Abstract
Background
Limited data are available on whether sampling from the penile shaft or urethra increases detection of penile HPV infection in men beyond that found in the glans and coronal sulcus.
Methods
Within a randomized clinical trial, a validation study of penile sampling was conducted in Kisumu, Kenya. Young men (18 to 24 years) were invited to provide penile exfoliated cells using pre-wetted Dacron swabs to determine the best site for HPV detection. β-globin gene PCR and HPV DNA type GP5+/6+ PCR status were ascertained from three anatomical sites.
Results
A total of 98 young HIV-seronegative, uncircumcised men participated. Penile HPV prevalence varied by anatomical site: 50% in penile exfoliated cells from the glans, coronal sulcus and inner foreskin tissue; 43% in the shaft and external foreskin tissue; and 18% in the urethra (p<0.0001). For each anatomical site, over 87% of samples were β-globin positive. Beyond that found in the glans/coronal sulcus, urethral sampling resulted in no increase in HPV positivity and shaft sampling resulted in an additional 7.3% of overall HPV positivity. The prevalence of high-risk HPV positivity varied by anatomical site: 39% in glans/coronal sulcus, 31% in shaft, and 13% in the urethra (p<0.0001). HPV 16 was the most common type identified.
Discussion
Penile HPV prevalence was approximately 50% among young men in Kisumu, Kenya. Urethral sampling for HPV detection in men added no sensitivity for HPV detection over that found from sampling the glans/coronal sulcus and penile shaft. These data will help inform studies on HPV transmission dynamics, and on the efficacy of HPV prophylactic vaccines on penile HPV carriage in men.
Summary
A validation study of penile sampling was conducted among young, uncircumcised men in Kenya and found that overall penile HPV DNA prevalence was approximately 50%. Beyond that found in the glans/coronal sulcus, urethral sampling resulted in no increase in HPV positivity and shaft sampling resulted in an additional 7.3% of overall HPV positivity.
Keywords: Human papillomavirus, men, validation, Kenya
Introduction
Years before human papillomavirus (HPV) infection was identified as the cause of invasive cervical cancer (ICC) in women (1), men were considered important in the etiology of ICC (2-3). Penile cancer rates have been correlated with ICC rates in women on the population-level (2) and a 4-fold increased risk of cervical cancer was found among women whose husbands had been previously married to a woman with cervical cancer (3). Given that men may transmit HPV infection to their female sexual partners, data on the prevalence of penile HPV infections in men and on transmission of HPV between sexual partners are needed to inform future cervical cancer prevention strategies, including the use of prophylactic HPV vaccines.
In determining the prevalence of genital HPV infections using highly-sensitive PCR-based laboratory detection, relatively few studies have been conducted in men as compared to women. The lack of studies on penile HPV infection has been due in part to the difficulty in identifying the most reliable sampling method and site to collect genital specimens in men. Recent PCR-based studies have increased our understanding of the burden of HPV infections in men by allowing identification of asymptomatic infections. We are no longer limited to the detection of clinical HPV-associated penile lesions using visual penoscopic inspection, or relatively insensitive detection methods (e.g. in-situ hybridization) (4-6). Sampling of penile exfoliated cells for HPV detection in most studies of men has been from the glans/coronal sulcus and sometimes urethra, generally without sampling from other penile anatomical sites (7;8). Limited data are thus available on whether sampling from penile sites other than the glans and coronal sulcus, such as the penile shaft, might increase detection of penile HPV infection in men (8;9) and whether this would impact HPV prevalence estimates for circumcised and uncircumcised men.
In the framework of a randomized clinical trial (RCT), a validation study of penile sampling was conducted in Kisumu, Kenya. Young, uncircumcised, HIV-seronegative men were asked to provide penile exfoliated cell samples to determine the best anatomical site for penile HPV detection.
Methods
Study population and enrollment
Uncircumcised men aged 18 to 24 years of age in Kisumu, Kenya were invited between February 4, 2002 and September 6, 2005 to participate in a randomized clinical trial (RCT) of male circumcision, coordinated by the Universities of Illinois at Chicago, Nairobi and Manitoba and RTI International. The primary aim of this RCT is to determine the effectiveness of male circumcision in reducing HIV incidence. Inclusion criteria included being uncircumcised, HIV seronegative, sexually active (defined as reporting sex within the last 12 months), and having hemoglobin ≥ 9.0 grams/100 ml. Men were excluded if their foreskin did not cover one-half or more of their glans, if they were not a resident in Kisumu or surroundings, were unlikely to remain in the study region for the 2-year follow-up period, or were unwilling to conform to the study follow-up protocol. Other exclusion criteria included hemophilia, other bleeding disorders, medical conditions for which a surgical procedure was contra-indicated, hypospadius, or an absolute indication for circumcision (e.g. urinary retention).
