Abstract
The nature, neural underpinnings, and etiology of deficits in verbal declarative memory in patients with schizophrenia remain unclear. To examine the contributions of genes and environment to verbal recall and recognition performance in this disorder, the California Verbal Learning Test was administered to a large population-based Finnish twin sample, which included schizophrenic and schizoaffective patients, their non-ill monozygotic (MZ) and dizygotic (DZ) co-twins, and healthy control twins. Compared with controls, patients and their co-twins showed relatively greater performance deficits on free recall compared with recognition. Intra-pair differences between patients and their non-ill co-twins in hippocampal volume and memory performance were highly positively correlated. These findings are consistent with the view that genetic influences are associated with reduced verbal recall in schizophrenia, but that non-genetic influences further compromise these abnormalities in patients who manifest the full-blown schizophrenia phenotype, with this additional degree of disease-related declarative memory deficit mediated in part by hippocampal pathology.
Keywords: CVLT, Twin, Neuropsychology, Memory, Genes, Environment, Hippocampus
1. Introduction
Deficits in verbal learning and memory are robust correlates of schizophrenia (Heinrichs & Zakzanis, 1998; Aleman et al., 1999; Kuperberg & Heckers, 2000; Cirillo & Seidman, 2003; Pelletier et al., 2005). Hypotheses about the cognitive underpinnings of these deficits include an inability to use efficient strategies spontaneously during encoding and/or retrieval, reduced conscious recollection and increased reliance on familiarity assessment as a basis for retrieval, and reduced monitoring processes during retrieval, processes thought to be mediated by prefrontal and medial temporal lobe regions (Achim & Lepage, 2005). A meta-analysis found that the degree of difference in performance on memory tests between schizophrenic patients and controls, expressed in terms of effect size, declines with an increase in the amount of contextual information provided at test (free recall < cued recall < recognition) (Aleman et al., 1999). Arguing against the interpretation that this differential level of deficit is an artifact of the discriminating power of the performance measure employed (Chapman & Chapman, 1973; Chapman & Chapman, 1978) is a report that chronic non-demented schizophrenic patients showed a larger deficit on a free recall compared to a performance matched recognition task (Calev, 1984a). This result was obtained despite the higher reliability for the recognition task, which would have predicted a higher discriminating power for the recognition versus the recall task, but also see (Calev, 1984b) and (Mohamed et al., 1999).
Several reviews have concluded that mean verbal recall performance is among the measures that show strong familial effects in relatives of schizophrenic patients (Sitskoorn et al., 2004; Whyte et al., 2005; Trandafir et al., 2006). A study that included non-ill relatives with either one or multiple first-degree relatives diagnosed with schizophrenia showed that deficits in story recall scaled with the level of genetic predisposition for the disorder (Faraone et al., 2000). Three twin reports including MZ twin pairs discordant for schizophrenia as well as control twins found that patients performed significantly worse than their co-twins, who performed significantly worse than the healthy twins on a story recall task, suggesting disease-related as well as familial influences on verbal memory performance in schizophrenia (Goldberg et al., 1990; Goldberg et al., 1993; Goldberg et al., 1995). Furthermore, one report showed that patients from concordant pairs did not differ from patients from discordant pairs, suggesting a similar etiology of the observed performance deficits in both groups (Goldberg et al., 1995). A previous report on this sample, which includes both MZ and DZ pairs discordant for schizophrenia as well as groups of healthy MZ and DZ twin pairs, examined free recall (story and verbal list items) as part of a canonical discriminant analysis, and showed that tests of verbal declarative memory contributed to the discrimination of patients from their own MZ co-twins (Cannon et al., 2000).
The majority of the studies including relatives of schizophrenic patients have examined performance on free recall of stories or word lists. Two recent meta-analytic studies concluded that too few studies have compared free recall, cued recall, and recognition for the same test in patients with schizophrenia and their relatives (Whyte et al., 2005; Trandafir et al., 2006) such that no firm conclusions can be drawn for the existence or not of retrieval deficits (Trandafir et al., 2006). Some studies have observed deficits in recognition hits among relatives of schizophrenic patients (Lyons et al., 1995), while others have not (Keri et al., 2001; Sponheim et al., 2004). One study showed that relatives performed worse on cued recall compared with controls (Sponheim et al., 2004).
To our knowledge, no study with schizophrenic patients' relatives at multiple levels of genetic predisposition has compared performance on free recall, cued recall, and recognition. The comparison of free recall, cued recall, and recognition provides a manipulation of the extent to which self-initiated strategic retrieval is needed to perform the task (Davidson et al., 2006). Encoding and consolidation are required in any of these conditions, but the conditions differ in the extent to which they require active retrieval (free recall > cued recall > recognition).
Evidence from neuroimaging studies of declarative memory in schizophrenia are consistent with reduced organizational processing at encoding as well as at retrieval and reduced post-retrieval monitoring, which may mainly involve the frontal lobes, and with less efficient associative encoding processes and a deficit in conscious recollection involving the hippocampus and medial temporal lobes (Achim & Lepage, 2005), regions that are known to be disrupted in patients with schizophrenia and their relatives.
The primary aim of the current paper was to determine the genetic and environmental influences on free recall, cued recall, and recognition performance in twins discordant for schizophrenia. Based on the foregoing, we predicted that patients and their co-twins would show relatively greater memory deficits compared with controls on conditions requiring active retrieval (free recall > cued recall > recognition), with the degree of deficit in co-twins varying in proportion to their genetic proximity to an affected individual. We also predicted that patients would show a greater deficit in recall and recognition compared with their own co-twins, possibly due to deficits in encoding and/or conscious recollection, and that these differences would be related to intra-pair differences in hippocampal volume.
2. Methods
The study protocol was reviewed and approved by the institutional review boards (IRBs) of the University of California (Los Angeles) and the National Public Health Institute of Finland, and all participants signed IRB-approved informed-consent forms.
2.1 Sample Ascertainment
Subjects were drawn from a twin cohort consisting of all same-sex twins born in Finland from 1940-1957 (N=9,562 pairs) part of the nationwide Finnish Twin Cohort (Kaprio & Koskenvuo, 2002) and their selection was as previously described (Cannon et al., 2000; van Erp et al., 2004). Because general intelligence and education level may be reduced in subjects with schizophrenia and their biological relatives due to disease and genetic predisposition, subject groups were not matched on these indices but rather on parental socioeconomic status (Meehl, 1970). Control twins did not have any schizophrenia spectrum disorder or Axis I psychosis, and their first-degree relatives were free of a history of psychosis, based on review of hospital and disability records. Patient's relatives with cluster A disorders were not removed from the final analyses given that these may constitute those relatives who have the highest genetic predisposition, though results from analyses with and without these relatives were similar.
