Abstract
Herpes simplex virus type-1 (HSV-1) is a neurotropic virus with significant potential as a viral vector for CNS gene therapy. This study provides visual evidence that recombinant green fluorescent protein (GFP) expressing HSV-1 travel down dendrites in differentiated P19 neuronal-like cells to efficiently reach the soma. The virus also promotes cytoskeletal rearrangements which facilitate viral spread in vitro, including often dramatic increases in dendritic filopodia. Viral movements, cell infection and filopodia induction were each reduced with the actin polymerization inhibitor cytochalasin D, suggesting the involvement of the actin cortex in these processes. The observation of neural cytoskeletal reorganization in response to HSV-1 may shed light on the mechanisms by which acute viral infection associated with herpes encephalitis produces cognitive deficits in patients.
Keywords: herpes, virus, dendritic filopodia, retrograde F-actin, surfing
INTRODUCTION
Recent reports have indicated some retroviruses have the ability to exploit long actin-rich structures like filopodia and their associated retrograde F-actin flow to travel or “surf” towards a target cell [19] and [28]. For a neurotropic virus like HSV-1, it appeared likely that viral particles might utilize the extensive network of long, actin rich processes found in neural cells [20]. Neural cells were represented by the P19 embryonal carcinoma line differentiated via treatment with retinoic acid as previously described [15]. These cells divide into three distinct populations: neurons, astroglia and microglia [22]. The neuronal cells have characteristic morphologies, test positive for neuron-specific markers like neurofilaments, and form functional synapses and normal central nervous system (CNS) neuronal polarity in vitro [14]. The glial cells similarly stain for glial fibrillar acidic protein, a major component of intermediate filaments in glial astrocytes [15]. Neuronal and glial populations can be distinguished from one another easily by cell morphology, providing a convenient in vitro model containing both cell types.
Through live cell imaging and confocal microscopy, we confirmed that HSV-1 does surf along the outer membrane of dendrites through retrograde F-actin flow to efficiently reach the soma. We also observed that administration of the virus caused extensive dendritic and glial filopodia formation and motility. Viral particles were able to bind to and surf along newly formed filopodia, thereby increasing the efficiency of viral spread.
Normal central nervous system function is critically dependent on both the number of synapses between neurons and their relative synaptic strengths. Given that dendritic filopodia play an early role in synaptogenesis [34], [13], [24] and [8] the observation of new filopodia formation and cytoskeletal rearrangement in response to acute HSV-1 infection could be linked with the cognitive deficits found in herpes-mediated encephalitis patients [16] and [27]. Glial cytoskeletal reorganization may similarly have some bearing on normal CNS function [2]. While it remains unclear whether these preliminary in vitro results can be extended to a physiological paradigm, our findings raise the intriguing possibility that acute HSV-1 infection may prompt some degree of synaptic dysfunction in the brain.
MATERIALS AND METHODS
Cell lines and reagents
Differentiated P19 cells were plated onto 35-mm glass-bottom dishes and allowed to recover for 2 days before live cell imaging. Cells were grown in alpha medium supplemented with 2.5% fetal calf serum and 7.5% calf serum (Sigma) and maintained at 37°C in a 5% CO2 environment. Nectin-1a antibody was obtained from Immunotech, while cytochalasin D and heparinase were both purchased from Sigma-Aldrich.
Viruses
The capsid-tagged GFP expressing recombinant HSV-1 (K26GFP-HSV-1) was used for all imaging work in this study. B-galactosidase expressing HSV-1 recombinant vectors were used during ONPG viral entry assays. Both viruses were grown in Vero cell flasks and purified by sucrose gradient as previously described [11].
Live cell imaging
Cells were seeded onto 35-mm glass bottom dishes (MatTek) for live cell imaging. After imaging the cells alone for several minutes, GFP-expressing HSV-1 was added to the dish. Signals were recorded on brightfield and FITC channels every 8 seconds on the 60X objective of a Nikon Eclipse TE2000-E microscope. All images and videos were processed using Metamorph (Molecular Devices) and Leica confocal software.
Immunocytochemistry
Differentiated P19 cells were plated at 50% confluency for 24 hours. GFP-tagged virus at 50 plaque forming units (PFU) per cell was added. Cells were fixed after 1 hour of infection. Actin staining was done using rhodamine conjugated phalloidin (Invitrogen). Images were collected using a Leica SP-2 confocal microscope at the 40X objective.
O-nitrophenyl-β-d-galactopyranoside (ONPG) viral entry assay
Cell flasks were grown to 100% confluency and then split via EDTA and trypsin as described above into 96 well plates. Cytochalasin D was added to the well plates by serial dilution and then incubated for 2 hours. Well plates were then rinsed with fresh media and infected with B-galactosidase expressing HSV-1 recombinant virus for 6 additional hours as described previously [9].