Male study participants were recruited from sexually transmitted infection (STI) clinics, workplaces, and community organizations. Most recruits were young men with high to moderate sexual risk-behavior. This HPV study is based on early participants to the randomized clinical trial who consented to HPV sample collection and shipment oversees for storage and tests. Inclusion required that baseline samples be available for shipment to the HPV DNA laboratory by mid-March, 2002. Specifically, between February 4 and March 7, 2002, 188 men completed an initial screening to participate in the RCT. Of these, 126 men eventually met inclusion criteria for the trial (HIV-seronegative, uncircumcised, sexually active, aged 18-24 years, having a hemoglobin of 9.0 grams per ≥ 100 ml, and a Kisumu resident) and were randomized. 115 of the 126 men had an enrollment visit by mid-March 2002 when HPV samples were shipped. Among the 115 men, 99 consented to HPV sample collection and shipment overseas for storage and HPV testing. One of these 99 individuals was subsequently excluded from the HPV study due to improper specimen collection.
The study protocol was reviewed and approved by the Institutional Review Boards of the Universities of Illinois at Chicago, Manitoba, Nairobi and North Carolina; RTI International and the VU University Medical Center.
Questionnaire, clinical examination and specimen collection
After undergoing informed consent, study participants were administered a standardized questionnaire on sociodemographic characteristics, and sexual behavior, by a trained male interviewer.
Participants also underwent a clinical examination by a trained physician or clinical officer. Penile exfoliated cells were collected for HPV DNA detection from three anatomical sites using pre-wetted Dacron swabs in separate conical tubes: shaft and external foreskin tissue (shaft specimen), the glans, coronal sulcus and inner foreskin tissue (glans specimen); and the urethra. Shaft and external foreskin cells were collected from each of the four sides of the external shaft tissue, from the proximal to distal penile shaft, using a type 3 Dacron swab pre-wetted in Tris buffer. From the glans and coronal sulcus, a second pre-wetted Type 3 Dacron swab was used as follows: the prepuce was gently retracted to collect exfoliated cells by swabbing the tip of the urethral opening, completely circling the urethral orifice 2-3 times; sampling the top to the bottom of the glans in a circular motion around the complete circumference of the penis; rotating the swab three times completely around the circumference of the coronal sulcus; and sampling from the inner foreskin tissue. A sample of the outer, external surface of the foreskin was also included in the glans sample as funding was not sufficient to test this specimen separately. A third specimen was collected from the distal urethra using a pre-wetted Type 1 Dacron swab, which was inserted approximately 2 cm into the urethra.
All three penile cell samples were placed in individual 15 ml centrifuge tubes containing 2 ml 10mM Tris-HCl, 0.01M 7.4 pH, buffer, and processed on the day of collection at the UNIM (Universities of Nairobi, Illinois and Manitoba) clinic laboratory by centrifugation at high speed (maximum, 3000 g) for 10 minutes. Excess Tris-HCl buffer was discarded using a Pasteur pipette, and the remaining cell pellet was resuspended in the same volume of 0.1 mM Tris-HCl buffer, and vortexed. Diluted cell pellets were then frozen at -75°C. All samples were sent using a dry shipper to the Department of Pathology, VU University Medical Center, Amsterdam, the Netherlands for HPV DNA laboratory testing.
HPV DNA testing
Crude extracts of penile exfoliated cell pellets obtained by consecutive freeze-thaw and boiling steps were evaluated for DNA quality by beta (β)-globin specific PCR followed by agarose gel electrophoresis according to protocols that have been detailed elsewhere (10-12). Of samples that were β-globin PCR negative on the crude extracts, the β-globin PCR was repeated following isolation of nucleic acids using magnetic beads and an EasyMag (Biomerieux) extractor. The samples that were β-globin PCR negative on isolated DNA as well were considered β-globin negative in this study. Of those that were β-globin PCR positive on the isolated DNA only, the DNA rather than the crude extract was used for subsequent HPV PCR analysis.
HPV positivity was assessed by GP5+/6+ PCR and an enzyme immunoassay (EIA) read-out system coupled thereto that, using HPV oligoprobe cocktails, detects the following 37 HPV types: HPV 6, 11, 16, 18, 26, 31, 33, 34, 35, 39, 40, 42, 43, 44, 45, 51, 52, 53, 54, 55, 56, 57, 58, 59, 66, 68, 61, 67, 70, 71 (equivalent to CP8061), 72, 73, 81 (equivalent to CP8304), 82 (IS39 and MM4 subtypes), 83 (equivalent to MM7), 84 (equivalent to MM8) and CP 6108 (10). Cycling and staining conditions were as described before (11)(12)
Subsequently, GP5+/6+-PCR positive samples were subjected to HPV typing by reverse line blot hybridization (11;12). HPV types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, 68, and 73 were considered high-risk (HR) types. The group of low-risk (LR) types included all other HPV types tested. HPV infections with more than one HPV type were considered as high-risk if any HPV type detected was high-risk.
