropsychology. 2001;15(2):230–243. [PubMed] [Google Scholar]
- Budson AE, Wolk DA, Chong H, Waring JD. Episodic memory in Alzheimer's disease: separating response bias from discrimination. Neuropsychologia. 2006;44(12):2222–2232. doi: 10.1016/j.neuropsychologia.2006.05.024. [DOI] [PubMed] [Google Scholar]
- Busse A, Hensel A, Guhne U, Angermeyer MC, Riedel-Heller SG. Mild cognitive impairment: long-term course of four clinical subtypes. Neurology. 2006;67(12):2176–2185. doi: 10.1212/01.wnl.0000249117.23318.e1. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Anderson ND, Locantore JK, McIntosh AR. Aging gracefully: compensatory brain activity in high-performing older adults. Neuroimage. 2002;17(3):1394–1402. doi: 10.1006/nimg.2002.1280. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Daselaar SM, Dolcos F, Prince SE, Budde M, Nyberg L. Task-independent and task-specific age effects on brain activity during working memory, visual attention and episodic retrieval. Cereb Cortex. 2004;14(4):364–375. doi: 10.1093/cercor/bhg133. [DOI] [PubMed] [Google Scholar]
- Chapman SB, Zientz J, Weiner M, Rosenberg R, Frawley W, Burns MH. Discourse changes in early Alzheimer disease, mild cognitive impairment, and normal aging. Alzheimer Dis Assoc Disord. 2002;16(3):177–186. doi: 10.1097/00002093-200207000-00008. [DOI] [PubMed] [Google Scholar]
- Curran T, Tepe KL, Piatt C. ERP explorations of dual processes in recognition memory. In: Zimmer HD, Mecklinger A, Lindenberger U, editors. ERP explorations of dual processes in recognition memory. Oxford: Oxford University Press; 2006. pp. 467–492. [Google Scholar]
- Dalla Barba G. Recognition memory and recollective experience in Alzheimer's disease. Memory. 1997;5(6):657–672. doi: 10.1080/741941546. [DOI] [PubMed] [Google Scholar]
- Daselaar SM, Fleck MS, Dobbins IG, Madden DJ, Cabeza R. Effects of healthy aging on hippocampal and rhinal memory functions: an event-related fMRI study. Cereb Cortex. 2006;16(12):1771–1782. doi: 10.1093/cercor/bhj112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson PS, Anaki D, Saint-Cyr JA, Chow TW, Moscovitch M. Exploring the recognition memory deficit in Parkinson's disease: estimates of recollection versus familiarity. Brain. 2006;129(Pt 7):1768–1779. doi: 10.1093/brain/awl115. [DOI] [PubMed] [Google Scholar]
- Davidson PS, Glisky EL. Neuropsychological correlates of recollection and familiarity in normal aging. Cogn Affect Behav Neurosci. 2002;2(2):174–186. doi: 10.3758/cabn.2.2.174. [DOI] [PubMed] [Google Scholar]
- Delacourte A, David JP, Sergeant N, Buee L, Wattez A, Vermersch P, et al. The biochemical pathway of neurofibrillary degeneration in aging and Alzheimer's disease. Neurology. 1999;52(6):1158–1165. doi: 10.1212/wnl.52.6.1158. [DOI] [PubMed] [Google Scholar]
- Donaldson W. The role of decision processes in remembering and knowing. Mem Cognit. 1996;24(4):523–533. doi: 10.3758/bf03200940. [DOI] [PubMed] [Google Scholar]
- Dubois B, Albert ML. Amnestic MCI or prodromal Alzheimer's disease? Lancet Neurol. 2004;3(4):246–248. doi: 10.1016/S1474-4422(04)00710-0. [DOI] [PubMed] [Google Scholar]
- Dubois B, Feldman HH, Jacova C, Dekosky ST, Barberger-Gateau P, Cummings J, et al. Research criteria for the diagnosis of Alzheimer's disease: revising the NINCDS-ADRDA criteria. Lancet Neurol. 2007;6(8):734–746. doi: 10.1016/S1474-4422(07)70178-3. [DOI] [PubMed] [Google Scholar]