2.2 Diagnostic Evaluation
Each co-twin was interviewed using the Structured Clinical Interview for DSM-III-R Disorders, SCID Patient or Non-Patient edition (Spitzer et al., 1989) by a different examiner who was blind to the zygosity and diagnostic status of their co-twin, and the twins were assigned diagnoses based on the DSM-IV (American Psychiatric Association, 1994). Personality disorder symptoms for co-twins and healthy subjects were rated (e.g., Cluster A) on the SCID-II (Spitzer & Williams, 1986). Final diagnoses were made by consensus among three independent raters after review of written case reports and diagnostic reliability was excellent (i.e., κ=0.94 ± .02) (Cohen, 1960). Subjects with a psychotic condition were also rated using the Scale for the Assessment of Positive Symptoms (SAPS) (Andreasen, 1984) and Scale for the Assessment of Negative Symptoms (SANS) (Andreasen, 1983). Classification of patients was based on best-estimate lifetime diagnoses. Among the 55 probands, 49 (27 MZ, 22 DZ) were diagnosed with schizophrenia and six with schizoaffective disorder (2 MZ, 4 DZ) (table 1).
Table 1.
Demographic Characteristics across Comparison Groups [Mean (SD) or N (%)]
| Probands | MZ co-twins | DZ co-twins | Control twins | F or χ2 | p | |
|---|---|---|---|---|---|---|
| (n=55) | (n=18) | (n=25) | (n=109) | |||
| Characteristic | ||||||
| Age (yrs) | 47.4 (6.2) | 48.8 (4.9) | 48.2 (4.6) | 49.1 (4.1) | 1.49 | .22 |
| Age at Onset | 24.7 (5.7) | |||||
| Duration of Illness | 22.8 (6.8) | |||||
| Males | 29 (53) | 9 (50) | 12 (48) | 58 (53) | .26 | .97 |
| Parental Social Classa | 6.8 (1.4) | 5.8 (1.3) | 7.0 (1.2) | 6.5 (1.4) | 1.2 | .31 |
| Right Handed | 51 (93) | 15 (83) | 22 (88) | 103 (95) | 3.4 | .33 |
| Years cohabitation | 19.5 (3.5) | 20.7 (3.0) | 18.8 (3.7) | 20.5 (4.3) | 1.6 | .19 |
| Substance Disorder | 12 (22) | 3 (17) | 3 (12) | 5 (5) | 11.7 | .009 |
| Affective Disorders | 1 (2) | 4 (22) | 4(16) | 10 (9) | 8.6 | .034 |
| Cluster A Disorder | … | 5 (28) | 1 (2) | 0 (0) | 37 | .001 |
| Educationb | 2.8 (1.4) | 2.8 (1.8) | 3.9(1.7) | 4.2(1.6) | 11.6 | <.0001 |
| Overall IQc | 8.3 (2.5) | 9.7 (2.4) | 10.6 (2.1) | 11.3 (1.7) | 26.3 | <.0001 |
MZ= Monozygotic; DZ = Dizygotic
Socioeconomic status on the Rahaula Scale, 9 = lowest and 1 = highest (Rauhala, 1970)
Educational scale from SCID (Spitzer et al., 1989) interview.
Estimated IQ based on average of age-corrected scaled scores of the Vocabulary, Similarities, Block Design, and Digit Symbol subtests of WAIS-R (Wechsler, 1981)
2.3 Zygosity
Zygosity was determined by DNA analysis using the following markers: DIS80 (20 alleles), DI7S30 (13 alleles), apoB (20 alleles), COL2A1 (10 alleles), vWA (9 alleles), and HUMTH01 (6 alleles). Assuming an average heterozygosity rate of 70% per marker, we estimate that this procedure will falsely classify a DZ pair as MZ in ∼ 1/482 cases.
2.4 Measurements
The Finnish version of the California Verbal Learning Test (CVLT) was administered in a quiet room by trained psychologists (ATH, TP) as part of a comprehensive neuropsychological battery (Cannon et al., 2000). The test was administered according to the instructions documented in the manual of the English version of the CVLT (Delis et al., 1987). As in the English version of the CVLT the test conditions included five free recall trials of list A (sixteen words from four semantic categories), followed by one free recall trial of list B (sixteen new words, half from categories shared with list A, and half from non-shared categories), a free and cued recall (semantic category cue) of list A, and a free recall, cued recall, and recognition test of List A words given after a 20-minute delay during which the subjects received the Continuous Performance Test, the WAIS-R Vocabulary and Similarities, and Trails A and B (Cannon et al., 2000). Because there were no significant group differences in forgetting at short and long delay intervals, recall performance data were averaged over delay (table 2). To allow for better comparison of performance across the different trial types (free recall, cued recall, and recognition), z-scores were computed by normalizing to the means and standard deviations observed in the control twins. The methods involved in the assessment of hippocampal volumes have been described in detail elsewhere (Van Erp et al., 2002; van Erp et al., 2004).
Table 2.
Absolute Means (Standard Deviation) across Comparison Groups
| Proband
(n=55) |
MZ-cotwin
(n=18) |
DZ-cotwin
(n=25) |
Control twins
(n=109) |
|
|---|---|---|---|---|
| Number Correct Free Recall | 6.3 (2.1) | 7.7 (2.0) | 9.0 (2.1) | 9.8 (2.0) |
| Number Correct Cued Recall | 7.6 (2.8) | 9.4 (3.0) | 10.6 (3.0) | 11.5 (2.4) |
| Number Correct Recognition | 13.0 (2.7) | 14.1 (1.6) | 14.4 (1.3) | 14.3 (1.7) |
| Left Hippocampal Volume | 4.12 (0.49) | 4.32 (0.52) | 4.33 (0.47) | 4.57 (0.52) |
The mean number correct is based on an average number correct per trial of the same type. The mean hippocampal volumes are reported in milliliters.