Statistical methods
Associations between categorical variables were assessed nonparametrically using Mantel-Haenszel (MH) methods to account for multiple measures from each study participant for the three anatomical sites (13). For example, a MH test for general association with stratification by participant was used to assess differences in HPV DNA positivity by anatomical sampling site. All MH tests were stratified by participant unless noted otherwise.
To determine possible factors associated with site-specific HPV infection, odds ratios (ORs) for HPV DNA detection and corresponding 95% confidence intervals (CIs) were estimated using logistic regression for each combination of site and possible risk factor. Logistic regression models were also employed to assess association between possible risk factors and overall HPV status wherein participants were categorized as positive if HPV infection was detected at one or more sites. All models included age as a covariate. No multiple comparison adjustments were made. Analyses were conducted using SAS (13)and R (14).
Results
β-globin and HPV prevalence, by anatomical collection site
A total of 98 uncircumcised men participated (median reported age=22, range=17-25). HPV prevalence varied by anatomical site (Table 1): 50% in penile exfoliated cells from the glans, 43% from the shaft, and 18% from the urethra (p<0.0001, MH test). β-globin positivity ranged from 88% in the shaft to 100% in the urethra (p=0.002, MH test). For β-globin positive samples, HPV DNA positivity was significantly lower in urethral than in the glans and in the shaft (p<0.0001, MH test). Analyses excluding urethral samples indicated no significant difference between glans and shaft sites in HPV prevalence (p=0.07, MH test), β-globin positivity (p=0.13, MH test), or HPV prevalence conditional on β-globin positivity (p=0.08, MH test). For β-globin negative samples, the observed prevalence was 33% for both the glans and shaft. In absolute terms, HPV DNA positivity was higher in β-globin positive samples compared to β-globin negative samples for both the glans and shaft (p=0.27, MH test stratified by sampling site). All subsequent analyses were thus limited to β-globin positive samples.
Table 1.
Positivity to human papillomavirus (HPV) according to β-globin status and sample collection site among young men in Kisumu, Kenya
| Site | N | HPV % | Beta-globin positive % | HPV % in Beta-globin positive | HPV % in Beta-globin negative |
|---|---|---|---|---|---|
| Glans/coronal sulcus | 98 | 50.0 (49/98) | 93.9 (92/98) | 51.1 (47/92) | 33.3 (2/6) |
| Shaft | 98 | 42.9 (42/98) | 87.8 (86/98) | 44.2 (38/86) | 33.3 (4/12) |
| Urethra | 98 | 18.4 (18/98) | 100 (98/98) | 18.4 (18/98) | - |
Samples from all three sites were β-globin positive for 81 participants. Of these men, 44 were HPV positive in at least one site (Table 2), representing a 54% HPV positivity overall. For men who were HPV positive in at least one site, N=41 were HPV positive in the glans (93% of overall HPV positives), N=35 were HPV positive in the shaft (80% of overall HPV positives) and N=17 were HPV positive in the urethra (39% of overall HPV positives). No samples were found to be HPV positive in the urethra that were not positive in either the glans or shaft. Shaft sampling resulted in an additional 7.3% of overall HPV positivity beyond that found in the glans/coronal sulcus.