- Dudas RB, Clague F, Thompson S, Graham KS, Hodges JR. Episodic and semantic memory in mild cognitive impairment. Neuropsychologia. 2005 doi: 10.1016/j.neuropsychologia.2004.12.005. In Press. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H. Hippocampus: cognitive processes and neural representations that underlie declarative memory. Neuron. 2004;44(1):109–120. doi: 10.1016/j.neuron.2004.08.028. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Yonelinas AP, Ranganath C. The medial temporal lobe and recognition memory. Annu Rev Neurosci. 2007;30:123–152. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Gallo DA, Sullivan AL, Daffner KR, Schacter DL, Budson AE. Associative recognition in Alzheimer's disease: Evidence for impaired recall-to-reject. Neuropsychology. 2004 doi: 10.1037/0894-4105.18.3.556. [DOI] [PubMed] [Google Scholar]
- Ganguli M, Dodge HH, Shen C, DeKosky ST. Mild cognitive impairment, amnestic type: an epidemiologic study. Neurology. 2004;63:115–121. doi: 10.1212/01.wnl.0000132523.27540.81. [DOI] [PubMed] [Google Scholar]
- Gardiner JM. Functional aspects of recollective experience. Memory and Cognition. 1988;16(4):309–313. doi: 10.3758/bf03197041. [DOI] [PubMed] [Google Scholar]
- Gauthier S, Reisberg B, Zaudig M, Petersen RC, Ritchie K, Broich K, et al. Mild cognitive impairment. Lancet. 2006;367(9518):1262–1270. doi: 10.1016/S0140-6736(06)68542-5. [DOI] [PubMed] [Google Scholar]
- Grady CL, McIntosh AR, Horwitz B, Maisog JM, Ungerleider LG, Mentis MJ, et al. Age-related reductions in human recognition memory due to impaired encoding. Science. 1995;269(5221):218–221. doi: 10.1126/science.7618082. [DOI] [PubMed] [Google Scholar]
- Guedj E, Barbeau EJ, Didic M, Felician O, de Laforte C, Ceccaldi M, et al. Identification of subgroups in amnestic mild cognitive impairment. Neurology. 2006;67(2):356–358. doi: 10.1212/01.wnl.0000225076.73312.d4. [DOI] [PubMed] [Google Scholar]
- Guillozet AL, Weintraub S, Mash DC, Mesulam MM. Neurofibrillary tangles, amyloid, and memory in aging and mild cognitive impairment. Archives of Neurology. 2003;60:729–736. doi: 10.1001/archneur.60.5.729. [DOI] [PubMed] [Google Scholar]
- Holdstock JS, Mayes AR, Roberts N, Cezayirli E, Isaac CL, O'Reilly RC, et al. Under what conditions is recognition spared relative to recall after selective hippocampal damage in humans? Hippocampus. 2002;12:341–351. doi: 10.1002/hipo.10011. [DOI] [PubMed] [Google Scholar]
- Howard MW, Bessette-Symons B, Zhang Y, Hoyer WJ. Aging selectively impairs recollection in recognition memory for pictures: evidence from modeling and receiver operating characteristic curves. Psychol Aging. 2006;21(1):93–106. doi: 10.1037/0882-7974.21.1.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudon C, Belleville S, Souchay C, Gely-Nargeot MC, Chertkow H, Gauthier S. Memory for gist and detail information in Alzheimer's disease and mild cognitive impairment. Neuropsychology. 2006;20(5):566–577. doi: 10.1037/0894-4105.20.5.566. [DOI] [PubMed] [Google Scholar]
- Ivnik RJ, Malec JF, Smith GE, Tangalos EG, Petersen RC. Neuropsychological tests' normsabove age 55: COWAT, BNT, MAE Token, WRAT-R Reading, AMNART, STROOP, TMT, and JLO. Clinical Neuropsychologist. 1996;10:262–278. [Google Scholar]
- Jacoby LL. A process-dissociation framework: separating automatic from intentional uses of memory. Journal of Memory and Language. 1991;30:513–541. [Google Scholar]
- Jacoby LL. Ironic effects of repetition: Measuring age-related differences in memory. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1999;25:3–22. doi: 10.1037//0278-7393.25.1.3. [DOI] [PubMed] [Google Scholar]
- Jennings JM, Jacoby LL. An opposition procedure for detecting age-related deficits in recollection: telling effects of repetition. Psychology and Aging. 1997;12(2):352–361. doi: 10.1037//0882-7974.12.2.352. [DOI] [PubMed] [Google Scholar]
- Johnson MK, O'Connor M, Cantor J. Confabulation, memory deficits, and frontal dysfunction. Brain and Cognition. 1997;34(2):189–206. doi: 10.1006/brcg.1997.0873. [DOI] [PubMed] [Google Scholar]
- Kaplan E, Goodglass H, Weintraub S. The Boston Naming Test. Philadelphia: Lea and Feibiger; 1983. [Google Scholar]