2.5 Statistical Analyses
For all regression models data were checked for homogeneity of variance (Levene, 1960) and residuals were checked for normality (Shapiro & Wilk, 1965). Because the number of correct was equivalent across proband groups (probands from concordant vs. discordant pairs, and from MZ and DZ index pairs), the probands were treated as a single group in the main analyses.
To test the first hypothesis, a mixed model regression analysis (Proc Mixed, SAS version 8.2, SAS Institute, Inc, Cary, NC) was performed, evaluating the z-scores for free recall, cued recall, and recognition performance as the dependent variable and treating risk group (proband, MZ-cotwin, DZ-cotwin, healthy), trial type (free recall, cued recall, recognition), and the risk group × trial type interaction as predictors. Trial type was modeled as a repeated measures factor. To test for differences in the effect of genetic predisposition between performance on free recall, cued recall, and recognition conditions of the CVLT, contrast analyses were performed to test whether the linear effects across the risk groups (proband, MZ-cotwin, DZ-cotwin, and healthy control, modeled as either –3, -1, 1, and 3 or 3, 1, -1, and –3) varied according to trial type (free recall > recognition, free recall > cued recall, and cued recall > recognition). Mixed model regression was also used to determine whether the differences between the z-scores of the number correct on recognition and the number correct on free recall were predicted by risk group.
Given reported effects of age (Smith, 1996) and sex (Maitland et al., 2004) on memory performance and the group differences in substance disorder, in all aforementioned analyses, substance disorder, sex, and age entered the model as covariates, and twin pair entered the model as a random variable, controlling for correlation among the co-twins by adjusting the model error terms accordingly (Satterthwaite option). The significance of each predictor was tested while accounting for all other model terms simultaneously. Whenever one of the terms contributed significantly to the prediction of the dependent variable, one-tailed contrast analyses were used to compare hypothesized mean differences within the term collapsing over non-significant terms in the model. This approach maintains the hypothesis-wise Type I error rate at .05 because a predictor's contribution to particular dependent measures is evaluated only if its effect is found to vary at the multivariate level. Where significant, contrast t-statistics, with their denominator degrees of freedom as estimated by the Satterthwaite procedure in subscript, are reported. All of the principal effects remained significant when co-varying for general intelligence and education, both of which also contributed significantly to recall performance. To allow for comparisons of the severity of the deficits across all the conditions and relative to other results reported in the literature, Hedge's g effect sizes (Hedges & Olkin, 1985) for number correct in probands, MZ co-twins, and DZ co-twins relative to control twins were computed (table 3).
Table 3.
Effect Sizes (Hedges g) Relative to the Control Twins (95% Confidence Intervals)
| Probands | MZ co-twins | DZ co-twins | |
|---|---|---|---|
| Free Recall Correct | 1.46 (1.10-1.82) | 0.84 (0.33-1.34) | 0.38 (-0.12-0.89) |
| Cued Recall Correct | 1.22 (0.87-1.57) | 0.62 (0.12-1.12) | 0.32 (-0.28-0.82) |
| Recognition Correct | 0.56 (0.23-0.89) | 0.07 (-0.43-.56) | 0.01 (-0.48-0.51) |
Hedges g adjust for sample size, though unlike the reported z-scores the Hedges g uses pooled variance.
To assess whether deficits in memory performance in patients relative to their non-ill co-twins are associated with hippocampal volume reduction in patients relative to their non-ill co-twins, the relationships between intra-pair differences in verbal recall and recognition and intra-pair differences in left hippocampal volumes were examined with Pearson's correlations (Hypothesis 2). This type of analysis is likely more powerful than performing regular correlations between the measures given that it controls for random genetic variance between pairs. Furthermore, the correlations of intra-pair differences among the monozygotic twins is particularly sensitive to variation due to environmental factors.
3. Results
3.1 Number Correct
There were significant effects of risk group [F(3,247)=42.13,P<0.0001], trial type [F(2,497)=20.11,P=<0.0001], risk group × trial type interaction [F(6,497)=7.12,P<0.0001], sex [F(1,103)=15.03,P=0.0001], and a marginally significant effect of age [F(1,104)=3.19,P=0.08] in predicting the number of words retrieved on the CVLT (Figure 1). The slope across risk groups (Proband < MZ-cotwin < DZ-cotwin < Healthy) was steeper for free recall [t497=33.41,P=<0.0001] and cued recall [t497=17.37,P=<0.0001] compared with recognition, and steeper for free recall compared to cued recall [t497=2.60,P=0.05, 1-tailed]. Group contrasts comparing free recall performance showed that probands performed worse than healthy twins (t300=-10.2,P<0.0001), MZ co-twins (t497=-3.2,P=0.001), and DZ co-twins (t497=-6.4,P<0.0001), that MZ co-twins performed worse than DZ co-twins (t497=-2.1,P=0.02) and healthy twins (t518=-4.3,P<0.0001), and that DZ co-twins performed worse than healthy twins (t446=-2.3,P=0.01). Group contrasts comparing cued recall performance showed that probands performed worse than healthy twins (t300=-8.6,P<0.0001), MZ co-twins (t542=-3.1,P=0.001), and DZ co-twins (t555=-5.4,P<0.0001), that MZ co-twins performed similarly to DZ co-twins (t582=-1.39,P=0.08) and worse than healthy twins (t518=-4.3,P<0.0001), and that DZ co-twins performed worse than healthy twins (t446=-1.9,P=0.03). Group contrasts comparing recognition performance showed that probands performed worse than healthy twins (t300=-3.9,P<0.0001), MZ co-twins (t542=-2.6,P=0.002), and DZ co-twins (t555=-3.3,P<0.0001), and that none of the other groups differed from each other. Females performed better than males (t103=3.9,P=0.0002, two-tailed) and performance showed a marginally significant decrease with age (t104=-1.79,P=0.08, two-tailed; slope=-0.015, standard error= 0.009).
Figure 1.