Table 2.
Penile human papillomavirus (HPV) by anatomical site for young men in Kisumu, Kenya with β-globin positive samples from all three sites
| Shaft | Glans | Urethra | N | % |
|---|---|---|---|---|
| - | - | - | 37 | 45.7 |
| + | + | + | 16 | 19.8 |
| + | + | - | 16 | 19.8 |
| - | + | - | 8 | 9.9 |
| + | - | - | 3 | 3.7 |
| - | + | + | 1 | 1.2 |
HPV type-specific distribution
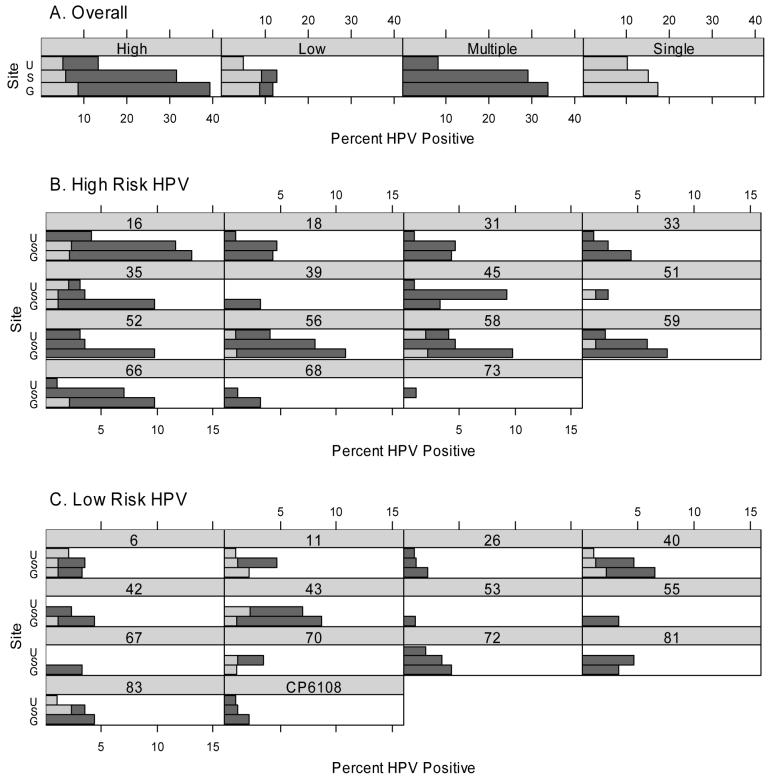
A total of 29 different HPV types (15 high-risk and 14 low-risk) were detected in either single or multiple infections (Figure 1). Most HPV-positive men had at least one high-risk HPV type detected (ranging from 71% in the shaft to 77% in the glans). Overall, the prevalence of high-risk infections varied by anatomical site: 39% in the glans, 31% in the shaft, and 13% in the urethra (p<0.0001, MH test). The prevalence of low-risk infections also varied by site: 12% in the glans, 13% in the shaft, and 5% in the urethra (p=0.02, MH test). Similarly, the prevalence of multiple HPV infections varied by site: 34% in the glans, 29% in the shaft, and 8% in the urethra (p<0.0001, MH test). Of note, over half of the 29 HPV types detected (52%) were found only within multiple infections, including high-risk HPV types 18, 31, 33, 39, 45, 52, 68, 73, and low-risk HPV types 26, 53, 55, 67, 72, 81, and CP6108. High-risk HPV type 16 was the most common type detected in all three sites: 13% of glans samples, 12% of shaft samples and 4% of urethra samples (types 56 and 58 also occurred in 4% of the urethra samples).
Figure 1.
Horizontal barplots of percent HPV positive among β-globin positive samples by anatomical site (G=glans, S=Shaft, U=Urethra) for different HPV types. For example, the upper left panel gives the percent HPV positive to at least one high-risk HPV type, with 13% of urethra samples positive, 32% of shaft samples positive, and 39% of glans samples positive. Dark shading indicates infection with multiple HPV types; light shading denotes infection with a single HPV type.
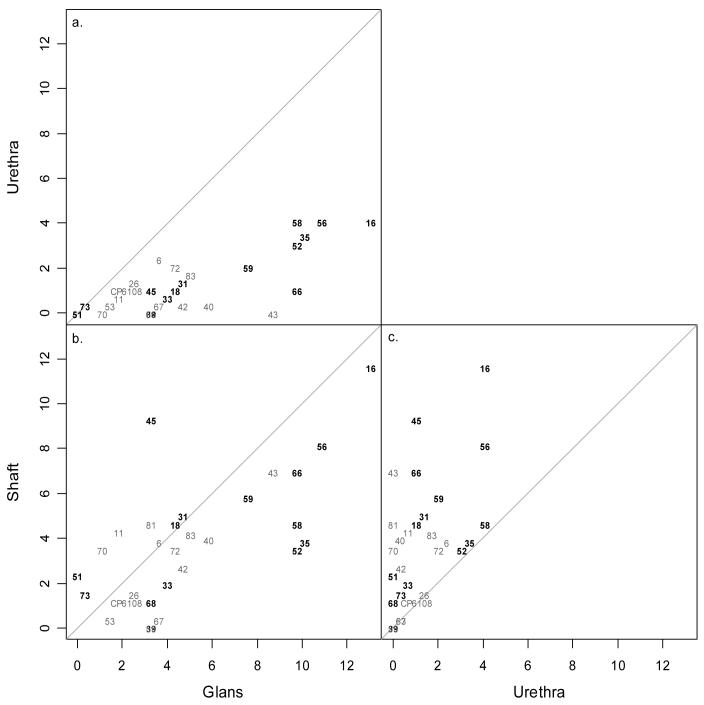
Figure 2 compares the observed HPV type-specific prevalence between each of the two pairs of the three sites. Overall, HPV positivity in the glans is close to the line of agreement with that detected from the shaft (Figure 2b). HPV 45 was more prevalent in the shaft than the glans whereas types 35, 58 and 52 were more likely detected in the glans than shaft. Two multiple (HPV 31/45, HPV 40/51/58/70) and one single (HPV 83) HPV infection were found in three shaft specimens that were HPV negative in the corresponding glans specimen. No additional HPV types were detected from the urethra that were not already detected in one or more of the remaining sites sampled as shown in Figures 2a and 2c.
Figure 2.
Scatterplots of percent HPV positive among β-globin positive samples across anatomical sites by HPV type. The gray diagonal line indicates perfect agreement in percent HPV positive between the two corresponding sites. High-risk HPV types are in bold.