- Klunk WE, Engler H, Nordberg A, Wang Y, Blomqvist G, Holt DP, et al. Imaging brain amyloid in Alzheimer's disease with Pittsburgh Compound-B. Ann Neurol. 2004;55(3):306–319. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- Kordower JH, Chu Y, Stebbins GT, DeKosky ST, Cochran EJ, Bennett D, et al. Loss and atrophy of layer II entorhinal cortex neurons in elderly people with mild cognitive impairment. Neurology. 2001;62:1317–1322. [PubMed] [Google Scholar]
- Larrieu S, Letenneur L, Orgogozo JM, Fabrigoule C, Amieva H, Le Carret N, et al. Incidence and outcome of mild cognitive impairment in a population-based prospective cohort. Neurology. 2002;59(10):1594–1599. doi: 10.1212/01.wnl.0000034176.07159.f8. [DOI] [PubMed] [Google Scholar]
- Lekeu F, Van der Linden M, Chicherio C, Collette F, Degueldre C, Franck G, et al. Brain correlates of performance in a free/cued recall task with semantic encoding in Alzheimer disease. Alzheimer Disease and Associated Disorders. 2003;17(1):35–45. doi: 10.1097/00002093-200301000-00005. [DOI] [PubMed] [Google Scholar]
- Light LL, Patterson MM, Chung C, Healy MR. Effects of repetition and response deadline on associative recognition in young and older adults. Mem Cognit. 2004;32(7):1182–1193. doi: 10.3758/bf03196891. [DOI] [PubMed] [Google Scholar]
- Logan JM, Sanders AL, Snyder AZ, Morris JC, Buckner RL. Under-recruitment and nonselective recruitment: dissociable neural mechanisms associated with aging. Neuron. 2002;33(5):827–840. doi: 10.1016/s0896-6273(02)00612-8. [DOI] [PubMed] [Google Scholar]
- Lopez OL, Becker JT, Klunk W, Saxton J, Hamilton RL, Kaufer DI, et al. Research evaluation and diagnosis of probable Alzheimer's disease over the last two decades: I. Neurology. 2000;55(12):1854–1862. doi: 10.1212/wnl.55.12.1854. [DOI] [PubMed] [Google Scholar]
- MacMillan N, Creelman C. Detection theory: a user's guide. New Jersey: Lawrence Erlbaum Associates; 2005. [Google Scholar]
- Mandler G. Recognizing: The judgement of previous occurrence. Psychological Review. 1980;87:252–271. [Google Scholar]
- Mayes A, Montaldi D, Migo E. Associative memory and the medial temporal lobes. Trends Cogn Sci. 2007;11(3):126–135. doi: 10.1016/j.tics.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Mayes AR, Holdstock JS, Isaac CL, Montaldi D, Grigor J, Gummer A, et al. Associative recognition in a patient with selective hippocampal lesions and relatively normal item recognition. Hippocampus. 2004;14(6):763–784. doi: 10.1002/hipo.10211. [DOI] [PubMed] [Google Scholar]
- Mitchell TW, Mufson EJ, Schneider JA, Cochran EJ, Nissanov J, Han L, et al. Parahippocampal tau pathology in healthy aging, mild cognitive impairment, and early alzheimer's disease. Annals of Neurology. 2002;51:182–189. doi: 10.1002/ana.10086. [DOI] [PubMed] [Google Scholar]
- Morris JC. Mild cognitive impairment is early-stage Alzheimer disease: time to revise diagnostic criteria. Arch Neurol. 2006;63(1):15–16. doi: 10.1001/archneur.63.1.15. [DOI] [PubMed] [Google Scholar]
- Morris JC, Heyman A, Mohs RC, Hughes JP, van Belle G, Fillenbaum G, et al. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer's disease. Neurology. 1989;39(9):1159–1165. doi: 10.1212/wnl.39.9.1159. [DOI] [PubMed] [Google Scholar]
- Morris JC, Storandt M, Miller JP, McKeel DW, Price JL, Rubin EH, et al. Mild cognitive impairment represents early-stage Alzheimer disease. Arch Neurol. 2001;58(3):397–405. doi: 10.1001/archneur.58.3.397. [DOI] [PubMed] [Google Scholar]
- Nunally JC, Bernstein IH. Psychometric Theory. New York: McGraw-Hill; 1994. [Google Scholar]
- Parkin AJ, Walter BM. Recollective experience, normal aging, and frontal dysfunction. Psychology and Aging. 1992;7:290–298. doi: 10.1037//0882-7974.7.2.290. [DOI] [PubMed] [Google Scholar]