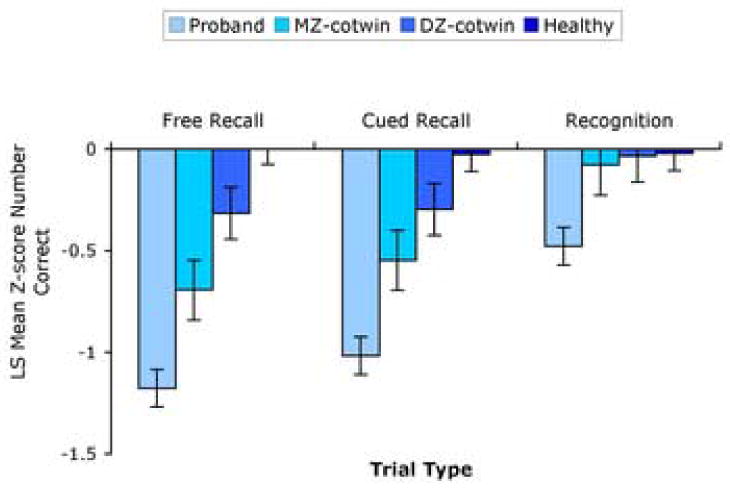
Least Square Mean Standard (Z) Scores on Free Recall, Cued Recall, and Recognition by Comparison Group +/- Standard Error.
3.2 Difference between Number Correct on Free Recall and Recognition
There were significant effects of risk group [F(3,150)=12.52,P<0.0001] and sex [F(1,99)=7.22,P=0.009] on the differences between the number correct on recognition and free recall. Contrast analyses revealed that the difference was larger in probands compared to DZ co-twins (t149=2.59,P=0.005) and healthy twins (t146=5.93,P<0.0001), and larger in MZ (t196=3.65,P=0.0002) and DZ (t186=2.7,P=0.007) co-twins than healthy twins. The difference between non-ill MZ and DZ co-twins was marginally significant (t146=2.15,P=0.07). The difference was larger in males than females (t99=2.69,P=0.009, two-tailed).
3.3 Relationship with Hippocampal Volume
Correlation analyses among intra-pair differences in left hippocampal volume and free recall, cued recall, and recognition performance showed significant correlations for discordant MZ pairs on recognition (r12=0.65; P=0.02), and for discordant DZ pairs on free recall (r19=0.74,P=0.0003 ), cued recall (r19=0.57,P=0.01), and recognition (r19=0.71; P=0.0006). Among the healthy twin pairs, the only significant correlation was between intra-pair differences in left hippocampal volume and cued recall performance (r22=.45,P=0.03). Two of these correlations survive a stringent Bonferroni correction for multiple comparisons with a corrected P-value of 0.004 based on the examination of twelve P-values (four groups (discordant MZ and DZ, and healthy MZ and DZ) by three trials types (free recall, cued recall, recognition).
4. Discussion
The principal finding of the study is that the effect of genetic predisposition to schizophrenia on verbal declarative memory performance is larger on the free recall compared with the recognition condition of the CVLT (figure 1 and table 3). Furthermore, intra-pair differences in left hippocampal volumes between patients and their co-twins correlate significantly with intra-pair differences in verbal declarative memory performance. Consistent with previous reports we found female superiority in verbal declarative memory performance (Maitland et al., 2004) and a decline with age (Smith, 1996).
Large deficits on free recall, intermediate deficits on cued recall, and relatively smaller deficits on recognition have been observed in frontal lobe lesion patients (Wheeler et al., 1995). Furthermore, frontal lobe gray matter deficits in schizophrenia appear to be influenced by genetic predisposition to the disorder, as they increase in severity in proportion with genetic proximity to an affected individual (MZ>DZ>control) (Cannon et al., 1998; Baare et al., 2001; Cannon et al., 2002). We therefore interpret these findings to be consistent with an inherited disturbance in prefrontal cortical circuits involved in memory retrieval (Cannon et al., 2000). The performance deficit on recognition appears to be mainly limited to the schizophrenic patients, and we interpret this finding to suggest a larger influence of environmental factors on verbal declarative memory acquisition, a function purportedly subserved by the medial temporal lobe including the hippocampus (Scoville & Milner, 2000). This interpretation is corroborated by the finding of significant correlations between intra-pair differences in left hippocampal volumes and recognition performance in both the discordant MZ and DZ twin pairs. The significant correlations between intra-pair differences in left hippocampal volumes and intra-pair differences in free recall and cued recall in the discordant DZ pairs may suggest that some additional shared genetic variance in hippocampal volume reduction contributes to retrieval deficits also, a finding in line with the observations of significant positive correlations between free recall performance and hippocampal volumes (Gur et al., 2000; O'Driscoll et al., 2001; Seidman et al., 2002), larger hippocampal activation for remember compared to know responses during retrieval in healthy subjects (Eldridge et al., 2000), and failed recruitment of the hippocampus during retrieval in patients with schizophrenia compared to controls (Heckers et al., 1998; Weiss et al., 2003). However, it is also possible that this result reflects a difference in power given that the correlations in the DZ twins were based on nineteen and those in MZ twins on only twelve pairs. Furthermore, It is important to note that response bias (false positives – misses / false positives + misses) did not differ significantly across the groups, which indicates that the deficit in recognition among the patients is not due to a negative response bias (saying no most of the time).
The reduced group differences on the number correct in recognition compared to free recall are unlikely due to a lower true score variance (estimated from the product of the reliability of the test and the variance of the observed scores) in the recognition compared to the free recall test (Chapman & Chapman, 1973; Chapman & Chapman, 1978), because the average estimated test-retest reliabilities for the free recall trials (.49) and recognition hits (.47) (Delis et al., 1987) are similar, as were the variances in free recall (SD=2.0) and recognition (SD=1.7) among the healthy twins (F[1,216]=1.68,P=0.20) (Levene, 1960). Furthermore, these findings corroborate those reported previously on psychometrically matched tests of free recall and recognition (Calev, 1984a), and those of a recent report showing a similar pattern of deficit for free recall and recognition in patients and their biological relatives compared to healthy controls (Sponheim et al., 2004).
We cannot fully exclude the possibility that a ceiling effect in recognition accuracy may preclude the stepwise genetic load effect on this measure as was observed on free recall since a substantial number of subjects (20-25% in each of the four groups) performed at ceiling (in this case, 16 items correct). However, because 75-80% of the subjects in each of the four groups did not perform at ceiling, and because the free recall and recognition variances in the healthy subjects do not differ significantly, it is reasonable to interpret the mean group differences as a valid reflection of central tendency.