Sample pooling
To determine whether the samples from the shaft and glans could be pooled (combined) for PCR purposes without losing information about β-globin or HPV status, we examined 41 combined samples (data not shown). Among 23 samples that were HPV positive on both the shaft and glans before pooling, all were HPV positive in the pooled sample. However, the pooled samples had consistently fewer detectable HPV types when compared to the results of adding the-globin positive after pooling. Among the 9 samples with discrepant HPV results before pooling, all were HPV DNA positive after pooling. Thus, PCR testing could be performed on pooled samples of the shaft and glans to obtain a reliable measurement of overall HPV positivity, but not to obtain reliable results on HPV type distribution.
Risk factors for penile HPV infection
The absolute prevalence of penile HPV DNA was higher in men of age in all three anatomical collection sites, although a significant association was only found in the glans (OR=2.7; 95% CI: 1.1-6.8). Trends towards increased overall prevalence of penile HPV positivity overall were noted with increasing number of reported lifetime sexual partners (OR=1.8, 95% CI: 0.5-5.8 for 3-5 partners, OR=2, 95% CI: 0.5-7.5 for 6 or more partners) and total number of sexual partners in the last 12-months (OR=2.2, 95% CI: 0.5-10 for 1 partner, OR=5.3, 95% CI: 1.2-24 for 2 or more partners). A trend towards lower overall HPV prevalence was observed with a history of condom use in the last 6-months with significant protection noted for consistent condom use (OR=0.74, 95% CI: 0.19-2.9 for use ≤ 50% of the time; OR=0.26, 95% CI: 0.08-0.9 for use >50% of the time). A non-significant trend of lower overall HPV prevalence was also noted with condom use with the last sexual partner (OR=0.6, 95% CI: 0.25-1.5). HPV positivity also tended to be more frequent among participants reporting lower educational attainment (OR= 0.11, 95% CI: 0.02-0.6 for secondary versus primary; OR=0.14, 95% CI: 0.02-1.1 for tertiary versus primary). Young men who reported to be salaried had a higher absolute HPV positivity in all three sites than those who were unemployed, although associations were only significant in shaft specimens (OR=4.4; 95% CI: 1.2-15). Penile HPV DNA detection did not appear to be associated with age at first intercourse or marital status (data not shown) (Table 3).
Table 3.
Observed prevalence and estimated odds ratios (ORs) of penile human papillomavirus (HPV) infection in young men in Kisumu, Kenya by selected characteristics
| Glans/Coronal Sulcus* | Shaft* | Urethra* | Overall*** | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HPV% | OR(CI) | HPV% | OR(CI) | HPV% | OR(CI) | HPV% | OR(CI) | ||
| Age (years) | >=21 | 59 (37/63) | 2.7 (1.1, 6.8) | 47 (28/60) | 1.4 (.55, 3.6) | 22 (15/67) | 2.7 (.72, 10) | 59 (33/56) | 1.8 (0.7, 4.7) |
| < 21 | 34 (10/29) | 1 | 38 (10/26) | 1 | 10 (3/31) | 1 | 44 (11/25) | 1 | |
| Educational Attainment | Tertiary | 40 (4/10) | .14 (.02, .84) | 25 (2/8) | .14 (.02, .96) | 20 (2/10) | 0.6 (.09, 3.9) | 50 (4/8) | .14 (.02, 1.1) |
| Secondary | 44 (28/64) | .15 (.04, .58) | 39 (24/61) | .27 (.08, .86) | 14 (10/69) | .38 (.11, 1.2) | 46 (26/57) | .11 (.02, .55) | |
| Primary | 83 (15/18) | 1 | 71 (12/17) | 1 | 32 (6/19) | 1 | 88 (14/16) | 1 | |
| Total # lifetime Sexual partners | 6 + | 59 (19/32) | 1.8 (0.5, 6.2) | 54 (14/26) | 1.7 (.47, 5.9) | 25 (8/32) | 1.7 (.35, 8.4) | 62 (16/26) | 2 (.52, 7.5) |
| 3-5 | 54 (20/37) | 1.6 (.51, 5.2) | 41 (15/37) | 1 (.33, 3.1) | 18 (7/40) | 1.2 (.26, 5.7) | 57 (20/35) | 1.8 (.54, 5.8) | |
| 0-2 | 35 (8/23) | 1 | 39 (9/23) | 1 | 12 (3/26) | 1 | 40 (8/20) | 1 | |
| Total # sexual partners in last 12 Months | 2 + | 62 (26/42) | 5.1 (1.2, 22) | 55 (22/40) | 3.5 (.81, 15) | 26 (12/46) | 4 (.46, 35) | 68 (25/37) | 5.3 (1.2, 24) |
| 1 | 49 (18/37) | 2.8 (.63, 12) | 38 (13/34) | 1.7 (.39, 7.7) | 13 (5/38) | 1.6 (.17, 16) | 48 (16/33) | 2.2 (.49, 10) | |
| 0 | 23 (3/13) | 1 | 25 (3/12) | 1 | 7 (1/14) | 1 | 27 (3/11) | 1 | |
| Condom use in last 6-months** | > 50% of time | 42 (15/36) | .39 (.13, 1.2) | 35 (12/34) | .49 (.16, 1.5) | 13 (5/38) | .64 (.16, 2.5) | 42 (14/33) | .26 (.08, 0.9) |
| ≤ 50% of time | 63 (15/24) | .81 (.24, 2.7) | 61 (14/23) | 1.4 (.43, 4.8) | 32 (8/25) | 1.8 (.48, 6.5) | 68 (15/22) | .74 (.19, 2.9) | |
| Never | 65 (15/23) | 1 | 52 (11/21) | 1 | 20 (5/25) | 1 | 74 (14/19) | 1 | |