F-actin fluorescence expression
F-actin expression was measured in chamber slides with constant (~50,000) cell populations and serial dilutions of a 100 PFU viral dose. After allowing incubation with the virus for 6 hours, cells were permeabilized, fixed and then treated with rhodamine-conjugated phalloidin (Invitrogen) at 2 nM for 45 minutes at room temperature. After washing, cells were mounted using Vectashield (Vector Laboratories, Inc.) and assessed for F-actin expression using a Teccan GENios Pro plate-reader.
RESULTS
Initial immunocytochemistry revealed that green fluorescent protein (GFP)-expressing HSV-1 virions were often attached to long, actin-rich dendritic networks and the localization could be observed almost immediately after addition of virus particles to cells (Fig 1). The fact that virions were localized on filopodia implied that viral surfing (itself an early event) may precede entry through the cell membrane. Live cell imaging of P19 neurons confirmed that HSV-1 virions tagged with GFP could surf along previously established dendrites to effectively reach the cell body (Fig 2, Supplemental video #1). Surfing usually began immediately after viral binding, although occasionally bound particles remained immobile for the duration of the live recording (~3 hours). Although viral movements were generally saltatory, average speeds were around 1.5 µm/min. The occasional inability of virus particles to surf may be related to their physiological condition, as UV-inactivated virus particles predominantly failed to surf (data not shown). The variable expression of viral receptors on dendritic filopodia could also be responsible for increased binding versus surfing activity, although this possibility was not examined in this study.
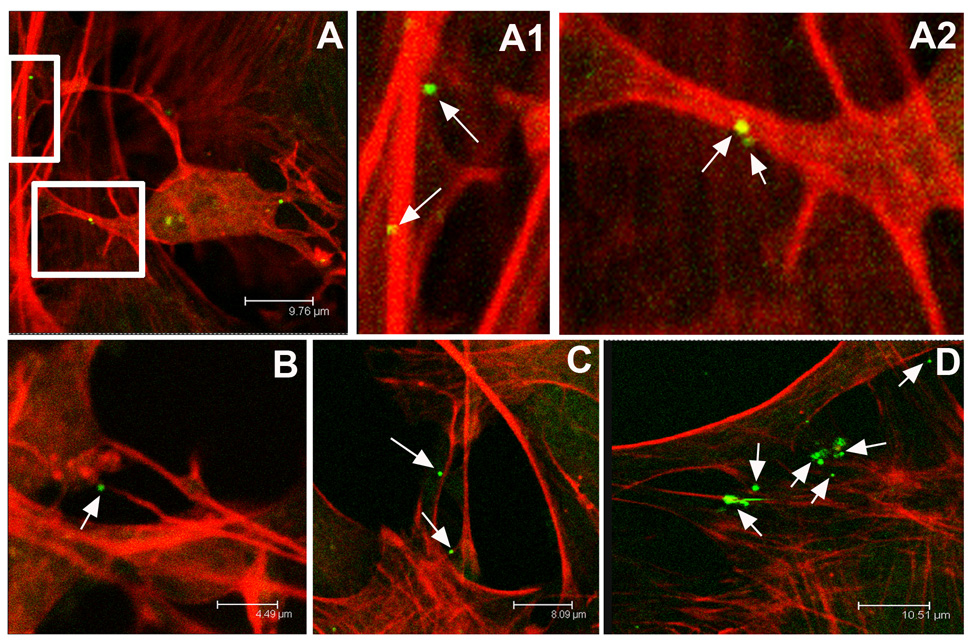
Figure 1.
Confocal microscope images of neurons infected with the neurotropic herpes simplex type 1 virus. Actin networks in P19 cell cultures are visualized in red through cell staining with rhodamine-conjugated phalloidin. Individual viral particles express green fluorescent protein. The virus preferentially associates with long, actin-rich dendritic processes. A, An infected P19 neuron and A1, A higher magnification of a dendrite revealing two green virions attached. A2, A higher magnification of the neuron pictured in A again showing virus attached to red actin-rich dendrites. B, Virus attached to an actin-rich process. C, Multiple HSV-1 virions adjacent to dendritic networks in P19 culture. D, The virus continues to appear bound to actin-rich processes even within complex dendritic networks.