While it can be argued that analysis of covariance is not a perfect method for establishing the independence of an effect from nuisance variables (Kahneman, 1965), the principal effects remained significant when co-varying for general intelligence and education, suggesting that, while to a certain extent common causes may underlie reduced general intelligence, education, and probably also memory performance in schizophrenia, they most likely do not completely overlap. This interpretation is further supported by observed memory deficits in other studies that have co-varied or matched for education or intellectual level when examining verbal declarative memory deficits in schizophrenia compared with healthy controls (Cirillo & Seidman, 2003).
While we cannot exclude the possibility that the verbal declarative memory deficits in the probands compared to their MZ co-twins are in part due to disease chronicity or medication effects, neither proband's hippocampal volumes (van Erp et al., 2004) nor free recall, cued recall, or recognition performance were associated with illness duration after co-varying for age or with years on neuroleptic treatment, suggesting that illness duration and treatment are not contributing significantly to the observed deficits. Furthermore, verbal declarative memory deficits (Saykin et al., 1994) and hippocampal volume reductions (Gur et al., 2000) have also been observed in never-medicated first-episode patients and can therefore not be fully accounted for by duration of illness or medication effects.
One implication for future research is that verbal declarative memory tasks that require active retrieval may be a useful endophenotypic measure in that can be used in the search for schizophrenia susceptibility genes. It must be noted that memory deficits (Gilbertson et al., 2002; Kieseppä et al., 2005) and lower hippocampal volumes (Geuze et al., 2005) have also been reported in numerous other neurological and psychiatric disorders, as well as in some of their co-twins (Gilbertson et al., 2002; Kieseppä et al., 2005), and it is still unclear to what extent these declarative memory and hippocampal deficits are unique vulnerability factors for schizophrenia and to what extent they may reflect vulnerability for psychiatric illness in general.
Nevertheless, patients have significantly more impaired declarative memory than their non-ill MZ co-twins, a finding that parallels the results observed for hippocampal volume reduction (van Erp et al., 2004). This pattern indicates that a non-genetic factor further compromises the declarative memory system in patients who manifest the full schizophrenia phenotype over and above the level of pathology that would be expected given presence of a schizophrenia-promoting genotype. Hippocampal volume has been associated with a history of obstetric complications (McNeil et al., 2000) and in particular with hypoxic events in subjects genetically predisposed to schizophrenia (Van Erp et al., 2002). Exposure to mild hypoxia has been shown to influence recollection but not familiarity (Yonelinas et al., 2002). Furthermore, there is evidence that twins from the same pair can differ in absolute nucleated red blood cell counts, which have been associated with relative fetal hypoxia (Mori et al., 2001; Green et al., 2004). Finally, left hippocampal gray matter reduction in adolescents with a history of prematurity (Gimenez et al., 2004), which is associated with increased nucleated red blood cell counts, have been associated with poor memory performance. Together these findings suggest that hypoxia may be such an environmental contributor to schizophrenia vulnerability and the hippocampal and memory deficits observed. In addition, it is possible that differential post-natal environmental changes during adolescence may be involved as suggested by a report that declarative memory deficits are progressive in prodromal high-risk subjects who experience symptoms (Cosway et al., 2000). A possible candidate environmental factor that may act more proximal to disease onset may be hypercortisolism (Corcoran et al., 2003), which has been associated with memory deficits (Het et al., 2005) and hippocampal volume reduction in schizophrenia (Walker et al., 2002).
A clinical implication of these findings, with importance for designing cognitive rehabilitation strategies, is that patients may do better on tasks that do not require active retrieval of information. Retrieval on the other hand can be a target for cognitive remediation, either through medication or cognitive therapy. While much attention has focused on elaborate encoding, the use of retrieval strategies has received little attention despite evidence that patients' poor performance on recall tasks is due to their failure to adopt efficient retrieval as well ass encoding strategies (McClain, 1983).
It is not clear to what extent the association of hippocampal volume with the memory deficits are due to a reduction in binding of information at encoding (Cohen et al., 1999) or reduced recollection at retrieval, both thought to involve the hippocampus (Cohen et al., 1999; Eldridge et al., 2000). Future studies using task matching and functional imaging may shed more light on this question.
Our results are consistent with those reported by Sponheim (2004) who examined memory deficits in patients and their siblings, in that patients and their co-twins show deficits in free recall, but that patients only show deficits in recognition also. An inherent problem in drawing conclusions about encoding and retrieval deficits based on free recall and recognition measures is that in most cases no task matching has been performed. Future work is needed examining patients and their co-twins across performance-matched free recall, cued recall and recognition conditions. In the mean time, the current data suggest that declarative memory deficits in schizophrenia are likely due to genetic influences on retrieval, possibly mainly reflective of frontal lobe dysfunction, and that they are further compromised by extra-genetic influences on encoding, possibly mainly reflective of medial temporal lobe dysfunction including the hippocampus. One must however keep in mind that brain regions do not function in isolation, and are part of memory systems allowing for localized effects as well as deficits in functional connectivity (Ragland et al., 2004).
In conclusion, genetic influences are associated with reduced verbal recall in schizophrenia and non-genetic influences further compromise these abnormalities in patients who manifest the full-blown schizophrenia phenotype, with this additional degree of disease-related declarative memory deficit mediated in part by hippocampal pathology.