- Petersen RC. Mild cognitive impairment as a diagnostic entity. Journal of Internal Medicine. 2004;256:183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Parisi JE, Dickson DW, Johnson KA, Knopman DS, Boeve BF, et al. Neuropathologic features of amnestic mild cognitive impairment. Arch Neurol. 2006;63(5):665–672. doi: 10.1001/archneur.63.5.665. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Stevens JC, Ganguli M, Tangalos EG, Cummings JL, DeKosky ST. Practice parameter: early detection of dementia: mild cognitive impairment (an evidence-based review). report of the quality standards subcommittee of the American Academy of Neurology. Neurology. 2001;56(9):1133–1142. doi: 10.1212/wnl.56.9.1133. [DOI] [PubMed] [Google Scholar]
- Pierce BH, Sullivan AL, Schacter DL, Budson AE. Comparing source-based and gist-based false recognition in aging and Alzheimer's disease. Neuropsychology. 2005;19(4):411–419. doi: 10.1037/0894-4105.19.4.411. [DOI] [PubMed] [Google Scholar]
- Rauchs G, Piolino P, Mezenge F, Landeau B, Lalevee C, Pelerin A, et al. Autonoetic consciousness in Alzheimer's disease: neuropsychological and PET findings using an episodic learning and recognition task. Neurobiol Aging. 2007;28(9):1410–1420. doi: 10.1016/j.neurobiolaging.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM. Differential aging of the brain: patterns, cognitive correlates and modifiers. Neurosci Biobehav Rev. 2006;30(6):730–748. doi: 10.1016/j.neubiorev.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitan R. Validity of the trail making test as an indicator of organic brain disease. Percept Motor Skills. 1958;8:271–276. [Google Scholar]
- Rosen AC, Prull MW, O'Hara R, Race EA, Desmond JE, Glover GH, et al. Variable effects of aging on frontal lobe contributions to memory. Neuroreport. 2002;13(18):2425–2428. doi: 10.1097/00001756-200212200-00010. [DOI] [PubMed] [Google Scholar]
- Rossion B, Pourtois G. Revisiting Snodgrass and Vanderwart's object pictorial set: the role of surface detail in basic-level object recognition. Perception. 2004;33(2):217–236. doi: 10.1068/p5117. [DOI] [PubMed] [Google Scholar]
- Rugg MD, Curran T. Event-related potentials and recognition memory. Trends Cogn Sci. 2007;11(6):251–257. doi: 10.1016/j.tics.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Slotnick SD, Dodson CS. Support for a continuous (single-process) model of recognition memory and source memory. Mem Cognit. 2005;33(1):151–170. doi: 10.3758/bf03195305. [DOI] [PubMed] [Google Scholar]
- Smith JA, Knight RG. Memory processing in Alzheimer's disease. Neuropsychologia. 2002;46(6):666–682. doi: 10.1016/s0028-3932(01)00137-3. [DOI] [PubMed] [Google Scholar]
- Spreen O, Strauss E. A compendium of neuropsychological tests: Administration, norms, and commentatory. 2nd ed. New York: Oxford University Press; 1998. [Google Scholar]
- Squire LR, Wixted JT, Clark RE. Recognition memory and the medial temporal lobe: a new perspective. Nat Rev Neurosci. 2007;8(11):872–883. doi: 10.1038/nrn2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki WA, Amaral DG. Where are the perirhinal and parahippocampal cortices? A historical overview of the nomenclature and boundaries applied to the primate medial temporal lobe. Neuroscience. 2003;120(4):893–906. doi: 10.1016/s0306-4522(03)00281-1. [DOI] [PubMed] [Google Scholar]
- Tendolkar I, Schoenfeld A, Golz G, Fernandez G, Kuhl K, Ferszt R, et al. Neural correlates of recognition memory with and without recollection in patients with Alzheimer's disease and healthy controls. Neuroscience Letters. 1999;263:45–48. doi: 10.1016/s0304-3940(99)00106-8. [DOI] [PubMed] [Google Scholar]