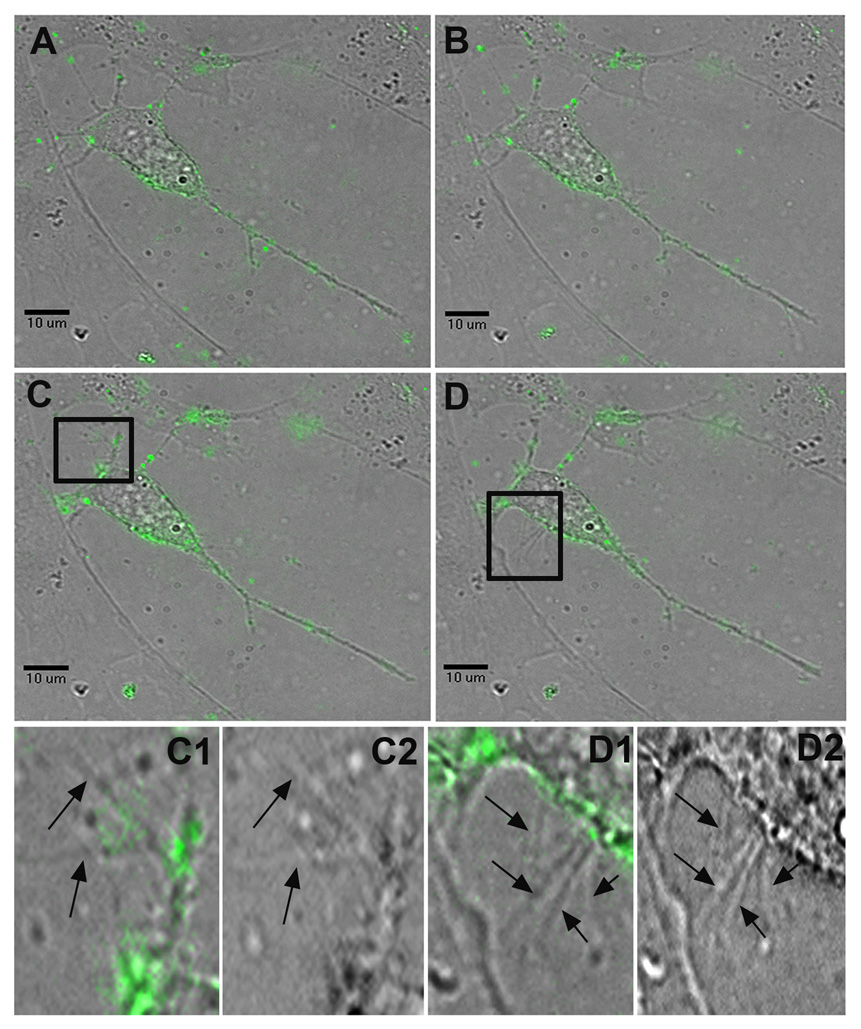
Figure 2.
Surfing of HSV-1 and the induction of dendritic filopodia formation. Still frames taken from a live-cell recording of multiple HSV-1 virions surfing on dendrites towards the soma of a P19 neuron (Supplementary video 1). A, At 5 minutes post-infection, the virus particles have begun to attach to the neuron and its dendrites. B, At 16 minutes post-infection, multiple virions begin surfing along dendrites towards the neuron’s cell body. C, At 25 minutes post-infection, viral particles continue to surf towards the cell body, while significant new dendritic filopodial growth emerges from a previously stable dendrite highlighted in C1, a FITC and brightfield composite image, and C2, a brightfield image alone. D, At 32 minutes post-infection, many viral particles originally found on the neuron’s dendrites have now reached the soma. Another burst of new filopodia appears near the axon hillock and the region highlighted in D1, a FITC and brightfield composite image, and D2, a brightfield image alone.
Whereas viral surfing on dendrites was observed within five minutes of exogenous HSV-1 application, filopodia induction was slower and usually occurred within 15 to 30 minutes post-infection. This was often dramatic, increasing the length of filopodia by up to 250% (Supplemental video 2). New filopodia were observed on established dendritic shafts, the axon hillock and the cell body itself. A peculiar writhing motion of stable processes in the region of interest tended to immediately precede the “bursting” of new filopodia (Fig 2, Supplemental video 1). In the case of previously established filopodia, a similar motion occurred before the subsequent increase in filopodia length. Presumably this represents a visual demonstration of live cell actin polymerization.
Glial cells in the P19 culture also responded strongly to HSV-1 application, exhibiting marked cytoskeletal movement and filopodia induction (Fig 3, Supplemental video 3). Similar to neuronal P19 cells, virus surfing along established filopodia eventually led to cell infection, inducing new filopodia that could in turn be exploited by other HSV-1 virions surfing towards the cell.