Acknowledgments
This research was supported by National Institute of Mental Health grant MH52857 (to T.D.C.). The authors wish to thank Ulla Mustonen, Pirjo Käki, and Eila Voipio for their contributions to subject recruitment and evaluation, AnttiTanskanen for his contributions to the register searches, Kauko Heikkilä for his contributions to data management of the Finnish Twin Cohort Study, and the Finnish twins for participation in the study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Achim AM, Lepage M. Episodic memory-related activation in schizophrenia: meta-analysis. British Journal of Psychiatry. 2005;187:500–509. doi: 10.1192/bjp.187.6.500. [DOI] [PubMed] [Google Scholar]
- Aleman A, Hijman R, de Haan EH, Kahn RS. Memory impairment in schizophrenia: a meta-analysis. American Journal of Psychiatry. 1999;156:1358–1366. doi: 10.1176/ajp.156.9.1358. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. DSM IV: diagnostic and statistical manual of mental disorders. American Psychiatric Association; Washington, DC: 1994. [Google Scholar]
- Andreasen N. The scale for the assessment of negative symptoms (SANS) University of Iowa; Iowa City: 1983. [Google Scholar]
- Andreasen N. The scale for the assessment of positive symp-toms (SAPS) University of Iowa; Iowa City: 1984. [Google Scholar]
- Baare WF, van Oel CJ, Hulshoff Pol HE, Schnack HG, Durston S, Sitskoorn MM, Kahn RS. Volumes of brain structures in twins discordant for schizophrenia. Archives of General Psychiatry. 2001;58:33–40. doi: 10.1001/archpsyc.58.1.33. [DOI] [PubMed] [Google Scholar]
- Calev A. Recall and recognition in chronic nondemented schizophrenics: use of matched tasks. Journal of Abnormal Psychology. 1984a;93:172–177. doi: 10.1037//0021-843x.93.2.172. [DOI] [PubMed] [Google Scholar]
- Calev A. Recall and Recognition in mildly disturbed schizophrenics: the use of matched tasks. Psychological Medicine. 1984b;14:425–429. doi: 10.1017/s0033291700003676. [DOI] [PubMed] [Google Scholar]
- Cannon TD, Huttunen MO, Lönnqvist J, Tuulio-Henriksson A, Pirkola T, Glahn D, Finkelstein J, Hietanen M, Kaprio J, Koskenvuo M. The inheritance of neuropsychological dysfunction in twins discordant for schizophrenia. American Journal of Human Genetics. 2000;67:369–382. doi: 10.1086/303006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon TD, Thompson PM, van Erp TG, Toga AW, Poutanen VP, Huttunen M, Lönnqvist J, Standerskjöld-Nordenstam CG, Narr KL, Khaledy M, Zoumalan CI, Dail R, Kaprio J. Cortex mapping reveals regionally specific patterns of genetic and disease-specific gray-matter deficits in twins discordant for schizophrenia. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:3228–3233. doi: 10.1073/pnas.052023499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon TD, van Erp TG, Huttunen M, Lönnqvist J, Salonen O, Valanne L, Poutanen VP, Standertskjöld-Nordenstam CG, Gur RE, Yan M. Regional gray matter, white matter, and cerebrospinal fluid distributions in schizophrenic patients, their siblings, and controls. Archives of General Psychiatry. 1998;55:1084–1091. doi: 10.1001/archpsyc.55.12.1084. [DOI] [PubMed] [Google Scholar]
- Chapman LJ, Chapman JP. Problems in the measurement of cognitive deficit. Psychological Bulletin. 1973;79:380–385. doi: 10.1037/h0034541. [DOI] [PubMed] [Google Scholar]
- Chapman LJ, Chapman JP. The measurement of differential deficit. Journal of Psychiatric Research. 1978;14:303–311. doi: 10.1016/0022-3956(78)90034-1. [DOI] [PubMed] [Google Scholar]
- Cirillo MA, Seidman LJ. Verbal declarative memory dysfunction in schizophrenia: from clinical assessment to genetics and brain mechanisms. Neuropsychology Review. 2003;13:43–77. doi: 10.1023/a:1023870821631. [DOI] [PubMed] [Google Scholar]
- Cohen J. A coefficient of agreement for nominal scales. Educational & Psychological Measurement. 1960;20:37–46. [Google Scholar]
- Cohen NJ, Ryan J, Hunt C, Romine L, Wszalek T, Nash C. Hippocampal system and declarative (relational) memory: summarizing the data from functional neuroimaging studies. Hippocampus. 1999;9:83–98. doi: 10.1002/(SICI)1098-1063(1999)9:1<83::AID-HIPO9>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Corcoran C, Walker E, Huot R, Mittal V, Tessner K, Kestler L, Malaspina D. The stress cascade and schizophrenia: etiology and onset. Schizophrenia Bulletin. 2003;29:671–692. doi: 10.1093/oxfordjournals.schbul.a007038. [DOI] [PubMed] [Google Scholar]
- Cosway R, Byrne M, Clafferty R, Hodges A, Grant E, Abukmeil SS, Lawrie SM, Miller P, Johnstone EC. Neuropsychological change in young people at high risk for schizophrenia: results from the first two neuropsychological assessments of the Edinburgh High Risk Study. Psychological Medicine. 2000;30:1111–1121. doi: 10.1017/s0033291799002585. [DOI] [PubMed] [Google Scholar]
- Davidson PS, Troyer AK, Moscovitch M. Frontal lobe contributions to recognition and recall: linking basic research with clinical evaluation and remediation. Journal of the International Neuropsychological Society. 2006;12:210–223. doi: 10.1017/S1355617706060334. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober BA. The California Verbal Learning Test. Psychological Coorperation; San Antonio: 1987. [Google Scholar]
- Eldridge LL, Knowlton BJ, Furmanski CS, Bookheimer SY, Engel SA. Remembering episodes: a selective role for the hippocampus during retrieval. Nature Neuroscience. 2000;3:1149–1152. doi: 10.1038/80671. [DOI] [PubMed] [Google Scholar]
- Faraone SV, Seidman LJ, Kremen WS, Toomey R, Pepple JR, Tsuang MT. Neuropsychologic functioning among the nonpsychotic relatives of schizophrenic patients: The effect of genetic loading. Biological Psychiatry. 2000;48:120–126. doi: 10.1016/s0006-3223(99)00263-2. [DOI] [PubMed] [Google Scholar]