| Condom use last partner | Yes | 45 (20/44) | 0.7 (0.3, 1.6) | 38 (15/40) | .61 (.26, 1.5) | 18 (8/45) | 1 (.36, 2.9) | 48 (19/40) | 0.6 (.25, 1.5) |
| No | 56 (27/48) | 1 | 50 (23/46) | 1 | 19 (10/53) | 1 | 61 (25/41) | 1 | |
| Employment | Salaried | 59 (10/17) | 1.9 (.58, 5.9) | 67 (10/15) | 4.4 (1.2, 15) | 28 (5/18) | 3.1 (.76, 13) | 71 (10/14) | 3.3 (.87, 12) |
| Self-employed | 60 (18/30) | 1.8 (.71, 4.8) | 52 (15/29) | 2.3 (.86, 6.3) | 25 (8/32) | 2.6 (.74, 8.8) | 63 (17/27) | 2.2 (.78, 5.9) | |
| Not employed | 42 (19/45) | 1 | 31 (13/42) | 1 | 10 (5/48) | 1 | 43 (17/40) | 1 | |
All estimates of ORs and 95% CIs based on logistic regression model adjusting for age. Separate models were fit for each combination of selected characteristic (age, educational attainment, etc) and site (glans, shaft, urethra, overall).
Site-specific models were fit to data from samples that were β-globin positive at the respective site.
Condom use for men having heterosexual sex in the last 6 months.
Participants were categorized as overall HPV positive if HPV infection was detected at one or more sites. The overall analysis only utilized data from 81 participants having β-globin positive samples at all three sites.
Discussion
This is a unique study which reports data on HPV infection among healthy uncircumcised men from Africa, and includes a comparison of individual, type-specific HPV DNA positivity at different anatomical sites. Penile HPV prevalence has varied notably across studies of young men using sensitive PCR-based methods, ranging from 1 to 73% (6;15-20). In comparison, the overall HPV positivity in the current study was relatively high (54%). The current study’s results are consistent with that found among sexually active males (20-29 years) from Cuernavaca, Mexico (50%) (16) and soldiers aged 21-24 in Mexico City (48.6%)(17), and higher than that found in similarly-aged university students in Seattle(35%)(9) and Korea (8.7%)(20), or in military conscripts from Finland (9.1%)(18) or Sweden (12%)(19). Reliable comparisons of the penile HPV prevalence across studies, however, are hampered by differences in populations surveyed, in anatomical sites sampled, in HPV DNA detection assays, and possibly in circumcision status.
In this study, β-globin positivity was high in specimens obtained using pre-wetted swabs from the glans/coronal sulcus (94%), shaft (87%) and urethra (100%). Although sampling of genital sites for HPV using emery paper has been successfully used by other US groups (9;21), the use of emory paper in this Kenyan population was not considered culturally acceptable. A strength of our study is that we were able to obtain a high β-globin positivity rate using a pre-wetted swabs alone. β-globin positivity based on sampling with a pre-wetted Dacron swab, has ranged from 74 to 100% in previous studies in men (6;7;22).
A low HPV DNA yield was obtained from urethral sampling. These results are consistent with a previous study of 820 Mexican soldiers, which concluded that urethral sampling did not markedly increase overall HPV positivity beyond that found in the glans/coronal sulcus (8). Taken together, these data consistently suggest that sampling from the urethra in men results in adequate cell yield, but a low sensitivity for HPV detection. Since no additional HPV infections are detected by urethral sampling, and since asymptomatic men tend not to agree to urethral sampling due to discomfort, urethral sampling should not be considered necessary for optimal penile HPV detection protocols.
Similar HPV DNA detection rates were found in penile exfoliated cells collected from the glans than in the penile shaft among young Kenyan men. The addition of the shaft specimen did not statistically increase overall HPV DNA detection rates beyond that found in the glans and coronal sulcus alone. However, the inclusion of the shaft specimen permitted the detection of three additional HPV positive samples (equating to 6.8% of HPV positive cases). These results are consistent with a previous study of 50 uncircumcised male university students in Seattle that found that 5.6% of HPV infections found in either the glans, foreskin or shaft would have been missed if shaft sampling had not been done(9). By comparing HPV type-specific detection rates by anatomical sites, data from this present study suggest an improved detection of carcinogenic HPV type 45 by the inclusion of shaft sampling in men, when compared to routine sampling of solely the glans and coronal sulcus.