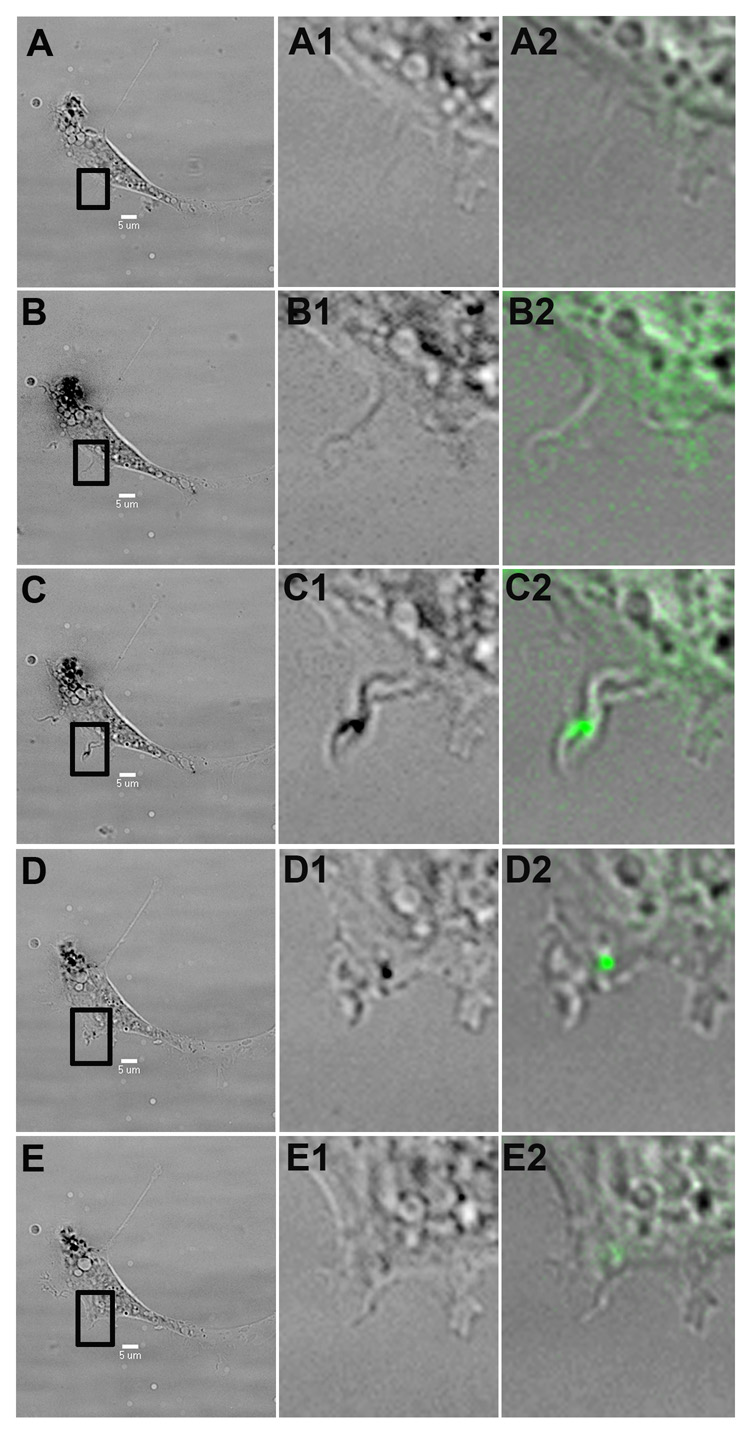
Figure 3.
Virus intake via surfing. A P19 cell exposed to HSV-1 begins to express filopodia, which are then used by HSV-1 virions (green) to surf into the cell. The first column shows a brightfield, low magnification cell image, while the second and third column represent closeups of the highlighted region in brightfield and FITC-brightfield composite, respectively. A, At 17 minutes post-infection, no filopodia are observed in the region of interest. B, At 20 minutes post-infection, a new filopodia emerges from the cell. C, At 22 minutes post-infection, a viral particle binds to the new filopodia. D, At 34 minutes, the virus is using the filopodia to surf towards the cell body. E, At 42 minutes post-infection, the virus appears to have reached the cell by surfing along the newly emerged filopodia.
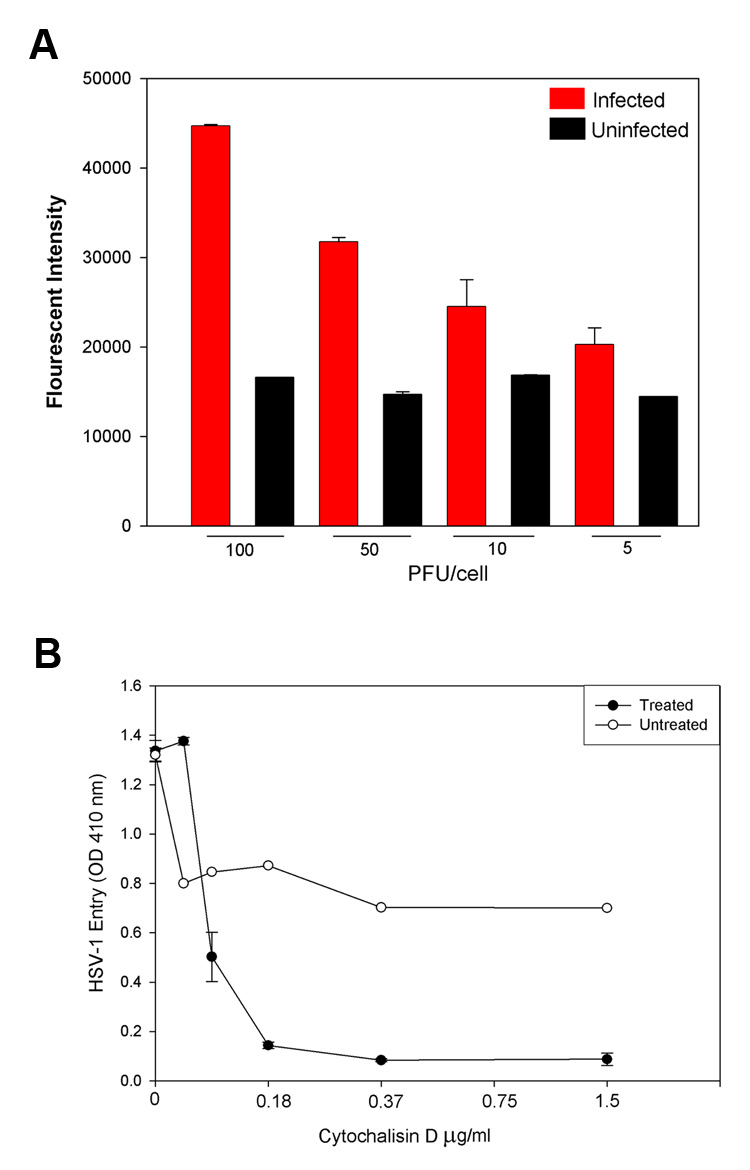
Increasing the viral dose caused an increase in the cell’s F-actin fluorescence, again implying filopodia formation and cytoskeletal reorganization of the cellular cortex in response to HSV-1 viral infection (Fig 4A). The central role of actin dynamics in viral spread was corroborated by treating cells with the actin polymerization inhibitor cytochalasin D, which completely inhibited filopodia induction and viral surfing at doses beyond 0.37 µM/mL (Supplemental video 4). This is in line with numerous previous studies delineating the relationship between the cytochalasins and actin structural motility and integrity (see review by Cooper, 1987). Cytochalasin D application also greatly reduced viral entry into cells (Fig 4B). Presumably virions moved on filopodia via retrograde F-actin flow, although closer examination sometimes revealed that viral particles could rapidly change direction (up to twice every minute) after binding to these long processes (Supplemental video 5). This was much more likely to occur in processes further from the cell body, where retrograde actin flow may be weaker than near the soma.
Figure 4.
Actin dynamics and HSV-1 viral spread. A, The F-actin fluorescence increases dramatically as a function of viral dose relative to uninfected controls. Viral dose was estimated in plaque-forming units (PFU) per P19 cell. A higher expression of F-actin implies filopodia formation and actin cortex reorganization in response to increasing HSV-1 concentrations. B, The actin polymerization inhibitor cytochalasin D greatly reduces viral entry, implicating actin dynamics in viral infection strategies in P19 neural cell cultures. Cytochalasin D prevented both surfing behavior and filopodia formation after HSV-1 application.
DISCUSSION