- Geuze E, Vermetten E, Bremner JD. MR-based in vivo hippocampal volumetrics: 2. Findings in neuropsychiatric disorders. Molecular Psychiatry. 2005;10:160–184. doi: 10.1038/sj.mp.4001579. [DOI] [PubMed] [Google Scholar]
- Gilbertson MW, Shenton ME, Ciszewski A, Kasai K, Lasko NB, Orr SP, Pitman RK. Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nature Neuroscience. 2002;5:1242–1247. doi: 10.1038/nn958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimenez M, Junque C, Narberhaus A, Caldu X, Salgado-Pineda P, Bargallo N, Segarra D. Hippocampal gray matter reduction associates with memory deficits in adolescents with history of prematurity. Neuroimage. 2004;23:869–877. doi: 10.1016/j.neuroimage.2004.07.029. [DOI] [PubMed] [Google Scholar]
- Goldberg TE, Ragland JD, Torrey EF, Gold JM. Neuropsychological assessment of monozygotic twins discordant for schizophrenia. Archives of General Psychiatry. 1990;47:1066–1072. doi: 10.1001/archpsyc.1990.01810230082013. [DOI] [PubMed] [Google Scholar]
- Goldberg TE, Torrey EF, Gold JM, Bigelow LB, Ragland RD, Taylor E, Weinberger DR. Genetic risk of neuropsychological impairment in schizophrenia: a study of monozygotic twins discordant and concordant for the disorder. Schizophrenia Research. 1995;17:77–84. doi: 10.1016/0920-9964(95)00032-h. [DOI] [PubMed] [Google Scholar]
- Goldberg TE, Torrey EF, Gold JM, Ragland JD, Bigelow LB, Weinberger DR. Learning and memory in monozygotic twins discordant for schizophrenia. Psychological Medicine. 1993;23:71–85. doi: 10.1017/s0033291700038861. [DOI] [PubMed] [Google Scholar]
- Green DW, Elliott K, Mandel D, Dollberg S, Mimouni FB, Littner Y. Neonatal nucleated red blood cells in discordant twins. American Journal of Perinatology. 2004;21:341–345. doi: 10.1055/s-2004-831883. [DOI] [PubMed] [Google Scholar]
- Gur RE, Turetsky BI, Cowell PE, Finkelman C, Maany V, Grossman RI, Arnold SE, Bilker WB, Gur RC. Temporolimbic volume reductions in schizophrenia. Archives of General Psychiatry. 2000;57:769–775. doi: 10.1001/archpsyc.57.8.769. [DOI] [PubMed] [Google Scholar]
- Heckers S, Rauch SL, Goff D, Savage CR, Schacter DL, Fischman AJ, Alpert NM. Impaired recruitment of the hippocampus during conscious recollection in schizophrenia. Nature Neuroscience. 1998;1:318–323. doi: 10.1038/1137. [DOI] [PubMed] [Google Scholar]
- Hedges LV, Olkin I. Statistical methods for meta-analysis. Academic Press; Orlando: 1985. [Google Scholar]
- Heinrichs RW, Zakzanis KK. Neurocognitive deficit in schizophrenia: a quantitative review of the evidence. Neuropsychology. 1998;12:426–445. doi: 10.1037//0894-4105.12.3.426. [DOI] [PubMed] [Google Scholar]
- Het S, Ramlow G, Wolf OT. A meta-analytic review of the effects of acute cortisol administration on human memory. Psychoneuroendocrinology. 2005;30:771–784. doi: 10.1016/j.psyneuen.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Kahneman D. Control of spurious association and the reliability of the controlled variable. Psychological Bulletin. 1965;64:326–329. doi: 10.1037/h0022529. [DOI] [PubMed] [Google Scholar]
- Kaprio J, Koskenvuo M. Genetic and environmental factors in complex diseases: the older Finnish Twin Cohort. Twin Research. 2002;5:358–365. doi: 10.1375/136905202320906093. [DOI] [PubMed] [Google Scholar]
- Keri S, Kelemen O, Benedek G, Janka Z. Different trait markers for schizophrenia and bipolar disorder: a neurocognitive approach. Psychological Medicine. 2001;31:915–922. doi: 10.1017/s0033291701004068. [DOI] [PubMed] [Google Scholar]
- Kieseppä T, Tuulio-Henriksson A, Haukka J, Van Erp T, Glahn D, Cannon TD, Partonen T, Kaprio J, Lönnqvist J. Memory and verbal learning functions in twins with bipolar-I disorder, and the role of information-processing speed. Psychological Medicine. 2005;35:205–215. doi: 10.1017/s0033291704003125. [DOI] [PubMed] [Google Scholar]
- Kuperberg G, Heckers S. Schizophrenia and cognitive function. Current Opinion in Neurobiology. 2000;10:205–210. doi: 10.1016/s0959-4388(00)00068-4. [DOI] [PubMed] [Google Scholar]
- Levene H. Contributions to Probability and Statistics: Essays in Honor of Harold Hotelling. Stanford University Press; Stanford: 1960. pp. 278–292. [Google Scholar]
- Lyons MJ, Toomey R, Seidman LJ, Kremen WS, Faraone SV, Tsuang MT. Verbal learning and memory in relatives of schizophrenics: preliminary findings. Biological Psychiatry. 1995;37:750–753. doi: 10.1016/0006-3223(94)00362-7. [DOI] [PubMed] [Google Scholar]
- Maitland SB, Herlitz A, Nyberg L, Backman L, Nilsson LG. Selective sex differences in declarative memory. Memory and Cognition. 2004;32:1160–1169. doi: 10.3758/bf03196889. [DOI] [PubMed] [Google Scholar]
- McClain L. Interval estimation: effect of processing demands on prospective and retrospective reports. Perception and Psychophysics. 1983;34:185–189. doi: 10.3758/bf03211347. [DOI] [PubMed] [Google Scholar]
- McNeil TF, Cantor-Graae E, Weinberger DR. Relationship of obstetric complications and differences in size of brain structures in monozygotic twin pairs discordant for schizophrenia. American Journal of Psychiatry. 2000;157:203–212. doi: 10.1176/appi.ajp.157.2.203. [DOI] [PubMed] [Google Scholar]
- Meehl PE. Nuisance variables and the ex post facto design. In: Radner M, Winoker S, editors. Minnesota Studies of the Philosophy of Science. University of Minnesota Press; Minneapolis: 1970. pp. 373–402. [Google Scholar]
- Mohamed S, Paulsen JS, O'Leary D, Arndt S, Andreasen N. Generalized cognitive deficits in schizophrenia: a study of first-episode patients. Archives of General Psychiatry. 1999;56:749–754. doi: 10.1001/archpsyc.56.8.749. [DOI] [PubMed] [Google Scholar]