High-risk HPV type 16 was the most common type detected in the current study, which is consistent with other studies in men from Sweden (23) and from a pooled analysis of men from Brazil, Colombia, Thailand, Philippines and Spain (7). In contrast, HPV type 59 was the most commonly detected type among men in two studies from Mexico (8;17). Among HPV positive men, data from the current study suggest that the prevalence of high and of low-risk HPV types in the shaft was similar to that found in the glans/coronal sulcus. These results are generally consistent with Mexican data suggesting that HPV types found in HPV DNA positive men did not notably differ by site of cell collection (glans/coronal sulcus, shaft, urethra)(17).
Results from our pilot study pooling samples from the shaft and glans/sulcus found that PCR testing can be performed on pooled samples in order to obtain a reliable measurement of overall HPV positivity, but not to obtain reliable results on HPV type-specific distribution. Given the high prevalence of penile HPV (∼50%) in this validation study, the pooling of samples was not considered efficient for penile HPV ascertainment in this cohort.
Younger age (≤21), lower educational status, and being salaried were consistently associated with a higher prevalence of penile HPV positivity in the three sampling sites, although our sample size was too small to detect meaningful differences. Our analyses also suggested a lower prevalence of HPV infection among male participants who reported consistent condom use, and fewer sexual partners, consistent with previous studies that have shown that markers of sexual behavior, such as lifetime and recent sexual partners, are associated with HPV infection prevalence(6;24). Penile HPV risk factors in this study appeared similar for samples collected from the glans/coronal sulcus and the shaft.
The current study’s advantages include the inclusion of uncircumcised men from a unique Kenyan population at a high-risk of HPV infection and central, type-specific DNA testing with an experienced HPV DNA laboratory using a highly sensitive, GP5+/6+ detection assay. Study limitations include not collecting male samples from either the scrotum or urine, although previous research suggests that sampling from either one of these sites is unlikely to result in higher HPV DNA positivity beyond that found in the penile shaft, glans coronal sulcus and inner foreskin tissue(9). Our data are also limited to uncircumcised men. Sampling from the shaft may notably increase HPV detection rates beyond the glans in circumcised men more than in uncircumcised men (9). Further comparisons of β-globin and HPV positivity among both circumcised and uncircumcised men, stratified by glans or shaft sampling, are planned in future study follow-up.
With recent improvements in the validity and range of sampling methods for HPV ascertainment in men, it will be possible to evaluate the potential efficacy of HPV prophylactic vaccines on penile HPV carriage in men and on HPV transmission dynamics between sexual partners using highly sensitive detection assays.
Acknowledgements
This study was supported by an RO1 grant from the National Cancer Institute, NIH, CA114773-01, by Grant No AI50440 from the Division of AIDS, National Institute of Allergies and Infectious Disease, NIH and by Grant No HCT 44180 from the Canadian Institutes of Health Research (CIHR). S. Moses is the recipient of an Investigator Award from the Canadian Institutes of Health Research. J. Smith was supported through the Centers for AIDS Research, National Institute of Allergy and Infectious Diseases (grant 5 P30 AI050410-07).
References
- 1.Skegg DCG, Corwin PA, Paul C. Importance of the Male Factor in Cancer of the Cervix. The Lancet. 1982;u:581–583. doi: 10.1016/s0140-6736(82)90661-4. [DOI] [PubMed] [Google Scholar]
- 2.Li JY, Li FP, Blot WJ, et al. Correlation between cancers of the uterine cervix and penis in China. J Natl Cancer Inst. 2004;69:1063–1065. [PubMed] [Google Scholar]
- 3.Kessler II. Human cervical cancer as a venereal disease. Cancer Res. 1976;36:783–791. [PubMed] [Google Scholar]
- 4.Brinton LA, Reeves WC, Brenes MM, et al. The male factor in the etiology of cervical cancer among sexually monogamous women. Int J Cancer. 1989;44:199–203. doi: 10.1002/ijc.2910440202. [DOI] [PubMed] [Google Scholar]
- 5.Strand A, Rylander E, Wilander E, et al. HPV infection in male partners of women with squamous intraepithelial neoplasia and/or high-risk HPV. Acta Derm Venereol. 1995;75:312–316. doi: 10.2340/0001555575312316. [DOI] [PubMed] [Google Scholar]