HSV-1 is a neurotropic virus that establishes life-long latency in the human nervous system. Given HSV-1’s potential as a viral vector for gene therapy in the CNS [4] it is important that the mechanisms involved in viral spread are clearly delineated before attempting large-scale therapeutic interventions. Efforts to use HSV-1 to trace multisynaptic pathways in vivo also rely heavily on our implicit understanding of viral movement strategies. Previous studies with the herpes virus family have focused on pathway tracing and internal axonal transport of viruses in the nervous system [32], [7], [5], [17], and [26]. Our live cell imaging of GFP-tagged HSV-1, however, has captured, for the first time, viral “surfing” behavior along the outer membrane of dendrites and dendritic filopodia. Viral infection also caused extensive cytoskeletal changes which may have some bearing on normal synaptogenesis in vivo. In addition, actin cytoskeletal hijacking may be a common viral invasion strategy [18].
Real-time viral surfing in dendritic networks was captured using GFP-expressing recombinant HSV-1. This behavior is apparently many times more efficient than simple diffusion while viruses are spreading in vivo. It seems that long dendritic connections between cells may act as “roadways” to guide viral movement efficiently towards other cell bodies. These results mirror recent findings that retroviruses can surf along filopodia from some cell types using retrograde F-actin flow [19] and [28]. In the context of a neurotropic virus like HSV-1, it appears that a similar mechanism has evolved to utilize actin-rich neuronal processes.
Filopodia in neurons and other cell types have been extensively associated with retrograde F-actin flow [21] and [20]. HSV-1 seemingly exploits this retrograde flow in existing actin-rich dendrites. Extensive filopodia formation was observed in neuronal and glial-like cells beyond 15 minutes post-infection. These were often dramatic cytoskeletal rearrangements creating numerous new dendritic filopodia and inducing some neurite motility. New filopodia extended from the axon hillock, dendritic shafts and the soma itself. Just prior to the emergence of new filopodial extensions, previously formed processes in the same region exhibited a marked writhing-like motion. These characteristic movements appeared to be related to the subsequent bursting of new filopodia from these locations, and may speak to the actin polymerization mechanism occurring, presumably, at the leading edge of filopodia and along dendritic shafts [1]. Alternatively, it may represent a reorganization of the dendritic network itself in order to facilitate new filopodia emergence [30].
The consequences of filopodia formation and elongation in glial P19 cells were not immediately clear. However, glial responses to HSV-1 infection may affect normal axonal myelination in vivo [2], and hence negatively influence action potential propagation in chronically infected neural systems. This could help explain previous reports of demyelination of CNS axons after herpes infection [31].