- Mori H, Mori K, Kojima Y, Ohkuchi A, Funamoto H, Minakami H, Sato I, Nakano T. Neonatal nucleated red blood cell counts in twins. Journal of Perinatal Medicine. 2001;29:144–150. doi: 10.1515/JPM.2001.019. [DOI] [PubMed] [Google Scholar]
- O'Driscoll GA, Florencio PS, Gagnon D, Wolff AV, Benkelfat C, Mikula L, Lal S, Evans AC. Amygdala-hippocampal volume and verbal memory in first-degree relatives of schizophrenic patients. Psychiatry Research. 2001;107:75–85. doi: 10.1016/s0925-4927(01)00095-6. [DOI] [PubMed] [Google Scholar]
- Pelletier M, Achim AM, Montoya A, Lal S, Lepage M. Cognitive and clinical moderators of recognition memory in schizophrenia: a meta-analysis. Schizophrenia Research. 2005;74:233–252. doi: 10.1016/j.schres.2004.08.017. [DOI] [PubMed] [Google Scholar]
- Ragland JD, Gur RC, Valdez J, Turetsky BI, Elliott M, Kohler C, Siegel S, Kanes S, Gur RE. Event-related fMRI of frontotemporal activity during word encoding and recognition in schizophrenia. American Journal of Psychiatry. 2004;161:1004–1015. doi: 10.1176/appi.ajp.161.6.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauhala U. The quantitaive strength of the social strate of Finnish society. Sosiaalinen-Aikakauskirja. 1970;63:347–362. [Google Scholar]
- Saykin AJ, Shtasel DL, Gur RE, Kester DB, Mozley LH, Stafiniak P, Gur RC. Neuropsychological deficits in neuroleptic naive patients with first-episode schizophrenia. Archives of General Psychiatry. 1994;51:124–131. doi: 10.1001/archpsyc.1994.03950020048005. [DOI] [PubMed] [Google Scholar]
- Scoville WB, Milner B. Loss of recent memory after bilateral hippocampal lesions. 1957. Journal of Neuropsychiatry and Clinical Neurosciences. 2000;12:103–113. doi: 10.1176/jnp.12.1.103. [DOI] [PubMed] [Google Scholar]
- Seidman LJ, Faraone SV, Goldstein JM, Kremen WS, Horton NJ, Makris N, Toomey R, Kennedy D, Caviness VS, Tsuang MT. Left hippocampal volume as a vulnerability indicator for schizophrenia: a magnetic resonance imaging morphometric study of nonpsychotic first-degree relatives. Archives of General Psychiatry. 2002;59:839–849. doi: 10.1001/archpsyc.59.9.839. [DOI] [PubMed] [Google Scholar]
- Shapiro SS, Wilk MB. An analysis of variance test for normality (complete samples) Biometrika. 1965;52:591–611. [Google Scholar]
- Sitskoorn MM, Aleman A, Ebisch SJ, Appels MC, Kahn RS. Cognitive deficits in relatives of patients with schizophrenia: a meta-analysis. Schizophrenia Research. 2004;71:285–295. doi: 10.1016/j.schres.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Smith AD. Memory. In: Birren JE, Schaie KW, editors. Handbook of the psychology of aging. Academic; San Diego: 1996. pp. 236–250. [Google Scholar]
- Spitzer RL, Williams JBW. Structured Clinical Interview for DSM-III-R Personality Disorders [SCID-II, 2-1-86] Biometrics Research Department, New York State Psychiatric Institute; New York: 1986. [Google Scholar]
- Spitzer RL, Williams JBW, Gibbon M, First MB. Instruction manual for the structured clinical interview for DSM-III-R SCID. Biometrics Research Department, New York State Psychiatric Institute; New York: 1989. [Google Scholar]
- Sponheim SR, Steele VR, McGuire KA. Verbal memory processes in schizophrenia patients and biological relatives of schizophrenia patients: intact implicit memory, impaired explicit recollection. Schizophrenia Research. 2004;71:339–348. doi: 10.1016/j.schres.2004.04.008. [DOI] [PubMed] [Google Scholar]
- Trandafir A, Meary A, Schurhoff F, Leboyer M, Szoke A. Memory tests in first-degree adult relatives of schizophrenic patients: a meta-analysis. Schizophrenia Research. 2006;81:217–226. doi: 10.1016/j.schres.2005.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Erp TG, Saleh PA, Huttunen M, Lönnqvist J, Kaprio J, Salonen O, Valanne L, Poutanen VP, Standertskjöld-Nordenstam CG, Cannon TD. Hippocampal volumes in schizophrenic twins. Archives of General Psychiatry. 2004;61:346–353. doi: 10.1001/archpsyc.61.4.346. [DOI] [PubMed] [Google Scholar]
- Van Erp TG, Saleh PA, Rosso IM, Huttunen M, Lönnqvist J, Pirkola T, Salonen O, Valanne L, Poutanen VP, Standertskjöld-Nordenstam CG, Cannon TD. Contributions of genetic risk and fetal hypoxia to hippocampal volume in patients with schizophrenia or schizoaffective disorder, their unaffected siblings, and healthy unrelated volunteers. American Journal of Psychiatry. 2002;159:1514–1520. doi: 10.1176/appi.ajp.159.9.1514. [DOI] [PubMed] [Google Scholar]
- Walker EF, Bonsall R, Walder DJ. Plasma hormones and catecholamine metabolites in monozygotic twins discordant for psychosis. Neuropsychiatry, Neuropsychology, and Behavioral Neurology. 2002;15:10–17. [PubMed] [Google Scholar]
- Wechsler D. Wechler adult intelligence scale-revised (WAIS-R) manual. Psychological Corporation; San Antonio, Texas: 1981. [Google Scholar]
- Weiss AP, Schacter DL, Goff DC, Rauch SL, Alpert NM, Fischman AJ, Heckers S. Impaired hippocampal recruitment during normal modulation of memory performance in schizophrenia. Biological Psychiatry. 2003;53:48–55. doi: 10.1016/s0006-3223(02)01541-x. [DOI] [PubMed] [Google Scholar]
- Wheeler MA, Stuss DT, Tulving E. Frontal lobe damage produces episodic memory impairment. Journal of the International Neuropsychological Society. 1995;1:525–536. doi: 10.1017/s1355617700000655. [DOI] [PubMed] [Google Scholar]
- Whyte MC, McIntosh AM, Johnstone EC, Lawrie SM. Declarative memory in unaffected adult relatives of patients with schizophrenia: a systematic review and meta-analysis. Schizophrenia Research. 2005;78:13–26. doi: 10.1016/j.schres.2005.05.018. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP, Kroll NE, Quamme JR, Lazzara MM, Sauve MJ, Widaman KF, Knight RT. Effects of extensive temporal lobe damage or mild hypoxia on recollection and familiarity. Nature Neuroscience. 2002;5:1236–1241. doi: 10.1038/nn961. [DOI] [PubMed] [Google Scholar]