- Tierney MC, Black SE, Szalai JP, Snow WG, Fisher RH, Nadon G, et al. Recognition memory and verbal fluency differentiate probable Alzheimer disease from subcortical ischemic vascular dementia. Archives of Neurology. 2001;58(10):1654–1659. doi: 10.1001/archneur.58.10.1654. [DOI] [PubMed] [Google Scholar]
- Van Hoesen GW, Augustinack JC, Dierking J, Redman SJ, Thangavel R. The parahippocampal gyrus in Alzheimer's disease. Clinical and preclinical neuroanatomical correlates. Ann N Y Acad Sci. 2000;911:254–274. doi: 10.1111/j.1749-6632.2000.tb06731.x. [DOI] [PubMed] [Google Scholar]
- Van Petten C. Relationship between hippocampal volume and memory ability in healthy individuals across the lifespan: review and meta-analysis. Neuropsychologia. 2004;42(10):1394–1413. doi: 10.1016/j.neuropsychologia.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Wais PE, Wixted JT, Hopkins RO, Squire LR. The hippocampus supports both the recollection and the familiarity components of recognition memory. Neuron. 2006;49(3):459–466. doi: 10.1016/j.neuron.2005.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale. WAIS-III. Administration and scoring manual. 3rd ed. San Antonio: The Psychological Corporation; 1997. [Google Scholar]
- Westerberg CE, Paller KA, Weintraub S, Mesulam MM, Holdstock JS, Mayes AR, et al. When memory does not fail: familiarity-based recognition in mild cognitive impairment and Alzheimer's disease. Neuropsychology. 2006;20(2):193–205. doi: 10.1037/0894-4105.20.2.193. [DOI] [PubMed] [Google Scholar]
- Wheeler MA, Stuss DT, Tulving E. Frontal lobe damage produces episodic memory impairment. Journal of the International Neuropsychological Society. 1995;1(6):525–536. doi: 10.1017/s1355617700000655. [DOI] [PubMed] [Google Scholar]
- Wilding EL, Rugg MD. Event-related potentials and the recognition memory exclusion task. Neuropsychologia. 1997;35(2):119–128. doi: 10.1016/s0028-3932(96)00076-0. [DOI] [PubMed] [Google Scholar]
- Winblad B, Palmer K, Kivipelto M, Jelic V, Fratiglioni L, Wahlund LO, et al. Mild cognitive impairment--beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med. 2004;256(3):240–246. doi: 10.1111/j.1365-2796.2004.01380.x. [DOI] [PubMed] [Google Scholar]
- Wixted JT. Dual-process theory and signal-detection theory of recognition memory. Psychol Rev. 2007;114(1):152–176. doi: 10.1037/0033-295X.114.1.152. [DOI] [PubMed] [Google Scholar]
- Wixted JT, Squire LR. Recall and recognition are equally impaired in patients with selective hippocampal damage. Cogn Affect Behav Neurosci. 2004;4(1):58–66. doi: 10.3758/cabn.4.1.58. [DOI] [PubMed] [Google Scholar]
- Yonelinas A. The nature of recollection and familiarity: A review of 30 years of research. Journal of Memory and Language. 2002;46:441–517. [Google Scholar]
- Yonelinas AP. Receiver-operating characteristics in recognition memory: Evidence for a dual-process model. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1994;20:1341–1354. doi: 10.1037//0278-7393.20.6.1341. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP, Jacoby LL. The relation between remembering and knowing as bases for recognition: effects of size congruency. Journal of Memory and Language. 1995;34:622–643. [Google Scholar]
- Yonelinas AP, Kroll NE, Quamme JR, Lazzara MM, Sauve MJ, Widaman KF, et al. Effects of extensive temporal lobe damage or mild hypoxia on recollection and familiarity. Nature Neuroscience. 2002a;5(11):1236–1241. doi: 10.1038/nn961. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP, Kroll NE, Quamme JR, Lazzara MM, Sauve MJ, Widaman KF, et al. Effects of extensive temporal lobe damage or mild hypoxia on recollection and familiarity. Nature Neuroscience. 2002b;5:1236–1241. doi: 10.1038/nn961. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP, Regehr G, Jacoby LL. Incorporating response bias in a dual-process theory of memory. Journal of Memory and Language. 1995;34(6):821–835. [Google Scholar]