Dendritic filopodia are extensively associated with synaptogenesis
Dendritic filopodia on stable dendritic shafts dynamically extend and retract before finding an appropriate contact site and transforming into dendritic spines [10] and [13]. Likewise, the formation of new synapses, especially in young cultures, is absolutely dependent on F-actin-enriched neuronal processes like dendritic filopodia [33]. These and other reports highlight the central importance of dendritic filopodia in sensing and forming new synaptic connections [24] and [8]. It seems possible that extensive filopodia induction by HSV-1 infection may disrupt the normal function of some of these processes, especially in brain structures that undergo high rates of synaptogenesis. Given the critical parts played by dendritic filopodia in finding and establishing synaptic contacts, HSV-1’s effects on the neuronal cytoskeleton may lead to functional deficits in chronically and/or acutely infected patients.
Acute HSV-1 infection can cause altered EEG patterns and long-term cognitive deficits
It is unclear why HSV-1 mediated encephalitis results in such severe cognitive deficits relative to other viral vectors. A previous study has found serologic evidence of infection with HSV-1 to be an independent predictor of cognitive dysfunction in schizophrenics [12]. Ex vivo investigations of mouse brain slices reveal infection limited primarily to leptomeningeal, periventricular and cortical brain regions and the hippocampus. Increasing viral inoculum tended to increase intensity in those regions while maintaining the same distinctive pattern of infection, one which differed from patterns typical of adenovirus and Vaccinia virus [6]. Magnetic resonance imaging studies of in vivo infection also showed hippocampal, parahippocampal, amygdala, insula and temporal lobe gyri damage [16]. These brain structures overlap heavily with those associated with memory and cognition and may be linked to long term symptoms like memory impairment, personality and behavioral abnormalities, epilepsy, anosmia and dysphasia reported in herpes encephalitis patients [23] and [27].
Previous clinical reports have documented altered electroencephalograms in acute stage encephalitis patients. These EEGs exhibited stereotyped, bilateral sharp-and-slow-wave complexes with transient episodes of electrographic seizure activity that temporarily suppressed periodic activity in that hemisphere [29]. The EEG changes were not considered pathognomic but could be used to distinguish herpes simplex-mediated encephalitis from inflammation induced by other viral vectors. It is currently unclear what the neural basis for these changes are in vivo.
Conclusion
This study provides evidence for actin-mediated “surfing” of the neurotropic HSV-1 virus. Viral surfing was observed on both glial filopodia and neuronal dendritic filopodia in differentiated P19 cell cultures. Our work is likely to extend the herpesvirus field by adding new information on how the filopodia can be induced and exploited during the initial stages of infection.
HSV-1 exposure increased filopodia expression in cultured neurons. Given that dendritic filopodia have been implicated as the dynamic precursors to stable dendritic spines and functional synapses, HSV-1 mediated cytoskeletal rearrangements raise the fascinating possibility that cognitive deficits observed in patients are linked to synaptic dysfunction in the brain. These preliminary observations provide the first in vitro morphological context for cognitive deficits in herpes-mediated encephalitis patients. Further study should determine whether these results occur in various other cell types.
Ultimately, there is a need to determine whether cellular responses to acute HSV-1 infection are also seen in living brains. Whether there is any effect on normal synaptogenesis and stable synaptic connections in the central nervous system also remains to be established. If the cause of altered filopodia dynamics in HSV-1 infected neural cells lies in cellular responses to viral envelope glycoproteins rather than viral DNA, this study could have implications for the use of the HSV-1 virion as a gene therapy vector.
Supplementary Material
The same neuron depicted in Fig. 2 between t=5 and t=35 minutes post-infection. GFP-tagged HSV-1 virions are observed surfing down dendrites towards the soma, often in a saltatory manner. Significant cytoskeletal changes are elicited from the infected neuron, including a dramatic increase in dendritic filopodia and movement in previously stable dendrites. Dendritic filopodia are seen bursting from the axon hillock, the soma and the dendrites themselves while HSV-1 surfs via retrograde F-actin flow towards the cell body.
Viral infection induces dramatic filopodia formation after 15 minutes post-infection.
The same P19 cell in Fig. 3 between t=17 and t=42 minutes post-infection. The P19 cell forms a new filopodial protrusion, to which a virus binds and subsequently surfs into the cell body.
Application of the actin polymerization-inhibitor cytochalasin D completely inhibits viral surfing and filopodia induction, but not viral binding.
Occasionally viral particles that attached to dendrites far removed from the soma could reverse directions while surfing. This viral behavior occurred up to twice per minute.