- 6.Partidge J, Koutsky L. Genital human papillomavirus infection in men. Lancet Infect Dis. 2006;6:21–31. doi: 10.1016/S1473-3099(05)70323-6. [DOI] [PubMed] [Google Scholar]
- 7.Franceschi S, Castellsague X, Dal Maso L, et al. Prevalence and determinants of human papillomavirus genital infection in men. Br J Cancer. 2002;86:705–711. doi: 10.1038/sj.bjc.6600194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aguilar L, Lazcano-Ponce E, Vaccarella S, et al. Human papillomavirus in men: comparison of different genital sites. Sex Transm Infect. 2006;82:31–33. doi: 10.1136/sti.2005.015131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weaver BA, Feng Q, Holmes KK, et al. Evaluation of genital sites and sampling techniques for detection of human papillomavirus DNA in men. J Infect Dis. 2004;189:677–685. doi: 10.1086/381395. [DOI] [PubMed] [Google Scholar]
- 10.Jacobs MV, Walboomers JM, Snijders PJ, et al. Distribution of 37 mucosotropic HPV types in women with cytologically normal cervical smears: the age-related patterns for high-risk and low-risk types. Int J Cancer. 2000;87:221–227. [PubMed] [Google Scholar]
- 11.van den Brule AJ, Pol R, Fransen-Daalmeijer N, et al. GP5+/6+ PCR followed by reverse line blot analysis enables rapid and high-throughput identification of human papillomavirus genotypes. J Clin Microbiol. 2002;40:779–787. doi: 10.1128/JCM.40.3.779-787.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Snijders PJ, van der Brule A, Jacobs MV, et al. HPV DNA detection and typing in cervical scrapes by general primer GP5+/6+ PCR. In: Davy CEDJE, editor. Method in Molecular Medicine; Human papillomaviruses-methods and protocols. Humana Press; Totowa, US: 2005. pp. 101–114. [DOI] [PubMed] [Google Scholar]
- 13.SAS/STAT™ software . Version Version 9.1 of the SAS System. SAS Institute, Inc.; Cary, NC: 2006. computer program. [Google Scholar]
- 14.R Development Core Team R A language and environment for statistical computing. 2005 Vienna, Austria ISBN 3-900051-07-0, URL http://www.R-project.org.
- 15.Dunne E, Nielson C, Stone KM, et al. Prevalence of HPV Infection among Men: A systematic review of the literature. J Infect Dis. 2006;194:1044–1057. doi: 10.1086/507432. [DOI] [PubMed] [Google Scholar]
- 16.Lazcano-Ponce E, Herrero R, Munoz N, et al. High prevalence of human papillomavirus infection in Mexican males: comparative study of penile-urethral swabs and urine samples. Sex Transm Dis. 2001;28:277–280. doi: 10.1097/00007435-200105000-00007. [DOI] [PubMed] [Google Scholar]
- 17.Lajous M, Mueller N, Cruz-Valdez A, et al. Determinants of Prevalence, Acquisition, and Persistence of Human Papillomavirus in Healthy Mexican Military Men. Cancer, Epidemiology, Biomarkers and Prevention. 2006;14:1710–1715. doi: 10.1158/1055-9965.EPI-04-0926. [DOI] [PubMed] [Google Scholar]
- 18.Hippelainen M, Syrjanen S, Hippelainen M, et al. Prevalence and risk factors of genital human papillomavirus (HPV) infections in healthy males: a study on Finnish conscripts. Sex Transm Dis. 1993;20:321–328. [PubMed] [Google Scholar]
- 19.Forslund O, Hansson BG, Rymark P, et al. Human papillomavirus DNA in urine samples compared with that in simultaneously collected urethra and cervix samples. J Clin Microbiol. 1993;31:1975. doi: 10.1128/jcm.31.8.1975-1979.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shin HR, Franceschi S, Vaccarella S, et al. Prevalence and determinants of genital infection with papillomavirus, in female and male university students in Busan, South Korea. J Infect Dis. 2004;190:468–476. doi: 10.1086/421279. [DOI] [PubMed] [Google Scholar]
- 21.Hernandez B, McDuffie K, Goodman M, et al. Comparison of Physician-and Self-Collected Genital Specimens for Detection of Human Papillomavirus in Men. Sex Transm Dis. 2006;44:513–517. doi: 10.1128/JCM.44.2.513-517.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Svare EI, Kjaer SK, Worm AM, et al. Risk factors for genital HPV DNA in men resemble those found in women: a study of male attendees at a Danish STD clinic. Sex Transm Infect. 2002;78:215. doi: 10.1136/sti.78.3.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wikstrom A, Popescu C, Forslund O. Asymptomatic penile HPV infection: a prospective study. Int J STD AIDS. 2000;11:80–84. doi: 10.1177/095646240001100203. [DOI] [PubMed] [Google Scholar]
- 24.Svare EI, Kjaer SK, Worm AM, et al. Risk factors for genital HPV DNA in men resemble those found in women: a study of male attendees at a Danish STD clinic. Sexually Transmitted Infections. 2004;78(3):215–218. doi: 10.1136/sti.78.3.215. [DOI] [PMC free article] [PubMed] [Google Scholar]