Acknowledgements
This investigation was supported by NIH grant (AI057860) and a Research to Prevent Blindness Career Award to D.S. VT was supported by American Heart Association Postdoctoral Fellowship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Abraham VC, Krishnamurthi V, Taylor DL, Lanni F. The actin-based nanomachine at the leading edge of migrating cells. Biophysical Journal. 1999;77:1721–1732. doi: 10.1016/S0006-3495(99)77018-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bacon C, Lakics V, Machesky L, Rumsby M. N-WASP regulates extension of filopodia and processes by oligodendrocyte progenitors, oligodendrocytes, and Schwann cells - implications for axon ensheathment at myelination. Glia. 2007;55:844–858. doi: 10.1002/glia.20505. [DOI] [PubMed] [Google Scholar]
- 3.Bentley D, Toroian-Raymond A. Disoriented pathfinding by pioneer neurone growth cones deprived of filopodia by cytochalasin treatment. Nature. 1996;323:712–715. doi: 10.1038/323712a0. [DOI] [PubMed] [Google Scholar]
- 4.Berges BK, Wolfe JH, Fraser NW. Transduction of brain by herpes simplex virus vectors. Molecular Therapy. 2007;15:20–29. doi: 10.1038/sj.mt.6300018. [DOI] [PubMed] [Google Scholar]
- 5.Billig I, Foris JM, Enquist LW, Card JP, Yates BJ. Definition of neuronal circuitry controlling activity of phrenic and abdominal motoneurons in the ferret using recombinant strains of pseudorabies virus. J. Neurosci. 2000;20:7446–7454. doi: 10.1523/JNEUROSCI.20-19-07446.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Braun E, Zimmerman T, Hur TB, Reinhartz E, Fellig Y, Panet A, Steiner I. Neurotropism of herpes simplex virus type 1 in brain organ cultures. J Gen Virol. 2006;87:2827–2837. doi: 10.1099/vir.0.81850-0. [DOI] [PubMed] [Google Scholar]
- 7.Card P. Exploring brain circuitry with neurotropic viruses: New horizons in neuroanatomy. The Anatomical Record. 1998;253:176–185. doi: 10.1002/(SICI)1097-0185(199812)253:6<176::AID-AR6>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 8.Chien CB, Rosenthal DE, Harris WA, Holt CE. Navigational errors made by growth cones without filopodia in the embryonic Xenopus brain. Neuron. 1993;11:237–251. doi: 10.1016/0896-6273(93)90181-p. [DOI] [PubMed] [Google Scholar]
- 9.Clement C, Tiwari V, Scanlan PM, Valyi-Nagy T, Yeu BY, Shukla D. Novel role for phagocytosis-like uptake in herpes simplex virus entry. Journal of Cell Biology. 2006;174:1009–1021. doi: 10.1083/jcb.200509155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dailey ME, Smith SJ. The Dynamics of Dendritic Structure in Developing Hippocampal Slices. J. Neurosci. 1999;16:2983–2994. doi: 10.1523/JNEUROSCI.16-09-02983.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Desai P, Person S. Incorporation of the green flouroscent protein into the herpes simplex virus type 1 capsid. Journal of Virology. 1998;72:7563–7568. doi: 10.1128/jvi.72.9.7563-7568.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dickerson Fb, Boronow JJ, Cstallings C, Origoni AE, Ruslanova I, Yolken RH. Association of serum antibodies to herpes simplex virus 1 with cognitive deficits in individuals with schizophrenia. Arch Gen Psychiatry. 2003;60:466–472. doi: 10.1001/archpsyc.60.5.466. [DOI] [PubMed] [Google Scholar]
- 13.Fiala JC, Feinberg M, Popov V, Harris KM. Synaptogenesis via dendritic filopodia in developing area CA1. J. Neurosci. 1998;18:8900–8911. doi: 10.1523/JNEUROSCI.18-21-08900.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Finley MFA, Kularni N, Huettner JE. Synapse formation and establishment of neuronal polarity in embryonic carcinoma cells and embryonic stem cells by P19. J. Neurosci. 1996;16:1056–1065. doi: 10.1523/JNEUROSCI.16-03-01056.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jones-Villeneuve EMV, McBurney MW, Rogers KA, Kalnins VI. Retinoic acid induces embryonal carcinoma cells to differentiate into neurons and glial cells. Journal of Cell Biology. 1982;94:253–262. doi: 10.1083/jcb.94.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kapur N, Barker S, Burrows EH, Ellison D, Brice J, Illis LS, Scholey K, Colbourn C, Wilson B, Loates M. Herpes simplex encephalitis: long term magnetic resonance imaging and neuropsychological profile. Journal of Neurology, Neurosurgery, and Psychiatry. 1994;57:1334–1342. doi: 10.1136/jnnp.57.11.1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kelly RM, Strick PL. Cerebellar Loops with Motor Cortex and Prefrontal Cortex of a Nonhuman Primate. J. Neurosci. 2003;23:8432–8444. doi: 10.1523/JNEUROSCI.23-23-08432.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kolesnikova L, Bohil AB, Cheney RE, Becker S. Budding of Marburgvirus is associated with filopodia. Cellular Microbiology. 2007;9:939–951. doi: 10.1111/j.1462-5822.2006.00842.x. [DOI] [PubMed] [Google Scholar]
- 19.Lehmann MJ, Sherer NM, Marks CB, Pypaert M, Mothes W. Actin and myosin driven movement of viruses along filopodia precedes their entry into cells. Journal of Cell Biology. 2005;170:317–325. doi: 10.1083/jcb.200503059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luo L. Actin cytoskeletal regulation in neuronal morphogenesis and structural plasticity. Annu. Rev. Cell Dev. Biol. 2002;18:601–635. doi: 10.1146/annurev.cellbio.18.031802.150501. [DOI] [PubMed] [Google Scholar]
- 21.Mallavarapu A, Mitchison T. Regulated actin cytoskeleton assembly at filopodium tips controls their extension and retraction. Journal of Cell Biology. 1999;146:1097–1106. doi: 10.1083/jcb.146.5.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McBurney MW. P19 embryonal carcinoma cells. Int. J. Dev. Bio. 1993;37:135–140. [PubMed] [Google Scholar]
- 23.McGrath N, Anderson NE, Croxson MC, Powell KF. Herpes simplex encephalitis treated with acyclovir: diagnosis and long term outcome. J Neurol Neurosurg Psychiatry. 1997;63:321–326. doi: 10.1136/jnnp.63.3.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O’Connor TP, Duerr JS, Bentley D. Pioneer growth cone steering decisions mediated by single filopodial contacts in situ. J. Neurosci. 1990;10:3935–3946. doi: 10.1523/JNEUROSCI.10-12-03935.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Okabe S, Hirokawa N. Actin dynamics in growth cones. J. Neurosci. 1991;11:1918–1929. doi: 10.1523/JNEUROSCI.11-07-01918.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rinaman L, Schwartz G. Anterograde transneuronal viral tracing of central viscerosensory pathways in rats. J. Neurosci. 2004;24:2782–2786. doi: 10.1523/JNEUROSCI.5329-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sartori G, Job R, Miozzo M, Zago S, Marchiori G. Category-specific formknowledge deficit in a patient with herpes simplex virus encephalitis. Journal of Clinical and Experimental Neuropsychology. 1993;15:280–299. doi: 10.1080/01688639308402563. [DOI] [PubMed] [Google Scholar]
- 28.Sherer NM, Lehmann MJ, Jimenez-Soto LF, Horensavitz C, Pypaert M, Mothes W. Retroviruses can establish filopodial bridges for efficient cell-to-cell transmission. Nature Cell Biology. 2007;9:310–315. doi: 10.1038/ncb1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith JB, Westmoreland BF, Reagan TJ, Sandok BA. A distinctive clinical EEG profile in herpes simplex encephalitis. Mayo Clin Proc. 1975;50:469–474. [PubMed] [Google Scholar]
- 30.Svitkina TM, Bulanova EA, Chaga OY, Vignjevic DM, Kojima S, Vasiliev JM, Borisy GG. Mechanism of filopodia initiation by reorganization of a dendritic network. J. Cell. Biol. 2003;160:409–421. doi: 10.1083/jcb.200210174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Turner SL, Jenkin FJ. The role of herpes simplex virus in neuroscience. Journal of Neurovirology. 1997;3:110–125. doi: 10.3109/13550289709015801. [DOI] [PubMed] [Google Scholar]
- 32.Zemanick MC, Strick PL, Dix RD. Direction of transneuronal transport of herpes simplex virus 1 in the primate motor system is strain-dependent. Proceedings of the National Academy of Sciences. 1991;88:8048–8051. doi: 10.1073/pnas.88.18.8048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang W, Benson DL. Stages of synapse development defined by dependence on Factin. J. Neurosci. 2001;15:5169–5181. doi: 10.1523/JNEUROSCI.21-14-05169.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ziv NE, Smith SJ. Evidence for a role of dendritic filopodia in synaptogenesis and spine formation. Neuron. 1996;17:91–102. doi: 10.1016/s0896-6273(00)80283-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The same neuron depicted in Fig. 2 between t=5 and t=35 minutes post-infection. GFP-tagged HSV-1 virions are observed surfing down dendrites towards the soma, often in a saltatory manner. Significant cytoskeletal changes are elicited from the infected neuron, including a dramatic increase in dendritic filopodia and movement in previously stable dendrites. Dendritic filopodia are seen bursting from the axon hillock, the soma and the dendrites themselves while HSV-1 surfs via retrograde F-actin flow towards the cell body.
Viral infection induces dramatic filopodia formation after 15 minutes post-infection.
The same P19 cell in Fig. 3 between t=17 and t=42 minutes post-infection. The P19 cell forms a new filopodial protrusion, to which a virus binds and subsequently surfs into the cell body.
Application of the actin polymerization-inhibitor cytochalasin D completely inhibits viral surfing and filopodia induction, but not viral binding.
Occasionally viral particles that attached to dendrites far removed from the soma could reverse directions while surfing. This viral behavior occurred up to twice per minute.